The effect of pH decline rate on the meat and eating quality of beef carcasses
D. L. Hopkins A E , E. N. Ponnampalam B , R. J. van de Ven C and R. D. Warner DA NSW Department of Primary Industries, Centre for Red Meat and Sheep Development, PO Box 129, Cowra, NSW 2794, Australia.
B Future Farming Systems Research Division, Department of Primary Industries, Werribee, Vic. 3030, Australia.
C NSW Department of Primary Industries, Orange Agricultural Institute, Forest Road, Orange, NSW 2800, Australia.
D CSIRO Animal, Food and Health Sciences, 671 Sneydes Road, Werribee, Vic. 3030, Australia.
E Corresponding author. Email: david.hopkins@dpi.nsw.gov.au
Animal Production Science 54(4) 407-413 https://doi.org/10.1071/AN12314
Submitted: 3 September 2012 Accepted: 7 March 2013 Published: 20 February 2014
Journal Compilation © CSIRO Publishing 2014 Open Access CC BY-NC-ND
Abstract
An experiment was undertaken to examine the effect of rapid pH fall at a high muscle temperature on meat and eating quality of two beef cuts (striploin and cube roll). From 115 beef steer carcasses of which the right side of each carcass was subjected to electrical stimulation, 25 carcasses which exhibited the largest difference in the rate of pH fall in the M. longissimus between sides were selected for subsequent sampling. All of the stimulated sides missed the ‘ideal’ pH/temperature window (defined as temperature at pH 6 in the M. longissimus <35°C and >12°C) at the upper end, as did several of the non-stimulated sides. The mean temperature at pH 6 for stimulated sides from modelling was 40.9 versus 33.3°C for non-stimulated sides. Despite the significant effect of stimulation on pH decline there was no statistically significant impact on shear force or sensory traits of the M. longissimus, but there was a significant effect of aging on these traits. There was no effect of stimulation or pH decline on drip loss of the striploin. After 14 days of aging there was no effect of stimulation or ultimate pH on striploin purge, but there was a significant effect of pH decline. This was not, however, evident for purge of the cube roll aged for either 4 or 42 days. The redness of the cube rolls as reflected by a* values declined with days of display, with the decline more rapid for samples aged for 42 days compared with those aged for 4 days. For meat aged and displayed identically, the a* values were on average significantly lower for meat from non-stimulated carcasses, but apart from aging there was no effect on the wavelength ratio 630/580 nm, an indicator of the formation of metmyoglobin. There was also evidence that a rapid decline in pH increased the onset of lipid oxidation.
Introduction
When the Meat Standards Australia (MSA) beef grading system was established, one of the initial specifications was the concept of an ideal pH/temperature window during chilling. This was such that to ‘hit’ the window the pH had to be above 6 if the muscle temperature was above 35°C and below 6 if the muscle temperature was below 12°C (Thompson 2002). This target was based on the classical studies of Locker and Hagyard (1963), which demonstrated that pre-rigor excised muscle would shorten more if exposed to high or low temperatures. In this case, minimum shortening occurred between 14 and 19°C and this is correlated with minimal toughness (Tornberg 1996). Related to this, at elevated temperatures a condition classified as ‘heat toughening’, has been observed. Not only does this condition lead to increased toughening, but also reduced tenderisation (Devine et al. 1999). Above 12−15°C, a contracture occurs at rigor and below this temperature a contracture occurs before rigor. It has also been shown in beef (Devine et al. 1999) that when shortening was prevented by tight wrapping and rigor mortis occurred at a range of temperatures from 15 to 35°C, that shear force was greater at the high rigor mortis temperatures. This difference was maintained with aging at 4°C (Devine et al. 1999). These conditions of low pH and high temperature are known to denature the contractile proteins, which are more stable at rigor mortis (Offer 1991). Such conditions, in conjunction with greater autolysis of calpains at high temperatures (Dransfield et al. 1992) would explain how aging enzymes are reduced in effectiveness so that both shear force increases and the aging potential is reduced.
Of interest is the finding by Hwang and Thompson (2001b) that the most tender beef after 14 days of aging was achieved when the temperature at pH 6.0 was 29−30°C under in situ conditions. This finding when contrasted with that of Devine et al. (1999) highlights that caution is needed when extrapolating from the in vitro (excised) to in situ states as the former commonly involves maintaining muscles at set temperatures during rigor development. So it seems there are differences in what might be considered the critical pH/temperature window. However, the work of Hwang and Thompson (2001b) suggested that the rate of pH decline had the greatest impact on eating quality. Given this and because there was evidence that a high proportion of carcasses from feedlot cattle exhibited a fast rate of pH decline (Hopkins et al. 2007; Warner et al. 2014b) it was considered important to verify the general validity of earlier work using the sensory testing regime developed for the MSA program. Therefore, the objective of this study was to examine the effect of rapid pH fall at a high muscle temperature on meat and eating quality of two beef cuts (striploin and cube roll). Electrical stimulation (– or +) was used to create differences in rates of pH decline within carcasses.
