Differential expression and localisation of heat shock protein 70 (HSP70) and glutathione peroxidase 5 (GPX5) in the testis and epididymis of Sonid Bactrian camels
Gaowa Hasi A , Liyasu Wu B , Tserennadmid Sodnompil A , Ruhan Yi A , Rihan Wu A , Rui Zhang A , Haya Na A , Hejie Liu A , Musi Ji A , Wangwei Xie A and Narenhua Nasenochir A C *
A C *
A College of Animal Science, Inner Mongolia Agricultural University, Hohhot, Inner Mongolia, China.
B College of Animal Breeding and Genetics, Norwegian University of Life Sciences, Ås, Akershus, Norway.
C Key Laboratory of Animal Genetics, Breeding and Reproduction, Inner Mongolia Agricultural University, Hohhot, Inner Mongolia 010018, China.
Reproduction, Fertility and Development 35(10) 552-562 https://doi.org/10.1071/RD23026
Published online: 9 June 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Heat shock protein 70 (HSP70) and glutathione peroxidase 5 (GPX5) are biomarkers of oxidative stress and stress in temperate, tropical environments, which are crucial for male reproduction. Their expression and distribution patterns in the testis and epididymis of Bactrian camels are still unknown.
Aims: This study aims to investigate the HSP70 and GPX5 expression and localisation in 3- and 6-year-old Bactrian camel testis and epididymis.
Methods: Reverse transcription quantitative polymerase chain reaction (qRT-PCR), Western blot and immunohistochemistry were used to detect HSP70 in the testis and epididymis (caput, corpus and cauda) and GPX5 in the epididymis at two developmental stages (3-year-old puberty group and 6-year-old adult group).
Key results: HSP70 was upregulated in the testis. Immunohistochemistry results indicated the HSP70 protein was mainly detected in spermatids and Leydig cells of testicular tissue. In the epididymis, HSP70 was located at the luminal spermatozoa, the epithelium lining the epididymal and the epididymal interstitium. GPX5 expression was significantly higher in the caput epididymis than in the corpus and cauda epididymis. GPX5 protein was observed in the epithelium lining the epididymal, interstitium and luminal spermatozoa in the epididymis by immunohistochemistry.
Conclusions: Bactrian camel HSP70 and GPX5 exhibited spatiotemporal expression specificity.
Implications: HSP70 and GPX5 may be essential for germ cell development and reproductive success after sexual maturation in Sonid Bactrian camels.
Keywords: Bactrian camels, epididymis, glutathione peroxidase 5, heat shock protein 70, immunohistochemistry, qRT-PCR, testis, Western blot.
Introduction
With global warming and the expansion of desertification, camels, suitable livestock for sustainable agriculture and animal production systems, have good prospects for survival under these harsh conditions. Dromedaries account for 95% of all Old-World camels. Most of them are found in the Horn of Africa, the Middle East, Pakistan, India and arid regions of North and West Africa (Zarrin et al. 2020), while most Bactrian camels (Camelus bactrianus) are located in Mongolia, China, Kazakhstan, Afghanistan and Uzbekistan (Hafez and Hafez 2001). Most Bactrian camels are considered domestic animals and are mainly distributed in the desert and semi-arid areas of Northwest China, such as Inner Mongolia, Xinjiang, Qinghai, Gansu, Ningxia and other provinces. They are widely used for labour and wool production. Based on their geographical distribution, Xinjiang, Alxa and Sonid camels have been described as the three main breeds of C. bactrianus in China (Zhao et al. 2015).
In China, the population of Bactrian camels has been in long-term decline since 1982. However, the population has slightly recovered since 2010 (Faye 2020). The reproductive capacity of male Bactrian camels is likely to be a contributing factor to this population increase. Spermatogenesis consists of three phases: (1) stem cell renewal and spermatogonia proliferation; (2) meiosis and differentiation; and (3) spermiogenesis (Walker 2010). Male camels begin puberty at approximately 3 years of age but do not start full reproductive activity until they are 6 to 7 years old (Al Eknah 2000); this is also strongly dependent on the breed (Marai et al. 2009). Spermatogonia, spermatocytes and Sertoli cells can be found in the testes of the 3-year-old camels (Gherissi et al. 2020). The total number of spermatogonia, primary spermatocytes and spermatids changes with slight variation between the age of 6 and 18 (Marai et al. 2009). Compared with other domestic animals, the Bactrian camel can survive better under extreme climatic conditions (Lamo et al. 2020). However, because of their biological characteristics, camels exhibit a lower reproductive efficiency than other domestic animals (Rateb et al. 2020). Reproductive efficiency is one of the most important factors affecting camel reproduction and is related to the regulation of gene expression during spermatogenesis.
In the last two decades, it has been recognised that molecular chaperones have positive effects in controlling the morphological differentiation of gametes during spermatogenesis. Heat shock protein 70 (HSP70) members constitute one of the most prevalent families of molecular chaperones (Zininga et al. 2017). Organisms must be able to maintain dynamic cellular homeostasis in a changing environment. Molecular chaperones are involved in this process (Hartl et al. 2011), protecting cellular proteins from damage due to extreme conditions, such as sudden increases in temperature, oxidative stress, exposure to heavy metals, hypoxia and metabolic dysfunction (Mendillo et al. 2020). The proteins originally identified as heat-inducible are termed heat shock proteins, and there are several families of chaperones (HSP110, HSP100, HSP90, HSP70, HSP60, HSP40 and sHSP) (Doyle et al. 2013). In humans, HSP70 is expressed in most tissues (Scieglinska et al. 2011), including reproductive organs (Légaré et al. 2004). It is located in three cellular compartments, including the cytoplasm (containing various organelles, such as mitochondria), the nucleus and the cytoplasmic membrane, and performs its tasks inside the cell (Shevtsov et al. 2020). These proteins are involved in various cellular processes, including protein folding and refolding, transport, translocation, depolymerisation and degradation (Balchin et al. 2020). They are also associated with many human pathophysiological diseases, such as neurodegenerative diseases, cancer (Murphy 2013) and biological aging (Walther et al. 2017).
HSPA2 is a testis-enriched protein that belongs to the HSP70 multi-gene family and is highly associated with men’s fertility (Scieglinska and Krawczyk 2015). In mice, targeted destruction of HSPA2 can prevent spermatogenesis, impair sperm maturation and inhibit fertilisation (Nixon et al. 2017). Changes in phosphorylation levels of serine residues in HSP70 are related to sperm cryo-tolerance during the holding time of cryopreservation in boar spermatozoa (Yeste et al. 2014). Moreover, levels of HSP70 proteins in the testes and epididymides are correlated to the different developmental phases in rams (Wang et al. 2020). Reduced expression of HSP70 is associated with the pathogenesis of male infertility (Feng et al. 2001). In juvenile mice, HSP70 is required for synaptonemal complex desynapsis. Its absence limits spermatogenic cells from undergoing late meiotic stages, resulting in apoptosis of the initial wave of germ cell development (Dix et al. 1997). Male mice with the mutant allele (Hsp70-/-), unable to synthesise HSP70, are infertile (Dix et al. 1996). They also lack mature sperm and postmeiotic spermatids. In contrast, female Hsp70-/- mice show no alterations in meiosis or fertility (Dix et al. 1996).
