Farm-scale nitrogen, phosphorus, potassium and sulfur balances and use efficiencies on Australian dairy farms
Cameron J. P. Gourley A F , Warwick J. Dougherty B , David M. Weaver C D , Sharon R. Aarons A , Ivor M. Awty A , Donna M. Gibson A , Murray C. Hannah A , Andrew P. Smith A and Ken I. Peverill EA Future Farming Systems Research Division, Ellinbank Centre, Department of Primary Industries, Ellinbank, Vic. 3821, Australia.
B NSW Department of Primary Industries, Richmond, NSW 2753, Australia.
C Department of Agriculture and Food, Albany, WA 6330, Australia.
D Centre of Excellence for Ecohydrology, The University of Western Australia, Crawley, WA 6009, Australia.
E KIP Consultancy Services Pty Ltd, 4 Collier Court, Wheelers Hill, Vic. 3150, Australia.
F Corresponding author. Email: cameron.gourley@dpi.vic.gov.au
Animal Production Science 52(10) 929-944 https://doi.org/10.1071/AN11337
Submitted: 8 December 2011 Accepted: 13 April 2012 Published: 3 July 2012
Journal Compilation © CSIRO Publishing 2012 Open Access CC BY-NC-ND
Abstract
Efficient and effective nutrient management decisions are critical to profitable and sustainable milk production on modern Australian dairy farms. Whole-farm nutrient balances are commonly used as nutrient management tools and also for regulatory assessment on dairy farms internationally, but are rarely used in Australia. In this study, nitrogen (N), phosphorus (P), potassium (K), and sulfur (S) imports and exports were measured during a standardised production year on 41 contrasting Australian dairy farms, representing a broad range of geographic locations, milk production, herd and farm size, reliance on irrigation, and soil types. The quantity of nutrients imported varied markedly – with feed and fertiliser generally the most substantial imports – and were principally determined by stocking rate and type of imported feed. Milk exports were the largest source of nutrient exports. Nitrogen balance ranged from 47 to 601 kg N/ha.year. Nitrogen-use efficiency ranged from 14 to 50%, with a median value of 26%. Phosphorus balance ranged from –7 to 133 kg P/ha.year, with a median value of 28 kg P/ha. Phosphorus-use efficiencies ranged from 6 to 158%, with a median value of 35%. Potassium balances ranged from 13 to 452 kg K/ha, with a median value of 74 kg K/ha; K-use efficiency ranged from 9 to 48%, with a median value of 20%. Sulfur balances ranged from –1 to 184 kg S/ha, with a median value of 27 kg S/ha; S-use efficiency ranged from 6 to 110%, with a median value of 21%. Nitrogen, P, K and S balances were all positively correlated (P < 0.001) with stocking rate and milk production per ha. Poor relationship between P, K and S fertiliser inputs and milk production from home-grown pasture reflected the already high soil fertility levels measured on many of these farms. The results from this study demonstrate that increasing milk production per ha will be associated with greater nutrient surpluses at the farm scale, with the potential for greater environmental impacts. We suggest that simplified and standardised nutrient balance methodologies should be used on dairy farms in Australia to help identify opportunities for improvements in nutrient management decisions and to develop appropriate industry benchmarks and targets.
Introduction
Nutrient imports onto dairy farms, mainly in the forms of feed, fertiliser and nitrogen (N) fixation by legumes, are usually much greater than the exports in milk, animals, and crops (Satter 2001; VandeHaar and St-Pierre 2006). These nutrient surpluses, or positive balances, tend to increase as farms intensify and stocking rates increase (Halberg et al. 2005).
The Australian dairy industry – like others around the world – has undergone major changes over the last 30 years. The number of dairy farms has declined from >22 000 in 1980 to ~8000 in 2010 (Dairy Australia 2011). Over the same period, average herd size has increased from 77 cows in 1980 to 220 in 2010 and average annual milk production per cow has increased from 2750 to 5500 L. A key driver of increased milk production has been the increase in supplementary feeding and increasing forage yields and quality due to fertiliser use, particularly N (Thorrold and Doyle 2007). This ongoing trend for increased intensification is likely to increase the importation and transformations of nutrients within dairy farm operations. It is therefore important to understand how much nutrient is removed in product and the overall efficiency of nutrient use on dairy farms, as this has both environmental and economic implications.
The risk of nutrient pollution from a dairy farm increases when nutrient inputs exceed the amount of nutrients leaving the farm in products. Excess phosphorus (P) on dairy farms can result in soil P increasing beyond agronomic requirements (Weaver and Reed 1998; Mekken et al. 2006; Gourley et al. 2007), which may also increase the concentration of P in surface runoff (Sharpley 1995), and leachate (Fortune et al. 2005). Nitrogen, unlike P is not significantly buffered by soils, and where N is applied in high concentrations such as in dung, urine or fertiliser, losses through volatilisation, denitrification, runoff and leaching can be high (Rotz et al. 2005). In addition to off-farm environmental impacts, excessive nutrient accumulation and plant uptake may impact on animal health and production. For example, excess potassium (K) can accumulate in soil and feed, and can cause severe metabolic disorders in ruminants (Caple 1989). Sulfur (S) is an important nutrient for plant production and changes in fertiliser practice and formulations in the last 20 years have seen an increase in marginal or deficient S status in soils (Glendinning 1999).
Over the past 20 years, a range of environmental policies have been developed and implemented in Europe and the US, and more recently in New Zealand, with the aim of reducing nutrient losses from dairy farms to the environment. Central to many of these policy approaches has been the development and on-farm implementation of nutrient balances. A range of nutrient balance approaches of varying complexity, which include whole-farm (also called farm-gate), soil surface and soil system, have been advocated (Oenema et al. 2003) to increase system understanding, measure nutrient-use efficiency, or as policy instruments (OECD 2008). Of these, the whole-farm balance is the most simple and easy to apply. It involves calculating the difference between total nutrients imported and those exported from the farm, presented on a per-ha basis. Nutrient-use efficiency is calculated as total exported nutrients in product divided by total imported nutrients, generally expressed as a percentage.
Nutrient balances have been widely adopted in the EU (Goodlass et al. 2003) and USA (Koelsch 2005), and more recently, has become a compulsory requirement to supply milk in New Zealand (Sneath and Furness 2006). Although there are currently fewer pressures or incentives within Australia to determine nutrient balances, and their use is low when compared with EU, the USA or New Zealand, there is growing interest from catchment management authorities and dairy companies, as a nutrient balance is viewed as a useful tool in helping to achieve voluntary environmental nutrient management standards. Moreover, the marked increase in some inorganic fertiliser prices over the past decade has generated further interest in nutrient-use efficiency by dairy consultants and fertiliser company advisors as part of more comprehensive nutrient management planning.
Nutrient balance data from international studies may have limited applicability for Australian dairy farms. Australian dairy farms are predominantly grazing-based enterprises supplemented with varying amounts of purchased grain-based concentrates (Dairy Australia 2011). In contrast, North American and European dairy enterprises have dairy cows confined to barns for a substantial part of the year and principally rely on home-grown harvested forage and to a lesser extent grains. These different systems may result in different flows of nutrients and potential differences in nutrient balances and use efficiencies.
The few nutrient balance studies that have been undertaken in Australia have generally concentrated on P or N on dairy farms within a geographically limited region (Lawrie et al. 2004; Ovens et al. 2008) or have quantified nutrient balances as part of smaller-scale farmlet or paddock studies (Eckard et al. 2007; Chataway et al. 2010; Staines et al. 2011). Additionally, there has been no systematic methodology development, and differences in methodology between these studies make comparison difficult.
The ongoing intensification of Australian dairy operations and increasing pressure for efficient resource use for improved financial and environmental outcomes requires refined nutrient management practices on Australian dairy farms. Whole-farm nutrient balances provide an effective and relatively simple method for estimating the efficiency of nutrient use and potential for nutrient losses to the environment. However, there is a need to develop standardised methodologies for nutrient balances for Australian dairy farms in order to improve their applicability, identify key sources of nutrient flows and opportunities to improve nutrient-use efficiencies at the farm level.
This paper reports on the results from a detailed dairy nutrient study that quantified N, P, K, and S flows, and whole-farm nutrient balances and nutrient-use efficiencies on a diverse array of pasture-based dairy farms across Australia. We also investigate farm characteristics that influence nutrient balances and efficiencies and discuss opportunities to improve the accuracy and efficiency of determining nutrient balances on dairy farms.
Methods and materials
Selection of farms
In total, 124 dairy farms were initially selected from the eight key dairy regions within Australia. In order to ensure the selected farms represented a broad diversity of dairy production systems, a stratified-random process was used, rather than a random sampling approach. Six key criteria were considered in the farm selection process, which reduced the number of eligible farms to 84. These were: (i) farms would be present in all dairy regions, with the number of selected farms in each region broadly representing the region’s relative contribution to total farm numbers in Australia; (ii) milk production (litres per grazed ha, MP), (iii) farm size (grazed ha), (iv) reliance on irrigation (% of grazed area irrigated); (v) soil P sorption, and (vi) the inclusion of a limited number of organic farms. An iterative optimisation routine was used within the regional constraints, and resulted in a selection of farms with a wide range of combinations of the desired key characteristics. Subject to fixed regional quotas, the routine maximised the criteria,

measuring evenness of farm numbers, n, in cells, and of all possible marginal cells, of the multidimensional contingency table with dimensions given by (ii) to (v) above, here indexed by i, j, k and l. Dimensions that were quantitative, (ii) to (v), were first grouped into ‘low’, ‘medium’ and ‘high’ classes, representing approximately the bottom 25%, mid 50% and top 25% respectively of the 8 dairy farms that meet the eligibility criteria. Intentionally, even selection into these groups biased the sample slightly away from ‘medium’ towards ‘low’ and ‘high’. A total of 44 dairy farms were selected (Fig. 1), of which four were practising organic production methods (Standards Australia 2009). As a result of this selection process, there were 14 low, 19 medium and 11 high P sorption farms, respectively; 15 farms with grazing areas <100 ha, 16 farms between 100 and 300 ha, and 13 farms with grazing areas >300 ha; 16 farms with no irrigation, 17 farms with 0–75% of the grazing land irrigated, and 11 farms with >75% of the grazing land irrigated; 16 farms with milk production <9200 L/ha, 19 farms with milk production between 9200 and 18 750 L/ha and 9 farms with milk production >18 750 L/ha.

