Thermal and physicochemical properties of red tilapia (Oreochromis niloticus) surimi gel as affected by microbial transglutaminase
Fariba Zad Bagher Seighalani A , Jamilah Bakar A D , Nazamid Saari B and Ali Khoddami CA Department of Food Technology, Faculty of Food Science and Technology, Universiti Putra Malaysia (UPM), Serdang, 43400, Selangor, Malaysia.
B Department of Food Science, Faculty of Food Science and Technology, Universiti Putra Malaysia (UPM), Serdang, 43400, Selangor, Malaysia.
C Department of Plant and Food Sciences, Faculty of Agriculture and Environment, University of Sydney, NSW 2006, Australia.
D Corresponding author. Email: jamilah@upm.edu.my
Animal Production Science 57(5) 993-1000 https://doi.org/10.1071/AN15633
Submitted: 19 September 2015 Accepted: 19 February 2016 Published: 2 June 2016
Journal Compilation © CSIRO Publishing 2017 Open Access CC BY-NC-ND
Abstract
Thermal and physicochemical properties of red tilapia (Oreochromis niloticus) surimi gel incorporated with different levels of microbial transglutaminase (MTGase) were investigated. Surimi samples mixed with various concentrations of MTGase were subjected to two-stages heating (at 45°C followed by 90°C) to prepare surimi gel. Samples formulated with 0.30 MTGase (units/g surimi) showed the highest breaking force and deformation, and lowest expressible water content among treatments. Highest storage modulus was found in the gels mixed with 0.30 MTGase (units/g surimi). Compared with control surimi gel, addition of microbial transglutaminase to levels 0.10, 0.20 and 0.30 (units/g surimi) increased the enthalpy and maximum transition temperature of myosin. Results suggest that up to 0.30 MTGase (units/g surimi) could improve texture, colour, water-holding capacity, elasticity and thermal stability of red tilapia surimi gel.
Additional keywords: differential scanning calorimetry, expressible water, storage modulus, textural properties, whiteness.
Introduction
Surimi is a pure protein paste made of minced and washed fish meat stabilised by cryoprotectants (Park and Lin 2005). Surimi products were first produced in Japan, but they are now known in many parts of the world through products such as fish balls, fish cake and crab meat analogues. Gel properties of surimi such as texture and gel-forming (gelation) ability are the major factors determining quality of surimi and related products (Yin and Park 2014). Gelation of fish proteins is the most important step in forming desired textures in surimi-based products. Depending on fish species, type of applied additives and preparation method, surimi gels with different properties and stabilities can be formed (Yongsawatdigul et al. 2002; Benjakul and Visessanguan 2003; Ramírez et al. 2007; Kaewudom et al. 2012).
Gelation process is associated with cross-linking of myosin heavy chains (MHC), induced by endogenous transglutaminase (TGase). Endogenous TGase is an enzyme catalysing covalent bonding between the ε-amino group of lysyl residues and the γ-carboxamide group of glutaminyl residues of adjacent protein molecules (Yongsawatdigul et al. 2002). Reactions catalysed by TGase result in significant changes in the physicochemical properties of gel such as changes in viscosity, thermal stability and elasticity (Kuraishi et al. 1997; Yokoyama et al. 2004). Endogenous TGase is a water-soluble enzyme and can be partially removed during washing step in surimi preparation process. Yongsawatdigul et al. (2002) investigated the residual TGase activity in threadfin bream surimi wash water, confirming that ~44% of original TGase activity remained in the final surimi after the washing process.
Thermal and physicochemical properties of surimi gel can be improved by the addition of food-grade ingredients and additives such as egg white (Yetim and Ockerman 1995), casein and beef plasma-thrombin (Baker et al. 2000) and whey protein concentrate (Rawdkuen et al. 2008) during surimi and surimi gel preparation. Dondero et al. (2006) reported that addition of 0.10% to 0.50% (surimi weight basis) of microbial TGase (MTGase) improved the textural quality of surimi gels produced from Chilean jack mackerel (Trachurus murphyi) by enhancement of ε-(γ-glutamyl) lysine bonds production.
MTGase has been widely used to improve protein gel texture including surimi gel (Cardoso et al. 2010; Hemung and Chin 2013). MTGase is a calcium independent enzyme that can be derived from microorganisms such as Streotoverticillium mobaraense and Streptomyces lydicus and can be used as a food-grade additive. Data from several sources indicate that the efficiency of MTGase-induced improvement in the properties of gel depends on fish species and the amount of MTGase added. A research done on surimi from lizardfish (Suarida tumbil) incorporated with MTGase revealed that the addition of MTGase (0.40 units/g surimi) was able to produce stronger gel (Chanarat et al. 2012), whereas the optimal amount of MTGase was 0.30 units/g for threadfin-bream (Nemipterus bleekeri) surimi, and 0.20 units/g surimi for pollack (Theragre chalcogramma) (Jiang et al. 2000). Red tilapia is one of the main economic freshwater fish species in Malaysia, but consumption of red tilapia is limited due to its muddy and fishy odour and its large number of intramuscular small bones (Rawdkuen et al. 2009). However, surimi and surimi products is a plausible choice for processing red tilapia meat. In the case of red tilapia surimi, there is insufficient information about the impact of different concentrations of MTGase on the surimi gel properties. Hence, this study aimed to investigate the effect of different levels of MTGase and determine the appropriate concentration of MTGase for improving the quality of red tilapia surimi gel.
