Bark traits, decomposition and flammability of Australian forest trees
Saskia Grootemaat A D , Ian J. Wright A , Peter M. van Bodegom B , Johannes H. C. Cornelissen C and Veronica Shaw AA Department of Biological Sciences, Macquarie University, North Ryde, NSW 2109, Australia.
B Institute of Environmental Sciences, Leiden University, Einsteinweg 2, 2333 CC Leiden, The Netherlands.
C System Ecology, Faculty of Earth and Life Sciences, VU University, 1081 HV Amsterdam, The Netherlands.
D Corresponding author. Email: saskia.grootemaat@live.nl
Australian Journal of Botany 65(4) 327-338 https://doi.org/10.1071/BT16258
Submitted: 16 December 2016 Accepted: 10 May 2017 Published: 4 July 2017
Abstract
Bark shedding is a remarkable feature of Australian trees, yet relatively little is known about interspecific differences in bark decomposability and flammability, or what chemical or physical traits drive variation in these properties. We measured the decomposition rate and flammability (ignitibility, sustainability and combustibility) of bark from 10 common forest tree species, and quantified correlations with potentially important traits. We compared our findings to those for leaf litter, asking whether the same traits drive flammability and decomposition in different tissues, and whether process rates are correlated across tissue types. Considerable variation in bark decomposability and flammability was found both within and across species. Bark decomposed more slowly than leaves, but in both tissues lignin concentration was a key driver. Bark took longer to ignite than leaves, and had longer mass-specific flame durations. Variation in flammability parameters was driven by different traits in the different tissues. Decomposability and flammability were each unrelated, when comparing between the different tissue types. For example, species with fast-decomposing leaves did not necessarily have fast-decomposing bark. For the first time, we show how patterns of variation in decomposability and flammability of bark diverge across multiple species. By taking species-specific bark traits into consideration there is potential to make better estimates of wildfire risks and carbon loss dynamics. This can lead to better informed management decisions for Australian forests, and eucalypt plantations, worldwide.
Additional keywords: decay, Eucalyptus, litter, wildfire.
Introduction
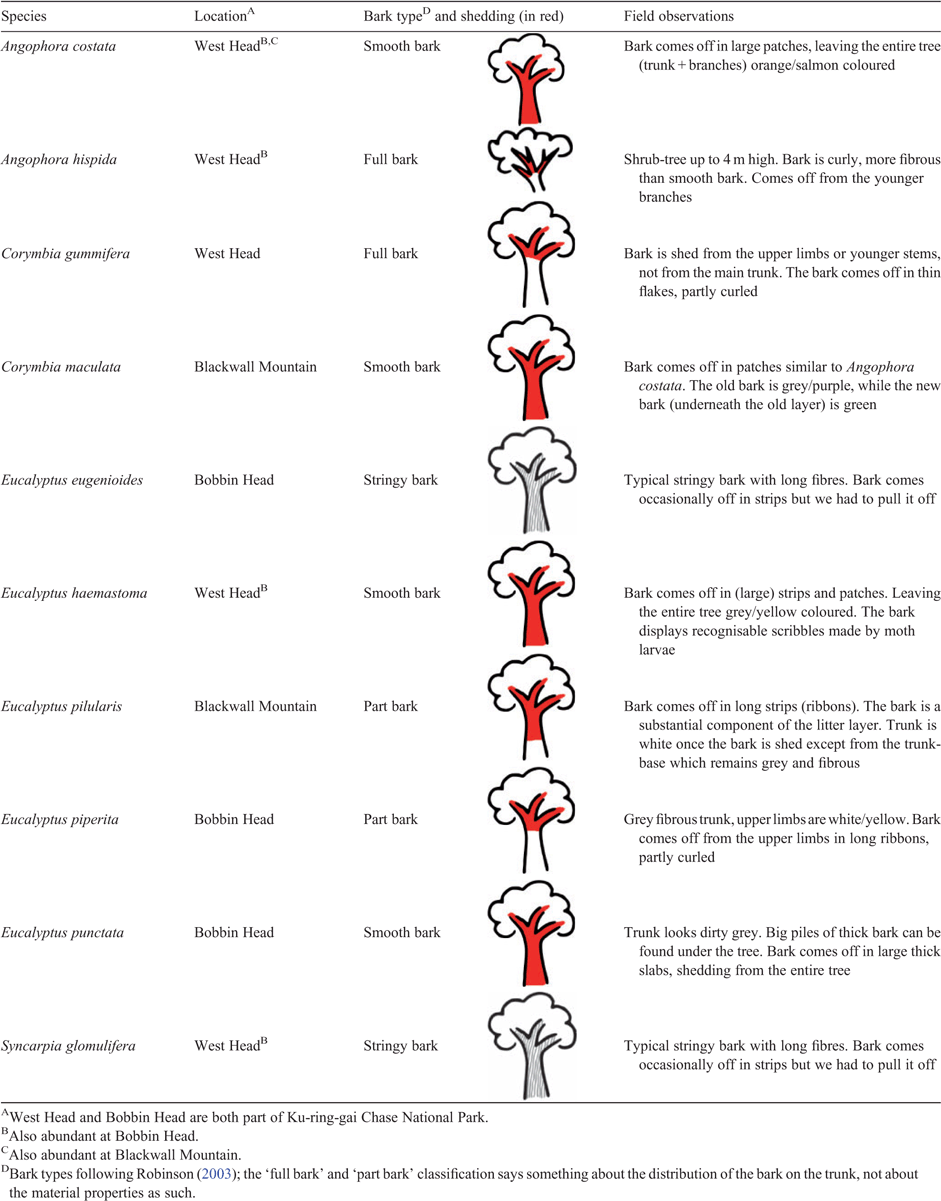
Bark is unequivocally a special feature in Australian forests. With a great variety of types (e.g. smooth bark, stringybark, ironbark; Table 1, Fig. 1), it is often used in floral keys as the first step for identifying Eucalyptus species (Millett 1969; Brooker and Kleinig 1990; Robinson 2003). Many species of Eucalyptus and other genera in the Myrtaceae family shed their bark annually, which leads to a spectacular accumulation of bark on the forest floor and long ribbons hanging down from the trunk and branches. This gives eucalypt forests, which cover 92 million ha of land surface in Australia (ABARES 2016), and 20 million ha of plantations around the world (Booth 2013), their unique appearance.
|
Bark shedding, especially of ‘smooth bark’ species, happens mainly in the Australian summer, often around December (Lamb 1985; Crockford and Richardson 1998). The ‘how and why’ of this bark shedding is still poorly understood, but is likely to depend on endogenous factors (e.g. tree size, growth, vigour) and on environmental factors, especially weather conditions (Crockford and Richardson 1998). The potential adaptive value of bark shedding in smooth bark species is thought to include promoting photosynthesis by the living tissues of the trunk (Aschan and Pfanz 2003; Cernusak and Hutley 2011), and eliminating pathogens and sap-feeding herbivores (Paine et al. 2010). It may also be a simple physical consequence of lateral stem growth, especially of thick-walled smooth bark (Jacobs 1955; Crockford and Richardson 1998). Some authors speculate that the loose hanging bark could promote fire spread from the surface layer up to the canopy by acting as ‘ladder fuels’ (Gould et al. 2011), thereby potentially benefitting pyrophytic tree species to promote their own competitive position by having a hot flammable burning strategy (the ‘kill thy neighbour’ effect, Bond and Midgley 1995).
Although we know relatively little about the causes of bark shedding, we know even less about the ecological effects. Bark contributes greatly to the litter layer on the forest floor. Depending on the age and composition of the tree species, bark can easily account for up to ~20–45% of the litter layer in Eucalyptus forests (McColl 1966; Woods and Raison 1983; Lamb 1985); the other chief components being leaves, twigs and fruits. This contribution of bark to the litter layer can have strong effects on upper soil properties and dynamics, like nutrient availability (Lamb 1985; Johnson et al. 2014) and microclimate (moisture retention and temperature regulation; Facelli and Pickett 1991). At the same time, the bark fraction affects the fuel availability and flammability of the litter layer in case of surface fires (Hines et al. 2010). Once the bark has dropped on the forest floor, several processes can potentially affect bark litter, namely: (i) leaching of water-soluble nutrients, (ii) breakdown of the woody material by UV light or fragmentation, (iii) decomposition by (micro-) organisms, or (iv) combustion in a fire (Cornwell et al. 2009). In this study we focussed on the latter two processes, as they are the dominant pathways for carbon loss from organic matter.