|
Data collection
The farm-scale nutrient balance approach used in this study involved calculating the difference between total nutrients imported and those exported at the farm scale, with results presented on a per-ha basis, and nutrient-use efficiency calculated as total exported nutrients divided by total imported nutrients expressed as a percentage. Similar techniques have been used in other studies (Mulier et al. 2003; Nevens et al. 2006; Fangueiro et al. 2008; Treacy et al. 2008) although we used modifications to these studies in order to suit Australian dairy farm operations (Gourley et al. 2007). Nutrient balance per unit of milk production (milk production balance; Schröder et al. 2003) was also determined. Data requirements included the mass and N, P, K, and S concentrations of all forms of purchased feed, bedding, inorganic and organic fertilisers, soil ameliorants, irrigation water, milk, animal purchases, sales and death of cows, and harvested forages exported off-farm. Estimates of inputs from N fixation and atmospheric deposition were also included. In calculating the whole-farm nutrient balance, internal transfers were not quantified.
Spatial analysis using GIS was used to quantify the total milking area and non-utilised areas on each dairy farm. Three land groups were defined as: (i) ‘contact land’ (land which was used by lactating animals for grazing and cow management), (ii) ‘home farm’ (the area within the boundary of the dairy farm, but also including areas where lactating animals were not in contact), and (iii) ‘total land’, including the home-farm area and other remote land areas used for forage production and/or keeping of dry stock.
Customised diaries were provided to all farmers at the commencement of the monitoring period for recording farm information and activities. Visits were undertaken to all 44 farms every 3 months between December 2007 and February 2009. Standard questionnaires were also used during each quarterly farm visit to collect further information and verify recorded information. A follow-up visit in July 2009 further collected any missing information and verified the compiled data for each farm. Nutrient imports and exports were then standardised for a 365-day calendar year between February 2008 and February 2009, on each farm, and expressed relative to the farm area. An inventory of stored feed, fertiliser and cattle numbers was also undertaken at the beginning and end of the study period to account for net changes in on-farm nutrient storage.
The mass of inorganic fertilisers and soil ameliorants imported was recorded and standard nutrient concentrations used as provided by commercial suppliers. Imported manure and organic fertilisers were sampled and analysed and actual nutrient concentrations from these samples were applied to the nutrient balance calculations.
Any forage grazed or harvested on the contact land and fed back on the contact land during the monitoring period contributed to home-grown forage consumed. Any forms of feed originating from non-contact areas and fed within the contact land were treated as imported feed. Imported feed types included whole and crushed grains, grain-based concentrates, ensiled or dried forages, a broad range of fresh or processed by-products, and mineral supplements. When a batch of feed was present at a quarterly visit, ~400 g of fresh material was collected and stored on ice before sample preparation for determining DM % and N, P, K, and S concentrations. The overall percentage of all feed and bedding imports which were directly sampled was 33%. This included 43% of bedding, 17% of by-products, 26% of pellets, 31% of grain, 31% of feed minerals, 53% of hay and 73% of silage imports. Consequently, when calculating nutrient imports and exports for particular sources and individual loads of feed or bedding on each farm, the following rules were applied and implemented in order: (i) actual nutrient concentrations were used when a batch of feed had been directly sampled, or (ii) nutrient concentrations from a similar source were used from that farm, or (iii) average nutrient concentrations were used from a similar source from any farm in the study, or (iv) standard nutrient concentrations from published Australian data were used.
Nutrients exported in milk were calculated from the determined N, P, K, and S concentrations of quarterly milk samples and the amount of bulk milk shipped off the farm over the corresponding period. The annual milk statement was also used to back-check the collected information from the quarterly visits. Milk samples for analysis were collected from the bulk milk vat after mixing, when both morning and afternoon milk was present. A 400-mL sample was stored on ice, and then stored frozen before chemical analysis.
Nutrients flows associated with animal purchases, sales and deaths were determined by multiplying liveweight based on breed and age class by the corresponding nutrient composition (i.e. N, 2.8%; P, 0.72%; K, 0.2%; and S, 0.8%) according to ARC (1994). Atmospheric deposition estimates were based on published figures which accounted for proximity to the coast or emissions from industry (Hutton and Leslie 1958; Hingston and Gailitis 1976; Probert 1976; Blackburn and McLeod 1983).
The N input from biological N fixation from legumes was based on the equation provided by Ledgard et al. (2001) that accounts for legume DM production and a negative effect of N fertiliser rate. Due to varying legume contents of pastures within a farm, each farm was divided into five zones (legume zone) reflecting potential differences in legume contents based on farmer opinion. Two paddocks representing each of the identified legume zones were then used to assess legume DM content using a dry weight ranking method (‘t Mannetje and Haydock 1963) during the spring growth period. Pasture production was determined for the farm as a whole, using a pasture production calculator (Heard et al. 2011) and then annual DM production was attributed to each legume zone based on the corresponding area and an assessment of relative potential pasture production by the farmer. Nitrogen fertiliser applications to each legume zone throughout the year were also recorded. The pasture production calculator (Heard et al. 2011) was also used to determine milk production from home-grown forage (MPhg L ha–1), representing the annual total milk production minus the estimated milk produced from imported feeds for each farm.
Data were collated and compiled within a relational database. Missing or questionable data were identified and a follow-up farm visit was undertaken with the participating farmers to review and fill data gaps and endorse the final data. At the end of this process, there were insufficient data from 3 out of the 44 participating farms to reliably determine balances and associated output/input efficiencies, so the data included in this paper relate to the remaining 41 dairy farms.
Analyses of feed, milk, fertilisers and irrigation water
Samples of each component of imported feed and organic fertiliser were oven-dried (60°C, 72 h) then ground to pass a 2-mm screen. Additional subsamples were oven-dried (100°C, 24 h) for calculating DM%. Samples of feed, milk and organic fertiliser were analysed by Weston Technologies, Sydney (http://www.georgewestontechnologies.com.au/, verified 31 May 2012) as follows: crude protein (CP) in feed and milk was measured according to AOAC methods (AOAC 2000; CP ÷ 6.25 was used to calculate total N concentration in feed; CP ÷ 6.38 was used to calculate total N concentration in milk); total P, K, and S in feed and organic fertiliser was measured by inductively coupled plasma optical emission spectrometry after digestion in hydrochloric and nitric acid. Irrigation water was analysed at the Monash Water Studies Centre (http://www.sci.monash.edu.au/wsc/, verified 31 May 2012) using standard methods for the examination of water and waste water (APHA-AWWA-WEF 2005).
Statistical analyses
A comparison of the two basic components of variation, namely within-farm and between-farms was undertaken for N, P, K and S concentrations of the main imported feed types. The within-farm variance is the pooled variance between all samples of that feed type within a farm. The between-farm variance estimates the additional variance between farms, unaccounted for by the variance within farms. The ratio of within-farm to total variance indicates the relative magnitude of the within-farm variance and a value greater than 60% was chosen to identify sample types and mineral contents where the within-farm variance is large.
The quarterly sampling and nutrient analysis of major feeds, organic fertilisers and milk for the individual farms provided a ‘best-case’ uncertainty distribution for measurement/sampling error alone. Measurement errors for concentrations and volume (or mass) from different sources were physically independent. Consequently the uncertainty calculations used repeated applications of the identity: Var(X + Y) = Var(X) + Var(Y), for sums of independent random variables (e.g. loads from difference sources), and the Taylor series approximation, CV2(XY) = CV2(X) + CV2(Y), for products of independent random variables (e.g. concentration by volume), to derive coefficients of variation for farm nutrient balances.
Although a more detailed variance decomposition (into components for within and between farm, time and region) were determined for specific feed types where there were large numbers of samples collected (e.g. pasture silage, pasture hay, ryegrass pasture, and milk), this was not possible with most feed types or other nutrient sources due to smaller overall datasets. Therefore, a more conservative, less detailed, approach of variance estimates was adopted for all measured feed, milk, bedding, organic fertiliser sources, where overall variance was determined from all pooled data generated within the project. Where no measured data were available, variance estimates from other published data were used. As no direct measurements of load weight were undertaken during the study, the variance of mass estimates was primarily based on previous research measurements or expert opinion.
Correlations between calculated nutrient indicators; i.e. whole-farm balance per ha, milk production balance per litre and nutrient efficiency, and key farm characteristics: i.e. farm stocking rate, % feed imported, MP, MPhg, and nutrient inputs from feed, fertiliser and N fixation were performed using S-PLUS 2000 (Systat Software, Inc., Chicago, IL, USA).
Results
Characteristics of participating dairy farms
The 41 dairy farms involved in this study represented the range of farm sizes, regional locations, livestock densities and irrigation practices typical of the Australian dairy industry (Table 1). Overall, the average total land, dairy farm land and contact land area was 336, 235, 194 ha, respectively, but varied widely, ranging from 67 to 1046, 47 to 612, and 40 to 460 ha, respectively (Table 1). Herd size across the farms ranged from 51 to 1263 cows, with an average of 296. The major breed of dairy cattle was Holstein-Friesian, with a smaller number of cross-bred herds, Jersey, Illawara and Australian Red. All dairy farms had effluent management systems; 16 farms used feed pads; 19 used sacrifice paddocks for feeding. All dairy farms used fertiliser, eight used organic fertilisers, and 40 used inorganic fertilisers. All but three dairy farms were milked twice a day, two milked once a day, the remaining farm milked three times per day. Fifteen of the dairy farms had different feeding strategies for different milking groups. Twelve of the 41 dairy farms did not use irrigation as a means of increasing pasture or crop production. The proportional area irrigated on each dairy farm ranged from 0 to 95% of the contact area, with a mean value of 34%.

