Factors contributing directly to platypus (Ornithorhynchus anatinus) mortality and implications for conserving populations in the wild
Melody Serena A * , Geoff A. Williams A and Jessica L. Thomas BA
B
Abstract
Based on details of more than 400 platypus deaths with an identifiable cause recorded since 1989, five main factors contributed directly to platypus mortalities: drowning in fish nets or enclosed crustacean traps (such as opera house traps), being killed by predators (especially canids), becoming accidentally hooked by recreational anglers who then cut the line, becoming entangled in discarded fishing line or other types of litter and being hit by motor vehicles (especially, though not exclusively, in Tasmania). Additional mortality factors included drought, severe flooding, being drawn into irrigation pumps and entrapment in manmade materials or infrastructure. Disease was implicated as being the likely causal agent in two platypus deaths, including a female that died late in lactation. Platypus mortalities were recorded throughout the year on the southeastern Australian mainland but peaked in early autumn, when many recently weaned juveniles are likely to be present. Given the very high number of platypus mortalities attributed to the use of fish nets or enclosed crustacean traps, their use should be banned in all waters where platypus population are known to occur.
Keywords: anthropogenic mortality, extinction drivers, monotreme, Ornithorhynchidae, platypus conservation, platypus management, predation risk, road trauma, wildlife disease.
Introduction
Effective conservation of mammals often relies on identifying the agents directly responsible for mortality. Populations can decline and even disappear due to impacts of a single dominant factor on survival, such as hunting or disease (Rosser and Manika 2002; Clavero and Garcia-Berthou 2005; de Castro and Bolker 2005). Proximate sources of mortality may adversely affect foraging efficiency, predator defence or evolutionary resilience by altering morphological, behavioural or demographic attributes on a population-wide scale (e.g. Coltman et al. 2003; McDougall et al. 2006; Milner et al. 2007; Allendorf et al. 2008; Darimont et al. 2009) or by reducing allelic diversity or genetic heterozygosity (Allendorf et al. 2008). Information pertaining to mortality factors is needed to develop meaningful management strategies, identify priority areas for population monitoring and provide a focus for community conservation activities.
The platypus (Ornithorhynchus anatinus) is a relatively long-lived animal: 24% of marked adults have been found to live to an age of ≥9 years in urban creeks (Serena et al. 2014), and both sexes can survive for >20 years in the wild (Grant 2004; Serena et al. 2024). Munday et al. (1998) listed car trauma, drowning in fish nets, canid predation and (in the case of dispersing juveniles) undernutrition as being risks that contribute routinely to platypus mortality. Additional deaths were attributed to shooting, trauma incurred in water pipes or power plant intakes, litter entanglement, raptor predation and infection by Mucor amphibiorum, an ulcerative fungus known to cause disease in Tasmania but not to date on the mainland (Gust et al. 2009; Macgregor et al. 2017). Based on 23 necropsies conducted in the 1990s, Connolly et al. (1998) reported that platypus deaths in Tasmania resulted from dog attack, car trauma, starvation/exposure (sometimes as an outcome of flooding) and M. amphibiorum infection.
Based on 124 platypus mortality reports recorded by the Australian Platypus Conservancy (APC) from 1989 to 2009, animals in Victoria most often died by drowning in fish nets and enclosed crustacean traps or being killed by predators, particularly canids. Other factors deemed to have contributed directly to two or more deaths included trauma incurred in irrigation pumps, being accidentally hooked by recreational anglers, litter entanglement, flooding, vehicle trauma, drought and being shot or bludgeoned (Serena and Williams 2010).
Details of an additional 153 platypus mortality reports have been shared with the APC since 2009, with 59 reports originating outside Victoria. Here we develop a consolidated set of records to characterise the entire range of factors known to have contributed directly to platypus mortality in recent decades. We also consider how the monthly distribution of mortality reports varies through the year, whether the ranking of platypus mortality factors has been consistent over time, and if major factors in Victoria are ranked the same as those elsewhere. In addition, we describe the factors responsible for platypus morbidity and eventual mortality in rescued individuals brought to Healesville Sanctuary for veterinary care in the last 20 years. Most of these cases were subject to detailed necropsies, increasing the likelihood that the immediate cause of death, including disease, was accurately identified.
Methods
From 1989 to mid-2024, research staff at the APC recorded details of 391 individual platypus mortalities with an identifiable cause in the wild (referred to hereafter as APC records), including 305 originating at sites in Victoria and 86 originating elsewhere (40 records for New South Wales, 25 for Queensland, 16 for Tasmania, 5 for Australian Capital Territory). More than half of the records (55%), including all of those originating outside Victoria, pertained to mortalities occurring from 2010 to 2024. Reports were submitted on an ad hoc basis by field biologists, veterinarians, natural resource managers, persons involved in wildlife rescue and reliable members of the public; supporting documentation (veterinary findings, direct observation by the authors and/or photographs of a carcass) was available for 46% of reports submitted before 2010 and 84% of reports submitted thereafter. Criteria for assigning mortalities to 10 causal factors (nets or traps targeting fish or decapod crustaceans, predation, angling, litter, vehicle trauma, flooding, drought, attack by humans, pumps or small turbines, other water supply infrastructure or related features) followed those in Serena and Williams (2010). An additional category (premature weaning) was defined as comprising severely underweight juveniles encountered in Victoria in summer, when there was no evidence that an animal’s poor condition was due to anything other than having become lost or orphaned prior to weaning (which normally occurs at the age of ~3.5–4 months in the wild: Grant et al. 1983, 2004a).
