Identification and characteristics of refuges for the threatened swamp antechinus (Antechinus minimus maritimus) under climate change; targeted surveys across the Otway Ranges, south-east Australia
Barbara A. Wilson A * , Kristen Agosta A , Mark J. Garkaklis B , Jemma K. Cripps C D , Marissa L. Parrott C E , Raylene Cooke A and John G. White AA
B
C
D
E
Abstract
There is growing evidence that persistence of mammal fauna under climate change is reliant on refuges protected from disturbances such as extreme drought and fire. During the ‘millennium drought’ (1996–2010), the swamp antechinus (Antechinus minimus maritimus) declined precipitously in the eastern Otways, resulting in restriction to coastal dune refuges. Here, we evaluated the species’ distribution across the extended Otway landscape to identify the localities and characteristics of refuges. Targeted surveys (cameras, live-trapping) were conducted at sites of previous healthy populations and in putative habitat refuges (2018–2023). Eleven micro-refuges (<500 ha), located in Coastal Dune Scrub, were identified but are subject to destruction due to sea level rise. Three mid-connected refuges (500–1000 ha), providing habitat connection features (e.g. gullies), and three macro-refuges (>1000 ha) of unfragmented, complex vegetation were identified. The swamp antechinus remains absent from previously inhabited heathy woodland in the eastern Otways, and although it was initially (2021) trapped with high success in heathy woodlands of the Carlisle Heath, it was not captured subsequently, possibly related to incompatible fire, introduced predators and Phytophthora dieback. Management of refuges to ensure the future of the swamp antechinus will require effective control of Phytophthora infestation and predators, and protection from inappropriate fire.
Keywords: climate, climate change, fire, population, rainfall, refuge, swamp antechinus.
Introduction
Climate change is impacting diverse ecosystems worldwide (Hughes 2000; Parmesan and Yohe 2003). Moreover, the anticipated escalation in climate change is expected to intensify both the frequency and severity of disturbance regimes including wildfire, heat waves and extreme weather events such as droughts and floods (IPCC 2013, 2023; Selwood et al. 2019; Collins et al. 2021; Jolly et al. 2022; Legge et al. 2022). Predictions for Mediterranean systems, such as southern Australia, anticipate reduced rainfall, rising temperatures and heightened occurrences of drought and fire (IPCC 2013, 2023; CSIRO and Australian Bureau of Meteorology 2022). These conditions present significant threats to Australia’s biodiversity, particularly vulnerable mammal species.
Increasing evidence suggests that the regional persistence of small mammal populations is reliant on the presence of refuges into which populations contract in the face of climate changes, such as during dry periods and increased fires (Milstead et al. 2007; Letnic and Dickman 2010; Keppel and Wardell-Johnson 2012; Wilson et al. 2012; Greenville et al. 2013; Pavey et al. 2017; White et al. 2022). These refuge areas enhance the resilience of mammal populations by providing more favourable environmental conditions or higher resource availability, and act as a source of animals when recolonisation occurs during and after subsequent resource increases across the landscape (Brandle et al. 1999; Milstead et al. 2007; Letnic and Dickman 2010; Dickman et al. 2011; Greenville et al. 2013; Reside et al. 2014; Pavey et al. 2017; White et al. 2022).
Although most of the ecological research on refuges has focussed on impacts from a single disturbance, such as climate warming (Keppel and Wardell-Johnson 2012) or fire (Robinson et al. 2013), many species are affected by multiple, interacting disturbances. These can accelerate population declines through additive or synergistic effects (Doherty et al. 2015). In many cases, refuges are likely to protect species from multiple interacting disturbances, so understanding the relative contribution of each to species declines can be difficult. For example, rocky gorges can provide animals with refuge from fire (Dobrowski 2011), and buffer them against thermal and hydric stress (Reside et al. 2014), and predation pressure (McDonald et al. 2013). It is thus important to understand how individual species use refuges and what disturbances they are protected from. This is particularly important for management efforts, as it allows for the implementation of scientifically backed and cost-effective actions.
The identification of locations that provide refuge from disturbances such as drought, increasing temperatures, fire and extreme weather events has been the focus of recent studies (Mackey et al. 2012; Robinson et al. 2013; Selwood et al. 2019; White et al. 2022). For example, landscape features such as gullies and rocky outcrops provide physical protection from fire (Robinson et al. 2013; Selwood and Zimmer 2020), while mesic locations characterised by high vegetation productivity, such as floodplains, drainage lines and riparian zones, support more stable animal assemblages during drought (Selwood et al. 2015, 2019; White et al. 2022). There is evidence, however, that high levels of predation from introduced predators, such as cats (Felis catus) and red foxes (Vulpes vulpes), can lead to the local extinction of species within refuges (Pavey et al. 2014). Therefore, the management and protection of refuges are recognised as essential conservation priorities for fauna, particularly as climate change intensifies (Letnic and Dickman 2010; Pavey et al. 2014, 2017).
While the Tasmanian swamp antechinus (Antechinus minimus minimus) is not currently at risk (Driessen 2024), the mainland swamp antechinus (Antechinus minimus maritimus) has a restricted, fragmented, coastal distribution in south-east Australia and is listed under the Environment Protection and Biodiversity Conservation Act (1999) as Vulnerable to extinction (Threatened Species Scientific Committee (2016). The mainland swamp antechinus extent of occurrence is estimated at 48,796 km2, and the area of occupancy estimated at 360 km2 (Threatened Species Scientific Committee 2016). It occurs from south-eastern South Australia to Wilson’s Promontory in Victoria and on nearby offshore islands (Menkhorst 1995; Bachmann and van Weenen 2001; Wilson et al. 2001). The species favours damp habitat, particularly dense heathlands and woodlands, tussock grasslands, sedgelands and gullies (Menkhorst 1995), often in landscape settings with little exposure to the sun (Wilson et al. 2001; Gibson et al. 2004). It prefers structurally complex, late post-fire successional habitat (Wilson et al. 2001; Gibson et al. 2004; Driessen 2024); however, this habitat is embedded within the wider landscape that is increasingly impacted by fire management and wildfire (Agosta, K. N., Cripps, J. K., Parrott, M. and Wilson, B. unpubl. data; Wilson et al. 1986, 2017; Wilson and Garkaklis 2020). After severe wildfire, local population extinctions have been recorded with some extinguished populations gradually recolonising 15 years post-fire. However, at some sites, no recolonisation has occurred even 33 years after the fire event (Wilson et al. 2001, 2017).
During long-term research (1975–2007) in Victoria, the swamp antechinus was recorded frequently and at high densities (17.5–27.9 per ha) in the eastern Otways region (Wilson et al. 1986, 2001; Aberton 1996; Reichl 1997; Hanley 1999; Gibson et al. 2004; Magnusdottir et al. 2008; Sale et al. 2008). However, recent surveys (2013–2017), revealed severe declines of the species including many local population extinctions and the capture of only eight individuals (Wilson, B. A., Garkaklis, M. J., Arnall, S., and Doherty, T. unpubl. data; Wayne et al. 2017; Wilson et al. 2017; Wilson and Garkaklis 2020). Swamp antechinuses were restricted to very small coastal sand dunes and gullies, indicating that these habitats represent significant refuges from disturbances occurring elsewhere (Wilson, B. A., Garkaklis, M. J., Arnall, S., and Doherty, T. unpubl. data; Wilson et al. 2017; Wilson and Garkaklis 2020).
Significant population declines of the swamp antechinus have been recorded in the eastern Otways during periods of below average rainfall and drought (Magnusdottir et al. 2008; Sale et al. 2008), especially during the ‘millennium drought’ (1996–2010) where much of the south-east of Australia experienced persistent drought (CSIRO and Australian Bureau of Meteorology 2015). Climate change predictions of declining rainfall and extended drought (CSIRO and Australian Bureau of Meteorology 2015, 2022) are likely to pose significant threats to this vulnerable species. Drought, which may decrease habitat structure and cover, and food and water availability, can negatively affect the body condition and survival of adult antechinuses, decrease fertility and litter size, and affect the survival of offspring (Parrott et al. 2007). Other potential threats to the swamp antechinus include long-term vegetation degradation due to the plant pathogen Phytophthora cinnamomi (Laidlaw and Wilson 2006; Annett 2008) and predation by foxes and cats (Wilson et al. 2001; van Weenen and Menkhorst 2008; Woinarski et al. 2014). Detailed information on the population trends for other populations of this subspecies in Victoria and South Australia, and the Tasmanian subspecies, is not available and should be investigated given the dire situation in the Otway Ranges (Bachmann and van Weenen 2001; Thompson and Bachmann 2021; Driessen 2024).
Regardless of the underlying causes of declines, more precise identification and better protection of refuges is considered crucial to maintain the species within the core of its distribution in the Otway region of southern Australia (Wilson, B. A., Garkaklis, M. J., Arnall, S., and Doherty, T. unpubl. data; Wayne et al. 2017; Wilson et al. 2017; Wilson and Garkaklis 2020). Therefore, the objectives of this study were to: evaluate the distribution of the swamp antechinus across the broad Otways landscape; identify the localities and characteristics of the refuges; assess the threats to the species; and provide management priorities for implementing cost-effective actions.