For leaves, which are another important component of the litter layer, decomposition and flammability are reasonably well understood. Leaf litter decomposition rates are a function of environmental conditions (air temperature, litter moisture content, UV radiation) as well as chemical composition of the leaf material (Adair et al. 2008; Cornwell et al. 2008; Makkonen et al. 2012). Higher initial concentrations of nitrogen (N) and phosphorus (P) lead to higher decomposition rates in the early stage of decomposition (Woods and Raison 1983; Berg and McClaugherty 2003), whereas recalcitrant structural compounds like lignin slow the decomposition process, especially in the later stages (Melillo et al. 1982; Berg and McClaugherty 2003). Under controlled conditions, leaf ignitibility (a measure of ease of ignition) is strongly driven by variation in specific leaf area (Murray et al. 2013; Grootemaat et al. 2015), whereas mass-standardised flame and smoulder durations are mainly determined by leaf chemistry (Grootemaat et al. 2015).
The growing understanding of leaf decomposition and flammability, and the underlying role of traits therein, contrasts with a lack of understanding of the trait-drivers of bark decomposition and flammability. Comparative studies on bark from multiple species are rare. The only study that we are aware of that explicitly included bark decomposition (Johnson et al. 2014) suggests substantial interspecific differences in bark decomposability amongst three tree species of the northern hemisphere. Previous work on bark flammability showed considerable differences among species (Gill and Ashton 1968, three species; Frejaville et al. 2013, eight species). However, these studies only looked at a limited set of bark traits.
The aim of this study was to lay a foundation for predicting surface litter (fuel) accumulation and flammability in Australian forests that are dominated by species of the Myrtaceae family. We investigated the relative decomposability and flammability (as described by ignitibility, fire sustainability and combustibility; Anderson 1970) of a range of tree species, while paying special attention to the bark. We examined the following specific questions and expectations:
-
How variable is bark decomposability among species, and how does bark decomposability compare to that of leaves? Specifically, although we expect substantial interspecific variation in bark decomposability based on their great visual differences in morphology, we also expect that bark will generally decompose more slowly than leaves because it contains more structural compounds such as lignin and cellulose (O’Connell 1997) and lower amounts of N and P (Lamb 1985).
-
How variable is bark flammability among species, and how does bark flammability compare to that of leaves? Based on field observations (S. Grootemaat, personal obs.) we expect ‘stringybarks’ to ignite more easily than smooth barks. When comparing mass-standardised combustion of bark and leaves, we expect longer flame and smoulder durations for bark, because bark is richer in structural compounds, which take more time to combust.
-
Is bark flammability correlated with bark decomposition rate? Based on previous work on leaves (Grootemaat et al. 2015) we hypothesise that decomposability and flammability are not correlated across species, starting from the premise that different traits underpin decomposability versus flammability (and its various parameters, i.e. ignitibility, fire sustainability and combustibility).
Materials and methods
Site description and species selection
Ten tree species with characteristic bark features were selected in the dry sclerophyll forests of Ku-ring-gai Chase National Park (33°37ʹ25ʹʹS, 151°14ʹ39ʹʹE) and Blackwall Mountain Reserve (33°30ʹ26ʹʹS, 151°20ʹ0ʹʹE), north of Sydney (New South Wales, Australia). The soils at these sites are predominantly sandy (derived from Hawkesbury Sandstone) and very low in P (30–80 mg kg–1; Leishman and Thomson 2005). Mean annual temperature was 17°C and mean annual rainfall 1332 mm for these sites over the past 50 years or more (http://www.bom.gov.au/climate/data/, accessed on 8 October 2015).
Tree species, all belonging to the Myrtaceae family, were selected based on their bark characteristics, abundance and their contribution of bark to the litter layer (a full list of species is provided in Table 1). We deliberately chose species with visually different bark types, including ‘smooth barks’ and ‘stringybarks’ (Fig. 1). Although the stringybark species (Eucalyptus eugenioides Sieber ex Spreng. and Syncarpia glomulifera Sm. Nied.) do not shed their bark like the other species, they were included for comparison and because of their importance in fire spread (see further discussion below).
Material collection and trait measurements
Bark
Bark from nine individual trees per species was collected in December 2012 and stored in paper bags under ambient laboratory conditions (21°C). Depending on the species, the bark came off in different sizes and shapes (flakes, slabs and ribbons). For comparison we roughly standardised the bark samples by splitting them into pieces of ~13 cm2 (one sided surface area). This size was practical to work with, both for the decomposition experiments (limited by the size of the litterbags) and for the experimental burns (limited by the size of the muffle furnace).
Leaves
Freshly senesced leaves of the same species were collected from the forest floor after windy days; these were easily distinguished from older (partially decomposed) senesced leaves by their yellow colour. Unlike the samples of bark, which were true replicates of nine individual trees, the leaf samples were taken from a bulk-sample from ~10–40 individual trees per species. The samples were stored in paper bags under ambient laboratory conditions (21°C).
Trait measurements
Subsamples of bark and leaves were kept aside for trait measurements (Table S1, available as Supplementary Material to this paper). Length and width were measured with a ruler. Thickness (mean of three measurements for bark) was measured with a thickness gauge. Dry mass was determined after oven drying at 60°C (when equilibrium was reached). One-sided surface area was estimated with a LI-3100C area meter (Li-Cor, Lincoln, NE, USA). Bark particle volume was estimated by using the gravimetric (water replacement) method. For leaf volume, leaf area was multiplied by mean leaf thickness. Tissue density was calculated as dry mass per volume. Bark tensile strength was measured by means of a 3 mm punch-test, using an Instron machine, model 5542 (Instron, Norwood, MA, USA). Fuel moisture contents were measured on air-dried subsamples just before the experimental burns. These samples were remeasured after 24 h of oven-drying at 105°C. Fuel moisture content was then expressed as a percentage of oven-dry weight. Energy content (MJ kg–1) of ground bark or leaf material was measured with a Parr 6400 calorimeter (Parr Instrument Co., Moline, IL, USA). Detailed extraction methods for analysing bark and leaf chemistry can be found in the Supplementary Material (Text S1). In short, carbon (C) and N were measured with a CHN combustion analyser (Rayment and Lyons 2011). Calcium (Ca), copper (Cu), potassium (K), magnesium (Mg) and P concentrations were quantified by acid digestion followed by spectrometry (Martinie and Schilt 1976). Tannins were quantified following Dalzell and Kerven (1998), and lignin, cellulose and ash concentrations were measured with an ADF extraction method (Rowland and Roberts 1994).
Decomposition experiment
Fibreglass litterbags (20 × 15 cm; 1.5–2 mm mesh) were filled with bark particles of ~13 cm2 each, or 1.0 g of intact senesced leaves, and placed in a common garden at Macquarie University Fauna Park, North Ryde (33°46ʹ9ʹʹS, 151°6ʹ46ʹʹE) in January 2013. This site consists mainly of S. glomulifera forest – a characteristic vegetation type of this region (Martyn 2010). Long-term climate data indicate a mean annual rainfall of 1397 mm (Turramurra weather station) and mean annual temperature of 17.3°C (Riverview Observatory; Australian Bureau of Meteorology), i.e. similar to the climate of the collection sites. An overview of the seasonal pattern of rainfall and temperature during our study period is shown in Fig. S1, available as Supplementary Material to this paper.
