Learning from past designs: improving amphibian fences using an adaptive management approach
John Gould A * , Alex Callen A , Gregory Knibb A , Rachael Donelly A , Kate Schmahl A , Cassandra Maynard A , Samantha Sanders A , Frank Lemckert B and Colin McHenry A
A * , Alex Callen A , Gregory Knibb A , Rachael Donelly A , Kate Schmahl A , Cassandra Maynard A , Samantha Sanders A , Frank Lemckert B and Colin McHenry A
A Conservation Science Research Group, University of Newcastle, Callaghan, NSW, Australia.
B Eco Logical Australia Pty Ltd, Newcastle, NSW, Australia.
Abstract
Fences have been widely used to exclude, manage, or monitor both native and invasive amphibian populations. Given that fences are artificial barriers that impact animal movements within the landscape, it is critical they do not allow for unwanted movement or lead to unintended animal welfare risks. We have carried out a literature review to identify features that have been used for amphibian fences, as well as aspects of fence design, installation, and maintenance that have limited their effectiveness. We also describe our own application of adaptive management to amphibian exclusion fences, in which we detected flaws and improved features, and monitored the effectiveness of these changes. Based on an exploration of the literature and our experiences, we found several key attributes to fences that must be considered when created for amphibians, including height, lip barriers, underground barriers, support frameworks, gates, seams, clearance zones, and moisture refuges. We found that studies commonly do not detail all of these aspects of their fences, and that few openly describe flaws in the design, installation, and subsequent maintenance of their fences. This is potentially concerning because it may limit chances to make improvements to fence designs that are specific for amphibians. We subsequently provide considerations and recommendations for each key fence attribute, along with maintenance and monitoring advice. These take into account intended fence purpose, desired fence permeability, and project constraints for a variety of amphibian types, life histories, and developmental stages. They are intended to be used by managers to assist in designing an effective fence for their target species. Some of our recommendations to reduce animal welfare risks are to minimise the use of: (1) fence materials that could cause abrasion injuries, (2) dry substrates that could lead to desiccation, (3) geofabrics that could lead to entanglement, and (4) fence aprons that animals could easily become trapped under. This is likely to be a valuable guide for practitioners who are required to install amphibian fences and for policy makers who prescribe fences for mitigation. This guide is applicable for projects managing threatened native species, as well as invasive species, such as the cane toad (Rhinella marina).
Keywords: amphibian, artificial barriers, cane toad, exclusion fence, fence design, landscape engineering, management tool, population management, terrestrial dispersal.
Introduction
Artificial barriers such as fences, roads, and dams can have a negative impact on wild populations by causing mortality (Hels and Buchwald 2001; Mbaiwa and Mbaiwa 2006; Beebee 2013), impeding movement (Forman and Alexander 1998; Cherney 2011), and isolating populations (Bennett 1991; Vos and Chardon 1998; Forman et al. 2003; Sillero 2008). However, some barriers, particularly fences, can also be effective management tools (Bergen et al. 2001; Hayward and Kerley 2009). Indeed, fences have been used widely in projects for a variety of environmental purposes, including to (1) protect native species from introduced species and for reintroductions (Moseby and Read 2006; Hayward and Kerley 2009; Chang et al. 2013; Anson 2018), (2) restrict access to areas of increased risk such as sites undergoing remediation (Chang et al. 2013), (3) permanently remove or retain wildlife from areas of conservation or economic value (Long and Robley 2004), and (4) guide movements between habitat patches (Schlupp and Podloucky 1994; Jones 2012; Bager and Fontoura 2013; Matos et al. 2019). The widespread use of fences in management has driven the need for low-cost, effective designs that have minimal environmental impact, minimise unintentional harm to wildlife, and have low maintenance requirements.
Fences have been used for monitoring amphibian populations (Schlupp and Podloucky 1994; Allaback and Laabs 2002), as well as to confine them to protected areas (Schlupp and Podloucky 1994; Homyack and Giuliano 2002), create exclusion zones (Hughes et al. 2021), and to manage invasive species such as the cane toad (Rhinella marina) (Florance et al. 2011). Yet, unwanted movement of target amphibians under, over, or through fences is a common issue (Dodd et al. 2004). This may have conservation implications for native species, allow for the dispersal of invasive species, or result in misinterpretation of research findings and breaches in ecological compliance for developments (Dodd 1991). Fences have also been shown to inadvertently but directly cause amphibian harm, such as via desiccation (Boyle et al. 2019). Thus, there is a need to assess the features of past fence designs that have limited their effectiveness and identify modifications that can improve their performance.
There is currently limited information on the most effective fence designs for amphibians (Arntzen et al. 1995; Woltz et al. 2008) and limited studies on aspects of fences that enhance their effectiveness (Dodd 1991; Hughes et al. 2021). Thus, there is the potential for future programs to repeatedly suffer the same flaws that could be costly and cause harm. Fences need to be customised to the requirements of the target species, taking into consideration their specific characteristics that may be challenging when preventing unwanted fence crossover. For example, some amphibians are better terrestrial dispersers and more likely to rapidly and repeatedly move towards fences (e.g. frogs and toads compared with salamanders and newts; Jehle and Arntzen 2000). Additionally, some amphibians have better climbing capacities and can bypass many barriers (e.g. tree versus ground frogs or toads; Hertwig and Sinsch 1995), or are adept burrowers and can move underneath barriers (e.g. fossorial versus arboreal species; Wells 2010; Keeffe and Blackburn 2020). Some amphibians are also of a size (e.g. small species or juvenile life stages) that allows for movement through holes and gaps. Despite these species-specific differences, recommendations are needed for amphibians in general so that managers can determine which features they may want to incorporate into their fence designs, particularly if ecological knowledge on the target species is limited, or to be effective against multiple target species.
The main objective of our review is to provide wildlife managers with a resource that can be used to assist in the creation of amphibian fences. To do so, we provide a first-hand account of the recent application of exclusion fences, as a case study, to explore potential pitfalls in fence design and how these were overcome using an adaptive management approach. We also carried out a literature review of amphibian fences to describe design features and potential flaws that limited their effectiveness. By considering these past projects, we provide a comprehensive list of fence features that can be incorporated into future designs, which practitioners can use to assist in customising an effective fence for their project. We also provide recommendations for fenceline construction, design, and maintenance, to improve their efficiency and reduce the potential for animal harm. We take into consideration fence components, project purpose and constraints, and amphibian size, life stage, and life history characteristics so that our recommendations are useful for all future projects requiring installation of an amphibian fence.
Case study: adaptive management of tree frog exclusion fences
Litoria aurea is an Australian tree frog that has experienced rapid declines from various threatening processes, particularly infection by the chytrid pathogen (Penman et al. 2008). Several populations have been subjected to intensive management within modified or degraded landscapes (Hamer et al. 2002; Darcovich and O’Meara 2008; Beranek et al. 2021). This includes one of the largest remanent populations that occurs on Kooragang Island, New South Wales (NSW), Australia, where temporary exclusion fences have been installed to manage terrestrial movements within an industrial landscape.
