Enteric methane emissions in response to ruminal inoculation of Propionibacterium strains in beef cattle fed a mixed diet
D. Vyas A , A. Alazzeh A D , S. M. McGinn A , T. A. McAllister A , O. M. Harstad B , H. Holo C and K. A. Beauchemin A EA Agriculture and Agri-Food Canada, Lethbridge Research Centre, Lethbridge, Alberta T1J 4B1, Canada.
B Department of Animal and Aquacultural Sciences, Norwegian University of Life Sciences, Ås, Norway.
C Department of Chemistry, Biotechnology and Food Science, Norwegian University of Life Sciences, Ås, Norway.
D Present address: Department of Clinical Nutrition, Faculty of Applied Medical Sciences, University of Hail, Hail, Saudi Arabia.
E Corresponding author. Email: karen.beauchemin@agr.gc.ca
Animal Production Science 56(7) 1035-1040 https://doi.org/10.1071/AN14801
Submitted: 9 September 2014 Accepted: 4 November 2014 Published: 20 February 2015
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
The objective of this study was to test the efficacy of Propionibacterium strains to mitigate enteric methane (CH4) emissions in beef heifers fed a mixed diet. An experiment was conducted with 16 ruminally cannulated beef heifers fed a basal diet consisting of 60 : 40 barley silage : barley grain (DM basis). Treatments included: (1) Control, (2) Propionibacterium freudenreichii T114, (3) P. thoenii T159, and (4) P. freudenreichii T54. Strains (1 × 1011 colony forming units) were administered daily directly into the rumen before feeding. No treatment effects were observed for DM intake (P = 0.90), mean ruminal pH (P = 0.50) and total volatile fatty acids (P = 0.44). However, compared with the Control, proportions of individual volatile fatty acids changed with acetate being less with Propionibacterium T159 (P = 0.02), whereas ruminal isobutyrate (P < 0.01) and acetate : propionate ratio (P = 0.04) were greater with Propionibacterium T114. Total daily enteric CH4 production averaged 188 g/day and was not affected by Propionbacterium strains (P = 0.51). Methane yield averaged 22 g/kg of DMI intake and tended to be greater with Propionibacterium strains (P = 0.08). The relative abundance of total Propionibacteria was greater with the inoculation of Propionibacterium T159 relative to the Control heifers (P = 0.04). In conclusion, inoculation of Propionibacterium T159 decreased ruminal acetate proportion and Propionibacterium T114 increased acetate : propionate ratio. However, inoculated strains failed to lower total CH4 emissions possibly due to the inability of Propionibacterium strains to elevate ruminal propionate concentrations.
Additional keywords: beef, methane, Propionibacterium.
Introduction
Some strains of Propionibacteria are natural propionate producers that inhabit the rumen and comprise 1.4% to 4.3% of the total microbial population (Mead and Jones 1981). The development of Propionibacterium strains as direct-fed microbials could offer an effective means of increasing ruminal propionate production and reducing enteric methane (CH4) emissions from cattle fed forage-based diets (Jeyanathan et al. 2014). Previous studies explored the potential to use Propionibacterium strains to mitigate CH4 emissions in beef cattle fed high-forage (Vyas et al. 2014a) and high-grain diets (Vyas et al. 2014b). However, no effects were observed on total CH4 emissions due to low persistency of the inoculated strains. Contrary to the previous in vivo studies (Vyas et al. 2014a, 2014b), in vitro batch culture studies with Propionibacterium strains showed promising results with significant reduction in CH4 production using both high-forage and high-grain diets (Alazzeh et al. 2013). The discrepancy between studies might be related to different strains of Propionibacteria used in the in vitro batch culture experiment compared with the in vivo study. The efficacy of Propionibacterium strains identified using an in vitro batch culture experiment as having CH4 mitigation potential (Alazzeh et al. 2013) needs to be validated in vivo before such a strategy can be recommended for lowering CH4 emissions from beef cattle. Hence, the primary objective of this study was to confirm in vivo the efficacy of Propionibacterium strains previously identified in vitro as having the potential to mitigate CH4 emissions in beef cattle.
