Root growth and N dynamics in response to multi-year experimental warming, summer drought and elevated CO2 in a mixed heathland-grass ecosystem
M. F. Arndal A E , I. K. Schmidt A , J. Kongstad A , C. Beier B D and A. Michelsen CA Department of Geosciences and Natural Resource Management, University of Copenhagen, Rolighedsvej 23, DK-1958 Frederiksberg C, Denmark.
B Department of Chemical and Biochemical Engineering, Technical University of Denmark, DTU, DK-2800 Kongens Lyngby, Denmark.
C Department of Biology, Terrestrial Ecology Section, Universitetsparken 15, University of Copenhagen, DK-2100 København Ø, Denmark.
D Present address: NIVA , Gaustadalléen 21,0349 Oslo, Norway.
E Corresponding author. Email: mfa@life.ku.dk
Functional Plant Biology 41(1) 1-10 https://doi.org/10.1071/FP13117
Submitted: 24 April 2013 Accepted: 18 July 2013 Published: 4 September 2013
Abstract
Ecosystems exposed to elevated CO2 are often found to sequester more atmospheric carbon due to increased plant growth. We exposed a Danish heath ecosystem to elevated CO2, elevated temperature and extended summer drought alone and in all combinations in order to study whether the expected increased growth would be matched by an increase in root nutrient uptake of NH4+-N and NO3– -N. Root growth was significantly increased by elevated CO2. The roots, however, did not fully compensate for the higher growth with a similar increase in nitrogen uptake per unit of root mass. Hence the nitrogen concentration in roots was decreased in elevated CO2, whereas the biomass N pool was unchanged or even increased. The higher net root production in elevated CO2 might be a strategy for the plants to cope with increased nutrient demand leading to a long-term increase in N uptake on a whole-plant basis. Drought reduced grass root biomass and N uptake, especially when combined with warming, but CO2 was the most pronounced main factor effect. Several significant interactions of the treatments were found, which indicates that the responses were nonadditive and that changes to multiple environmental changes cannot be predicted from single-factor responses alone.
Additional keywords: Calluna vulgaris, CLIMAITE, Deschampsia flexuosa, excised roots, ingrowth core, 15N-assay.
Introduction
Most climate change scenarios project that greenhouse gas concentrations will increase over the next decades with a continued increase in average global temperatures (Solomon et al. 2007). Along with increasing CO2 concentrations and a projected warming of ~0.2°C per decade, changes in precipitation patterns are also expected. These three factors are drivers for many important ecosystem processes, and changes in ecosystem function are therefore expected.
A substantial amount of carbon assimilated by plants is transported underground and transferred to the soil; in temperate grasslands, the largest fraction of total ecosystem carbon is stored below the ground (Mokany et al. 2006). The calculated root surface area and fine root length is often greater than leaf area, and in grasslands, it is more than an order of magnitude larger (Jackson et al. 1997). Hence soils are often the critical C sink in grasslands, in contrast to the large aboveground biomass sink in forests (Gill et al. 2006).
Regulation of the ability to take up nutrients such as nitrogen might have a great influence on carbon sequestration (Bassirirad 2000), and nutrient uptake kinetics are expected to be stimulated under high CO2 (Jackson and Reynolds 1996). Both nutrient uptake capacity and root growth generally increase in response to increasing temperature, but not always (Bassirirad 2000). This can be due to increased photosynthesis at higher temperatures, where the extra fixed carbon is allocated belowground to sustain new root growth (Pregitzer and King 2005). However, high temperatures often increase evapotranspiration and lead to drought, and low soil moisture reduces nutrient availability and nutrient uptake in the rooting zone, thus decreasing nutrient uptake rates (Gutschick and Pushnik 2005).
Even though elevated CO2 is expected to increase plant growth, this increase may not be sustained in the long term if nutrients or water are limited. The Progressive Nitrogen Limitation hypothesis suggests that without additional N input or reduced N loss, the N availability decreases over time at elevated CO2 (Luo et al. 2004), leading to reduced C uptake in the long term. The direct effects of warming and CO2 on plant growth in natural ecosystems can be limited if nutrient stocks are gradually depleted (Arnone et al. 2000; Gill et al. 2006). However, several examples exist that show no evidence of progressive nitrogen limitation even after several years of treatment with elevated CO2 in forests (Barnard et al. 2006; Norby and Iversen 2006; Phillips et al. 2006).
Plant responses to elevated CO2 are very dependent on plant N acquisition; however, studies of potential changes in root uptake capacity in response to the projected climate changes are few and inconsistent. It is critical to know, if the plants can positively adjust to the expected increased demand for nitrogen under elevated CO2 by increasing root growth or physiological properties. There are, to our knowledge, no longer term investigations of the effects of combined temperature, drought and elevated CO2 on root dynamics and root–plant–nutrient interactions.
Plants adapted to acid soils, like heathlands, have a preference for ammonium (Marschner 1995), but NO3– and organic N is also taken up by the heathland plants. Plant uptake of organic nitrogen (glycine) was studied at our experimental site 1 year after treatments started (Andresen et al. 2009). Here, we report the response of root growth and uptake of ammonium (NH4+-N) and nitrate (NO3– -N) to changes in atmospheric CO2, temperature and prolonged summer drought in all combinations. The objectives of this study were to study the inorganic N uptake and root production in a future climate, and determine whether an expected higher root growth would be followed by increased nutrient acquisition.
We hypothesised that root growth would be stimulated in response to elevated CO2 due to increased C uptake (Albert et al. 2011a; Albert et al. 2011b), and that therefore there would be greater C allocation to the roots. Drought was expected to slow down root growth, whereas the temperature treatment was expected to increase root growth, depending on soil moisture content. We hypothesised that nitrogen uptake would increase in response to elevated CO2, demonstrating a larger nutrient deficiency, but it would decrease under warming due to higher mineralisation and nutrient availability (Andresen et al. 2010b; Larsen et al. 2011). Drought, by contrast, was expected to decrease N acquisition. The single-factor effects were expected to be simply additive, when looking at the full combination of warming, drought and elevated CO2.
