Does tree hollow volume influence use by small arboreal mammals?
William Terry A * and Ross L. Goldingay AA
Abstract
Identifying the specific tree hollow requirements of hollow dependent fauna is central to managing these shelter resources, as well as determining where habitat restoration is required. We installed pairs of carved tree hollows of different cavity volume at 14 sites to investigate whether preferences were shown by the brush-tailed phascogale (Phascogale tapoatafa), inland sugar glider (Petaurus notatus) and agile antechinus (Antechinus agilis). Small (1500 cm3) cavities should be of sufficient size to support non-breeding individuals, whereas large (9000 cm3) cavities should be required for breeding. Camera traps showed that the probability of visitation was highly seasonal but did not differ between hollow types for any species. Despite a high probability of visitation per week by all species (0.45–0.75, in summer), inspection of the hollows showed that few were occupied. Two phascogale nests were observed in large hollows and one in a small hollow. Nesting sugar gliders were seen in two large hollows and a nest was seen in another. No antechinus nests were recorded. Low occupancy of artificial hollows relative to high visitation, and compared with the results of other studies, is attributed to abundant natural hollows within our study area. Management of nest box programs requires careful planning that should include an evaluation of the existing tree hollow resource prior to any intervention.
Keywords: arboreal, artificial tree hollow, glider, nest boxes, occupancy modelling, phascogale, tree cavity, tree hollow.
Introduction
Tree hollows are an essential natural resource that are used by over 300 species of Australian vertebrate fauna (Gibbons et al. 2000; Gibbons and Lindenmayer 2002). Birds, mammals, reptiles and amphibians rely on hollows for shelter, protection from predators and for breeding (Lindenmayer et al. 1991, 2014; Gibbons et al. 2000; Goldingay 2011; Stojanovic et al. 2012). Hollows typically form naturally in trees that are at least 100 years old (Mackowski 1984; Gibbons et al. 2000; Whitford 2002; Wormington et al. 2003; Koch et al. 2008). Widespread deforestation and forest degradation from land use have resulted in a shortage of tree hollows. The loss of hollow-bearing trees is considered a key threatening process for Australian wildlife (Manning et al. 2013; Lindenmayer et al. 2014; Le Roux et al. 2016). Understanding the specific hollow requirements of hollow-dependent species is critical for protecting the appropriate tree hollows of these species in old-growth forest as well as managing tree hollows in restored or timber production forests (Gibbons and Lindenmayer 2002; Goldingay 2011; Stojanovic et al. 2021a; Thompson et al. 2023; Lindenmayer et al. 2024).
Artificial cavities, including nest boxes and carved tree hollows, have been shown to provide important refuge opportunities for fauna in forests where natural hollow availability is low (Harley 2006; Rueegger 2017; Goldingay et al. 2020a, 2024; Griffiths et al. 2020; Macak 2020; Quin et al. 2021; Stojanovic et al. 2021b). However, their success in mitigating poor availability relies on understanding the factors that increase their use. Whereas much is known about the influence of cavity entrance size (Saunders et al. 1982; Goldingay et al. 2007, 2020b; LeRoux et al. 2016), less is known about cavity volume (e.g. Best et al. 2022). Cavities need to be large enough to contain at least one individual when used only as shelter, but may need to be substantially larger for breeding individuals with developing young or group living species simply because additional individuals require more space. Few studies have investigated such influences on hollow selection (e.g. Goldingay 2020).
In this study, we investigated whether three species of arboreal marsupial (Fig. 1) showed a preference for carved hollows of two sizes, namely, small (1500 cm3) and large (9000 cm3). Our main target species was the brush-tailed phascogale (Phascogale tapoatafa), an arboreal marsupial insectivore that is threatened with extinction in Victoria and New South Wales (NSW Government 2024; Flora and Fauna Guarantee Act 1988, Threatened List, Victorian Government). A key threat for this species is the loss of tree hollows required for shelter and raising young. Following mating in early winter, all male phascogales die (Cuttle 1982). Females raise four to eight young in a hollow until they disperse in early summer (Soderquist and Lill 1995). The maternal hollow has a volume of at least 8000 cm3 (i.e. 20 cm × 20 cm × 20 cm) and contains a complex, well-insulated nest of bark, interspersed with feathers, to provide warmth for developing young (Goldingay and Thomas 2023; Soderquist 1993a). Outside the maternal period, much smaller hollows, and even other structures may be used (Soderquist 1993a; Traill and Coates 1993). Consequently, habitat restoration for this species may benefit from provisioning of small and large volume cavities. The other mammal species we investigated were the inland sugar glider (Petaurus notatus) and the agile antechinus (Antechinus agilis). We predicted that the group-living sugar glider would favour the large cavity because the small cavity would be of insufficient size for more than two individuals. The small-bodied antechinus could use either size.