|
The organic farms were smaller and more reliant on home-grown feed. The total land used for dairy production on the four organic dairy farms ranges from 135 to 344 ha, while the home-farm land areas ranged from 135 to 330 ha (Table 1). In contrast, the total land used for dairy production on the 37 conventional dairy farms ranges from 67 to 1046 ha, while the home-farm land areas ranged from 47 to 612 ha. Outblocks or leased land contributed ~15% total land on the organic farms and ~32% on the conventional farms. Herd size across the four organic dairy farms was generally smaller than the conventional herds, and ranged from 100 to 180 cows with an average of 129 cows, compared with the conventional farms which ranged from 51 to 1263 cows with an average of 313 cows (Table 1). Stocking rates, determined as home-farm stocking rates, averaged 0.74 cows/ha on the organic farms and 1.49 on the conventional farms.
Variability of key nutrient flows
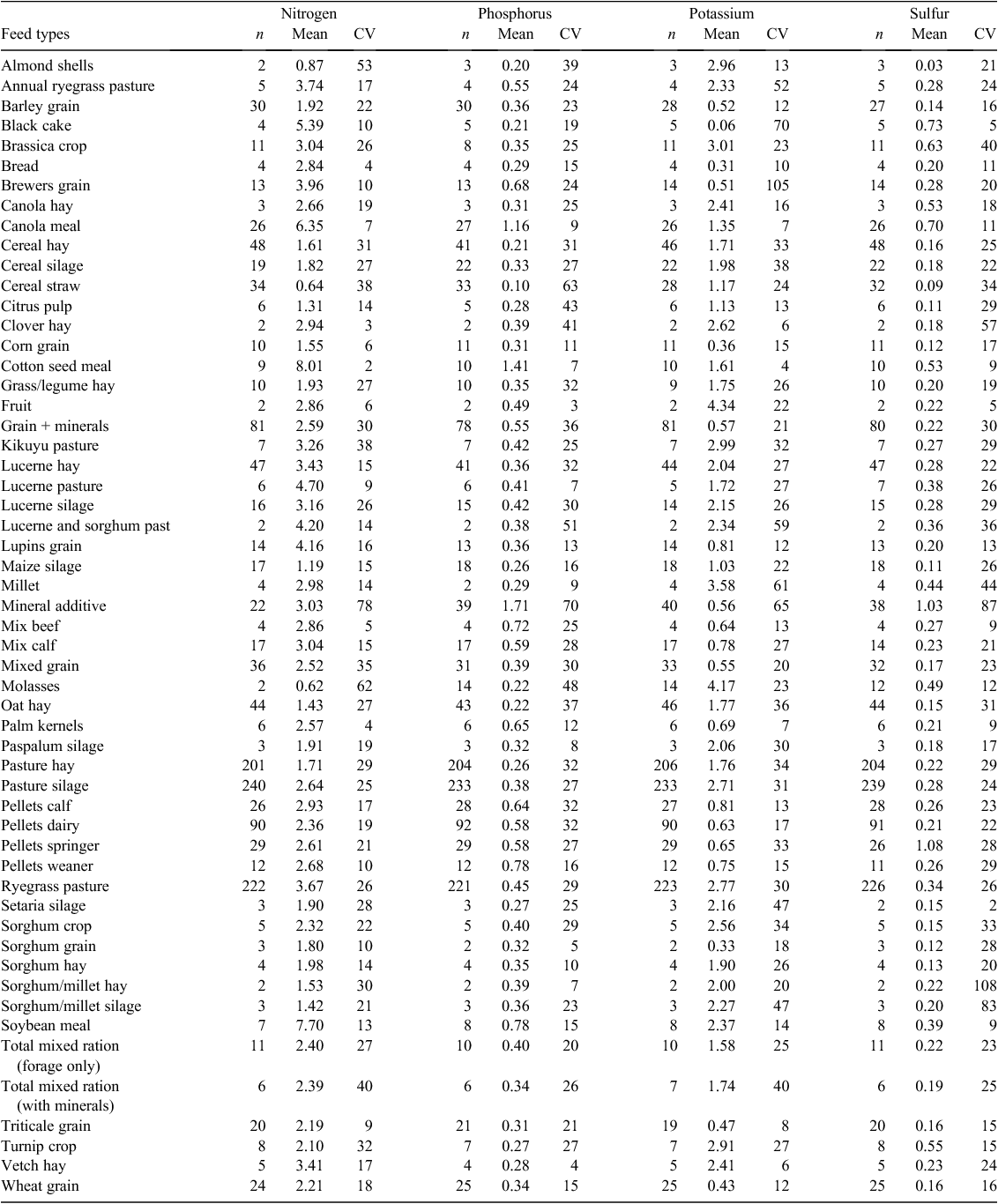
The feed used or purchased on all 41 dairy farms varied in terms of types and amounts, with a broad variety of grain, grain-based concentrates, hay, silage and by-products imported (Table 2). Grain types included wheat, barley, corn, lupins, triticale and sorghum. Grain-based concentrates were generally pelletised blends of grains with additives such as minerals and salts. Silage types included lucerne, sorghum, millet, and a variety of pasture-based silages. By-products included black cake (sugar by-product), brewers grain, canola meal, citrus pulp, cotton seed meal, tomatoes, mash, palm kernels, pea pollard and soybean meal.
|
Over the 12-month monitoring period more than 1500 separate feed samples were collected and analysed. Raw data were initially reviewed by segmenting sample analysis into broad and specific feed types and then plotting distributions. Outliers were investigated through rechecking data entry and descriptions and where deemed appropriate, reallocated to different feed types. Robust Z scores were calculated according to the NATA standards (>4 standard deviations from the mean) and therefore used to identify outlying values. Where these data points were unexplainable, they were excluded from the dataset. This applied to only 74 of the 9480 sample analyses (0.8% of sample analysis). The number of samples, N, P, K and S concentrations and CV (%) for each feed type identified during the five quarterly farm visits is provided in Table 2.
When the variance estimates for N, P, K, and S concentrations for the 19 main feed types were assessed (barley grain, brewers grain, canola meal, cereal hay, cereal silage, cereal straw, grain and minerals, lucerne hay, minerals, mixed grain, oat hay, pasture hay, pasture silage, calf pellets, dairy pellets, springer pellets, ryegrass pasture, triticale grain, and wheat grain), 64 of the 93 ‘feed type × nutrient content’ combinations had within-farm variance estimates >60% of the total variance. However, there was a distinct difference between feed types. Imported grains, pellets and minerals almost always had within-farm variation as a smaller component of the total variance (<50%), with these feed types making up 26 of the 29 remaining feed type by nutrient content combinations, while forages almost always had a greater within-farm variance. These results are not surprising considering that particular farms are likely to have a regular supplier of grains, pellets and minerals with a consequent more uniform nutrient content.
In general, the N, P and K concentrations of forages such as hay, silage and pasture were lower on organic dairy farms (P < 0.05). These farms had low stocking rates and did not import grain-based concentrates. Consequently, no comparison of the nutrient concentrations of grains or pellets between organic and conventional dairy farms was possible.
Legume contents in pastures were generally low. Of the 205 legume zones assessed, the median legume content (% DM) was 6% (mean 11%, CV 137%), with a highest legume content of 68%. While only one dairy farm had no measurable legume, 71% of legume zones had legume contents of <10% and only 8% of the legume zones had legume contents >40%. Legume contents were often heterogeneous within each farm, varying by >30% between legume zones on 37% of the farms studied. There was no influence of dairy region on legume content (P > 0.10). Correspondingly, the estimated N fixation from legumes was also generally low but often variable both within and between farms. Of the 205 legume zones assessed, the median estimated input from N fixation was 17 kg/ha (mean 36 kg/ha, CV 138%), while the highest input was 290 kg N/ha. Seventy-eight percent of legume zones had estimated N inputs from legumes of <50 kg N/ha and only 10% of the pastures assessed had N inputs from legumes of >100 kg N/ha.
There were 57 independent water samples collected from irrigation channels, rivers and bores from the 29 farms that used irrigation water. Recycled water used for irrigation was not included in this summary as this was not considered an external input. Nutrient concentrations from these varying water sources varied substantially with CV values all above 30% (Table 3).

|
The N, P, K, and S concentrations of milk were similar across all the participating dairy farms and between sampling times (Table 4). Apart from the milk S levels, variations in nutrient concentrations were small (CV <15%), demonstrating a general consistency of milk nutrient levels across the different farms, regions and seasons.

|
Nutrient flows, balances and efficiencies
The various sources of N, P, K and S imports and exports (kg/ha), and the minimum, maximum and median values of each of these (on a contact land basis) for the 41 participating farms, are provided in Table 5. The median value is presented rather than the mean, as high input farms resulted in a skewed data distribution. Almost all of the identified inputs and outputs contribute substantially in at least some dairy operations.
The single largest source of N imported was generally inorganic fertiliser, most commonly as urea and accounted for 43% of total N imports, but ranged from 0 to 88% for individual farms. Imported feed, as grain, by-products, hay and silage contributed 40% of total N imports and ranged from 4 to 79%. Interestingly, N fixation by legumes contributed 16% of total N inputs, but ranged from 0 to 88%. Feed minerals, irrigation water and atmospheric deposition contributed only a relatively small amount of imported N (on average 0.1, 0.4 and 1 kg N/ha, respectively). Milk sales were the single largest source of exported N and accounted for 82% of N exports, ranging from 53 to 99% for individual farms.