The factor contributing most directly to platypus mortality could not be reliably assigned in 50 Victorian records submitted from 1989 to 2009 (28%), 44 Victorian records submitted from 2010 to 2024 (34%) and 22 non-Victorian (referred to hereafter as interstate) records submitted from 2010 to 2024 (26%). This was most commonly due to a carcass being found on land (generally within 20 m of the water) with no obvious signs of trauma or other evidence indicating the likely cause of death (48%), followed by a carcass being too decomposed to provide useful information (22%), a necropsy generating no significant findings (17%) or a carcass being seen but not recovered from the water (13%).
Twenty-three records relating to severely unwell individuals (all older than nestlings) that died from 2005 to mid-2024 after being brought to Healesville Sanctuary by members of the public were extracted in June 2024 from the online Zoological Information Management System maintained by Species360 (zims.Species360.org) (referred to hereafter as HS records). An individual’s sex and age class (first-year juvenile male, second-year subadult male, adult male >2 year, first-year juvenile female or subadult/adult female >1 year) was inferred from the appearance of calcaneal spurs and spur sheaths (Williams et al. 2013; Grant et al. 2024) and/or internal reproductive anatomy.
Summary statistics (mean ± standard error) and statistical tests were calculated using Systat 13.0, with significance set at 0.05. Tests were performed using Chi-square analysis (to determine if the number of platypus mortality reports differed in the first and second halves of the year) and non-parametric Spearman rank correlation (to determine if the ranking of major platypus mortality factors differed in Victoria in two time periods, or varied between Victorian and interstate locations). Two-sample t-tests were used to compare how many platypus deaths occurred respectively in fish nets and enclosed crustacean traps, and to ascertain if the mean number of mammals (platypus and rakali/Australian water-rat Hydromys chrysogaster) dying in enclosed crustacean traps differed before and after Victoria banned their deployment.
Results
How were platypus mortalities distributed by month through the year?
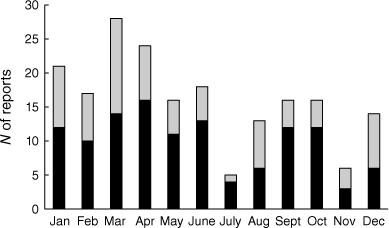
Based on 194 reports that reliably documented the month when an animal died (APC records), platypus deaths in Victoria occurred throughout the year but were noted most often in March and April, and least often in July and November (Fig. 1). A disproportionately high number of deaths (n = 124, 64%) was reported in the first half of the year, with the frequencies of reports in the first and second halves of the year differing significantly from those expected if mortalities were distributed randomly (Χ2 = 15.0309, P < 0.001).
What factors contributed to platypus mortalities in Victoria and elsewhere?
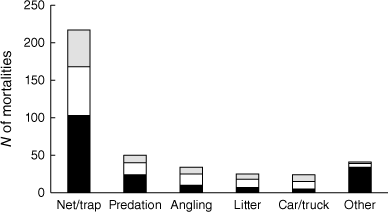
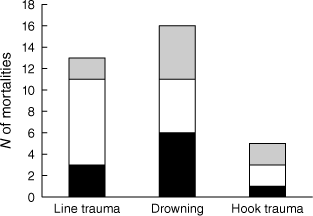
Five factors collectively accounted for nearly 90% of the 391 APC platypus mortality records with an identifiable cause: drowning in fish nets (mainly mesh gill nets, fyke nets and drum nets) or enclosed crustacean traps (mainly opera house traps) (55%); being killed by predators, especially canids but occasionally raptors (13%); dying as an outcome of inappropriate angling practices (9%); becoming entangled in items of litter other than fishing line (6%); and being hit by motor vehicles (6%) (Fig. 2). Angling-related mortalities comprised 13 records in which a carcass was found tightly encircled by fishing line (often with deep associated lesions) and 21 records in which an animal died after being snagged by a fishing hook, most often when loose line trailing from the hook became tangled around an object in the channel so the platypus drowned or died of exhaustion (Fig. 3). The relative rankings of the five main factors contributing directly to platypus deaths in Victoria were identical in 1989–2009 and 2010–2024 (rs = 1.000, P = 0.020). In contrast, the five main mortality factors identified in Victoria from 2010 to 2024 were ranked differently compared to interstate locations in the same period (rs = 0.410, P = 0.590). This was largely due to a high frequency of vehicle-related trauma reported for Tasmania (7 of 10 records with an identifiable cause), coupled with the absence of predation-related mortalities for this state.
N of reported platypus mortalities caused by fish nets/enclosed crustacean traps, predation, angling practices, litter, motor vehicles or other known factors. Black (Victoria, 1989–2009), white (Victoria, 2010–2024), grey (interstate, 2010–2024).
N of platypus angling-related mortalities due to line-related trauma, drowning/exhaustion or direct hook-related trauma. Black (Victoria, 1989–2009), white (Victoria, 2010–2024), grey (interstate, 2010–2024).
More than 70% of the remaining 41 APC mortalities were recovered from irrigation pumps or (in one case) a domestic-scale hydroelectric turbine, had become entrapped in a manmade structure or natural feature, or were found in a severely drought-affected part of a channel or after major flooding (Table 1). Platypus entrapment mainly occurred in manmade structures: a bathtub used as a livestock watering point, a homemade flow gauge, overlapping wire mesh panels used to exclude floating vegetation from a small weir pool or stabilise a bank, and components of irrigation or other water supply networks (a narrow pipe, an instream gate, a Dethridge water wheel and an enclosed section of concrete channel near a large dam wall). In addition, one platypus died after falling into a natural sinkhole located in a rock outcrop near a waterfall. Four other mortalities occurred when a platypus was inadvertently shot (after bubbles were seen rising to the surface of a river) or purposefully bludgeoned to death, most recently in 2004. In addition, three nestlings died after being accidentally dug up from nesting burrows in November or December, and one emaciated and very weak juvenile female died soon after being found near a river in mid-February, presumably after becoming orphaned or lost prior to weaning.