Materials and methods
Study area
This study was undertaken in the Otway Ranges, approximately 100 km south-west of Melbourne, Victoria (Fig. 1). The area (186,377 ha) is predominantly public land including the Great Otway National and Otway Forest Park (140,000 ha) and the Carlisle State Park (5600 ha), comprising heathland, woodland and forest (Parks Victoria 1998). The vegetation of the study area includes a diverse mosaic of Ecological Vegetation Communities (EVCs; the standard unit for classifying vegetation types in Victoria, based on floristics, lifeforms and ecological characteristics) predominantly of Wet Forest (42,357 ha) and Shrubby Wet Forest (32,858 ha), distributed inland from Lorne to Cape Otway, (Wilson, B. A., Burns, P., and Garkaklis, M. J. unpubl. data). Dry forest consisting of Lowland Forest (41,301 ha) and Shrubby Foothill Forest (29,910 ha) extends from the eastern Otways on a plateau, and coastally from Anglesea to Lorne. Heathy Woodland covers 23,198 ha and is distributed in an arc from the east (Anglesea) to central Otways north of Forrest, and southwest of Carlisle. Within the woodlands are areas of Riparian Scrub or Swampy Scrub/Woodlands (14,595 ha) and limited areas of Wet Heathlands (2158 ha).
Study area across the Otways including eastern (Anglesea–Lorne), central (Lorne–Cape Otway) and western (Lorne–Port Campbell) areas, showing location in Victoria, and historical records for swamp antechinus (1950s to 2020).
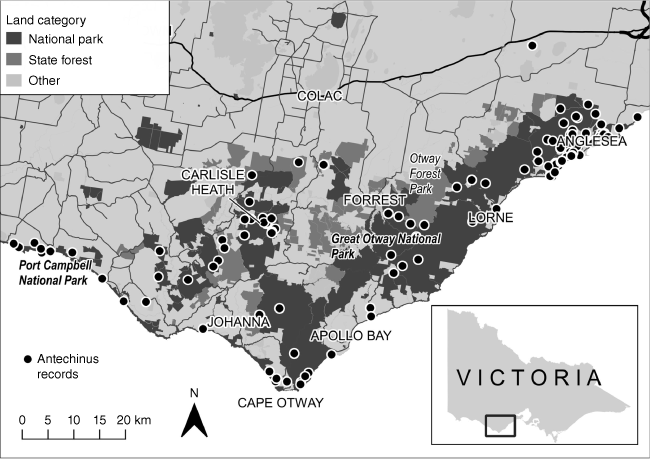
The most extensive recent fire in the area was the ‘Ash Wednesday’ wildfire (1983), which burnt 40,000 ha and left few unburnt small patches of vegetation (Wilson et al.1990, 2001). Fuel reduction burns have been undertaken since 1986 and an increase in burning has been implemented following recommendations arising from the 2009 Victorian Bushfires Royal Commission (Coates, F., Loyn, R., Ibbett, M., and Brown, G. unpubl. data; Parliament of Victoria 2010; Department of Environment and Primary Industry 2014). The total area of heathlands burnt by management between 1987 and 2008 (21 years) was 2266 ha (mean = 107.9 ha per annum), and between 2009 and 2012 (3 years) 3095 ha (mean = 1031.67 ha per annum), an annual increase of 900% (Wilson et al. 2017). In 2013, 42% of the Anglesea heathland was below its ‘minimum tolerable fire interval’, and 37% was at an early growth stage (Department of Environment and Primary Industry 2014). Regular fox baiting has occurred since 2005 (Parks Victoria and Department of Sustainability and Environment 2009).
Historical occurrence of the swamp antechinus across the Otways (1950s–2021)
To assess the historical distribution of the swamp antechinus across the Otways, all available records for the species were obtained for the period 1957–2021 (Wilson, B. A., Burns, P., and Garkaklis, M. J. unpubl. data). This included records from the Victorian Biodiversity Atlas (VBA) (as of 27 January 2021) together with all other known databases containing historical small mammal occurrence records from research institutes, universities, State Government authorities and individual researchers. Data were carefully audited to ensure spatial accuracy and to remove duplicate or unreliable records (Wilson, B. A., Burns, P., and Garkaklis, M. J. unpubl. data).
A Geographic Information System (GIS) approach was employed to map and calculate areas of all EVCs across the study area (Department of Environment, Land, Water and Planning 2019a). Swamp antechinus observations were mapped for each EVC, and calculations of swamp antechinus records per ha of each EVC was recorded as an indication of species’ preferred habitats. Accessibility to locations may have influenced location of the swamp antechinus records.
Assessment of current distribution and abundance of the swamp antechinus (2018–2023)
Surveys for the swamp antechinus targeted sites of notable healthy populations at long-term monitoring sites in the eastern Otways (1975–2007), historic records and suitable habitat identified with suitable EVCs and Habitat Distribution Models (NatureKit; Gibson et al. 2004). Furthermore, the surveys targeted sites downslope in gullies (Wilson, B. A., Garkaklis, M. J., Arnall, S., and Doherty, T. unpubl. data; Wilson, B. A., Burns, P., and Garkaklis, M. J. unpubl. data) and sites with higher Normalised Difference Vegetation Index (NDVI) values (Garkaklis and Wilson 2021) representing higher moisture and vegetation productivity, respectively, and indicative of more drought resistant refuges. Due to the targeted surveys and restrictions on time and resources, not all areas of potentially suitable habitat were surveyed.
Surveys were conducted between 2018 and 2023 with camera data obtained from 121 sites (518 cameras, 17,454 trap nights) and live trapping from 99 sites (5888 trap nights) (Supplementary Table S1). Nineteen of the sites had both camera and live trapping, and sampling sites were considered independent, being from 2 to 109 km apart. Sampling was conducted across three main areas, broadly delineated by differing EVCs: (1) the eastern Otways from Anglesea to Lorne coastally and inland to the Coastal Forest Park; (2) the central Otways from Lorne to Cape Otway coastally and inland to Forrest (dominated by wetter EVCs including Wet and Shrubby Wet Forest), and Carlisle Heath (dominated by Heathy Woodland and Wet Heathland); and (3) the western Otways from Cape Otway to Port Campbell Heath (dominated by Coastal Heathland Scrub and Damp Heath Scrub) (Fig. 1). Trapping was undertaken using Elliott traps (325 × 90 × 100 mm; Elliott Scientific Equipment, Upwey, Victoria) baited with a mixture of rolled oats, honey/golden syrup and peanut butter. At most sites, 30 Elliott traps were set in a transect formation in lines of 10 at 10–15-m intervals between lines and traps (0.3–0.5 ha), except at sites where the habitat patch was very small (<0.5 ha) necessitating only 4–10 traps being set (<0.5 ha). Traps were set for three to four nights. Species, sex, reproductive condition and body weight (g) were recorded for each animal and these data were used for multiple studies. Captured swamp antechinus individuals were identified or marked with ear notch before release at their capture point.
At each camera trapping site, predominantly one to four (and up to 10 at some sites) remote infrared cameras (Reconyx Hyperfire HC600 Passive Infrared, HP2W Hyperfire 2) were positioned approximately 100 m apart, for 3–6 weeks. Cameras were set to high sensitivity with a 0–15-s interval between triggers and three to five images taken per trigger. Bait lures of peanut butter and rolled oats were employed to attract animals. Cameras were secured vertically 0.2–1 m from ground level, at 0.5–0.9 m from the bait station, and dense vegetation was cleared from the field of view where required. Photographs of individuals were identified to species level using reference photographs from the area (B.A. Wilson pers. obs.) and species descriptions (Van Dyck and Strahan 2008; Van Dyck et al. 2013). Although identifying small mammals to species level from infrared (black and white) images can be challenging, we identified only five dusky antechinuses (Antechinus mimetes), recognised by their very contiguous dark fur, thickset body (especially the rump), long slender rostrum, very long claws, and tail which is 80% of body length. In contrast, the swamp antechinus has a shorter nose and ears, a lighter grizzled brown fur on upperparts (particularly on the rump), with very pale underparts, shorter claws, and a tail which is 70% of body length. Such differences provide confidence of correct identifications.
Trapping and camera data analyses
Although mark–recapture and other analyses have been used to derive estimates of populations (number of individuals known to be alive; density) for swamp antechinuses at particular sites (Wilson et al. 1986, 1990, 2001; Annett 2008; Magnusdottir et al. 2008; Sale et al. 2008), this precise population information was not available at the survey sites. To ensure consistent comparison, we used trapping rate as a proxy measure of abundance: Trap success = (total captures (including recaptures)/total number of nights) × 100. For camera trapping data, the total number of independent captures (visits) was used to calculate trapping success at each site by: Trap success = (total captures/total number of nights) × 100. An independent capture (visit) was defined as a 30-min interval between images of the same species (DeBondi et al. 2010; Claridge et al. 2010).
Identification and assessment of refuges for swamp antechinus
The results of recent surveys (2018–2023) were used to identify refuges based on several criteria: (1) survival of species (>20 years) during long-term monitoring; or (2) live capture success of >2.2 (2018–2023); or (3) the presence of swamp antechinus (live or camera) at least two trap sites (2018–2023); together with presence of suitable habitat (>30 ha). The results were used to map the location and extent of refuges supporting the species across the Otways, including refuges such as coastal dunes, gullies and waterways connecting coastal dunes and inland habitat (Wilson et al. 2017; Wilson and Garkaklis 2020). The refuges were delineated by incorporating the location records and mapping these in suitable habitat to topographic features (gully, road, beach) of unsuitable habitat. The location, shape, topographic features (creek, gully, dune), area (ha) and connectivity of refuges was estimated in GIS, together with the EVC.