Methods
Study area
Our study area consisted of a combination of small roadside reserves and patches of forest in central Victoria near the township of Lancefield, approximately 60 km north of Melbourne (Fig. 2). The area is known for supporting large numbers of brush-tailed phascogales (Flora and Fauna Guarantee Act 1988, Threatened List, Victorian Government). We established 14 study sites spaced at least 500 m apart. Sites contained a mixture of eucalypt species, dead trees, large volumes of fallen timber, and an intact ground cover consisting of native grasses and herbs.
Installation of hollows
In April 2020, artificial hollows (see Rueegger 2017; Griffiths et al. 2018; Honey et al. 2021) were created in suitable trees at the 14 study sites. We used the Matthecks tree ratio (Mattheck et al. 1994) to select suitable trees at each site to protect against breakages. This includes ensuring that a tree retains a minimum of two-thirds of stem thickness following construction of a chainsaw carved hollow. From a group of pre-selected trees, we selected two trees of the same species within 50 m of each other. We randomly determined whether to construct a small (15 cm × 10 cm × 10 cm; 1500 cm3) or large hollow (15 cm × 15 cm × 40 cm; 9000 cm3) in the two pre-selected trees (Fig. 3). Phascogales are known to utilise tree hollows low to the ground (Soderquist 1993a; Quin et al. 2021), so all hollows were installed at 1.5 m. This allowed for easier access for monitoring. All hollows included an external faceplate created from hardwood, with an entrance hole of 4.5 cm (Fig. 3).
(a) Large carved hollow, measuring 15 cm × 15 cm × 40 cm; 9000 cm3. (b) Small carved hollow, measuring 15 cm × 10 cm × 10 cm; 1500 cm3. (c) The internal view of a large hollow carved into tree. (d) Camera mounted on a bracket and directed at the hollow entrance. All entrance holes measured 45 mm in diameter. Images: William Terry.

Trees used for the hollow carving were based on the availability of each species at each site. Only rough barked eucalypts were selected as they are known to be preferred by phascogales (Traill and Coates 1993). Species included messmate stringybark (Eucalyptus obliqua), narrow-leaved peppermint (E. radiata subsp. radiata), broad-leaved peppermint (E. dives), bundy (E. goniocalyx), grey box (E. microcarpa), and yellow box (E. melliodora).
Our monitoring extended over a 4.5-year period, which provided the opportunity to investigate whether the carved hollows required any maintenance. A record of maintenance requirements for each carved hollow was kept, including repairs required to the faceplate or internal cavity (e.g. faceplates can warp or crack) (Terry et al. 2021). The pole camera was used to investigate any obvious changes to the condition of the tree within each cavity and build-up of water. The callous regrowth was measured to the nearest centimetre on the sides of each hollow at the end of Year 4. The faceplate condition was also noted for any obvious signs of cracking or damage. Signs of water ingress were also recorded.
Monitoring of hollows
Two methods (a pole camera and camera trap) were used to monitor the use of the carved hollows. An initial check was undertaken within 1 month of installation. Checks of hollows using a pole camera (Arborcam Mk2) were then undertaken annually from 2020 to 2024. Subsequent checks were undertaken between June and October when female phascogales have young and are likely to construct complex nests (Soderquist 1993a). Signs of hollow use included animals present or the presence of nesting material such as leaves, bark strips, feathers or scats, which can be used to identify the species (Goldingay et al. 2020b). The freshness of nesting material indicated whether it was placed in the year of the observation. A limitation to the pole camera was that animals under nesting material can be obscured and therefore go undetected (Goldingay et al. 2020a).
Camera traps (Reconyx Hyperfire 2) were mounted using a custom bracket (Fig. 3), which allowed for entrances of each hollow to be monitored. Cameras were mounted upside down, facing directly at the hollow entrance at approximately 1 m. Cameras were rotated around to the different study locations for differing amounts of time between April 2020 and June 2021. Cameras were set to the highest sensitivity, with no quiet period between triggers and to record in rapid fire mode. The cameras were serviced every 4–8 weeks. Cameras monitored hollow pairs (large and small) for between 59 and 347 nights (mean = 198 ± 36.9). We defined a visit to a hollow as being any animal photographed inspecting the exterior or interior of the hollow. The series of images we obtained could not be used reliably to determine whether animals remained in the hollows during the day because of their ability to exit the hollow without the camera being triggered. We recorded only one observation per hollow for each species per 24 h.