The largest source of P and K was generally imported feed, with a median contribution of 47% (range 4–98%) and 55% (range 8–98%), respectively of total P and K imports. Inorganic fertiliser accounted for 46% (range 0–92%) and 32% (range 0–84%), of total P and K imports, respectively. Sulfur was largely imported in inorganic fertiliser with a median contribution of 43% (range 0–88%), while 30% was also imported in feed (range 2–90%). Milk sales were the single largest source of exported P, K and S and accounted for 74, 94 and 52%, respectively.
Total imports of N, K and S for organic farms were generally at much lower levels per ha than on conventional farms, reflecting the smaller farm sizes, lower cow numbers and stocking rates, and limited use of manufactured fertiliser. Most N was imported either through N fixation, which ranged between 32 and 88% of the total imported N, or purchased feed, which ranged between 7 and 58%. In contrast, the application of high rates of rock phosphate (52–66 kg P/ha) on three of the organic farms resulted in high P imports. All other sources delivered very little P, K and S on organic dairy farms.
The uncertainty estimates (CV) of each ‘grouped source’ of imports (i.e. imported feed, fertiliser) or exports (i.e. milk, animals) were in general relatively small and usually less than 10%. In contrast, a high degree of uncertainty (137%) was attributed to N inputs from legumes based on the variation determined in this study. The magnitudes of these uncertainties for various nutrient inputs and export estimates are similar to those determined in other studies (Mulier et al. 2003; Oenema et al. 2003; Ledgard et al. 2004). However, as these collective variance estimates were further integrated, the uncertainty of N, P, K and S balances for the 41 farms ranged between 2 and 11%, with a median uncertainty of 4%.
The median, minimum and maximum values for N, P, K and S balances and efficiencies are provided in Table 5. It should be noted that the median balances are not the same as the sum of the median import and export values presented in the same table. Whole-farm N balances were always in surplus (imports > exports) and ranged from 47 to 601 kg N/ha. Fourteen farms had N surplus values less than 150 kg N/ha, while five farms had N surplus values above 300 kg N/ha (Fig. 2a). The median N surplus was 193 kg N/ha. The milk production N surplus ranged from 9 to 45 g N/L, with a median value of 17 g N/L. The overall N-use efficiency across the 41 dairy farms ranged from 14 to 50%, with a median value of 25%.

|
Phosphorus balances ranged from –7 to 133 kg P/ha (Table 5), with a median P balance of 26 kg P/ha. Five dairy farms were in net deficit (negative balance), with P balances <0 kg P/ha, while in contrast eight farms had P surplus values >50 kg P/ha. The milk production P balance ranged from –0.6 to 17 g P/l, with a median surplus value of 2 g P/L. The overall P-use efficiency ranged from 6 to 158%, with a median value of 32%.
All K balances were also in net surplus, ranging from 13 to 452 kg K/ha (Table 5). Eleven of the farms had K surplus values <40 kg K/ha and 14 farms had K surplus values >100 kg K/ha. The median K surplus was 74 kg K/ha. The milk production K surplus ranged from 0.1 to 25 g K/L, with a median value of 6 g K/L. The K-use efficiency ranged from 9 to 48%, with a median value of 20%. Sulfur balances ranged from –1 to 184 kg S/ha, with a median S surplus of 27 kg S/ha (Table 5). One farm had a small S deficit, while 16 farms had S surpluses <20 kg S/ha and seven farms had surplus values >50 kg S/ha. The milk production S balance ranged from 0.1 to 11 g S/L, with a median value of 2 g S/L. The overall S-use efficiency ranged from 6 to 110%, with a median value of 21%.
Relationships between nutrient balances and milk production
Key relations between milk production (L/ha) and nutrient balance (kg/ha and g/L) and nutrient use-efficiency are shown in Fig. 2. Other correlations are not shown. Nitrogen balance per ha was positively (P < 0.001) related to stocking rate, MP (Fig. 2a), MPhg, and inputs of feed and N fertiliser (P < 0.001) but not N fixation (P > 0.05). Nitrogen milk production balance per litre was negatively related to MP (Fig. 2b) and MPhg (P < 0.001), and also to stocking rate and inputs of fertiliser plus N fixation (P < 0.05). Nitrogen-use efficiency was positively related to MPhg, and negatively related to inputs via fertiliser and N fixation combined (P < 0.05). Phosphorus balance per ha was positively related to stocking rate, MP (Fig. 2d), and feed inputs (P < 0.001). Phosphorus milk production balance per litre was negatively related to MPhg (P < 0.05), and positively related to fertiliser P inputs (P < 0.001), but not to MP (P > 0.05) (Fig. 2e). Phosphorus-use efficiency was negatively related (P < 0.01) to % feed imported. Potassium balance per ha was positively related to stocking rate, % feed imported, MP (Fig. 2g), MPhg, and inputs of feed and fertiliser K (P < 0.001). Potassium milk production balance per litre was positively related to % feed imported, and negatively related to MPhg and fertiliser K inputs (P < 0.05), but not to MP (Fig. 2h). Potassium-use efficiency was negatively related to % feed imported, and inputs of feed (P < 0.05) and fertiliser K (P < 0.01). Sulfur balance per ha was positively related to stocking rate, % feed imported, MP (Fig. 2j), MPhg, and inputs of feed and fertiliser S (P < 0.001). Sulfur milk production balance per litre and S-use efficiency were positively related to fertiliser S inputs (P < 0.001). There was no relationship (P > 0.05) between N-, P-, K- and S-use efficiencies and stocking rate or MP (Fig. 2c, f, i, l).
Feed N, P, K and S inputs were higher (P < 0.01) on farms with higher stocking rates and MP. There was a positive correlation between N fertiliser input and MPhg (Fig. 3a), which was further improved by the inclusion of N input from N fixation (Fig. 3b). In contrast, there were poorly defined relationships between P and K fertiliser inputs and MPhg (Fig. 3c, d), with substantial variation in the amount of P and K fertiliser applied on farms with similar levels of MPhg.

|
Impact of different dairy land bases and standardised nutrient concentrations
The home-farm area for each farm was between 4 and 119% (median 13%) larger than the contact area, while the total-farm area was between 6 and 302% (median 59%) larger than the contact land. The relative increase in area was <10% for 18 farms and >20% for 11 farms when home-farm area was compared with contact land, while the relative increase in area was <10% for only four farms and >20% for 32 farms, when total farm was compared with contact land. Not surprisingly, as the land area increased, the net nutrient balances per ha decreased. For example, N surplus ranged from 47 to 601 kg N/ha (median 194 kg N/ha) for contact land, 40 to 301 kg N/ha (median 179 kg N/ha) for home-farm and 14 to 301 kg N/ha (median 127 kg N/ha) for total-farm land. Nitrogen-use efficiencies were not influenced by land area.
The difference in whole-farm balances, when using farm-derived nutrient concentrations for feed, milk, irrigation water and organic fertiliser, or standardised ‘book values’ for these components, were in most cases small, both in absolute and relative terms. For example, the N surplus increased for 20 farms and decreased for 21 farms. The relative difference in N surplus was <5% for 21 of the 41 farms, >10% for 5 farms, and >15% for only two farms. Not surprisingly, the magnitude of these differences was largely driven by the reliance of imported feed onto each farm. There were negligible changes in nutrient exports. Similar results were determined for P, K and S.
Discussion
Nutrient balances and use efficiencies
The farms involved in this study included a broad range of grazed dairy systems that reflect the diversity of the Australian dairy industry. Milk production for the 41 dairy farms ranged from 2948 to 36 637 L/ha (median 10 866 L/ha; mean 12 388 L/ha), which was similar to the national average of ~12 000 L/ha (Dairy Australia 2011). The dairy systems studied also had a broad range of feed and fertiliser inputs, reflecting the climatic and seasonal differences between regions as well as milk production goals.
The integrated uncertainty of the whole-farm nutrient balances was relatively small (median 4%) and provided a high degree of confidence in the estimates of nutrient balance (surplus or deficit) and nutrient-use efficiency for the individual farms studied. Milk was the single largest source of N, P, K and S export. Fertiliser and imported feed provided substantial inputs, while bedding, atmospheric deposition and irrigation water made relatively minor nutrient contributions. Similar results have been found in other studies (Nevens et al. 2006; Fangueiro et al. 2008; Ovens et al. 2008; Treacy et al. 2008). It is interesting to note that the organic farms included in this study imported relatively high rates of P, largely as rock phosphate. There was a generally low legume content in most of the dairy pastures studied, potentially due to either regular N fertiliser inputs (McKenzie et al. 2003a), competition from other species such as ryegrass, and/or dry seasonal conditions. However, N fixation by legumes provided a substantial source of N on some farms, and the variation was not related to region. Additionally, the movement of animals and feed minerals also provided substantial nutrient inputs or exports on some farms. These results indicate that it is essential to include estimates of N fixation and all types of feed and fertiliser imports, as well as changes in animal numbers, when determining nutrient balances across the diversity of Australian dairy production systems.
The degree of variation in nutrient contents of imported feeds suggests that greater accuracy in nutrient balances could be achieved through direct sampling from individual farms or segmenting dairy feeds based on regions, seasons and organic or conventional status. In contrast milk nutrient concentrations were highly consistent and supported the use of standardised concentrations (NRC 2001; Mulier et al. 2003). In the broader context of whole-farm nutrient balances however, the variability in nutrient concentrations of inputs and exports became less important. The substitution of book values for measured values of feed imports and milk exports in the calculations resulted in only relatively small changes to whole-farm balances and efficiencies on almost all the farms studied. Not surprisingly, the magnitude of these differences was largely driven by the relative contribution from imported and exported feed, as other key nutrient sources such as inorganic fertiliser imports were already determined from published nutrient concentrations. While using the most accurate data are clearly desirable, few farms undertake mineral analysis of feeds (Dairy Australia 2010), and limited data are available specific to dairy regions or sampling times. Consequently, the use of industry-wide nutrient concentrations for particular feed and other nutrient sources is a more practical approach when nutrient concentrations specific for an individual farm are not available. An important exception to this could be made when considering organic dairy farms. These farms use specifically selected feeds and fertilisers that meet strict organic or biodynamic standards (Standards Australia 2009). The significant differences (P < 0.05) in N, P and K concentrations between organic and conventional dairy farms for a range of feed types would support the use of a separate set of nutrient concentration standards for this part of the industry.