In the initial assessment of potential refuges, three distinctive characteristics of refuges emerged: size, shape and connectivity, the latter being areas connecting small or large refuges, where suitable habitat for the swamp antechinus was present and the species was recorded at both ends indicating likely population connectivity. Three categories of refuges were then adopted: (1) micro-refuges consisting of smaller refuges (<500 ha) which were frequently linear; (2) mid-connected refuges which were medium-sized refuges (500–1000 ha) providing habitat connection features (e.g. creeks, gullies) which can connect populations; and (3) macro-refuges which were large refuges (>1000 ha) consisting of interconnected key habitats likely to support self-sustaining long-term populations of the species.
An assessment of threats was undertaken at each refuge. An estimation of extent of fragmentation of sites was based on the habitat patch size delineated by roads or tracks and scored as high (<340 ha, linear), medium (60–300 ha, not linear) and low (700–4800 ha, not linear). The presence of weeds and the plant pathogen, P. cinnamomi, were based on site observations, and scored as high (>30%), medium (20–30%) and low (<20%), and for P. cinnamomi classed as not applicable where vegetation present was non-susceptible such as coastal scrub. The presence of people, domestic dogs (Canis familiaris) and introduced herbivores including European rabbits (Oryctolagus cuniculus) and predators (fox, cat), were based on camera results and site observations. The erosion vulnerability of refuges was assessed based on the Victorian Coastal Hazard Assessment as Very High, High or Medium (CoastKit, www.marineandcoasts.vic.gov.au). The threat of wildfire was assessed as High for all refuges based on the Otway bushfire risk assessment (The State of Victoria Department of Environment, Land, Water and Planning 2020), and for fuel reduction burning as Low, if habitat was not included in spatial and temporal fuel management and ecological fire regime burn plans (e.g. coastal dunes), Medium if included in burn plans, and High if included in burn plans to be implemented in the next 3 years (The State of Victoria Department of Environment, Land, Water and Planning 2020, 2024).
Assessment of population abundance and habitat in the Carlisle Heath macro-refuge (2021)
Following preliminary survey work that identified the swamp antechinus in the Carlisle Heath (2019–2020), intensive live trapping was undertaken (April–June 2021) at 40 sites selected within suitable habitat. Trapping was conducted for four consecutive nights along two parallel 90-m transects (20 m apart) per site with 10 Elliott traps placed along each transect at 10-m spacings (total of 3744 trap nights). General trapping procedures described above were followed. The burn history at the study sites ranged from 1 to 82 years (obtained from Department of Environment, Land, Water and Planning 2019b). The abundance of swamp antechinuses in time since fire intervals of early (0–9 years), medium (10–20 years) and long (≥21 years) were calculated. In 2022, subsequent live trapping was conducted at eight of the 40 sites trapped in 2021, of which four were in recently burnt habitat (2022) and four were in long unburnt habitat (25 to ≥82 years post-fire). In 2023, repeat live trapping was conducted across four unburnt sites.
Comparison of capture success and density of swamp antechinus to previous studies
Estimates of trap success were compared to previous studies in the Otway region (Wilson et al. 2001, 2017). Density estimates for the trapping at Carlisle Heath, the number of individuals ‘known to be alive’ (Krebs 1989) divided by the trapping area, were compared to previous studies of the species including in the Otway Ranges.
Results
Historical distribution of the swamp antechinus across the Otways (1950s–2021)
A total of 684 records for swamp antechinus were obtained between 1957 and 2021, primarily clustered in the eastern Otways, coastal Cape Otway and inland Carlisle areas (Fig. 1). The high numbers of records in the eastern Otways is related to intensive trapping studies on the species between the 1980s and 2007 (Gibson et al. 2004; Wilson et al. 1986, 2001, 2017; Magnusdottir et al. 2008; Wilson and Garkaklis 2020). The species has been recorded in the eastern Otways since the 1950s, Cape Otway since the 1970s, Carlisle heathlands since the 1980s, and Port Campbell since the 1990s.
Swamp antechinus was recorded predominantly in Heathy Woodlands, Coastal Scrub Grasslands and Woodlands, Heathland, Sand Heathland, Wet or Damp Forests, Lowland Forests, Coastal Headland Scrub, and Riparian Scrub/Swampy Riparian Woodland complex (Fig. 2). The highest percentage of swamp antechinus observations per ha EVC occurred in Sand Heathland (55%) followed by Coastal Tussock Grassland (10%) (Fig. 2).
Historic observation records of Swamp antechinus (1950s to 2020) in Ecological Vegetation Classes (EVCs) across the Otway Ranges.
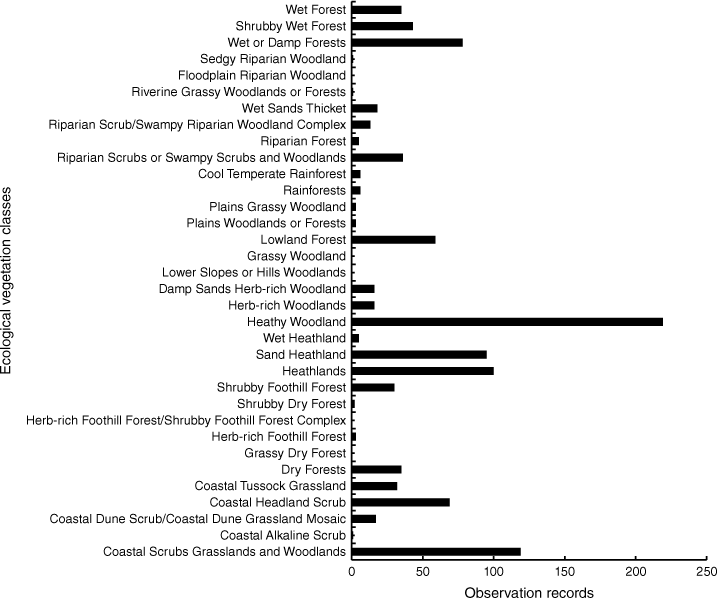
The distribution records of swamp antechinus in Heathy Woodland and Heathland were predominantly in the east Otways, particularly Anglesea heath, and the west Otways in Carlisle heath. The species also occurred in Coastal Headland Scrub, Coastal Dune Scrub/Dune Grass mosaic and Sand Heathland along the coast in the eastern Otways and in Riparian Scrub/Swampy Riparian Woodland complex in Carlisle heath. The records in Wet Forest and Shrubby Wet Forest were largely across the central south Otways, north of Kennett River to Lavers Hill and central north Otways south of Forrest. The species was predominantly recorded in Coastal Dune Scrub, Coastal Tussock Grassland and Damp Sand Herb-rich Woodlands in the southwest near Princetown and Cape Otway. The swamp antechinus was also located in Riparian Scrub/Swampy Riparian Woodland complex.
Survey results for assessment of current distribution of the swamp antechinus (2018–2023)
Swamp antechinuses were recorded during camera surveys at 19 sites (15.1%), 10 sites in the eastern Otways, five in the central Otways and four in the western Otways (Table 1, Supplementary Table S1). Live survey trapping resulted in the recording of the species at 12 sites (17.1%) (Table 1), two in the eastern Otways (one inland and one at Painkalac Creek), and five in the central Otways (two inland at Carlisle Heath and three on coastal dunes at Cape Otway and Skenes Creek). In the western Otways, the species was trapped at five sites (four in coastal heath at Port Campbell National Park, and one in dunes at Princetown) (Table 1).
| Region | Live | Camera | |
|---|---|---|---|
| Eastern otways | N = 2 (9) (1718 TN) | N = 10 (41) (245 cameras set) | |
| Trap success | 7.9 ± 3.3, (1.7–16.7) | 21.8 ± 6.9, (3.9–70.0) (14.5% of cameras) | |
| Central otways | N = 5 (38) (1950 TN) | N = 5 (20) (100 cameras set) | |
| Trap success | 4.2 ± 2.1, (1.1–12.5) | 100.2 ± 27.3, (2.8–480.0) (25% of cameras) | |
| Western otways | N = 6 (16) (1159 TN) | N = 4 (8) (52 cameras set) | |
| Trap success | 10.0 ± 3.1, (4.4–18.8) | 8.33 ± 2.96 (4.2–12.5) (12.5% of cameras) | |
| 12/70 sites (17.1%) | 19/69 sites (27.53%) |
Live and camera trapping. N = sites the species was present (nos. sites trapped). TN = Total trap nights. Trap success at sites species was present; mean ± s.e. (range).
The species was located at 141 unique trap stations (individual live and camera traps) over the study period, including the survey results and population abundance assessment in the Carlisle heath refuge (Fig. 3). The largest percentage (36%) of locations was recorded inland in the central Otways in Heathy Woodland and Heathland. In the far eastern Otways, there were records at only three inland locations: two were in unburnt gullies (Heathy Woodland and Heathland) following fuel management burns (Watchorn et al. 2024), and one was located in Estuarine Woodland (Painkalac Creek) (Fig. 3). Further to the west, the species was recorded inland in Shrubby Wet Forest and Shrubby Foothills Forest in the confluence of several creeks.