Materials and methods
Experimental design
A total of 115 beef steer carcasses were selected at a commercial abattoir from cattle that had been grain fed for 120 days as a lot. In order to avoid any interaction with stimulation, the post-slaughter immobiliser was turned off for all cattle in this study. At ~30 min post-slaughter, the right side of each carcass was subjected to electrical stimulation (2-A peak, 2-ms pulse width, 15-Hz frequency) for 35 s using a medium voltage stimulation unit, before chiller entry. This stimulation system delivers constant current and variable voltage. Additionally on the unstimulated sides some of the subcutaneous fat was removed from over the M. longissimus thoracis et lumborum (LL) using a knife to further increase the rate of cooling. The decline in pH in the LL was measured using pH meters in both sides of each carcass at the site used by MSA between the 2nd and 5th lumbar vertebrae (Anon. 2006). Meters with temperature compensation (WP-80, TPS Pty Ltd, Brisbane, Qld, Australia) and a polypropylene spear-type gel electrode (Ionode IJ 44) were calibrated at ambient temperature. The pH and temperature were measured as soon as the sides entered the chiller and then hourly for 4 h. From this dataset 25 carcasses (mean weight of 322 ± 26.8 kg ranging from 276 to 390 kg), which exhibited the largest difference in the rate of pH fall between sides were selected for subsequent sampling. This was based on linear interpolation of pH data over time.
Carcass measurement and processing
Carcasses were weighed hot. After chilling overnight at an air temperature of 0−1°C the fat depth at the 10th/11th rib site was measured with a ruler after quartering at this site (Anon. 2005). Visually assessed meat colour of the LL was assessed using AUS-MEAT colour chips 1A (very pale) to 7 (very dark purple) (Anon. 2005) as was the texture (1 = firm to 7 = coarse) and firmness (1 = firm to 7 = soft). Subsequently the striploin and cube roll (Anon. 2005; AUS-MEAT product identification numbers 2140 and 2244) were removed from each carcass side with mean weights of 1366 ± 230 and 2978 ± 364 g, respectively. A cranial (9 cm) and a caudal (9 cm) section of the striploin were removed for sensory assessment and the epimysium was removed. One portion was frozen (−20°C) at Day 1 after being sliced into 2.5-cm steaks and the other portion was vacuum packed and aged for 14 days at 0−1°C after which it was sliced into 2.5-cm steaks and frozen. The allocation to aging days was randomised between positions. Samples (8 cm) used for shear force testing were taken from the medial section of the striploin and randomised between 1 and 14 days of aging.
Meat quality measures
The final pH of the LL (pHu) was measured on the medial section at 24 h post-mortem using a pH meter as previously described, calibrated at chiller temperature (0−2°C). Drip loss was determined on duplicate 80 g samples of striploin taken from the medial section at 24 h post-mortem. The samples were weighed and then suspended for 48 h in plastic bags at 4°C and reweighed again after the 48-h period. Shear force samples aged for 14 days were weighed before vacuum packing and then removed from the vacuum pack after the aging period, patted dry and weighed to determine purge. Purge was also determined for cube rolls by weighing them before vacuum packing and then removing them from the vacuum pack after 4 days, patting dry and reweighing. At this time the cube roll was cut in half and one-half was vacuum packed and aged for another 38 days after which purge was determined again.
Samples of LL were prepared into 100-g blocks for shear testing. These blocks were thawed overnight at 0−1°C and then cooked in a water bath in plastic bags at 70°C for 1h. The shear force samples were prepared to give five replicate samples of 1-cm2 cross-sectional area. The force required to shear these was measured perpendicular to fibre orientation using a Lloyd texture analyser (Model LRX, Lloyd Instruments, Hampshire, UK) fitted with a vee-shaped cutting blade that sheared down through the samples. The cross head speed of the analyser was 300 mm/min.
After 4 or 42 days of aging, 2.5-cm-thick slices of cube roll (one per tray) were placed on foam trays and over-wrapped using polyvinylchloride film. Trays were maintained at a refrigerated temperature of 3−4°C for the evaluation of colour stability of meat under fluorescent light (1000 lux). The light was provided by NEC Biolux high grade tri phos BR-B meat display pink 58-W tubes (NEC Pty Ltd, Japan). Packs were placed into the display cabinet in randomised order. Data loggers (Escort Junior, Escort Data Logging Systems Ltd, Auckland, New Zealand) were placed in the display cabinet and temperature was recorded every 10 min. The average temperature of the display cabinet was 4.76°C (s.d. = ± 0.10).
The surface colour of the meat was measured at 0, 0.5, 1, 2, 3, 4, 7 and 10 days using a HunterLab colour meter (Hunterlab Miniscan, TM XE Plus 45/0-S, model 4500S, with small viewing area, aperture size of 5 mm, Reston, VA, USA) with light source set at D65/10. The change in the colour of the meat surface during simulated retail display was calculated as the ratio of reflectance at 630/580 nm, which is an indicator of the ratio of oxymyoglobin to metmyoglobin as noted by others (MacDougall 1995). Additionally visual colour scores were given to each slice on each measurement day by 2, 3, 4 or 5 (of 7) scorers using the following scoring system: 0 = pale, 1 = bright cherry red, 2 = red, 3 = slightly dark and 4 = dark. The lipid oxidation in the cube roll slices was assessed by the thiobarbituric acid reactive substances (TBARS) procedure (Witte et al. 1970) expressed in mg of malondialdehyde (MDA) per kg of muscle. Samples for TBARS analysis (30 g) were collected and frozen at 0, 1, 3 and 10 days of display.