| Mortality factor | N | |
|---|---|---|
| Irrigation pumps, small hydro-turbine | 11 | |
| Drought/lack of surface water | 7 | |
| Major flood | 6 | |
| Entrapment (manmade structures) | 8 | |
| Entrapment (natural sinkhole) | 1 | |
| Shooting/attack by humans | 4 | |
| Nestling(s) dug up from burrow | 3 | |
| Premature weaning | 1 |
A causal factor could be reliable assigned to 18 of 23 cases in which a non-nestling platypus was brought to Healesville Sanctuary for veterinary assessment from 2005 to 2024 and died or was humanely euthanased. An age class was assigned to 15 cases, comprising 9 first-year juveniles and six adults or subadults (Table 2). Stemming from the fact that Healesville Sanctuary is widely known to have facilities and expertise needed to raise orphaned wildlife, five cases (28%) involved severely underweight juveniles found in January without evidence of associated physical trauma, presumably after having been weaned prematurely. As in the case of APC records, other mortalities were largely due to canid predation, litter entanglement, inappropriate angling practices or motor vehicle trauma. Disease was the likely causal agent in two mortalities: a lactating female that died in January following lung haemorrhage and extensive ulceration of the small intestine and stomach (though the identity of disease agents could not be confirmed) and a non-lactating adult or subadult female that died in May with evidence of coccidial infection (intestines), possible salmonellosis (lungs) and infection by an unidentified fungus (skin and lungs). The absence of any cases involving drowning in nets or traps reflects the fact that Healesville Sanctuary normally receives rescued living animals rather than carcasses.
| Mortality factor | Adult/subadult | Juvenile | Unspecified age | |
|---|---|---|---|---|
| Premature weaning | 0 | 5 | 0 | |
| Predation | 1 | 3 | 1 | |
| Litter | 1 | 0 | 1 | |
| Angling | 2 | 0 | 0 | |
| Vehicle trauma | 1 | 1 | 0 | |
| Disease | 1 | 0 | 1 |
See text for animal age criteria.
Apart from when fish nets or enclosed crustacean traps were deemed to be responsible, most APC records (96%) documented single platypus mortalities, with a maximum of two animals dying in a given incident. In contrast, multiple mortalities were routinely reported in incidents involving fishing gear, particularly when two or more nets or traps were set at a given location or when such gear was abandoned in the water (so-called ‘ghost fishing’). Based on records obtained since 2009, the mean number of animals killed in an incident involving fish nets (4.0 ± 1.2, n = 10) significantly exceeded the mean number killed in an incident involving enclosed crustacean traps (1.5 ± 0.1, n = 48) (t = 3.990, P < 0.001), though up to five individuals died in just two opera house traps set at the same site.
Did the number of trap-related mortalities drop after use of enclosed traps was banned in Victoria?
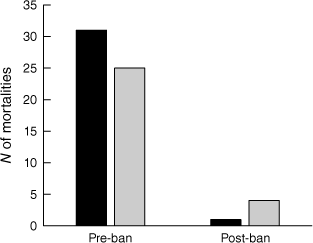
Victoria enacted a statewide ban on recreational use of opera house traps (and other similar types of enclosed crustacean traps) that came into effect on 30 June 2019. The combined number of platypus and rakali deaths reportedly caused by drowning in such traps was an order of magnitude greater in the 60 months before the ban came into effect compared to the 60 months afterwards (Fig. 4). The mean annual number of pre-ban and post-ban mortalities differ significantly in the case of both the platypus (t = 2.774, P = 0.024) and rakali (t = 2.537, P = 0.035).
Discussion
Major factors contributing to platypus mortality
Human activity is the dominant force driving virtually every aspect of contemporary environmental change (Lewis and Maslin 2015). Reflecting this ascendancy, an analysis of factors contributing to mortality of adult mammals in North America found that humans were directly responsible for more than half (52%) of deaths with an identifiable cause (Collins and Kays 2011). Similarly, a study of mortality factors affecting radio-tagged vertebrates globally concluded that 28% of deaths were attributable to anthropogenic causes (Hill et al. 2019). In Australia, factors such as motor vehicle trauma, being attacked by pets, and entanglement in wire fencing or nets protecting fruit trees are major contributors to morbidity and mortality of Queensland wildlife (Taylor-Brown et al. 2019). In our study, after excluding nestlings and unweaned juveniles, 56% of APC platypus mortality records with an identifiable cause were due to animals drowning in nets or traps and 27% were attributed to other anthropogenic factors. Similarly, after again excluding unweaned juveniles and cases where no cause was assigned, anthropogenic factors were deemed to be responsible for 46% of platypus deaths involving rescued wild individuals brought to Healesville Sanctuary for veterinary assessment.
Although a platypus can avoid breathing for up to 11 min by resting quietly underwater and reducing its cardiac rate to as little as 1.2 beats min−1 (Evans et al. 1994), active animals must breathe at intervals of ≤2.3–2.6 min (McLeod 1993; Bethge et al. 2003; Grant et al. 2004b). They are, therefore, highly vulnerable to drowning in weighted mesh gill nets that prevent an entangled platypus from reaching the water surface (Grant and Temple-Smith 2003; Serena and Williams 2010) or wholly submerged fyke nets, drum nets or enclosed crustacean traps such as opera house traps (Grant et al. 2004b; Serena and Williams 2010; Serena et al. 2016). The lethal nature of these designs reflects the presence of inwardly directed funnels (or ‘valves’) around the entry points that effectively mask the central opening from the perspective of a platypus held inside (Serena et al. 2016). Up to five individuals have died in incidents involving just one or two opera house traps (Grant et al. 2004b; this paper). Similarly, five platypus carcasses have been recovered from two licensed fyke nets located ~1 km upstream of an area legally allocated to commercial eel fishing, and a single abandoned (unlicensed) fyke net contained the remains of 17 carcasses (Serena and Williams 2010).