Glutathione peroxidase 5 (GPX5) is an H2O2-scavenging enzyme identified in boar seminal plasma (Barranco et al. 2016). The mammals’ glutathione peroxidase (GPX) gene family encodes bifunctional enzymes that eliminate reactive oxygen species (ROS) and generate disulfide bridges in thiol-containing proteins (Noblanc et al. 2011). GPX5 is released from the epididymis and binds to the spermatozoa plasma membrane (Taylor et al. 2013). It is thought to shield sperm against peroxide-mediated damage by interacting with them through their epididymis transit (Taylor et al. 2013). Thus, GPX5 knockout mice receive greater oxidative damage to sperm DNA than wild-type mice (Taylor et al. 2013). As a result, the offspring born from the GPX5-/- mice have a high rate of spontaneous abortions and developmental defects. However, it occurs only when GPX5-/- mice are older than one year (Taylor et al. 2013). These findings imply that GPX5 is closely associated with male reproduction.
The previous study analysed the antioxidant biomarkers and the anti-freeze-associated gene expression in the post-thawed sperm of rooster (Mehaisen et al. 2022). Testes are the site of spermatozoa production, while the epididymis has stored spermatozoa (Hedger 2011). Therefore, the aim of this study was to investigate whether HSP70 and GPX5 is associated with testis and epididymis development in the Bactrian camel. In this study, we examined the expression and localisation of HSP70 in the testis and epididymis and the GPX5 gene in the epididymis of Sonid Bactrian camels at the age of 3 and 6 years.
Materials and methods
Animals and sample acquisition
All animal experiments were reviewed and approved by the Experimental Animal Ethics Committee of Inner Mongolia Agricultural University (Approval No. [2020]056). A total of 12 healthy male Bactrian camels (Inner Mongolia, China) were used in this study. Camels were divided into two age groups: 3-year-old and 6-year-old groups (n = 6 for each group). Following castration, tissue samples of the testis and epididymis (caput, corpus and cauda) from all camels were instantly snap-frozen in liquid nitrogen and stored for RNA and protein extraction or were fixed in 4% paraformaldehyde for approximately 48 h before paraffin embedding.
Reverse transcription quantitative polymerase chain reaction (qRT-PCR)
Total RNA was extracted according to the TRIzol (Invitrogen, Waltham, MA, USA) solubilisation and extraction method. The purity and concentration of extracted RNA were detected using a NanoDrop ND-2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The RNA samples were then reverse transcribed into cDNA using the PrimeScript RT Master Mix according to manufacturer’s instructions (Takara, Kusatsu, Japan). The cDNA was amplified by reverse transcription PCR using 2× Taq Plus PCR MasterMix (TianGen, Beijing, China). The primer sequences used for HSP70, GPX5 and GAPDH genes are shown in Table 1. qRT-PCR was performed using the Takara TB Green Premix Ex Taq II (Takara). A 7500 Fast Real-time PCR System (Thermo Fisher Scientific) was used to perform qRT-PCR analysis. The optimised qRT-PCR protocol was as followed: 94°C for 30 s; 40 cycles at 94°C for 5 s; and 60°C for 30 s. The expression of target gene mRNA was calculated relating to that of GAPDH used for a housekeeping gene by the 2−ΔΔCt method (Livak and Schmittgen 2001).
Western blot
Total protein of the selected testis and epididymis (caput, corpus and cauda) was extracted using radioimmunoprecipitation assay buffer (Solarbio, Beijing, China). The concentration of protein extract was detected via a BCA (bicinchoninic acid) assay kit (Boster Biological, China). The denatured samples with equivalent total protein (30 μg) were separated by 12% (w/v) sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred onto 0.45 μm polyvinylidene difluoride membranes for Western blot. Transferred blots were blocked in 5% (g/mL) skim milk powder for 2 h at 37°C and incubated overnight with either rabbit anti-HSP70 (Abcam, Shanghai, China, 1:1000), anti-GPX5 (Abcam, 1:1000), anti-β-ACTIN antibody (Abcam, 1:1000), followed by incubation with a goat anti-rabbit horseradish peroxidase conjugated IgG antibody (Abcam, 1:5000) for 2 h at room temperature. The used antibodies information is summarised in Table 2. The signals of bands were visualised using an enhanced chemiluminescence kit (Boster Biological, China). The band intensities were assessed by ImageJ software (ver. 1.46r, National Institutes of Health, Bethesda, MA, USA).
| Protein | Antibody | Source | Dilution | Website |
| HSP70 | Ab79852 | Rabbit | WB: 1:1000 IHC:1:100 | https://www.abcam.cn/HSP70-antibody-ab79852.html |
| GPX5 | PA5-102342 | Rabbit | WB: 1:1000 IHC:1:100 | https://www.thermofisher.cn/cn/zh/antibody/product/GPX5-Antibody-Polyclonal/PA5-102342 |
| β-ACTIN | PA1-32416 | Rabbit | WB: 1:5000 | https://www.thermofisher.cn/cn/zh/antibody/product/beta-Actin-Antibody-Polyclonal/PA1-32416 |
Immunohistochemistry
After dewaxing, hydration and microwave antigen retrieval, endogenous peroxidase activity from paraffin sections was eliminated by 3% H2O2, blocking for 1 h with 1% bovine serum albumin. Sections were then incubated with polyclonal rabbit anti-HSP70 primary antibody (Abcam, 1:100) or GPX5 (Abcam, 1:100) overnight at 4°C. Negative controls were generated with phosphate-buffered saline in place of the primary antibody. Peroxidase-conjugated goat anti-rabbit IgG (Abcam, 1:200) was used as the secondary antibody for 1 h at room temperature and visualised with Diaminobenzidine (Maixin, China). Slides were counterstained with hematoxylin. Localisation of HSP70 and GPX5 was then evaluated using a microscope (ZEISS Axio Imager A2).
Statistical analysis
The data were statistically analysed using one-way ANOVA in SPSS 26.0 (IBM Corp., USA) with the least significant difference (l.s.d.). Data were expressed as mean ± s.d. The relative changes in gene expression from qRT-PCR experiments were calculated by the 2−ΔΔCT method. Data were analysed graphically with GraphPad Prism 8 software (San Diego, CA, USA). *P < 0.05 was regarded as statistically significant and **P < 0.01 was regarded as highly significant.
