Effects of different dietary concentrate to forage ratio and thiamine supplementation on the rumen fermentation and ruminal bacterial community in dairy cows
Hongrong Wang A D , Xiaohua Pan A B , Chao Wang C , Mengzhi Wang A and Lihuai Yu AA College of Animal Science and Technology, Yangzhou University, Yangzhou 225009, China.
B Faculté de Médecine vétérinaire, Université de Liège, Liège 4000, Belgique.
C School of Clinical Medicine, Jiangsu University, Zhenjiang 212001, China.
D Corresponding author. Email: hrwang@yzu.edu.cn
Animal Production Science 55(2) 189-193 https://doi.org/10.1071/AN14523
Submitted: 24 February 2014 Accepted: 7 November 2014 Published: 19 December 2014
Journal Compilation © CSIRO Publishing 2015 Open Access CC BY-NC-ND
Abstract
A subacute ruminal acidosis (SARA) model was induced gradually by increasing the proportion of dietary concentrate to evaluate the effect of thiamine supplementation on the structure of bacterial community in dairy cows. Three Holstein dairy cows with rumen cannula were randomly assigned to a replicated 3 × 3 Latin square design trial and received three diets during three successive 21-day periods in each square. The three dietary treatments were as follows: a low-concentrate diet (control), a high-concentrate SARA-induced diet (SARA) and a high-concentrate SARA-induced diet with 180 mg thiamine/kg DM (SARA+thiamine). Real-time–polymerase chain reaction assay was used to quantify the population variation of SARA-related ruminal bacteria in these cows. The results showed that SARA was induced gradually when cows were fed with the high-concentrate diets. The mean ruminal pH value was higher in the control cows than in those of SARA and SARA+thiamine groups, the mean was decreased in cows fed on SARA diet, and the depression was alleviated by supplemented thiamine and the difference was significant (P < 0.05) especially at 9-h and 12-h sample times (or 1 h and 4 h after the second feeding). The populations of Streptococcus bovis and genus Lactobacillus in cows from the SARA group were increased in log copies/µL by 3.62% and 4.65%, respectively, compared with the control group (P < 0.05). In contrast, in log copies/µL, populations of Butyrivibrio fibrisovens and Megasphaera elsdenii were decreased by 1.14% and 4.90%, respectively (P < 0.05). Thiamine supplementation led to an obvious reduction of Strepococcus bovis and Lactobacillus (P < 0.05), whereas the number of log copies/µL of Megasphaera elsdenii was dramatically increased (P < 0.05). There was no significant effect of thiamine supplementation on the number of log copies/µL of Butyrivibrio fibrisovens and Selenomonas ruminantium (P > 0.05). It was concluded that thiamine supplementation to high-concentrate diets at concentrations of 180 mg/kg DM could help alleviate SARA by increasing rumen pH and balancing the population of lactic acid-producing and -consuming bacteria.
Additional keywords: dietary NFC : NDF, SARA.
Introduction
It is well established that thiamine is an essential cofactor required for carbohydrate metabolism, and the production of thiamine by the rumen microflora is normally adequate to supply ruminant’s requirements (Breves et al. 1981; Miller et al. 1986). Subacute ruminal acidosis (SARA) is a common chronic digestive disorder in high-yielding dairy cows that receive highly digestible grain diets, and is defined as periods of moderately depressed ruminal pH, namely, lower than 5.8 (Penner et al. 2007). Current feeding practices in feedlot beef and high-producing dairy cattle use highly fermentable grain diets to increase growth rate and milk production, but because of microbial disturbances, they predispose cattle to digestive disorders such as SARA. It is estimated that 20% of early lactating dairy cows can be affected by SARA in intensive feeding systems, and this results in a decrease of feed efficiency and milk production, and SARA may even develop into chronic metabolic acidosis, causing hepatic abnormalities, diarrhoea and laminitis (Plaizier et al. 2008). Therefore, it is a major concern to prevent or alleviate the occurrence of SARA in dairy herds. In the past decade, several dietary strategies proposed for use in preventing SARA, such as sodium bicarbonate buffer and monensin iono-phones as well as probiotics, have been found to stabilise ruminal pH and improve animal production (Mutsvangwa et al. 2002; Paton et al. 2006; Chaucheyras-Durand et al. 2008; Desnoyers et al. 2009; Packer et al. 2011). However, none of these approaches has consistently maintained higher and stable ruminal pH. De Oliveira et al. (1997) and Tafaj et al. (2006) observed that the adequate status of thiamine in ruminants can be altered by SARA conditions and this can lead to cerebrocortical necrosis, which is particularly associated with intensive feeding systems and high-concentrate diets in dairy cattle. Our previous research found some evidence that adding 180 mg thiamine/kg could alleviate SARA by increasing the rumen pH and decreasing the lactate concentration by regulating the structure of rumen microbial community in vitro (Pan et al. 2013). Moreover, effects of thiamine on the microbial community in the rumen of dairy cows during high-concentrate induced SARA are less understood, and there is very little information on use of thiamine for preventing SARA. Therefore, the objective of the present study was to determine the effects of different dietary concentrate levels and thiamine supplementation on the rumen fermentation and ruminal bacterial community in dairy cows.