Another important part of standardising nutrient balances in Australia is to ensure that the corresponding land base used to estimate per-ha nutrient balances is well defined and uniformly applied. Many dairy production systems in Australia have separate land areas that contribute to milk production through the production of forage and grain or feeding of young or dry stock (Dairy Australia 2010), and which was reflected in the land base of the farms associated with this study. The assessment of three different land use categories highlights the difficulties in comparing nutrient balance information presented on a per-ha basis from different studies when the land base used is different or poorly defined. We recommend that contact-land area (land which was used by lactating animals for grazing and cow management, including laneways and holding areas) be used as the basis of standardising land area for nutrient balance determination in Australia and elsewhere. This land represents the principal management area contributing to milk production, is the major land area where nutrient cycling and deposition is occurring, and is the likely area contributing to nutrient accumulation and losses.
Previous studies have considered N balances and efficiencies more commonly than other nutrients (Goodlass et al. 2003; Halberg et al. 2005). This is because of the greater magnitude of N flows and surpluses at the farm scale, the lack of reliable soil N tests and the recognition of measured surplus as a quantifiable loss to the broader environment (Oenema et al. 2009; Jarvis et al. 2011). This does not however reduce the importance of other nutrient balance assessments, which are important both as part of an environmental performance assessment (particularly in the case of P) and as production/economic factors in improving management decisions for fertiliser and manure.
Nitrogen surpluses in this study ranged from 47 to 601 kg N/ha and 9 to 45 g N/L with N-use efficiency ranging from 14 to 50%. Although there is a wide variation in N balances and efficiencies, they are consistent with results from other comparable dairy studies in Australia and internationally. For example, a large study involving 130 commercial dairy systems across Western Europe reported average regional N surpluses between 93 and 502 kg N/ha, 15 and 28 g N/L and N-use efficiencies ranging between 19 and 40% (Raison et al. 2006). Similar ranges in N surpluses and use efficiencies have been reported on commercial dairy farms in New Zealand (Ledgard et al. 2004), the USA (Hristov et al. 2006), and Europe (i.e. Van der Meer 2001; Nevens et al. 2006; Fangueiro et al. 2008; Treacy et al. 2008). In Australia, there have been few studies involving commercial farms. In an assessment of 44 dairy farms in south-west Western Australia, Ovens et al. (2008) reported N surpluses ranging from 40 to 700 kg N/ha (median 128 kg N/ha) and N-use efficiencies between 8 and 50% (median 19%). In a paddock-scale N fertiliser study in south-east Victoria, Eckard et al. (2007) reported N surpluses of between 48 and 229 kg N/ha and N-use efficiency between 30 and 50%. In a 4-year farmlet study with differing stocking rates and feed and fertiliser inputs in south-east Queensland, Chataway et al. (2010) reported N surpluses between 178 and 600 kg N/ha and use efficiencies between 16 and 25%. In south-west Western Australia, Staines et al. (2011) reported N surpluses from a dairy farmlet study, which ranged between 72 and 779 kg N/ha and use efficiencies between 17 and 50%.
The results obtained in our study demonstrate a strong correlation between total N imported and milk production per ha. Nitrogen surplus was also strongly related to stocking rate and milk production (Fig. 2a) with the slope of this linear relationship (0.0121; s.e. = 0.0015) providing a national industry estimate of the milk production N surplus, equivalent to 12.1 g N/L milk produced. Despite year-round grazing and the contrasting climatic conditions of Australian dairy systems, the same relationship (slope = 0.012) has been described for Western European dairy farms with milk production ranging from 3000 to 50 000 L/ha (Raison et al. 2006). Similar positive relationships between stocking rate and N surplus, and milk production and N surplus have also been described for dairy farms in Flanders (Nevens et al. 2006), Ireland (Treacy et al. 2008), Northern Portugal (Fangueiro et al. 2008), and Western Australia (Staines et al. 2011). Interestingly, there was no significant relationship between N surplus/ha and reliance on imported feed. This may be explained by a counter-balancing of N inputs from fertiliser N resulting in higher forage yields which support greater milk production and consequently export of N (King and Stockdale 1980; McKenzie et al. 2003b).
There was also no significant relationship between stocking rate and whole-farm N-use efficiency and milk production per ha and whole-farm N-use efficiency (Fig. 2c). While a decrease in whole-farm N-use efficiency on dairy farms would be expected to occur with increased stocking rates when other influences remain similar (Staines et al. 2011), factors such as the biological potential of cows to transform feed N into milk and soil and climatic conditions which affect the utilisation of applied fertiliser and recycled N in manure by pastures and crops (Powell et al. 2010) may be quite different between farms. This suggests that within-farm management practices across the diversity of farms studied, as well as soil and climatic characteristics, are likely to be key drivers of N-use efficiency, rather than prescriptive farm characteristics alone.
Whole-farm P balances ranged from small net deficits to surpluses in excess of 100 kg P/ha with P balance poorly related to milk production per ha (Fig. 2d). Interestingly, three of the organic dairy farms with relatively low milk production had P surpluses >50 kg P/ha, while farms with milk production around the national average (12 000–15 000 L/ha) had wide ranging P balances between –8 and 90 kg P/ha. Similarly broad ranges of P balances have been reported for commercial dairy farms in Northern Portugal (5–72 kg P/ha; Fangueiro et al. 2008), Western Europe (4–36 kg P/ha; Raison et al. 2006), New South Wales (1–127 kg P/ha; Lawrie et al. 2004) and Western Australia (3–200 kg P/ha; Ovens et al. 2008). A recent meta-analysis of P balances for sheep, beef, dairying and cropping in Australia (Weaver and Wong 2011) calculated a median P surplus for dairy farms of 18.1 kg P/ha, which was significantly greater than the other enterprises, and a median P-use efficiency of 19%.
The overall trend of increasing K and S surpluses with increasing milk production (Fig. 2g, j) reflects greater imports of both feed and fertiliser as dairy systems intensify. The increasing reliance on imported feed on many dairy farms and relatively high K concentration in forages in particular, can result in substantial K (and to a lesser extent S) imports. Potassium surpluses were generally of a similar order to those determined for N. In turn, S surpluses tend to be similar to those determined for P. Very few studies have considered K balances on dairy farms and none appear to have measured S balances. Fangueiro et al. (2008) reported K surpluses between 52 and 107 kg K/ha for a range of commercial dairy farms in Northern Portugal, while Chataway et al. (2010) found that K surpluses ranged between 22 and 156 kg K/ha in five contrasting farmlets in south-east Queensland; both lower than those determined in this study.
Environmental and management implications
A key driver of increased milk production in Australia over the past three decades has been the increase in supplementary feeding (Dairy Australia 2011) and increasing forage yields due to fertiliser use, particularly N (Eckard et al. 2004). While farm numbers are expected to continue to decline, milk production per farm and per ha is expected to increase (Dairy Australia 2011). This ongoing intensification is likely to further exacerbate nutrient surpluses at the farm scale and create further environmental challenges in relation to water quality, particularly for excess N and P. Fangueiro et al. (2008) argue that lower N surpluses per litre of milk occur in higher input systems. However, in this study we did not find any relationship between milk production and N milk production surplus (Fig. 2b) although the farms with lower milk production had a wider range of milk production surpluses. As environmental impacts are quantified principally on an area basis, surplus per ha is more widely recognised as being the more relevant metric (Nevens et al. 2006).
All other things being equal, a greater whole-farm N surplus is recognised as resulting in higher losses of N to the broader environment (Ledgard et al. 1999; Jarvis et al. 2011). Pathways and forms of N loss from dairy farms include the volatilisation of ammonia, emission of nitrous oxide and dinitrogen, and surface runoff, sub-surface lateral flow and leaching of dissolved nitrate and organic forms of N. The magnitude of these N loss pathways will largely be determined by system characteristics such as livestock management and housing, N fertiliser rates and timing, soil conditions, urinary N loads, and manure collection and application practices (Jarvis et al. 2011). Particular management strategies are often directed to reduce N losses in particular forms, i.e. nitrate (Ledgard et al. 2004), nitrous oxide (de Klein and Eckard 2008) and ammonia (Hristov et al. 2011), and while these may assist in meeting particular environmental targets, they may also result in pollution ‘swapping’ (Stevens and Quinton 2008), i.e. a decrease in one loss pathway may increase another. This is particularly so when these strategies are not accompanied by attempts to improve N-use efficiency. The diversity of dairy farming systems and climatic and soil conditions experienced in Australia, makes it difficult to make general predictions about the forms and amounts of N losses through these different pathways. In general, it is agreed that improved farm and fertiliser management practices that increase the overall utilisation of N will be the most effective method of reducing gaseous and non-gaseous N losses without simple pollution swapping (Jarvis et al. 2011). Consequently whole-farm N surpluses and N-use efficiency provide a simple way to quantify and differentiate the utilisation of N, and when combined with information on key components of N cycling on dairy farms can greatly assist in targeting improvements in management (Powell et al. 2010; Gourley et al. 2012).
In contrast, whole-farm balances and efficiencies of P, K and S are not as useful unless they are also considered in combination with existing soil fertility levels, the potential for accumulation or depletion and potential environmental impacts. Moreover, the cycling of P, K, S and N will be spatially and temporally heterogeneous within a farm, with some areas having high nutrient surpluses due to animal excreta patterns and differential fertiliser applications while others may be in net deficit (Gourley et al. 2007).