Results
Expression of HSP70 mRNA in testis and epididymis of Bactrian camel
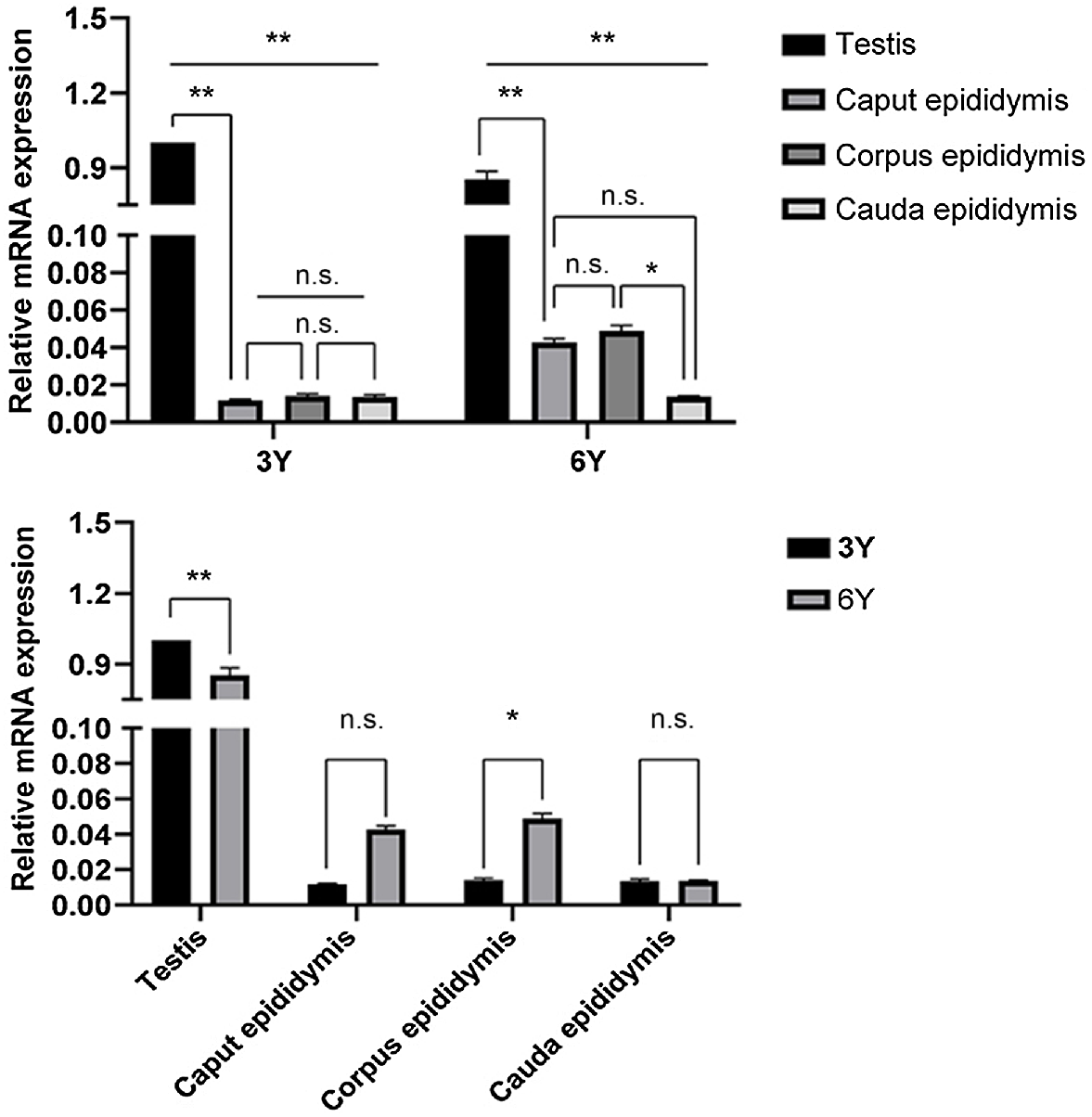
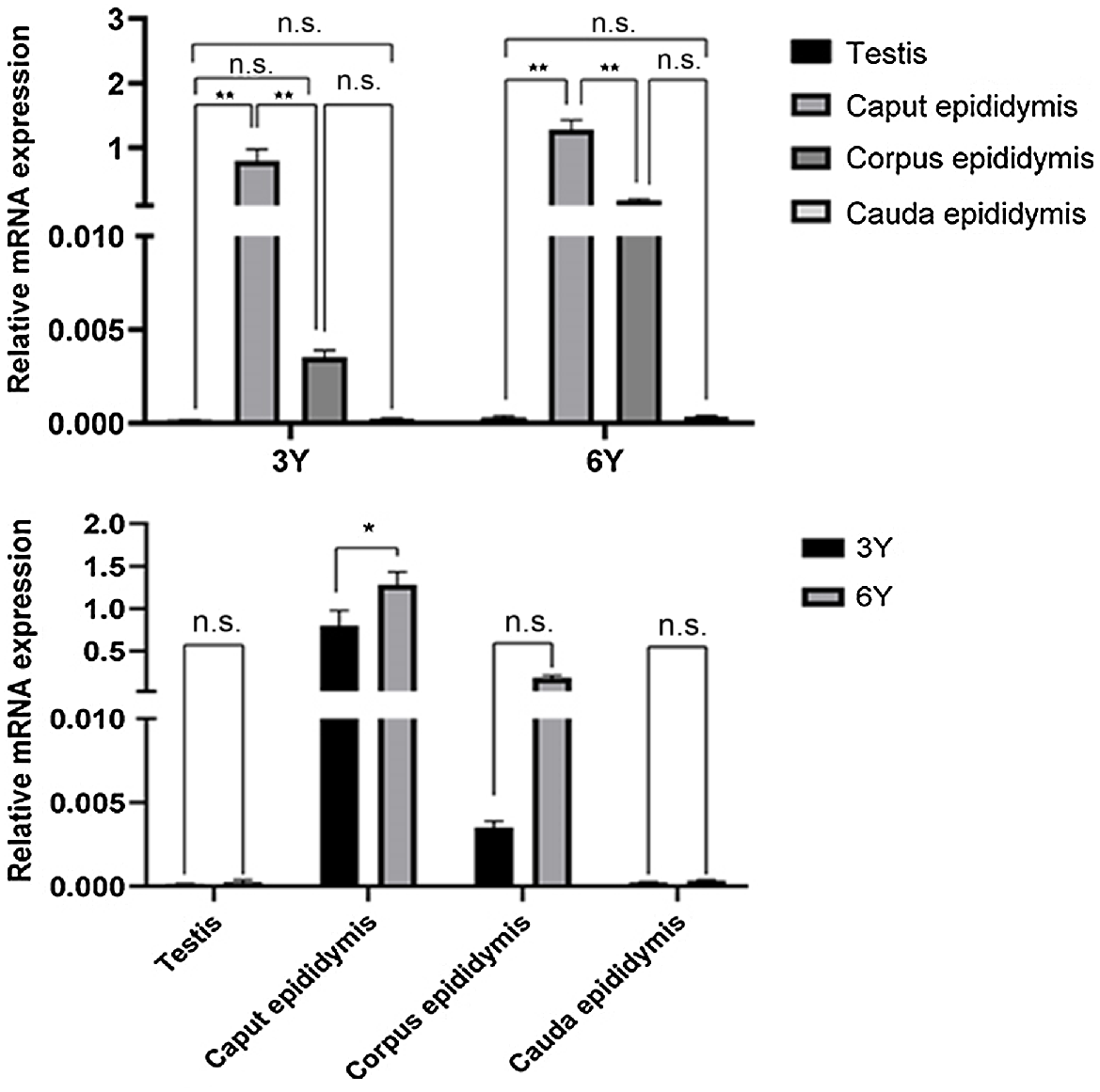
Transcriptional expression of HSP70 was first detected in the testis and the entire epididymis (caput, corpus and cauda) by qRT-PCR. We found that the HSP70 transcript was expressed in the testis and the caput, corpus and cauda epididymis in two developmental stages but was mainly expressed in the testis (P < 0.01; Fig. 1). The expression of HSP70 transcripts in the testis was significantly higher at puberty (3 years old) than at adulthood (6 years old) (P < 0.01; Fig. 1). There was no significant difference in the expression of HSP70 in the epididymal caput and cauda between the two stages, and the epididymal corpus HSP70 expression was significantly higher at adult than at puberty (P < 0.05; Fig. 1). For amplification curves and dissolution curves, see Supplementary Figs S1–S4.