Other important factors contributing to platypus mortality after the age of milk-dependency in Victoria included predation, recreational angling, litter entanglement and motor vehicle trauma (as inferred from both APC and HS records). The rankings of the five leading mortality factors were concordant when APC records for 1989–2009 and 2010–2024 were compared, implying that their relative impacts have remained unchanged over this time. In contrast, major mortality factors were ranked differently at Victorian and interstate sites in the last 15 years, mainly due to the large number of vehicle-related deaths and absence of predation-related deaths reported for Tasmania since 2009. Although pet dogs contribute to platypus mortality in Tasmania (Connolly et al. 1998; Munday et al. 1998), fewer canid-related deaths are expected to occur there than on the mainland given that red foxes Vulpes vulpes apparently first entered Tasmania in 2001 and were eradicated or nearly so by 2013 (Marks et al. 2014; Caley et al. 2015). Vehicle-related platypus mortalities in Tasmania have been described by Tyson (1980), Taylor et al. (1991), Otley and le Mar (1998) and Mooney and Spencer (2000), particularly at sites where use of a road culvert was impeded by an overhanging or stepped entrance (if rising vertically more than ~15 cm: Musser et al. 2024). Contributing to the likelihood of vehicle-related risk, platypus dispersal across land appears to occur more often in Tasmania than on the mainland (Furlan et al. 2013).
Predation was the second most important mortality factor identified in both APC and HS records, respectively accounting for 13% and 38% of post-milk-dependent platypus deaths. Although canids have consistently been deemed to be mainly responsible (Connolly et al. 1998; Munday et al. 1998; Serena and Williams 2010; this paper), other platypus predators include spotted-tail quoll Dasyurus maculatus (Dawson et al. 2007), Tasmanian devil Sarcophilus harrisii (Munday et al. 1998), carpet python Morelia spilota (Burrell 1927) and sizable raptors such as eagles (Munday et al. 1998; Rakick et al. 2001). Compared to adults, juvenile mammals belonging to a wide range of species are more likely to be killed by predators (Hill et al. 2019). In the case of the platypus, first-year juveniles comprised nearly two-thirds of 16 known-age APC predator mortalities from 1989 to 2009 (Serena and Williams 2010) and three of four known-age HS predator mortalities since 2005. More generally, the March–April peak in APC platypus mortalities plausibly reflects the peak occurrence of recently weaned juveniles in early autumn on the southeastern Australian mainland (Grant et al. 1983, 2004a). Creek and river flow at this time of year is also often low, presumably compounding platypus predation risk (Serena and Williams 2010).
Litter entanglement and recreational angling together accounted for 15% of APC post-milk-dependent platypus mortality records, and 31% of corresponding HS records. Platypus live-trapping studies in Victoria have found that litter items encircle the neck or body of 4% of individuals occupying catchments near Melbourne and 0.5% of those in rural waterways, often causing lesions that become progressively more life-threatening. Virtually any ring or loop measuring ~10–24 cm in circumference can be problematic to a platypus, including such diverse items as engine gaskets, cable-ties, tamper-proof food jar rings, a hospital identification wristband, a child’s plastic bracelet, the rim from a bicycle headlamp and (very commonly) elastic hair-ties (Serena and Williams 1998, 2022).
The spectre of anthropogenic climate change has focused scientific attention on mechanisms by which more extreme or variable weather patterns may adversely affect Australian wildlife (Hoffman et al. 2019). In our study, both drought and flooding were found to contribute directly to platypus mortality, respectively accounting for 1.8% and 1.6% of APC records. The platypus feeds exclusively in the water, mainly on benthic macroinvertebrates (McLachlan-Troup et al. 2010; Marchant and Grant 2015; Hawke et al. 2022), and so will starve if its habitat dries up entirely during drought. In theory, drought may also exacerbate platypus predation risk if animals are forced to cross substantial dry land to access burrows or reach disjunct feeding areas (Grant and Bishop 1998; Grant 2007). In practice, differences in the trajectories of stable and declining platypus populations during an extended drought have been found to be best explained by variation in juvenile recruitment rather than adult mortality, with a significant positive relationship evident between surface discharge in the months before breeding and reproductive success (Serena et al. 2014; Serena and Grant 2017). Similarly, though adults are sometimes killed by severe flooding (Grant 2007; Serena and Williams 2010), juveniles are considered to be much more vulnerable to high flows, particularly in the weeks before or shortly after they first exit the nesting burrow (Serena and Williams 2010; Serena et al. 2014; Serena and Grant 2017).
A relatively small number of infectious agents and parasites have been identified in the platypus, few of which are known to result in morbidity (Connolly et al. 1998; Munday et al. 1998; Booth and Connolly 2008; Macgregor et al. 2017). Disease was deemed to be responsible for only two platypus mortalities in the current study, both described at Healesville Sanctuary. Both animals died while hospitalised, contributing to the likelihood that disease was successfully diagnosed. One individual was a lactating female recorded in January, towards the end of lactation when female energy requirements more than double compared to those of non-lactating individuals (Thomas et al. 2020). Fat stores of wild females assessed through the year in Victoria are also typically lowest in mid-to-late summer during the final weeks of lactation (Handasyde et al. 2003), with exceptionally thin females sometimes encountered at this time of year (Connolly et al. 2016). However, though the substantial energy allocated by a female vertebrate to reproduction may increase her risk of infection (Martin 2009), the actual onset of disease and its outcome are likely to depend on the specific individual, her environment and the pathogens to which she is exposed (Martin 2009; Hing et al. 2016). The potentially complex nature of disease aetiology in the platypus is illustrated by a case study involving a prematurely weaned (likely orphaned) juvenile female who failed to survive (Kessell et al. 2014). When rescued, she still had moderately high fat stores but was suffering from anaemia and multiple infections, most likely in response to a high tick burden and underlying stress-related immunosuppression. The absence in our study of deaths caused by the fungus Mucor amphibiorum presumably reflects the relatively low number of Tasmanian mortality records included in the analysis, all of which date from 2010. The frequency of Mucor-related platypus disease declined significantly from the late 1990s to the late 2000s, with a mean prevalence of just 0.07 cases in affected catchments by 2008–2009 (Gust et al. 2009).