Materials and methods
Site description
The experimental site is situated in a dry mixture of heathland and grassland ~50 km north-west of Copenhagen (55°53′N, 11°58′E), Denmark, on a hilly nutrient-poor acid sandy deposit. The soil consists of 71.5% sand, 20.5% coarse sand, 5.8% silt and 2.2% clay (Nielsen et al. 2009). The soil is well drained with a pHCaCl2 in the topsoil of 3.3 increasing to 4.5 in the B-horizon and an organic top layer of 2–5 cm (O-horizon).
The yearly mean temperature is 8°C with 613 mm of precipitation (Danish Meteorological Institute, 2009, http://www.dmi.dk). The site had a relatively low atmospheric N bulk deposition of 1.35 ± 0.04 g N m–2 year–1 in 2007 (Larsen et al. 2011).
The dominant and studied plant species are an evergreen dwarf shrub Calluna vulgaris Hull. (~30% cover) and a perennial grass Deschampsia flexuosa Trin. (~70% cover). A few other grasses, herbs and mosses that are common in acidic grasslands are also present at the site (Kongstad et al. 2012).
Experimental manipulations
The manipulations in the CLIMAITE (CLIMate change effects on biological processes In Terrestrial Ecosystems) experiment were designed according to the climate predictions for Denmark in year 2075. The climate scenario was an elevated atmospheric CO2 concentration at 510 parts per million (ppm), elevated temperatures of 1−2°C above current mean air temperatures and a prolonged summer drought period (4–6 weeks).
The experiment consisted of 12 octagons (7 m in diameter) laid out pairwise in six blocks. Each block consisted of two octagons with one octagon receiving elevated CO2 (CO2, 510 ppm) using the free air CO2 enrichment technique and the other receiving ambient CO2. The CO2 concentration is highest at the edge, where the CO2 is released (the position of this depends upon the wind direction); the concentration sharply declines within the first 20 cm and towards the middle of the octagon where the mean target value of 510 ppm is reached. Within each octagon, there were four subplots with the following treatments: summer drought (D, exclusion of rain by automatic shelters), elevated temperature (T, passive night-time warming by reflective curtains, which covered the experimental plots 50 cm above the ground during the night, reducing the loss of heat and thereby increasing the temperature), a combination of drought and elevated temperature (TD) and an untreated control for reference (A, i.e. ambient CO2 and neither temperature- nor drought-treated) in a split-plot design.
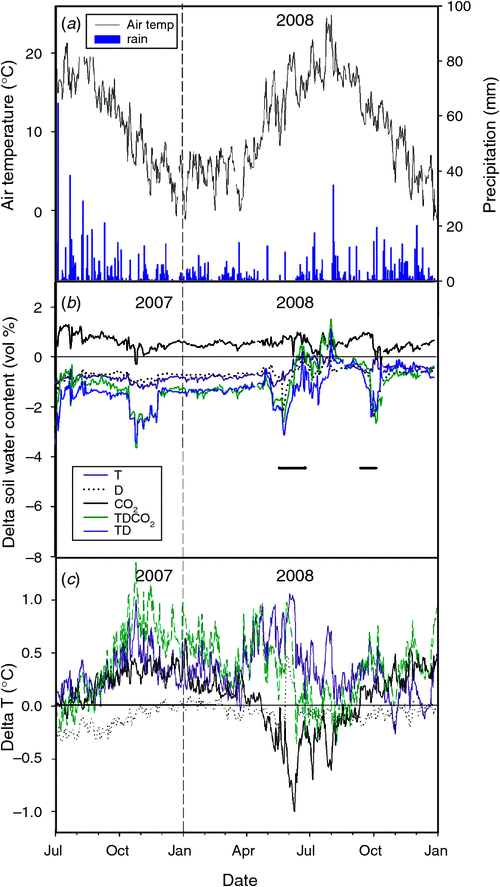
The experiment provided a full factorial design replicated 6 times with the treatments and combinations: A, T, D, CO2, TD, TCO2, DCO2 and TDCO2, giving a total of 48 plots. The treatments were initiated in October 2005. See Mikkelsen et al. (2008) and Larsen et al. (2011) for further details of the experimental design and set-up. Fig. 1 shows the climatic data from the site and the change in soil water content due to treatments and temperature in some of the treatments from 2007 to 2008.
|
The imposed drought periods were in 2008: 21 May to 22 June and again from 16 September to 2 October. The experimental drought treatment in 2008 was weak due to an extremely dry summer.
Root biomass
Soil samples were taken in October 2008 to give an estimate of root biomass in undisturbed areas under both Calluna and Deschampsia vegetation. One core was taken under each species per experimental plot. The soil auger was 4.5 cm in diameter and the soil core was divided into horizons: O-horizon, 0–5 cm deep and 5–10 cm deep. Fine roots (<1 mm in diameter) were separated from the soil in the soil laboratory by use of sieves and hand picking the roots with forceps, and then washed carefully. The roots were dried in the oven at ~60°C and weighed.
Root ingrowth cores and N pool size estimation
Annual net root productivity was estimated by in-growth cores, covering a full year of root growth from October 2007 to November 2008. In each treatment plot, two soil cores were sampled under patches of Calluna and Deschampsia, respectively. Roots were separated from the soil by the use of sieves and hand picking the roots with forceps. A tube of plastic mesh (mesh size: ~2 mm) lined the soil pit to mark the original hole (6 cm in diameter) and this was then filled up with root-free soil in the same original order of depth, divided into O-horizon, 0–5 cm deep and 5–10 cm deep, separated from each other by a small cloth inside the bag. The majority of roots in this ecosystem are found in the upper 10 cm of soil. After 13 months (November 2008), a soil auger of 4.5 cm was used to take a sample inside the original hole. The 96 samples were brought back to the laboratory and put into a fridge until the time of root sorting. Roots were not separated into species, but we assumed that the majority of the roots would belong to the species under which the in-growth cores were placed due to the patchy distribution of species. In an earlier in-growth core study, we found that some roots from Deschampsia grew into the mesh bags of Calluna (especially in the O-horizon), whereas the opposite was rarely seen. A mean estimate of Deschampsia roots growing into Calluna mesh bags indicated ~20% grass roots in the O-horizon, 12% in soil 0–5 cm deep and 15% in soil 5–10 cm deep under the dwarf shrubs, but little or no ingrowth of Calluna roots into the mesh bags under Deschampsia (unpublished results). In-growth bag samples are therefore referred to as ‘under’ Calluna or Deschampsia, due to the assumption that the majority of the root samples taken belonged to the aboveground plant species directly above.