Litterbags were distributed at random on the cleared forest floor and staked down with 10 cm long nails. Large trees surrounding our plots (semi-)shaded the litterbags for most of the time. We deliberately left the samples uncovered, so natural processes like revegetation and litterfall could continue as per normal. We started the decomposition experiment with 270 bark litterbags (10 species × nine replicates × three retrieval times) and 180 leaf litter bags (10 species × six replicates × three retrieval times). After 3 months one replicate of each species (both for bark and leaf samples) was harvested to obtain a preliminary estimate of the decomposition rate. Based on the latter, we decided to harvest the first batch of leaves after 3.5 months. Since bark decomposition was slower, we left the bark for a later harvest. The two other harvest moments were after 12 and 24 months (bark and leaves). After harvesting, samples were dried at 60°C for ≥10 days until equilibrium mass. After brushing off any dirt, the remaining sample mass was weighed.
Experimental burns
Leaves were burned as described by Grootemaat et al. (2015). Samples were horizontally inserted into an open-doored muffle furnace (Charles Moloney, Sydney, NSW, Australia) set at 400°C. The leaves were held by tongs on the petiole, in a parallel direction to the furnace door. A high frequency electrical spark gun was held ~8 mm above the centre of each sample to provide a source of ignition (Gill and Moore 1996). Three thermocouples (type K, chromel-alumel) were attached to a stainless steel grill perpendicular to the opening of the furnace. When the average temperature value of the three thermocouples was ~400°C (± 10°C) leaf samples were inserted.
A similar set-up was used for the experimental burns of bark, with a few adjustments: the bark samples were inserted by tongs and then left on the grill. The remaining ash was collected in a stainless steel dish (99 × 99 × 18 mm) underneath the grill. However, the mass of the remaining ash was undetectable on a scale with three digits and therefore we considered the burns as complete combustions.
The combustion process was filmed and subsequently analysed by using the digital video editor ‘VideoPad’ (NCH Software, Canberra, ACT, Australia). This allowed us to measure time-to-ignition (TTI) as a proxy for ignitibility, while sustainability was registered both by flame duration (FD) and smoulder duration (SD) (Grootemaat et al. 2015). Combustibility was expressed as initial sample mass (corresponding approximately with total mass burnt) divided by total burning time (sum of FD and SD).
Statistical analyses
We chose to report the percentage mass lost at a given (standard) point in time rather than a k-value (Olson 1963), because (i) there was no exponential trend in our data, which forms the basis of such decomposition models; and (ii) the decomposition constant k averages the decomposition rate over the different stages (months) and is strongly dependent on variations in temperature and moisture content (Woods and Raison 1983). Several other decomposition models have been proposed for leaves and coarse woody debris (Harmon et al. 2004; Adair et al. 2008; Cornwell and Weedon 2014) but they cannot always account for the complexity of the litter, nor for the complex heterotrophic interactions (Facelli and Pickett 1991).
Variance component analyses were used to partition the total variance in decomposability and flammability into within-species and among-species components. Since the variation in bark FD and SD was strongly driven by sample mass (88 and 97% respectively), we standardised by dry mass, and then used these new variables, i.e. ‘FD/mass’ and ‘SD/mass’, in subsequent analyses. For comparison, we also mass-standardised the leaves.
Next, measurements were averaged per species and the flammability parameters and trait measurements were log10-transformed to satisfy the assumptions of normality and homogeneity of variance. Linear regressions were used to compare (i) decomposability (as % mass loss) of leaves and bark, (ii) flammability (as ignitibility, sustainability and combustibility) of leaves and bark, and (iii) decomposability and flammability for a given material. To test for correlations with bark SD/mass we used non-parametric Spearman’s rho because bark SD/mass was not normally distributed. One-way ANOVAs were used to compare the ignitibility and sustainability of the non-shedding stringybarks with the other (smooth) bark types.
Bivariate regressions were used to quantify trait effects on decomposition and flammability of bark and leaves. Wherever appropriate, ANCOVAs were used to test if the slopes from the bivariate regressions differed for bark and leaves. Stepwise multiple linear regressions were used to determine whether the measured traits together could account for the variation in bark and leaf decomposability and flammability. Because of the likelihood of collinearity between certain traits, the variance inflation factor (VIF) was calculated. Results with VIF ≥ 4 are highlighted in the results section.
All statistical analyses were carried out using SPSS Statistics ver. 22.0 (IBM Corp., Armonk, NY, USA).
Results
Bark versus leaf decomposition
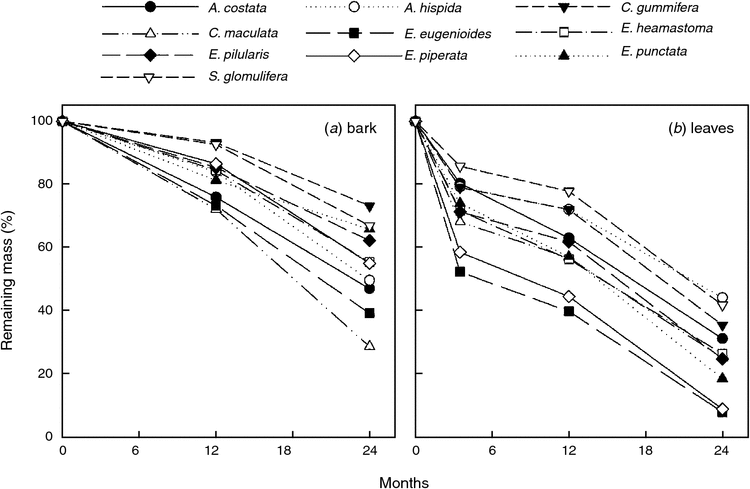
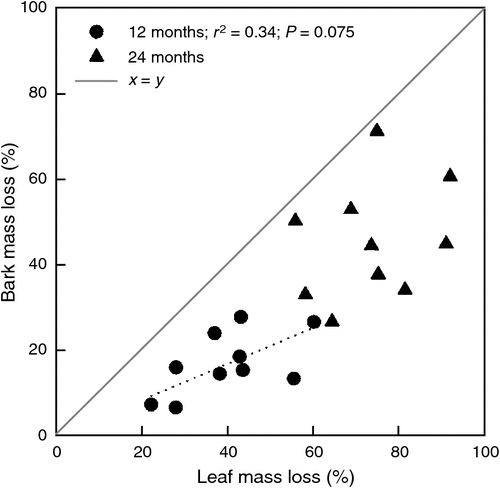
In the first year bark material decomposed much slower than leaves. After 12 months 7–28% of the initial bark mass was lost (species means) compared with 22–60% for leaves (Fig. 2). In the second year the rate of bark decomposition approached that of leaves, which (depending on species) led to 27–72% mass loss for bark and 56–92% mass loss for leaves after 24 months (Fig. 2). Percentage mass loss after 12 and 24 months were strongly correlated (r2 = 0.77 for bark; r2 = 0.92 for leaves). Still, for completeness, we report decomposability as percentage mass loss at both harvest times throughout this paper. After 12 months, there was a weak tendency for leaf decomposition to be correlated with bark decomposition (r2 = 0.34, P = 0.075; Fig. 3), implying that species with easily decomposable leaves also have easily decomposable bark. However, after 24 months there was no relationship at all (P = 0.411).
|
Bark versus leaf flammability
The most widely recognised trait influencing fuel flammability is moisture content. Because all leaf and bark samples were air-dried before the experimental burns, they varied little in moisture content (species means ranging from 11.8 to 14.4% for bark and 7.3 to 10.0% for leaves). Apart from a significant correlation with bark TTI (r2 = 0.40, P = 0.049; Table S2), interspecific variation in fuel moisture content had no strong effects on the measured flammability parameters (all P ≥ 0.05; Table S2; data not shown for leaves). Because all samples were stored under similar conditions, we consider it fair to make the comparison between bark and leaf flammability.