Sensory measures
The steaks from the LL portions to be used for sensory testing after either 1 or 14 days of aging were kept frozen (−20°C) until testing. Sample preparation for consumer testing has been outlined by Watson et al. (2008) and was based on grill cooking steaks using a Silex clam grill. The cooking was controlled by a timer to produce a medium degree of doneness (internal temperature of ~65°C). Each untrained consumer tested 10 samples and was asked to assess each steak for tenderness, juiciness, liking of flavour, and overall liking on a continuous 100-point scale from 0 to 100. The 10 tastings for each LL sample were averaged to give the final eating quality scores for the muscle. In addition each person was asked to score (rate) each sample as: awful, unsatisfactory, good every day (3 star), better than every day (4 star) or premium (5 star).
Statistical analyses
The trends in pH for carcass sides against temperature were modelled using an approach similar to that used in van de Ven et al. (2014). The average pH trend with temperature within each stimulation treatment (–ES and +ES) were modelled as separate spline models. Then each carcass side was allowed to deviate from the average trend for its stimulation treatment group as the sum of a spline associated with the particular carcass and an independent spline associated with the particular side within the carcass. The model also included a random error component and was fitted using the package ASReml (VSN International, Hemel Hempstead, UK) under R (R Development Core Team 2010). The fitted model was used to estimate mean temperature at pH 12 (pH@Temp12) and mean temperature at pH 6 (Temp@pH6) for each side, and bootstrap methods (Efron and Tibshirani 1993) were used to estimate standard errors of these estimates. An analysis of pHu included stimulation as a fixed effect with carcass as a random effect.
The analyses of shear force, transformed on the loge scale, and the sensory traits were initially based on the following model with factor levels stimulation and aging, and the covariates pHu, Temp@pH6 and pH@Temp12 fitted as fixed effects and carcass and side within carcass (Carcass : Side) fitted as random effects;

Y denotes the dependent variable being analysed. Non-significant (P > 0.05) fixed effect terms were dropped from the model. The covariates Temp@pH6 and pH@Temp12 were included so that any effect of stimulation independent of the rate of pH decline could be detected.
To analyse the drip loss, purge and TBARS data, the following model was used, which included the pHu, Temp@pH6 and pH@Temp12 as covariates, with carcass as a random term;
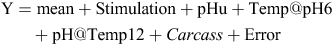
A linear mixed model was used to analyse the instrumental colour data generated by display and included, as fixed effects, separate linear regressions on display time across the four combinations of stimulation × aging. Also included were possible smooth deviations from linearity for each stimulation × aging combination, with these deviations modelled using splines. Random effects included, in addition to random error, random regressions on display time for each carcass and separate random regressions on display time for each carcass × side. For visual colour scores, averaged over graders, the fixed effects were the same as for instrumental colour data. Random effects included random regressions on display time for each carcass; random regressions for each carcass × side, and discrete effects for display time which were allowed to interact with stimulation, aging and stimulation × aging, carcass and side. The random error variances were weighted according to the number of graders averaged to give the visual colour score. For both instrumental and visual colour data Temp@pH6, pH@Temp12 and pHu were also tested as covariates in the models. Subjective firmness scores were analysed using logistic regression, modelling the proportion of carcasses scoring ≤3, and including stimulation level as a fixed effect and carcass as a random effect in the model.
Results
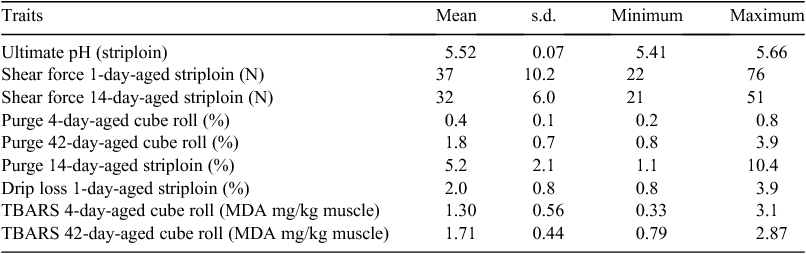
There was a difference (P < 0.001) in fat depth at the rib site with unstimulated sides having a mean of 7.5 ± 0.96 mm and the stimulated sides having a mean of 11.4 ± 0.96 mm. This reflects the removal of subcutaneous fat in the former sides. A list of the traits measured and the mean values and range is given in Table 1.