While not considered as an immediate and directly quantifiable indicator of loss, P surplus appear to be resulting in increased soil P levels on Australian dairy farms, often well above agronomic levels (Gourley et al. 2010; Weaver and Wong 2011), which pose an increased risk of greater P losses in surface water (Sharpley 1995). In general, dairy farms that did not apply P fertiliser in any form had lower surpluses and higher P-use efficiencies. There were a small number of farms with whole-farm P deficits, indicated a net removal of P, presumably largely from the soil. This appears to be warranted in some systems where existing soil P levels are above recommended thresholds of adequacy (Weaver and Reed 1998; Gourley et al. 2006; Weaver and Wong 2011). Staines et al. (2011) achieved P surpluses of 0–7 kg/ha.year and P efficiencies of 89–115% (average over 4 years) in a farmlet study when fertiliser P was applied only to paddocks where soil tests indicated a requirement for P fertiliser. However, P deficits may also be potentially limiting pasture growth and milk production on several low input farms when soil P is already below adequate levels. As K and S losses are not usually associated with environmental impacts, whole-farm balances are more useful in determining potential fertiliser requirements. However, subsequent increases in soil K levels and luxury uptake of K by pastures or crops can also cause serious metabolic disorders in dairy cows such as grass tetany and milk fever (Caple 1989).
In the present study, high nutrient surpluses were generally associated with high milk production per ha and high imports of fertiliser and feed. However, the efficiency of nutrient use was generally variable irrespective of milk production. While similar ranges of surpluses and efficiencies also exist internationally, strict regulations in parts of Europe and USA have forced dairy farmers to improve nutrient efficiencies and reduce whole-farm surpluses. Key management strategies have included a reduction in, or more strategic use of, inorganic fertilisers, optimising the use of home-produced manure, reduced grazing time and lowering nutrient concentrations in the ration (Oenema et al. 2011), which have resulted in substantial reductions in N and P surpluses and increases in use efficiencies in the Netherlands (Groot et al. 2006), Flanders (Nevens et al. 2006), south-west England (Cherry et al. 2012), Northern Portugal (Fangueiro et al. 2008) as well as contrasting dairy systems in the USA (Kohn et al. 1997; Jonker et al. 2002).
Improvements in nutrient-use efficiency should also be expected on Australian dairy farms. For example, while we found that MPhg increased with increasing N fertiliser inputs (Fig. 3a), there was a high degree of variation around the effectiveness of N fertiliser applications. Although factors outside of management control, such as climate and soil characteristics, will undoubtedly be influencing the efficiency of N use by pastures and crops, there is likely to be further improvements through better management of applied N (McKenzie et al. 2003b). The potential milk production benefits of applying P, K and S fertilisers should also be strongly scrutinised as limited milk production gains appear to result from further fertiliser inputs. This in large part may be explained by the generally high levels of soil P, K and S measured on these dairy farms (Gourley et al. 2010), suggesting that soil P, K and S reserves can be utilised for a period of time without a resulting decline in milk production. Levels of soil P in excess of agronomic requirements have also been reported recently in a range of agricultural industries across Australia (Simpson et al. 2011; Weaver and Wong 2011).
As a result of the common practice of year-round grazing, a much smaller proportion of dairy manure is usually collected in Australia than from housing systems overseas (Gourley et al. 2012) and generally from concreted areas such as the dairy parlour, holding yards and feed pads. Collected manure in grazing-based systems is more frequently applied to readily accessible paddocks adjacent to the holding dams (Gourley et al. 2007) and as cow numbers and reliance on manual feeding systems increase, continued poor redistribution of collected manure has the potential to result in greater nutrient losses in the future. Consequently, further investment in collection, storage and redistribution systems may be required to overcome current and future inefficiencies in the recycling of manure nutrients.
Improving nutrient intakes and reducing the concentration of excreted nutrients may be more difficult on grazing-based dairy farms, particularly when pasture comprises the majority of the diet. Nutrient intakes in pasture can vary significantly between farms and seasons, and excess levels of dietary N, P and K intake are common, particularly during spring (Jacobs and Rigby 1999) due to regular use of fertilisers to optimise milk production and the application of dairy effluent. McKenzie et al. (2003c) found in Victorian dairy pastures that increasing rates of N fertiliser consistently elevated whole sward CP content, with this effect still evident 3 months after the last N application. Better balanced diets can result from improved selections of imported feeds. For example, the use of by-products such as brewer’s grain has the potential to increase nutrient concentrations in the diet, while in contrast, the use of concentrates and cereal and maize silage presents opportunities to better balance energy and CP levels in dairy feeds.
Conclusions and recommendations
Despite a lack of regulatory policy approaches to deal with diffuse pollution from agriculture in Australia, the need for ongoing productivity improvements, and the increasingly stringent environmental standards of international markets, justifies the need for reductions in nutrient surpluses and increased nutrient-use efficiency on Australian dairy farms.
Our data suggest that increasing milk production per ha will increase nutrient surpluses at the farm scale and consequently increase the risk of adverse environmental impacts from Australian dairy farms. Consequently, simple and effective assessment methods are needed to understand the potential efficiency of nutrient use in Australian dairy systems and to set realistic goals for improved nutrient balances and efficiencies. Information relating to whole-farm nutrient balances continues to be well received by farmers and policy makers internationally, due to the relative accessibility of information used and ability to integrate farm-based information into simple and easy to understand outputs. A similar approach appears well justified for the Australian dairy industry. At the farm level, the greater use of nutrient balances will enable more targeted mitigation strategies, improving both profitability and environmental outcomes. At the industry and government level, industry wide nutrient balances will provide an evidence-based approach to improved environmental standards and help shape strategic policy directions.
Further research is needed to better quantify the environmental, productivity and economic gains from improved on-farm practices which capture more nutrients in milk production. This information can then be used to develop and apply recommendations that have a greater probability of being implemented on commercial dairy farms.
Acknowledgements
The authors would like to thank the Accounting for Nutrients Project Advisory Committee for their constructive contributions throughout the project. We would also like to acknowledge the following individuals and their organisations for providing substantial in-kind support to farm data collection and processing: Martin Clarke, John Grant (DAF Western Australia), Rob Chataway (Queensland DPI), Nigel Fleming, Phil Lewis (South Australia RDI), John Lindsay, Dick Bryant, Ashley Senn, Rick Jennings, Hayden Kingston, Craig Muir, Michael Davy, Scott Richards, Ken Giddings (New South Wales DPI), Jessica Coad, Lucy Burkitt (Tasmanian IAR), Scott McDonald, Leah DeVries, Paul Durling, Jenny Collins and Lianne Dorling (DPI Victoria) for their contributions. We would also like to thank Professor Mark Powell (USDA – ARS) for his contribution to the development of project methodology and data interpretation. In particular we would like to thank the 44 farmers who participated in this project. Their time and effort in collecting and providing farm information was essential to achieve successful project outcomes. Financial support was provided by the Department of Primary Industries Victoria (MIS project 06854), Dairy Australia, Incitec-Pivot Limited, Megafert and Impact Fertilisers, and Land and Water Australia.
References
APHA-AWWA-WEF (2005) ‘Standard methods for the examination of water and wastewater.’ 21st edn. (American Public Health Association: Washington DC)ARC (1994) ‘The nutrient requirements of ruminant livestock.’ Technical review by an Agricultural Research Council Working Party. (CAB International: Oxon, UK)
AOAC (2000) ‘Official methods of analysis, vol. II.’ 17th edn. (AOAC: Arlington, VA)
Blackburn G, McLeod S (1983) Salinity of atmospheric precipitation in the Murray-Darling drainage division, Australia. Australian Journal of Soil Research 21, 411–434.
| Salinity of atmospheric precipitation in the Murray-Darling drainage division, Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXhtVOjsLg%3D&md5=d64891a6c189b01303b5e9ed9dba8f12CAS |
Caple IW (1989) Nutritional problems affecting calcium and magnesium metabolism in grazing ruminants. In ‘Recent advances in animal nutrition in Australia’. (Ed. DJ Farrell) pp. 37–46. (University of New England: Armidale)
Chataway RG, Walker RG, Callow MN (2010) Development of profitable milk production systems for northern Australia: a field assessment of the productivity of five potential farming systems using farmlets. Animal Production Science 50, 246–264.
| Development of profitable milk production systems for northern Australia: a field assessment of the productivity of five potential farming systems using farmlets.Crossref | GoogleScholarGoogle Scholar |
Cherry K, Mooney SJ, Ramsden S, Shepherd MA (2012) Using field and farm nitrogen budgets to assess the effectiveness of actions mitigating N loss to water. Agriculture Ecosystems & Environment 147, 82–88.
| Using field and farm nitrogen budgets to assess the effectiveness of actions mitigating N loss to water.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs1yrsrjK&md5=8ae2b85a3883dcd34481b1c3ccbf0665CAS |
Dairy Australia (2010) Dairy feeding update. Available at http://www.dairyaustralia.com.au/Standard-Items/News/Dairy-News/Dairy-feeding-2010-update.aspx [verified 4 November 2011]
Dairy Australia (2011) The Australian dairy industry in focus. Available at http://www.dairyaustralia.com.au/Statistics-and-markets/Dairy-Situation-and-Outlook-September-2011.aspx [verified 4 November 2011]
de Klein CAM, Eckard RJ (2008) Targeted technologies for nitrous oxide abatement from animal agriculture. Australian Journal of Experimental Agriculture 48, 14–20.
| Targeted technologies for nitrous oxide abatement from animal agriculture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXovVKi&md5=f81b863f6145ba4db3ca8176ab8ae94aCAS |
Eckard RJ, Chapman DF, White RE, Chen D (2004) The environmental impact of nitrogen fertilizer use on dairy pastures. Australian Journal of Dairy Technology 59, 145–148.