Materials and methods
Animal, diets and experimental design
The protocol for the study was approved by Lethbridge Research Centre Animal Care Committee before the experiment began and animals were cared for according to the guidelines of the Canadian Council on Animal Care (1997). Sixteen ruminally cannulated crossbred beef heifers were used in this study. The heifers were grouped on the basis of pre-experimental bodyweight (mean ± s.d.: Group 1 = 602 ± 31 kg, Group 2 = 570 ± 60 kg, Group 3 = 590 ± 50 kg and Group 4 = 620 ± 27 kg). Dietary treatments included: (1) Control, (2) Propionibacterium freudenreichii T114, (3) P. thoenii T159, and (4) P. freudenreichii T54. Strains were grown daily in sodium lactate broth under anaerobic conditions according to the method described earlier (Alazzeh et al. 2013) and were administered daily (1 × 1011 colony forming units) at the time of feeding directly into the rumen. Treatments were randomly allotted within each group. All heifers were fed the basal diet (60 : 40 forage to concentrate [(dry matter (DM) basis); Table 1] formulated to provide adequate metabolisable energy and protein for 600 kg growing beef cattle with an average daily gain of 1 kg/day (NRC 2000). Heifers were fed for ad libitum intake once daily at 1300 hours, housed in a ventilated tie-stall barn, and exercised daily in an open dry lot. Diets were supplemented with melengesterol acetate (1.3 mg/head.day; MGA-100 premix, Pfizer Animal Health, Pfizer Canada Inc., Kirkland, QC, Canada) to suppress oestrus and prevent ovulation in the beef heifers.

|
Data and sample collection
During the experiment, Days 1 to Day 14 were used to adapt heifers to their treatments. Ruminal contents were collected on Day 15 and Day 18 at 0, 3, 6 and 9 h post feeding. Ruminal pH was measured continuously from Day 15 to Day 21, and enteric CH4 emissions were measured from Day 19 to Day 21. Daily intakes and orts of the diets for individual heifers were recorded. Diets and orts were sampled daily during days of CH4 measurement and were pooled for each animal at the end of the period of CH4 measurement. Dietary ingredients were sampled once weekly and analysed for DM by drying at 55°C for 72 h. Ingredients and total mixed ration samples were stored at −20°C until analysed.
Methane emissions were measured from individual heifers for 3 days using environmental chambers as described earlier (Beauchemin and McGinn 2006). Briefly, chambers were calibrated before and after each period by sequentially releasing 0, 0.2, and 0.4 L/min of CH4 (Praxair Canada Inc., Mississauga, ON, Canada) separately into each empty chamber using a mass-flow meter (Omega Engineering, Stamford, CT, USA). A 3-point regression was developed by plotting actual against calculated CH4 emission (R2 = 0.99). The slopes of these best fit linear relationships were used to correct for between-chamber variability. Conditions of air circulation and sampling procedures were as described by Avila-Stagno et al. (2013).
To determine the effect of Propionibacteria on ruminal pH, daily pH profiles were measured starting at feeding on Day 15 using an indwelling pH data acquisition system (LRC pH dataloggers, Dascor, Escondido, CA, USA) that was retained in the rumen for 7 days (includes the period of CH4 measurement). The system was standardised using pH 4 and 7 buffers before insertion on the first day and then upon removal on the last day as described earlier (Penner et al. 2006). On Days 15 and 18, at 0, 3, 6, and 9 h post feeding, ruminal contents were sampled from four different sites (cranial, caudal, ventral, and dorsal sacs), composited and strained through a double layer of polyester monofilament fabric (Pecap 7–1180/59, mesh opening 1180 µm, Tetko Inc., Scarborough, ON, Canada). Two samples of filtered ruminal fluid (5 mL) were preserved by adding 1 mL of 25% (wt/vol) phosphoric acid for volatile fatty acids (VFA) and lactate determination, and 1 mL of 1% (wt/vol) sulfuric acid for ammonia-N (NH3-N) determination. The samples were stored at −20°C until analysed.
Rumen samples collected at 0, 3, and 9 h were processed for microbial analysis, separately for each heifer. Microbial pellet was extracted based on a method described earlier (Vyas et al. 2014a). Quantitative real-time PCR assays were performed with a 7900 HT Fast Real-time PCR system (Applied Biosystems, Foster City, CA, USA) using POWER SYBR Green PCR Master Mix (Applied Biosystems, Warrington, UK), forward and reverse primers (500 nM of each primer/reaction), and ~20 ng of template DNA in a final volume of 25 μL per reaction. The primers for universal bacteria (forward primer: 5ʹ-TCCTACGGGAGGCAGCAGT-3ʹ; reverse primer: 5ʹ-GGACTACCAGGGTATCTAATCCTGTT-3ʹ; Nadkarni et al. 2002) and total Propionibacteria (forward primer: 5ʹ-RGTGGCGAAGGCGGTTCTCTGGA-3ʹ; reverse primer: 5ʹ-TGRGGTCGAGTTGCAGACCCCAAT-3ʹ; Rossi et al. 1999) were used. Amplifications were performed under the conditions described earlier (Vyas et al. 2014a). The relative population size of total Propionibacteria was determined as the ratio of the amplification of total Propionibacteria 16S rRNA to the amplification of the universal bacteria. PCR efficiency was calculated using the formula E = [10(–1/slope) – 1]. The slopes ranged from –3.37 to –3.40 for total bacterial primer and –3.29 to –3.30 for total Propionibacteria.