Habitat measurements
Habitat data were collected at each site within a 50 m × 20 m transect adjacent to the artificial hollows. Tree species (>5 cm DBH) and their size (DBH) were recorded. Phascogales are known to utilise small entrance hollows (Soderquist 1993b; Traill and Coates 1993). The number of suitable tree hollows (entrance size of 2.5–9 cm) observed within the transect was recorded. A pair of binoculars (Nikon Monarch) was used to estimate hollow entrance size of higher hollows that could not be closely inspected from the ground. Where possible, internal measurements were taken of some of the hollows. Only the data on tree hollow abundance were used in the analysis below.
Data analysis
Detection of animals within hollows by the pole camera was too infrequent to be able to analyse. However, we had ample data from the camera monitoring to analyse. These records represent visitation, which we assume represents animals inspecting the hollows to assess their suitability. We used an occupancy modelling approach to analyse our data. We did not investigate the probability of occupancy specifically because the two hollow types were co-located at the same sites. Instead, we analysed our data using the multi-method occupancy approach of Nichols et al. (2008) as implemented in program Presence (ver. 12.24; USGS Patuxent Wildlife Research Centre, Laurel, MD, USA). This approach allows the use of repeat observations at sites containing two or more detection methods. For our study, we equate our two hollow types to different detection methods. We assume that if a species prefers one type over the other, this will lead to higher probabilities of detection. If no preference is shown, these probabilities will be equivalent. The multi-method occupancy approach estimates three parameters, a method-specific (e.g. hollow type) probability of detection (m), the probability of site occupancy (ψ), and the probability of presence, given a site is occupied (i.e. available for detection) on a specific occasion (theta, θ). Our focus was on comparing different detection models. Therefore, the parameters for occupancy and theta were kept constant.
From the camera monitoring, we constructed separate detection histories for the phascogale, sugar glider and antechinus to reflect whether a species was detected (1) or not (0), or a camera was not in place (–) at each pair of cavities across sample occasions (e.g. H = 00 01 10 11 -- …). Our monitoring produced nightly records that we aggregated into weekly detection histories. Pairs of hollows were monitored concurrently, but we did not have sufficient cameras to monitor all hollows concurrently. Missing samples are readily accommodated and do not have an influence on the modelling outputs (MacKenzie and Nichols 2004). Our monitoring extended over 66 weeks (April 2020 to August 2021).
For each mammal species, we tested the following detection models. A null model where the probability of detection was estimated as equal across the two hollow types and sample occasions. A model that estimated detection as different across hollow types, but equal across sample occasions. A model that estimated detection as different across seasons. A model that estimated detection as influenced by the local abundance of tree hollows. All species undergo seasonal breeding, which leads to seasonal variation in local abundance (Suckling 1984; Cockburn and Lazenby-Cohen 1992; Soderquist 1993b), and, consequently, we predict will influence detection. All require tree hollows for shelter and for breeding (Suckling 1984; Cockburn and Lazenby-Cohen 1992; Soderquist 1993b). They will use artificial hollows (Goldingay et al. 2020a, 2024) but may prefer tree hollows if such hollows are locally abundant. Models were ranked by Akaike’s information criterion corrected for small sample size (AICc) (Burnham and Anderson 2004) and ranked from the lowest to the highest AICc value. These values indicate the plausibility of competing models to explain the data. Any model within 2AICc of the top model is considered equally plausible to it, whereas differences of >4 suggest less plausible models (Burnham and Anderson 2004). The relative support for a model is indicated by its model weight (w).
Results
Camera-trap monitoring
A variety of mammals and birds were photographed at the hollows (Fig. 4). Camera traps installed at the time of construction showed that the carved hollows were quickly discovered by mammals and birds. Sugar gliders and antechinuses were observed on the first night exploring some of the hollows. Phascogales and common brushtail possum (Trichosurus vulpecula) were detected within 4 days, and feather-tailed glider (Acrobates spp.) after 7 weeks. Unidentified microbats were observed visiting the hollows on five occasions across three sites and a small unidentified skink used the large and small hollow at one site. The introduced black rat (Rattus rattus) was observed visiting one hollow on three occasions.
Animals investigating the newly carved tree hollows. (a, b) Brush-tailed phascogale. (c) Inland sugar glider. (d, e) Agile antechinus. (f) White-throated treecreeper. (g) Crimson rosella. (h) Striated pardalote. Images: William Terry.

Several bird species inspected the hollows, including the crimson rosella (Platycercus elegans), white-throated treecreeper (Cormobates leucophaea), spotted pardalote (Pardalotus punctatus) and striated pardalote (P. striatus). Birds showed slightly more interest in the smaller hollows (n = 130 daily inspections) than the large hollows (n = 100). Treecreepers accounted for 80% of bird visits and were detected at all but one site.