The standing pool of belowground plant nitrogen was calculated as the root biomass from the in-growth core multiplied by the nitrogen concentrations (see next section).
Nitrogen uptake
The study of nitrogen uptake was done by 15N assay (Jones et al. 1991) and used roots from the in-growth cores. Hence, all the roots had been exposed to the experimental treatments and had a maximum age of 13 months.
Due to the large number of samples and the time-consuming root sorting procedure, sampling was done sequentially in three campaigns in autumn 2008 (four octagons per campaign: 24 November, 30 November and 7 December). The soil samples were separated into O-horizon, 0–5 cm deep and 5–10 cm deep. Each horizon was sorted by hand and fine roots were washed in demineralised water. The roots were put in a plastic bag with a moist hand towel and kept in the dark at 5°C until the assay was conducted.
The N bioassay was performed using the method described by Jones et al. (1991). Fresh roots from each species and soil layer were divided into two subsamples for analysis of absorption of 15NH4+-N and 15NO3–-N, respectively. Root samples from the O-horizon were not sufficient for the two assays and were therefore used for 15NH4+-N uptake assay only. All roots of both Deschampsia and Calluna were approximately of same diameter (<1 mm in diameter) and were not separated into different size classes. Hence all roots were considered active and able to take up nutrients.
The fresh root bundles were marked with a name tag and presoaked for 30 min (5 × 10−4 M CaCl2 solution) to maintain root cell membrane integrity and to remove ammonium, nitrate and nitrite ions from the cell-free space (Rosengren et al. 2003). After the presoaking treatment, roots were transferred to the uptake solution (5 × 10−4 M CaCl2 labelled with 2 mL 15N-NH4Cl or -KNO3 and 8 mL of 14N-NH4Cl or -KNO3, to get a 20% enrichment of 15N) and soaked for 2 h, washed in running demineralised water for 15 min, put in paper bags, dried in the oven at 80°C, ground and weighed and finally analysed for 15N, %N and %C (Eurovector elemental analyser, (Milan, Italy) coupled to an Isoprime mass spectrometer (Cheadle Hulme, UK)). Excess 15N (atom %) was converted to absorption rate of N (in µg N g–1 root DW 2 h–1). The total N concentration was also calculated, subtracting the N that had been taken up during the bioassay (Michelsen et al. 1999).
The nitrogen deficiency test was developed as a tool to relate root nitrogen levels to the fertiliser regime of trees in excised roots (Jones et al. 1991). High 15N uptake demonstrates nitrogen limitation (Jones et al. 1991; Rosengren et al. 2003). The results obtained with excised roots represent a relative measurement of the root net uptake capacity at different soil depths and not the actual uptake rate, as would have been found with the roots still attached to the plant (Göransson et al. 2007).
Statistical analysis
Statistical analyses were conducted by using the PROC MIXED procedure of SAS (SAS Institute, Cary, NC, USA). The same model was used for all variables, with all main factor effects and their interactions. The main effects (factors) of the statistical model were drought (D), warming (T) and elevated CO2 (CO2), and their interactions (T × D, T × CO2, D × CO2, T × D × CO2). This means, for instance, that with a significant main effect for CO2, all treatments with CO2 (CO2, TCO2, DCO2, TDCO2) will be different overall from all treatments with ambient CO2 (A, T, D, TD). The statistical model chosen included a random statement that accounted for the experimental design (random block octagon, octagon × D, octagon × T). The same model was used for all variables, with all main factor effects and their interactions, except when the differences between soil depths were studied. Soil sample identification was also included in the random statement to account for possible correlations between biomasses at different depths in the same soil sample.
Data were transformed in case requirements for the normality and homogeneity of variances were not met. P-values <0.05 were considered significant, but trends at P < 0.1 are also reported. Model reduction was done to obtain the best model by sequential removal of the terms in the model with the highest P-value. Differences of least-squares means were used to interpret significant interactions.
Results
Fine root biomass and annual net root productivity
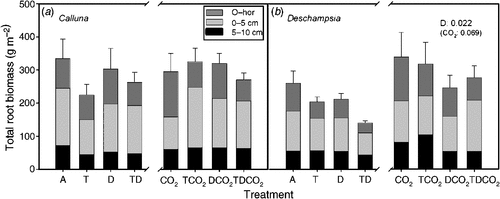
The total root biomass of Deschampsia sampled in October was significantly lower in the drought treatment (Fig. 2). Prolonged summer drought reduced Deschampsia root biomass in the O-horizon (P = 0.031), and at 0–5 cm deep, the combined warming and drought treatment reduced the biomass compared with the combination with elevated CO2 (T × D × CO2, P = 0.039). Elevated CO2 tended to increase the root biomass across all temperature and drought treatments by an average of 43%. Elevated CO2 increased the root biomass of Deschampsia significantly in the upper 5 cm (0–5 cm, P = 0.044); at 5–10 cm deep, there was only a tendency (P < 0.1) towards increased biomass in both Calluna and Deschampsia. We observed no significant effects of any treatment on the standing root biomass of Calluna (Fig. 2).
|
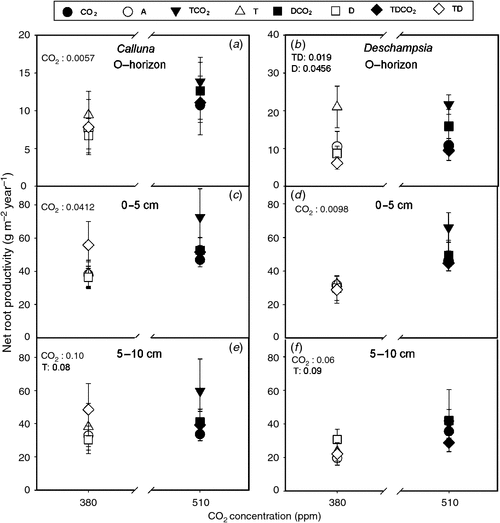
Elevated CO2 stimulated the total root production of both plant species in the in-growth cores by 28% in Calluna and by 55% in Deschampsia compared with plots under ambient CO2 (Fig. 3). The response to elevated CO2 was significant or tended to be in both species and in all three soil depths. Furthermore, there was an interaction where drought in combination with either T or TCO2 decreased the biomass in Deschampsia, mainly in the organic layer (D, P = 0.046 and T × D, P = 0.019). These results indicate that the Deschampsia roots are more affected by the drought treatment compared with Calluna roots.
