Gravel-associated organic material is important to quantify soil carbon and nitrogen stocks to depth in an agricultural cropping soil
Clive A. Kirkby A , John A. Kirkegaard A and Alan E. Richardson A *
A *
A CSIRO Agriculture & Food, GPO Box 1700, Canberra, ACT 2601, Australia.
Soil Research 60(3) 224-233 https://doi.org/10.1071/SR21140
Submitted: 1 June 2021 Accepted: 7 September 2021 Published: 16 November 2021
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Gravel is a common constituent in soil and is routinely excluded when estimating soil carbon (C) and nitrogen (N) stocks.
Aims: We investigated the contribution that the gravel fraction (>2 mm) makes to C and N stocks in an agricultural soil.
Methods: The amount of gravel and the C and N content of gravel-associated organic matter (OM) was assessed to 180 cm in a long-term cropping soil with differing nutrient treatments.
Key results: Gravel-associated C and N accounted for ∼5% of the total C and N stocks in the upper layers (0–30 cm) of soil and up to 40% below 100 cm. The C:N ratio of the gravel-associated OM was similar to that in fine earth fraction (FEF) soil, with C:N ratio of ∼13 in surface layers to ∼8 at depth.
Conclusions: We estimated that 19% and 23% of the total stock of C and N, respectively, were associated with gravel over the whole soil profile. In the two nutrient treatments, with differing C and N stocks in the FEF, gravel-associated OM accounted for 9.3–10.6 t C ha−1 and 1.1–1.3 t N ha−1.
Implications: Our work highlights the significance of gravel in contributing to soil OM and the importance of sampling to depth to estimate soil C and N stocks. Importantly, disregard of the gravel fraction results in an underestimation of total soil C and N, which has implications for the accounting of C in agricultural soils and for the development of strategies to sequester soil C.
Keywords: carbon, C:N ratio, coarse fraction, gravel, sequestration, soil, SOM, stoichiometry.
Introduction
Soils are the largest sink of the global terrestrial carbon (C) cycle. It is estimated that soils across the globe hold approximately 2200 Gt of C, of which 1500 Gt is contained in soil organic matter (SOM), an amount three times that in the terrestrial biomass and twice that in the atmosphere (Corti et al. 2002; Scharlemann et al. 2014). SOM and the C it contains, is critical to maintain soil quality as it affects many of the soil chemical, physical and biological properties that contribute to soil function. Despite this, approximately half of all soil organic carbon (SOC) in managed ecosystems has been lost to the atmosphere during the last two centuries, which is a major factor leading to soil degradation and declining soil quality (Schlesinger 1984; Lal 2004, 2006). Although most estimates of global SOC stocks only consider total C content to 1 m depth or less, it is now well established that there are substantial amounts of SOC below 1 m that need to be accounted for to achieve more accurate estimates of global C stocks (Harrison et al. 2011; Rumpel and Kögel-Knabner 2011; James et al. 2014). Soil C associated with the coarse mineral fraction (CMF) of soil (i.e. the fraction >2 mm diameter including coarse sand, gravel and stones) is also generally not considered in estimates of C stocks. Standardised procedures to measure stocks of SOC routinely use sieving to remove coarse material (including the CMF), based on it being greater than 2 mm in diameter (ISRIC 2002; Department of the Environment 2014; FAO 2020; Smith et al. 2020). Historically, it has been assumed that the amount of SOC associated with gravel is small and chemically inert, making a negligible contribution to soil function (Ugolini et al. 1996). Most estimates of SOC have therefore almost exclusively measured C in the fine earth fraction (FEF) only, which excludes the CMF (i.e. gravel and stones) along with the removal of identifiable plant material. For example, FAO (2020) and Wang et al. (2014) explain that the coarse fraction of the soil has negligible capacity to store C, therefore it is removed before analysis and the SOC content is measured in the FEF. In addition, several procedures for whole soils specifically exclude the gravel component in the calculation of SOC (Perruchoud et al. 2000; Stolbovoy et al. 2007; Liu et al. 2013; Department of the Environment 2014; FAO 2020). Several justifications are put forward for this based on (i) agricultural soils generally have low amounts of gravel, so techniques for soil analysis primarily focus on the FEF only, (ii) it is generally considered that being inert, gravel does not contribute to the soil chemistry, (iii) it is often difficult to obtain a homogeneous sample for analysis, especially if small samples are required for chemical analysis in soils with appreciable amounts of gravel and (iv) specialist equipment is required to pulverise the gravel prior to analysis (Ugolini et al. 1996; Harrison et al. 2003).
Several studies, mainly in forest soils have shown that the CMF may be more involved in biogeochemical nutrient cycling than has been commonly assumed. For example, Cromack et al. (1999) showed that ∼60% of the C, nitrogen (N) and phosphorus (P) was in the CMF for a coastal forest site in Oregon. Although distinction between organic and inorganic C was not made, the amount of N and P in the fraction suggested a substantial amount was likely to be organic. Corti et al. (2002) similarly sampled forest and orchard sites across Europe and the Canadian Arctic and found that up to 55% of C that was associated with gravel was organic. Gravel (>2 mm) from soils in seven USA Pacific Northwest forest sites contained up to 46% of the whole-soil C and averaged 23% for the soils that had C in that fraction (Homann et al. 2004). Even after sodium hexametaphosphate treatment to disaggregate soil material, up to 20% of the whole-soil C was shown to remain in the >2 mm fraction. In two related studies of 17 forest sites in northern and central America, Whitney and Zabowski (2004) and Zabowski et al. (2011) found that 1–25% of the soil C and 0.3–37% of the soil N was contained in the CMF. For some Australian forest sites, Bauhus et al. (2002) has shown that while a substantial amount of >2 mm C was present as charcoal, C in the >2 mm fraction contributed 7.3–11.4% of the total C.