Laboratory analyses
Dry matter for all samples was determined by oven drying at 55°C for 72 h. Dried samples were ground in a Wiley mill (A. H. Thomas, Philadelphia, PA, USA) through a 1-mm screen. Analytical DM content of the ground sample was determined by drying at 135°C for 2 h (method 930.15; AOAC 2005), followed by hot weighing. The organic matter content was calculated as the difference between 100 and the percentage ash (method 942.05; AOAC 2005). The neutral detergent fibre and acid detergent fibre contents were determined according to Van Soest et al. (1991) with heat stable amylase and sodium sulfite used in the neutral detergent fibre procedure. Samples were ground using a ball mill (Mixer Mill MM2000, Tetsch, Haan, Germany) for the determination of crude protein. Total N was quantified by flash combustion and thermal conductivity detection (Carlo Erba Instuments, Milan, Italy). Ruminal VFA, lactate and NH3-N concentration were quantified as described earlier (Vyas et al. 2014a). Gross energy concentration was determined using a bomb calorimeter (model E2k, CAL2k, Johannesburg, South Africa).
Statistical analyses
The data were analysed using the MIXED procedure of SAS with heifer as the experimental unit. For data that were collected serially [DM intake (DMI), CH4, and ruminal fermentation] the model included the fixed effect of treatment, sampling time and their interaction, with sampling time considered as a REPEATED effect in the model. Group was used in the RANDOM statement. Variance components were estimated by the restricted maximum likelihood method. Kenward–Roger’s option was used in the MODEL statement to estimate denominator degrees of freedom. Time-series covariance structure was modelled using the options of autoregressive order-one, compound symmetry, and unstructured order-one. Best time-series covariance structure for each variable was selected based on lowest Akaike and Bayesian information criteria. CONTRAST statement was used to evaluate differences between means of Control and Propionibacterium treatments. Data are presented as least-squares means ± standard error of the means. Statistical significance was declared at P ≤ 0.05 and trends are discussed at P ≤ 0.10.
Results
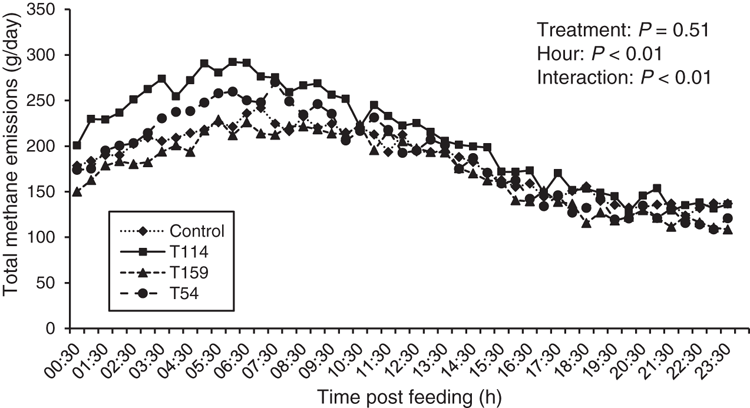
No treatment effects were observed on DMI (P = 0.90; Table 2) or ruminal pH variables. Likewise, total VFA production was similar across all treatments (P = 0.44; Table 3). Ruminal acetate proportion was reduced with Propionibacterium T159 (P = 0.02) whereas no effects were observed with other strains. Correspondingly, no treatment effects were observed on proportion of ruminal propionate (P = 0.12). The proportion of ruminal isobutyrate (P < 0.01) and acetate : propionate ratio was increased (P = 0.04) with Propionibacterium T114. Ruminal NH3-N concentration was similar for all the treatments (P = 0.79).