Materials and methods
Animals and sample collection
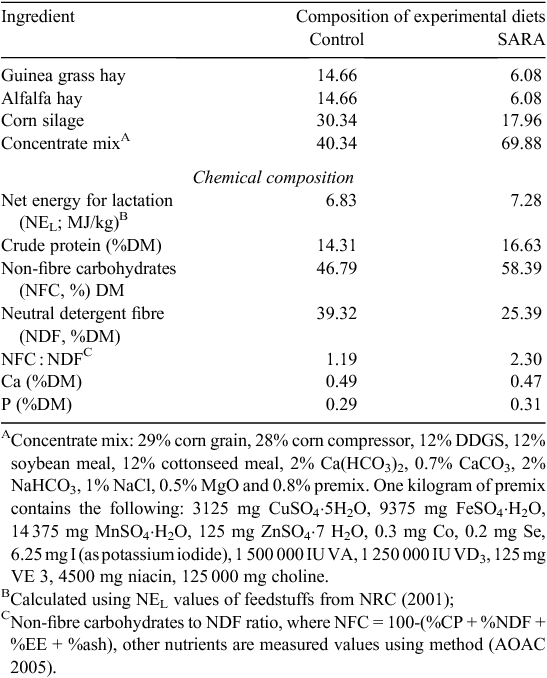
Three Holstein cows (650 ± 20 kg) fitted with ruminal cannula were allotted to a replicated 3 × 3 Latin square design (n = 6). The animals were fed with the following three diets: a low-concentrate diet [control: roughage to concentrate (R : C) ratio of 60 : 40, non-fibre carbohydrate (NFC) : neutral detergent fibre (NDF) = 1.19], a high-concentrate SARA diet (SARA: R : C ratio of 30 : 70, NFC : NDF = 2.30), and a high-concentrate SARA diet with 180 mg/kg thiamine (SARA+thiamine), where SARA was induced gradually by a high-concentrate diet. The composition and nutrient concentrations of diets are shown in Table 1. Cows were housed in individual tie-stalls and provided feed ad libitum at 8-h intervals daily (0600 hours, 1400 hours and 2200 hours) and free access to drinking water. All animals were cared for and handled in accordance with the protocol approved by Animal Care and Ethical Committee of Yangzhou University (No. 201206118). The trial was replicated with three successive 21-day periods in each square. At the end of each period, rumen digesta samples were collected from the three fistula cows at 0-h, 3-h, 6-h sample times (0 h, 3 h and 6 h after the first feeding), and 9-h and 12-h sample times (1 h and 4 h after second feeding) for pH determination. At each sampling time, the pH was measured immediately after collection, with a handheld pH electrode (Model B-4, Shanghai Chemical, China). Other rumen samples collected immediately 1 h after the morning feeding were used for DNA extraction.
|
DNA extraction
Community DNA was extracted from 0.5 mL aliquots of rumen fluid and digesta by the method described by Yu and Morrison (2004).