Unique trap station records for Swamp antechinus from camera and live trapping surveys (2018–2023).
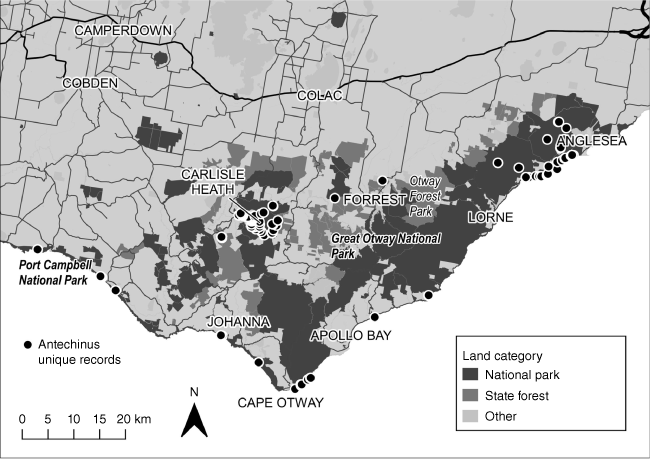
The swamp antechinus occurred in Coastal Headland Scrub, Coastal Dune Scrub/Dune Grass mosaic and Sand Heathland along the coast in the eastern Otways between Jan Juc and Spout Creek, in the central Otways (Grey River, Skenes Creek), in the western Otways (Aire River, Johanna River), and Cape Otway where a high percentage of locations (15%) were recorded. In the western Otways (Port Campbell), the species was recorded in Coastal Headland Scrub and Damp Heath Scrub (Fig. 3). Our re-surveys (camera and trapping) of known sites occupied by the swamp antechinus in the eastern Otways resulted in detection in Estuarine Woodland at Painkalac Creek where the species was last recorded in 2002; at Hutt Gully coastal dunes and Hutt Gully, last recorded in 2015; at Urquharts Bluff coastal headlands, last recorded in 2007; and inland at Edwards Creek Track Gully, last recorded in 2018 (Table 2, Supplementary Table S3). Success for live trapping ranged from 1.5 to 35.0 (mean = 9.1). Maximum success was recorded at Painkalac (35.0), Carlisle (21.5), Cape Otway, Port Campbell National Park, Gibsons Steps (18.8) and Parker Inlet (12.5) (Table 2, Supplementary Table S3).
| Location | Sites surveyed | Sites Amin detected at (%) | Mean trap success range (x–y) | |
|---|---|---|---|---|
| Eastern otways | ||||
| 1975–2007 | 120 | 21 (17.5) | 7.4 (1.1–20.7) | |
| 2013–2016 | 46 | 6 (13.9) | 1.26 (0.33–2.22) | |
| 2018–2023 | 35 | 2 (5.7) | 13.8 (1.5–35.0) | |
| Central otways | ||||
| 1975–2007 | 1 | 1 | 17.0 | |
| 2013–2016 | – | – | – | |
| 2018–2023 | 39 | 5 (12.8) | 8.3 (1.7–21.5) | |
| Western otways | ||||
| 1975–2007 | 6 | 1(16.6) | 5.2 | |
| 2013–2016 | – | – | – | |
| 2018–2023 | 16 | 6 (37.5) | 8.2 (2.2–18.8) | |
Characteristics of refuges across the Otways for the swamp antechinus
There were 11 micro-refuges of between 39 and 339 ha, three mid-connected refuges of between 718 and 942 ha, and three macro-refuges of 1170, 4395 and 4859 ha, respectively, identified, mapped and assessed (Fig. 4, Supplementary Table S4).
Refuges identified for swamp antechinus in Otways (2018–2023). Numbers are refuges micro (1, 2, 4–9, 17), mid connected (3, 10, 13) and macro (14–16).
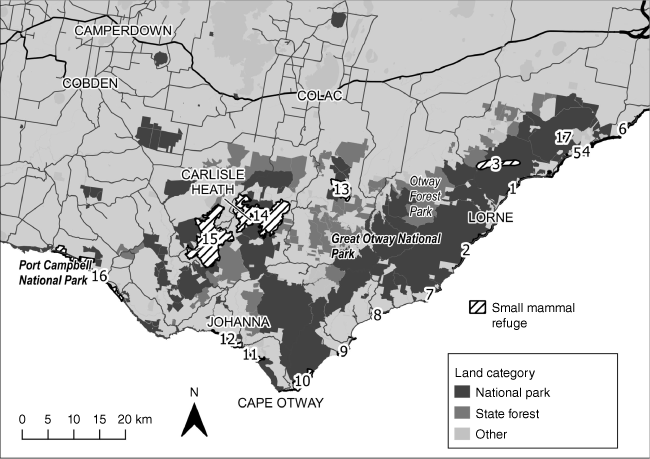
Micro-refuges were predominantly located in, or partially in, coastal dunes from Anglesea in the east to Johanna in the west (e.g. 1, 2, 5, 7, 8, 9, 12) (Fig. 4, Supplementary Table S4). The vegetation is predominantly Coastal Scrub and Estuarine Woodland which is characteristically long unburnt (since 1983), with structurally complex vegetation, high moisture and nutrients (Wilson, B. A., Garkaklis, M. J., Arnall, S., and Doherty, T. unpubl. data). The refuge at Aire River, Hordern Valley, is fragmented and surrounded by agriculture. There were few inland micro-refuges, and these were predominantly in Heathy Woodlands, Heathy Lowland Forest with several located in unburnt gullies subjected to fuel management burns, and another in a deep dissected gully.
Mid-connected refuges included the inland headwaters of creeks (Moggs, Coalmine, Spout, Grassy and Reedy) that provide connectivity between the creek systems and the coastal dune systems (Fig. 4, Supplementary Table S4). The vegetation is predominantly Shrubby Wet Forest, Shrubby Foothills Forest and Heathy Lowland Forest. Another mid-connected refuge provides connectivity along the coast between Cape Otway and Marengo and is predominantly Coastal Scrub/Grassland Mosaic and Lowland Forest, another, the Porcupine Reference area, is in Heathy Woodland/Lowland Forest (Fig. 4, Supplementary Table S4).
Macro-refuges such as Carlisle Heath include areas of poorly drained, Wet Heathlands (micro-refuges), interconnected by draining gullies and surrounded by Heathy Woodland (Fig. 4, Supplementary Table S4). The Port Campbell macro-refuge occurs on Coastal Headland interspersed with Damp Heath Scrub in deeper gullies that flow coastally from inland (Fig. 4, Supplementary Table S4).
Significant threats to refuges
An assessment of disturbances and threats occurring at each swamp antechinus refuge recognised erosion vulnerability due to sea level rise and tidal surges as of medium to very high risk in coastal micro-refuges (Supplementary Table S5). Further threats include habitat fragmentation, fire, weeds (coast wattle, Acacia sophorae, coastal tea-tree, Leptospermum laevigatum and sweet hakea, Hakea drupacea), foxes, cats, rabbits and recreation, including threats from dogs (especially off-leash dogs) and mountain bikes. The assessment found that inland micro-refuges are threatened predominantly by inappropriate fire regimes, the impacts of Phytophthora dieback, and the presence of foxes (Supplementary Table S5), and inland mid-connected refuges are threatened by wildfire, inappropriate fuel reduction burning and, where susceptible Heathy Woodland habitat is present, the impacts of Phytophthora dieback (Supplementary Table S5). Threatening processes in the macro-refuges are predominantly inappropriate fire regimes and the impacts of Phytophthora dieback, which contribute to severe habitat loss, while the presence of foxes under these conditions are considered likely to severely impact mammal prey (Supplementary Table S5).
Trapping results for the swamp antechinus at the Carlisle Heath macro-refuge (2021–2023)
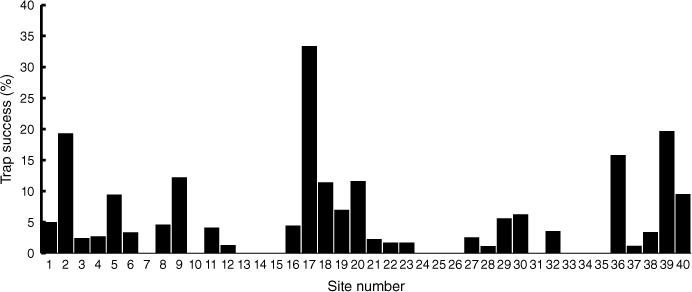
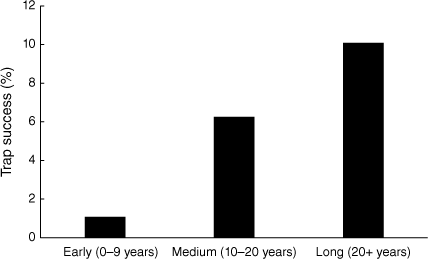
In 2021, the swamp antechinus was the most frequently trapped small mammal species in the Carlisle Heath (152 captures) and was detected at 28 of the 40 trap sites with total trap success ranging from 1.2 to 33.4 and averaging 5.2% (Fig. 5). The total number of swamp antechinus individuals captured was 74 and ranged from one to eight animals at surveyed sites. The trap success was 10-times greater in long post-fire aged habitat (>20 years) and six-times greater in medium post-fire aged habitat (10–20 years) compared with early post-fire age (0–9 years) (Fig. 6). When live trapping was repeated (2022–2023) at a selection of the 40 sites trapped in 2021, no swamp antechinuses were recorded.