Relative abundance of HSP70 transcript in puberty and adult Bactrian camel testis and epididymis. Each sample was analysed in triplicate. The results indicate the mean ± s.d. GAPDH was used as a reference gene. **P < 0.01; *P < 0.05; n.s. (no significance), P > 0.05. 3Y, 3-year-old group; 6Y, 6-year-old group.
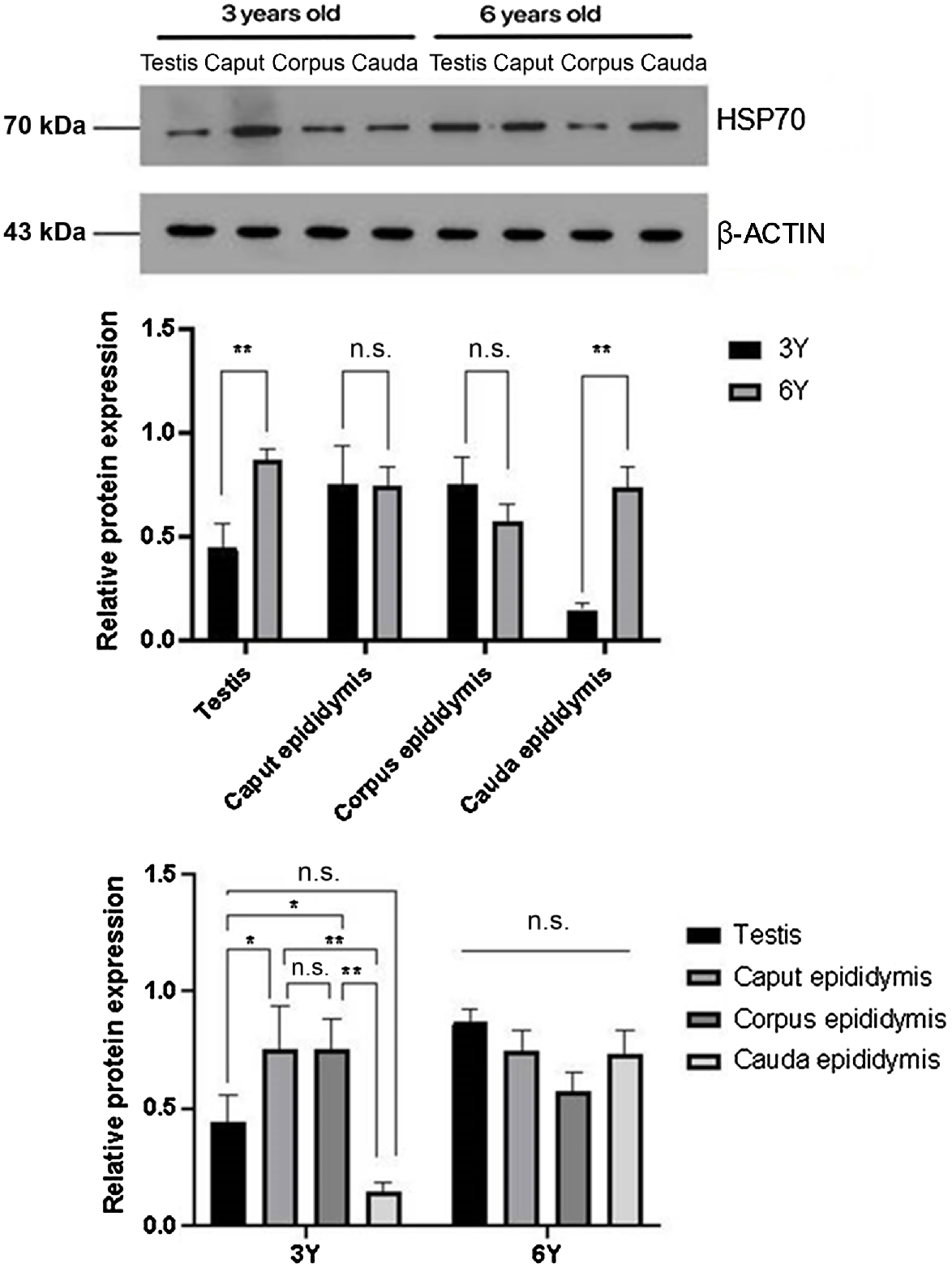
Expression of HSP70 protein in testis and epididymis of Bactrian camel
Expression levels of HSP70 protein in the testis and cauda epididymis were significantly higher in the 6-year-old adult group compared with the 3-year-old puberty group (P < 0.01), whereas no significant differences were observed in the caput and corpus (Fig. 2).
Localisation of HSP70 protein in testis and epididymis of Bactrian camel
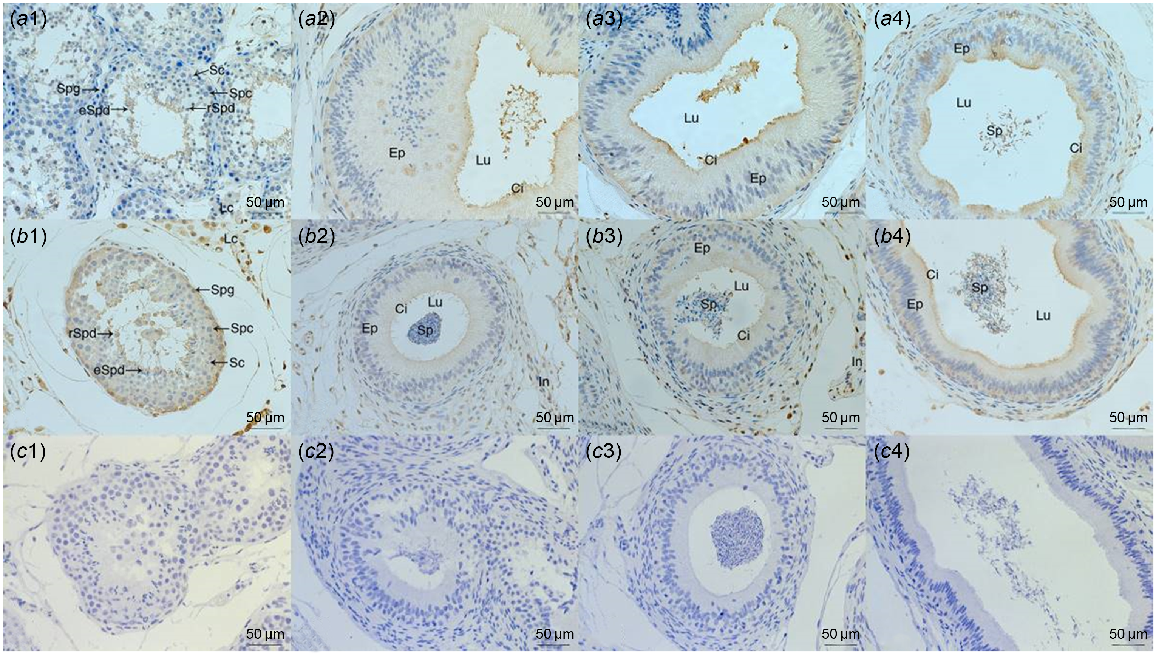
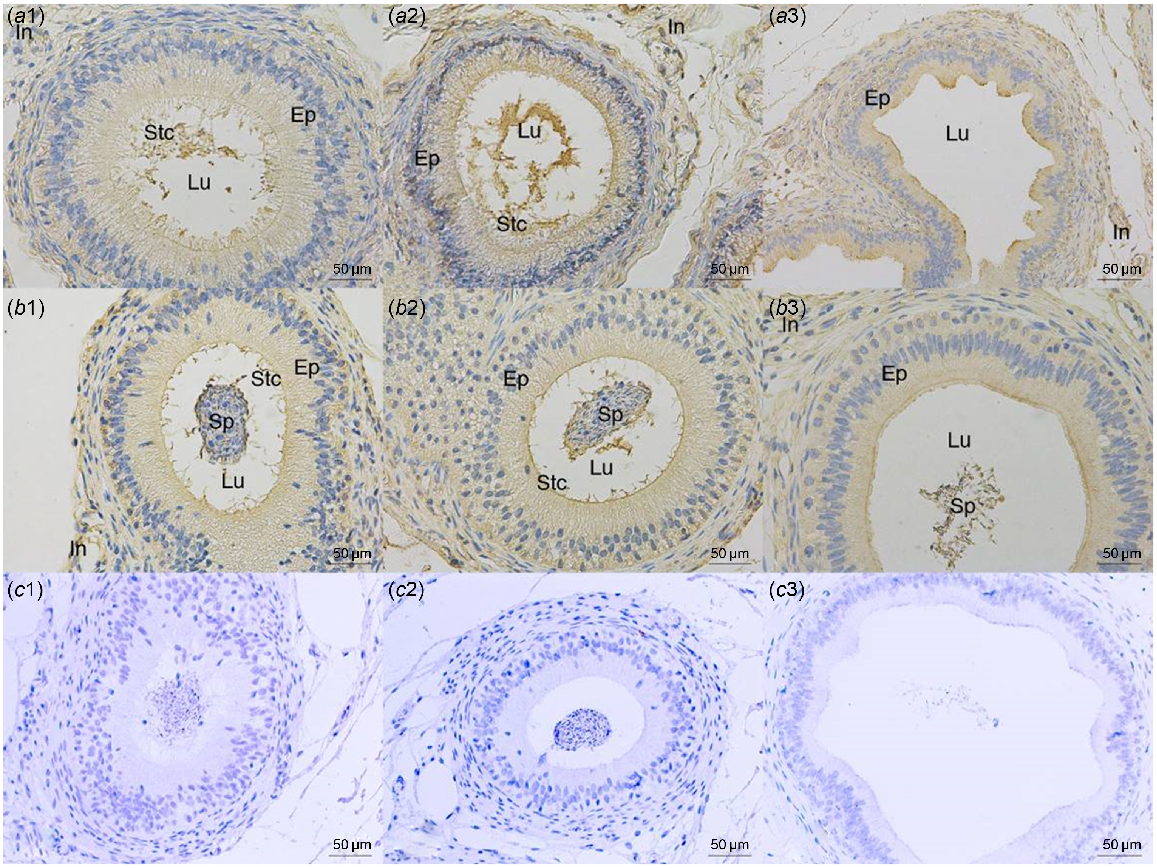
HSP70 protein was mainly distributed in Leydig cells in the testis of pubertal and adult Sonid Bactrian camels; namely, in spermatogonia and spermatids. In caput and corpus epididymis, HSP70 was present on the epididymal epithelium static cilia and epididymal interstitium for both age groups. In the cauda epididymis of 3-year-old and epididymis (caput, corpus and cauda) of 6-year-old camels, positive staining for the HSP70 protein was present in the epididymal epithelium and interstitium. It was also observed in spermatozoa inside the lumen of the epididymis (Fig. 3).