Eckard RJ, Chapman DF, White RE (2007) Nitrogen balances in temperate perennial grass and clover dairy pastures in south-eastern Australia. Australian Journal of Agricultural Research 58, 1167–1173.
| Nitrogen balances in temperate perennial grass and clover dairy pastures in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsVentrbJ&md5=4cf0276018f1b7a0fac47cb668569a06CAS |
Fangueiro D, Pereira J, Coutinho J, Moreira N, Trindade H (2008) NPK farm-gate nutrient balances in dairy farms from Northwest Portugal. European Journal of Agronomy 28, 625–634.
| NPK farm-gate nutrient balances in dairy farms from Northwest Portugal.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXktFClt7o%3D&md5=b4d177c814e8e29e2c2415f8b43275d0CAS |
Fortune S, Lu J, Addiscott TM, Brookes PC (2005) Assessment of phosphorus leaching losses from arable land. Plant and Soil 269, 99–108.
| Assessment of phosphorus leaching losses from arable land.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXks1Oisr0%3D&md5=8a57ed505eb76490cb64c9e657bfd512CAS |
Glendinning JS (1999) ‘Australian soil fertility manual.’ (CSIRO Publishing: Melbourne)
Goodlass G, Halberg N, Verschuur G (2003) Input output accounting systems in the European community – an appraisal of their usefulness in raising awareness of environmental problems. European Journal of Agronomy 20, 17–24.
| Input output accounting systems in the European community – an appraisal of their usefulness in raising awareness of environmental problems.Crossref | GoogleScholarGoogle Scholar |
Gourley CJP, Melland AM, Waller RA, Awty IM, Smith AP, Peverill KI, Hannah MC (2006) Making better fertiliser decisions for grazed pastures in Australia. Available at http://www.asris.csiro.au/themes/nutrients [verified 4 November 2011]
Gourley CJP, Powell JM, Dougherty WJ, Weaver DM (2007) Nutrient budgeting as an approach for improving nutrient management on Australian dairy farms. Australian Journal of Experimental Agriculture 47, 1064–1074.
| Nutrient budgeting as an approach for improving nutrient management on Australian dairy farms.Crossref | GoogleScholarGoogle Scholar |
Gourley CJP, Aarons SR, Hannah MD, Dougherty WJ (2010) Soil nutrient concentrations and variations on dairy farms in Australia. In ‘Proceedings of the 19th World Congress of Soil Science, Soil Solutions for a Changing World. 1–6 August 2010, Brisbane, Australia’. (Eds RJ Gilkes, N Prakongkep) (DVD) (IUSS: Brisbane)
Gourley CJP, Aarons SR, Powell JM (2012) Nitrogen use efficiency and manure management in contrasting dairy production systems. Agriculture Ecosystems & Environment 147, 73–81.
| Nitrogen use efficiency and manure management in contrasting dairy production systems.Crossref | GoogleScholarGoogle Scholar |
Groot JC, Rossing WAH, Lantinga EA (2006) Evolution of farm management, nitrogen efficiency and economic performance on Dutch dairy farms reducing external inputs. Livestock Science 100, 99–110.
| Evolution of farm management, nitrogen efficiency and economic performance on Dutch dairy farms reducing external inputs.Crossref | GoogleScholarGoogle Scholar |
Halberg N, Verschuur G, Goodlass G (2005) Farm level environmental indicators; are they useful? An overview of green accounting systems for European farms. Agriculture Ecosystems & Environment 105, 195–212.
| Farm level environmental indicators; are they useful? An overview of green accounting systems for European farms.Crossref | GoogleScholarGoogle Scholar |
Heard JW, Doyle PT, Francis SA, Staines MVH, Wales WJ (2011) Calculating dry matter consumption of dairy herds in Australia: the need to fully account for energy requirements and issues with estimating energy supply. Animal Production Science 51, 605–614.
| Calculating dry matter consumption of dairy herds in Australia: the need to fully account for energy requirements and issues with estimating energy supply.Crossref | GoogleScholarGoogle Scholar |
Hingston FJ, Gailitis V (1976) The geographic variation of salt precipitated over Western Australia. Australian Journal of Soil Research 14, 319–335.
| The geographic variation of salt precipitated over Western Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2sXns1ehsA%3D%3D&md5=dc2c5fb20764a68baa73b20a4ed9d8e1CAS |
Hristov AN, Hazen W, Ellsworth JW (2006) Efficiency of use of imported nitrogen, phosphorus and potassium and potential for reducing phosphorus imports on Idaho dairy farms. Journal of Dairy Science 89, 3702–3712.
| Efficiency of use of imported nitrogen, phosphorus and potassium and potential for reducing phosphorus imports on Idaho dairy farms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xos1Kht7g%3D&md5=42cc062a7c732541486c31886117a5b3CAS |
Hristov AN, Hanigan M, Cole A, Todd R, McAllister TA, Ndegwa PM, Rotz A (2011) Review: ammonia emissions from dairy farms and beef feedlots. Canadian Journal of Animal Science 91, 1–35.
| Review: ammonia emissions from dairy farms and beef feedlots.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmtF2itLw%3D&md5=a22b8ac3a4ca8ba1f92a983def264981CAS |
Hutton JT, Leslie TI (1958) Accession of non-nitrogenous ions dissolved in rainwater to soils in Victoria. Australian Journal of Agricultural Research 9, 492–507.
| Accession of non-nitrogenous ions dissolved in rainwater to soils in Victoria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaG1cXhtVWgtbY%3D&md5=7da7d4cbe56fb40a0f7b6843d77a082cCAS |
Jacobs JL, Rigby SE (1999) ‘Minerals in dairy pastures in Victoria.’ (Department of Natural Resources and Environment: Melbourne)
Jarvis S, Hutchings N, Brentrup F, Olesen JE, van der Hoek KW (2011) Nitrogen flows in farming systems across Europe. In ‘The European nitrogen assessment: sources, effects and policy perspectives’. (Eds MA Sutton, CA Howard, JW Erisman, A Bleeker, P Greennfelt, H van Grinsven, B Grizzetti) pp. 211–228. (Cambridge University Press: Cambridge)
Jonker JS, Kohn RA, High J (2002) Dairy herd management practices that impact nitrogen utilization efficiency. Journal of Dairy Science 85, 1218–1226.
| Dairy herd management practices that impact nitrogen utilization efficiency.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XktFWjsrs%3D&md5=82f78ce5e0cc39a609eef3bd67305924CAS |
King KR, Stockdale CR (1980) The effects of stocking rate and nitrogen-fertiliser on the productivity of irrigated perennial pasture grazed by dairy-cows. 2. Animal production. Australian Journal of Experimental Agriculture 20, 537–542.
| The effects of stocking rate and nitrogen-fertiliser on the productivity of irrigated perennial pasture grazed by dairy-cows. 2. Animal production.Crossref | GoogleScholarGoogle Scholar |
Koelsch R (2005) Evaluating livestock system environmental performance with whole-farm nutrient balance. Journal of Environmental Quality 34, 149–155.
Kohn RA, Dou Z, Ferguson JD, Boston RC (1997) A sensitivity analysis of nitrogen losses from dairy farms. Journal of Environmental Management 50, 417–428.
| A sensitivity analysis of nitrogen losses from dairy farms.Crossref | GoogleScholarGoogle Scholar |
Lawrie RA, Havilah EJ, Eldridge SM, Dougherty WJ (2004) Phosphorus budgeting and distribution on dairy farms in coastal New South Wales. In ‘Supersoil 2004. Program and Abstracts for the 3rd Australian New Zealand Soils Conference’. (Ed. B Singh). Available at http://www.regional.org.au/au/asssi/supersoil2004/s13/oral/1619_lawrier.htm#TopOfPage [verified 19 June 2012]
Ledgard SF, Penno JW, Sprosen MS (1999) Nitrogen inputs and losses from clover/grass pastures grazed by dairy cows, as affected by nitrogen fertiliser application. The Journal of Agricultural Science 132, 215–225.
| Nitrogen inputs and losses from clover/grass pastures grazed by dairy cows, as affected by nitrogen fertiliser application.Crossref | GoogleScholarGoogle Scholar |
Ledgard SF, Sprosen MS, Penno JW, Rajendram GS (2001) Nitrogen fixation by white clover in pastures grazed by dairy cows: temporal variation and effects of nitrogen fertilization. Plant and Soil 229, 177–187.
| Nitrogen fixation by white clover in pastures grazed by dairy cows: temporal variation and effects of nitrogen fertilization.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXit1aqu7o%3D&md5=89752c3d63cf9c0345a98d3d96c7df5eCAS |
Ledgard SF, Journeaux PR, Furness H, Petch RA, Wheeler DM (2004) Use of nutrient budgeting and management options for increasing nutrient use efficiency and reducing environmental emissions from New Zealand farms. In ‘OECD expert meeting on farm management indicators and the environment. Session 5, Palmerston North, New Zealand’. pp. 8–12.