Conservation implications
Platypus abundance is typically limited by the availability and production of benthic prey species (Marchant and Grant 2015). However, direct mortality could potentially jeopardise population survival, particularly in isolated groups that have limited potential to attract migrants to offset natural or anthropogenic losses. Juvenile males will travel >40 km during presumed dispersal (Serena and Williams 2013), and adults sometimes move >15 km (Gardner and Serena 1995) or nearly 19 km (Bino et al. 2018) over a few weeks. Observational studies have also confirmed that the platypus will leave the water to bypass weir walls measuring up to at least 10 m high (Musser et al. 2024), with genetic studies indicating that overland dispersal may occur reasonably often between neighbouring watersheds (Kolomyjec et al. 2009; Furlan et al. 2013). However, genetic evidence also suggests that manmade barriers above a certain size (e.g. dam walls greater than 400 m long or 70 m high: Furlan et al. 2013; Mijangos et al. 2022) may deter effective platypus population exchange along a given water course, potentially increasing the vulnerability of one or both moieties to extinction. For example, Khurana et al. (2024) posited that the absence of O. anatinus upstream but not downstream of two sizable weirs (29 and 43 m high) was most likely due to animals originally found above the weirs (in each case, occupying ≤25 km of river channel) being unwilling to move downstream past the weirs to access refuge pools during protracted cease-to-flow periods, most recently during a drought from 2017 to 2020. However, this hypothesis requires further testing to confirm that these weirs genuinely deter platypus passage around them, including the possibility that habitats located upstream of the weirs will eventually be recolonised once populations recover farther downstream.
The major contribution made by fish nets and enclosed crustacean traps to platypus mortality suggests that a high premium should be placed on eliminating use of problematic designs wherever platypus populations occur. In Victoria, the frequency of platypus deaths in enclosed crustacean traps continued to rise after their use was banned in public waters (but not privately owned ponds or farm dams) in mid-2001, due to ongoing availability of inexpensive opera house traps in retail outlets and widespread confusion about where these traps could be deployed (Serena and Williams 2010). However, a subsequent statewide ban on recreational use of enclosed crustacean traps in all waters, both public and private, that came into effect in mid-2019 has had much better success. Importantly, it followed research that both quantified the very high degree of associated risk to air-breathing mammals and demonstrated that edible crustaceans can be captured just as effectively in wildlife-friendly trap designs (Brown et al. 2015; Serena et al. 2016). The later legislation also had broad community support following a decade of widely publicised images of animals killed in opera house traps, and the number of trap-related platypus and rakali mortalities has since declined markedly (this paper). Commercial use of nets or traps known to present a platypus mortality risk should also be phased out as a matter of priority in all habitats supporting this species – for example, some locations in Victoria where fyke nets can currently be deployed legally to capture eels (Victorian Fisheries Authority 2017).
A number of the mortality factors identified in this study are likely to be mitigated most effectively through community engagement. Given the diverse nature of hazardous litter items and the many ways they enter waterbodies, litter entanglement is likely to be best addressed through ongoing education focusing on the widespread adoption of new habits (Heimlich and Ardoin 2008), such as cutting through closed loops or rings before disposing of them responsibly (Serena and Williams 1998, 2022). Similarly, platypus mortality arising from recreational angling could largely be avoided if anglers routinely retrieved tangled line and refrained from fishing when a platypus is nearby to reduce the risk of snagging it with a hook. Likewise, the simplest way to avoid platypus injuries caused by domesticated dogs will be for owners to keep pets on leads near creeks and rivers, particularly in the hours near dawn or dusk when a platypus is most likely to enter or leave a burrow (e.g. McLeod 1993; Gardner and Serena 1995; Gust and Handasyde 1995; Roberts and Serena 2024). Even more importantly, community action to strengthen vegetation cover along watercourses should both reduce platypus predation risk from a range of predators (Serena et al. 1998; Brunt 2022) and improve environmental quality for this and other aquatic species (e.g. Pusey and Arthington 2003; Lind et al. 2019).
Data availability
Appropriately annotated details of APC mortality records have been lodged with the Atlas of Living Australia. HS mortality records will be shared upon reasonable request to J.L.T.
Declaration of funding
J.L.T.’s involvement in this project was facilitated by funding provided to Healesville Sanctuary by Dr. Audrey Harvey, Dr. Dennis Wilson and Helen Wilson. Funds donated by Chocolatier Australia to the Australian Platypus Conservancy supported Open Access publication of this paper.
Acknowledgements
We (M.S. and G.A.W.) are grateful to Wildlife Victoria and the many individuals who have reported details of platypus mortalities over the years, along with the veterinary practices and individual veterinarians who have shared relevant necropsy findings. In particular, we would like to acknowledge the information provided by Cameron Edge (Goulburn–Murray Water), Luke Gregory (Bonorong Wildlife Sanctuary), Bruce Jackson (Otway Eco Tours), Mike Sverns (former Senior Investigator with the Victorian Conservation Regulator) and Pam Whiteley (Wildlife Health Victoria), along with staff of Alpine Veterinary Hospital (Porepunkah) and Rose City Veterinary Hospital (Benalla). We also sincerely thank the veterinary team at the Australian Wildlife Health Centre, Healesville Sanctuary, for assisting with the review of the Sanctuary’s platypus necropsy records.