Discussion
This research has provided recent survey data on the distribution of the swamp antechinus across the Otway Ranges landscape and has identified refuge locations for the species. It highlights the growing concern for small terrestrial mammals posed by changes including declining rainfall, increased temperatures and fire, and coastal erosion due to intensifying climate change conditions in south-eastern Australia. Prior to the Millennium Drought, the swamp antechinus was distributed across much of the eastern Otway Ranges where suitable habitat types occurred. However, during and after the drought, the species distribution declined substantially, and in many areas it became locally extinct (Wilson et al. 2017; Wilson and Garkaklis 2020). There may also be a link between increased fuel reduction burning following the 2009 Victorian Bushfires Royal Commission, and the swamp antechinus declines. Despite experiencing high annual rainfall and recent La Niña conditions (2020–2022), the species has not recolonised many previously occupied sites that supported high-abundance populations (Wilson et al. 2017; Wilson and Garkaklis 2020). Declines in small mammal distribution and abundance associated with declining rainfall have also been documented elsewhere in southeast and southwest Australia (Lunney et al. 1987; Wooller et al. 1998, 2000; Parrott et al. 2007; Tokushima and Jarman 2008; Tokushima et al. 2008; Lada et al. 2013; Kelly et al. 2020). In the Grampians, landscape scale mammal declines were recorded during drought; however, recovery after major rains was widespread in this landscape (Hale et al. 2016).
The long-term survival of species like the swamp antechinus during periods of extremely low rainfall is likely contingent on them being able to access areas of refuge within landscapes where they can maintain populations during drought periods. These refuges often coincide with wetter vegetation types or areas that can maintain soil moisture, active growth and food resources (such as invertebrates), even when the rest of the landscape is dry and lacking in vegetation cover (Milstead et al. 2007; Letnic and Dickman 2010; Greenville et al. 2013; Keppel and Wardell-Johnson 2012; Wilson et al. 2012; Pavey et al. 2017; White et al. 2022). Such refuge areas often occupy only a small portion of landscapes, for example, refuge habitats for rodents in Chile occupied only approximately 2% of one landscape studied (Milstead et al. 2007). In this current work, we have identified refuges across the Otways as being important for the swamp antechinus, and locations that have supported populations of the species following the end of the Millennium Drought. While they may occupy only a small proportion of the area, these refuges can play an important role in maintaining the species across the landscape, particularly under supportive management regimes.
Refuge characteristics and ability to sustain populations
The findings of this investigation have highlighted the variability in refuges supporting the swamp antechinus, including size, shape, connectivity and habitat. Some native mammal species rely upon shifting refuges that are variable in space and move across the landscape through stochastic processes, such as rainfall or fire (Newsome et al. 1975; Pavey et al. 2017). In contrast, the swamp antechinus refuges identified here can be classified as fixed refuges as they occur in predictable locations that are used over time (Reside et al. 2014). Fixed refuges can arise through patchiness and be products of topographic complexity, including mountain ranges, rocky gorges, boulder piles, gullies or slopes (Reside et al. 2014; McDonald et al. 2015). They may be regions of reliable water (e.g. riparian zones, persistent waterholes or drainage lines), that have high vegetation productivity and support more stable animal assemblages during drought (Nimmo et al. 2015; Selwood et al. 2015, 2019; Selwood and Zimmer 2020; White et al. 2022). The swamp antechinus refuges identified across the Otways include similar mesic locations, including riparian zones, gullies and dune areas that have high structural vegetation complexity, moisture and nutrient levels (Wilson, B. A., Garkaklis, M. J., Arnall, S., and Doherty, T. unpubl. data). They range from linear micro-refuges of coastal dunes to interconnecting mid-connected refuges providing habitat connection features and larger macro-refuges of interconnected key habitats that are very likely to support large, self-sustaining populations of the species. The vegetation communities identified within the refuges are characteristically long unburnt and structurally complex, offering protection from predators by increasing shelter for swamp antechinus and decreasing predator activity and hunting efficiency (Doherty et al. 2022; von Takach et al. 2022).
The results provide evidence of survival of swamp antechinuses in dune systems over a 30-year period in the eastern Otways (Wilson et al. 1990, 2017; Wilson and Garkaklis 2020) and Cape Otway (Wilson 1983; J. White and A. Rendall pers. comm.), highlighting the resilience of these locations as refuge sites. Despite the presence of foxes and cats in these refuges, there is evidence that in areas where habitat structural diversity and complexity is maintained, small mammal communities, including swamp antechinus, are resilient (Wilson et al. 2017; Wilson and Garkaklis 2020). Allochthonous inputs such as marine wrack and seabird guano have been linked to increased nutrients and high food availability (e.g. invertebrates) in other similar coastal sites. Such inputs support dense vegetation cover, increased reproductive success and higher abundance including swamp antechinuses on coastal islands (Wolfe et al. 2004; Bancroft et al. 2005; Sale et al. 2006, 2008; Sale and Arnould 2012).
Many of the coastal dune micro-refuges have been identified as of very high erosion vulnerability and are highly unlikely to support mammals in the near future. Thus, there is a need to plan for rescue of threatened mammals, such as the swamp antechinus, prior to habitat loss and population extinctions, and undertake translocations to more suitable and sustainable habitats. The identification of Painkalac Creek as a micro-refuge for the swamp antechinus was an important result as monitoring had failed to locate the species here since 2002 (Wilson et al. 2001, 2017). Further, more recent surveys have recorded swamp antechinus 3 km upstream, confirming the significance of this site as a ‘source population’.
The swamp antechinus was recorded in a number of micro-refuges located inland in gullies, that connect with refuge sites within the coastal dunes. The results are significant because they provide evidence that the species continues to use the habitat on both sides of the Great Ocean Road. While the road is considered a significant barrier to movements of the species, many do have water management culverts and drains under the road. The utilisation of these by the swamp antechinus needs to be assessed to determine their use, and the potential to improve the connectivity of populations by provision of cover and refuge structures within the underpasses (Soanes et al. 2015; Goldingay et al. 2019).
The population of swamp antechinus in the Carlisle Heath no longer appears to be at high density, at least at the sites trapped in 2022–2023, indicating the need for caution on relying on this macro-refuge as a constant source of the species. The dramatic decline of the species in such a short timeframe and in a period of high rainfall is concerning. Such declines likely impact the genetic diversity of the populations, making them prone to inbreeding effects in the future, and less resilient to a changing climate. Threatening processes acting on the species in this location include incompatible fire regimes, introduced predators, changes in vegetation structure due to Phytophthora, and changes in rainfall patterns with climate change. Further investigation into the direct and indirect effects of fuel reduction burns and associated changes is required as limited data are available.
Management implications for swamp antechinus refuges in a drying climate
A key question in conservation management remains: once refuges for key species are identified, how do we use that knowledge to benefit their survival? The key threats identified for the Otways refuges were coastal erosion vulnerability, wildfire, inappropriate fuel reduction burning, the impacts of Phytophthora dieback, weeds, foxes and cats. Appropriate management to mitigate these threats will increase the survival and resilience of the species. However, such threats need to be consistently reassessed in the different refuges as they will likely change with altered conditions. For example, in La Nina years, the impacts of Phytophthora dieback and growth of weeds are much greater, and in El Nino years, wildfire risk is much higher.
There is an important need to refine the risk assessment of swamp antechinus in vulnerable coastal dunes and consider management intervention including timely translocations of swamp antechinuses to more stable refuges to prevent their extirpation and aid genetic management. Previous trial translocations of the swamp antechinus have been conducted and, recently, an evaluation has been completed of the feasibility of translocating the species within the Otway Ranges, to improve population viability based on genetic diversity (Aberton 1996; Wilson et al. 2017; Cripps and Parrott 2023). This work provides strong guidance for translocation of the species to avoid imminent loss of animals and important genetic diversity remaining in coastal dune refuges.
The need to implement appropriate fire management for wildfire and fuel reduction burning for this late successional species has long been identified (Wilson, B. A., Burns, P., and Garkaklis, M. J. unpubl. data; Threatened Species Scientific Committee 2016; Woinarski et al. 2014; Wilson et al. 2017). Strong protection of refuges from wildfire and fuel reduction burning is imperative. Detailed spatial fire data is required to inform optimum burning regimes, ensure burning is undertaken at a suitable time (e.g. outside times when young antechinus are in nests), and to maintain suitable successional aged habitat for survival, dispersal and recolonisation of the species (Bradstock et al. 2005; Clarke 2008; MacHunter et al. 2009; Driscoll et al. 2010). The fuel and ecological fire management strategy for the Otways region identifies the level of activity to reduce fuel hazard levels and/or optimise ecological outcomes and broadly prioritises minimising impacts to human settlements over the environment and wildlife (Department of Environment and Primary Industry 2014; Gazzard et al. 2020; The State of Victoria Department of Environment, Land, Water and Planning 2020). Mitigation of impacts on the swamp antechinus includes operational controls that reduce planned burn coverage to maintain refuge areas (The State of Victoria Department of Environment, Land, Water and Planning 2020). This has recently been implemented in practice with burning conducted to protect some of the refuges we have identified.