Annual monitoring
Annual inspections of hollows by using a pole camera over 4.5 years showed that few hollows were occupied by any animals. We recorded two phascogale nests consisting of stripped bark and feathers in a large hollow and one in a small hollow. Phascogale scat was observed inside a different large hollow, suggesting that it had been used by a dispersing individual. Three large hollows contained active sugar glider nests, but individuals were recorded only at two (Fig. 5). A white-throated treecreeper nest was recorded in a large hollow with a nest of stripped bark and green moss.
Visitation to hollows by target species
Overall, we recorded 130 daily visits to large hollows and 68 to small hollows by brush-tailed phascogales at 10 sites, but sampling was uneven across sites. Our modelling suggested that a null model, with the probability of detection equivalent between hollow types, fit the data better than a model that included hollow type (Table 1). A model that included hollow abundance in the surrounding habitat showed a poorer fit than did the null model. The probability of phascogale detection was influenced by season, being highest in summer and autumn, and lowest in winter and spring (Fig. 6a). The probability of occupancy was estimated as 0.86 ± 0.09, and the probability of presence as 0.46 ± 0.06.
| Model | AICc | ΔAICc | W | K | |
|---|---|---|---|---|---|
| p(2 seasons) | 599.60 | 0.00 | 0.99 | 4 | |
| p(seasons) | 610.12 | 10.52 | 0.01 | 6 | |
| p(.) | 655.59 | 55.99 | 0.00 | 3 | |
| p(hollows) | 656.00 | 56.40 | 0.00 | 4 |
(.), no covariates; 2 seasons, autumn/summer and winter/spring; seasons, 4 seasons; W, model weight; K, number of parameters.
Seasonal probability of detection of for (a) brush-tailed phascogale, (b) inland sugar glider and (c) agile antechinus. Values are estimated from a model with all seasons different.
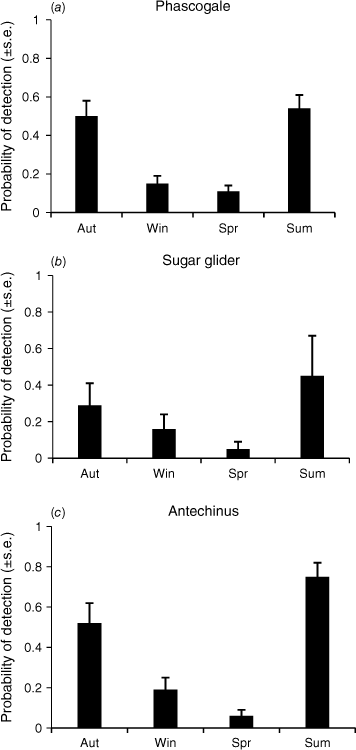
We recorded 23 daily visits to large hollows and 14 to small hollows by inland sugar gliders at 10 sites. The null detection model showed a better fit to the data than did a model that included hollow type (Table 2). A model that included surrounding hollow abundance showed a poorer fit than did the null model. The probability of sugar glider detection was influenced by season, being highest in summer and lowest in spring (Fig. 6b). The probability of occupancy was estimated as 0.78 ± 0.13, and the probability of presence as 0.29 ± 0.11.
| Model | AICc | ΔAICc | W | K | |
|---|---|---|---|---|---|
| p(3 seasons) | 248.29 | 0.00 | 0.67 | 5 | |
| p(.) | 251.30 | 3.01 | 0.15 | 3 | |
| p(hollows) | 251.62 | 3.33 | 0.13 | 4 | |
| p(m) | 253.21 | 4.92 | 0.06 | 4 |
(m), method (i.e. hollow type); (.), no covariates; 3 seasons, autumn and summer equal; W, model weight; K, number of parameters.
There were 92 daily visits to large hollows and 81 to small hollows by antechinuses at nine sites. The null detection model showed a better fit to the data than did a model that included hollow type differing by 3.8 AICc. A model that included hollow abundance also showed a poorer fit than did the null model (Table 3). The probability of antechinus detection was influenced by season, being highest in autumn and summer and lowest in spring (Fig. 6c). The probability of occupancy was estimated as 0.65 ± 0.13, and the probability of presence as 0.44 ± 0.05.
