Identification of Planigale ingrami and Planigale tenuirostris from mandibles deposited in eastern barn owl (Tyto javanica delicatula) pellets
C. L. Charley A * , E. L. Gray
A * , E. L. Gray  A and A. M. Baker
A and A. M. Baker  A B
A B
A
B
Abstract
Distinguishing Planigale ingrami from P. tenuirostris skeletal remains currently requires an intact skull. However, mandibular material is much more commonly encountered, particularly in owl pellets. Here, we show an identification feature for these planigales based solely on mandibles found in eastern barn owl (Tyto javanica delicatula) pellets collected from Toorak, north-western Queensland, Australia. Despite considerable overlap between taxa, our measurements of the angle between the molar row and ascending ramus suggested both planigale species were present. This has implications for mammal species richness estimates made from owl pellets where planigales would otherwise remain identifiable only to genus, and for our understanding of planigale species distributions.
Keywords: barn owl, dasyurid, detection, dietary analysis, diversity, mammal, morphology, species identification.
Introduction
In his landmark revision of the genus Planigale, Archer (1976) described Planigale ingrami (long-tailed planigale) and Planigale tenuirostris (narrow-nosed planigale) as often ‘not clear that they are separate species, (p. 358)’, on the basis of similarities in craniodental features. Subsequent genetic studies have clearly supported Planigale ingrami and Planigale tenuirostris as distinct (Painter et al. 1995; Blacket et al. 2000; Umbrello et al. 2023). Nevertheless, morphological similarities between the two often make confident identification difficult, especially when dealing with skeletal remains, which typically rely on intact skulls (via cranium depth:width ratios) (Blacket et al. 2008; Van Dyck et al. 2013). This presents a challenge for confident identification where skeletal material is incomplete, such as owl pellet content analysis, which is increasingly used as a detection tool to generate small mammal species richness estimates (Heisler et al. 2016; Schoenefuss et al. 2024; Charley et al. 2025).
Planigale craniodental material sourced from owl pellets mostly consists of mandibles, albeit well preserved, with the cranium typically crushed and fragmented. This currently limits confident identification of planigales in owl pellets to genus level (Charley et al. 2025). The inability to accurately attribute species-level identification to planigales found in owl pellets means that species richness estimates may be underestimated, and knowledge of planigale species distributions remains unclear. Here, with reference to registered, identified specimens held at Australian museums, we undertake an analysis of eastern barn owl (Tyto javanica delicatula) pellets by using a mandibular identification feature in an attempt to discriminate between Planigale ingrami and Planigale tenuirostris.
Materials and methods
This research was conducted under the auspices of Queensland Department of Environment, Science and Innovation (DESI) Permit P-PTUKI-100171210 and QUT Research Ethics Permit 5154.
Planigale mandibles assessed in the present study (n = 68) consisted of both Queensland and South Australian Museum reference specimen mandibles (total n = 30; P. ingrami n = 16; P. tenuirostris n = 14) for which correct identification could be strongly attributed by museum curators [i.e. with an intact skull (Blacket et al. 2008)] (see Appendix 1), and a representative subset of Planigale spp. mandibles (n = 38, designated as Unknown) collected from eastern barn owl pellet material taken from an abandoned wool shed at Toorak, north-western Queensland, Australia (21.03290 S, 141.79958 E). Toorak is located ~40 km south of Julia Creek and lies within the semi-arid region of the Mitchell Grass Downs bioregion (Fig. 1). For full details of the owl pellet collection and processing methodology, see Charley et al. (2025). Only left mandibles were assessed in the present study to ensure that each individual contributed only one set of measurements to the analysis. Only adult specimens were used, because some measurements could be affected by bone lengths. Adult specimens were designated by full emergence of the lower (M4) molar tooth (Blacket et al. 2008).
Map of Australia with state and territory borders. Points represent planigale specimen collection locations used in the present study. Shaded areas represent the respective species distributions (generated from Marsh et al. 2022).