There was considerable variation in bark and leaf flammability, both within and among species (Fig. 4). Overall, bark took longer to ignite than leaves (species means: 2.6–14.0 s versus 1.9–4.2 s, respectively, Fig. 4a). Bark flamed and smouldered for longer than leaves, even after standardisation by mass (Fig. 4b, c) and consequently, bark was less combustible (4.9–7.5 mg s–1) than leaves (9.8–18.3 mg s–1) (Fig. 4d). Bark from the stringybark species (E. eugenioides and S. glomulifera) had shorter ignition times than the bark from the other species (P < 0.001), but the two species-groups did not differ in FD/mass, SD/mass or combustibility (all P ≥ 0.392). Bark from Eucalyptus punctata, which is characterised by very thick slabs, had the longest time-to-ignition and highest SD/mass. This means that although E. punctata bark does not ignite easily, it can still play an important role in wildfires (and their effects) due to its prolonged smouldering. Bark and leaf flammability were unrelated for all four flammability parameters (all P ≥ 0.322). For example, species whose leaves ignited easily and quickly did not necessarily have easily ignitable bark.
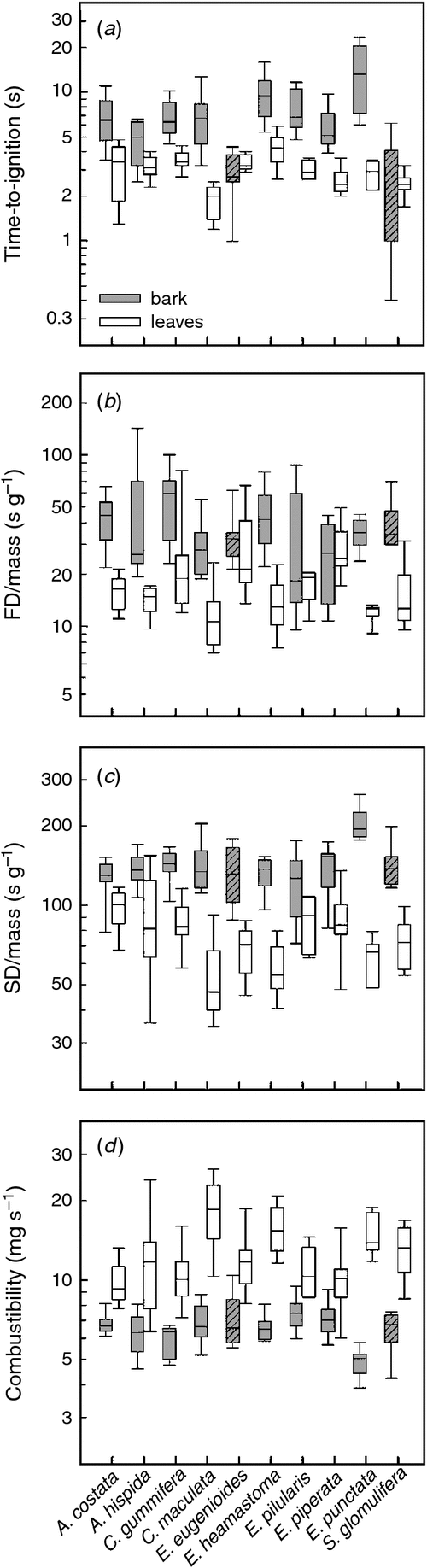
|
Decomposition and flammability explained by different drivers
The rate of bark decomposition (after either 12 or 24 months) was unrelated to any measure of bark flammability, and the same was true for leaf decomposition and flammability (all P ≥ 0.180; Table S3). The relevant traits, associated with the two turnover processes, will be discussed below.
Lignin concentration drives decomposition
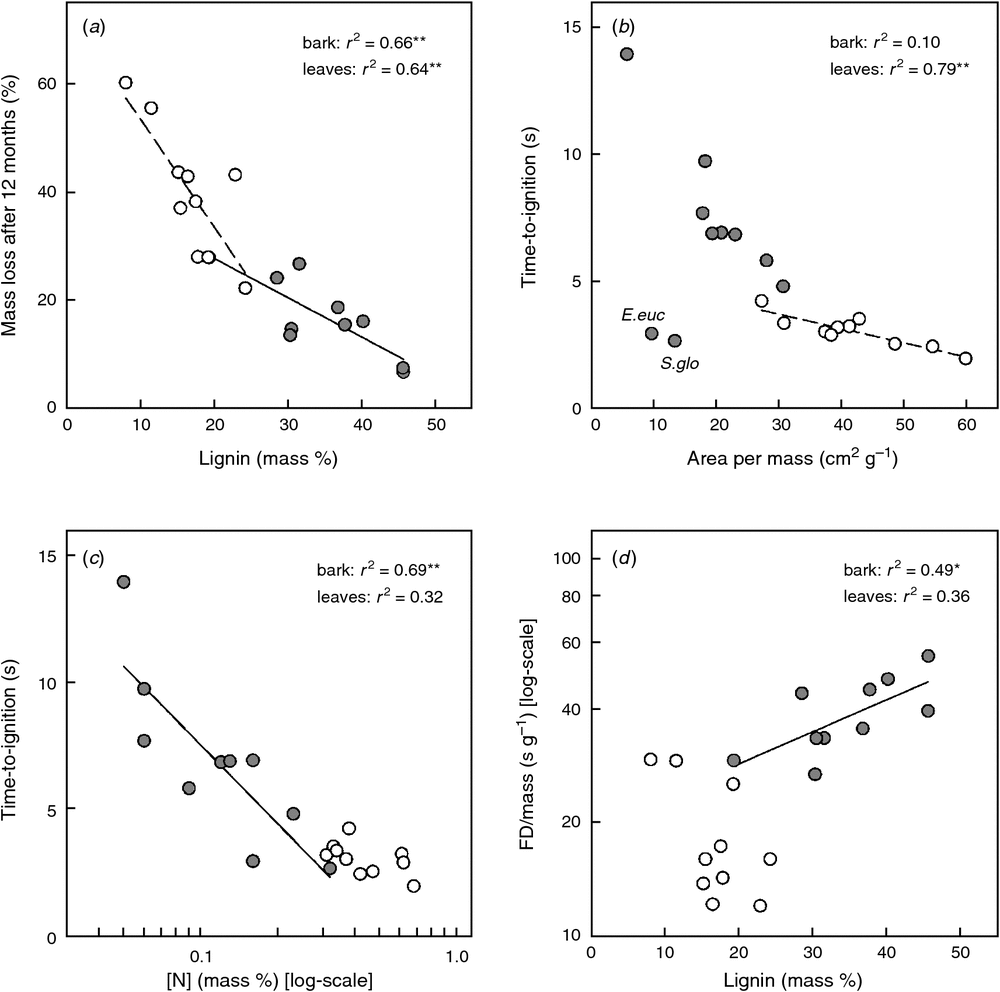
After both 12 and 24 months, initial lignin concentration was the strongest predictor of bark decomposition (r2 = 0.66 and 0.64, respectively, Fig. 5a), followed by cellulose concentration (r2 = 0.57 and 0.43; Table S2). Lignin and tannins are recalcitrant compounds which impede decomposition. Higher values of cellulose, instead, make the plant material more decomposable. After 12 months, the energy content and tissue carbon concentration also accounted for a substantial portion of the variation in bark decomposition (r2 = 0.55 and 0.56; both negatively); but after 24 months these trends were no longer significant. Stepwise multiple regressions showed that tannin concentration accounted for 21% (12 months) and 18% (24 months) of the variation in bark decomposability in addition to the 66 and 64% already accounted for by lignin (Table S4). The high VIF of 11.9 between initial lignin and cellulose concentrations, reflecting that these compounds constitute the main components of woody material, suggests that the relationship between decomposition and lignin also indirectly includes the effects of cellulose on mass loss. However, lignin had a stronger relationship with decomposition than cellulose (both after 12 and 24 months), and was therefore chosen by the stepwise regression.
As for bark, initial lignin concentration was also the most important (negative) driver for leaf decomposition (r2 = 0.64 and 0.55; Fig. 5a) with an additional 27% accounted for by leaf thickness (negative correlation) after 24 months (i.e. total r2 = 0.82; Table S4; lignin concentration and leaf thickness were not correlated). Even though the decomposability of both bark and leaves was driven by initial lignin concentration, the slopes were different (e.g. after 12 months, ANCOVA interaction term P = 0.023; Fig. 5a). Therefore, we conclude that there is no common decomposability-lignin function across tissue types.