Although the exclusion fences on Kooragang Island were constructed using best practice guidelines, flaws were detected and corrective actions taken. In this case study, we describe the steps we took to improve our fences after they were installed. This case study demonstrates the level of detail regarding fence design, monitoring, and flaw correction that should be provided in publications. We also show the extent of monitoring required to ensure animal safety around fences.
Exclusion fence I – design and survey method
An exclusion fence was created around a terrestrial area earmarked for ground remediation in late 2019 to prevent L. aurea access. The fence followed designs previously used for L. aurea management and currently considered best practice (Muir 2008; Eco Logical Australia 2017). It was made of polyethylene netting approximately 90 cm in height to prevent frogs jumping over, and was buried to a depth of 10 cm to prevent frogs burrowing underneath. The netting was draped over a framework of metal stakes positioned along the fenceline at an interval of 5 m to create a continuous horizontal wall, with metal wiring laid horizontally between the metal stakes at the top to stabilise the lip and along the mid-section to provide further support for the netting. The open edges of the fence netting were sewn together using a series of metal pins.
An L. aurea juvenile dispersal event occurred on Kooragang Island following significant April rainfall at the end of the 2019–2020 breeding season. Many juveniles breached the fence and moved into the exclusion zone where there was limited vegetation cover and no water sources to prevent moisture stress. An intensive monitoring and removal program was subsequently carried out in the evenings from 7 to 12 April, 2020 to prevent animal harm. Individuals found within the exclusion zone were translocated to nearby wetlands known to be used by the species.
Exclusion fence I – findings
Fencing around the exclusion zone was found to be in poor condition. This included the presence of holes in the netting, gaps where the edges of netting were sewn together (Fig. 1a), sections not anchored against the ground, and encroaching vegetation. These issues with the fence all had the potential to contribute to the unwanted movement of juvenile frogs into the construction area.
Design and maintenance issues for polyethylene amphibian exclusion fence on Kooragang Island, Australia installed in 2019 to keep Litoria aurea out of a construction zone. Images show gaps caused by (a) spaces between metal pins used to connect fence sections together, and (b) a tree frog stuck between folds of fence material near the ground.

All L. aurea detected during evening surveys were small juveniles (<40 mm snout–vent length) with similar abundances around the outside (N = 1795) and inside (N = 2007) of the fence. Juveniles were found wedged in folds of fence material near the ground (Fig. 1b) or observed climbing and at the top of the fence (Fig. 2), the latter indicating that movement into the zone was possible even if the fences were in good condition. Inspection showed that, although apparently tight, there was also enough looseness in the fence fabric between the metal pins to allow juvenile frogs to push through the fence seams.
Exclusion fence II – design and survey method
In September, 2020, an exclusion fence was installed around a contaminated wetland complex on Kooragang Island to prevent L. aurea from entering the area during remediation. The fence was constructed while frogs still occupied the extant wetland habitat within the exclusion zone, so frogs were present inside the fenced area at the start of the project. Lessons learned from the previous construction of exclusion fencing were incorporated into this design. The fence was constructed to approximately 100–120 cm in height and composed of polyethylene netting (Fig. 3a). It was perpendicular to the ground (90°) and supported on a framework of vertical metal posts spaced 5 m apart (Fig. 3b). A section of loose netting at the base was buried to a depth of 20 cm and curved to prevent animals from getting through underneath. The fence also featured a unidirectional design, with a fence lip to prevent entry over the top (Fig. 3b) and ramps on the inside to allow frogs to exit the site (Fig. 3c). The fence lip was outward facing and made of netting draped over an additional series of metal posts, extending the top of the fence horizontally by 25 cm before hanging down over the edge of the outer posts. The ramps were made of polyethylene material installed along the inside of the fenceline every 50 m. Fence seams were folded and sewn together using a series of metal pins (Fig. 3d). The exclusion zone had one partially removable section of netting that functioned as a gate that could be dropped and pulled to one side to allow vehicle access (Fig. 3e). Because this section of gate fencing could not be buried, a series of sand-filled bags were placed over the material on the ground to restrict frog movement from underneath.
Design features included for polyethylene amphibian exclusion fence constructed on Kooragang Island, Australia in 2020, to keep Litoria aurea out of a construction zone. (a) Fence with geofabric laid along the outside of the fenceline, with the inside covered in short vegetation. (b) Framework of paired metal posts used to support the polyethylene fence and external lip. (c) Polyethylene ramp to allow frogs to escape the exclusion zone. (d) Metal pins used to connect the folded edges of sections of fence material, creating a seam. (e) Fence gate with bottom surface covered with sandbags to prevent frog ingress below the material that could not be buried. (f) Artificial ponds installed along the outside of the fenceline to act as aquatic refuge.

During the initial installation phase, the area directly underneath and within 2 m of the outside of the fenceline was cleared and covered in crusher dust (a material made from crushed rock and/or concrete). This material was found to pose a significant desiccation risk for frogs moving across it so the outside of the fenceline was later covered in a synthetic geofabric made of non-woven polyester short filaments to act as a protective barrier, which prevented direct contact by frogs moving over this surface (Fig. 3a, b, f); the inside of the fenceline contained vegetation and water to protect against desiccation. A series of 80 small, artificial ponds were also installed at 10–40 m intervals around the outside of the fenceline to act as ‘rehydration stations’ to further reduce desiccation risk (Fig. 3f). These were lined with black Aquapro PVC lining and manually filled weekly with tank water. The ponds also contained vegetation, including grass and sticks, to act as cover from sunlight and predation. Midway through the L. aurea breeding season in December 2020, a sprinkler system was installed around the outside of the fenceline to allow for automated wetting of ponds and the geofabric material when air temperature reached 26°C. Fence maintenance, primarily the closing of rips in the polyethylene fabric with metal wiring or cable ties, occurred whenever such issues were detected during surveys. Vegetation maintenance, including the mowing of grass/weeds in proximity to the fence, was performed every 6 months.
Visual surveys were conducted around the fenceline from September 2020 to March 2021, during the height of the L. aurea breeding season. This monitoring was carried out to collect animals near the fenceline and move them to nearby wetlands for their protection. Surveys were initially performed during the day – to collect animals when they were at their most vulnerable to heat stress and desiccation. Night surveys were performed three nights a week between the end of February and the start of May 2021. The time it took to survey the entire fenceline was dependent on the number of frogs detected, ranging anywhere from 30 min to 3 h. All captured animals were marked with a coded passive integrated transponder (PIT) tag for identification purposes. All images obtained during surveys and presented in this review are available for download at Gould (2023).