Immunohistochemistry staining of the HSP70 protein in testis and epididymis. Testis (a1, b1), caput epididymis (a2, b2), corpus epididymis (a3, b3) and cauda epididymis (a4, b4) tissues of 3-year-old (a1, a2, a3, a4) and 6-year-old (b1, b2, b3, b4) Sonid Bactrian camels. Negative controls of immunohistochemistry (c1, c2, c3, c4). Spg, spermatogonia; Spc, spermatocytes; rSpd, round spermatids; eSpd, elongated spermatids; LC, Leydig cells; SC, Sertoli cells; Lu, lumen; Ep, epithelium; In, interstitium; Ci, cilia; Sp, spermatozoa.
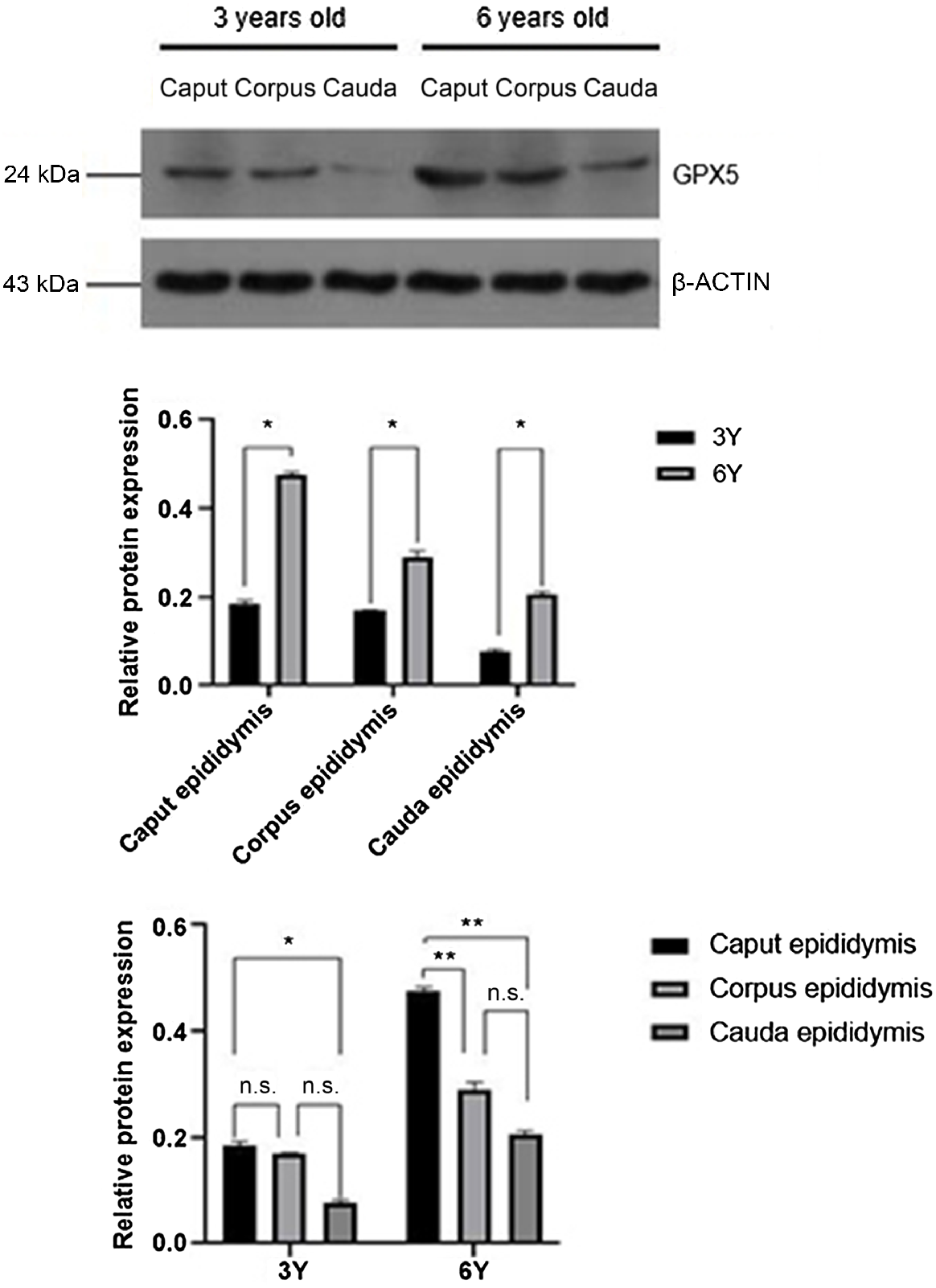
Expression of GPX5 mRNA in testis and epididymis of Bactrian camel
Relative quantification at the transcriptional level of RNA extracted from the testis and the entire epididymal (caput, corpus and cauda) tissues of camels at different developmental stages showed that the main expression region of the GPX5 gene was the caput epididymis (Fig. 4). There were also significant differences in epididymal expression between developmental stages. The caput epididymis was the main expression region of GPX5 mRNA in camels at both stages; the expression was significantly higher than in the corpus and cauda of the epididymis (P < 0.01). In addition, the expression in the epididymal caput in adult Bactrian camels was significantly higher than in pubescent camels (P < 0.01). In contrast, only a small amount of GPX5 gene expression was detected in the epididymal corpus and epididymal cauda, and the differences between the age groups were not significant (P > 0.05).