McKenzie FR, Jacobs JL, Riffkin P, Kearney G, McCaskill M (2003a) Long-term effects of multiple applications of nitrogen fertiliser on grazed dryland perennial ryegrass/white clover dairy pastures in south-west Victoria. 1. Nitrogen fixation by white clover. Australian Journal of Agricultural Research 54, 461–469.
| Long-term effects of multiple applications of nitrogen fertiliser on grazed dryland perennial ryegrass/white clover dairy pastures in south-west Victoria. 1. Nitrogen fixation by white clover.Crossref | GoogleScholarGoogle Scholar |
McKenzie FR, Jacobs JL, Kearney G (2003b) Long-term effects of multiple applications of nitrogen fertiliser on grazed dryland perennial ryegrass/white clover dairy pastures in south-west Victoria. 2. Growth rates, dry matter consumed, and nitrogen response efficiencies. Australian Journal of Agricultural Research 54, 471–476.
| Long-term effects of multiple applications of nitrogen fertiliser on grazed dryland perennial ryegrass/white clover dairy pastures in south-west Victoria. 2. Growth rates, dry matter consumed, and nitrogen response efficiencies.Crossref | GoogleScholarGoogle Scholar |
McKenzie FR, Jacobs JL, Kearney G (2003c) Long-term effects of multiple applications of nitrogen fertiliser on grazed dryland perennial ryegrass/white clover dairy pastures in south-west Victoria. 3. Botanical composition, nutritive characteristics, mineral content, and nutrient selection. Australian Journal of Agricultural Research 54, 477–485.
| Long-term effects of multiple applications of nitrogen fertiliser on grazed dryland perennial ryegrass/white clover dairy pastures in south-west Victoria. 3. Botanical composition, nutritive characteristics, mineral content, and nutrient selection.Crossref | GoogleScholarGoogle Scholar |
Mekken JC, Swink SN, Ketterings QM (2006) ‘Statewide and county-based phosphorus balances for New York State. First Release. Department of Crop and Soil Sciences Extension Series E06–3.’ (Cornell University: Ithaca, NY) Available at http://nmsp.cals.cornell.edu/publications/extension.html [verified 19 June 2012]
Mulier A, Hofman G, Baecke E, Carlier L, De Brabander D, De Groote G, De Wilde R, Fiems L, Janssen G, Van Cleemput O, Van Herck A, Van Huylenbroeck G, Verbruggen I (2003) A methodology for the calculation of farm level nitrogen and phosphorus balances in Flemish agriculture. European Journal of Agronomy 20, 45–51.
| A methodology for the calculation of farm level nitrogen and phosphorus balances in Flemish agriculture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXpt1Sgtbg%3D&md5=e108edab3b54e3d75ab09aeea922282bCAS |
Nevens F, Verbruggen I, Reheul D, Hofman G (2006) Farm gate nitrogen surpluses and nitrogen use efficiency of specialized dairy farms in Flanders: evolution and future goals. Agricultural Systems 88, 142–155.
| Farm gate nitrogen surpluses and nitrogen use efficiency of specialized dairy farms in Flanders: evolution and future goals.Crossref | GoogleScholarGoogle Scholar |
NRC (2001) ‘Nutrient requirements of dairy cattle.’ 7th edn. (National Academy Press: Washington, DC)
OECD (2008) Environmental performance of agriculture in OECD countries since 1990, Paris, France. Available at www.oecd.org/tad/env/indicators [verified 25 November 2011]
Oenema O, Kros H, de Vries W (2003) Approaches and uncertainties in nutrient budgets: implications for nutrient management and environmental policies. European Journal of Agronomy 20, 3–16.
| Approaches and uncertainties in nutrient budgets: implications for nutrient management and environmental policies.Crossref | GoogleScholarGoogle Scholar |
Oenema O, Witzke HP, Klimont Z, Lesschen JP, Velthof GL (2009) Integrated assessment of promising measures to decrease nitrogen losses from agriculture in EU-27. Agriculture Ecosystems & Environment 133, 280–288.
| Integrated assessment of promising measures to decrease nitrogen losses from agriculture in EU-27.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXptl2ht7o%3D&md5=74b59f831df494289b74659b3ceeeed5CAS |
Oenema J, van Keulen H, Schils RLM, Aarts HFM (2011) Participatory farm management adaptations to reduce environmental impact on commercial pilot dairy farms in the Netherlands. NJAS Wageningen Journal of Life Sciences 58, 39–48.
| Participatory farm management adaptations to reduce environmental impact on commercial pilot dairy farms in the Netherlands.Crossref | GoogleScholarGoogle Scholar |
Ovens R, Weaver DM, Keipert N, Neville SN, Summers RN, Clarke MF (2008) Farm gate nutrient balances in south-west Western Australia – an overview. In ‘12th International Conference on Integrated Diffuse Pollution Management (IWA DIPCON 2008). Research Center for Environmental and Hazardous Substance Management (EHSM), Khon Kaen University, Thailand; 25–29 August 2008’. Available at http://www.ecohydrology.uwa.edu.au/__data/page/149281/Farm_gate_nutrient_balances_in_south_west_Western_Australia.pdf [verified 19 June 2012]
Powell JM, Gourley CJP, Rotz CA, Weaver DM (2010) Nitrogen use efficiency: a measurable performance indicator for dairy farms. Environmental Science & Policy 13, 217–228.
| Nitrogen use efficiency: a measurable performance indicator for dairy farms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXlsFOgsr0%3D&md5=5ae87c796ec6bc0c83bc5ff2c9ecf608CAS |
Probert ME (1976) The composition of rainwater at two sites near Townsville, Qld. Australian Journal of Soil Research 14, 397–402.
| The composition of rainwater at two sites near Townsville, Qld.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2sXns1ehsQ%3D%3D&md5=bd90ff37fa97dfcff411c8fda13a6be2CAS |
Raison C, Pflimlin A, Le Gall A (2006) ‘Optimisation of environmental practices in a network of dairy farms of the Atlantic area.’ (Institut de l’Elevage: Paris, France)
Rotz CA, Taube F, Russelle MP, Oenema J, Sanderson MA, Wachendorf M (2005) Whole-farm perspectives of nutrient flows in grassland agriculture. Crop Science 45, 2139–2159.
| Whole-farm perspectives of nutrient flows in grassland agriculture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht1OktbzE&md5=ea92fba0d61594a11556c90a38f0a72aCAS |
Satter L (2001) Nutrient management in dairy production systems. In ‘Nutrient management challenge in livestock and poultry operations: international and national perspectives’. (Eds K Kanwar, R Baggett) pp. 38–53. (Babcock Institute for International Dairy Research and Development Publisher, Babcock Institute, University of Wisconsin: Madison, WI)
Schröder JJ, Aarts HFM, ten Berge HFM, van Keulen H, Neeteson JJ (2003) An evaluation of whole-farm nitrogen balances and related indices for efficient nitrogen use. European Journal of Agronomy 20, 33–44.
| An evaluation of whole-farm nitrogen balances and related indices for efficient nitrogen use.Crossref | GoogleScholarGoogle Scholar |
Sharpley AN (1995) Dependency of runoff phosphorus on extractable soil phosphorus. Journal of Environmental Quality 24, 920–926.
| Dependency of runoff phosphorus on extractable soil phosphorus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXot1Wks7c%3D&md5=5431aac304102ad981479a4676055574CAS |
Simpson RJ, Oberson A, Culvenor RA, Ryan MH, Veneklaas EJ, Lambers H, Lynch JP, Ryan PR, Delhaize E, Smith A, Smith SE, Harvey PR, Richardson AE (2011) Strategies and agronomic interventions to improve the phosphorus-use efficiency of farming systems. Plant and Soil 349, 89–120.
| Strategies and agronomic interventions to improve the phosphorus-use efficiency of farming systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsFKntb7M&md5=c20589872ece90953e10fa1f9ac549ceCAS |
Sneath G, Furness H (2006) Progress in developing and implementing nutrient management tools and systems for New Zealand agriculture. Implementing sustainable nutrient management strategies in agriculture. In ‘Proceedings of the Fertilizer and Lime Workshop’. (Eds LD Currie, JA Hanly) (Massey University: Palmerston North, New Zealand).
Staines M, Morris R, Bolland M, Bennett D, Casson T, Fillery I, Lucey J, Russell B (2011) Greener pastures: profitable and sustainable use of nitrogen in dairy grazing systems. Final Report to Dairy Australia, Project DAW12101. Department of Agriculture and Food WA, South Perth.
Standards Australia (2009) ‘Organic and biodynamic products AS 6000.’ (Council of Standards Australia: Sydney)
Stevens CJ, Quinton JN (2008) Policy implications of pollution swapping. Journal Physical Chemistry of the Earth 34, 589–594.
t Mannetje L, Haydock KP (1963) The dry weight rank method for botanical analysis of pasture. Journal of British Grassland Society 18, 268–275.
| The dry weight rank method for botanical analysis of pasture.Crossref | GoogleScholarGoogle Scholar |
Thorrold B, Doyle P (2007) Nature or nurture – forces shaping the current and future state of dairy farming in New Zealand and Australia. In ‘Meeting the challenges for pasture-based dairying’. (Eds DF Chapman, DA Clark, KL Macmillan, DP Nation) pp. 450–460. (National Dairy Alliance: Melbourne)
Treacy M, Humphreys J, McNamara K, Browne R, Watson CJ (2008) Farm-gate nitrogen balances on intensive dairy farms in the south west of Ireland. Irish Journal of Agricultural and Food Research 47, 105–117.
Van der Meer HG (2001) Reduction of nitrogen losses in dairy production systems: the Dutch experience. In ‘Nutrient management challenge in livestock and poultry operations: international and national perspectives’. (Eds K Kanwar, R Baggett) pp. 82–97. (Babcock Institute for International Dairy Research and Development Publisher, Babcock Institute, University of Wisconsin: Madison, WI)
VandeHaar MJ, St-Pierre N (2006) Major advances in nutrition: relevance to the sustainability of the dairy industry. Journal of Dairy Science 89, 1280–1291.
| Major advances in nutrition: relevance to the sustainability of the dairy industry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xjt1Sjsb4%3D&md5=4323b1716fdee2aa26edb5a102c90887CAS |
Weaver DM, Reed AEG (1998) Patterns of nutrient status and fertiliser practice on soils of the south coast of Western Australia. Agriculture Ecosystems & Environment 67, 37–53.
| Patterns of nutrient status and fertiliser practice on soils of the south coast of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Weaver DM, Wong MF (2011) Scope to improve phosphorus (P) management and balance efficiency of crop and pasture soils with contrasting P status and buffering indices. Plant and Soil 349, 37–54.
| Scope to improve phosphorus (P) management and balance efficiency of crop and pasture soils with contrasting P status and buffering indices.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsFKntb3J&md5=20096a120c77eef5cc1f2209a5f71871CAS |