Exclusion fence II – findings
We detected a variety of issues with the fence during surveys. We found that some sections of fence were not properly buried underground (Fig. 4a) – an easily rectified mistake during the installation phase. Holes within the fence were detected across survey events, ranging in size from 1 to 15 cm (Fig. 4b). These holes primarily formed at the base of the fence near the ground due to natural wear, stress from the piling of crusher dust after rainfall events, and sections that had torn because they were too taut. Holes were also formed by rats chewing through the polyethylene material or during vegetation maintenance if the blades of garden tools accidently hit the fence. Holes were patched immediately upon detection by sewing additional material over the hole or with cable ties. In addition, we noticed that the metal posts used to support the fence material often made direct contact with the lip of the fence netting, which may have allowed animals to cross over the top. Fences were susceptible to nearby vegetation growing through them, indicating that vegetation maintenance (twice yearly) was too infrequent for spring and summer weed growth. Furthermore, the geofabric material began to deteriorate within 12 months, with holes forming across the surface (Fig. 4c). The artificial ponds surrounding the fenceline often dried throughout the survey period. This was due to issues with pond liner integrity, their relatively small size, and high exposure to sun and wind that caused rapid evaporation rates. Ramps running perpendicular to the fence on the inside did not perform as an exit route for frogs as predicted, with no evidence they were used. The automated sprinkler system did not prevent pond desiccation, partly because the sprinkler heads were not in close enough proximity to the ponds and they sprayed water into the air and not directly into the depressions.
Issues with design and maintenance of polyethylene amphibian exclusion fence constructed on Kooragang Island, Australia in 2020, to keep Litoria aurea out of a construction zone. (a) Section of fence not buried underground. (b) Horizontal cut in fence material caused by mower blades during vegetation maintenance. (c) Degradation of geofabric material used to line the outside of the fenceline.

In total, 610 individuals were recorded during 55 daytime surveys events from October to March, with the majority being juveniles (83%). Animals were only found around the outside of the fenceline, primarily within or less than 10 cm from artificial ponds (68%). Along the outside of the fenceline, 748 individuals were detected over 23 night surveys from March to the start of May. The majority of these animals were adults (74%) and found on the geofabric between the artificial pools (95%). In contrast to daytime surveys, we also found a relatively large number of frogs along the inside of the fenceline during the night surveys (N = 327). The majority of these animals were adults (87%), with more than half (52%) found at the base or within 20 cm of the fenceline. We detected previously captured animals within the fenced area at night, showing that animals had made their way back into the exclusion zone over or through the fence. Our intense monitoring revealed a considerable increase in the total number of animals detected when surveys were changed to night-time and the location of animal detection. This change indicated that the timing of surveys had a critical impact on the potential outcomes of monitoring, with day surveys allowing frogs with a higher risk of desiccation to be collected, and night surveys allowing for higher detectability of frogs for translocation.
Frogs were observed scaling the exclusion fence, including on the lip at fence gates that had become slack over time through repeated use (Fig. 5). Fence climbing occurred more often on the non-construction outside of the fence (14% of all captures) compared with the inside of the fence line (4% of all captures).
Adult tree frogs (Litoria aurea) scaling polyethylene amphibian exclusion fence (a) midway up the height of the fence and (b) on the lip of a gate that has sagged.

We also detected multiple animal behaviours during surveys. Predation events were observed on two occasions along the outside of the fenceline, including an L. aurea juvenile with wounds to the chest believed to be caused by a bird attack, and a black-bellied swamp snake (Hemiaspis signata) consuming a Limnodynastes peronii adult. Breeding was detected at the refuge ponds on at least 10 occasions, as evidenced by the presence of Limnodynastes tasmaniensis spawns and tadpoles. We also detected foraging behaviour along the inside of the fenceline, with L. aurea adults found consuming Limnodynastes adults on three occasions.
We recorded L. aurea individuals that presented with abrasions to the nose, including blackened skin or, in more severe cases, open wounds (Fig. 6a, b). This was detected more commonly in frogs captured along the outside of the fence compared with the inside, with some indication that animals showed progressive deterioration with repeated captures. Similar damage was also recorded in other frog species, particularly L. peronii and Litoria fallax. We hypothesise that these abrasions were the result of repeated rubbing against the fence material as frogs attempted to push their way in to get to the ponds located within the construction site. Indeed, observations were made of L. aurea adults hitting into the fenceline repeatedly, suggesting that their movement patterns increased the risk of injury.
The potential damage caused by materials used to construct amphibian exclusion fences. Nose abrasion on (a) a tree frog (Litoria aurea) and (b) a ground frog (Limnodynastes peronii) collected from the outside of a polyethylene amphibian exclusion fence, as well as frogs stuck within the fibres on the surface of geofabric sheeting used to (c) line the ground beneath the same exclusion zone fence and (d) pad the lower sections of the fence.

Because of natural deterioration, the geofabric at the base of the fence was replaced after 12 months. In contrast to the previous instalment where the geofabric was laid only along the ground, the material on this occasion was also laid halfway up the bottom third of the fence to prevent nose abrasions (Fig. 3b). However, we observed frogs becoming stuck within the fibres of this new geofabric material (Fig. 6c, d), which was subsequently removed altogether to prevent any further harm.
Review of amphibian fence projects
Our case study highlights how, even when based on best practice guidelines, our fence design and maintenance was not adequate to prevent ingress into an exclusion zone and that certain design features had the potential to cause harm. This first-hand account shows that unforeseen issues arise with the application of amphibian fences, which are only discovered through intense monitoring. We subsequently wanted to understand how our fence differed from others described in the literature, and whether there were any features we could have included to achieve better outcomes. We also wanted to determine whether past projects openly explored flaws in fence design, construction, and maintenance, and whether the issues we experienced were generally faced by other managers.
Literature search
We conducted a literature search of fencelines established for the management or monitoring of amphibian populations. Published literature was found on Google Scholar using key search terms (e.g. fence, fenceline, drift fence, exclusion zone) with ‘And (amphibian, salamander, newt, anuran, frog)’ to filter for fencelines that were installed/used specifically by amphibians. We also reviewed any relevant literature cited within these original articles. In total, we obtained data on 51 fence designs across 41 studies. We recorded whether fences were established specifically for amphibians, and whether aspects of fence design (height, length, material, framework, buried, sloped, and lip) or trespass were stated (Table 1). We recorded exact measures of features when they were defined.
| Fence aspect | Aspect type | Defined in study (%) | Detail | |
|---|---|---|---|---|
| Form | Material | 88 | Plastic (53%) metal (35%), mixture (7%), netlon (2%) | |
| Height | 75 | Range: 10–100 cm, mean: 58 cm | ||
| Slope | 9 | Straight (50%), incline (50%) | ||
| Lip | 18 | Range: 10–15 cm, mean: 12 cm | ||
| Buried | 51 | Range: 0–20 cm, mean: 10 cm | ||
| Framework | 24 | Wood (60%), metal (20%), plastic (10%), non-descript (10%) | ||
| Framework interval | 11 | Range: 100–1000 cm, mean: 430 cm | ||
| Function | Trespass occurrence | 47 | Over (88%), through (12%), under (12%) |
The percentage of studies that clearly state each aspect of fence design is provided, along with details of fence aspects.