Relative abundance of GPX5 transcript in puberty and adult Bactrian camel testis and epididymis. Each sample was analysed in triplicate. The results indicate the means ± s.d. GAPDH was used as a reference gene. **P < 0.01; *P < 0.05; n.s. (no significance), P > 0.05. 3Y, 3-year-old group; 6Y, 6-year-old group.
Expression of GPX5 protein in epididymis of Bactrian camel
In 3-year-old camels (Fig. 5), the caput epididymis expressed the highest amount of GPX5 protein, which was not significantly different from the corpus epididymis (P > 0.05) but was significantly higher than the cauda epididymis (P < 0.01). In 6-year-old camels (Fig. 5), the caput epididymis was the main expression site of GPX5 protein, and the relative expression was significantly higher than the corpus and cauda of the epididymis (P < 0.01), while there was no significant difference between the corpus and cauda epididymis (P > 0.05).
Localisation of GPX5 protein in epididymis of Bactrian camel
The GPX5 protein was observed by immunohistochemistry in paraffin sections of the epididymis (caput, corpus, cauda) from both groups (Fig. 6). A positive signal for GPX5 protein was found on the epididymal epithelium and stereocilia of the epididymal caput in the group of 3-year-old camels and the epididymal epithelium and interstitium of the cauda epididymis. In the group of 6-year-old camels, there was a positive signal for GPX5 protein in the epididymis epithelium’s caput, corpus and cauda, as well as the interstitium and sperm cells inside the epididymis lumen (Fig. 6).
Immunohistochemistry staining of the GPX5 protein in the epididymis. Caput epididymis (a1, b1), corpus epididymis (a2, b2) and cauda epididymis (a3, b3) tissues of 3-year-old (a1, a2, a3) and 6-year-old (b1, b2, b3) Sonid Bactrian camels. Negative controls of immunohistochemistry (c1, c2, c3). Lu, lumen; Ep, epithelium; In, interstitium; Stc, stereocilia; Sp, spermatozoa.
Discussion
It is well known that HSP70 expression is upregulated in the testis (Scieglinska and Krawczyk 2015). Previous studies showed that in human testes with abnormal spermatogenesis, HSPA2 mRNA expression is reduced (Son et al. 2000). Failed meiosis, germ cell apoptosis and male infertility were brought on by the targeted mutation of the mouse HSP70 gene (Dix et al. 1996). In this work, we identified the expression patterns of HSP70 at both the transcript and protein levels in different age groups of Sonid Bactrian camel testis and epididymis. We found that HSP70 mRNA and protein were expressed in the testes and epididymis of both the 3-year-old and 6-year-old Bactrian camel groups, with significantly higher levels in puberty than adulthood. These results are consistent with the upregulation of HSP70 expression in adult testis of yak (Liu et al. 2018), Tibetan sheep (Wang et al. 2020) and pig (Huang et al. 2005). In Bactrian camels, there is an age-related increase in HSP70 mRNA in the corpus epididymis. HSP70 protein levels in the caput epididymis increased with age. In Tibetan sheep, with aging, the expression of HSP70 in the caput epididymis gradually increased (Wang et al. 2020); the expression first increased and then reduced to varied degrees in corpus and cauda epididymis (Wang et al. 2020). While this difference in expression pattern could be attributed to different mechanisms depending on the species, a trend emerges that HSP70 is differentially expressed between adult and juvenile animals.
Spermatozoa acquire fertility and motility during the passage of the epididymal pathway. This process is called sperm maturation. Other functions attributed to the epididymis include sperm concentration and transport, immune defence of male germ cells and acting as a reservoir for sperm (Sullivan and Mieusset 2016). However, studies have yet to be conducted on the expression pattern of HSP70 in the epididymis. To further clarify the HSP70 protein localisation in different regions of the Bactrian camel testis and epididymis, the HSP70 protein was visualised by immunohistochemistry. HSP70 staining was mainly located in spermatogonia, spermatids, Sertoli cells and Leydig cells in the Bactrian camel testis. Similar findings have been previously reported, with HSP70 being abundant in rabbit spermatogonia, spermatids and Sertoli cells (Wu et al. 2011). In Tibetan sheep, the positive HSP70 protein signals were primarily present in the round and luminal spermatids in post-pubescent testis (Wang et al. 2020).
In caput and corpus epididymis, immunohistochemical signals were distributed in the epididymal epithelium and interstitium of the two age groups. In cauda epididymis the HSP70 protein was not only present in the epididymal epithelium and interstitium, but it was also observed in spermatozoa within the lumen in the epididymis for both age groups. This corresponds to a previous study, showing that HSP70 proteins were mostly localised in epithelial cells in mice and Tibetan sheep epididymis (Wang et al. 2020).