Material and methods
Raw material and additive
Red tilapia fish were purchased from the wholesale fish market, Selangor, Malaysia. The fish was kept on ice at a fish : ice ratio of 1 : 2 (w/w) and transported within 2 h to the Fish Laboratory of the Department of Food Technology, Universiti Putra Malaysia. The fish was gutted, headed and washed to use for surimi and surimi gel preparation.
MTGase from Streptoverticillium mobaraense (TG-K) with activity of 100 units/g dry matter was supplied by Ajinomoto, Malaysia.
Surimi preparation
The surimi preparation procedure was performed according to the method of Benjakul et al. (2005). The washed and gutted fish was mechanically deboned using a deboning machine (Fish meat separator, Model FD 6, Kedah, Malaysia) to obtain minced fish meat. The mince was washed with cold water (5°C) in the holding tank at a mince: water ratio of 1 : 3 (w/v). The mixture was manually stirred for 5 min and the water was removed by tilting the tank and squeezing manually. The washing and dewatering process were repeated three times. After the third washing, the mince was filtered using a nylon screen. The mince was mixed with 4% sucrose and 4% sorbitol in a domestic blender (KitchenAid, Model 5K5SS, Greenville, OH, USA) for 5 min and then packed into a 1-kg polyethylene bag and kept at −18°C for further treatment and analysis.
Surimi gel processing and treatments
Frozen surimi was thawed with running water (25°C) until the temperature of the surimi block reached 5°C. After thawing, surimi was cut into small pieces (~1 cm cube), blended with 2.5% sodium chloride, and mixed with crushed ice to adjust surimi moisture content to 80% [monitored using moisture analyser (Precisa Gravimetrics AG, CH-8953, Dietikon, Switzerland]. MTGase at different levels (0.10, 0.20, 0.30, 0.40 and 0.50 units/g surimi) was added and the mixture was homogenised in a domestic blender (Pensonic, Model PB-326, Johor Bahru, Malaysia) at a speed of 7000g at 5°C for 5 min. Surimi with no additive was used as the control. The obtained paste (500 g) was placed in a cylindrical polyvinylidene chloride casing (3 cm diameter and 15 cm height) and sealed tightly at both ends. The surimi gel was prepared by using two-stage heat treatment in a water bath, first at 45°C for 30 min and then at 90°C for 20 min. The heated surimi gel was cooled at room temperature and kept for 24 h at 4°C before analysis.
Texture analyses
Determination of the breaking force (gel strength), and deformation (elasticity/deformability) of surimi gels were accomplished according to the method described by Zhou et al. (2006) using a texture analyser TA.XT2i (Stable Micro Systems, Surrey, UK). The cylinder-shaped samples were cut to a height of 2.5 cm and subjected to a double compression test by a spherical plunger (5 mm diameter) to 75% of the original height at a speed of 60 mm/min. The breaking force [the force (g) to puncture into the gel] and deformation [the distance (mm) at which the probe punctured into the gel] were measured and reported.
Expressible water content
The expressible water (EW) for each treatment was measured according to the method of Mao and Wu (2007). Approximately 3 ± 0.20 g of samples were weighed and put between two filter papers (Whatman No.1). Samples were placed at the bottom of 50-mL centrifuge tubes and centrifuged at 5000g for 20 min at 15°C. Immediately after centrifugation, the samples were weighted and the EW was calculated as follows:

where Wi was the initial weight of surimi gel and Wf was the final weight of surimi gel.
Whiteness
The reflected colour of the surimi gels from red tilapia surimi formulated with different levels of MTGase was determined using a Minolta Croma Meter Model CR-300 (Minolta Co., Tokyo, Japan). The colourimeter was standardised with a standard-white reflection plate using a standard 3–4 white tile (L* value of 96.35, a* value of 0.28 and b* value of 1.68). Before measuring, the surface of samples was lightly dried with paper towels. L* (lightness), a* (redness/greenness) and b* (yellowness/blueness) were measured in five replications for each sample and whiteness was calculated as described by Shie and Park (1999) as follows:

Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE)
Protein patterns of all treatments were analysed on SDS-PAGE according to the method of Laemmli (1970). Protein sample was prepared according to the method of Benjakul et al. (2006). To prepare the sample, 27 mL of 5% SDS solution was heated to 85°C for 1 h. The heated solutions were added to 3 g of the sample and homogenised for 2 min using a domestic homogeniser (Pensonic, Model PB-326) at a speed of 7000 rpm. The mixture was then incubated at 85°C for 1 h to dissolve proteins. The samples were centrifuged at 5000g at 5°C for 15 min to remove undissolved residues. Protein concentration in the supernatant was determined as per the method of Lowry et al. (1951). Samples (15 µg protein) were loaded on the polyacrylamide gel (12.50% separation gel and 4% stacking gel). Electrophoresis was carried out at 20-mA constant current in 1xTAE (TRIS-acetate-EDTA) buffer using a Mini Protein II unit (BioRad Laboratories, Berkeley, CA, USA). Subsequently, the polyacrylamide gels were stained with Coomasie brilliant blue R-250 overnight and then destained in a solution containing 15% methanol (v/v) and 10% (v/v) acetic acid. A protein standard (Bio-Rad Laboratories) ranged from 10 to 250 kDa was used as the marker.