|
Root nitrogen concentration and N pool sizes
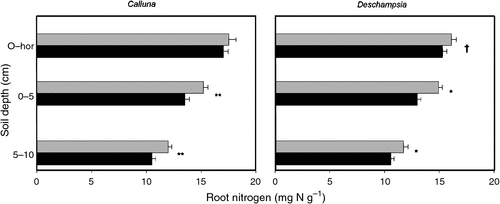
Elevated CO2 significantly reduced fine root N concentration under both Calluna (0–5 cm, P = 0.004; 5–10 cm P = 0.004) and Deschampsia (0–5 cm, P = 0.011; 5–10 cm, P = 0.016) by almost 10% when viewed across all depths (Fig. 4). The root N concentration also decreased with increasing soil depth. Reductions in N concentration were associated with corresponding increases in the C : N ratio, significantly in Calluna roots (0–5 cm deep and 5–10 cm deep, P = 0.003 for both depths; data not shown).
|
The root N pool size was increased in elevated temperature in the Deschampsia O-horizon, but when combined with drought, the N pool decreased (T× D interaction) (Table 1). Elevated CO2 stimulated the root N pool size in Deschampsia at 0–5 cm deep and tended to do so at 5–10 cm deep, and in Calluna in the organic horizon. Hence, overall root N pool size was increased per unit of area under elevated CO2, due to the increased root growth that compensated for decreases in root N concentration.
Nitrogen uptake
The highest uptake rate of NH4+-N in Calluna roots (per unit of root biomass) was found at 0–5 cm and 5–10 cm deep (Fig. 5). In Deschampsia roots, uptake in soil 0–5 cm deep was the highest. The uptake of NH4+-N was similar in the two species, with only a few treatment effects. Drought reduced the uptake of NH4+-N in Deschampsia roots especially in the deeper layers (5–10 cm, P = 0.014), except in the full treatment combination (T × D × CO2, P = 0.031). Elevated CO2 interacted with both T and TD, and increased the uptake of NH4+-N (T × CO2, P = 0.047). The uptake of NH4+-N in Deschampsia roots in the TD treatment was generally low at all three depths (not significant).
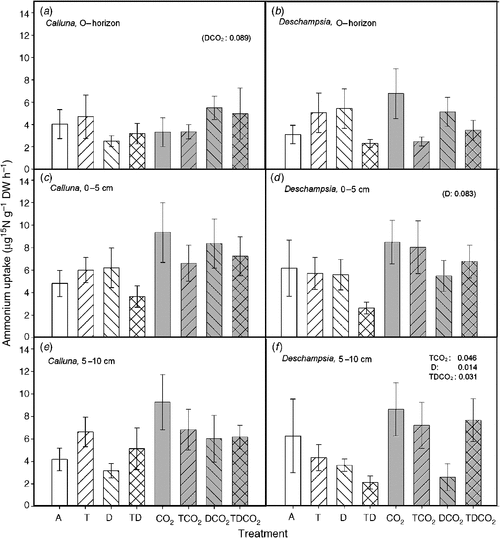
|
The uptake rate of NH4+-N was three to four times larger than the uptake rate of NO3–-N for both species. The NO3–-N uptake showed a similar response pattern to that observed for NH4+-N uptake with respect to treatment effects in Deschampsia (Fig. 6). In Deschampsia, drought reduced or tended to reduce the uptake, and the TD treatment generally decreased the NH4+-N uptake in all depths, but not significantly.
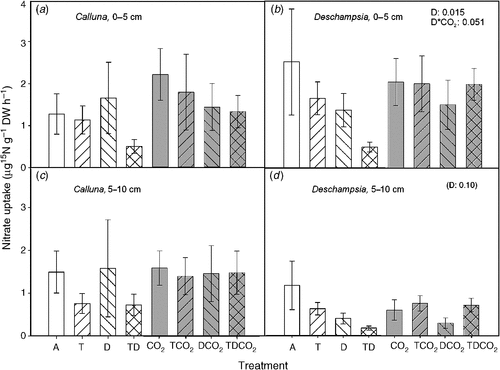
|
NH4+-N was the preferred nitrogen form, reflecting the differential abundance of the two inorganic N forms (Andresen et al. 2010a).
Discussion
Net root growth and standing root biomass
Increased root growth and biomass under elevated CO2 was observed, similar to other grassland studies (Jackson and Reynolds 1996; Sindhøj et al. 2004). In Calluna, a clear CO2 response was only seen in the in-growth cores, and a response in undisturbed areas might become pronounced only after a disturbance or after a longer time period.
In the CLIMAITE experiment, Albert et al. (2011a, 2011b) found increased net photosynthesis and increased leaf C : N ratios in response to elevated CO2. This is comparable to our higher root C : N ratio, which supports our hypothesis of the improved carbon status of the plant and a higher C allocation to roots grown under elevated CO2. Hence our increased net root production under elevated CO2 would result in more C-enriched root litter to the soil when the roots die. Due to the slower decomposition rate of roots compared with leaves (Franck et al. 1997; Gorissen and Cotrufo 2000), this might have a positive effect on soil carbon storage, especially in soils under Calluna due the recalcitrant compounds of the litter.
The few drought effects seen were found mostly in the O-horizon, where the drought effect on soil moisture is also most pronounced and the combined treatment of drought and elevated temperature seemed to reduce soil moisture enough to reduce root biomass under Deschampsia but not under Calluna (Fig. 2). The standing root biomass from Deschampsia was negatively affected in the TD treatment, but the opposite was true for Calluna. The explanation for this could be the leaf dieback that Deschampsia experiences during drought (Kongstad et al. 2012); the same adjustment in root biomass stocks has been reported. Many grass species cope with dry soil by rapidly shedding the fine lateral roots (Eissenstat and Yanai 1997), a strategy Deschampsia might also use. This may have given Calluna a competitive advantage, with its roots being able to grow without competition from Deschampsia’s roots (the drought effect was apparently sustained until autumn and winter).