References
Allendorf, F. W., England, P. R., Luikart, G., Ritchie, P. A., and Ryman, N. (2008). Genetic effects of harvest on wild animal populations. Trends in Ecology and Evolution 23, 327-337.
| Crossref | Google Scholar | PubMed |
Bethge, P., Munks, S., Otley, H., and Nicol, S. (2003). Diving behaviour, dive cycles and aerobic dive limit in the platypus Ornithorhynchus anatinus. Comparative Biochemistry and Physiology A 136, 799-809.
| Crossref | Google Scholar | PubMed |
Bino, G., Kingsford, R. T., Grant, T., Taylor, M. D., and Vogelnest, L. (2018). Use of implanted acoustic tags to assess platypus movement behaviour across spatial and temporal scales. Scientific Reports 8, 5117.
| Crossref | Google Scholar | PubMed |
Brown, P., Hunt, T. L., and Khageswor, G. (2015). Effects of gear type, entrance size and soak time on trap efficiency for freshwater crayfish Cherax destructor and C. albidus. Marine and Freshwater Research 66, 989-998.
| Crossref | Google Scholar |
Caley, P., Ramsey, D. S. L., and Barry, S. C. (2015). Inferring the distribution and demography of an invasive species from sighting data: the red fox incursion into Tasmania. PLoS One 10, e0116631.
| Crossref | Google Scholar | PubMed |
Clavero, M., and Garcia-Berthou, E. (2005). Invasive species are a leading cause of animal extinctions. Trends in Ecology and Evolution 20, 110.
| Crossref | Google Scholar |
Collins, C., and Kays, R. (2011). Causes of mortality in North American populations of large and medium-sized mammals. Animal Conservation 14, 474-483.
| Crossref | Google Scholar |
Coltman, D. W., O’Donoghue, P., Jorgenson, J. T., Hogg, J. T., Strobeck, C., and Festa-Bianchet, M. (2003). Undesirable evolutionary consequences of trophy hunting. Nature 426, 655-658.
| Crossref | Google Scholar | PubMed |
Connolly, J. H., Obendorf, D. L., Whittington, R. J., and Muir, D. B. (1998). Causes of morbidity and mortality in platypus (Ornithorhynchus anatinus) from Tasmania, with particular reference to Mucor amphibiorum infection. Australian Mammalogy 20, 177-187.
| Crossref | Google Scholar |
Connolly, J. H., Claridge, T., Cordell, S. M., Nielsen, S., and Dutton, G. H. (2016). Distribution and characteristics of the platypus (Ornithorhynchus anatinus) in the Murrumbidgee catchment. Australian Mammalogy 38, 58-67.
| Crossref | Google Scholar |
Darimont, C. T., Carlson, S. M., Kinnison, M. T., Paquet, P. C., Teimchen, T. E., and Wilmers, C. C. (2009). Human predators outpace other agents of trait change in the wild. Proceedings of the National Academy of Sciences of the USA 106, 952-954.
| Crossref | Google Scholar | PubMed |
Dawson, J. P., Claridge, A. W., Triggs, B., and Paull, D. J. (2007). Diet of a native carnivore, the spotted-tail quoll (Dasyurus maculatus), before and after an intense wildfire. Wildlife Research 34, 342-351.
| Crossref | Google Scholar |
de Castro, F., and Bolker, B. (2005). Mechanisms of disease-induced extinction. Ecology Letters 8, 117-126.
| Crossref | Google Scholar |
Evans, B. K., Jones, D. R., Baldwin, J., and Gabbott, G. R. J. (1994). Diving ability of the platypus. Australian Journal of Zoology 42, 17-27.
| Crossref | Google Scholar |
Furlan, E. M., Griffiths, J., Gust, N., Handasyde, K. A., Grant, T. R., Gruber, B., and Weeks, A. R. (2013). Dispersal patterns and population structuring among platypuses, Ornithorhynchus anatinus, throughout south-eastern Australia. Conservation Genetics 14, 837-853.
| Crossref | Google Scholar |
Gardner, J. L., and Serena, M. (1995). Spatial organisation and movement patterns of adult male platypus, Ornithorhynchus anatinus (Monotremata: Ornithorhynchidae). Australian Journal of Zoology 43, 91-103.
| Crossref | Google Scholar |
Grant, T. R. (2004). Captures, capture mortality, age and sex ratios of platypuses, Ornithorhynchus anatinus, during studies over 30 years in the upper Shoalhaven River in New South Wales. Proceedings of the Linnean Society of New South Wales 125, 217-226.
| Crossref | Google Scholar |
Grant, T. R., and Bishop, K. A. (1998). Instream flow requirements for the platypus (Ornithorhynchus anatinus): a review. Australian Mammalogy 20, 267-280.
| Crossref | Google Scholar |
Grant, T. R., and Temple-Smith, P. D. (2003). Conservation of the platypus, Ornithorhynchus anatinus: threats and challenges. Aquatic Ecosystem Health and Management 6, 5-18.
| Crossref | Google Scholar |
Grant, T. R., Griffiths, M., and Leckie, R. M. C. (1983). Aspects of lactation in the platypus, Ornithorhynchus anatinus (Monotremata), in waters of eastern New South Wales. Australian Journal of Zoology 31, 881-889.
| Crossref | Google Scholar |
Grant, T. R., Griffiths, M., and Temple-Smith, P. D. (2004a). Breeding in a free-ranging population of platypuses, Ornithorhynchus anatinus, in the upper Shoalhaven River, New South Wales – a 27 year study. Proceedings of the Linnean Society of New South Wales 125, 227-234.