Dynamic test
The storage (elastic) modulus (Gʹ) of the surimi from red tilapia mixed with different levels of MTGase in a course of heating from 20°C to 90°C were measured using a Thermo Electro Corporation Rheostress (Haake, RT 20, Rotovisco, Berlin, Germany) according to the method of Rawdkuen et al. (2008). The Rheostress was equipped with C35/2°Ti cone and plate geometry. An oscillation of 0.1 Hz with a resistance stress of 3 Pa was used for the testing. A plastic cover supplied by the manufacturer was used to prevent moisture evaporation during analysis.
Differential scanning calorimetry
Thermal properties of surimi gels were studied using a differential scanning calorimetry (DSC7, Perkin-Elmer, Norwalk, CT, USA) by monitoring of the maximum temperature of denaturation and enthalpy, according to the method of Karayannakidis et al. (2008) with slight modification. Samples (surimi paste mixed with different levels of MTGase) of 10 ± 0.01 mg were weighted and placed in a 50-µL Perkin-Elmer aluminium pans, then capped and sealed. The prepared sample was scanned from 27°C to 100°C at a heating rate of 5°C/min. An empty pan was used as a reference. Maximum temperature of denaturation and changes in enthalpy were measured from the trace.
Statistical design and analyses
Completely randomised design was employed for statistical design of this study. All data were analysed by one-way ANOVA using a Minitab software (Version 16.0 software, Minitab Inc., State College, PA, USA) to determine the effect of MTGase at different levels on the surimi gel. Tukey’s test was used to carry out the difference of means between pairs with 95% confidence interval.
Results and discussion
Textural properties
Breaking force and deformation of surimi gels containing various levels of MTGase are shown in Table 1. Different concentrations of MTGase generally had significant effect (P < 0.05) on breaking force and deformation. MTGase addition at the level of 0.1 and 0.2 units/g surimi did not significantly affect the breaking force and deformation in comparison with control gel. The breaking force and deformation significantly increased with the addition of MTGase at 0.3 units/g surimi and decreased significantly with the addition of more than 0.3 MTGase (units/g surimi). Among all samples mixed with MTGase, those containing 0.30 MTGase (units/g surimi) showed the highest breaking force (763.70 ± 10.76) and deformation (14.79 ± 0.40), whereas those added with 0.10 MTGase (units/g surimi) had the lowest breaking force (435.17 ± 2.60) and deformation (10.60 ± 0.08). Several reports have shown that the use of MTGase above an optimum concentration caused a detrimental effect on the textural properties of surimi gel (Lee et al. 1997; Ramírez et al. 2000). These results are in accord with those of obtained by Jiang et al. (2000) who reported that breaking force and deformation of surimi gels from threadfin-bream (Nemipterus virgatus) and pollock (Rastrelliger kanagurta) increased when MTGase increased up to a certain level (0.30 unit MTGase/g surimi) and further increase in MTGase decreased the gel strength of surimi gels. Dondero et al. (2006) reported maximum of 364% increase in gel strength in surimi made from jack mackerel (T. murphyi) when 0.20% TGase applied. The addition of MTGase at up to 2 g/kg to red tilapia surimi showed the highest breaking force, which was increased by 240% compared with surimi with no additive (Duangmal and Taluengphol 2010). MTGase induces the formation of non-disulfide covalent bonds (Tammatinna et al. 2007), which results in the formation of MHC cross-linking and subsequently, a strong gel (Chaijan and Panpipat 2010). Addition of TGase to a gelatin from megrim (Lepidorhombus boscii) skins beyond a certain concentration (0.30%) resulted in a decrease in the gel strength (Jongjareonrak et al. 2006). Decrease in breaking force and deformation of surimi gel with more than 0.30 MTGase (units/g surimi) might be due to excessive cross-linking, which lower the gel strength through impeding intermolecular aggregation that reduced the gel network formation (Jongjareonrak et al. 2006).