Real-time–polymerase chain reaction (PCR) assays
As demonstrated in Table 2, a set of PCR primers were designed according to GenBank accession numbers and validated for specific detection and quantification of Strepococcus. bovis, Butyrivibrio fibrisolvens, Lactobacillus, Selenomonas ruminantium and Megasphaera elsdenii. Plasmids were constructed and verified by a real-time–PCR standard curve, using 10-fold serial dilutions. Real-time–PCR amplification and detection were performed using ABI 7500 system (Applied Biosystems, Foster City, CA, USA). The reaction was conducted in a final volume of 20 μL, containing the following: 10.4 μL SYBR® Premix ExTaq™ (TaKaRa, Dalian, China), 0.8 μL forward primer, 0.8 μL reverse primer, 6.0 μL distilled water, and 2.0 μL of DNA solution (50 ng/μL). PCR conditions for rumen bacteria above were as follows: DNA was initially denatured at 94°C for 5 min, followed by 30 cycles (denaturing at 94°C for 30s, annealing 56°C for 34s and extension at 72°C for 40 s). After the last cycle of amplification, an analysis of the product melting curve was performed to determine the specificity of amplification.

|
Statistical analyses
Statistical analysis was performed for data evaluation. Variables, least square means for rumen pH and bacterial population data were generated and tested at a significance level of P = 0.05. An analysis was performed by ANOVA using the mixed procedure of SAS version 9.2 (2002; SAS Institute Inc., Cary, NC, USA). The experimental data were analysed in a replicated 3 × 3 Latin square design, using following model:
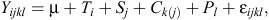
where Yijkl is the dependent variable, µ is the general mean, Ti is fixed effect of the treatments (i = 1, 2 and 3 for control, SARA, and SARA+thiamine, respectively), Sj is random effect of square (j = 1, 2 and 3), Ck(j) is the effect of cow within square, Pl is period within square, and εijkl is the random residual error. Means were separated using Duncan’s multiple range tests.
Results
Induction of SARA model
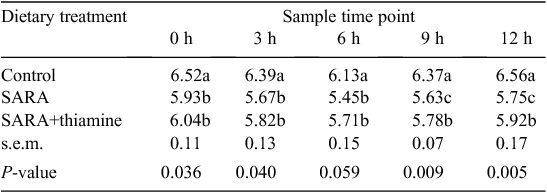
The results in Table 3 show that ruminal pH in cows fed with a low-concentrate diet (control) was in the normal physiological range at 0- to ~12-h sample times after feeding. Lower pH values were observed in cows that received treatment SARA and SARA+thiamine than in the control. Ruminal pH fell below 5.8 in cows fed with SARA (high-concentrate) diets and this lasted more than 6 h (from 3-h to 12-h sampling times), indicating that SARA was induced successfully in cows receiving the SARA diet. In addition, ruminal pH in cows fed with SARA+thiamine diet was higher than that with SARA treatment and the differences were significant (P < 0.05) at 9-h and 12-h sample times (1 h and 4 h after second feeding).
|
Verification of recombinant plasmid
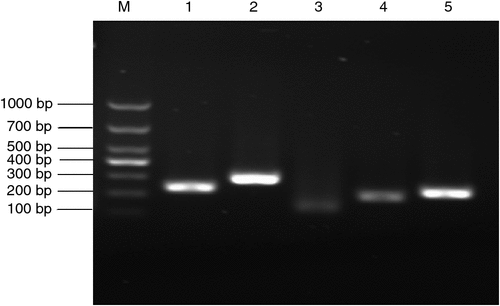
The recombinant plasmids were verified by PCR amplification, with incubating medium as a template directly. As shown in Fig. 1, the gel electrophoresis results of PCR products were satisfactory with clear bands, correct placement and specific amplification, and were used for the construction of the calibration curve (data not shown).
|
Calibration curve and quantitative analysis of rumen bacteria
External standards for real-time–PCR were prepared from bacterial plasmids. For each standard, linear regressions derived from the threshold cycle [Ct] of each DNA dilution versus the log quality were calculated. Logarithms of the DNA concentration (copies/μL) were plotted against the calculated means, obtaining a straight line of equations Y = –3.05X + 36.25 (S. bovis, R2 = 0.996); Y = –3.35X + 36.77 (B. fibrisolvens, R2 = 0.985); Y = –3.18X + 37.45 (Lactobacillus, R2 = 0.987); Y = –2.93X + 36.25 (S. ruminantium, R2 = 0.993) and Y = –2.99X + 42.51 (M. elsdenii, R2 = 0.930), where Y is the log of DNA concentration and X is the Ct, the equations above were used to quantify DNA from rumen fluid samples.