| Crossref | Google Scholar |
Grant, T. R., Lowry, M. B., Pease, B., Walford, T. R., and Graham, K. (2004b). Reducing the by-catch of platypuses (Ornithorhynchus anatinus) in commercial and recreational fishing gear in New South Wales. Proceedings of the Linnean Society of New South Wales 125, 259-272.
| Crossref | Google Scholar |
Grant, T., Serena, M., Williams, G. A., and Temple-Smith, P. (2024). Age determination in the platypus (Ornithorhynchus anatinus) using spur sheath and spur developmental stages: a review. Australian Mammalogy 46, AM24020.
| Crossref | Google Scholar |
Gust, N., and Handasyde, K. (1995). Seasonal variation in the ranging behaviour of the platypus (Ornithorhynchus anatinus) on the Goulburn River, Victoria. Australian Journal of Zoology 43, 193-208.
| Crossref | Google Scholar |
Gust, N., Griffiths, J., Driessen, M., Philips, A., Stewart, N., and Geraghty, D. (2009). Distribution, prevalence and persistence of mucormycosis in Tasmanian platypuses (Ornithorhynchus anatinus). Australian Journal of Zoology 57, 245-254.
| Crossref | Google Scholar |
Handasyde, K. A., McDonald, I. R., and Evans, B. K. (2003). Plasma glucocorticoid concentrations in free-ranging platypuses (Ornithorhynchus anatinus): response to capture and patterns in relation to reproduction. Comparative Biochemistry and Physiology A 136, 895-902.
| Crossref | Google Scholar | PubMed |
Hawke, T., Bino, G., Shackleton, M. E., Ross, A. K., and Kingsford, R. T. (2022). Using DNA metabarcoding as a novel approach for analysis of platypus diet. Scientific Reports 12, 2247.
| Crossref | Google Scholar | PubMed |
Heimlich, J. E., and Ardoin, N. M. (2008). Understanding behavior to understand behavior change: a literature review. Environmental Education Research 14, 215-237.
| Crossref | Google Scholar |
Hill, J. E., DeVault, T. L., and Belant, J. L. (2019). Cause-specific mortality of the world’s terrestrial vertebrates. Global Ecology and Biogeography 28, 680-689.
| Crossref | Google Scholar |
Hing, S., Narayan, E. J., Thompson, R. C. A., and Godfrey, S. S. (2016). The relationship between physiological stress and wildlife disease: consequences for health and conservation. Wildlife Research 43, 51-60.
| Crossref | Google Scholar |
Hoffman, A. A., Rymer, P. D., Byrne, M., Ruthrof, K. X., Whinam, J., McGeoch, M., Bergstrom, D. M., Guerin, G. R., Sparrow, B., Joseph, L., Hill, S. J., Andrew, N. R., Camac, J., Bell, N., Riegler, M., Gardner, J. L., and Williams, S. E. (2019). Impacts of recent climate change on terrestrial flora and fauna: some emerging Australian examples. Austral Ecology 44, 3-27.
| Crossref | Google Scholar |
Kessell, A. E., Boulton, J. G., Dutton, G. J., Woodgate, R., Shamsi, S., Peters, A., and Connolly, J. H. (2014). Haemolytic anaemia associated with Theileria sp. in an orphaned platypus. Australian Veterinary Journal 92, 443-449.
| Crossref | Google Scholar | PubMed |
Khurana, J., Bino, G., and Hawke, T. (2024). Impacts of river regulation and fragmentation on platypuses in the northern Murray-Darling Basin. Marine and Freshwater Research 75, MF24037.
| Crossref | Google Scholar |
Kolomyjec, S. H., Chong, J. Y. T., Blair, D., Gongora, J., Grant, T. R., Johnson, C. N., and Moran, C. (2009). Population genetics of the platypus (Ornithorhynchus anatinus): a fine-scale look at adjacent river systems. Australian Journal of Zoology 57, 225-234.
| Crossref | Google Scholar |
Lewis, S. L., and Maslin, M. A. (2015). Defining the Anthropocene. Nature 519, 171-180.
| Crossref | Google Scholar | PubMed |
Lind, L., Hasselquist, E. M., and Laudon, H. (2019). Towards ecologically functional riparian zones: a meta-analysis to develop guidelines for protecting ecosystem functions and biodiversity in agricultural landscapes. Journal of Environmental Management 249, 109391.
| Crossref | Google Scholar | PubMed |
Macgregor, J. W., Holyoake, C. S., Munks, S. A., Connolly, J. H., Robertson, I. D., Fleming, P. A., and Warren, K. S. (2017). Investigations into individual health and exposure to infectious agents of platypuses (Ornithorhynchus anatinus) in two river catchments in northwest Tasmania. Journal of Wildlife Diseases 53, 258-271.
| Crossref | Google Scholar | PubMed |
Marchant, R., and Grant, T. R. (2015). The productivity of the macroinvertebrate prey of the platypus in the upper Shoalhaven River, New South Wales. Marine and Freshwater Research 66, 1128-1137.
| Crossref | Google Scholar |
Marks, C. A., Obendorf, D., Pereira, F., Edwards, I., and Hall, G. P. (2014). The dispersion and detection patterns of mtDNA-assigned red fox Vulpes vulpes scats in Tasmania are anomalous. Journal of Applied Ecology 51, 1033-1040.
| Crossref | Google Scholar | PubMed |
Martin, L. B. (2009). Stress and immunity in wild vertebrates: timing is everything. General and Comparative Endocrinology 163, 70-76.
| Crossref | Google Scholar | PubMed |
McDougall, P. T., Réale, D., Sol, D., and Reader, S. M. (2006). Wildlife conservation and animal temperament: causes and consequences of evolutionary change for captive, reintroduced and wild populations. Animal Conservation 9, 39-48.