The plant pathogen Phytophthora cinnamomi has been identified as a major threat to mammals due to long-term vegetation degradation resulting in loss of susceptible plant species and simplification of vegetation structure, particularly in damp heathlands, and heathy woodlands (Laidlaw and Wilson 2006; Annett 2008; Wilson et al 2024). The swamp antechinus is at significant risk due to loss of susceptible keystone plant species (e.g. Xanthorrhoea australis) that contribute to complex vegetation structure and associated food resources (invertebrates, fungi) Woinarski et al. 2014; Threatened Species Scientific Committee 2016; Wilson et al 2024). Recent work has identified priority areas to protect high biodiversity values, including the presence of swamp antechinus, from Phytophthora dieback across the Otways and has developed management strategies for dealing with the threat and plans to contain the pathogen (Walshe, T., Wilson, B. A., Bridges, D., and Garkaklis, M. J. unpubl. data ; Wilson and Garkaklis (2023). These include increased hygiene procedures (e.g. disinfecting equipment, shoes, vehicles), and the application of phosphite (hand and aerial spraying) to reduce spread of the pathogen and enhance vegetation survival (Shearer and Tippett 1989; Hardy et al. 2001; Barrett and Rathbone 2018; Commonwealth of Australia 2018).
While there is little direct evidence of the impacts of introduced predators on the swamp antechinus, there is evidence of fox and cat predation on other mammals in the eastern Otways (Hutchings 1996, 2003; Wilson and Wolrige 2000; Wilson et al. 2001; Hradsky et al. 2017; Rees et al. 2023). Although fox baiting is regularly undertaken by land managers (Parks Victoria 2015), the effectiveness of the current program is likely low depending on the duration and intensity of baiting (Robley et al. 2019; Rees et al. 2023). There is little landscape-targeted cat control in the region. Recent management of foxes and cats has, however, been implemented in and around the coastal and estuary swamp antechinus refuges we have identified (Great Ocean Road Coast and Parks Authority 2022; Parks Victoria pers. comm., Surf Coast Shire pers. comm., M. Loughnan pers. comm.).
Further management is required to control invasive weeds, such as coast wattle and coastal tea-tree which outcompete all understorey plant species resulting in severely depauperate mammal habitats (Mitchell 2007). In coastal micro-refuges located close to human habitation and recreation, the presence of dogs, cats, human activities and littering are also threats to the swamp antechinus, requiring community education on responsible pet ownership, habitat protection areas and litter removal programs to assist the survival of the swamp antechinus.
The swamp antechinus is affected by multiple, likely interacting disturbances, that can accelerate population declines through additive or synergistic effects (Doherty et al. 2015). Fire can increase the activity of foxes and cats by removing vegetation which normally provides prey with refuge (McGregor et al. 2014, 2015; Leahy et al. 2015; Hradsky et al. 2017; Miritis et al.2023). There is evidence that increased predator activity is more likely when areas were burnt more recently (Doherty et al. 2023). For the swamp antechinus, there may be a critical period immediately post-fire when it is most vulnerable to an elevated risk of predation and within which management interventions are likely to be most impactful. The impacts of severe habitat loss and cover for swamp antechinus due to Phytophthora dieback is also likely to result in increased activity of foxes and cats. Integrated management of predators and fire is strongly recommended (Doherty et al. 2015, 2023) and should be paired with the application of phosphite to reduce spread of the pathogen and enhance the survival of vegetation.
It is recommended that refuges are monitored regularly (2–4 years) to confirm loss or recovery of populations and that the monitoring be linked to an assessment of management effectiveness, to aid adaptive management (Threatened Species Scientific Committee 2016; Wilson et al. 2017). Climate change will continue to have major impacts upon landscapes and species, and identifying critical refuges and protecting them to maintain and increase populations is essential.
Data availability
The data that support this study are available in the article and accompanying online supplementary material.
Declaration of funding
Financial and in-kind support from the Deakin University, and Parks Victoria is gratefully acknowledged. The work conducted between 2020 and 2023 was supported by Corangamite Catchment Management Authority, through funding from the Australian Government’s Environmental Restoration Fund.
Acknowledgements
We gratefully acknowledge our colleagues, postgraduates and undergraduates for the contributions to the studies and their continued support including Dr T. Doherty, D. Watchorn, D. Lees (Deakin University), J. Miller, H. Wiggs (Corangamite Catchment Management Authority), Dr M. Antos, K. Lovett (Parks Vic) and T. Miller (Department of Energy, Environment and Climate Action, Victoria). Many thanks to our excellent GIS, field and data technicians (D. Bridges, A. Garkaklis, M. Omerod). All work was conducted under the Victorian Wildlife Act and National Parks Act, and scientific permits approved by the Department of Environment, Land, Water and Planning/Department of Energy, Environment and Climate Action, Victoria and Parks Victoria (10007838, 10009183 10010395), under approval of the Deakin University (B01-2016 B06-2019) and Zoos Victoria (ZV22003) Animal Ethics Committees.
References
Bancroft, W. J., Roberts, J. D., and Garkaklis, M. J. (2005). Burrowing seabirds drive decreased diversity and structural complexity, and increased productivity in insular-vegetation communities. Australian Journal of Botany 53, 231-241.
| Crossref | Google Scholar |
Barrett, S., and Rathbone, D. (2018). Long-term phosphite application maintains species assemblages, richness and structure of plant communities invaded by Phytophthora cinnamomi. Austral Ecology 43, 360-374.
| Crossref | Google Scholar |
Bradstock, R. A., Bedward, M., Gill, A. M., and Cohn, J. S. (2005). Which mosaic? A landscape ecological approach for evaluating interactions between fire regimes, habitat and animals. Wildlife Research 32, 409-423.
| Crossref | Google Scholar |
Brandle, R., Moseby, K. E., and Adams, M. (1999). The distribution, habitat requirements and conservation status of the plains rat, Pseudomys australis (Rodentia: Muridae). Wildlife Research 26, 463-477.
| Crossref | Google Scholar |
Clarke, M. F. (2008). Catering for the needs of fauna in fire management: science or just wishful thinking? Wildlife Research 35, 385-394.
| Crossref | Google Scholar |
Collins, L., Bradstock, R. A., Clarke, H., Clarke, M. F., Nolan, R. H., and Penman, T. D. (2021). The 2019/2020 mega-fires exposed Australian ecosystems to an unprecedented extent of high-severity fire. Environmental Research Letters 16, 044029.
| Crossref | Google Scholar |
Claridge, A. W., Paull, D. J, and Barry, S. C. (2010). Detection of medium-sized ground-dwelling mammals using infrared digital cameras: an alternative way forward? Australian Mammalogy 32, 165-171.
| Crossref | Google Scholar |
Commonwealth of Australia (2018) ‘Threat abatement plan for disease in natural ecosystems caused by Phytophthora cinnamomi.’ http://www.environment.gov.au/biodiversity/threatened/publications/threat-abatement-plan-disease-natural-ecosystems-caused-phytophthora-cinnamomi-2018
CSIRO Australian Bureau of Meteorology (2022). State of the Climate 2022. National Climate Statement (Commonwealth of Australia).(climatechangeinaustralia.gov.au)
DeBondi, N., White, J. G., Stevens, M., and Cooke, R. (2010). A comparison of the effectiveness of camera trapping and live trapping for sampling terrestrial small-mammal communities. Wildlife Research 37, 456-465.
| Crossref | Google Scholar |
Department of Environment, Land, Water and Planning (2019a). Native Vegetation - Modelled 2005 Ecological Vegetation Classes (with Bioregional Conservation Status). Available at https://discover.data.vic.gov.au/dataset/native-vegetation-modelled-2005-ecological-vegetation-classes-with-bioregional-conservation-sta [accessed 2 September 2021].
Department of Environment, Land, Water and Planning (2019b). Fire history overlay of most recent fires only showing scars. Available at https://discover.data.vic.gov.au/dataset/fire-history-overlay-of-most-recent-fires-only-showing-scars [accessed 2 September 2021].
Dickman, C. R., Greenville, A. C., Tamayo, B., Wardle, G. M., and Blake, B. H. (2011). Spatial dynamics of small mammals in central Australian desert habitats: the role of drought refugia. Journal of Mammalogy 92, 1193-1209.
| Google Scholar |
Dobrowski, S. Z. (2011). A climatic basis for microrefugia: the influence of terrain on climate. Global Change Biology 17, 1022-1035.
| Crossref | Google Scholar |
Doherty, T. S, Chris, R., Dickman, C. R., Dale, G., Nimmo, D. G., and Ritchie, E. G. (2015). Multiple threats, or multiplying the threats? Interactions between invasive predators and other ecological disturbances. Biological Conservation 190, 60-68.
| Crossref | Google Scholar |
Doherty, T. S., Geary, W. L., Jolly, C. J., Macdonald, K. J., Miritis, V., Watchorn, J. W., Cherry, M. J., Conner, L. M., González, T. M., Legge, S. M., Ritchie, E. G., Stawski, C., and Dickman, C. R. (2022). Fire as a driver and mediator of predator-prey interactions. Biological Reviews 97(4), 1539-1558.
| Crossref | Google Scholar | PubMed |
Doherty, T. S., Watchorn, J. W., Miritis, V., Pestell, A. J., and Geary, W. L. (2023). Cats, foxes and fire: quantitative review reveals that invasive predator activity is most likely to increase shortly after fire. Fire Ecology 19, 22.
| Crossref | Google Scholar |
Driessen, M. M. (2024). Small mammal succession following low severity planned burns with different fire intervals. Australian Mammalogy 46, AM23016.
| Crossref | Google Scholar |
Driscoll, D. A., Lindenmayer, D. B., Bennett, A. F., Bode, M., Ross, A., Bradstock, R. A., Cary, G. J., Clarke, M. F., Dexter, N., Fensham, R., Friend, G., Gill, A. M., James, S., Kay, G., Keith, D., MacGregor, C., Russel- Smith, J., Salt, D., Watson, J. E. M., Williams, R. J., and York, A. (2010). Fire management for biodiversity conservation: key research questions and our capacity to answer them. Biological Conservation 143, 1928-1939.
| Crossref | Google Scholar |
Garkaklis, M. J. and Wilson, B. A (June 2021). Wild Otways Initiative – Project 3: Protecting Plant and Animal biodiversity in the Otway Ranges, Bells Beach (Ironbark Basin) and Great Ocean Road hinterland from Phytophthora dieback. 6 months Progress Report. Unpublished Report to the Corangamite Catchment Management Authority, Victoria. June 2021.