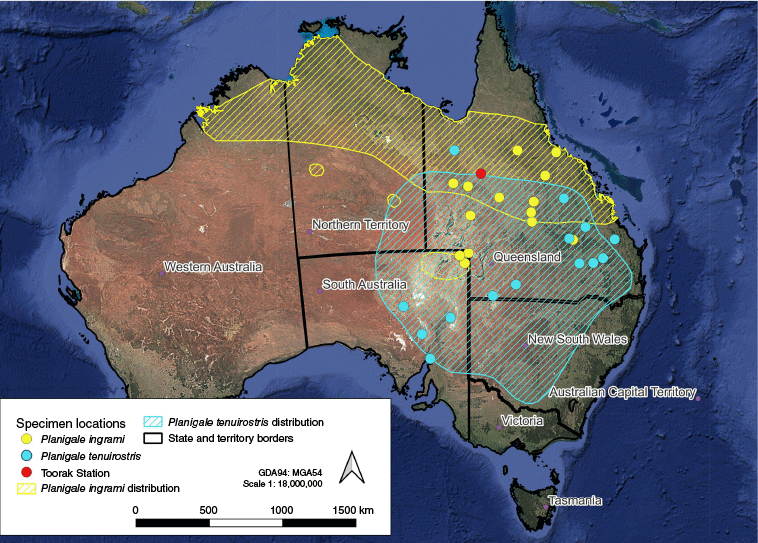
Planigale ingrami and Planigale tenuirostris have potentially overlapping distributions at Toorak (Baker and Gynther 2023). Two other species of planigale occur in Queensland, Planigale gilesi and Planigale maculata. Although these two species were unlikely to be present at the surveyed site, they are in any case relatively easily identified because the former has only two premolar teeth and the latter is notably larger in size than are congeners (Baker and Gynther 2023).
On the basis of expert recommendation (S. Van Dyck, pers. comm.), the angle the ascending ramus makes at the emergence of the lower molar tooth row (henceforth, LMR-AR angle) was suggested as a possible diagnostic feature for distinguishing between Planigale ingrami and Planigale tenuirostris. This feature was measured using a disconnected angle tool (using Zeiss labscope software ver. 4.3.1, https://www.zeiss.com/microscopy/en/products/software/zeiss-labscope.html, although any angle software would be applicable) using a Zeiss Stemi 305 stereo microscope with attached Axiocam 208 colour microscopy camera, placing the lower angle arm to intersect both the anterior molar emergence point on M1 and the posterior molar emergence point on M4, and the upper angle arm following the anterior border of the ascending ramus (Archer 1976) (Fig. 2).
The LMR-AR angle measurement for planigales assessed in the present study. QLD Museum specimen (a) JM18198, identified by museum curators as Planigale ingrami, and (b) JM2192, identified by museum curators as Planigale tenuirostris.

Analyses were performed in R (R Core Team 2024). A Shapiro–Wilk test was first conducted on the dataset to test for normality. Then, a one-way ANOVA was used to assess for differences in planigale angle measurements among the three assessed groups (Unknown, Planigale ingrami and Planigale tenuirostris). Tukey’s honest significant differences (TukeyHSD) test was then used to compare pairwise differences between the groups. Tukey’s HSD test is used when performing multiple simultaneous comparisons to ensure that the P-value achieves an 0.05 error rate (Agbangba et al. 2024).
Results and discussion
Complete data are presented in Appendix 1. Data were deemed to be normally distributed (Shapiro–Wilk normality test, W = 0.99, P-value = 0.82). ANOVA testing demonstrated a significant difference between species (d.f. = 2, f-value = 7.54, P-value = 1.14 × 10−3). Post hoc testing (TukeyHSD) demonstrated a significant difference between Planigale tenuirostris and Planigale ingrami museum specimens (diff = −4.45, lower = −7.57, upper = −1.33, adjusted P-value = 3.03 × 10−3) (Fig. 3). Overlap in the molar row-ascending ramus angle measurement between Planigale ingrami and Planigale tenuirostris was common (Table 1, Fig. 3), although there was clear separation in the upper and lower extremes for both species. Any angle measurement in the assessed museum registered mandibles that was ≥120° was attributable to Planigale ingrami, whereas any angle of ≤112° was attributable to Planigale tenuirostris.
Boxplot comparison of the LMR-AR angle for various Planigale ingrami and Planigale tenuirostris museum reference specimens and owl pellet collection mandibles (Unknown). Boxes represent the interquartile ranges, the vertical lines the median and the horizontal lines the total range.