| Crossref | Google Scholar |
McLachlan-Troup, T. A., Dickman, C. R., and Grant, T. R. (2010). Diet and dietary selectivity of the platypus in relation to season, sex and macroinvertebrate assemblages. Journal of Zoology 280, 237-246.
| Crossref | Google Scholar |
Mijangos, J. L., Bino, G., Hawke, T., Kolomyjec, S. H., Kingsford, R. T., Sidhu, H., Grant, T., Day, J., Dias, K.N., Gongora, J., and Sherwin, W.B. (2022). Fragmentation by major dams and implications for the future viability of platypus populations. Communications Biology 5, 1127.
| Crossref | Google Scholar | PubMed |
Milner, J. M., Nilsen, E. B., and Andreassen, H. P. (2007). Demographic side effects of selective hunting in ungulates and carnivores. Conservation Biology 21, 36-47.
| Crossref | Google Scholar | PubMed |
Mooney, N., and Spencer, C. (2000). Why did the platypus cross the road? Australian Mammalogy 21, 264-267.
| Google Scholar |
Munday, B. L., Whittington, R. J., and Stewart, N. J. (1998). Disease conditions and subclinical infections of the platypus (Ornithorhynchus anatinus). Royal Society of London Philosophical Transactions: Biological Sciences 353, 1093-1099.
| Crossref | Google Scholar | PubMed |
Musser, A., Grant, T., and Turak, E. (2024). Movements of platypuses around and through instream structures and natural barriers in the Jenolan Karst Conservation Reserve, New South Wales. Australian Mammalogy 46, AM23031.
| Crossref | Google Scholar |
Otley, H. M., and le Mar, K. (1998). Observations on the avoidance of culverts by platypus. The Tasmanian Naturalist 120, 48-50.
| Google Scholar |
Pusey, B. J., and Arthington, A. H. (2003). Importance of the riparian zone to the conservation and management of freshwater fish: a review. Marine and Freshwater Research 54, 1-16.
| Crossref | Google Scholar |
Rakick, R., Rakick, B., Cook, L., and Munks, S. (2001). Observations of a platypus foraging in the sea and hunting of a platypus by a wedge-tailed eagle. The Tasmanian Naturalist 123, 2-4.
| Google Scholar |
Roberts, S., and Serena, M. (2024). Use of consolidated time-lapse camera imagery to detect and monitor platypus (Ornithorhynchus anatinus) activity. Australian Mammalogy 46, AM23045.
| Crossref | Google Scholar |
Rosser, A. M., and Manika, S. A. (2002). Overexploitation and species extinctions. Conservation Biology 16, 584-586.
| Crossref | Google Scholar |
Serena, M., and Grant, T. R. (2017). Effect of flow on platypus (Ornithorhynchus anatinus) reproduction and related population processes in the upper Shoalhaven River. Australian Journal of Zoology 65, 130-139.
| Crossref | Google Scholar |
Serena, M., and Williams, G. A. (1998). Rubber and plastic rubbish: a summary of the problem posed to platypus Ornithorhynchus anatinus in suburban habitats. The Victorian Naturalist 115, 47-49.
| Google Scholar |
Serena, M., and Williams, G. (2010). Factors contributing to platypus mortality in Victoria. The Victorian Naturalist 127, 178-183.
| Google Scholar |
Serena, M., and Williams, G. A. (2013). Movements and cumulative range size of the platypus (Ornithorhynchus anatinus) inferred from mark-recapture studies. Australian Journal of Zoology 60, 352-359.
| Crossref | Google Scholar |
Serena, M., and Williams, G. A. (2022). Factors affecting the frequency and outcome of platypus entanglement by human rubbish. Australian Mammalogy 44, 81-86.
| Crossref | Google Scholar |
Serena, M., Thomas, J. L., Williams, G. A., and Officer, R. C. E. (1998). Use of stream and river habitats by the platypus, Ornithorhynchus anatinus, in an urban fringe environment. Australian Journal of Zoology 46, 267-282.
| Crossref | Google Scholar |
Serena, M., Williams, G. A., Weeks, A. R., and Griffiths, J. (2014). Variation in platypus (Ornithorhynchus anatinus) life-history attributes and population trajectories in urban streams. Australian Journal of Zoology 62, 223-234.
| Crossref | Google Scholar |
Serena, M., Grant, T. R., and Williams, G. A. (2016). Reducing bycatch mortality in crustacean traps: effect of trap design on platypus and yabby retention rates. Fisheries Research 175, 43-50.
| Crossref | Google Scholar |
Serena, M., Snowball, G., Thomas, J. L., Williams, G. A., and Danger, A. (2024). Platypus longevity: a new record in the wild and information on captive life span. Australian Mammalogy 46, AM23048.
| Crossref | Google Scholar |
Taylor, R., Mooney, N., and Lange, K. (1991). Observations on platypus. The Tasmanian Naturalist 105, 1-3.
| Google Scholar |
Taylor-Brown, A., Booth, R., Gillett, A., Mealy, E., Ogbourne, S. M., Polkinghorne, A., and Conroy, G. C. (2019). The impact of human activities on Australian wildlife. PloS One 14, e0206958.
| Crossref | Google Scholar | PubMed |
Thomas, J. L., Parrott, M. L., Handasyde, K. A., and Temple-Smith, P. (2020). Maternal care of platypus nestlings. Australian Mammalogy 42, 283-292.
| Crossref | Google Scholar |
Tyson, R. M. (1980). Road killed platypus. The Tasmanian Naturalist 60, 8.
| Google Scholar |
Williams, G. A., Serena, M., and Grant, T. R. (2013). Age-related change in spurs and spur sheaths of the platypus (Ornithorhynchus anatinus). Australian Mammalogy 35, 107-114.
| Crossref | Google Scholar |