|
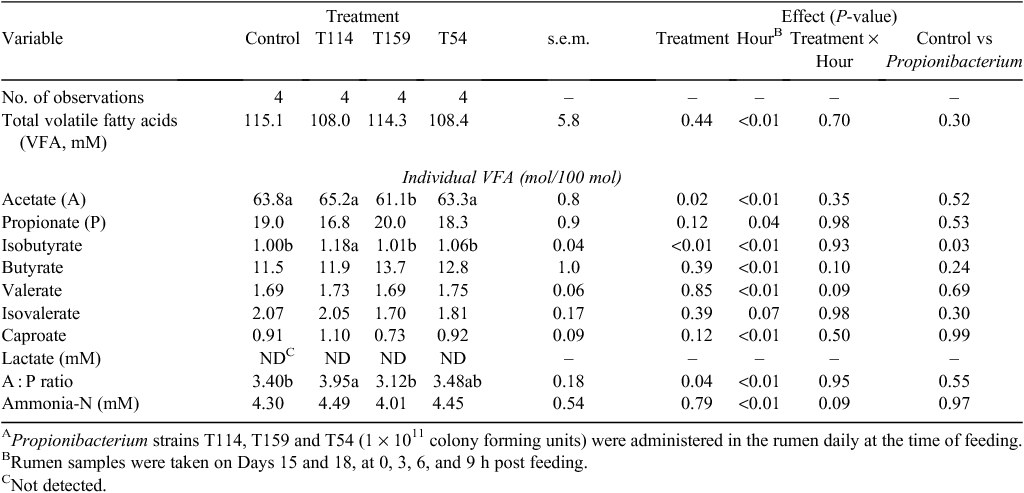
No treatment differences were observed for DMI on the days of CH4 measurement in chambers (P = 0.65; Table 4). However, DMI in chambers was reduced by 5–21% compared with DMI measured during the metabolism experiment, with a greater decline observed in the animals receiving Propionibacterium strains, primarily Propionibacterium T159. Total enteric CH4 production was not affected by treatments and averaged 188 g/day (P = 0.51). Methane yield adjusted for DMI (P = 0.19) and gross energy intake (P = 0.17) were similar across all the treatments. However, contrary to our hypothesis, CH4 yield adjusted for DMI and gross energy intake tended to increase when means were compared between Control and Propionibacterium treatments (P = 0.08). The numerical differences in total CH4 emissions with the inoculation of Propionibacterium strains were driven by changes observed during the initial 0–10 h post feeding (Fig. 1).
Inoculation of Propionibacterium T159 increased the relative abundance of total Propionibacteria probably due to the greater prevalence of the respective strain (P = 0.04; Fig. 2). However, no effects were observed on the relative abundance of total Propionibacteria with the inoculation of other strains. Relative abundance of total Propionibacteria was not affected by sampling time (P = 0.27).

|
Discussion
Strategies to mitigate CH4 emissions in cattle fed a mixed diet are desirable as emissions are higher from feedlot cattle during the growing, as compared with the finishing, phase of beef production (Beauchemin and McGinn 2005). Recently, the role of Propionibacterium species in reducing CH4 emissions was explored in beef cattle fed a high-forage diet (Vyas et al. 2014a) and a high-grain diet (Vyas et al. 2014b); however, inoculated strains failed to increase ruminal propionate proportion and mitigate total CH4 emissions. It is possible that the lack of effect of Propionibacterium species in those studies was due to the strains selected; thus, the present study examined additional Propionibacterium strains. The Propionibacterium strains used in the present study were previously screened for their CH4 mitigation potential in vitro using both forage- and grain-based diets (Alazzeh et al. 2013), unlike in the studies of Vyas et al. (2014a, 2014b). Animals used in the present study had no previous exposure to Propionibacterium strains thereby ruling out the possibility of any carry-over effects that might have confounded results in the present study.
The present in vivo study showed no significant treatment effects on total CH4 production for any of the strains used, in contrast to observations from a previous in vitro study (Alazzeh et al. 2013). Moreover, total enteric CH4 emissions corrected for DM and gross energy intake tended to be greater with the inoculation of Propionibacterium strains. The results observed in the present study are contrary to the suppression of CH4 yield observed earlier with Propionibacterium strains inoculated under similar dietary conditions (Vyas et al. 2014a). The inconsistency between results might be attributed to the use of different strains or species of Propionibacterium across the two studies and their differential effects on DMI under stressful conditions when animals were in chambers. For some unknown reason, in the present study the drop in the DMI in chambers was more prominent for animals inoculated with Propionibacterium strains than Control cattle. Given that intake affects ruminal passage rate of digesta (Sniffen et al. 1992), reduced intake with Propionibacterium strains might have increased retention time of substrates in the rumen resulting in greater fermentation and thereby greater CH4 emissions. A similar inverse relationship between CH4 production and ruminal passage rates was observed previously where CH4 production was decreased by 29% with 63% increase in the fractional passage rate of particulate matter in steers (Okine et al. 1989).