Habitat assessment
Tree species recorded included messmate stringybark (E. obliqua), bundy (E. goniocalyx) broad-leaved peppermint (E. dives), narrow-leaved peppermint (E. radiata subsp. radiata), swamp gum (E. ovata), manna gum (E. viminalis), grey box (E. microcarpa), yellow box (E. melliodora), silver wattle (Acacia dealbata), and blackwood (Acacia melanoxylon). No ironbarks were recorded in the habitat plots. Large trees (>60 cm DBH) were abundant across sites (Table 4).
| Variable | Mean (±s.e.) | Range | |
|---|---|---|---|
| All stems >5 cm DBH | 535.7 ± 55.6 | 260–1010 | |
| Smallest stems 5–10 cm DBH | 201.0 ± 41.9 | 40–410 | |
| Largest stems >60 cm DBH | 41.4 ± 10.3 | 10–130 | |
| Dead trees | 93.5 ± 15.1 | 0–230 | |
| Dead trees >30 cm DBH | 14.2 ± 3.5 | 0–20 | |
| Dead trees >60 cm DBH | 5.0 ± 2.5 | 0–15 | |
| Tree hollows | 30.0 ± 7.5 | 0–100 |
The study sites were characterised by a very high density of hollows (Table 4). Hollow-bearing trees had a mean DBH of 62.5 ± 22.6 cm (range 12–114). Hollows had a mean height of 9.6 ± 0.7 m (range 0.8–22). Three hollows were well used, showing signs of wear from frequent movement of animals. Phascogale scats and nesting material were recorded near the entrance of two hollows.
Discussion
Visitation to hollows
The camera traps recorded widespread interest in the newly carved hollows by a range of hollow dependent mammals and birds. Our principal target species, the brush-tailed phascogale, was the most frequently recorded species inspecting the hollows, and was detected visiting hollows within 4 days of installation. Our other target species, the sugar glider and agile antechinus, were recorded inspecting hollows rapidly following installation, including within the first night at one site. Our modelling suggested that there was no difference in the probability of detection (i.e. visitation) by any of our target species at the small or large hollows.
The camera-trap monitoring within 1.5 years of hollow installation did not show any nesting attempts by our target species. Our more extended monitoring (4.5 years) with a pole camera confirmed that use was low. Three of the large hollows contained active sugar glider nests during the monitoring. One of these hollows was at a site where no hollows were recorded in the habitat plot, but the others were at sites with abundant hollows. Although no hollows were recorded in plots at two of our study sites, the density of hollows was high across the study region and is presumed to have influenced occupation of the carved hollows.
Detection of target species in hollows
Our study was not able to determine the preferences by our target species for large versus small hollows because our visitation rates did not differ and we had too few occupancies to draw a conclusion.
Our hollow occupancy was low compared with that in other studies involving carved hollows or nest boxes (Goldingay et al. 2018, 2024; Terry et al. 2021; Best et al. 2022). These other studies were conducted where there was a shortage of natural tree hollows. In contrast, many natural hollows were located within close proximity to our carved hollows. Our study sites consisted of small conservation reserves and rural roadsides, which contain remnant vegetation including multiple large trees that have been protected from fuel reduction burns and historical timber collection. These roadsides make up valuable habitat for species such as phascogales, which depend on the hollows contained within these areas (van der Ree et al. 2006).
Goldingay et al. (2024) recorded high occupancy of nest boxes by brush-tailed phascogales in the box-ironbark forests of central Victoria. That study recorded 6.8 hollows per hectare, with a mean height of only 0.20 ± 0.03 m. They recorded high occupancy rates of 20% for phascogales and 32% for sugar gliders. In contrast, we recorded a mean of 30 hollows per hectare. It is likely that the much higher density of natural hollows in our study was a contributing factor to the low occupancy of our carved hollows.
Another factor that may have influenced hollow use was the height at which the hollows were installed. Menkhorst (1984) found that sugar gliders, when given a choice, preferred higher hollows. High hollows would provide occupants with greater levels of protection from ground-based predators such as the introduced red fox (Vulpes vulpes) and feral cat (Felix catus). However, phascogales are known to use low hollows in stumps and basal trunks, but perhaps it is more likely to occur when hollows are uncommon (Traill and Coates 1993). We have installed nest boxes at similar low heights at other locations in central Victoria with a high use by phascogales and sugar gliders (W. Terry, unpubl. data). The benefit of supplementary hollows at a lower height is the ease of monitoring and maintenance. Further research is needed to understand the height preference of arboreal mammals, particularly of phascogales.
Seasonal visitation
Our three target mammals showed pronounced seasonal patterns in detectability (i.e. visitation), with a strong contrast of detectability being lowest in spring and highest in summer and autumn. This finding contrasts with previous findings (Terry et al. 2021) where detection in carved hollows (by using a pole camera) of the phascogale was lowest in spring (0.5 ± 0.02) but highest only in autumn (0.31 ± 0.08), whereas a model that included season for the sugar glider (0.22 ± 0.08) had poor support. The difference between studies may reflect different measures of activity (visitation versus occupation).