|
pH decline and ultimate pH
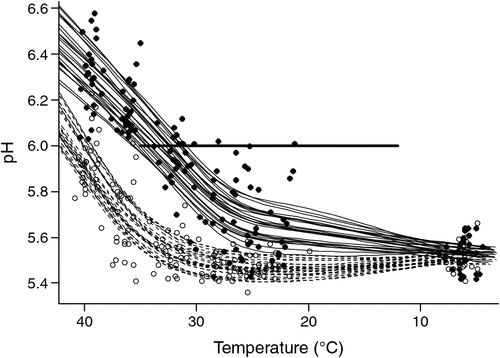
The fitted lines based on splines for pH versus temperature are shown in Fig. 1 and these indicate clearly the significant difference in the rate of pH fall achieved by the use of the experimental selection procedure. The ‘ideal’ pH/temperature ‘window’ based on MSA work is also shown as a solid line indicating that all of the stimulated sides missed the window as did several of the non-stimulated sides. The non-stimulated sides, which passed through the window, did so at the ‘hot’ end. The mean Temp@pH6 for stimulated sides was 40.9 versus 33.3°C for non-stimulated sides with a standard error of 0.14 and the corresponding pH@Temp12 values were 5.49 versus 5.57, respectively, with a standard error of 0.006. Stimulation had no significant effect (P = 0.32) on pHu, with means for stimulated and non-stimulated being 5.53 and 5.51, respectively, with a standard error of 0.013. It is worth noting that for carcasses not selected for the study from the 115 tested for pH decline the difference between sides (stimulated and non-stimulated) for Temp@pH6 based on linear interpolation was only on average 2.5°C, with means of 39 and 37°C, respectively.
|
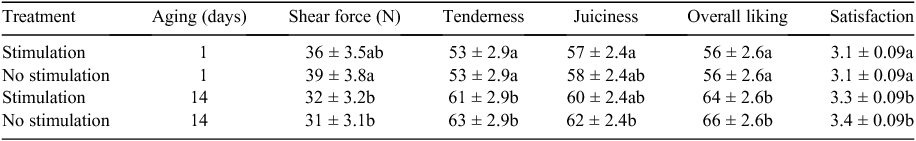
Shear force and sensory traits
Despite the difference in the rate of pH decline (reflected as either Temp@pH6 or pH@Temp12) there was no impact on shear force or sensory traits (P > 0.05; Table 2), but there was an effect of aging (P < 0.001; Table 2) on these traits. Additionally pHu did not significantly impact on shear force or sensory traits. Thus means shown in Table 2 are not adjusted for the covariates Temp@pH6, pH@Temp12 or pHu.
Subjective traits
Appraisal of the distribution of meat colour or texture scores indicated no requirement for analysis with no apparent difference due to stimulation (Table 3). There was no effect (P > 0.05) of stimulation on the distribution of firmness scores.
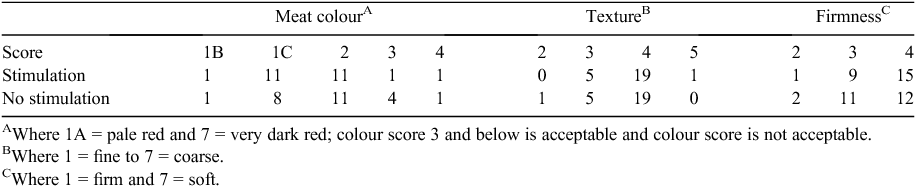
|
Drip loss and purge
There was no effect (P > 0.05) of stimulation or pH decline on drip loss of the striploin, but there was an effect (P < 0.05) of pHu, such that as pHu increased the drip loss was reduced;

For purge of the striploin after 14 days of aging there was no effect of pHu, but there was a significant joint effect of level of stimulation, Temp@pH6 and pH@Temp12. For the data analysed, the prediction model for purge (%) for striploin after 14 days under no stimulation is;

For samples from stimulated carcasses having the same pH@Temp12 and Temp@pH6, purge loss (%) for the striploin after 14 days of aging is estimated to be reduced by 5.79 ± 2.56. This model is clearly not intended to predict purge (%) for the striploin after 14 days in general, but it indicates at least for the observed data, that a faster rate of pH decline, corresponding to reduced values of Temp@pH6 and pH@Temp12, will lead to increased purge loss.
The purge for the cube roll measured after 4 or 42 days of aging was not significantly influenced by any combination of level of stimulation, pHu, or rate of pH decline. The predicted means for each level of stimulation at the average values for pHu, pH@Temp12 and Temp@pH6 are given in Table 4.

|
Colour stability and lipid oxidation
The redness of the cube rolls as reflected by a* values declined with days of display (P < 0.001), with the decline more rapid for samples aged for 42 days compared with those aged for 4 days (P < 0.001; Fig. 2). For meat aged and displayed identically, the a* values were on average significantly (P < 0.05) lower for meat from non-stimulated carcasses with the difference estimated to equal 0.44 (s.e. = 0.14) units.