| Species | n | x̄ | s.e.m. | Median | Min | Max | σ | CV | |
|---|---|---|---|---|---|---|---|---|---|
| Planigale ingrami | 16 | 116.8 | 0.68 | 116.4 | 113.1 | 121.9 | 2.71 | 0.02 | |
| Planigale tenuirostris | 14 | 112.3 | 1.08 | 113.2 | 105.5 | 119.3 | 4.03 | 0.04 | |
| Unknown | 38 | 113.1 | 0.60 | 113.8 | 104.2 | 121.3 | 3.67 | 0.03 |
Abbreviations: n, sample size; x̄, sample mean; s.e.m., standard error of the mean; σ, s.d.; CV, coefficient of variation.
There was a significant difference between Unknown and Planigale ingrami (diff = −3.66, lower = −6.20, upper = −1.12, adjusted P-value = 2.72 × 10−3), but no significant difference between Unknown and Planigale tenuirostris (diff = 0.79, lower = −1.87, upper = 3.45, adjusted P-value = 0.76). The measured angles for Planigale tenuirostris had a markedly larger range (105.5°–119.3°) than did those of Planigale ingrami (113.1°–121.9°). This range showed notable overlap with the Unknown sample (104.15°–121.32°), which may explain the lack of significant difference between Planigale tenuirostris and the Unknown samples (Fig. 3).
One unknown mandible specimen could be attributed to Planigale ingrami (≥120°), and 13 unknown mandible specimens could be attributed to Planigale tenuirostris (≤112°) by using the proposed LMR-AR angle. Despite this, the observed interquartile range (IQR) of both species was largely separate (Planigale tenuirostris 109.9°–115.6°; Planigale ingrami 115.1°–118.0°; Fig. 3), with 20 unknown specimens falling within the IQR of Planigale tenuirostris, and seven unknown specimens falling within the IQR of Planigale ingrami. Interestingly, one unknown specimen (104.15°) fell outside of the observed minimum of Planigale tenuirostris (105.5°), suggesting possible further variability in the measurement.
The Unknown sample measurements showed notable overlap with both Planigale ingrami and Planigale tenuirostris. This suggests the presence of both planigale species at Toorak, despite prior owl pellet collections from this location being assumed to consist solely of Planigale ingrami (Woolley 2010). Further field-based detection methodologies [pitfall trapping; Catling et al. (1997)] should be employed to corroborate this finding with live planigales at Toorak and surrounds. Voucher specimens would permit comparative examination of both DNA and a full suite of craniodental features.
Implications
Although confident species-level identification could not be made for all assessed planigale mandibles collected at Toorak, the suggested identification feature has practical application. Owl pellet content analysis is an effective methodology for sampling small mammal community composition (Torre et al. 2004; Heisler et al. 2016) and being unable to identify individuals to species may result in underestimates of diversity (Kutt et al. 2021).
Our planigale mandible identification feature is relevant to owl pellet species richness sampling, particularly where mandibles are the only intact craniodental material present. Indeed, it has general application to taxonomic identification of planigales. Being able to confidently attribute even a percentage of individuals to species level offers further insight into small mammal community composition and planigale species distribution patterns that would otherwise remain unknown.
Data availability
The data that support this study are available in the article and accompanying appendix.
Declaration of funding
This research was principally funded by Multicom Resources as part of an Offset Management Plan conceptualised by Multicom Resources and Epic Environmental and approved under the Environment Protection and Biodiversity Conservation Act 1999 (EPBC Act) for the Saint Elmo Vanadium project (EPBC 2017/8007). Additional funding and resources were provided by QUT.
Acknowledgements
We thank Dr Steve Van Dyck for providing the initial suggestion for the mandible feature examined in the present study. We are grateful to Heather Janetzki (Queensland Museum) for valuable discussion and for facilitating access to the Queensland Museum reference collection. Thanks go to David Stemmer (South Australian Museum) for providing use of the South Australian Museum specimens. We thank Dr Linette Umbrello for providing pictures of South Australian Museum reference specimens and for valuable insight into planigale diversity and identification. We are grateful to the various field assistants who collected pellets, and members of the Baker Mammal Ecology Lab for additional guidance and suggestions regarding the project. We thank two anonymous reviewers and the handling editor for their comments, which improved the paper.