Gazzard, T., Walshe, T., Galvin, P., Salkin, O., Baker, M., Cross, B., and Ashton, P. (2020). What is the ‘appropriate’ fuel management regime for the Otway Ranges, Victoria, Australia? Developing a long-term fuel management strategy using the structured decision-making framework. International Journal of Wildland Fire 29(5), 354-370.
| Crossref | Google Scholar |
Gibson, L. A., Wilson, B. A., Cahill, D. M., and Hill, J. (2004). Modelling habitat suitability of the swamp antechinus (Antechinus minimus maritimus) in the coastal heathlands of southern Victoria, Australia. Biological Conservation 117, 143-150.
| Crossref | Google Scholar |
Goldingay, R. L., Taylor, B. D., and Parkyn, J. L. (2019). Use of tall wooden poles by four species of gliding mammal provides further proof of concept for habitat restoration. Australian Mammalogy 41, 142-146.
| Crossref | Google Scholar |
Great Ocean Road Coast, Parks Authority. (2022). Coastal Vegetation Strategy. Available at www.greatoceanroadauthority.vic.gov.au
Greenville, A. C., Wardle, G. M., and Dickman, C. R. (2013). Extreme rainfall events predict irruptions of rat plagues in central Australia. Austral Ecology 38, 754-764.
| Crossref | Google Scholar |
Hale, S., Nimmo, D. G., Cooke, R., Holland, G., James, S., Stevens, M., De Bondi, N., Woods, R., Castle, M., Campbell, K., Senior, K., Cassidy, S., Duffy, R., Holmes, B., and White, J. G. (2016). Fire and climatic extremes shape mammal distributions in a fire-prone landscape. Diversity and Distributions 22, 1127-1138.
| Crossref | Google Scholar |
Hardy, G. E. S., Barrett, S., and Shearer, B. L. (2001). The future of phosphite as a fungicide to control the soilborne plant pathogen Phytophthora cinnamomi in natural ecosystems. Australasian Plant Pathology 30, 133-139.
| Crossref | Google Scholar |
Hradsky, B. A. K., Mildwaters, C., Ritchie, E. G., Christie, F., and Di Stefano, J. (2017). Responses of invasive predators and native prey to a prescribed forest fire. Journal of Mammalogy 98, 835-847.
| Crossref | Google Scholar |
Hughes, I. I. (2000). Biological consequences of global warming: is the signal already apparent? Trends in Ecology & Evolution 15, 56-61.
| Crossref | Google Scholar | PubMed |
IPCC. (2013). Climate Change 2013: The Physical Science Basis. In ‘Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change’. (Eds T. F. Stocker, D. Qin, G.-K. Plattner, M. Tignor, S. K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex, P. M. Midgley.) 1535 pp. (Cambridge University Press, Cambridge: UK and New York, NY, USA.)
IPCC. (2023). Climate Change 2023: Synthesis Report. In ‘Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change’. (Eds Core Writing Team, H. Lee, J. Romero.) 184 pp. (IPCC: Geneva, Switzerland.) 10.59327/IPCC/AR6-9789291691647
Jolly, C. J., Dickman, C. R., Doherty, T. S., van Eeden, L. M., Geary, W. L., Legge, S. M., Woinarski, J., and Nimmo, D. G. (2022). Animal mortality during fire. Global Change Biology 28(6), 2053-2065.
| Crossref | Google Scholar | PubMed |
Kelly, L. T., Giljohann, K. M., Duane, A., Aquilué, N., Archibald, S., Batllori, E., Bennett, A. F., Buckland, S. T., Canelles, Q., Clarke, M. F., Fortin, M.-J., Hermoso, V., Herrando, S., Keane, R. E., Lake, F. K., McCarthy, M. A., Morán-Ordóñez, A., Parr, C. L., Pausas, J. G., and Brotons, L. (2020). Fire and biodiversity in the anthropocene. Science 370, 6519.
| Crossref | Google Scholar | PubMed |
Keppel, G., and Wardell-Johnson, G. W. (2012). Refugia: keys to climate change management. Global Change Biology 18, 2389-2391.
| Crossref | Google Scholar |
Lada, H., Thomson, J. R., Cunningham, S. C., and Mac Nally, R. (2013). Rainfall in prior breeding seasons influences population size of a small marsupial. Austral Ecology 38, 581-591.
| Crossref | Google Scholar |
Laidlaw, W. S., and Wilson, B. A. (2006). Habitat utilisation by small mammals in a coastal heathland exhibiting symptoms of Phytophthora cinnamomi infestation. Wildlife Research 33, 639-649.
| Crossref | Google Scholar |
Leahy, L., Legge, S. M., Tuft, K., McGregor, H., Barmuta, L., Jones, M. E., and Johnson, C. N. (2015). Amplified predation after fire suppresses rodent populations in Australia’s tropical savannas. Wildlife Research 42, 705-716.
| Crossref | Google Scholar |
Legge, S., Rumpff, L., Woinarski, J. C., Whiterod, N. S., Ward, M., Southwell, D. G., Scheele, B. C., Nimmo, D. G., Lintermans, M., Geyle, H. M., and Garnett, S. T. (2022). The conservation impacts of ecological disturbance: Time-bound estimates of population loss and recovery for fauna affected by the 2019–2020 Australian megafires. Global Ecology and Biogeography 31, 2085-2104.
| Crossref | Google Scholar |
Letnic, M., and Dickman, C. R. (2010). Resource pulses and mammalian dynamics: Conceptual models for hummock grasslands and other Australian desert habitats. Biological Reviews 85, 501-521.
| Crossref | Google Scholar | PubMed |
Lunney, D., Cullis, B., and Eby, P. (1987). Effects of logging and fire on small mammals in Mumbulla State Forest, near Bega, New South Wales. Wildlife Research 14, 163-181.
| Crossref | Google Scholar |
Mackey, B., Berry, S., Hugh, S., Ferrier, S., Harwood, T. D., and Williams, K. J. (2012). Ecosystem greenspots: identifying potential drought, fire, and climate-change micro-refuges. Ecological Applications 22, 1852-64.
| Crossref | Google Scholar | PubMed |
Magnusdottir, R., Wilson, B. A., and Hersteinsson, P. (2008). Dispersal and the influence of rainfall on a population of swamp antechinus (Antechinus minimus maritimus). Wildlife Research 35, 446-454.
| Crossref | Google Scholar |
McDonald, P. J., Pavey, C. R., Knights, K., Grantham, D., Ward, S. J., and Nano, C. E. M. (2013). Extant population of the critically endangered central rock-rat Zyzomys pedunculatus located in the Northern Territory, Australia. Oryx 47, 303-306.
| Crossref | Google Scholar |
McDonald, P. J., Griffiths, A. D, Nano, C. E. M., Dickman, C. R., Ward, S. J., and Luck, G. W. (2015). Landscape-scale factors determine occupancy of the critically endangered central rock-rat in arid Australia: the utility of camera trapping. Biological Conservation 191, 93-100.
| Crossref | Google Scholar |
McGregor, H. W., Legge, S., Jones, M. E., and Johnson, C. N. (2014). Landscape management of fire and grazing regimes alters the fine-scale habitat utilisation by feral cats. PLoS One 9, e109097.
| Crossref | Google Scholar | PubMed |
McGregor, H., Legge, S., Jones, M. E., and Johnson, C. N. (2015). Feral cats are better killers in open habitats, revealed by animal-borne video. PLoS One 10, e0133915.
| Crossref | Google Scholar | PubMed |
Milstead, W. B., Meserve Peter, L., Campanella, A., Previtali, M. A., Kelt Douglas, A., and Gutiérrez Julio, R. (2007). Spatial ecology of small mammals in north-central Chile: role of precipitation and refuges. Journal of Mammalogy 88, 1532-1538.
| Google Scholar |
Miritis, V., Dickman, C. R., Nimmo, D. G., and Doherty, T. S. (2023). After the ‘Black Summer’ fires: Faunal responses to megafire depend on fire severity, proportional area burnt, and vegetation type. Journal of Applied Ecology 61, 63-75.