Spermatozoa rely heavily on the antioxidant properties of the fluids surrounding them for protection against ROS damage. Therefore, the epididymis and seminal plasma are rich in antioxidants, including small molecules that destroy free radicals (vitamin C, uric acid, taurine, thioredoxin) (Bibov et al. 2018) and highly specialised extracellular antioxidant enzymes such as superoxide dismutase and unique isoforms of GPX, especially GPX5 (Aitken 2009). GPX5 was previously thought to protect sperm membranes from the deleterious effects of lipid peroxidation (Hall et al. 1998). Histological analysis of the epididymis and spermatozoa from GPX5-/- mice showed no obvious defects (Chabory et al. 2009). There were also no obvious differences in the fertilisation rate of adult GPX5-/- mice compared with wild-type (WT) mice. When female WT mice were paired with GPX5-/- males older than one year, more miscarriages and developmental defects caused by DNA oxidation were observed compared with crosses with WT males of the same age (Chabory et al. 2009). Consequently, GPX5 mRNA and protein expression are likely to be essential for successful reproduction. Here, we found that the expression of GPX5 is most enriched in caput in camel epididymis. Similarly, a previous study reported that GPX5 in caput was significantly higher than corpus and cauda epididymis in Small Tail Han sheep (Li et al. 2018). In mice, when compared with the cauda epididymis, GPX5 expression was abundant in the caput (Rejraji et al. 2002). GPX5 is expressed on epithelial cells, some cell cytoplasm and stereocilia of rat epididymis (Kolasa-Wołosiuk et al. 2019). The immunohistochemical results showed positive signals on the epithelium lining the epididymal duct and stereocilia of the caput epididymis at both developmental stages of the Bactrian camel and GPX5 was also present in the epithelium lining the epididymal duct of the cauda epididymis.
Based on this, we hypothesise that GPX5 protein may be synthesised and secreted from the epithelial cells of the epididymis into the lumen to bind to the spermatozoa and protect the spermatozoa during the process of sperm transport and storage in Sonid Bactrian camels. GPX5 from cattle appeared to be poorly secreted and is not present in spermatozoa of cauda epididymis (Li et al. 2018). However, Vernet et al. (1997) reported that in mice, GPX5 expression was localised at the apical border of the epithelial cells of the caput epididymis and can subsequently be detected in the lumen of the epithelial ducts of the corpus and cauda epididymis. We hypothesise that GPX5 plays a critical role in Bactrian camel epididymis by promoting sperm maturation and protecting sperm from ROS damage.
Conclusions
In the testis and epididymis of Bactrian camels, HSP70 displayed spatiotemporal expression specificity and GPX5 displayed the same property in the epididymis. HSP70 was abundantly expressed in the testis of Sonid Bactrian camels. Moreover, GPX5 was shown to be highly expressed in the caput epididymis. In the epididymis, HSP70 and GPX5 were distributed in the epithelial lining of the epididymal duct, the interstitium and the luminal spermatozoa of the epididymis.
This suggests that these two genes play a vital role in the reproductive organs of Sonid Bactrian camels at different developmental stages, similar to other animals. However, the elucidation of the precise regulatory mechanism involving these two genes during Bactrian camel spermatogenesis, sperm maturation and involvement in the regulation of sperm motility still requires in-depth investigation.
Acknowledgements
We are incredibly grateful to Dr. Narenhua, Ms. Liyasu Wu and Ms. Yu Wang for their critical reading of the manuscript and thoughtful feedback.
References
Aitken RJ (2009) Gpx5 protects the family jewels. The Journal of Clinical Investigation 119(7), 1849-1851.
| Crossref | Google Scholar |
Al Eknah MM (2000) Reproduction in old world camels. Animal Reproduction Science 60–61, 583-592.
| Crossref | Google Scholar |
Balchin D, Hayer-Hartl M, Hartl FU (2020) Recent advances in understanding catalysis of protein folding by molecular chaperones. FEBS Letters 594(17), 2770-2781.
| Crossref | Google Scholar |
Barranco I, Tvarijonaviciute A, Perez-Patiño C, Vicente-Carrillo A, Parrilla I, Ceron JJ, Martinez EA, Rodriguez-Martinez H, Roca J (2016) Glutathione peroxidase 5 is expressed by the entire pig male genital tract and once in the seminal plasma contributes to sperm survival and in vivo fertility. PLoS ONE 11(9), e0162958.
| Crossref | Google Scholar |
Bibov MY, Kuzmin AV, Alexandrova AA, Chistyakov VA, Dobaeva NM, Kundupyan OL (2018) Role of the reactive oxygen species induced DNA damage in human spermatozoa dysfunction. AME Medical Journal 3(19), 1-12.
| Crossref | Google Scholar |
Chabory E, Damon C, Lenoir A, Kauselmann G, Kern H, Zevnik B, Garrel C, Saez F, Cadet R, Henry-Berger J, Schoor M, Gottwald U, Habenicht U, Drevet JR, Vernet P (2009) Epididymis seleno-independent glutathione peroxidase 5 maintains sperm DNA integrity in mice. Journal of Clinical Investigation 119(7), 2074-2085.
| Crossref | Google Scholar |
Dix DJ, Allen JW, Collins BW, Mori C, Nakamura N, Poorman-Allen P, Goulding EH, Eddy EM (1996) Targeted gene disruption of Hsp70-2 results in failed meiosis, germ cell apoptosis, and male infertility. Proceedings of the National Academy of Sciences 93(8), 3264-3268.
| Crossref | Google Scholar |
Dix DJ, Allen JW, Collins BW, Poorman-Allen P, Mori C, Blizard DR, Brown PR, Goulding EH, Strong BD, Eddy EM (1997) HSP70-2 is required for desynapsis of synaptonemal complexes during meiotic prophase in juvenile and adult mouse spermatocytes. Development 124(22), 4595-4603.
| Crossref | Google Scholar |
Doyle SM, Genest O, Wickner S (2013) Protein rescue from aggregates by powerful molecular chaperone machines. Nature Reviews Molecular Cell Biology 14(10), 617-629.
| Crossref | Google Scholar |
Faye B (2020) How many large camelids in the world? A synthetic analysis of the world camel demographic changes. Pastoralism 10(1), 25.
| Crossref | Google Scholar |
Feng HL, Sandlow JI, Sparks AET (2001) Decreased expression of the heat shock protein hsp70-2 is associated with the pathogenesis of male infertility. Fertility and Sterility 76(6), 1136-1139.
| Crossref | Google Scholar |
Gherissi DE, Boukhili M, Gherissi A (2020) Genital histomorphometrical evaluation and survey on reproductive traits of male camel (Camelus dromedarius) in relation to the pubertal age under extreme arid conditions. Asian Journal of Agriculture and Biology 8, 436-446.
| Crossref | Google Scholar |
Hafez ESE, Hafez B (2001) Reproductive parameters of male dromedary and bactrian camels. Archives of Andrology 46(2), 85-98.
| Crossref | Google Scholar |
Hall L, Williams K, Perry ACF, Frayne J, Jury JA (1998) The majority of human glutathione peroxidase type 5 (GPX5) transcripts are incorrectly spliced: implications for the role of GPX5 in the male reproductive tract. Biochemical Journal 333(1), 5-9.
| Crossref | Google Scholar |
Hartl FU, Bracher A, Hayer-Hartl M (2011) Molecular chaperones in protein folding and proteostasis. Nature 475(7356), 324-332.
| Crossref | Google Scholar |
Hedger MP (2011) Immunophysiology and pathology of inflammation in the testis and epididymis. Journal of Andrology 32(6), 625-640.
| Crossref | Google Scholar |
Huang S-Y, Tam M-F, Hsu Y-T, Lin J-H, Chen H-H, Chuang C-K, Chen M-Y, King Y-T, Lee W-C (2005) Developmental changes of heat-shock proteins in porcine testis by a proteomic analysis. Theriogenology 64(9), 1940-1955.
| Crossref | Google Scholar |
Kolasa-Wołosiuk A, Tarnowski M, Baranowska-Bosiacka I, Chlubek D, Wiszniewska B (2019) Antioxidant enzyme expression of mRNA and protein in the epididymis of finasteride-treated male rat offspring during postnatal development. Archives of Medical Science 15(3), 797-810.