Bark flammability is driven by bark area per mass and chemical composition
Several traits were important for the ignitibility, sustainability and combustibility of bark. Bark area per mass (BAM; cm2 g–1), analogous to specific leaf area (SLA; cm2 g–1), did not affect ignitibility when all species were included (Fig. 5b, r2 = 0.10, P = 0.385). However, as already noted, the two stringybark species (E. eugenioides and S. glomulifera) are quite different from the other species in their morphology and ignitibility, and are not classified as bark shedding species. Once these were excluded, BAM showed a strong negative relationship with time-to-ignition of the smooth bark species (r2 = 0.89, P < 0.001). Taking all 10 species into account, [N] was the most important driver of bark ignitibility (Fig. 5c, r2 = 0.69, P = 0.003). At higher N concentrations, bark samples took less time to ignite. Calcium concentration added another 27% (negative correlation) to the explained variance of bark ignitibility (Table S4). Since [Ca] was positively correlated with [Mg] (VIF = 6.651), these results indirectly also include effects of [Mg]. In fact, a similar model built with [N] and [Mg] gave similar but slightly weaker results (total r2 = 0.93, P = 0.001) and the retarding effects of [Ca] and [Mg] on ignitibility can be considered as similar. Higher fuel moisture contents delayed the bark ignition (r2 = 0.40, Table S2), but this moisture effect was not strong enough, or sufficiently independent of the [N] and [Ca] effects to influence the multiple regression model results.
Bark sample mass accounted for 88 and 97% of the variation in flame and smoulder durations respectively. When we standardised FD and SD by dry mass, within-species variance accounted for a far larger proportion than among-species variance (88 and 71% within species, 12 and 29% among species; Table S5). Also, bark combustibility was more variable within than among species (73% vs 27%; Table S5). Thus, despite the clear morphological and chemical differences of the bark from different species, once the samples were alight, the combustibility, flame and smoulder durations varied relatively little among species (at least, compared with variation within any given species).
Variation among species in bark FD/mass was most strongly positively associated with both copper concentration (r2 = 0.54) and lignin concentration (r2 = 0.49; Table S2, Fig. 5d). Phosphorus concentration accounted for 26% on top of the explained variance by [Cu] (Table S4); at a given [Cu], bark samples with higher [P] had shorter flame durations per gram material. Through a correlation with [Cu], i.e. VIF = 5.078, lignin effects on FD/mass are implicitly included through the effects of [Cu].
Calcium concentration and cellulose together accounted for 79% of the variation in combustibility among species (Table S4). Higher [Ca] lowered the combustibility. However, as seen before, the significant effects of [Ca] also include indirect effects of [Mg] since [Ca] and [Mg] are quite strongly correlated.
Drivers of leaf flammability
Specific leaf area was the most important correlate of leaf TTI. Species with higher SLA ignited more quickly (r2 = 0.79, P = 0.001; Table S4; Fig. 5b). Similarly but less markedly than was the case for bark, more variation in leaf FD/mass, SD/mass and combustibility was found within species than among species (Table S5). Leaf FD/mass was negatively correlated with potassium concentration (r2 = 0.44; Table S4). None of the measured leaf traits were correlated with SD/mass or with combustibility. This suggests that standardising by mass explains most variation in leaf SD and combustibility. The more material there is to start with, the longer the smouldering phase will take, and the slower the combustibility.
Discussion
Slow decomposition of bark
Higher lignin concentrations slowed the decomposition of both leaf material (see also Melillo et al. 1982; Adair et al. 2008; Freschet et al. 2012) and bark. The higher initial lignin concentrations in bark compared with leaves might explain the slower decomposition rates of the former, especially in the first year. Other reasons for the lag-phase of bark decomposition could be local nutrient limitation (since bark had lower [N] than leaves; Table S1) and/or priority effects among decomposers (i.e. the decomposers prefer leaves over bark).
The lower decomposability of bark may cause a long-term slow release of nutrients to the forest soil (Lamb 1985; O’Connell 1997; Johnson et al. 2014) and accumulation of fuel for potential fires (Hines et al. 2010). In contrast, the rapid initial decomposition in leaves, likely associated with leaching of water-soluble compounds (Woods and Raison 1983; Berg and McClaugherty 2003) and higher initial concentrations of N and P (Cornwell et al. 2008), releases nutrients relatively quickly. The different rates of bark and leaf decomposition thus provide important information for estimating the carbon and nutrient balance of the forest.
Ignitibility of bark is lower than that of leaves, but bark burns for longer
Previous studies of bark flammability have typically focussed on bark that is still attached to the tree, e.g. quantifying the ability of bark to protect the vascular cambium (Uhl and Kauffman 1990; Pinard and Huffman 1997; Lawes et al. 2011), but our study compared the flammability of bark chunks as components of the litter layer. Bark flammability varied 1.5- to 5.4-fold among the 10 tree species examined in this study. In general, bark took longer to ignite than leaves, and burned more slowly. The sustainability of fire in bark (and leaves) was strongly mass dependent; the more mass available, the longer the flame and smoulder duration, and therefore the lower the combustibility. Thus, the species-specific contribution of bark to the litter layer can be very important for the duration of a surface fire. When high temperatures (≥70°C) prevail, this can lead to extensive thermal damage to the local flora and soil fauna (Neary et al. 1999; Gagnon et al. 2010).
FD/mass and SD/mass varied mostly within species, prompting two observations: (i) despite huge variety in bark morphology and chemistry, once ignited, the flame and smoulder durations among species hardly varied; and (ii) even after standardising by mass, the bark samples still flamed and smouldered longer than the leaf samples. The latter is explained by bark being richer in structural compounds (e.g. lignin; Fig. 5d), which have higher thermal stability and therefore require more time, or higher temperatures, for combustion (Philpot 1970; Di Blasi 2008). In addition, the greater thickness of bark compared with leaves may also have contributed to the lower combustibility by constraining heat supply to the inner parts of bark chunks by means of conduction (Lawes et al. 2011).
Drivers of flammability
The strongest driver of interspecific bark ignitibility in our dataset was N concentration; bark samples with a higher N concentration ignited more quickly. This was contrary to expectation because N, at least in ammonium phosphates, has fire retardant properties (Duquesne et al. 2003). However, we have little understanding of the relationship between [N] and ignitibility when it is part of other chemical compounds.
Leaf ignitibility was strongly correlated with SLA (leaf area per mass) (Murray et al. 2013; Grootemaat et al. 2015). Similarly, at least when the stringybarks were excluded from the analysis, a strong relationship was found between the SLA-analogue BAM (bark area per mass) and ignitibility. This predictive power of surface area per mass could improve fire prediction models that take plant traits into account (e.g. Zylstra et al. 2016).
The remaining interspecific variation in bark FD/mass was mostly correlated with [Cu] and secondly with [P]. Although P has known flame retardant properties (Green 1992; Scarff et al. 2012), we only have an indirect explanation for the apparent flame-prolonging effects of [Cu]. A possible explanation may lie in the correlation of [Cu] with lignin, and lignin has more complex carbohydrate chains which take longer to disintegrate during the depolymerisation phase (pyrolysis) than, for example, cellulose or volatiles (Sullivan and Ball 2012). The lower combustibility of bark at higher concentrations of Ca or Mg agrees with the common perception that the presence of cations, or higher nutrient concentrations in general, promotes the formation of chars during the pyrolysis, at the expense of volatile tar formation. This makes the fuel less flammable (King and Vines 1969; Mak 1982).
Despite the large variation in bark morphological and chemical traits (Paine et al. 2010; Poorter et al. 2014; Rosell et al. 2014), relatively little is known about how this variation affects the ecological functions of bark. Pausas (2015) suggested that fire regimes can explain a large portion of the variance in bark thickness (e.g. thicker bark in ecosystems with frequent low intensity fires), although thin and thick barked plants can co-exist under a given fire regime (Rosell 2016; present study). Most likely, different plant strategies (such as serotiny and resprouting) have evolved as fire survival traits, and thicker bark is only one of them (Pausas 2017).