The higher visitation rates in different seasons relate to the ecology of these three species. Phascogale and antechinus males die shortly after mating, so that there are fewer individuals in winter and spring to be detected, and therefore less visitation to hollows would be predicted. The number of individuals foraging and exploring changes after weaning and dispersal in summer (Cuttle 1982; Rhind 2002; Fisher 2005), which would account for higher visitation to hollows in summer. The pattern in the sugar glider is harder to explain. Sugar glider dispersal occurs during autumn prior to the onset of breeding (Suckling 1984). If females have dependent young in spring, they may be less likely to explore unoccupied hollows. The subsequent increase in hollow visitation in summer may relate to an increase in activity outside the nest by weaned young.
Maintenance requirements
Previous research (Terry et al. 2021; Best et al. 2022) on carved hollows showed that callous regrowth can rapidly grow over the faceplate of carved hollows, blocking the entrance and creating a maintenance issue. We found that such growth was more extensive on coarse bark types such as messmate, but much slower on other fine bark trees such as peppermint and box. The faceplates on some of the hollows also became loose and occasionally had to be repaired. Water ingress was observed (see also Terry et al. 2021), but water eventually leaked out of the sides of the faceplate and evaporated.
Management considerations and next steps
The installation of artificial hollows can be costly and time consuming. Our study has provided evidence from sites where such installations appear not to be necessary. We recorded frequent visitations, confirming the presence of our target species, but the low occupation over an extended period suggests that there was no benefit to the target species. The results suggest that supplementary hollows may be an effective tool only where natural hollows are in low supply and confirmed the findings of other studies (Best et al. 2022). Further research is needed to explore the apparent preference for larger hollows, as suggested by Best et al. (2022) for the sugar glider. We recommend that our study be replicated in an area where few natural hollows occur, to build on this research.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
Ross Goldingay is Editor-in-Chief for Australian Mammalogy but was not involved in the peer review or decision-making process for this paper. The author(s) have no further conflicts of interest to declare.
Declaration of funding
Partial funding was obtained from a Federal Government Community Funding Grant.
Acknowledgements
The authors acknowledge Deep Creek Landcare Group (Ken Allender and Phil Severs) for providing access to their camera traps, which were used in the monitoring and assistance in setting and collecting camera traps. Deep Creek Landcare Group also assisted in locating suitable sites within the region. Private landowners including Iain Woxvold, David and Maria Glenn, Emma Stevens, Ken Allender and Andy Moore are also thanked for providing access to bushland where some of the research was conducted. Ironbark Environmental Arboriculture (Grant Harris) are thanked for carving the hollows that were used in this study.
References
Best, K., Haslem, A., Maisey, A. C., Semmens, K., and Griffiths, S. R. (2022). Occupancy of chainsaw-carved hollows by an Australian arboreal mammal is influenced by cavity attributes and surrounding habitat. Forest Ecology and Management 503, 119747.
| Crossref | Google Scholar |
Burnham, K. P., and Anderson, D. R. (2004). Multimodel inference: understanding AIC and BIC in model selection. Sociological Methods & Research 33, 261-304.
| Crossref | Google Scholar |
Cockburn, A., and Lazenby-Cohen, K. A. (1992). Use of nest trees by Antechinus stuartii, a semelparous lekking marsupial. Journal of Zoology 226, 657-680.
| Crossref | Google Scholar |
Fisher, D. O. (2005). Population density and presence of the mother are related to natal dispersal in male and female Antechinus stuartii. Australian Journal of Zoology 53, 103-110.
| Crossref | Google Scholar |
Gibbons, P., Lindenmayer, D. B., Barry, S. C., and Tanton, M. T. (2000). Hollow formation in eucalypts from temperate forests in southeastern Australia. Pacific Conservation Biology 6, 218-228.
| Crossref | Google Scholar |
Goldingay, R. L. (2011). Characteristics of tree hollows used by Australian arboreal and scansorial mammals. Australian Journal of Zoology 59, 277-294.
| Crossref | Google Scholar |
Goldingay, R. L. (2020). Sex and age differences in tree cavity dependence in a small arboreal marsupial. Journal of Zoology 312, 53-62.
| Crossref | Google Scholar |
Goldingay, R. L., and Thomas, K. J. (2023). Temperature variation in nest boxes occupied by arboreal mammals during winter in southern Australia. Australian Mammalogy 45, 24-31.
| Crossref | Google Scholar |
Goldingay, R. L., Grimson, M. J., and Smith, G. C. (2007). Do feathertail gliders show a preference for nest box design? Wildlife Research 34, 484-490.
| Crossref | Google Scholar |
Goldingay, R. L., Thomas, K. J., and Shanty, D. (2018). Outcomes of decades long installation of nest boxes for arboreal mammals in southern Australia. Ecological Management & Restoration 19, 204-211.
| Crossref | Google Scholar |
Goldingay, R. L., Rohweder, D., and Taylor, B. D. (2020a). Nest box contentions: are nest boxes used by the species they target? Ecological Management and Restoration 21, 115-122.