An earlier study at our experimental site showed no persistent changes in the aboveground biomass under any of the treatments (Kongstad et al. 2012). This could indicate that the higher root growth in the CO2-treated plots is a response to limited nitrogen supply, as larger root systems relative to shoots are common when N is more limited than C (Poorter and Nagel 2000).
Root N concentration and N uptake
Our results support the hypothesis that N concentration decreases in plants grown under elevated CO2, as has been found by many others (for example Cotrufo et al. 1998). Elevated CO2 commonly results in lower tissue N concentration, for reasons that are not fully understood but include the dilution effect due to the accumulation of nonstructural carbohydrates (Bassirirad et al. 1997; Taub and Wang 2008). The stimulated root growth results in more roots that can exploit soil nutrients better than root systems in plots maintained at ambient CO2 concentrations.
Our results from the 15N-assay indicate that nitrogen uptake rate per unit of root mass would not increase under elevated CO2. This means that if our results are consistent with no increases in nutrient uptake per unit of root mass, other compensatory adjustments by the plants may take place. If a greater proportion of the additional carbon resulting from growth under elevated CO2 is allocated to root growth, then a high CO2 concentration can lead to an increased N uptake on a whole-plant basis, although the uptake rate per unit of root mass remains unchanged (Bassirirad et al. 1999). We also observed that the belowground N pool increased under elevated CO2, indicating an increased N allocation below the ground.
Different studies have provided highly variable results with increasing, decreasing or unchanged uptake under elevated CO2 (Rogers et al. 1994; Newbery et al. 1995; Jackson and Reynolds 1996; Bassirirad 2000; Gavito et al. 2001; Bielenberg and Bassirirad 2005; Taub and Wang 2008). Dijkstra et al. (2010) found that an increase in plant biomass with CO2 enrichment was mostly a result of increased nitrogen use efficiency, whereas increased plant N uptake contributed to the increase in biomass with increased soil moisture. Furthermore, both root age and diameter of individual roots have a large effect on the N uptake capacity (Volder et al. 2009), which also results in large variability.
Since the availability of NH4+-N is most often highest at the top of the soil where mineralisation is greatest, we expected a decrease in NH4+-N and NO3–-N uptake with depth. This was, however, not the case and corresponds to the results of Göransson et al. (2006), who did not find any significant differences in the uptake rate of NH4+-N at different soil depths in a beech (Fagus sylvatica L.) and a spruce (Picea abies (L.) Karst.) stand in Denmark.
The highest N uptake was found at a depth of 0–5 cm due to the large root biomass here. The N concentration in the roots decreased with increasing soil depth but no clear correlation was found between the uptake rate of NH4+-N or NO3–-N, and the root nitrogen concentration, as also seen in the study of Göransson et al. (2007).
Although Deschampsia and Calluna acquire some form of organic N, as amino acids (Andresen et al. 2010a, 2011), NH4+-N and NO3–-N are probably the most important sources of nitrogen at the site, and many grasses and ericoid species from heathlands prefer NH4+-N as their main N source (de Graaf et al. 1998; Falkengren-Grerup and Schöttelndreier 2004).
The roots of both species and at all depths were able to absorb both forms of N, but NH4+-N was always taken up at a greater rate: at a 4-fold higher rate, on average. The uptake ratio of NH4+-N to NO3–-N corresponds well with that of inorganic N in the soil, with NH4+-N roughly 4 and 10 times higher than NO3–-N in soil under Calluna and Deschampsia, respectively (Andresen et al. 2010a). Jin and Evans (2007) found increased NH4+-N and NO3–-N uptake under elevated CO2 in arid shrub lands, with NO3–-derived N taken up at the greatest rate. However, our results did not indicate that elevated CO2 increased the relative preference of NO3–-N to NH4+-N, similar to Jackson and Reynolds (1996).
In the combined treatment TD, NO3–-N uptake was low (although not significantly) in both species and at both depths, whereas the respective nitrogen concentration in TD was not lower than in the other treatments. As a low uptake of 15N indicates lack of nutrient limitation in the plant, the explanation for low uptake in the TD treatment could be due to higher nutrient availability, caused by delayed mineralisation after an intensive drought during summer combined with lysis of microbial nitrogen. In most plant roots, the kinetics of N uptake are regulated by demand, which is likely to exhibit a seasonal pattern (Gessler et al. 1998). The seasonal activities of soil organisms produce substantial changes in the availability of required nutrients (Glass 2005). This winter study may, therefore, not fully describe the overall CO2 effects on N uptake in this mixed heathland and grassland.
In summary, the CO2 response in the measured root parameters was not significantly moderated by the full treatment combination (TDCO2) and we did not observe an additive effect of the manipulations, as the effects of T and D were moderate. The response to TDCO2, which mimics the future climate, was not straightforward to interpret from single-factor effects. The root growth and nitrogen concentration in this study showed a clear effect of CO2, with no antagonistic effects of temperature and drought. Calluna root production seems to be less affected by drought, which could be an advantage when competing for nutrients in a future climate with longer drought events. The increased root growth in elevated CO2 will add more belowground litter to the soil, and C could be accumulated in a pool with relatively slow turnover, at least for the non-woody Deschampsia.
The increased root growth in elevated CO2 plots might be a strategy for the plants to cope with low nutrient supply, leading to an increased N pool size on a whole-plant basis, although the N concentration in the roots decreased.
In conclusion, root growth was stimulated by elevated CO2, leading to a higher biomass, but with a lower N concentration. However, the overall N content per unit of area was increased demonstrating an increased N allocation belowground. The nutrient uptake was not much affected by the treatments after 3 years of experimental manipulations, which suggest that both plant species are not strongly N-limited at present.
Acknowledgements
The authors thank The CLIMAITE project, funded by the Villum Kann Rasmussen foundation and further supported by Air Liquide Denmark A/S, DONG and the participating institutions. The authors also thank Nina Thomsen, Preben Jørgensen and Svend Danbæk for keeping the CLIMAITE facilities running and constantly ready for field work. Mette Sustman Carter is thanked for her help with statistical analysis, and Karen Thirslund and other students are thanked for their assistance in the field and Laboratory. Two anonymous referees are thanked for suggestions to improve earlier version of the manuscript.