Bark flammability and spotting
Ignitibility is of special importance for wildfires, since material with shorter ignition times is likely to start or propagate a wildfire more easily, and fire spread can be seen as an accumulation of ignition steps (Rothermel 1972; Grootemaat et al. 2017). Bark ignitibility plays a particularly important role in Australian ecosystems because loose bark may form firebrands (large embers) which can travel through the air and start new fires in unburnt forests far ahead of the actual fire front (so-called ‘spotting’) (Hines et al. 2010; Ellis 2011; Cruz et al. 2012). Stringybarks are known for short to medium distance spotting (up to 4 km) and the shorter ignition times of E. eugenioides and S. glomulifera found in this study could be relevant information for estimating spotting potential. Also, the morphology of ‘ribbon bark’, especially the longitudinal curvature, is a key factor in determining the spotting distance due to the burnout time (FD + SD) of the bark (Hall et al. 2015). Firebrands of ribbon bark have been found to travel tens of kilometres (Cruz et al. 2012). Two of our smooth bark species, E. pilularis and E. piperata, could be classified as ribbon barks. In our study, however, we did not examine the longitudinal curvature, and used samples with different levels of curvature - although we tried to avoid cylinders with multiple rotations of bark. This may explain the larger variation in FD and SD found for these species.
Turnover processes were unrelated, comparing across tissue types
The decomposition rate of bark was unrelated to that of leaves. Similarly, the various flammability properties were unrelated across tissue type. Can we explain this lack of correlation by a lack of functional coordination between plant tissues? For example, there is a general tendency for species with leaf traits promoting fast growth to also have stem and root traits that promote fast growth (Freschet et al. 2010; Reich 2014). Further, Freschet et al. (2012, 2013) found that, as an ‘afterlife’ consequence, the decomposability of leaves, stems and roots were positively correlated. This has given rise to the idea of a ‘fast-slow’ plant economics spectrum (Reich 2014). The present study suggests that this same pattern does not extend to bark versus leaves. Presumably this reflects the very different functions these tissues fulfil. The main function of leaves is carbon acquisition by means of photosynthesis. By contrast, although some species have weakly photosynthetic bark (Aschan and Pfanz 2003; Cernusak and Hutley 2011), the main functions of bark, besides photosynthate transport, are protection from pests and fire, water storage, and biomechanical support (Niklas 1999; Rosell et al. 2014) – functions that are not directly related to growth rate.
Turnover processes were unrelated, within each tissue type
For any given material (i.e. for bark or for leaves), decomposability and flammability were unrelated across our 10 study species. This is a confirmation of our previous findings, for leaves only, for a different set of 32 species (Grootemaat et al. 2015). The results here suggest a similar ‘decoupling’ for bark: at a given bark decomposition rate, a full range of bark ignitibility, fire sustainability and combustibility values is possible. Presumably this lack of correlation reflects the fact that variation in decomposition and flammability are driven by different traits, as quantified in this study.
Concluding remarks
This study examined (mostly) bark shedding species, because of the substantial contribution of bark to the litter layer and fuel load in Australian forests. In most forests there will be a mix of relatively fast and slow decomposing species, and species with high and low flammability characteristics. This leads to variation in litter turnover rates and litter accumulation, both for bark and for leaves. The contribution of bark of some species to the litter layer in dry sclerophyll forests can be substantial (up to ~45%; McColl 1966; Lamb 1985). Combined with differences in decomposability and flammability, our findings suggest that it is important to consider the species composition of the litter, and relative contribution of leaf and bark tissues, when estimating carbon and nutrient loss rates, fuel loads and fire risks.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgements
This study was possible thanks to permission of the NSW National Park and Wildlife Service. A special thanks goes out to Allyson Eller who helped us with the experimental design and fabrication of the litterbags. David Appleton performed the chemical analyses. We are grateful that we could use part of the Macquarie University fauna park for our decomposition experiment for such an extended period of time. This project was funded by a Scholarship from Macquarie University to SG and by ARC funding to IJW (FT100100910).
References
ABARES (2016) ‘Australian forest profiles: Australia’s forests.’ (Australian Bureau of Agricultural and Resource Economics and Sciences: Canberra, ACT)Adair EC, Parton WJ, Del Grosso SJ, Silver WL, Harmon ME, Hall SA, Burke IC, Hart SC (2008) Simple three-pool model accurately describes patterns of long-term litter decomposition in diverse climates. Global Change Biology 14, 2636–2660.
Anderson HE (1970) Forest fuel ignitibility. Fire Technology 6, 312–319.
| Forest fuel ignitibility.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE3MXhsVent7g%3D&md5=212bdfbd63ac6e0460dfe8e198dedcfcCAS |
Aschan G, Pfanz H (2003) Non-foliar photosynthesis – a strategy of additional carbon acquisition. Flora – Morphology, Distribution, Functional Ecology of Plants 198, 81–97.
| Non-foliar photosynthesis – a strategy of additional carbon acquisition.Crossref | GoogleScholarGoogle Scholar |
Berg B, McClaugherty C (2003) ‘Plant litter: decomposition, humus formation, carbon sequestration.’ (Springer: Heidelberg, Germany)
Bond WJ, Midgley JJ (1995) Kill thy neighbour: an individualistic argument for the evolution of flammability. Oikos 73, 79–85.
| Kill thy neighbour: an individualistic argument for the evolution of flammability.Crossref | GoogleScholarGoogle Scholar |
Booth TH (2013) Eucalypt plantations and climate change. Forest Ecology and Management 301, 28–34.
| Eucalypt plantations and climate change.Crossref | GoogleScholarGoogle Scholar |
Brooker MIH, Kleinig DA (1990) ‘Field guide to eucalypts: south-eastern Australia.’ (Inkata Press: Melbourne, Vic.)
Cernusak LA, Hutley LB (2011) Stable isotopes reveal the contribution of corticular photosynthesis to growth in branches of Eucalyptus miniata. Plant Physiology 155, 515–523.
| Stable isotopes reveal the contribution of corticular photosynthesis to growth in branches of Eucalyptus miniata.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXksFagtLw%3D&md5=1e5e3e83a5c42377690f01815ff94844CAS |
Cornwell WK, Weedon JT (2014) Decomposition trajectories of diverse litter types: a model selection analysis. Methods in Ecology and Evolution 5, 173–182.
| Decomposition trajectories of diverse litter types: a model selection analysis.Crossref | GoogleScholarGoogle Scholar |
Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Pérez-Harguindeguy N, et al (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecology Letters 11, 1065–1071.
| Plant species traits are the predominant control on litter decomposition rates within biomes worldwide.Crossref | GoogleScholarGoogle Scholar |
Cornwell WK, Cornelissen JHC, Allison SD, Bauhus J, Eggleton P, Preston CM, Scarff F, Weedon JT, Wirth C, Zanne AE (2009) Plant traits and wood fates across the globe: rotted, burned, or consumed? Global Change Biology 15, 2431–2449.
| Plant traits and wood fates across the globe: rotted, burned, or consumed?Crossref | GoogleScholarGoogle Scholar |
Crockford RH, Richardson DP (1998) Litterfall, litter and associated chemistry in a dry sclerophyll eucalypt forest and a pine plantation in south-eastern Australia: 1. Litterfall and litter. Hydrological Processes 12, 365–384.
| Litterfall, litter and associated chemistry in a dry sclerophyll eucalypt forest and a pine plantation in south-eastern Australia: 1. Litterfall and litter.Crossref | GoogleScholarGoogle Scholar |
Cruz M, Sullivan A, Gould J, Sims N, Bannister A, Hollis J, Hurley R (2012) Anatomy of a catastrophic wildfire: the Black Saturday Kilmore East fire in Victoria, Australia. Forest Ecology and Management 284, 269–285.
| Anatomy of a catastrophic wildfire: the Black Saturday Kilmore East fire in Victoria, Australia.Crossref | GoogleScholarGoogle Scholar |
Dalzell SA, Kerven GL (1998) A rapid method for the measurement of Leucaena spp. proanthocyanidins by the proanthocyanidin (butanol/HCl) assay. Journal of the Science of Food and Agriculture 78, 405–416.