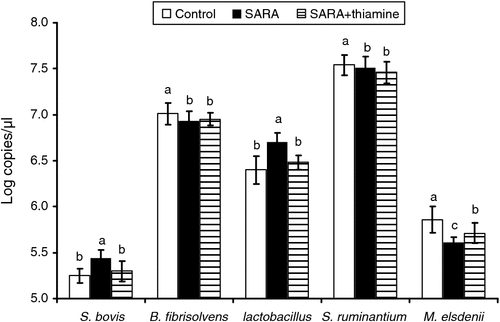
The profiles of related rumen bacterial population are illustrated in Fig. 2. The population of genus Streptococcus bovis and Lactobacillus of cows from the SARA treatment were increased (P < 0.05) in log copies/µL by 3.62% and 4.65%, respectively, compared with control. In contrast, log copies/µL of B. fibrisolvens and M. elsdenii were decreased by 1.14% and 4.90% (P < 0.05), respectively. Supplementation with thiamine led to a reduction of S. bovis profile (P < 0.05). However, the population of M. elsdenii was dramatically enhanced in the cows receiving the SARA+thiamine diet, compared with that of the cows receiving SARA without thiamine diet (P < 0.05). There was no significant (P > 0.05) effect of thiamine supplementation on the population of B. fibrisolvens and S. ruminantium in cows.
|
Discussion
SARA is the consequence of feeding grain diets to ruminant animals. The ruminal pH observed in the SARA diet corresponded to a higher rate of degradation and fermentation of the high-grain diet that produced more organic acids in the rumen, and a decline in ruminal pH occurred during SARA accordingly. Current definitions of SARA are based on the low rumen pH typically generated on high-grain diets (Oetzel 2003; Plaizier et al. 2008). However, there is no general agreement on the pH threshold that defines SARA, and moreover, rumen pH may not even be highly correlated with disease symptoms (Enemark et al. 2004; Khafipour et al. 2009). According to the definition of experimental SARA, a pH threshold value of 5.8 was used to define SARA as suggested by Kleen and Cannizzo (2012) and Penner et al. (2007). The pH values of cows on the SARA diet were below 5.8 for at least 9 h, and 6 h in cows on SARA+thiamine diet in the current experiment, this suggesting that SARA occurred in cows fed both the SARA and SARA+thiamine diets.
One of the most interesting findings of the present study was the bacterial population variation. The present real-time–PCR analysis shows distinct changes in the bacterial population profile in the rumen of cows fed different treatment diets. There was a more abundant population of S. bovis and Lactobacillus in cows fed the SARA diet than either the control or SARA+thiamine diets. Such changes in bacterial population profile and abundance may be due to the increased fermentable substrate present in the diet, favouring the growth of amylolytic and other starch-digesting bacterial species (Goad et al. 1998). The results suggest that supplementation with thiamine could alleviate SARA, and supports our previous findings in vitro (Pan et al. 2013). One possible reason for this is that thiamine promotes carbohydrate metabolism, where thiamine was in the form of thiamine pyrophosphatase (TPP) as the coenzyme of pyruvate dehydrogenase and α-ketoglutarate dehydrogenase in the pathway of citric acid cycle, and, consequently, to reduce the accumulation of pyruvate and lactate. Also, SARA may be a result of decreasing profile of lactate-consuming bacteria (Russell and Hino 1985), where the growth of lactate-consuming bacteria such as M. elsdenii and S. ruminantium may be suppressed when SARA occurs, and the unbalanced rumen microflora may eventually result in SARA (Russell et al. 1981). Particularly, the real-time–PCR data also indicated that thiamine supplementation significantly reduced S. bovis and increased the M. elsdenii population profile compared with that of the SARA diet (Fig. 2). This implies that thiamine may have a function to stabilise ruminal pH value by improving the growth of lactate-consuming bacteria (such as M. elsdenii) and suppressing that of lactate-producing bacteria (such as S. bovis), which may help maintain the balance in the rumen bacteria and contribute to the remission of SARA. The pathway of thiamine regulating the bacterial growth and their metabolism is still unclear and further research is needed. Overall, this evidence may provide an alternative and method to manage SARA in the future.