|
Expressible water content
Content of EW is associated with the water-holding capacity of the surimi gel. There is a close relationship between gel texture and water-holding capacity and subsequently gel textural properties of cooked gel (Lee 1984). Expressible moisture content of surimi gels from red tilapia treated with MTGase at different levels is shown in Table 2. EW value of control surimi gel was 11.70 ± 0.05% whereas the lowest (10.50 ± 0.02%) EW was found in surimi gels containing 0.30 MTGase (units/g surimi). Surimi gels showed a continuous decrease in EW content when the levels of MTGase increased up to 0.30 units/g surimi (P < 0.05). However, increase in EW content was noticeable when higher levels of MTGase were added (P < 0.05). A lower EW indicates a higher water-holding capacity of the gel matrix (Ramírez et al. 2000; Tammatinna et al. 2007). MTGase-induced protein cross-linking may result in more water being retained inside the gel matrix. Chaijan and Panpipat (2010) investigated the effect of TGase on gel-forming ability of mackerel (Rastrelliger branchysoma) protein and indicated that water-holding capacity of surimi gels increased when the concentration of TGase increased up to 0.25 (unit/g of surimi). The addition of MTGase to red tilapia surimi gel led to an increase in the observed EW compared with the control, especially with 3 g/kg MTGase additive (Duangmal and Taluengphol 2010). The stronger gel network was possibly linked with its capacity to hold water. However, TGase can also increase protein–protein interactions, leading to a decrease in protein–water interaction. Therefore, water-holding capacity of gels can decrease when high concentrations of MTGase are used (Kaewudom et al. 2013).

|
Whiteness
Colour is an important indicator in surimi gel quality and generally, high whiteness commands better value back as a result of higher demand. Different levels of MTGase had significant (P < 0.05) effect on red tilapia surimi gel whiteness compared with the control (Table 2). Whiteness of surimi gels increased significantly (P < 0.05) when MTGase concentration increased. However, there were slight differences in the whiteness of surimi gels mixed with 0.30–0.50 MTGase (units/g surimi). Surimi gels with 0.50 MTGase (units/g surimi) showed the highest value (80.20 ± 0.12) of whiteness compare to that of the control (76.53 ± 0.20).
This result was in accordance with Karayannakidis et al. (2008) who reported that the addition of MTGase had beneficial effect on whiteness of surimi gels from sardine (Sardinella gibbosa). Duangmal and Taluengphol (2010) also showed that MTGase at levels of 1, 2 and 3 g/kg increased whiteness of surimi. As transglutaminase catalyses the cross-linking reaction of myosin and leads to the formation of protein intra- and intermolecular covalent bonds (Lee 1984), it was concluded that differences in the whiteness value could correspond to the increased turbidity of gels as a result of TGase activity. Moreover, increase in whiteness could be caused by other compounds (lactose, maltodextrin) in commercial MTGase powder, which cause light scattering effect (Chanarat et al. 2012).
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis
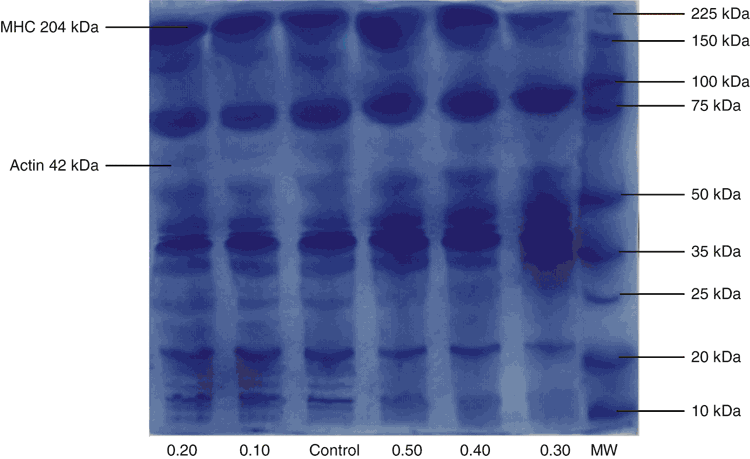
The protein profiles of surimi gels incorporated with MTGase at various concentrations are shown in Fig. 1. Surimi gel contained MHC and actin as the major proteins. Two bands ~43 kDa and 205 kDa range were those of actin and MHC, respectively (Thorarinsdottir et al. 2002).
|
The visual presence of MHC band intensity decreased as the amount of MTGase increased. The MHC distinct band nearly disappeared with 0.30 MTGase (units/g surimi). This might be associated with formation of non-disulfide covalent cross-linking, which is contributed partially to gel strength enhancement (Ko et al. 2007; Yin and Park 2014). These findings are in agreement with the findings of Dondero (2006), who reported that the content of MHC decreased and the cross-linked protein amount increased with increasing setting time and addition of TGase in Chilean jack mackerel (Trachurus murphyi) surimi. SDS-PAGE pattern from surimi gels of threadfin-bream (Nemipterus virgatus) and pollack (Rastrelliger kanagurta) surimi with TGase showed that inter- and/or intra-molecular cross-linking formed in MHC band cross-linked during heating (Benjakul et al. 2006; Chanarat et al. 2012). Moreover, study conducted by Duangmal and Taluengphol (2010) on surimi showed MTGase addition decreased the intensity of the MHC band significantly. The decrease in MHC band could be due to of protein bonds formation of the ε-(γ-glutamyl) lysineiso in cross-linked MHC in the presence of MTGase. Polymerised myosin could not go into the SDS gel, leading to the decline of the band intensity (Benjakul et al. 2002). There is no marked difference in actin band intensity of surimi gels formulated with different levels of MTGase. It can be suggested that actin was not a preferred substrate of TGase or actin affected gel functionality to a lesser extent (Yin and Park 2014).