| A rapid method for the measurement of Leucaena spp. proanthocyanidins by the proanthocyanidin (butanol/HCl) assay.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXnvVejsLY%3D&md5=d2f149679eb61c9a34ff0b28bc833c8cCAS |
Di Blasi C (2008) Modeling chemical and physical processes of wood and biomass pyrolysis. Progress in Energy and Combustion Science 34, 47–90.
| Modeling chemical and physical processes of wood and biomass pyrolysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsVCksbjN&md5=432db5d547ad16ec93c026578df40de6CAS |
Duquesne S, Le Bras M, Delobel R, Camino G, Gengembre L (2003) X-ray photoelectron spectroscopy study of the ammonium polyphosphate – polyurethane system used as fire-retardant additive in EVA. Journal of Fire Sciences 21, 89–115.
| X-ray photoelectron spectroscopy study of the ammonium polyphosphate – polyurethane system used as fire-retardant additive in EVA.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXltlKht7w%3D&md5=1d9d7d626c646977d8ab36dd6f9c35d5CAS |
Ellis PFM (2011) Fuelbed ignition potential and bark morphology explain the notoriety of the eucalypt messmate ‘stringybark’ for intense spotting. International Journal of Wildland Fire 20, 897–907.
| Fuelbed ignition potential and bark morphology explain the notoriety of the eucalypt messmate ‘stringybark’ for intense spotting.Crossref | GoogleScholarGoogle Scholar |
Facelli J, Pickett SA (1991) Plant litter: its dynamics and effects on plant community structure. Botanical Review 57, 1–32.
| Plant litter: its dynamics and effects on plant community structure.Crossref | GoogleScholarGoogle Scholar |
Frejaville T, Curt T, Carcaillet C (2013) Bark flammability as a fire-response trait for subalpine trees. Frontiers in Plant Science 4, 1–8.
| Bark flammability as a fire-response trait for subalpine trees.Crossref | GoogleScholarGoogle Scholar |
Freschet GT, Cornelissen JHC, van Logtestijn RSP, Aerts R (2010) Substantial nutrient resorption from leaves, stems and roots in a subarctic flora: what is the link with other resource economics traits? New Phytologist 186, 879–889.
| Substantial nutrient resorption from leaves, stems and roots in a subarctic flora: what is the link with other resource economics traits?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXotVWhur8%3D&md5=1164ae4ca22bcce3dc0e2e89aca23e17CAS |
Freschet GT, Aerts R, Cornelissen JHC (2012) A plant economics spectrum of litter decomposability. Functional Ecology 26, 56–65.
| A plant economics spectrum of litter decomposability.Crossref | GoogleScholarGoogle Scholar |
Freschet GT, Cornwell WK, Wardle DA, Elumeeva TG, Liu W, Jackson BG, Onipchenko VG, Soudzilovskaia NA, Tao J, Cornelissen JHC (2013) Linking litter decomposition of above- and below-ground organs to plant–soil feedbacks worldwide. Journal of Ecology 101, 943–952.
| Linking litter decomposition of above- and below-ground organs to plant–soil feedbacks worldwide.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtVaks7fI&md5=b00452c568507afa3b18fd6366875ce7CAS |
Gagnon PR, Passmore HA, Platt WJ, Myers JA, Paine CET, Harms KE (2010) Does pyrogenicity protect burning plants? Ecology 91, 3481–3486.
| Does pyrogenicity protect burning plants?Crossref | GoogleScholarGoogle Scholar |
Gill AM, Ashton DH (1968) The role of bark type in relative tolerance to fire of three central Victorian eucalypts. Australian Journal of Botany 16, 491–498.
| The role of bark type in relative tolerance to fire of three central Victorian eucalypts.Crossref | GoogleScholarGoogle Scholar |
Gill AM, Moore PHR (1996) ‘Ignitibility of leaves of Australian plants.’ (CSIRO Division of Plant Industry: Canberra, ACT)
Gould JS, Lachlan McCaw W, Phillip Cheney N (2011) Quantifying fine fuel dynamics and structure in dry eucalypt forest (Eucalyptus marginata) in Western Australia for fire management. Forest Ecology and Management 262, 531–546.
| Quantifying fine fuel dynamics and structure in dry eucalypt forest (Eucalyptus marginata) in Western Australia for fire management.Crossref | GoogleScholarGoogle Scholar |
Green J (1992) A review of phosphorus-containing flame retardants. Journal of Fire Sciences 10, 470–487.
| A review of phosphorus-containing flame retardants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXkt12jt70%3D&md5=3c08d5360d86d6f20659a99801b98f43CAS |
Grootemaat S, Wright IJ, van Bodegom PM, Cornelissen JHC, Cornwell WK (2015) Burn or rot: leaf traits explain why flammability and decomposability are decoupled across species. Functional Ecology 29, 1486–1497.
| Burn or rot: leaf traits explain why flammability and decomposability are decoupled across species.Crossref | GoogleScholarGoogle Scholar |
Grootemaat S, Wright IJ, van Bodegom PM, Cornelissen JH (2017) Scaling up flammability from individual leaves to fuel beds. Oikos
| Scaling up flammability from individual leaves to fuel beds.Crossref | GoogleScholarGoogle Scholar |
Hall J, Ellis PF, Cary GJ, Bishop G, Sullivan AL (2015) Long-distance spotting potential of bark strips of a ribbon gum (Eucalyptus viminalis). International Journal of Wildland Fire 24, 1109–1117.
| Long-distance spotting potential of bark strips of a ribbon gum (Eucalyptus viminalis).Crossref | GoogleScholarGoogle Scholar |
Harmon ME, Franklin JF, et al. (2004) Ecology of coarse woody debris in temperate ecosystems. In ‘Advances in ecological research. Vol. 34’. (Ed. H Caswell) pp. 59–234. (Academic Press: New York)
Hines F, Tolhurst KG, Wilson AAG, McCarthy GJ (2010) ‘Overall fuel hazard assessment guide.’ (4th edn) (Victorian Government, Department of Sustainability and Environment: Melbourne, Vic.)
Jacobs MR (1955) ‘Growth habits of the eucalypts.’ (Forestry and Timber Bureau, Commonwealth of Australia: Canberra, ACT)
Johnson CE, Siccama TG, Denny EG, Koppers MM, Vogt DJ (2014) In situ decomposition of northern hardwood tree boles: decay rates and nutrient dynamics in wood and bark. Canadian Journal of Forest Research 44, 1515–1524.
| In situ decomposition of northern hardwood tree boles: decay rates and nutrient dynamics in wood and bark.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhvVKru7%2FL&md5=7da48b72949fe91338c6c5a2c63802c1CAS |
King NK, Vines RG (1969) ‘Variation in the flammability of the leaves of some Australian forest species.’ (CSIRO Division of Applied Chemistry: Melbourne, Vic.)
Lamb R (1985) Litter fall and nutrient turnover in two eucalypt woodlands. Australian Journal of Botany 33, 1–14.
| Litter fall and nutrient turnover in two eucalypt woodlands.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXhtlWjtbk%3D&md5=25296a6b74fe8e8f9c24ae076641898fCAS |
Lawes M, Richards A, Dathe J, Midgley J (2011) Bark thickness determines fire resistance of selected tree species from fire-prone tropical savanna in north Australia. Plant Ecology 212, 2057–2069.
| Bark thickness determines fire resistance of selected tree species from fire-prone tropical savanna in north Australia.Crossref | GoogleScholarGoogle Scholar |
Leishman MR, Thomson VP (2005) Experimental evidence for the effects of additional water, nutrients and physical disturbance on invasive plants in low fertility Hawkesbury Sandstone soils, Sydney, Australia. Journal of Ecology 93, 38–49.
| Experimental evidence for the effects of additional water, nutrients and physical disturbance on invasive plants in low fertility Hawkesbury Sandstone soils, Sydney, Australia.Crossref | GoogleScholarGoogle Scholar |
Mak EHT (1982) The relationship between the nutrient status and flammability of forest fuels. PhD thesis. Department of Forestry, Australian National University, Canberra, ACT.