| Crossref | Google Scholar |
Newsome, A. E., McIlroy, J., and Catling, P. (1975). The effects of an extensive wildfire on populations of twenty ground vertebrates in south-east Australia. Proceedings of the Ecological Society of Australia 9, 107-123.
| Google Scholar |
Nimmo, D. G., Mac Nally, R., Cunningham, S. C., Haslem, A., and Bennett, A. F. (2015). Vive la résistance: reviving resistance for 21st century conservation. Trends in Ecology & Evolution 30, 516-523.
| Crossref | Google Scholar | PubMed |
Parmesan, C., and Yohe, G. (2003). A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37-42.
| Crossref | Google Scholar | PubMed |
Parrott, M. L., Ward, S. J., Temple-Smith, P. D., and Selwood, L. (2007). Effects of drought on weight, survival and breeding success of agile antechinus (Antechinus agilis), dusky antechinus (A. swainsonii) and bush rats (Rattus fuscipes). Wildlife Research 34, 437-442.
| Crossref | Google Scholar |
Pavey, C. R., Cole, J. R., McDonald, P. J., and Nano, C. E. M. (2014). Population dynamics and spatial ecology of a declining desert rodent, Pseudomys australis: the importance of refuges for persistence. Journal of Mammalogy 95, 615-625.
| Crossref | Google Scholar |
Pavey, C., Addison, J., Brandle, R., Dickman, C., McDonald, P., Moseby, K., and Young, L. (2017). The role of refuges in the persistence of Australian dryland mammals. Biological Reviews 92, 647-664.
| Crossref | Google Scholar | PubMed |
Rees, M. W., Wintle, B. A., Robley, A., Pascoe, J. H., Le Pla, M., Birnbaum, E. K., and Hradsky, B. A. (2023). Fox control and fire influence on the occurrence of invasive predators and threatened native prey. Biological Invasions 26, 685-703.
| Crossref | Google Scholar |
Reside, A. E., Welbergen, J. A., Phillips, B. L., Wardell-Johnson, G. W., Keppel, G., Ferrier, S., Williams, S. E., and VanDerWal, J. (2014). Characteristics of climate change refugia for Australian biodiversity. Austral Ecology 39, 887-897.
| Crossref | Google Scholar |
Robinson, N. M., Leonard, S. W. J., Ritchie, E. G., Bassett, M., Chia, E. K., Buckingham, S., Gibb, H., Bennett, A. F., and Clarke, M. F. (2013). REVIEW: Refuges for fauna in fire-prone landscapes: their ecological function and importance. Journal of Applied Ecology 50, 1321-1329.
| Crossref | Google Scholar |
Sale, M. G., Ward, S. J., and Arnould, J. P. Y. (2006). Aspects of the ecology of swamp antechinus (Antechinus minimus maritimus) on a Bass Strait Island. Wildlife Research 33, 215-221.
| Crossref | Google Scholar |
Sale, M. G., Wilson, B. A., and Arnould, J. P. Y. (2008). Factors influencing population dynamics in island and mainland populations of swamp antechinus (Antechinus minimus; Marsupialia). Australian Journal of Zoology 56, 187-194.
| Crossref | Google Scholar |
Sale, M. G., and Arnould, J. P. Y. (2012). Inflated population density of island antechinus: a case of allochthonous marine inputs leading to increased food availability? Australian Journal of Zoology 60, 343-351.
| Crossref | Google Scholar |
Selwood, K. E., and Zimmer, H. C. (2020). Refuges for biodiversity conservation: A review of the evidence. Biological Conservation 245, 108502.
| Crossref | Google Scholar |
Selwood, K. E., McGeoch, M. A., and Mac Nally, R. (2015). The effects of climate change and land‐use change on demographic rates and population viability. Biological Reviews 90, 837-853.
| Crossref | Google Scholar | PubMed |
Selwood, K. E., Cunningham, S. C., and Mac Nally, R. (2019). Beyond refuges: Identifying temporally dynamic havens to support ecological resistance and resilience to climatic disturbances. Biological Conservation 233, 131-138.
| Crossref | Google Scholar |
Soanes, K., Vesk, P. A., and van der Ree, R. (2015). Monitoring the use of road-crossing structures by arboreal marsupials: insights gained from motion-triggered cameras and passive integrated transponder (PIT) tags. Wildlife Research 42, 241-256.
| Crossref | Google Scholar |
Tokushima, H., and Jarman, P. J. (2008). Ecology of the rare but irruptive Pilliga mouse, Pseudomys pilligaensis. II. Demography, home range and dispersal. Australian Journal of Zoology 56, 375-387.
| Crossref | Google Scholar |
Tokushima, H., Green, S. W., and Jarman, P. J. (2008). Ecology of the rare but irruptive Pilliga mouse (Pseudomys pilligaensis). I. Population fluctuation and breeding season. Australian Journal of Zoology 56, 363-373.
| Crossref | Google Scholar |
van Weenen, J., and Menkhorst, P. (2008). Antechinus minimus. In ‘IUCN red list of threatened species’. Version 2012.1. Available at www.iucnredlist.org [accessed 5 April 2017].
von Takach, B., Jolly, C. J., Dixon, K. M., Penton, C. E., Doherty, T. S., and Banks, S. C. (2022). Long-unburnt habitat is critical for the conservation of threatened vertebrates across Australia. Landscape Ecology 37, 1469-1482.
| Crossref | Google Scholar |
Watchorn, D. J., Doherty, T. S., Wilson, B. A., Garkaklis, M. J., and Driscoll, D. A. (2024). Patchy prescribed fire has variable effects on invasive predators and their native prey. Ecology and Evolution 14, e11450.
| Crossref | Google Scholar |
Wayne, A. F., Wilson, B. A., and Woinarski, J. C. Z. (2017). Falling apart? Insights and lessons from three recent studies documenting rapid and severe decline in terrestrial mammal assemblages of northern, south-eastern and southwestern Australia. Wildlife Research 44, 114-126.
| Crossref | Google Scholar |
White, J. G., Sparrius, J., Robinson, T., Hale, S., Lupone, L., Healey, T., Cooke, R., and Rendall, A. R. (2022). Can NDVI identify drought refugia for mammals and birds in mesic landscapes? Science of the Total Environment 851, 158318.
| Crossref | Google Scholar | PubMed |
Wilson, B. A., and Garkaklis, M. J. (2020). Patterns of decline of small mammal assemblages in vegetation communities of coastal south-east Australia: identification of habitat refuges. Australian Mammalogy 43, 203-220.
| Crossref | Google Scholar |
Wilson, B. A., and Wolrige, J. (2000). Assessment of the diet of the fox, Canis vulpes in habitats of the eastern Otway Ranges, Victoria. Australian Mammalogy 22, 201-211.
| Google Scholar |
Wilson, B. A., Bourne, A. R., and Jessop, R. E. (1986). Ecology of small mammals in coastal heathland at Anglesea, Victoria. Australian Wildlife Research 13, 397-406.
| Crossref | Google Scholar |
Wilson, B. A., Robertson, D., Moloney, D. J., Newell, G. R., and Laidlaw, W. S. (1990). Factors affecting small mammal distribution and abundance in the eastern Otway Ranges, Victoria. Proceeding of the Ecological Society of Australia 16, 379-96.
| Google Scholar |
Wilson, B. A., Aberton, J., and Reichl, T. (2001). Effects of fragmented habitat and fire on the distribution and ecology of the Swamp Antechinus (Antechinus minimus maritimus) in the Eastern Otways, Victoria. Wildlife Research 28, 527-536.
| Crossref | Google Scholar |
Wilson, B. A., Valentine, L. E., Reaveley, A., Isaac, J., and Wolfe, K. M. (2012). Terrestrial mammals of the Gnangara Groundwater System, Western Australia: history, status, and the possible impacts of a drying climate. Australian Mammalogy 34, 202-216.
| Crossref | Google Scholar |
Wilson, B. A., Zhuang-Griffin, L., and Garkaklis, M. J. (2017). Decline of the dasyurid marsupial Antechinus minimus maritimus in south-east Australia: implications for recovery and management under a drying climate. Australian Journal of Zoology 65, 203-216.
| Crossref | Google Scholar |
Wilson, B. A., Casey, S. P., Garkaklis, M. J., Learmonth, C., and Wevill, T. (2024). Impact of Phytophthora dieback on a key heathland species Xanthorrhoea australis (Asphodelaceae) (austral grasstree) and floristic composition in the eastern Otways, Victoria. Australian Journal of Botany 72, BT23076.
| Crossref | Google Scholar |
Wolfe, K. M., Mills, H. R., Garkaklis, M. J., and Bencini, R. (2004). Post-mating survival in a small marsupial is associated with nutrient input from seabirds. Ecology 85, 1740-1746.
| Crossref | Google Scholar |
Wooller, R. D., Richardson, K. C., Garavanta, C. A. M., Saffer, V. M., Anthony, C., and Wooller, S. J. (1998). The influence of annual rainfall upon capture rates of a nectar-dependent marsupial. Wildlife Research 25, 165-169.
| Crossref | Google Scholar |
Wooller, R. D., Richardson, K. C., Garavanta, C. A. M., Saffer, V. M., and Bryant, K. A. (2000). Opportunistic breeding in the polyandrous honey possum, Tarsipes rostratus. Australian Journal of Zoology 48, 669-680.
| Crossref | Google Scholar |