Results
The majority of fences were established specifically for amphibians (88%) and were either fences used to monitor populations (55%), to guide movement near roads (31%), or for the purpose of exclusion (14%) (Table 1). The actual design features of fences were often not clearly described in detail or not described at all (Table 1). The most commonly used fence materials were plastic (53%) or metal (35%), with few made of netlon (2%) or a mixture of materials (7%). Fences had a mean height of 59 cm (s.d. = 25), buried to a mean depth of 10 cm (s.d. = 6), and had a mean lip length of 12 cm (s.d. = 3). Only half (47%) of all studies specifically addressed whether fence trespass occurred or not. We found limited cases where harm to target and non-target species did or did not occur, and no recommendations to prevent potential harm for future projects.
Fence issues, solutions, and recommendations
Our review indicates that there is a lack of available information on past fence designs. The implication of this finding is that fences are being installed based solely on human judgement and without evidence for their effectiveness and safety, which could lead to sub-optimal solutions for such complex infrastructure problems. Additionally, there is a lack of sufficient warning for future projects to avoid known yet undivulged issues, which could lead to animal harm. Below, we detail fence features and issues that should be considered, along with potential solutions and recommendations that take into account different fence purposes, project constraints, and species traits (Table 2).
| Feature | Options | Detail | Schematic | |
|---|---|---|---|---|
| Material | Plastic/fibreglass | Less expensive but less durable Prone to hole formation, UV damage, animal chewing Easier to manipulate into desired shape Lighter |  | |
| Metal | Durable but more expensive Difficult to manipulate into desired shape Heavier | |||
| Pores | Yes (woven plastic, metal mesh) | Allows air/water passage: reduces strain on fence Rough surface: more likely to cause abrasion injuries May impede animal climbing |  | |
| No (plastic or metal sheeting) | Does not allow air/water passage: increases strain on fence and risk of fence breakage Smoother surface: less likely to cause abrasion injuries May not impede animal climbing | |||
| Colour | Black | Blends into environment Commercially common May increase temperature of fence surface and heat-related injuries |  | |
| Coloured | Only some colours commercially common or available May reduce fence surface temperature Less inconspicuous in the environment | |||
| Layers | Single layer | Animal passage more likely if holes form Animal entrapment within fence not possible |  | |
| Multiple layers | Animal passage less likely if holes form Chance of animal entrapment if holes form in outer layers | |||
| Panels | No | Damaged sections of fence cannot be easily replaced but can be patched with additional material Less expensive due to simpler design and installation |  | |
| Yes | Damaged units are more easily replaceable More expensive due to increased design complexity and installation | |||
| Angle | Straight (180°) | Movement from either fence side is easier if there is no barrier at the top (e.g. lip) Easy installation | 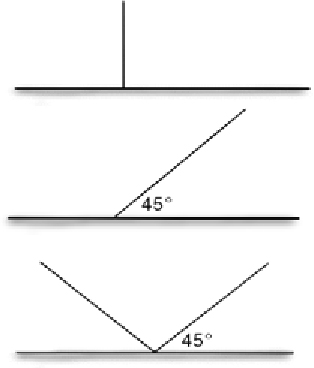 | |
| Slanted ‘I’ shaped (45°) | Only unidirectional movement over fence is possible More complex installation | |||
| ‘V’ shaped fence | No movement from either side of fence is possible More expensive due to increased material required More complex installation | |||
| Height | Minimum height of 50 cm 80 cm if fence is slanted | Prevents all amphibian types jumping over fence so long as there is no elevated land near the fence | 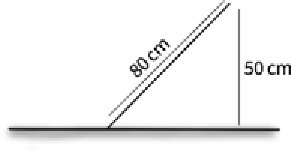 | |
| Lip | Additional material at the top of fence Freely hanging Weighted seam | Prevents trespass over fence Weight at the end of the lip removes the need for supporting wires On a slanted fence there is less chance of lip contacting lower sections Straight fences may require additional support at the top to prevent the lip making contact with lower sections | 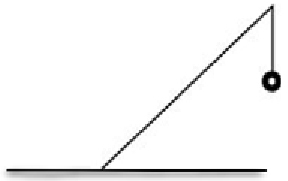 | |
| Below ground fence | Additional material buried Curved ‘J’ shaped end | Stabilises fence at ground Prevents trespass under fence Removes the need for fence apron that may cause animal entrapment Curved end prevents fence lifting |  | |
| Support | Framework of metal posts Interval length 4–5 m On one side of fence Horizontal arm connects posts to fence for slanted designs | Metal posts: durable, can be dug into wet soil near water Interval length supports fence without need for supporting wires Posts on one side (for I shaped fence) or middle (for V shaped fence) prevents animals climbing over fence from that side | 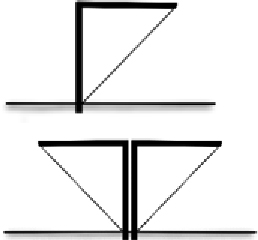 | |
| Seam | Plastic or metal mesh material is rolled, then closed with glue and metal pins Metal sheets closed with glue or soldered together | Creates seamless connections between fence edges Prevent gaps that allow animal trespass through fence | 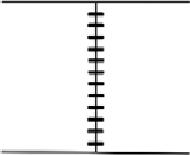 | |
| Gate | Plastic material: fence section that can be temporarily pulled down using zippers installed along vertical edges Metal material: section adapted using hinges down one side | Zipper system prevents plastic fences sagging, allows gate section to be buried, prevents unwanted gaps between fence and gate Gates are more difficult to construct for metal sheet fences as the section near the ground must still be buried. |  | |
| Clear zone | Vegetation on both sides of fence cleared Remaining vegetation kept below fence height Fence positioned away from tall structures (e.g. trees) | Prevents unwanted animal movement over fence Prevents vegetation growing through fence and forming holes Remaining vegetation provides moisture, stabilises topsoil to prevent erosion, and provides novel foraging area Cleared ground allows animals to be detected during monitoring |  | |
| Refuge | Aquatic shelter near fence Installed on at least one side of the fence where animal crossing is restricted Shelter filled with rocks/vegetation | Act as rehydration stations for animals stuck at fence, preventing desiccation and overheating Rocks/vegetation provide refuge from sun and predators | 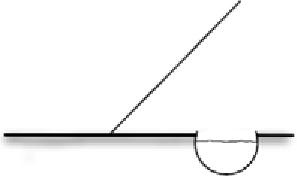 |
A description of each aspect of fence design is provided, along with a schematic.