References
Albert KR, Ro-Poulsen H, Mikkelsen TN, Michelsen A, Van Der Linden L, Beier C (2011a) Effects of elevated CO2, warming and drought episodes on plant carbon uptake in a temperate heath ecosystem are controlled by soil water status. Plant, Cell & Environment 34, 1207–1222.| Effects of elevated CO2, warming and drought episodes on plant carbon uptake in a temperate heath ecosystem are controlled by soil water status.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXpsVKhtbg%3D&md5=7de76ee0dd0b3cc6823dd9c5b5f535efCAS |
Albert KR, Ro-Poulsen H, Mikkelsen TN, Michelsen A, Van Der Linden L, Beier C (2011b) Interactive effects of elevated CO2, warming, and drought on photosynthesis of Deschampsia flexuosa in a temperate heath ecosystem. Journal of Experimental Botany 62, 4253–4266.
| Interactive effects of elevated CO2, warming, and drought on photosynthesis of Deschampsia flexuosa in a temperate heath ecosystem.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVeit7jL&md5=7e3d6461615799be813ba953c2897c6eCAS | 21586430PubMed |
Andresen LC, Michelsen A, Jonasson S, Beier C, Ambus P (2009) Glycine uptake in heath plants and soil microbes responds to elevated temperature, CO2 and drought. Acta Oecologica 35, 786–796.
| Glycine uptake in heath plants and soil microbes responds to elevated temperature, CO2 and drought.Crossref | GoogleScholarGoogle Scholar |
Andresen LC, Michelsen A, Jonasson S, Schmidt IK, Mikkelsen TN, Ambus P, Beier C (2010a) Plant nutrient mobilization in temperate heathland responds to elevated CO2, temperature and drought. Plant and Soil 328, 381–396.
| Plant nutrient mobilization in temperate heathland responds to elevated CO2, temperature and drought.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXit1Wqs7k%3D&md5=db8b2dedd9146bb57d39e73fde9ed2efCAS |
Andresen L, Michelsen A, Ambus P, Beier C (2010b) Belowground heathland responses after 2 years of combined warming, elevated CO2 and summer drought. Biogeochemistry 101, 27–42.
| Belowground heathland responses after 2 years of combined warming, elevated CO2 and summer drought.Crossref | GoogleScholarGoogle Scholar |
Andresen LC, Michelsen A, Jonasson S, Strom L (2011) Seasonal changes in nitrogen availability, and root and microbial uptake of 15N13C-phenylalanine and 15N-ammonium in situ at a temperate heath. Applied Soil Ecology 51, 94–101.
| Seasonal changes in nitrogen availability, and root and microbial uptake of 15N13C-phenylalanine and 15N-ammonium in situ at a temperate heath.Crossref | GoogleScholarGoogle Scholar |
Arnone JA, Zaller JG, Spehn EM, Niklaus PA, Wells CE, Korner C (2000) Dymamics of root systems in native grasslands, effects of elevated atmospheric CO2. New Phytologist 147, 73–85.
| Dymamics of root systems in native grasslands, effects of elevated atmospheric CO2.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXms1ylt7g%3D&md5=22b6639288f6867534e8b9bec5743ba5CAS |
Barnard R, Barthes L, Leadley PW (2006) Short-term uptake of N15 by a grass and soil micro-organisms after long-term exposure to elevated CO2. Plant and Soil 280, 91–99.
| Short-term uptake of N15 by a grass and soil micro-organisms after long-term exposure to elevated CO2.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhslOrs78%3D&md5=6c29133c0810490bac9f4da7a9c2d586CAS |
Bassirirad H (2000) Kinetics of nutrient uptake by roots: responses to global change. New Phytologist 147, 155–169.
| Kinetics of nutrient uptake by roots: responses to global change.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXms1yltL8%3D&md5=9d898896002c8ccb0376bf95e5e8ad24CAS |
Bassirirad H, Reynolds JF, Virginia RA, Brunelle MH (1997) Growth and root NO3– and PO4 3– uptake capacity of three desert species in response to atmospheric CO2 enrichment. Australian Journal of Plant Physiology 24, 353–358.
| Growth and root NO3– and PO4 3– uptake capacity of three desert species in response to atmospheric CO2 enrichment.Crossref | GoogleScholarGoogle Scholar |
Bassirirad H, Prior SA, Norby RJ, Rogers HH (1999) A field method of determining NH4+ and NO3– uptake kinetics in intact roots: Effects of CO2 enrichment on trees and crop species. Plant and Soil 217, 195–204.
| A field method of determining NH4+ and NO3– uptake kinetics in intact roots: Effects of CO2 enrichment on trees and crop species.Crossref | GoogleScholarGoogle Scholar |
Bielenberg DG, Bassirirad H (2005) Nutrient acquisition of terrestrial plants in a changing climate. In ‘Nutrient acquisition by plants. An ecological perspective. Vol. 181’. (Ed. H Bassirirad H) pp. 311–330. (Springer-Verlag, Berlin)
Cotrufo MF, Ineson P, Scott A (1998) Elevated CO2 reduces the nitrogen concentration of plant tissues. Global Change Biology 4, 43–54.
| Elevated CO2 reduces the nitrogen concentration of plant tissues.Crossref | GoogleScholarGoogle Scholar |
de Graaf MCC, Bobbink R, Roelofs JGM, Verbeek PJM (1998) Differential effects of ammonium and nitrate on 3 heathland species. Plant Ecology 135, 185–196.
| Differential effects of ammonium and nitrate on 3 heathland species.Crossref | GoogleScholarGoogle Scholar |
Dijkstra FA, Blumenthal D, Morgan JA, LeCain DR, Follett RF (2010) Elevated CO2 effects on semi-arid grassland plants in relation to water availability and competition. Functional Ecology 24, 1152–1161.
| Elevated CO2 effects on semi-arid grassland plants in relation to water availability and competition.Crossref | GoogleScholarGoogle Scholar |
Eissenstat DM, Yanai RD (1997) The ecology of root lifespan. Advances in Ecological Research 27, 1–60.
| The ecology of root lifespan.Crossref | GoogleScholarGoogle Scholar |
Falkengren-Grerup U, Schöttelndreier M (2004) Vascular plants as indicators of nitrogen enrichment in soil. Plant Ecology 172, 51–62.