| Crossref | Google Scholar |
Goldingay, R. L., Quin, D. G., Talamo, O., and Mentiplay-Smith, J. (2020b). Nest box revealed habitat preferences of arboreal mammals in box-ironbark forest. Ecological Management & Restoration 21, 131-142.
| Crossref | Google Scholar |
Goldingay, R. L., Quin, D. G., and Thomas, K. J. (2024). Habitat preferences of arboreal mammals in box-ironbark forest during maternal and non-maternal periods. Australian Mammalogy 46, AM24010.
| Crossref | Google Scholar |
Griffiths, S., Lentini, P., Semmens, K., Watson, S., Lumsden, L., and Robert, K. (2018). Chainsaw-carved cavities better mimic the thermal properties of natural tree hollows than nest boxes and log hollows. Forests 9, 235.
| Crossref | Google Scholar |
Griffiths, S. R., Semmens, K., Watson, S. J., and Jones, C. S. (2020). Installing chainsaw‐carved hollows in medium‐sized live trees increases rates of visitation by hollow‐dependent fauna. Restoration Ecology 28, 1225-1236.
| Crossref | Google Scholar |
Harley, D. K. (2006). A role for nest boxes in the conservation of Leadbeater’s possum (Gymnobelideus leadbeateri). Wildlife Research 33, 385-395.
| Crossref | Google Scholar |
Honey, R., McLean, C. M., Murray, B. R., Callan, M. N., and Webb, J. K. (2021). Choice of monitoring method can influence estimates of usage of artificial hollows by vertebrate fauna. Australian Journal of Zoology 69, 18-25.
| Crossref | Google Scholar |
Koch, A., Munks, S., and Driscoll, D. (2008). The use of hollow-bearing trees by vertebrate fauna in wet and dry Eucalyptus obliqua forest, Tasmania. Wildlife Research 35, 727-746.
| Crossref | Google Scholar |
Le Roux, D. S., Ikin, K., Lindenmayer, D. B., Bistricer, G., Manning, A. D., and Gibbons, P. (2016). Effects of entrance size, tree size and landscape context on nest box occupancy: considerations for management and biodiversity offsets. Forest Ecology and Management 366, 135-142.
| Crossref | Google Scholar |
Lindenmayer, D. B., Cunningham, R. B., Tanton, M. T., Smith, A. P., and Nix, H. A. (1991). Characteristics of hollow-bearing trees occupied by arboreal marsupials in the montane ash forests of the Central Highlands of Victoria, south-east Australia. Forest Ecology and Management 40, 289-308.
| Crossref | Google Scholar |
Lindenmayer, D. B., Laurance, W. F., Franklin, J. F., Likens, G. E., Banks, S. C., Blanchard, W., Gibbons, P., Ikin, K., Blair, D., McBurney, L., and Manning, A. D. (2014). New policies for old trees: averting a global crisis in a keystone ecological structure. Conservation Letters 7, 61-69.
| Crossref | Google Scholar |
Lindenmayer, D. B., Bowd, E., Youngentob, K., and Evans, M. J. (2024). Quantifying drivers of decline: a case study of long-term changes in arboreal marsupial detections. Biological Conservation 293, 110589.
| Crossref | Google Scholar |
Macak, P. V. (2020). Nest boxes for wildlife in Victoria: an overview of nest box distribution and use. The Victorian Naturalist 137, 4-14.
| Google Scholar |
MacKenzie, D. I., and Nichols, J. D. (2004). Occupancy as a surrogate for abundance estimation. Animal Biodiversity and Conservation 27, 461-467.
| Crossref | Google Scholar |
Manning, A. D., Gibbons, P., Fischer, J., Oliver, D. L., and Lindenmayer, D. B. (2013). Hollow futures? Tree decline, lag effects and hollow-dependent species. Animal Conservation 16, 395-403.
| Crossref | Google Scholar |
Mattheck, C., Bethge, K., and West, P. W. (1994). Breakage of hollow tree stems. Trees 9, 47-50.
| Crossref | Google Scholar |
Menkhorst, P. W. (1984). Use of nest boxes by forest vertebrates in Gippsland: acceptance, preference and demand. Australian Wildlife Research 11, 255-264.
| Crossref | Google Scholar |
Nichols, J. D., Bailey, L. L., O’Connell, A. F., Talancy, N. W., Campbell Grant, E. H., Gilbert, A. T., Annand, E. M., Husband, T. P., and Hines, J. E. (2008). Multi-scale occupancy estimation and modeling using multiple detection methods. Journal of Applied Ecology 45, 1321-1329.