Fence material
The materials used to create amphibian fences, including metal flashing, metal mesh, plastic sheeting and woven material are all readily available at hardware stores. Fences made of metal sheeting have been favoured for longer-term studies due to their durability and minimal maintenance (Hughes et al. 2021). However, they are likely the most expensive option and prevent wind and water passage, which makes them more vulnerable to weather damage. Metal mesh allows water and wind passage and is similarly expensive, but may also allow passage of small amphibians if the pore size is too large. Plastic sheeting or ‘film’ is less costly than metal, but may be less effective because it's more prone to wind damage and may degrade with UV exposure, and because mammals can chew through it (Arntzen et al. 1995; Poole 2002). Woven materials such as polyethylene ‘shade’ cloth or metal/fibreglass window screening (‘insect flyscreen’) are also affordable options that are cheaper than metal and easy to transport, install, and repair. However, although the long-term effectiveness of woven materials has been previously reported (Zug et al. 2001), they are prone to breakdown over time and suffer from hole formation within months.
The most optimal fence material to select should be based on project funding and longevity, and the durability of material required based on environmental conditions. More durable options, such as metal, should be selected for projects where longevity will be important. Cheaper options (such as polyethylene) are viable, but only if regular maintenance is completed to ensure holes do not allow unwanted animal movements.
The likelihood and/or rate of fence degradation is dependent on material, but also on the location of fence installation because this determines sun exposure, vegetation growth, substrate type, and presence/density of animals that have a propensity to chew through the chosen material. Certain materials that are more likely to degrade, such as plastic sheeting, may have an impact on the surrounding ecosystem as a form of non-biodegradable pollution. This is unlikely to be an issue with polyethylene woven material, which does not easily break down into small pieces or tear away from the body of the fence, or with metal. Biodegradable materials made from plant fibres, wood or bamboo are available but are not effective for long-term projects and have not been readily tested for amphibian fencing purposes.
To reduce the capacity for animal trespass, a three-layered fence design may be advantageous, similar to Arntzen et al. (1995), in which external layers of plastic or woven material covers an internal layer of metal wire meshing. The multi-layered design ensures that breaches in the external material do not provide access points before they can be repaired, with the internal wire mesh adding strength to prevent the fence from drooping without the need for supporting wires. However, using multiple layers increases project costs, and because amphibians are known to seek shelter underneath surfaces and in confined spaces (Forti et al. 2022), the risk of individuals getting stuck within multi-layered fencing also needs to be considered.
We recommend that plastic sheeting or woven materials are not pulled tight as a means of preventing fence sagging, because this increases the chance of hole formation. This can be achieved by producing ‘panels’ composed of the selected fence material, which become easily replaceable units placed side-by-side to form the fence. This could also be achieved for metal fences. However, producing replaceable units may increase installation costs and lead to more joining points where frogs could move through gaps, with no one fence type better in this regard.
Scaling of fence
The climbing tendency and ability of amphibians is species-specific, dependent on a variety of characteristics such as animal size and toe pad surface area (Smith et al. 2006; Labonte et al. 2016). Climbing ability is also influenced by properties of the material being climbed, including surface moisture and substrate roughness (Dodd 1991; Hou et al. 2010; Crawford et al. 2016). Smoother surfaces may provide amphibians with better adhesion and promote climbing (Emerson and Diehl 1980), which will need to be remedied by incorporating other fence designs that impede trespass. Sheet metal and plastic surfaces provide the smoothest surfaces, which may allow for easier climbing when compared with mesh fences of any type.
We have observed that Australian tree frog juveniles and adults have the capacity to scale vertical polyethylene and insect flyscreen fences. Vertical fences are therefore not likely to impede the natural climbing tendency or ability of many amphibians, particularly tree frogs, but may be effective for non-climbing species, such as ground frogs and toads.
Fences installed at an angle to the ground allow for unidirectional movement. A slanted ‘I’ cross-sectional design causes one side of the fence to overhang the ground. This design can be achieved by connecting a slanted fence to a framework of posts using supporting arms located on the fence side where movement is allowed – an important consideration because amphibians may be able to climb the supporting framework. We suggest a slope angle of between 40° and 50°, based on previous effective fence designs and the general declining climbing ability of amphibians with increasing gradient (Schlupp and Podloucky 1994; Hou et al. 2010). From one side of the fence, this creates a gentle slope that will allow animals to move over it with friction alone, without the need for installing ramps that are not easily discoverable by frogs unless installed regularly along fencelines. On the other side, the opposing slope angle is steep, making it difficult for animals to traverse because they will be reliant on wet adhesion and/or suction to hang upside down (Emerson and Diehl 1980). If movement from both sides needs to be prevented, then a slanted ‘I’ design should be replaced with a ‘V’ cross-sectional design, in which two separate fences are hung off either side of a shared, internal framework of posts. This would be more effective and easier to install from commercially available materials than an ‘S’ cross-sectional design in which the fence is curved at two positions along its height (Swan 1986). Slanted fences are more complex than vertical fences and may require additional installation time.
A lip of additional fence material should be included at the top of the fenceline irrespective of fence design, given how effective this is at limiting amphibian crossovers (Hughes et al. 2021). Typically, a strip of non-supported, hanging material is the simplest design, either a single lip for ‘I’ shaped fences or two lips facing outward for ‘V’ shaped fences, and weighted along the end seam to minimise movement during strong winds. A slanted fence design is likely to allow for an effective lip because there is less chance of the end of the lip making contact with the fence or supporting framework of posts, which we suspect is a flaw in some vertical fence designs. The inclusion of a lip for vertical fences made of netting commonly requires the material to be draped over an additional series of supporting posts to prevent the lip from making contacting with the fence below, unless the posts can be curved into a hooked ‘candy cane’ shape.
A minimum fence height greater than 80 cm has been shown to reduce nearly all amphibian crossovers in previous studies, even in some fences without a lip (Woltz et al. 2008; Beebee 2013). The corresponding height of an 80-cm tall fence that is slanted by 45° is approximately 50–60 cm. This is shorter in overall height, but it is still above amphibian jumping heights reported in the literature (Hou et al. 2010). This is the minimum height that will be effective for preventing crossovers by all amphibians, including the cane toad (Florance et al. 2011), as long as there aren’t elevated land or vegetation surfaces near the fence that could be used at jumping points. Even shorter fences may be effective for smaller target species or those with less jumping capacity, such as ground frogs and toads, reducing the total material costs for the project.
Burrowing beneath fences
A weakness in fence installation occurs at the base of the fence where the material meets the ground. This is because the fence may be improperly buried or lift and create an opening if not properly anchored, thereby allowing even non-climbing amphibians to move through.
We propose that additional fence material is included so that the base can be buried underground, which is in agreement with Dodd et al. (2004). The bottom of this material should be curved to the side in a ‘J’ shaped design to prevent lifting. This action will prevent most movement under the fence, which needs to be considered because some amphibians are fossorial or show burrowing capabilities (Wells 2010; Keeffe and Blackburn 2020), and to counteract other animals that may provide underground holes for passage (Dodd 1991). Of course, the capacity for amphibians to dig is dependent on substrate compactness (Dodd 1991). We suggest that more fence material should be buried in locations of loose, sandy soil because this is easier to dig through. Furthermore, additional fence material should not be left above ground, because small frogs may wedge themselves between folds and become stuck. This could be a reason to avoid installing horizontal foot aprons around the base of fences, which have been used previously to prevent animals from digging (Moseby and Read 2006; Florance et al. 2011), but are not required if fences are buried.