Makkonen M, Berg MP, Handa IT, Hättenschwiler S, van Ruijven J, van Bodegom PM, Aerts R (2012) Highly consistent effects of plant litter identity and functional traits on decomposition across a latitudinal gradient. Ecology Letters 15, 1033–1041.
| Highly consistent effects of plant litter identity and functional traits on decomposition across a latitudinal gradient.Crossref | GoogleScholarGoogle Scholar |
Martinie GD, Schilt AA (1976) Wet oxidation efficiencies of perchloric acid mixtures for various organic substances and the identities of residual matter. Analytical Chemistry 48, 70–74.
| Wet oxidation efficiencies of perchloric acid mixtures for various organic substances and the identities of residual matter.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE28XltlKgsA%3D%3D&md5=fa9af402abc612eee6e29d76a85c1c61CAS |
Martyn J (2010) ‘Field guide to the bushland of the Lane Cove Valley.’ (STEP Incorporated, Turramurra, NSW, Australia)
McColl JG (1966) Accession and decomposition of litter in spotted gum forests. Australian Forestry 30, 191–198.
| Accession and decomposition of litter in spotted gum forests.Crossref | GoogleScholarGoogle Scholar |
Melillo JM, Aber JD, Muratore JF (1982) Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63, 621–626.
| Nitrogen and lignin control of hardwood leaf litter decomposition dynamics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL38XkvFWjurk%3D&md5=4128d4a4c805f784071074c5cc978784CAS |
Millett M (1969) ‘Australian Eucalyptus.’ (Periwinkle Books, Melbourne, Vic., Australia)
Murray BR, Hardstaff LK, Phillips ML (2013) Differences in leaf flammability, leaf traits and flammability–trait relationships between native and exotic plant species of dry sclerophyll forest. PLoS One 8, e79205
| Differences in leaf flammability, leaf traits and flammability–trait relationships between native and exotic plant species of dry sclerophyll forest.Crossref | GoogleScholarGoogle Scholar |
Neary DG, Klopatek CC, DeBano LF, Ffolliott PF (1999) Fire effects on belowground sustainability: a review and synthesis. Forest Ecology and Management 122, 51–71.
| Fire effects on belowground sustainability: a review and synthesis.Crossref | GoogleScholarGoogle Scholar |
Niklas KJ (1999) The mechanical role of bark. American Journal of Botany 86, 465–469.
| The mechanical role of bark.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MnhtVKjsw%3D%3D&md5=e8c40d20ed5ba86d85c7b3e9ee1593e8CAS |
O’Connell AM (1997) Decomposition of slash residues in thinned regrowth eucalypt forest in Western Australia. Journal of Applied Ecology 34, 111–122.
| Decomposition of slash residues in thinned regrowth eucalypt forest in Western Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXjs1Sisbw%3D&md5=32153c3b3956798a00829b0f8e4fd5e0CAS |
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44, 322–331.
| Energy storage and the balance of producers and decomposers in ecological systems.Crossref | GoogleScholarGoogle Scholar |
Paine CET, Stahl C, Courtois EA, Patiño S, Sarmiento C, Baraloto C (2010) Functional explanations for variation in bark thickness in tropical rain forest trees. Functional Ecology 24, 1202–1210.
| Functional explanations for variation in bark thickness in tropical rain forest trees.Crossref | GoogleScholarGoogle Scholar |
Pausas JG (2015) Bark thickness and fire regime. Functional Ecology 29, 315–327.
| Bark thickness and fire regime.Crossref | GoogleScholarGoogle Scholar |
Pausas JG (2017) Bark thickness and fire regime: another twist. New Phytologist 213, 13–15.
| Bark thickness and fire regime: another twist.Crossref | GoogleScholarGoogle Scholar |
Philpot CW (1970) Influence of mineral content on the pyrolysis of plant materials. Forest Science 16, 461–471.
Pinard MA, Huffman J (1997) Fire resistance and bark properties of trees in a seasonally dry forest in eastern Bolivia. Journal of Tropical Ecology 13, 727–740.
| Fire resistance and bark properties of trees in a seasonally dry forest in eastern Bolivia.Crossref | GoogleScholarGoogle Scholar |
Poorter L, McNeil A, Hurtado V-H, Prins HHT, Putz FE (2014) Bark traits and life-history strategies of tropical dry- and moist forest trees. Functional Ecology 28, 232–242.
| Bark traits and life-history strategies of tropical dry- and moist forest trees.Crossref | GoogleScholarGoogle Scholar |
Rayment GE, Lyons DJ (2011) ‘Soil chemical methods: Australasia.’ (CSIRO Publishing: Melbourne, Vic.)
Reich PB (2014) The world-wide ‘fast–slow’ plant economics spectrum: a traits manifesto. Journal of Ecology 102, 275–301.
| The world-wide ‘fast–slow’ plant economics spectrum: a traits manifesto.Crossref | GoogleScholarGoogle Scholar |
Robinson L (2003) ‘Field guide to the native plants of Sydney.’ (Kangaroo Press, Cammeray, NSW, Australia)
Rosell JA (2016) Bark thickness across the angiosperms: more than just fire. New Phytologist 211, 90–102.
| Bark thickness across the angiosperms: more than just fire.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XoslOqt7Y%3D&md5=b1c49481db0320f1618cb84e5c7fb7ceCAS |
Rosell JA, Gleason S, Méndez-Alonzo R, Chang Y, Westoby M (2014) Bark functional ecology: evidence for tradeoffs, functional coordination, and environment producing bark diversity. New Phytologist 201, 486–497.
| Bark functional ecology: evidence for tradeoffs, functional coordination, and environment producing bark diversity.Crossref | GoogleScholarGoogle Scholar |
Rothermel RC (1972) A mathematical model for predicting fire spread in wildland fuels. Research Paper INT-115. USDA, Forest Service, Ogden, UT, USA.
Rowland AP, Roberts JD (1994) Lignin and cellulose fractionation in decomposition studies using acid‐detergent fibre methods. Communications in Soil Science and Plant Analysis 25, 269–277.
| Lignin and cellulose fractionation in decomposition studies using acid‐detergent fibre methods.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXitFGqtrw%3D&md5=3e51da322dc6127a29364a81ce0e7578CAS |
Scarff FR, Gray BF, Westoby M (2012) Exploring phosphate effects on leaf flammability using a physical chemistry model. International Journal of Wildland Fire 21, 1042–1051.
| Exploring phosphate effects on leaf flammability using a physical chemistry model.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhslKrsbbF&md5=bd96a0174f1ed9037104aa7b882091deCAS |
Sullivan AL, Ball R (2012) Thermal decomposition and combustion chemistry of cellulosic biomass. Atmospheric Environment 47, 133–141.
| Thermal decomposition and combustion chemistry of cellulosic biomass.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs1KrtbzI&md5=24df974dfd4307ec963f15cacc3f6d47CAS |
Uhl C, Kauffman JB (1990) Deforestation, fire susceptibility, and potential tree responses to fire in the eastern Amazon. Ecology 71, 437–449.
| Deforestation, fire susceptibility, and potential tree responses to fire in the eastern Amazon.Crossref | GoogleScholarGoogle Scholar |
Woods PV, Raison RJ (1983) Decomposition of litter in sub-alpine forests of Eucalyptus delegatensis, E. pauciflora and E. dives. Australian Journal of Ecology 8, 287–299.
| Decomposition of litter in sub-alpine forests of Eucalyptus delegatensis, E. pauciflora and E. dives.Crossref | GoogleScholarGoogle Scholar |
Zylstra P, Bradstock RA, Bedward M, Penman TD, Doherty MD, Weber RO, Gill AM, Cary GJ (2016) Biophysical mechanistic modelling quantifies the effects of plant traits on fire severity: species, not surface fuel loads, determine flame dimensions in eucalypt forests. PLoS One 11, e0160715
| Biophysical mechanistic modelling quantifies the effects of plant traits on fire severity: species, not surface fuel loads, determine flame dimensions in eucalypt forests.Crossref | GoogleScholarGoogle Scholar |