The absence of treatment effects on total CH4 emissions observed in a previous study by Vyas et al. (2014a) was attributed to the lack of survival and persistence of inoculated Propionibacterium strains as the abundance of the inoculated bacteria returned to pre-treatment levels within 9 h post inoculation. In contrast, in the present study, the relative abundance of total Propionibacteria was greater with the inoculation of Propionibacterium T159, with greater levels of abundance at every time point post inoculation, relative to the Control. Discrepancy between studies might be due to the use of different Propionibacterium strains and better adaptability of Propionibacterium T159 to ruminal conditions in animals fed mixed diets. The lack of survival and persistence of Propionibacterium strains in the rumen observed previously (Vyas et al. 2014a) was attributed to absence of ruminal lactate, a preferred substrate for the growth of Propionibacterium spp. Although ruminal lactate was not detectable in the present study, better persistence of Propionibacterium T159 could have been due to utilisation of alternative substrates including glucose as well as amino acids to produce propionate and acetate (Piveteau 1999). The presence of metabolically active Propionibacterium T159 might have accounted for the reduced molar proportion of ruminal acetate; however, lack of significant effect on total CH4 emissions might be attributed to the inefficacy of Propionibacterium T159 to increase ruminal propionate.
Contrary to the effects on VFA profile observed with Propionibacterium T159, inoculation of Propionibacterium T114 increased acetate : propionate ratio. The relative abundance of total Propionibacteria in the rumen of heifers inoculated with Propionibacterium T114 suggested a lack of persistence of the inoculated strain making it difficult to explain the induced changes in ruminal VFA profile. It is possible that the relative abundance of total Propionibacteria with the inoculation of Propionibacterium T114 was below the detection limit, yet sufficient enough to influence and induce corresponding changes in ruminal VFA profile.
The lack of response on ruminal fermentation and CH4 emissions with the inoculation of Propionibacterium T54 might be attributed to the absence of metabolically active Propionibacterium T54 given that there was no significant increase in relative abundance of total Propionibacteria post inoculation of the respective strain. The effects on ruminal fermentation and CH4 emissions with Propionibacterium T114 and T54 are contrary to the in vitro results observed earlier (Alazzeh et al. 2013). The discrepancy between studies could be attributed to the use of different methods for studying ruminal fermentation. The in vivo method used in the present experiment is more representative of the biological system as compared with the in vitro batch culture experiment used earlier (Alazzeh et al. 2013).
It should also be acknowledged that failure to detect numerical differences observed on total CH4 emissions and CH4 yield could also be attributed to the lack of statistical power of the experiment. Hence, results observed from the present study might definitively dismiss the role of Propionibacterium strains on mitigating CH4 emissions; however, future studies with greater replication are required to validate the results observed in this study.
In conclusion, Propionibacterium strains induced changes in ruminal VFA profile but failed to elicit significant treatment differences in total CH4 emissions probably due to the inability of Propionibacterium strains to significantly alter ruminal propionate concentrations. When results of this study are examined together with previous in vivo studies, it appears that supplementing cattle diets with Propionibacterium has limited potential to mitigate enteric CH4 emissions.
Acknowledgements
This study was financially supported by Norwegian – Canadian BILAT project. We would also like to thank B. Farr and K. Andrews for sampling and laboratory assistance, D. Vedres for GC analyses, K. Munns for assisting in growing Propionibacterium strains in the laboratory and staff at the Metabolism Unit of the Lethbridge Research Centre (Agriculture and Agri-Food Canada, Lethbridge, Alberta, Canada) for animal care.