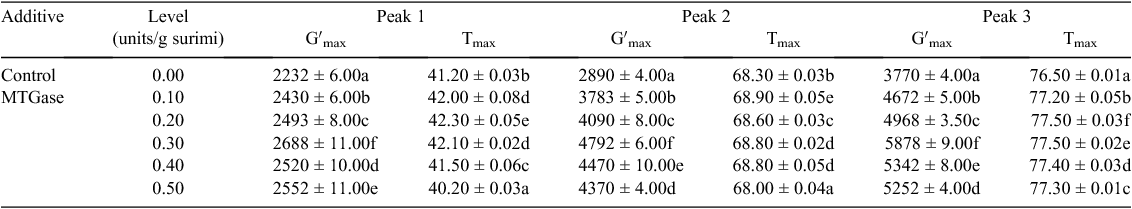
Storage modulus
Storage modulus (elastic modulus) has been used to study the heat-induced gelation of myofibrillar proteins. Changes in maximum storage modulus (Gʹmax) of red tilapia surimi gels affected by different concentrations of MTGase are shown in Table 3. Three obvious peaks of storage modulus were observed during heating. The first peak of Gʹ at ~40°C may have resulted from the tail portion of the myosin molecule via hydrophobic interactions, bringing the cross-linking between the protein molecules. This is believed to be associated with the high-temperature setting called suwari phenomenon (Chen et al. 2005).
The second peak was observed ~68°C. This step was referred as gel strengthening and was accompanied by an increase in the number of cross-links between protein aggregates and a deposition of additional denatured proteins in the existing protein network to strengthen the gel matrix (Chen et al. 2005). Xiong (1997) also reported that the increase in Gʹ up to 70°C indicates the formation of a highly elastic myofibrillar protein gel in modori stage. By increasing the temperature over 76°C (third peak), transformation of the samples to final stage called kamaboko phenomenon happened which was associated with a sharp increase in Gʹ values (Benjakul et al. 2002).
Values of Gʹ of the peaks (Gʹmax) were significantly (P < 0.05) different in all samples. The highest Gʹ for the three peaks was observed in the surimi gels mixed with 0.30 MTGase (units/g surimi), which were 2688 ± 11.00 Pa at 42.10°C, 4792 ± 6.00 at 68.80°C and 5878 ± 9.00 Pa at 77.50°C. Greater Gʹmax values indicated higher elasticity of formulated surimi gels. This behaviour could be due to slower protein aggregation than denaturation, resulting in higher elasticity in gels during heating (Xiong 1997). Higher Gʹmax could also be due to more cross-linking of proteins, which also increases the amount of energy stored in the gel matrix (Kyaw et al. 2001).
Decrease in maximum storage modulus in surimi gels with higher levels MTGase (0.40 and 0.50 units/g surimi) might be due to the increase in formation of transglutaminase-mediated covalent bonds, thus delaying the intermolecular aggregation leading to reduced elasticity of the gel (Yongsawatdigul and Piyadhammaviboon 2005).
Differential scanning calorimetry
Enthalpy and maximum temperature of denaturation are two main factors in determination of thermal properties of fish and fish products, which can be measured by DSC. Maximum temperature of denaturation (Tm) is protein’s heat uptake that is required to denature protein structure (Norziah et al. 2009). The enthalpy value is evaluated from the space under the transition peak, which is associated with the secondary structure of protein (Spink 2008). Maximum temperature of denaturation and enthalpy of peaks from the control and samples mixed with MTGase at various levels were recorded by DSC and shown in Table 4. Thermograms of all samples showed two endothermic peaks (Fig. 2), which are related to the thermal denaturation of myosin (P1) and actin (P2).

|
Addition of MTGase had significant effect (P < 0.05) on ΔH and Tm of the myosin and ΔH of actin. Maximum temperature of denaturation for myosin significantly (P < 0.05) increased from 43.30 ± 0.20°C to 45.00 ± 0.10°C when the MTGase concentration was increased from 0.10 to 0.30 (units MTGase/g). The Tm of the actin for surimi gel treated with 0.30 MTGase (units/g surimi) was 75.00 ± 0.10°C, which was significantly higher than that of control surimi gel (74.00 ± 0.10°C), although there were no significant differences (P > 0.05) in Tm of other formulated surimi gels.
There is no significant difference between the enthalpy of myosin in gels with 0.40 and 0.50 MTGase (P > 0.05). A significant increase (from 1.54 ± 0.60 to 1.92 ± 0.08) in ΔH of actin was observed when the MTGase concentration increased from 0.10 to 0.30. This indicated that the higher MTGase concentration produced more protein denaturation and thus better texture characteristics (Park 2005). Obtained results are in agreement with the findings obtained from breaking force and deformation measurements.