Fence support
A supporting framework is required for woven or mesh fences (because they are not free-standing) and for metal sheet fences to provide additional support. Fences can also be supported using wires that run horizontally at the mid and top sections of the fence. There are a variety of framework types that can be used, each with their own durability and cost constraints. Posts made of metal are the most expensive option when compared with those made of wood, but they are much stronger and more durable. We have observed multiple instances of wooden posts breaking when fences are exposed to strong winds for extended periods, and degradation of wooden fences once exposed to moisture. Metal posts can be secured into the ground using a post driver, whereas wooden posts can be secured using a hammer. The depth that posts need to be driven into the ground is dependent on soil substrate, with greater depths required for loose, sandy soils to achieve post stability.
We suggest that metal stakes should be used because these are stronger and less likely to degrade over time when compared with wooden posts. Few studies have provided detail on the distance that posts should be placed along fencelines. The mean interval between posts used in previous studies is 4–5 m, which has been found to provide sufficient stability for single-layer fences. A multi-layered design will require posts to be set closer together to carry the additional load (Arntzen et al. 1995), and we suggest that distance should be between 3 and 4 m.
Fence gaps
Fence gaps primarily result from poor initial fence installation. Although they are likely for any project (Malt 2012), we have found that gaps usually occur along the seams for plastic or fabric-based fences, often because the metal pins or wiring used to join two sections of material are spaced too far apart or as a result of the seam partially coming undone over time. Gaps may also form in metal sheeting fences if sheets are not perfectly aligned or attached together.
Fence gaps must be eliminated as best as possible during the installation phase. The risk of gap formation can be reduced by ensuring the entire length of any seams within the fenceline are sealed. For plastic or woven material fences, this can be achieved by initially rolling the edges of the separate sections together and then using a combination of an adhesive, such as pure silicone, and metal pins to bind them to create a perfect seal. For fences made of metal mesh, seams can be joined by looping or stapling additional wire along the two sections of material. For fences made of metal sheeting, seams can be glued or soldered together.
Fence gates
Partially removable sections of fence may be required to allow vehicle movement across a fenceline. These gates can be produced by keeping a free edge of fence material that can be opened and moved horizontally to the side when in use. However, the need for these sections to be periodically separated from the fenceline prevents them from being properly anchored to the ground and buried. Animal trespass under the gate when closed is therefore commonly prevented using temporary solutions, such as sandbags or other heavy objects along the bottom edge of the gate, which typically do not form a perfect barrier. Furthermore, fence gates are difficult to connect to the surrounding fenceline without creating gaps and can stretch overtime due to repeated use, leading to eventual slack in the material that may increase the chance of animal trespass.
The section of fence being used as a gate must be permanently buried to prevent animal trespass from underneath. To achieve this on a plastic fence, zippers could be installed along the vertical edges of the section of fence that is acting as a gate. This gate could thus be unzipped and lowered entirely to the ground and flattened when necessary. The installation of zippers would avoid the creation of gaps while preventing the fence material from becoming stretched and saggy over time. For metal sheet fences, gates could be formed by adapting a section of panelling using hinges down one side to allow that section to be opened to the side. However, it will be critical for a section below the metal panelling to be buried.
Animal injury
We have observed frogs with abrasions to their noses in close proximity to polyethylene fences, which we hypothesise are the result of repeated rubbing against the material. Similar injury types have not been noted in other studies that have used woven material (Schlupp and Podloucky 1994). Furthermore, black fencing materials have higher heat capacity than other coloured materials, which may increase the risk of burn injuries and hyperthermia for amphibians that come in direct contact (Boyle et al. 2019).
Hughes et al. (2021) tested the use of sandpaper on fencelines to promote animal slippage, but we suggest that rough surfaces in general, including polyethylene, are avoided or tested prior to the project to reduce the chance of causing injury. A potential replacement for polyethylene includes insect flyscreen made of fibreglass or aluminium, which are both smoother and thus less likely to be abrasive. Insect flyscreen generally possesses a small pore diameter (<2 mm), making it effective for even the smallest amphibian species. Other fence materials, such as plastic or metal sheeting, may not require replacement because they are already non-abrasive.
Black fencing material should be avoided when possible, to reduce the risk of heat-related injuries. Painting black fencing should also be avoided, because this may cause the surface to become toxic. The risk of heat-related injuries from black fencing is reduced if the material is highly porous, given that the pores reduce the total heat capacity of the fence per area. Black materials are likely to be more commercially available than certain colours but more inconspicuous in the environment, which may be beneficial by reducing the risk of attracting unwanted human attention.
Nearby vegetation
Vegetation growing in close proximity to the fence is a constant issue that needs to be managed. If vegetation is allowed to grow out of control, it may allow animals to move across the fence or create holes in the fence material that go unnoticed.
We suggest that a buffer zone is established on both sides of the fence where vegetation is maintained to below the height of the fence. This may require the pruning of overhanging branches for trees close to the fenceline that cannot be removed, or movement of the fenceline to account for this. Although vegetation needs to be maintained regularly, it is a much less damaging solution than the use of herbicides (Dodd et al. 2004). Inspections of the vegetation must be regular enough to ensure that it does not grow sufficiently to allow amphibians to breach the fence. Regular inspections must be followed immediately by remedial actions. Depending on vegetation type and growth rate, clearance will need to be conducted anywhere from once a month to every 6 months. Caution must be taken to ensure machinery used to clear vegetation does not damage the fence, with mowing avoided directly underneath the fence unless a protective board is used to prevent cutting into the fence material. Animal clearance surveys are required prior to vegetation management, which should be completed during a period of low animal activity.
Keeping low-cut vegetation rather than no vegetation near the fenceline is beneficial because it: (1) provides moist refuge for animals moving towards the fence and possibly establishing a novel foraging area; and (2) stabilises the topsoil, which helps reduce erosion and prevents soil from piling against the fence after intense rainfall events. If vegetation cannot be kept near the fence, we suggest that a silt fence be installed close to the fence to act as a sediment barrier in areas where loose topsoil has been detected. The loss of vegetation cover in proximity to fencelines may increase the vulnerability of amphibians to predation by removing cover, which must be considered.
Lack of moisture near fences
The installation of fences within the landscape often acts as a barrier that may restrict animal movement or access to shelter and water sources. Indeed, previous studies have suggested that fences subject herpetofauna to increased exposure (Mark Peaden et al. 2017; Boyle et al. 2019), yet this is not often considered in fenceline designs. Furthermore, fencelines where the surrounding ground has been covered with soil or other materials to suppress vegetation growth have the potential to be drying, resulting in moisture stress on animals.