Conclusions
The results of this study indicated that thiamine supplementation to high-concentrate diets at concentrations of 180 mg/kg DM could help alleviate SARA by increasing rumen pH and balancing the population of lactic acid-producing and -consuming bacteria.
Acknowledgements
This study was supported by a grant from NSFC (Grant No. 31072051), a China government-funded project (Studies on dynamics of microbial thiamine metabolism in the rumen and its related to subacute ruminal acidosis) and a project funded by National Science and Technology Support Program (Project No. 2012BAD12B02).
References
AOAC (2005)’Official method of analysis.’ 18th edn. (Association of Official Analytical Chemists: Washington, DC)Breves GM, Hoeller HQ, Rohr K (1981) Flow of thiamine to the duodenum in dairy cows fed different rations. Journal of Animal Science 96, 587–591.
Chaucheyras-Durand F, Walker DN, Bach A (2008) Effect of active dry yeasts on the rumen microbial ecosystem: past, present and future. Animal Feed Science and Technology 145, 5–26.
| Effect of active dry yeasts on the rumen microbial ecosystem: past, present and future.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXpsVKrtLo%3D&md5=8588b7a415d4552fd537e5b4f5f312c5CAS |
De Oliveira LA, Jean-Blain C, Komisarczuk-Bony S, Durix A, Durier C (1997) Microbial thiamin metabolism in the rumen simulating fermenter: the effect of acidogenic conditions, a high sulfur level and added thiamin. The British Journal of Nutrition 78, 599–613.
| Microbial thiamin metabolism in the rumen simulating fermenter: the effect of acidogenic conditions, a high sulfur level and added thiamin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXmslentro%3D&md5=27f61a28bff5cda762a2acb762521eb4CAS |
Desnoyers M, Giger-Reverdin S, Bertin G, Duvaux-Ponter C, Sauvant D (2009) Meta-analysis of the influence of Saccharomyes cerevisiae supplementation on ruminal parameters and milk production of ruminants. Journal of Dairy Science 92, 1620–1632.
| Meta-analysis of the influence of Saccharomyes cerevisiae supplementation on ruminal parameters and milk production of ruminants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjvFamtLw%3D&md5=b736395207374104f7d69ab1ba9918f0CAS | 19307644PubMed |
Enemark JMD, Jørgensen RJ, Kristensen NB (2004) An evaluation of parameters for the detection of subclinical rumen acidosis in dairy herds. Veterinary Research Communications 28, 687–709.
| An evaluation of parameters for the detection of subclinical rumen acidosis in dairy herds.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2cnjsVWmug%3D%3D&md5=4e2e81969744981046ce6fcb6b6a6314CAS |
Goad DW, Goad CL, Nagaraja TG (1998) Ruminal microbial and fermentative changes associated with experimentally induced subacute acidosis in steers. Journal of Animal Science 76, 234–241.
Khafipour E, Li S, Plaizier JC, Krause DO (2009) Rumen microbiome composition determined using two nutritional models of subacute ruminal acidosis. Applied and Environmental Microbiology 75, 7115–7124.
| Rumen microbiome composition determined using two nutritional models of subacute ruminal acidosis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFSit7vN&md5=1e602155e1243dbc5b71e1e9ef3e7036CAS | 19783747PubMed |
Kleen JL, Cannizzo C (2012) Incidence, prevalence and impact of SARA in dairy herds. Animal Feed Science and Technology 172, 4–8.
| Incidence, prevalence and impact of SARA in dairy herds.Crossref | GoogleScholarGoogle Scholar |
Miller BL, Meiske JC, Groodrich RD (1986) Effect of grain source and concentrate level on B-vitamin production and absorption in steers. Journal of Animal Science 62, 473–483.