Conclusion
This study indicated that MTGase addition up to 0.3 units/g surimi can be used to improve red tilapia surimi gel quality. Deformation, breaking force and whiteness increased in surimi gels incorporated with MTGase at 0.1 and 0.2 and 0.3 (units/g surimi), whereas EW and intensity of MHC decreased compared with the control. Storage modulus correlated with the texture and showed an increase in gel elasticity, indicating enhanced thermal gelation of red tilapia surimi. Result of DSC confirmed higher stability of the protein after MTGase treatment. However, protein stability slightly decreased in gels with more than 0.30 MTGase (units/g surimi). The present study contributes additional evidence that suggests the addition of 0.30 MTGase (units/g surimi) to red tilapia surimi gels improved surimi gel quality, whereas exceeding this optimal level did not provide further improvements.
Acknowledgement
The authors acknowledge the financial support for the project by the Department of Fishery, Malaysian Ministry of Agriculture.
References
Baker KH, Lanier TC, Green DP (2000) Cold restructuring of seafood using transglutaminase mediated binding. In ‘2000 IFT annual meeting book of abstracts’. pp. 75–76. (IFT Press: Chicago, IL)Benjakul S, Visessanguan W (2003) Transglutaminase-mediated setting in bigeye snapper surimi. Food Research International 36, 253–266.
| Transglutaminase-mediated setting in bigeye snapper surimi.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXkvVymsg%3D%3D&md5=b8c5e23545e6ac1d4d0ea59cc7fefe48CAS |
Benjakul S, Visessanguan W, Riebroy S, Ishizaki S, Tanaka M (2002) Gel-forming properties of bigeye snapper, Priacanthus tayenus and P. macracanthus, stored in ice. Journal of the Science of Food and Agriculture 82, 1442–1451.
| Gel-forming properties of bigeye snapper, Priacanthus tayenus and P. macracanthus, stored in ice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xnsl2lu74%3D&md5=9600063ac72a346ff43be6fe272abb8eCAS |
Benjakul S, Visessanguan W, Thongkaew C, Tanaka M (2005) Effect of frozen storage on chemical and gel forming properties of fish commonly used for surimi production in Thailand. Food Hydrocolloids 19, 197–207.
| Effect of frozen storage on chemical and gel forming properties of fish commonly used for surimi production in Thailand.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXptl2htrc%3D&md5=1af2a8f59796f619640ccb59c22afc33CAS |
Benjakul S, Julavittayanukul O, Visessanguan W (2006) Effect of phosphate compounds on gel-forming ability of surimi from bigeye snapper (Priacanthus tayenus). Journal of Food Hydrology 20, 1153–1163.
Cardoso C, Mendes R, Vaz-Pires P, Nunes ML (2010) Effect of salt and MTGase on the production of high quality gels from farmed sea bass. Journal of Food Engineering 101, 98–105.
| Effect of salt and MTGase on the production of high quality gels from farmed sea bass.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXpsVGhsr0%3D&md5=b93336f1ee7e8d8b7d960adc66286939CAS |
Chaijan M, Panpipat W (2010) Gel-Forming Ability of Mackerel (Rastrelliger Branchysoma) Protein Isolate as Affected by Microbial Transglutaminas. Walailak Journal of Science and Technology 7, 41–49.
Chanarat S, Benjakul S, H-Kittikun A (2012) Comparative study on protein cross-linking and gel enhancing effect of microbial transglutaminase on surimi from different fish. Journal of the Science of Food and Agriculture 92, 844–852.
| Comparative study on protein cross-linking and gel enhancing effect of microbial transglutaminase on surimi from different fish.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVSns74%3D&md5=bba723707cc4f34ba7762a30d3da9e41CAS | 22413145PubMed |
Chen NJ, Morikawa J, Hashimoto T (2005) Effect of amino acids on the eutectic behavior of NaCl solutions studied by DSC. Cryobiology 50, 264–272.
| Effect of amino acids on the eutectic behavior of NaCl solutions studied by DSC.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXltVOktrc%3D&md5=9a297f1c3d74c46776dcf6984e2fe7a3CAS | 15925578PubMed |
Dondero M, Figueroa V, Morales X, Curotto E (2006) Transglutaminase effects on gelation capacity of thermally induced beef protein gels. Food Chemistry 99, 546–554.
| Transglutaminase effects on gelation capacity of thermally induced beef protein gels.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XksF2js70%3D&md5=4462a4c3550f533aaae771add1e088b5CAS |
Duangmal K, Taluengphol A (2010) Effect of protein additives, sodium ascorbate, and microbial transglutaminase on the texture and colour of red tilapia surimi gel. International Journal of Food Science & Technology 45, 48–55.
| Effect of protein additives, sodium ascorbate, and microbial transglutaminase on the texture and colour of red tilapia surimi gel.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXksVyqsQ%3D%3D&md5=950614a5e521903d1e0e94faaa210878CAS |
Hemung BO, Chin KB (2013) Effects of fish sarcoplasmic proteins on the properties of myofibrillar protein gels mediated by microbial transglutaminase. Food Science and Technology (Campinas.) 53, 184–190.
Jiang S-T, Hsieh J-F, Ho M-L, Chung Y-C (2000) Microbial Transglutaminase Affects Gel Properties of Golden Threadfin-bream and Pollack Surimi. Journal of Food Science 65, 694–699.