Dry substrates should not be placed around fencelines to purposely reduce unwanted crossings or prevent vegetation regrowth, because the risk to animal welfare is high. Indeed, it should not be assumed that amphibians will avoid crossing dry surface types (but see Lesbarrères et al. 2004; Mazerolle and Desrochers 2005). Likewise, synthetic geofabrics should not be used as protective barriers to cover surfaces that have the potential to be drying, because small frogs could get caught within the threads of this material.
Aquatic refuges with added shelter (e.g. submerged and surrounding rocks) should be established along fencelines. These features provide sources of moisture near the fenceline and prevent animals left exposed during the day from desiccating. Artificial ponds also provide refugia, which may be important for protecting amphibians that concentrate near the fenceline, particularly if vegetation cover is being controlled, from predators using fences as prey traps (Wallander et al. 2006). We have also found that fencelines provide novel but temporary foraging sites for amphibians, a phenomenon that has been observed in other fence programs (Aresco 2005; Matos et al. 2019). Furthermore, water features along fencelines have been observed to be used as breeding sites for some amphibian species, which may benefit populations until areas being remediated are completed and fencelines are removed.
We suggest that permanent aquatic systems are installed by burying open top metal buckets. These are readily available at hardware stores and reduce the extent of maintenance required to keep them wet or the need for irrigation compared with smaller ponds dug into the ground and lined with plastic lining (which is susceptible to being punctured). However, buckets should not be deep because there is a chance of them becoming animal traps if water levels fall. If irrigation is required, sprinkler heads should be replaced by feeder lines that are placed directly into water, reducing water wastage.
Fence monitoring
Regular monitoring needs to be conducted after fences have been installed, to maintain integrity and assess if they are functioning as intended. Monitoring is also important to detect issues concerning animal welfare, which may include animals getting stuck within fence material or within exclusion zones.
Fences should be monitored at times that correspond with peak activity levels of the target species, to maximise the number of animals that can be encountered in order to detect animal welfare risks. However, monitoring should also be performed during periods where animals in need are more likely to be detected, such as during the day when they could be stuck at fences and at greater risk of desiccation.
If animals are found at fences during times when they could be at greater risk of injury if they remain at the fence, managers should put in place protocols for animal collection and removal. This should include the identification of nearby habitat that could be used as translocation points.
Conclusion
Amphibian fences previously reported in the literature, and those observed by us, do not always perform as desired due to issues in design, installation, and subsequent maintenance. We have provided recommendations to minimise these flaws in future fences. Our recommendations are general and applicable to a wide range of functions, amphibian taxa, life history stages, and characteristics, including both native and in troduced species. Of course, each recommendation comes with its own potential benefits and limitations, and the most appropriate solution will depend on the target species, objectives of the project, budget, and the extent to which the fenceline must be impermeable (Bode and Wintle 2010). What must also be considered is the potential impacts fences may have on non-target species (Long and Robley 2004; Wallander et al. 2006), particularly because fences designed specifically for amphibians are likely to be impermeable to many other small-sized animals.
Acknowledgements
This work was conducted under ethics number A-2014-137, approved by the University of Newcastle. All experimental procedures were performed in accordance with the Australian code for the care and use of animals for scientific purposes. This study was assisted by funding from Port Waratah Coal Services (PWCS), Newcastle Coal Infrastructure Group (NCIG), Hunter and Central Coast Development Corporation (HCCDC), and Port of Newcastle (PoN). We thank Ben Lowder, Haley Ardagh, Mike Bardsley, Grant Moylan, Cheslyn Africa, Jacqui Spiteri, Brigid Kelly, and Jennifer Anderson.
References
Allaback ML, Laabs DM (2002) Effectiveness of road tunnels for the Santa Cruz long-toed salamander. Transactions of the Western Section of the Wildlife Society 38/39, 5-8.
| Google Scholar |
Anson JR (2018) Predator proofing for conservation: an AWC perspective. Australian Zoologist 39, 352-358.
| Crossref | Google Scholar |
Aresco MJ (2005) Mitigation measures to reduce highway mortality of turtles and other herpetofauna at a north Florida lake. Journal of Wildlife Management 69, 549-560.
| Crossref | Google Scholar |
Arntzen JW, Oldham RS, Latham DM (1995) Cost effective drift fences for toads and newts. Amphibia-Reptilia 16, 137-145.
| Crossref | Google Scholar |
Bager A, Fontoura V (2013) Evaluation of the effectiveness of a wildlife roadkill mitigation system in wetland habitat. Ecological Engineering 53, 31-38.
| Crossref | Google Scholar |
Beebee TJC (2013) Effects of road mortality and mitigation measures on amphibian populations. Conservation Biology 27, 657-668.
| Crossref | Google Scholar |
Beranek CT, Maynard C, McHenry C, Clulow J, Mahony M (2021) Rapid population increase of the threatened Australian amphibian Litoria aurea in response to wetlands constructed as a refuge from chytrid-induced disease and introduced fish. Journal of Environmental Management 291, 112638.
| Crossref | Google Scholar |
Bergen SD, Bolton SM, Fridley JL (2001) Design principles for ecological engineering. Ecological Engineering 18, 201-210.
| Crossref | Google Scholar |
Bode M, Wintle B (2010) How to build an efficient conservation fence. Conservation Biology 24, 182-188.
| Crossref | Google Scholar |
Boyle SP, Dillon R, Litzgus JD, Lesbarrères D (2019) Desiccation of herpetofauna on roadway exclusion fencing. The Canadian Field-Naturalist 133, 43-48.
| Crossref | Google Scholar |
Chang Y-H, Wu B-Y, Lu H-L (2013) A study on the use of ecological fences for protection against Polypedates megacephalus. Ecological Engineering 61, 161-165.
| Crossref | Google Scholar |
Cherney DN (2011) Securing the free movement of wildlife: lessons from the American west’s longest land mammal migration. Environmental Law 41, 599-617.
| Google Scholar |
Crawford N, Endlein T, Pham JT, Riehle M, Barnes WJP (2016) When the going gets rough – studying the effect of surface roughness on the adhesive abilities of tree frogs. Beilstein Journal of Nanotechnology 7, 2116-2131.
| Crossref | Google Scholar |
Darcovich K, O’Meara J (2008) An olympic legacy: green and golden bell frog conservation at Sydney Olympic Park 1993-2006. Australian Zoologist 34, 236-248.
| Crossref | Google Scholar |
Dodd CK, Jr (1991) Drift fence-associated sampling bias of amphibians at a Florida sandhills temporary pond. Journal of Herpetology 25, 296-301.
| Crossref | Google Scholar |
Dodd CK, Jr, Barichivich WJ, Smith LL (2004) Effectiveness of a barrier wall and culverts in reducing wildlife mortality on a heavily traveled highway in Florida. Biological Conservation 118, 619-631.