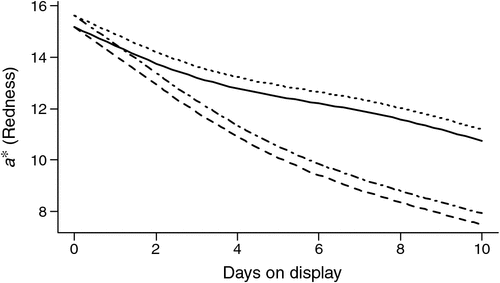
|
On average the oxymyoglobin/metmyoglobin ratio (630/580 nm) declined for 4-day-aged samples over the display period 0–10 days (P < 0.001; Fig. 3). For 42-day-aged samples there was a decline over the display period 3–7 days and then an increase in the ratio value as display increased to 10 days. The 42-day-aged samples were not monitored over the period 0–3 days of display, due to an equipment failure, but there was a difference (P < 0.001) in the change in the ratio values over display time compared with samples aged for 4 days. There was no effect (P > 0.05) of stimulation or the covariates pH@Temp12 or Temp@pH6 on the ratio values.
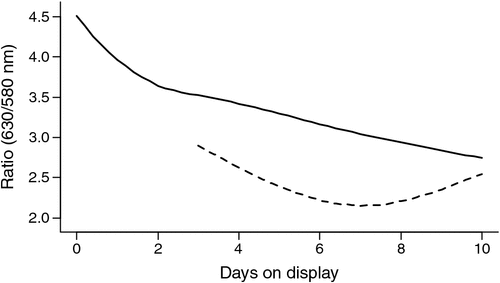
|
Of the fixed effect terms, only days on display (P < 0.05), aging (P < 0.05) and stimulation (P < 0.001) effects were significant for visual colour scores. Plots of the predicted average colour scores for each stimulation × aging combination for display periods in the interval 0–10 days are given in Fig. 4. None of the three covariates, Temp@pH6, pH@Temp12 or pHu, individually accounted for significant variation in results after fitting the above final model.

|
The extent of lipid oxidation as indicated by the level of MDA (mg/kg muscle) from the TBARS assay was not affected by either stimulation or pH@Temp12 after adjusting for pHu and Temp@pH6. However, pHu and pH@Temp6 jointly did have an effect (P < 0.05) in cube rolls aged for 4 days and then displayed for 3 days and this indicates that as Temp@pH6 decreases for a given pHu there is less formation of MDA and thus less lipid oxidation, with adjusted means for stimulated and non-stimulated meat being 1.5 ± 0.27 and 1.1 ± 0.27 MDA mg/kg muscle, respectively. By contrast there was no significant effect on MDA levels for cube rolls aged for 42 days and then displayed for 3 days with means for stimulated and non-stimulated meat being 2.0 ± 0.24 and 1.4 ± 0.24 MDA mg/kg muscle, respectively.
Discussion
Tenderness and eating quality
There was on average a 7°C difference in the Temp@pH6 between treatments, and the stimulated sides ‘missed’ the MSA temperature/pH window, but there was no adverse effect on shear force or eating quality. Based on the assertions by Thompson (2002) that ‘hitting’ the window was important this was an unexpected result. However, it is of interest that the MSA model for beef does not include measurement of pH decline as a predictive trait (Watson et al. 2008), even though a temperature/pH window is recommended. This is because there is not presently a suitable method for routinely measuring the pH-temperature fall in carcasses in a robust way. Hwang and Thompson (2001a) showed that pH decline had the largest effect on eating quality of the measured traits, but inclusion of other traits in the MSA model probably led to the exclusion of the importance of pH decline in terms of eating quality. Whether there is an effect beyond 14 days cannot be established from the current results. In fact, for shear force in the current experiment the rapid decline in pH actually meant that there was no significant further reduction from aging stimulated meat, whereas for non-stimulated meat there was a much larger response due to aging in line with expectations from previous work (Hwang et al. 2003).
In the current study electrical stimulation was used as the method to manipulate the rate of pH decline in relation to temperature as was the removal of subcutaneous fat from the non-stimulated sides. Based on these results and those for the carcasses not selected for intensive study it is apparent that the rate of pH decline would have been largely unacceptable according to the MSA temperature/pH window in the cattle studied. Similarly Warner et al. (2014b) showed that this abattoir has recorded ~40% heat-toughening overall and for the class of cattle used in the current study, the incidence of heat-toughening could exceed 70%. Likewise without stimulation Hopkins et al. (2007) showed that the majority of heavier carcasses they measured had rates of pH decline that were too fast and that compliance with the MSA temperature/pH window was poor. In using stimulation to manipulate pH it is accepted that this may give rise to subtle differences to muscle structure (Rosenvold et al. 2008) that are not found in non-stimulated meat that also undergoes a rapid decline in pH. The impacts of stimulation have been extensively covered elsewhere (Hwang et al. 2003) and this is not the primary focus of the work reported here. It is worth noting however, that timing and type of stimulation will impact on the response in pH decline, enzyme activity and thus the effect on shear force (Pike et al. 1993; Hwang and Thompson 2001b).