Jongjareonrak A, Benjakul S, Visessanguan W, Prodpran T, Tanaka M (2006) Characterization of edible films from skin gelatin of brown stripe red snapper and bigeye snapper. Food Hydrocolloids 20, 492–501.
| Characterization of edible films from skin gelatin of brown stripe red snapper and bigeye snapper.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1GitLg%3D&md5=261d70f8a5e0d209fa80e1a1a2d689ceCAS |
Kaewudom P, Benjakul S, Kijroongrojana K (2012) Effect of bovine and fish gelatin in combination with microbial transglutaminase on gel properties of threadfin bream surimi. International Aquatic Research 4, 12
| Effect of bovine and fish gelatin in combination with microbial transglutaminase on gel properties of threadfin bream surimi.Crossref | GoogleScholarGoogle Scholar |
Kaewudom P, Benjakul S, Kijroongrojana K (2013) Properties of surimi gel as influenced by fish gelatin and microbial transglutaminase. Food Bioscience 1, 39–47.
| Properties of surimi gel as influenced by fish gelatin and microbial transglutaminase.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhtVOlsbw%3D&md5=b41890f1411622d440a87045b14eeb34CAS |
Karayannakidis PD, Zotos A, Petridis D, Taylor KDA (2008) The Effect of Washing, Microbial Transglutaminase, Salts and Starch Addition on the Functional Properties of Sardine (Sardina Pilchardus) Kamaboko Gels. Food Science and Technology International 14, 167–177.
Ko WC, Yu CC, Hsu KC (2007) Changes in conformation and sulfhydryl groups of tilapia actomyosin by thermal treatment. LWT - Food Science and Technology (Campinas.) 40, 1316–1320.
Kuraishi C, Sakamoto J, Yamazaki K, Susa Y, Kuhara C, Soeda T (1997) Production of restructured meat using microbial transglutaminase without salt or cooking. Journal of Food Science 62, 488–490.
| Production of restructured meat using microbial transglutaminase without salt or cooking.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXjvVGrs7s%3D&md5=d409730722ee82507d368d85c9f3820fCAS |
Kyaw ZY, Cheow CS, Yu SY, Dzulkifly MH (2001) The effect of pressure cooking on the microstructure and expansion of fish cracker (keropok). Journal of Food Quality 24, 181–194.
| The effect of pressure cooking on the microstructure and expansion of fish cracker (keropok).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXmtVSlsL8%3D&md5=7e287aaa0bc4cecedca8a458da6dd680CAS |
Laemmli UK (1970) Cleavage of structural protein during the assembly of head bacteriophage T4. Nature 227, 680–685.
| Cleavage of structural protein during the assembly of head bacteriophage T4.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXlsFags7s%3D&md5=0be8db2782a3a038d29de9c9807e0776CAS | 5432063PubMed |
Lee CM (1984) Surimi process technology. Journal of Food Technology 38, 69–80.
Lee HG, Lanier TC, Hamann DD, Knopp JA (1997) Transglutaminase effects on low temperature gelation of fish protein sols. Journal of Food Science 62, 20–24.
| Transglutaminase effects on low temperature gelation of fish protein sols.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXhtFWgsL8%3D&md5=3c9e82543d5a2f14aed7bf7a1069614dCAS |
Lowry QH, Rosebrough NJ, Farr LA, Randall RJ (1951) Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry 193, 256–275.
Mao L, Wu T (2007) Gelling properties and lipid oxidation of kamaboko gels from grass carp (Ctenopharyn godonidellus) influenced by chitosan. Journal of Food Engineering 82, 128–134.
| Gelling properties and lipid oxidation of kamaboko gels from grass carp (Ctenopharyn godonidellus) influenced by chitosan.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXntFOms74%3D&md5=3317335a659c1c58dbda2a43ecf859e9CAS |
Norziah MH, Al-Hassan A, Khairulnizam Mordi MN, Norita M (2009) Characterization of fish gelatin from surimi processing wastes: thermal analysis and effect of transglutaminase on gel properties. Food Hydrocolloids 23, 1610–1616.
| Characterization of fish gelatin from surimi processing wastes: thermal analysis and effect of transglutaminase on gel properties.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjtFanurk%3D&md5=a0e44201beace9aed71e641adace719dCAS |
Park JW (2005) Ingredient technology for surimi and surimi seafood. In Surimi and surimi seafood’. 2nd edn. (Ed. JW Park) pp. 107–140. (CRC Press: Boca Raton, FL, USA).
Park JW, Lin TM (2005) Surimi: manufacturing and evaluation. In Surimi and Surimi Seafood. (Ed. JW Park) pp. 33–105. (Taylor and Francis: Boca Raton, FL, USA).