Mutsvangwa T, Walton JP, Plaizier JC, Dufield TF, Bagg R, Dick P, Vessie G, McBride BW (2002) Effects of monensin controlled-release capsule or premix on attenuation of subacute ruminal acidosis in dairy cows. Journal of Dairy Science 85, 3454–3461.
| Effects of monensin controlled-release capsule or premix on attenuation of subacute ruminal acidosis in dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXht1eisw%3D%3D&md5=c0bb49d425da50af075ccf6d3eacf6daCAS | 12512618PubMed |
NRC (2001) ‘Nutrient requirements of dairy cattle.’ 7th edn. (National Academy Press: Washington, DC)
Oetzel GR (2003) Subacute ruminal acidosis in dairy cattle. Advances in Dairy Technology 15, 307–317.
Packer El, Clayton EH, Cusack PMV (2011) Rumen fermentation and liveweight gain in beef cattle treated with monensin and grazing lush forage. Australian Veterinary Journal 89, 338–345.
Pan XH, Wang MZ, Wang HR (2013) Effects of dietary concentrate to forage ratio and thiamine supplementation on in vitro rumen fermentation parameters and microbial community structure in dairy cows. Chinese Journal of Animal Nutrition 25, 88–99.
Paton LJ, Beauchemin KA, Veria DM, von Keyserlingk MAG (2006) Use of sodium bicarbonate, offered free choice or blended into the ration, to reduced the risk of ruminal acidosis in cattle. Canadian Journal of Animal Science 86, 429–437.
| Use of sodium bicarbonate, offered free choice or blended into the ration, to reduced the risk of ruminal acidosis in cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXksFKlurs%3D&md5=c17b1671f18547eac6a4b63765a6de48CAS |
Penner GB, Beauchemin KA, Mutsvangwa T (2007) Severity of ruminal acidosis in primiparous Holstein cows during the periparturient period. Journal of Dairy Science 90, 365–375.
| Severity of ruminal acidosis in primiparous Holstein cows during the periparturient period.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD28jksVWnsA%3D%3D&md5=681085cdb705980e40c6013a8a0fdd36CAS | 17183105PubMed |
Plaizier JC, Krause DO, Gozho GN, McBride BW (2008) Subacute ruminal acidosis in dairy cows: the physiological causes, incidence and consequences. Veterinary Journal (London, England) 176, 21–31.
| Subacute ruminal acidosis in dairy cows: the physiological causes, incidence and consequences.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXjs1SrsbY%3D&md5=606fca5b3a81b0e6ea62bc9370eb387dCAS |
Russell JB, Hino T (1985) Regulation of lactate production in Streptococcus bovis: a spiraling effect that contributes to rumen acidosis. Journal of Dairy Science 68, 1712–1721.
| Regulation of lactate production in Streptococcus bovis: a spiraling effect that contributes to rumen acidosis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXkvVCrsrs%3D&md5=0b455db30902dd2ea05c5ced4b9391f1CAS |
Russell JB, Cotta MA, Dombrowski DB (1981) Rumen bacterial competition in continuous culture: Streptococcus bovis versus Megasphaera elsdenii. Applied and Environmental Microbiology 41, 1394–1399.
Tafaj M, Schollenberger M, Feofilowa J (2006) Relationship between thiamine concentration and fermentation patterns in the rumen fluid of dairy cows fed with graded concentrate levels. Journal of Animal Physiology and Animal Nutrition 90, 335–343.
| Relationship between thiamine concentration and fermentation patterns in the rumen fluid of dairy cows fed with graded concentrate levels.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XosFequrk%3D&md5=2a8ef1f589ae412c2337491ceffafe0fCAS | 16867079PubMed |
Yu Z, Morrison M (2004) Improved extraction of PCR-quality community DNA from digesta and fecal samples. BioTechniques 36, 808–812.