| Vascular plants as indicators of nitrogen enrichment in soil.Crossref | GoogleScholarGoogle Scholar |
Franck VM, Hungate BA, Chapin FS, Field CB (1997) Decomposition of litter produced under elevated CO2: dependence on plant species and nutrient supply. Biogeochemistry 36, 223–237.
| Decomposition of litter produced under elevated CO2: dependence on plant species and nutrient supply.Crossref | GoogleScholarGoogle Scholar |
Gavito ME, Curtis PS, Mikkelsen TN, Jakobsen I (2001) Interactive effects of soil temperature, atmospheric carbon dioxide and soil N on root development, biomass and nutrient uptake of winter wheat during vegetative growth. Journal of Experimental Botany 52, 1913–1923.
| Interactive effects of soil temperature, atmospheric carbon dioxide and soil N on root development, biomass and nutrient uptake of winter wheat during vegetative growth.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXms12ksbo%3D&md5=b983cb686b1d989ac864f1a4d37cbae3CAS | 11520880PubMed |
Gessler A, Schneider S, Von Sengbusch D, Weber P, Hanemann U, Huber C, Rothe A, Kreutzer K, Rennenberg H (1998) Field and laboratory experiments on net uptake of nitrate and ammonium by the roots of spruce (Picea abies) and beech (Fagus sylvatica) trees. New Phytologist 138, 275–285.
| Field and laboratory experiments on net uptake of nitrate and ammonium by the roots of spruce (Picea abies) and beech (Fagus sylvatica) trees.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXitlOlsrw%3D&md5=fa303d409e74e9873b8403d5e63c01cbCAS |
Gill RA, Anderson LJ, Polley HW, Johnson HB, Jackson RB (2006) Potential nitrogen constraints on soil carbon sequestration under low and elevated atmospheric CO2. Ecology 87, 41–52.
| Potential nitrogen constraints on soil carbon sequestration under low and elevated atmospheric CO2.Crossref | GoogleScholarGoogle Scholar | 16634295PubMed |
Glass AD (2005) Homeostatic processes for the optimization of nutrient absorption: physiology and molecular biology. In ‘Nutrient acquisition by plants. An ecological perspective. Vol. 181’. (Ed. H Bassirirad) pp. 117–146. (Springer-Verlag, Berlin)
Göransson H, Wallander H, Ingerslev M, Rosengren U (2006) Estimating the relative nutrient uptake from different soil depths in Quercus robur, Fagus sylvatica and Picea abies. Plant and Soil 286, 87–97.
| Estimating the relative nutrient uptake from different soil depths in Quercus robur, Fagus sylvatica and Picea abies.Crossref | GoogleScholarGoogle Scholar |
Göransson H, Fransson AM, Jonsson-Belyazid U (2007) Do oaks have different strategies for uptake of N, K and P depending on soil depth? Plant and Soil 297, 119–125.
| Do oaks have different strategies for uptake of N, K and P depending on soil depth?Crossref | GoogleScholarGoogle Scholar |
Gorissen A, Cotrufo MF (2000) Decomposition of leaf and root tissue of three perennial grass species grown at two levels of atmospheric CO2 and N supply. Plant and Soil 224, 75–84.
| Decomposition of leaf and root tissue of three perennial grass species grown at two levels of atmospheric CO2 and N supply.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXnsVejsbk%3D&md5=1a732ebd4465e4712528bc503bd60198CAS |
Gutschick VP, Pushnik JC (2005) Internal regulation of nutrient uptake by relative growth rate and nutrient-use efficiency. In ‘Nutrient acquisition by plants. An ecological perspective. Vol. 181. (Ed. H Bassirirad) pp. 63–88. (Springer-Verlag, Berlin)
Jackson RB, Reynolds HL (1996) Nitrate and ammonium uptake for single- and mixed-species communities grown at elevated CO2. Oecologia 105, 74–80.
| Nitrate and ammonium uptake for single- and mixed-species communities grown at elevated CO2.Crossref | GoogleScholarGoogle Scholar |
Jackson RB, Mooney HA, Schulze ED (1997) A global budget for fine root biomass, surface area, and nutrient contents. Proceedings of the National Academy of Sciences of the United States of America 94, 7362–7366.
| A global budget for fine root biomass, surface area, and nutrient contents.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXksFOmtL4%3D&md5=208b2ce3d1222830d1670a255106e8d9CAS | 11038557PubMed |
Jin VL, Evans RD (2007) Elevated CO2 increases microbial carbon substrate use and nitrogen cycling in Mojave Desert soils. Global Change Biology 13, 452–465.
| Elevated CO2 increases microbial carbon substrate use and nitrogen cycling in Mojave Desert soils.Crossref | GoogleScholarGoogle Scholar |
Jones HE, Quarmby C, Harrison AF (1991) A root bioassay test for nitrogen deficiency in forest trees. Forest Ecology and Management 42, 267–282.
| A root bioassay test for nitrogen deficiency in forest trees.Crossref | GoogleScholarGoogle Scholar |
Kongstad J, Schmidt IK, Riis-Nielsen T, Beier C, Arndal MF, Mikkelsen TN (2012) High resilience in heathland plants to changes in temperature, drought and CO2 in combination: results from the CLIMAITE experiment. Ecosystems 15, 269–283.
| High resilience in heathland plants to changes in temperature, drought and CO2 in combination: results from the CLIMAITE experiment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjtFOhsb8%3D&md5=9ba0a50a96b1fea4cf8e310a3e503050CAS |
Larsen KS, Andresen LC, Beier C, Jonasson S, Albert KR, Ambus P, Arndal MF, Carter MS, Christensen S, Holmstrup M, Ibrom A, Kongstad J, Van Der Linden L, Maraldo K, Michelsen A, Mikkelsen TN, Pilegaard K, Prieme A, Ro-Poulsen H, Schmidt IK, Selsted MB, Stevnbak K (2011) Reduced N cycling in response to elevated CO2, warming, and drought in a Danish heathland: synthesizing results of the CLIMAITE project after two years of treatments. Global Change Biology 17, 1884–1899.