| Crossref | Google Scholar |
NSW Government (2024). Brush-tailed Phascogale (Phascogale tapoatafa): saving our species strategy. Available at https://www.environment.nsw.gov.au/savingourspeciesapp/project/738. [accessed 13 September 2024]
Quin, B. R., Goldingay, R. L., Quin, D. G., Collins, E., Bartlett, N., Jerome, R., Murnane, T., Marsh, T., and Jessup, S. (2021). Long-term monitoring of nest boxes and nest logs in a tree-hollow depleted box–ironbark forest in north-eastern Victoria. Australian Journal of Zoology 68, 150-166.
| Crossref | Google Scholar |
Rhind, S. G. (2002). Reproductive demographics among brushtailed phascogales (Phascogale tapoatafa) in south-western Australia. Wildlife Research 29, 247-257.
| Crossref | Google Scholar |
Rueegger, N. (2017). Artificial tree hollow creation for cavity-using wildlife: trialling an alternative method to that of nest boxes. Forest Ecology and Management 405, 404-412.
| Crossref | Google Scholar |
Saunders, D. A., Smith, G. T., and Rowley, I. (1982). The availability and dimensions of tree hollows that provide nest sites for cockatoos (Psittaciformes) in Western Australia. Australian Wildlife Research 9, 541-556.
| Crossref | Google Scholar |
Soderquist, T. R. (1993a). Maternal strategies of Phascogale tapoatafa (Marsupialia: Dasyuridae). II. Juvenile thermoregulation and maternal attendance. Australian Journal of Zoology 41, 567-576.
| Crossref | Google Scholar |
Soderquist, T. R. (1993b). Maternal strategies of Phascogale tapoatafa (Marsupialia, Dasyuridae). 1. Breeding seasonality and maternal investment. Australian Journal of Zoology 41, 549-566.
| Crossref | Google Scholar |
Soderquist, T., and Lill, A. (1995). Natal dispersal and philopatry in the carnivorous marsupial Phascogale tapoatafa (Dasyuridae). Ethology 99, 297-312.
| Crossref | Google Scholar |
Stojanovic, D., Webb, M., Roshier, D., Saunders, D., and Heinsohn, R. (2012). Ground-based survey methods both overestimate and underestimate the abundance of suitable tree-cavities for the endangered Swift Parrot. Emu - Austral Ornithology 112, 350-356.
| Crossref | Google Scholar |
Stojanovic, D., Rayner, L., Cobden, M., Davey, C., Harris, S., Heinsohn, R., Owens, G., and Manning, A.D. (2021a). Suitable nesting sites for specialized cavity dependent wildlife are rare in woodlands. Forest Ecology and Management 483, 118718.
| Crossref | Google Scholar |
Stojanovic, D., Owens, G., Young, C. M., Alves, F., and Heinsohn, R. (2021b). Do nest boxes breed the target species or its competitors? A case study of a critically endangered bird. Ecological Restoration 29, e13319.
| Crossref | Google Scholar |
Suckling, G. C. (1984). Population ecology of the sugar glider, Petaurus breviceps, in a system of fragmented habitats. Australian Wildlife Research 11, 49-75.
| Crossref | Google Scholar |
Terry, W., Goldingay, R., and van der Ree, R. (2021). Can chainsaw carved hollows provide an effective solution to the loss of natural tree cavities for arboreal mammals? Forest Ecology and Management 490, 119122.
| Crossref | Google Scholar |
Thompson, E. K., Keenan, R. J., and Kelly, L. T. (2023). The use of nest boxes to support bird conservation in commercially managed forests: a systematic review. Forest Ecology and Management 550, 121504.
| Crossref | Google Scholar |
Traill, B. J., and Coates, T. D. (1993). Field observations on the brush-tailed phascogale Phascogale tapoatafa (Marsupialia: Dasyuridae). Australian Mammalogy 16, 61-65.
| Crossref | Google Scholar |
van der Ree, R., Bennett, A. F., and Soderquist, T. R. (2006). Nest-tree selection by the threatened brush-tailed phascogale (Phascogale tapoatafa) (Marsupialia: Dasyuridae) in a highly fragmented agricultural landscape. Wildlife Research 33, 113-119.
| Crossref | Google Scholar |
Whitford, K. R. (2002). Hollows in jarrah (Eucalyptus marginata) and marri (Corymbia calophylla) trees I. Hollow sizes, tree attributes and ages. Forest Ecology and Management 160, 201-214.
| Crossref | Google Scholar |
Wormington, K. R., Lamb, D., McCallum, H. I., and Moloney, D. J. (2003). The characteristics of six species of living hollow-bearing trees and their importance for arboreal marsupials in the dry sclerophyll forests of southeast Queensland, Australia. Forest Ecology and Management 182, 75-92.
| Crossref | Google Scholar |