Given the results of previous studies that have examined the impact of elevated temperature on meat with a lower pH it may have been expected that stimulated meat in the current study would be more tender when un-aged (Thomson et al. 2008; Kim et al. 2012; Warner et al. 2014a). Additionally with aging the meat could have been expected to not tenderise to the same extent as meat that had experienced a slower rate of rigor onset (Thomson et al. 2008; Kim et al. 2012; Warner et al. 2014a). However, two of these previous studies were undertaken with excised meat that had been held at set pre-rigor temperatures (37 and 38°C, respectively), until rigor was attained (pH 5.6 and 5.55, respectively). This created a very different pre-rigor environment in which the temperature profile of the LL would be very different to that in the carcass. There is an obvious interplay between temperature, pH and enzyme activity and if the conditions allow early activation of the calpains before denaturation then there will be a commensurate improvement in tenderness (e.g. Strydom et al. 2005), with calcium having an integral role in tenderisation (Hopkins and Thompson 2001).
Liquid fraction
It could be expected that when muscle experiences a rapid decrease in pH at high temperatures that this would impact on protein functionality, with a reduction in the ability of muscle to retain water (den Hertog-Meischke et al. 1997; Bee et al. 2007). There was some evidence to support this contention in the current study as evidenced by an increase in purge in the striploin, but this did not occur in the cube roll section of the LL and drip loss was only impacted by pHu, not the rate of pH decline. A phenomenon like pale, soft exudative meat is not considered common in beef (Shen et al. 2009), but has been reported in the hindleg muscles (Aalhus et al. 1998) where presumably the decline in temperature under chilling is much slower. This suggests that the focus should be on these muscles in future studies.
Colour and oxidation
There was an effect of rate of pH decline seen by a stimulation effect on the redness of the displayed meat with some minor advantage from this treatment. There was not however an impact on the ratio (630/580 nm) values, which is an indicator (proxy) of oxidation of metmyglobin (Hunt 1980). Rate of redness deterioration was faster in meat aged for 42 days than meat aged for 4 days and the significantly shorter display life was consistent with the visual colour scores. If consumer benchmark values for lamb (Khliji et al. 2010) translate to beef then the short aged meat (4 days) was still acceptable after 10 days on display. By comparison, acceptability based on ratio values was for shorter periods of display life especially the 42-day-aged meat compared with acceptability based on redness. The colour of meat is an extremely important factor that influences a consumer’s purchase decision as it is deemed a visual measure of freshness and quality (Faustman and Cassens 1990) so the fact that rate of pH decline did not have a detrimental effect on colour is a notable result. Previous reports have found that reflectance data (instrumental) and visual scores for colour were initially better for stimulated meat aged for 6 days and displayed, but by 3 days of display had deteriorated more than non-stimulated meat (Unruh et al. 1986). Clearly in the LL colour deterioration is not an issue under accelerated rigor conditions as applied in this study.
There was some evidence that lipid oxidation was advanced by a more rapid rate of pH decline, however the reason for this is not obvious apart from a differential effect on oxygen consumption (Faustman et al. 2010).
Conclusions
An accelerated onset of rigor achieved by electrical stimulation did not have a deleterious effect on shear force or eating quality traits, but accelerated onset of rigor achieved by electrical stimulation did lead to small increases in purge. If anything the increase in pH fall had beneficial effects on meat colour under retail display, but increased the onset of lipid oxidation.
Acknowledgements
This work was supported with funding from Meat & Livestock Australia, NSW Department of Primary Industries and the Department of Primary Industries, Victoria. The authors acknowledge the assistance of Tracy Lamb and Megan Moppett (NSW Department of Primary Industries), Matthew Kerr, Athula Naththarampatha, Paul Weston and Stuart Baud (Department of Primary Industries, Victoria), Janine Lau, (Meat Standards Australia), Alan Gee (Co-Sign Pty Ltd, Coffs Harbour, NSW) and the cooperation of the collaborating abattoir.
References
Aalhus JL, Best DR, Murray AC, Jones SDM (1998) A comparison of the quality characteristics of pale, soft and exudative beef and pork. Journal of Food Science 9, 267–280.Anon. (2005) ‘Handbook of Australian meat.’ 7th edn. (AUS-MEAT Limited: Brisbane)
Anon. (2006) ‘pH measurement and decline in beef carcasses. Module 4B. Version 4.0.’ (Meat and Livestock Australia: Sydney)
Bee G, Anderson AL, Lonergan SM, Huff-Lonergan E (2007) Rate and extent of pH decline affect proteolysis of cytoskeletal proteins and water-holding capacity in pork. Meat Science 76, 359–365.
| Rate and extent of pH decline affect proteolysis of cytoskeletal proteins and water-holding capacity in pork.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXit12ltro%3D&md5=a10aca66eaf5a12bd3a7b5bcddf29d79CAS | 22064307PubMed |
den Hertog-Meischke MJA, Smulders FJM, van Logtestijn JG, van Knapen F (1997) Effect of electrical stimulation on the water-holding capacity and protein denaturation of two bovine muscles. Journal of Animal Science 75, 118–124.