| Reduced N cycling in response to elevated CO2, warming, and drought in a Danish heathland: synthesizing results of the CLIMAITE project after two years of treatments.Crossref | GoogleScholarGoogle Scholar |
Luo Y, Su B, Currie WS, Dukes JS, Finzi AC, Hartwig U, Hungate B, McMurtrie RE, Oren R, Parton WJ, Pataki DE, Shaw MR, Zak DR, Field CB (2004) Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. Bioscience 54, 731–739.
| Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide.Crossref | GoogleScholarGoogle Scholar |
Marschner H (1995) ‘Marschner’s mineral nutrition of higher plants.’ 2nd edn. (Academic Press, London)
Michelsen A, Graglia E, Schmidt IK, Jonasson S, Sleep D, Quarmby C (1999) Differential responses of grass and a dwarf shrub to long-term changes in soil microbial biomass C, N and P following factorial addition of NPK fertilizer, fungicide and labile carbon to a heath. New Phytologist 143, 523–538.
| Differential responses of grass and a dwarf shrub to long-term changes in soil microbial biomass C, N and P following factorial addition of NPK fertilizer, fungicide and labile carbon to a heath.Crossref | GoogleScholarGoogle Scholar |
Mikkelsen TN, Beier C, Jonasson S, Holmstrup M, Schmidt IK, Ambus P, Pilegaard K, Michelsen A, Albert K, Andresen LC, Arndal MF, Bruun N, Christensen S, Danbaek S, Gundersen P, Jorgensen P, Linden LG, Kongstad J, Maraldo K, Prieme A, Riis-Nielsen T, Ro-Poulsen H, Stevnbak K, Selsted MB, Sorensen P, Larsen KS, Carter MS, Ibrom A, Martinussen T, Miglietta F, Sverdrup H (2008) Experimental design of multifactor climate change experiments with elevated CO2, warming and drought: the CLIMAITE project. Functional Ecology 22, 185–195.
Mokany K, Raison RJ, Prokushkin AS (2006) Critical analysis of root : shoot ratios in terrestrial biomes. Global Change Biology 12, 84–96.
| Critical analysis of root : shoot ratios in terrestrial biomes.Crossref | GoogleScholarGoogle Scholar |
Newbery RM, Wolfenden J, Mansfield TA, Harrison AF (1995) Nitrogen, phosphorus and potassium uptake and demand in Agrostis capillaris – the influence of elevated CO2 and nutrient supply. New Phytologist 130, 565–574.
| Nitrogen, phosphorus and potassium uptake and demand in Agrostis capillaris – the influence of elevated CO2 and nutrient supply.Crossref | GoogleScholarGoogle Scholar |
Nielsen PL, Andresen LC, Michelsen A, Schmidt IK, Kongstad J (2009) Seasonal variations and effects of nutrient applications on N and P and microbial biomass under two temperate heathland plants. Applied Soil Ecology 42, 279–287.
| Seasonal variations and effects of nutrient applications on N and P and microbial biomass under two temperate heathland plants.Crossref | GoogleScholarGoogle Scholar |
Norby RJ, Iversen CM (2006) Nitrogen uptake, distribution, turnover, and efficiency of use in a CO2-enriched sweetgum forest. Ecology 87, 5–14.
| Nitrogen uptake, distribution, turnover, and efficiency of use in a CO2-enriched sweetgum forest.Crossref | GoogleScholarGoogle Scholar | 16634292PubMed |
Phillips DL, Johnson MG, Tingey DT, Catricala CE, Hoyman TL, Nowak RS (2006) Effects of elevated CO2 on fine root dynamics in a Mojave Desert community: a FACE study. Global Change Biology 12, 61–73.
| Effects of elevated CO2 on fine root dynamics in a Mojave Desert community: a FACE study.Crossref | GoogleScholarGoogle Scholar |
Poorter H, Nagel O (2000) The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review. Australian Journal of Plant Physiology 27, 595–607.
| The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXlslars7w%3D&md5=a3d2f27999e5246c93b798e253182489CAS |
Pregitzer KS, King JS (2005) Effects of soil temperature on nutrient uptake. In ‘Nutrient acquisition by plants. An ecological perspective. Vol. 181’. (Ed. H Bassirirad) pp. 277–310. (Springer-Verlag, Berlin)
Rogers HH, Runion GB, Krupa SV (1994) Plant responses to atmospheric CO2 enrichment with emphasis on roots and the rhizosphere. Environmental Pollution 83, 155–189.
| Plant responses to atmospheric CO2 enrichment with emphasis on roots and the rhizosphere.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2c7psFOlsw%3D%3D&md5=ff859787d9e8f7b36d9c23f08116704aCAS | 15091762PubMed |
Rosengren U, Sleep D, Jones HE, Thelin G (2003) Increasing the sensitivity of the 15N root bioassay technique: suggested procedures. Communications in Soil Science and Plant Analysis 34, 2363–2373.
| Increasing the sensitivity of the 15N root bioassay technique: suggested procedures.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXlvVKgsbw%3D&md5=9c6befa010f238615865ed1317875791CAS |
Sindhøj E, Andrén O, Kätterer T, Marissink M, Pettersson R (2004) Root biomass dynamics in a semi-natural grassland exposed to elevated atmospheric CO2 for five years. Acta Agriculturae Scandinavica, Section B – Soil & Plant Science 54, 50–59.
| Root biomass dynamics in a semi-natural grassland exposed to elevated atmospheric CO2 for five years.Crossref | GoogleScholarGoogle Scholar |
Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (Eds) (2007) Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, 2007. (Cambridge University Press: Cambridge, UK and New York)
Taub DR, Wang XZ (2008) Why are nitrogen concentrations in plant tissues lower under elevated CO2? A critical examination of the hypotheses. Journal of Integrative Plant Biology 50, 1365–1374.
| Why are nitrogen concentrations in plant tissues lower under elevated CO2? A critical examination of the hypotheses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVyltb7M&md5=bf9571602e061c14e68c915828f22544CAS | 19017124PubMed |
Volder A, Anderson LJ, Smart DR, Bloom AJ, Lakso AN, Eissenstat DM (2009) Estimating nitrogen uptake of individual roots in container- and field-grown plants using a 15N-depletion approach. Functional Plant Biology 36, 621–628.
| Estimating nitrogen uptake of individual roots in container- and field-grown plants using a 15N-depletion approach.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXotVegu74%3D&md5=e8092c67073e9c6b63ee51f09903b416CAS |