References
Agbangba, C. E., Sacla Aide, E., Honfo, H., and Glèlè Kakai, R. (2024). On the use of post-hoc tests in environmental and biological sciences: a critical review. Heliyon 10(3), e25131.
| Crossref | Google Scholar |
Archer, M. (1976). Revision of the marsupial genus Planigale Troughton (Dasyuridae). Memoirs of the Queensland Museum 17, 341-365.
| Google Scholar |
Blacket, M. J., Adams, M., Krajewski, C., and Westerman, M. (2000). Genetic variation within the dasyurid marsupial genus Planigale. Australian Journal of Zoology 48(5), 443-459.
| Crossref | Google Scholar |
Blacket, M. J., Kemper, C., and Brandle, R. (2008). Planigales (Marsupialia:Dasyuridae) of eastern Australias interior: a comparison of morphology, distributions and habitat preferences, with particular emphasis on South Australia. Australian Journal of Zoology 56(3), 195-205.
| Crossref | Google Scholar |
Catling, P. C., Burt, R. J., and Kooyman, R. (1997). A comparison of techniques used in a survey of the ground-dwelling and arboreal mammals in forests in north-eastern New South Wales. Wildlife Research 24(4), 417-432.
| Crossref | Google Scholar |
Charley, C. L., Gray, E. L., and Baker, A. M. (2025). Owl pellet content analysis proves an effective technique to monitor a population of threatened Julia Creek dunnarts (Sminthopsis douglasi) throughout a native rodent plague. Ecology and Evolution 15(2), e70922.
| Crossref | Google Scholar |
Heisler, L. M., Somers, C. M., and Poulin, R. G. (2016). Owl pellets: a more effective alternative to conventional trapping for broad-scale studies of small mammal communities. Methods in Ecology and Evolution 7(1), 96-103.
| Crossref | Google Scholar |
Kutt, A. S., Kern, P. L., Schoenefuss, P., Moffatt, K., Janetzki, H., Hurwood, D., and Baker, A. M. (2021). Diet of the eastern barn owl (Tyto delicatula) in the Simpson Desert reveals significant new records and a different mammal fauna to survey data. Australian Mammalogy 43(2), 248-251.
| Crossref | Google Scholar |
Marsh, C. J., Sica, Y. V., Burgin, C. J., Dorman, W. A., Anderson, R. C., del Toro Mijares, I., Vigneron, J. G., Barve, V., Dombrowik, V. L., Duong, M., Guralnick, R., Hart, J. A., Maypole, J. K., McCall, K., Ranipeta, A., Schuerkmann, A., Torselli, M. A., Lacher, T., Jr, Mittermeier, R. A., Rylands, A. B., Sechrest, W., Wilson, D. E., Abba, A. M., Aguirre, L. F., Arroyo-Cabrales, J., Astúa, D., Baker, A. M., Braulik, G., Braun, J. K., Brito, J., Busher, P. E., Burneo, S. F., Camacho, M. A., Cavallini, P., de Almeida Chiquito, E., Cook, J. A., Cserkész, T., Csorba, G., Cuéllar Soto, E., da Cunha Tavares, V., Davenport, T. R. B., Deméré, T., Denys, C., Dickman, C. R., Eldridge, M. D. B., Fernandez-Duque, E., Francis, C. M., Frankham, G., Franklin, W. L., Freitas, T., Friend, J. A., Gadsby, E. L., Garbino, G. S. T., Gaubert, P., Giannini, N., Giarla, T., Gilchrist, J. S., Gongora, J., Goodman, S. M., Gursky-Doyen, S., Hackländer, K., Hafner, M. S., Hawkins, M., Helgen, K. M., Heritage, S., Hinckley, A., Hintsche, S., Holden, M., Holekamp, K. E., Honeycutt, R. L., Huffman, B. A., Humle, T., Hutterer, R., Ibáñez