Devine CE, Wahlgren NM, Tornberg E (1999) Effect of rigor temperature on muscle shortening and tenderisation of restrained and unrestrained beef m. longissimus thoracis et lumborum. Meat Science 51, 61–72.
| Effect of rigor temperature on muscle shortening and tenderisation of restrained and unrestrained beef m. longissimus thoracis et lumborum.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbnsVWqtA%3D%3D&md5=0fa670a2c0be3f3e48dbef00926751beCAS | 22061537PubMed |
Dransfield E, Etherington DJ, Taylor MAJ (1992) Modelling post-mortem tenderisation-II: enzyme changes during storage of electrically stimulated and non-stimulated beef. Meat Science 31, 75–84.
| Modelling post-mortem tenderisation-II: enzyme changes during storage of electrically stimulated and non-stimulated beef.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38Xht1Ohtrc%3D&md5=c36d0a3830ca544f80333d2f13a3e657CAS | 22059511PubMed |
Efron B, Tibshirani RJ (1993) ‘An introduction to the Bootstrap.’ (Chapman & Hall: New York)
Faustman C, Cassens RG (1990) The biochemical basis for discoloration in fresh meat: a review. Journal of Muscle Foods 1, 217–243.
| The biochemical basis for discoloration in fresh meat: a review.Crossref | GoogleScholarGoogle Scholar |
Faustman C, Sun Q, Mancini R, Suman SP (2010) Myoglobin and lipid oxidation interactions: mechanistic bases and control. Meat Science 86, 86–94.
| Myoglobin and lipid oxidation interactions: mechanistic bases and control.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXos1Cntrk%3D&md5=762efcf84fdb9955d219e456f67fbd4aCAS | 20554121PubMed |
Hopkins DL, Thompson JM (2001) Inhibition of protease activity 2. Degradation of myofibrillar proteins, myofibril examination and determination of free calcium levels. Meat Science 59, 199–209.
| Inhibition of protease activity 2. Degradation of myofibrillar proteins, myofibril examination and determination of free calcium levels.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXlt1Sht78%3D&md5=5e4179107a02bc800d0ca10ce94c5bddCAS | 22062679PubMed |
Hopkins DL, Cassar JA, Toohey ES, Wynn PC (2007) Examination of pH in lot fed beef for Japan. Proceedings of the New Zealand Society of Animal Production 67, 436–440.
Hunt MC (1980) Meat colour measurements. Proceedings Reciprocal Meat Conference 33, 41–46.
Hwang IH, Thompson JM (2001a) The effect of time and type of electrical stimulation on the calpain system and meat tenderness in beef longissimus dorsi muscle. Meat Science 58, 135–144.
| The effect of time and type of electrical stimulation on the calpain system and meat tenderness in beef longissimus dorsi muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXit1Wgurg%3D&md5=270f7e9f2075e49cd0428af203c46a10CAS | 22062108PubMed |
Hwang IH, Thompson JM (2001b) The interaction between pH and temperature decline early postmortem on the calpain system and objective tenderness in electrically stimulated beef longissimus dorsi muscle. Meat Science 58, 167–174.
| The interaction between pH and temperature decline early postmortem on the calpain system and objective tenderness in electrically stimulated beef longissimus dorsi muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXit1Wgurc%3D&md5=40939faba202cd6c32db4d2d60e5d1ecCAS | 22062112PubMed |
Hwang IH, Devine CE, Hopkins DL (2003) The biochemical and physical effects of electrical stimulation on beef and sheep meat tenderness – a review. Meat Science 65, 677–691.
| The biochemical and physical effects of electrical stimulation on beef and sheep meat tenderness – a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXktVOnsLg%3D&md5=33d6c703a2229fe4940cd9db220c0b32CAS | 22063428PubMed |
Khliji S, van de Ven R, Lamb TA, Lanza M, Hopkins DL (2010) Relationship between consumer ranking of lamb colour and objective measures of colour. Meat Science 85, 224–229.
| Relationship between consumer ranking of lamb colour and objective measures of colour.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3c3ks1amuw%3D%3D&md5=d2512e15f78e38b8bb91a6cf85d1d933CAS | 20374889PubMed |
Kim BYH, Stuart A, Nygard G, Rosenvold K (2012) High pre rigor temperature limits the ageing potential of beef that is not completely overcome by electrical stimulation and muscle restraining. Meat Science 91, 62–68.
| High pre rigor temperature limits the ageing potential of beef that is not completely overcome by electrical stimulation and muscle restraining.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjtVCmsLg%3D&md5=1f902cbe6afe74ff48f6d5b2ac116177CAS |
Locker RH, Hagyard CJ (1963) A cold shortening effect in beef muscles. Journal of the Science of Food and Agriculture 14, 787–793.
| A cold shortening effect in beef muscles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2cXlsFOiug%3D%3D&md5=ebcef65dee72d68932c06451127cd7e1CAS |
MacDougall DB (1995) Colour of meat. In ‘Quality attributes and their measurement in meat, poultry and fish products. Advances in Meat Research Vol. 9’. (Eds AM Pearson, DR Dutson) pp. 79–93. (Springer: Berlin)
Offer G (1991) Modelling of the formation of pale, soft and exudative meat: effects of chilling regime and rate and extent of glycolysis. Meat Science 30, 157–184.