Ramírez JA, Rodríguez-Sosa R, Morales OJ, Vázquez M (2000) Surimi gels from striped mullet (Mugil cephalus) employing microbial transglutaminase. Food Chemistry 70, 443–449.
| Surimi gels from striped mullet (Mugil cephalus) employing microbial transglutaminase.Crossref | GoogleScholarGoogle Scholar |
Ramírez JA, Velazquez G, Echevarría GL, Torres JA (2007) Effect of adding insoluble solids from surimi wash water on the functional and mechanical properties of Pacific Whiting grade A surimi. Bioresource Technology 98, 2148–2153.
| Effect of adding insoluble solids from surimi wash water on the functional and mechanical properties of Pacific Whiting grade A surimi.Crossref | GoogleScholarGoogle Scholar | 17071079PubMed |
Rawdkuen S, Benjakul S, Visessanguan W, Lanier TC (2008) Rheological and textural properties of pacific whiting surimi gels as influenced by chicken plasma. International Journal of Food Properties 11, 820–832.
| Rheological and textural properties of pacific whiting surimi gels as influenced by chicken plasma.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVSjsrnN&md5=5a0bd7279272f9fccbfed76da3a9432eCAS |
Rawdkuen S, Sai-Ut S, Khamsorn S, Chaijan M, Benjakul S (2009) Biochemical and gelling properties of tilapia surimi and protein recovered using an acid alkaline process. Food Chemistry 112, 112–119.
| Biochemical and gelling properties of tilapia surimi and protein recovered using an acid alkaline process.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXovFyjtrY%3D&md5=f58ef27fa78bbcb64e084ca9f9f4cb9fCAS |
Shie JS, Park JW (1999) Physical characteristics of surimi seafood as affected by thermal processing conditions. Journal of Food Science 64, 287–290.
| Physical characteristics of surimi seafood as affected by thermal processing conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXjvVShsr0%3D&md5=f2d592a56b71992089cd1bd7c57fee9aCAS |
Spink CH (2008) Differential scanning calorimetry. Methods in Cell Biology 84, 115–141.
| Differential scanning calorimetry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXht1amtLw%3D&md5=b2448a0a187fa55648adfe0db6f841cbCAS | 17964930PubMed |
Tammatinna A, Benjakul S, Visessanguan W, Tanaka M (2007) Gelling properties of white shrimp (Penaeus vannamei) meat as influenced by setting condition and microbial tranglutaminase. LWT- Food Science and Technology (Campinas.) 40, 1489–1497.
Thorarinsdottir KA, Arason S, Geirsdottir M, Bogason SG, Kristbergsson K (2002) Changes in myofibrillar proteins during processing of salted cod (Gadus morhua) as determined by electrophoresis and differential scanning calorimetry. Food Chemistry 77, 377–385.
| Changes in myofibrillar proteins during processing of salted cod (Gadus morhua) as determined by electrophoresis and differential scanning calorimetry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XjslaqsLs%3D&md5=026c9db401c037ed474d97a3d1fbe94aCAS |
Xiong YL (1997) Structure-functionality relationships of muscle proteins. In ‘Food proteins and their applications’. (Eds S Damoradan, A Paraf) pp. 341–392. (Marcel Dekker, Inc.: New York, NY)
Yetim H, Ockerman HW (1995) The effects of egg white, tumbling and storage time on proximate composition and protein fractions or restructured fish products. Journal of Aquatic Food Product Technology 4, 65–78.
| The effects of egg white, tumbling and storage time on proximate composition and protein fractions or restructured fish products.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXotlOkt78%3D&md5=eef8110bcf04c6919dffa8263fd2910fCAS |
Yin T, Park JW (2014) Effects of nano-scaled fish bone on the gelation properties of Alaska pollock surimi. Food Chemistry 150, 463–468.
Yokoyama K, Nio N, Kikuchi Y (2004) Properties and applications of microbial transglutaminase. Applied Microbiology and Biotechnology 64, 447–454.
| Properties and applications of microbial transglutaminase.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjtFyis7w%3D&md5=80a64a23c197e747c03b6acfdbc7eafdCAS | 14740191PubMed |
Yongsawatdigul J, Piyadhammaviboon P (2005) Effect of microbial transglutaminase on outolysis and gelation of lizardfish surimi. Journal of the Science of Food and Agriculture 85, 1453–1460.
| Effect of microbial transglutaminase on outolysis and gelation of lizardfish surimi.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXmtVenuro%3D&md5=32184e61c85b04cdf8af7a82559431a9CAS |
Yongsawatdigul J, Worratao A, Park JW (2002) Effect of endogenous transglutaminase on threadfin bream surimi gelation. Journal of Food Science 67, 3258–3263.
| Effect of endogenous transglutaminase on threadfin bream surimi gelation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjs1Gi&md5=deb0078f9eae78a47352d76a0dbe3ae2CAS |
Zhou A, Benjakul S, Pan K, Gong J, Liu X (2006) Cryoprotective effects of trehalose and sodium lactate on tilapia (Sarotherodonnilotica) surimi during frozen storage. Food Chemistry 96, 96–103.
| Cryoprotective effects of trehalose and sodium lactate on tilapia (Sarotherodonnilotica) surimi during frozen storage.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFGht7zE&md5=caac38c0e31a94084c81fedaf58d52f7CAS |