Ulargui, C., Jackson, S. M., Janecka, J., Janecka, M., Jenkins, P., Juškaitis, R., Juste, J., Kays, R., Kilpatrick, C. W., Kingston, T., Koprowski, J. L., Kryštufek, B., Lavery, T., Lee, T. E., Jr, Leite, Y. L. R., Novaes, R. L. M., Lim, B. K., Lissovsky, A., López-Antoñanzas, R., López-Baucells, A., MacLeod, C. D., Maisels, F. G., Mares, M. A., Marsh, H., Mattioli, S., Meijaard, E., Monadjem, A., Morton, F. B., Musser, G., Nadler, T., Norris, R. W., Ojeda, A., Ordóñez-Garza, N., Pardiñas, U. F. J., Patterson, B. D., Pavan, A., Pennay, M., Pereira, C., Prado, J., Queiroz, H. L., Richardson, M., Riley, E. P., Rossiter, S. J., Rubenstein, D. I., Ruelas, D., Salazar-Bravo, J., Schai-Braun, S., Schank, C. J., Schwitzer, C., Sheeran, L. K., Shekelle, M., Shenbrot, G., Soisook, P., Solari, S., Southgate, R., Superina, M., Taber, A. B., Talebi, M., Taylor, P., Vu Dinh, T., Ting, N., Tirira, D. G., Tsang, S., Turvey, S. T., Valdez, R., Van Cakenberghe, V., Veron, G., Wallis, J., Wells, R., Whittaker, D., Williamson, E. A., Wittemyer, G., Woinarski, J., Zinner, D., Upham, N. S., and Jetz, W. (2022). Expert range maps of global mammal distributions harmonised to three taxonomic authorities. Journal of Biogeography 49(5), 979-992.
| Crossref | Google Scholar |
Painter, J., Krajewski, C., and Westerman, M. (1995). Molecular phylogeny of the marsupial genus Planigale (Dasyuridae). Journal of Mammalogy 76(2), 406-413.
| Crossref | Google Scholar |
Schoenefuss, P., Kutt, A. S., Kern, P. L., Moffatt, K. A., Bon, J., Wardle, G. M., Dickman, C. R., Hurwood, D. A., and Baker, A. M. (2024). An investigation into the utility of eastern barn owl pellet content as a tool to monitor small mammal diversity in an arid ecosystem. Austral Ecology 49(3), e13503.
| Crossref | Google Scholar |
Torre, I., Arrizabalaga, A., and Flaquer, C. (2004). Three methods for assessing richness and composition of small mammal communities. Journal of Mammalogy 85(3), 524-530.
| Crossref | Google Scholar |
Umbrello, L. S., Cooper, N. K., Adams, M., Travouillon, K. J., Baker, A. M., Westerman, M., and Aplin, K. P. (2023). Hiding in plain sight: two new species of diminutive marsupial (Dasyuridae: Planigale) from the Pilbara, Australia. Zootaxa 5330(1), 1-46.
| Crossref | Google Scholar |
Woolley, P. A. (2010). The Julia Creek dunnart and other prey of the barn owl in Mitchell grass downs of north-western Queensland. Memoirs of the Queensland Museum-Nature 55, 107-117.
| Google Scholar |
Appendix 1.Angle measurements (rounded to one decimal place) used in the present study.
| Species | Collection ID | ID | Sex | Angle (°) | |
|---|---|---|---|---|---|
| Unknown | Toorak station | TWS453 | Unknown | 104.2 | |
| Unknown | Toorak station | TWS386 | Unknown | 106.7 | |
| Unknown | Toorak station | TWS380 | Unknown | 107.6 | |
| Unknown | Toorak station | TWS570 | Unknown | 107.8 | |
| Unknown | Toorak station | TWS657 | Unknown | 108.1 | |
| Unknown | Toorak station | TWSJUL | Unknown | 108.3 | |
| Unknown | Toorak station | TWSJUL | Unknown | 109.4 | |
| Unknown | Toorak station | TWS631 | Unknown | 109.6 | |
| Unknown | Toorak station | TWSJUL | Unknown | 110.6 | |
| Unknown | Toorak station | TWSJUL | Unknown | 111.3 | |
| Unknown | Toorak station | TWS580 | Unknown | 111.4 | |
| Unknown | Toorak station | TWS627 | Unknown | 111.6 | |
| Unknown | Toorak station | TWS510 | Unknown | 111.7 | |
| Unknown | Toorak station | TWS420 | Unknown | 112.1 | |