| Modelling of the formation of pale, soft and exudative meat: effects of chilling regime and rate and extent of glycolysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXmslygu7Y%3D&md5=ab78c2132e3b69fa280d2bb8d1e81744CAS | 22061833PubMed |
Pike MM, Ringkob TP, Beekman DD, Koh YO, Gerthoffer WT (1993) Quadratic relationship between early-post-mortem glycolytic rate and beef tenderness. Meat Science 34, 13–26.
| Quadratic relationship between early-post-mortem glycolytic rate and beef tenderness.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXisFeltrk%3D&md5=4e8f2f89c9df9b4b2b21d0dec19eddf1CAS | 22060264PubMed |
R Development Core Team (2010) ‘R: a language and environment for statistical computing.’ (R Foundation for Statistical Computing: Vienna). Available at http://www.R-project.org [Verified 15 April 2013]
Rosenvold K, North M, Devine C, Micklander E, Hansen P, Dobbie P, Wells R (2008) The protective effect of electrical stimulation and wrapping on beef tenderness at high pre-rigor temperatures. Meat Science 79, 299–306.
| The protective effect of electrical stimulation and wrapping on beef tenderness at high pre-rigor temperatures.Crossref | GoogleScholarGoogle Scholar | 22062758PubMed |
Shen QW, Du M, Means WJ (2009) Regulation of post-mortem glycolysis and meat quality. In ‘Applied muscle biology and meat science’. (Eds M Du, R McCormick) pp. 175–194. (CRC Press/Taylor & Francis Group: Boca Raton, FL)
Strydom PE, Frylinck L, Smith MF (2005) Should electrical stimulation be applied when cold shortening is not at risk? Meat Science 70, 733–742.
| Should electrical stimulation be applied when cold shortening is not at risk?Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3Mbns1Wmsw%3D%3D&md5=482601dd9c927aeddabee0fbdddf1da7CAS | 22063900PubMed |
Thompson JM (2002) Managing meat tenderness. Meat Science 62, 295–308.
| Managing meat tenderness.Crossref | GoogleScholarGoogle Scholar |
Thomson KL, Gardner GE, Simmons N, Thompson JM (2008) Length of exposure to high post-rigor temperatures affects the tenderisation of the beef M. longissimus dorsi. Australian Journal of Experimental Agriculture 48, 1442–1450.
| Length of exposure to high post-rigor temperatures affects the tenderisation of the beef M. longissimus dorsi.Crossref | GoogleScholarGoogle Scholar |
Tornberg E (1996) Biophysical aspects of meat tenderness. Meat Science 43, S175–S191.
| Biophysical aspects of meat tenderness.Crossref | GoogleScholarGoogle Scholar |
Unruh JA, Kastner CL, Kropf DH, Dikeman ME, Hunt MC (1986) Effects of low-voltage electrical stimulation during exsanguination on meat quality and display colour stability. Meat Science 18, 281–293.
| Effects of low-voltage electrical stimulation during exsanguination on meat quality and display colour stability.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbmvFalsA%3D%3D&md5=a8aa37a737e6f2adf88ed75b5a983df2CAS | 22055733PubMed |
van de Ven RJ, Pearce KL, Hopkins DL (2014) Post-mortem modelling of pH and temperature in related lamb carcases. Meat Science 96, 1034–1039.
| Post-mortem modelling of pH and temperature in related lamb carcases.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvV2gu7jP&md5=8935549e17fae2bf1a5340bea5b609b0CAS | 23102639PubMed |
Warner RD, Thompson JM, Polkinghorne R, Gutzke D, Kearney GA (2014a) A consumer sensory study of the influence of rigor temperature on eating quality and ageing potential of beef striploin and rump. Animal Production Science 54, 396–406.
| A consumer sensory study of the influence of rigor temperature on eating quality and ageing potential of beef striploin and rump.Crossref | GoogleScholarGoogle Scholar |
Warner RD, Dunshea FR, Gutzke D, Lau J, Kearney G (2014b) Factors influencing the incidence of high rigor temperature in beef carcasses in Australia. Animal Production Science 54, 363–374.
| Factors influencing the incidence of high rigor temperature in beef carcasses in Australia.Crossref | GoogleScholarGoogle Scholar |
Watson R, Polkinghorne R, Thompson JM (2008) Development of the Meat Standards Australia (MSA) prediction model for beef palatability. Australian Journal of Experimental Agriculture 48, 1368–1379.
| Development of the Meat Standards Australia (MSA) prediction model for beef palatability.Crossref | GoogleScholarGoogle Scholar |
Witte VC, Krause GF, Bailey ME (1970) A new extraction method for determining 2-thiobarbituric acid values of pork and beef during storage. Journal of Food Science 35, 582–585.
| A new extraction method for determining 2-thiobarbituric acid values of pork and beef during storage.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE3MXmtlahsQ%3D%3D&md5=0b7387b82668e2f93f80dfb62b7e26c8CAS |