| Crossref | Google Scholar |
Emerson SB, Diehl D (1980) Toe pad morphology and mechanisms of sticking in frogs. Biological Journal of the Linnean Society 13, 199-216.
| Crossref | Google Scholar |
Florance D, Webb JK, Dempster T, Kearney MR, Worthing A, Letnic M (2011) Excluding access to invasion hubs can contain the spread of an invasive vertebrate. Proceedings of the Royal Society B: Biological Sciences 278, 2900-2908.
| Crossref | Google Scholar |
Forman RTT, Alexander LE (1998) Roads and their major ecological effects. Annual Review of Ecology and Systematics 29, 207-231.
| Crossref | Google Scholar |
Forti LR, Pontes MR, Augusto-Alves G, Martins A, Hepp F, Szabo JK (2022) Data collected by citizen scientists reveal the role of climate and phylogeny on the frequency of shelter types used by frogs across the Americas. Zoology 155, 126052.
| Crossref | Google Scholar |
Gould J (2023) Tree frog exclusion zone fencing. figshare, Online resource. 10.6084/m9.figshare.22817459
Hamer AJ, Lane SJ, Mahony MJ (2002) Management of freshwater wetlands for the endangered green and golden bell frog (Litoria aurea): roles of habitat determinants and space. Biological Conservation 106, 413-424.
| Crossref | Google Scholar |
Hayward MW, Kerley GIH (2009) Fencing for conservation: restriction of evolutionary potential or a riposte to threatening processes? Biological Conservation 142, 1-13.
| Crossref | Google Scholar |
Hels T, Buchwald E (2001) The effect of road kills on amphibian populations. Biological Conservation 99, 331-340.
| Crossref | Google Scholar |
Hertwig I, Sinsch U (1995) Comparative toe pad morphology in marsupial frogs (genus Gastrotheca): arboreal versus ground-dwelling species. Copeia 1995, 38-47.
| Crossref | Google Scholar |
Homyack JD, Giuliano WM (2002) Effect of streambank fencing on herpetofauna in pasture stream zones. Wildlife Society Bulletin 30, 361-369.
| Google Scholar |
Hou W-S, Chang Y-H, Wang H-W, Tan Y-C (2010) Using the behavior of seven amphibian species for the design of banks of irrigation and drainage systems in Taiwan. Irrigation and Drainage 59, 493-505.
| Crossref | Google Scholar |
Hughes DF, Green ML, Warner JK, Davidson PC (2021) Evaluating exclusion barriers for treefrogs in agricultural landscapes. Wildlife Society Bulletin 45, 305-311.
| Crossref | Google Scholar |
Jehle R, Arntzen JW (2000) Post-breeding migrations of newts (Triturus cristatus and T. Marmoratus) with contrasting ecological requirements. Journal of Zoology 251, 297-306.
| Crossref | Google Scholar |
Jones CG (2012) Grand challenges for the future of ecological engineering. Ecological Engineering 45, 80-84.
| Crossref | Google Scholar |
Keeffe R, Blackburn DC (2020) Comparative morphology of the humerus in forward-burrowing frogs. Biological Journal of the Linnean Society 131, 291-303.
| Crossref | Google Scholar |
Labonte D, Clemente CJ, Dittrich A, Kuo C-Y, Crosby AJ, Irschick DJ, Federle W (2016) Extreme positive allometry of animal adhesive pads and the size limits of adhesion-based climbing. Proceedings of the National Academy of Sciences 113, 1297-1302.
| Crossref | Google Scholar |
Lesbarrères D, Lodé T, Merilä J (2004) What type of amphibian tunnel could reduce road kills? Oryx 38, 220-223.
| Crossref | Google Scholar |
Mark Peaden J, Justin Nowakowski A, Tuberville TD, Buhlmann KA, Todd BD (2017) Effects of roads and roadside fencing on movements, space use, and carapace temperatures of a threatened tortoise. Biological Conservation 214, 13-22.
| Crossref | Google Scholar |
Matos C, Petrovan SO, Wheeler PM, Ward AI (2019) Short-term movements and behaviour govern the use of road mitigation measures by a protected amphibian. Animal Conservation 22, 285-296.
| Crossref | Google Scholar |
Mazerolle MJ, Desrochers A (2005) Landscape resistance to frog movements. Canadian Journal of Zoology 83, 455-464.
| Crossref | Google Scholar |
Mbaiwa JE, Mbaiwa OI (2006) The effects of veterinary fences on wildlife populations in Okavango Delta, Botswana. International Journal of Wilderness 12, 17-41.
| Google Scholar |
Moseby KE, Read JL (2006) The efficacy of feral cat, fox and rabbit exclusion fence designs for threatened species protection. Biological Conservation 127, 429-437.
| Crossref | Google Scholar |
Muir G (2008) Design of a movement corridor for the green and golden bell frog Litoria aurea at Sydney Olympic Park. Australian Zoologist 34, 297-302.
| Crossref | Google Scholar |
Penman T, Muir G, Magarey E, Burns E (2008) Impact of a chytrid-related mortality event on a population of the green and golden bell frog Litoria aurea. Australian Zoologist 34, 314-318.
| Crossref | Google Scholar |
Poole D (2002) Effectiveness of two types of electric fence for excluding the red fox (Vulpes vulpes). Mammal Review 32, 51-57.
| Crossref | Google Scholar |
Schlupp I, Podloucky R (1994) Changes in breeding site fidelity: a combined study of conservation and behaviour in the common toad Bufo bufo. Biological Conservation 69, 285-291.
| Crossref | Google Scholar |
Sillero N (2008) Amphibian mortality levels on Spanish country roads: descriptive and spatial analysis. Amphibia-Reptilia 29, 337-347.
| Crossref | Google Scholar |
Smith JM, Barnes WJP, Downie JR, Ruxton GD (2006) Structural correlates of increased adhesive efficiency with adult size in the toe pads of hylid tree frogs. Journal of Comparative Physiology A 192, 1193-1204.
| Crossref | Google Scholar |
Vos CC, Chardon JP (1998) Effects of habitat fragmentation and road density on the distribution pattern of the moor frog Rana arvalis. Journal of Applied Ecology 35, 44-56.
| Crossref | Google Scholar |
Wallander J, Isaksson D, Lenberg T (2006) Wader nest distribution and predation in relation to man-made structures on coastal pastures. Biological Conservation 132, 343-350.
| Crossref | Google Scholar |
Woltz HW, Gibbs JP, Ducey PK (2008) Road crossing structures for amphibians and reptiles: informing design through behavioral analysis. Biological Conservation 141, 2745-2750.
| Crossref | Google Scholar |
Zug GR, Lhon WZ, Min TZ, Kyaw K, Thin T, Win H, Nyein MTD, Aung K, Tin KT (2001) Durability of silt-fencing for drift-fence arrays at a tropical site. Herpetological Review 32, 235 236.
| Google Scholar |