| Unknown | Toorak station | TWS641 | Unknown | 112.8 | |
| Unknown | Toorak station | TWS386 | Unknown | 113.1 | |
| Unknown | Toorak station | TWS655 | Unknown | 113.1 | |
| Unknown | Toorak station | TWS611 | Unknown | 113.2 | |
| Unknown | Toorak station | TWSOCT | Unknown | 113.8 | |
| Unknown | Toorak station | TWS427 | Unknown | 113.9 | |
| Unknown | Toorak station | TWS657 | Unknown | 114.0 | |
| Unknown | Toorak Station | TWS536 | Unknown | 114.1 | |
| Unknown | Toorak station | TWS388 | Unknown | 114.1 | |
| Unknown | Toorak station | TWS96 | Unknown | 114.2 | |
| Unknown | Toorak station | TWS297 | Unknown | 114.2 | |
| Unknown | Toorak station | TWS335 | Unknown | 114.2 | |
| Unknown | Toorak station | TWS214 | Unknown | 114.3 | |
| Unknown | Toorak station | TWS655 | Unknown | 114.6 | |
| Unknown | Toorak station | TWS386 | Unknown | 115.7 | |
| Unknown | Toorak station | TWS337 | Unknown | 115.7 | |
| Unknown | Toorak station | TWS627 | Unknown | 116.2 | |
| Unknown | Toorak station | TWS386 | Unknown | 116.7 | |
| Unknown | Toorak station | TWS471 | Unknown | 117.0 | |
| Unknown | Toorak station | TWS631 | Unknown | 117.3 | |
| Unknown | Toorak station | TWS631 | Unknown | 117.4 | |
| Unknown | Toorak station | TWS124 | Unknown | 118.5 | |
| Unknown | Toorak station | TWS657 | Unknown | 118.8 | |
| Unknown | Toorak station | TWS670 | Unknown | 121.3 | |
| Planigale tenuirostris | QM | JM14296 | Unknown | 105.5 | |
| Planigale tenuirostris | SAM | M11028 | Unknown | 107.2 | |
| Planigale tenuirostris | SAM | M26276 | Unknown | 109.9 | |
| Planigale tenuirostris | QM | JM2192 | Unknown | 109.9 | |
| Planigale tenuirostris | QM | J15873 | Unknown | 110.0 | |
| Planigale tenuirostris | QM | JM11212 | Unknown | 110.3 | |
| Planigale tenuirostris | SAM | M18480 | Unknown | 111.2 | |
| Planigale tenuirostris | SAM | M8405 | Unknown | 111.4 | |
| Planigale tenuirostris | QM | JM12668 | Male | 113.1 | |
| Planigale tenuirostris | QM | JM2831 | Unknown | 114.7 | |
| Planigale tenuirostris | QM | JM14990 | Male | 115.9 | |
| Planigale tenuirostris | QM | JM14789 | Unknown | 116.5 | |
| Planigale tenuirostris | QM | JM14694 | Unknown | 117.8 | |
| Planigale tenuirostris | QM | J3824 | Male | 119.3 | |
| Planigale ingrami | SAM | M17886 | Unknown | 113.1 | |
| Planigale ingrami | SAM | M17892 | Unknown | 113.2 | |
| Planigale ingrami | QM | JM13259 | Unknown | 113.5 | |
| Planigale ingrami | SAM | M17907 | Unknown | 115.0 | |
| Planigale ingrami | QM | JM10550 | Unknown | 115.1 | |
| Planigale ingrami | SAM | M17888 | Unknown | 115.2 | |
| Planigale ingrami | SAM | M6066 | Unknown | 116.0 | |
| Planigale ingrami | QM | JM4944 | Male | 116.1 | |
| Planigale ingrami | QM | JM12631 | Male | 116.6 | |
| Planigale ingrami | QM | JM13265 | Unknown | 117.4 | |
| Planigale ingrami | QM | JM22680 | Male | 117.4 | |
| Planigale ingrami | QM | JM11042 | Male | 117.9 | |
| Planigale ingrami | QM | JM10079 | Unknown | 118.4 | |
| Planigale ingrami | QM | JM18198 | Male | 120.7 | |
| Planigale ingrami | QM | JM13341 | Male | 120.9 | |
| Planigale ingrami | QM | JM11136 | Male | 121.9 |
Collection ID of QM refers to the Queensland Museum reference collection, and SAM to the South Australian Museum reference collection.