References
Alazzeh A, Sultana H, Beauchemin K, Wang Y, Holo H, Harstad O, McAllister T (2013) Using strains of Propionibacteria to mitigate methane emissions in vitro. Acta Agriculturae Scandinavica, Section A– Animal Science 62, 263–272.Association of Official Analytical Chemists (2005) ‘Official methods of analysis. Vol. 2.’ 18th edn. (AOAC: Arlington, VA)
Avila-Stagno J, Chaves AV, He MM, Harstad OM, Beauchemin KA, McGinn SM, McAllister TA (2013) Effects of increasing concentrations of glycerol in concentrate diets on nutrient digestibility, methane emissions, growth, fatty acid profiles, and carcass traits of lambs. Journal of Animal Science 91, 829–837.
| Effects of increasing concentrations of glycerol in concentrate diets on nutrient digestibility, methane emissions, growth, fatty acid profiles, and carcass traits of lambs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXlvVKmsrg%3D&md5=30c71d09fdbb9f0a6f7281407b1849d0CAS | 23148243PubMed |
Beauchemin KA, McGinn SM (2005) Methane emissions from feedlot cattle fed barley or corn diets. Journal of Animal Science 83, 653–661.
Beauchemin KA, McGinn SM (2006) Methane emissions from beef cattle: effects of fumaric acid, essential oil, and canola oil. Journal of Animal Science 84, 1489–1496.
Canadian Council on Animal Care (1997) ‘Guide to the care and use of experimental animals.’ (CCAC: Ottawa, ON)
Jeyanathan J, Martin C, Morgavi DP (2014) The use of direct-fed microbials for mitigation of ruminant methane emissions: a review. Animal 8, 250–261.
| The use of direct-fed microbials for mitigation of ruminant methane emissions: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXptlSisA%3D%3D&md5=831149728402dfe7e710eac3889d245cCAS | 24274095PubMed |
Mead LJ, Jones GA (1981) Isolation and presumptive identification of adherent epithelial bacteria (‘epimural’ bacteria) from the ovine rumen wall. Applied and Environmental Microbiology 41, 1020–1028.
Nadkarni MA, Martin FE, Jacques NA, Hunter N (2002) Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primer set. Microbiology 148, 257–266.
National Research Council (2000) ‘Nutrient requirements of beef cattle.’ 7th rev. edn. (National Academy Press: Washington, DC)
Okine EK, Mathison GW, Hardin RT (1989) Effects of changing in frequency of reticular contractions on fluid and particulate passage rates in cattle. Journal of Animal Science 67, 3388–3396.
Penner GB, Beauchemin KA, Mutsvangwa T (2006) An evaluation of the accuracy and precision of a stand-alone submersible continuous ruminal pH measurement system. Journal of Dairy Science 89, 2132–2140.
| An evaluation of the accuracy and precision of a stand-alone submersible continuous ruminal pH measurement system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xlt1eqs7k%3D&md5=4a2d0a7e5e285423a60e1aa0a0c271ceCAS | 16702280PubMed |
Piveteau P (1999) Metabolism of lactate and sugars by dairy propionibacteria: a review. Le Lait 79, 23–41.
| Metabolism of lactate and sugars by dairy propionibacteria: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXisFKqtbs%3D&md5=2a552fbb813d4e8d819863ee8f584047CAS |
Rossi F, Torriani S, Dellaglio F (1999) Genus-and species-specific PCR-based detection of dairy propionibacteria in environmental samples by using primers targeted to the genes encoding 16S rRNA. Applied and Environmental Microbiology 65, 4241–4244.
Sniffen CJ, O’Connor JD, Van Soest PJ, Fox DG, Russell JB (1992) A net carbohydrate and protein system for evaluating cattle diets: II. Carbohydrate and protein availability. Journal of Animal Science 70, 3562–3577.
Van Soest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. Journal of Dairy Science 74, 3583–3597.
Vyas D, McGeough EJ, McGinn SM, McAllister TA, Beauchemin KA (2014a) Effect of Propionibacterium spp. on ruminal fermentation, nutrient digestibility and methane emissions in beef heifers fed a high forage diet. Journal of Animal Science 92, 2192–2201.
| Effect of Propionibacterium spp. on ruminal fermentation, nutrient digestibility and methane emissions in beef heifers fed a high forage diet.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXot12itLs%3D&md5=efceeb4a30ca245216fc1ecd263e7761CAS | 24663192PubMed |
Vyas D, McGeough EJ, Mohammed R, McGinn SM, McAllister TA, Beauchemin KA (2014b) Effect of Propionibacterium strains on ruminal fermentation, nutrient digestibility and methane emissions in beef cattle fed a corn grain finishing diet. Animal 8, 1807–1815.
| Effect of Propionibacterium strains on ruminal fermentation, nutrient digestibility and methane emissions in beef cattle fed a corn grain finishing diet.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhslGqsLjM&md5=02570711b981d4c0083aba165442f252CAS | 25322788PubMed |