| Crossref | Google Scholar |
Lamo D, Gahlawat G, Kumar S, Bharti VK, Ranjan P, Kumar D, Chaurasia OP (2020) Morphometric, haematological and physio-biochemical characterization of Bactrian (Camelus bactrianus) camel at high altitude. BMC Veterinary Research 16(1), 291.
| Crossref | Google Scholar |
Légaré C, Thabet M, Sullivan R (2004) Expression of heat shock protein 70 in normal and cryptorchid human excurrent duct. Molecular Human Reproduction 10(3), 197-202.
| Crossref | Google Scholar |
Li R, Fan X, Zhang T, Song H, Bian X, Nai R, Li J, Zhang J (2018) Expression of selenium-independent glutathione peroxidase 5 (GPx5) in the epididymis of Small Tail Han sheep. Asian-Australasian Journal of Animal Sciences 31(10), 1591-1597.
| Crossref | Google Scholar |
Liu P, Yu S, Cui Y, He J, Zhang Q, Liu J, Song L, Xu Y, Wang T, Zou S, Li H (2018) Regulation by HSP70/90 in the different tissues and testis development of male cattle (Cattle-yak and Yak) [Preprint]. bioRxiv 393371.
| Crossref | Google Scholar |
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4), 402-408.
| Crossref | Google Scholar |
Marai IFM, Zeidan AEB, Abdel-Samee AM, Abizaid A, Fadiel A (2009) Camels’ reproductive and physiological performance traits as affected by environmental conditions. Tropical and Subtropical Agroecosystems 10(2), 129-149.
| Google Scholar |
Mehaisen GMK, Elomda AM, Hamad SK, Ghaly MM, Sun Y, Li Y, Zong Y, Chen J, Partyka A, Nazmi A, Abbas AO, Stino FKR (2022) Effect of dimethylacetamide concentration on motility, quality, antioxidant biomarkers, anti-freeze gene expression, and fertilizing ability of frozen/thawed rooster sperm. Animals 12(20), 2739.
| Crossref | Google Scholar |
Murphy ME (2013) The HSP70 family and cancer. Carcinogenesis 34(6), 1181-1188.
| Crossref | Google Scholar |
Noblanc A, Kocer A, Chabory E, Vernet P, Saez F, Cadet R, Conrad M, Drevet JR (2011) Glutathione peroxidases at work on epididymal spermatozoa: an example of the dual effect of reactive oxygen species on mammalian male fertilizing ability. Journal of Andrology 32(6), 641-650.
| Crossref | Google Scholar |
Rateb SA, El-Bahrawy KA, Khalifa MA, El-Hamid A, Zaghloul AA, Kamel AM, El-Hassanein EE (2020) Recent achievements for improving reproductive efficiency of dromedary camels in Egypt. Egyptian Journal of Animal Production 57(Suppl. Issue), 61-65.
| Google Scholar |
Rejraji H, Vernet P, Drevet JR (2002) GPX5 is present in the mouse caput and cauda epididymidis lumen at three different locations. Molecular Reproduction and Development 63(1), 96-103.
| Crossref | Google Scholar |
Scieglinska D, Krawczyk Z (2015) Expression, function, and regulation of the testis-enriched heat shock HSPA2 gene in rodents and humans. Cell Stress and Chaperones 20(2), 221-235.
| Crossref | Google Scholar |
Scieglinska D, Piglowski W, Chekan M, Mazurek A, Krawczyk Z (2011) Differential expression of HSPA1 and HSPA2 proteins in human tissues; tissue microarray-based immunohistochemical study. Histochemistry and Cell Biology 135(4), 337-350.
| Crossref | Google Scholar |
Shevtsov M, Balogi Z, Khachatryan W, Gao H, Vígh L, Multhoff G (2020) Membrane-associated heat shock proteins in oncology: from basic research to new theranostic targets. Cells 9(5), 1263.
| Crossref | Google Scholar |
Son W-Y, Han C-T, Hwang S-H, Lee J-H, Kim S, Kim YC (2000) Repression of hspA2 messenger RNA in human testes with abnormal spermatogenesis. Fertility and Sterility 73(6), 1138-1144.
| Crossref | Google Scholar |
Sullivan R, Mieusset R (2016) The human epididymis: its function in sperm maturation. Human Reproduction Update 22(5), 574-587.
| Crossref | Google Scholar |
Taylor A, Robson A, Houghton BC, Jepson CA, Ford WCL, Frayne J (2013) Epididymal specific, selenium-independent GPX5 protects cells from oxidative stress-induced lipid peroxidation and DNA mutation. Human Reproduction 28(9), 2332-2342.
| Crossref | Google Scholar |
Vernet P, Faure J, Dufaure J-P, Drevet JR (1997) Tissue and developmental distribution, dependence upon testicular factors and attachment to spermatozoa of GPX5, a murine epididymis-specific glutathione peroxidase. Molecular Reproduction and Development 47(1), 87-98.
| Crossref | Google Scholar |
Walker WH (2010) Non-classical actions of testosterone and spermatogenesis. Philosophical Transactions of the Royal Society B: Biological Sciences 365(1546), 1557-1569.
| Crossref | Google Scholar |
Walther DM, Kasturi P, Zheng M, Pinkert S, Vecchi G, Ciryam P, Morimoto RI, Dobson CM, Vendruscolo M, Mann M, Hartl FU (2017) Widespread proteome remodeling and aggregation in aging C. elegans. Cell 168(5), 944.
| Crossref | Google Scholar |
Wang X, Li T, Yin D, Chen N, An X, Zhao X, Ma Y (2020) Distinct expression and localization patterns of HSP70 in developmental reproductive organs of rams. Gene 760, 145029.
| Crossref | Google Scholar |
Wu Y, Pei Y, Qin Y (2011) Developmental expression of heat shock proteins 60, 70, 90, and A2 in rabbit testis. Cell and Tissue Research 344(2), 355-363.
| Crossref | Google Scholar |
Yeste M, Estrada E, Rivera del Álamo M-M, Bonet S, Rigau T, Rodríguez-Gil J-E (2014) The increase in phosphorylation levels of serine residues of protein HSP70 during holding time at 17°C is concomitant with a higher cryotolerance of boar spermatozoa. PLoS ONE 9(3), e90887.
| Crossref | Google Scholar |
Zarrin M, Riveros JL, Ahmadpour A, de Almeida AM, Konuspayeva G, Vargas-Bello-Pérez E, Faye B, Hernández-Castellano LE (2020) Correction to: Camelids: new players in the international animal production context. Tropical Animal Health and Production 52(6), 3931-3932.
| Crossref | Google Scholar |
Zhao D-B, Bai Y-H, Niu Y-W (2015) Composition and characteristics of Chinese Bactrian camel milk. Small Ruminant Research 127, 58-67.
| Crossref | Google Scholar |
Zininga T, Anokwuru CP, Sigidi MT, Tshisikhawe MP, Ramaite IID, Traoré AN, Hoppe H, Shonhai A, Potgieter N (2017) Extracts obtained from Pterocarpus angolensis DC and Ziziphus mucronata exhibit antiplasmodial activity and inhibit heat shock protein 70 (Hsp70) function. Molecules 22(8), 1224.
| Crossref | Google Scholar |