It is generally accepted that ‘stony soils’ (or gravel soils) are widely distributed throughout the world (Corti et al. 2002), although the profile distribution and quantification of the gravel content of soils remain relatively poorly documented. Poesen (1990) reported that soils rich in coarse material are widespread in Italy where they may constitute more than 60% of the land in the Mediterranean region. In Australia, gravelly soils are common and occur across many cropping regions and pasture soils where variable amounts of gravel occur within the soil profile, and generally show increases with soil depth most often being higher in deeper soil layers (i.e. below 1 m) but still within the rooting zone of most annual agricultural crops. At a global scale, gravel soils are clearly not only confined to forest soil but are also widespread in agricultural regions. Given that the CMF of these soils is likely to be variable and contain a more significant amount of C than previously recognised, it is important to understand the potential contribution of the CMF to total soil C stock.
We utilised a long-term tillage experiment at a single field site in the wheatbelt of eastern Australia to investigate the amount of gravel-associated OM in soil and its contribution to total soil C stocks throughout the profile of a cropping soil with differing nutrient input. Previously we had sampled this soil extensively to monitor changes in soil C levels to a depth of 160 cm in the FEF only in response to tillage management and nutrient inputs (Kirkby et al. 2016). In the course of the work, gravel was routinely removed from soil samples prior to analysis, but the existence of a distinct ‘brownish’ coating on the gravel suggested it may contain organic material and thus contribute to the stock of total soil C. In this paper we specifically test the hypothesis that gravel makes an important contribution to the total soil C over the depth profile of an arable agricultural soil with a history of long-term cropping.
Materials and methods
Site description
The study was carried out at a field site located at ‘Oxton Park West’, a mixed farming enterprise near Harden, NSW, in the south-eastern wheatbelt of Australia (34°30′S, 148°17′E). The site is on a well-drained, elevated (497 m above sea level) and sloping (3%) position with a typical Red Chromosol soil (Isbell 2002) with strong texture contrast between soil horizons. The surface texture of the soil was classified as a sandy-loam (15% clay, 10% silt and 75% sand after removal of the coarse fraction) as described in Table 1. Throughout the profile the soil had negligible carbonate content. Such soils (often termed duplex soils) are widespread in eastern Australia and are typically used for annual crops as described by Isbell (2002). The site was part of a long-term field experiment established in 1990 to assess the effects of different tillage and stubble management treatments on soil fertility and crop performance in a continuous cropping system, as first reported by Kirkegaard et al. (1994) and more recently by Kirkby et al. (2016). Over the past 30 years the site had been subject to continuous cropping with annual planting of wheat (Triticum aestivum), canola (Brassica napus) or occasional pulse legumes grown in rotation. Crop management treatments with variable nutrient input and diverse crop residue management systems each year were replicated four times in a randomised block design and individual treatment plots (30 m × 6 m) comprised two paired sub-plots (30 m × 2 m), side by side, separated by a central 1-m buffer to allow controlled-traffic management. At this site, Kirkby et al. (2016) previously reported soil C stocks in the FEF to a depth of 160 cm over a 5-year study (2007–2012) that investigated the positive role of supplementary nutrients in promoting the sequestration of soil C in response to the incorporation of C-rich crop residues.

|
Preliminary assessment of gravel-associated organic matter
During the studies on C-sequestration (2007–2012; Kirkby et al. 2016) the coarse fraction (>2 mm; hereafter referred to as gravel) was routinely removed from the samples using a standard 2 mm sieving procedure as recommended for soil water and nutrient measurements on the FEF (ISRIC 2002). However, observations of a suspected ‘organic coating’ on the removed material prompted an investigation into the nature of the coating. Gravel from two separate soil samples (0–10 cm) collected in 2013 and 2014 was initially assessed, whereby sub-samples were progressively subjected to a series of cleaning procedures in order to determine how strongly the ‘coated material’ was associated with the gravel and to better understand its composition. The procedures included the standard 2 mm sieving as routinely carried out for soil C analysis to remove gravel from the FEF, and then steps to progressively remove the coating with physical and mild or more severe chemical treatments (Table 2).

|
The initial separation of the gravel from the FEF yielded ∼150 g of gravel from several kilograms of soil. From this ∼50 g was retained for direct analysis and ∼100 g was subjected to physical disruption by processing in a puck mill for 30 s without rings or pucks to dislodge any loosely attached fine material (Table 2), whereby both the treated gravel and removed material were retained for analysis (procedure 2). Separate ∼25 g sub-samples were then subjected to washing in either water with a detergent added (procedure 3), or by washing in a 30% hydrogen peroxide solution (procedure 4). Following washing, the samples were rinsed with deionised water and then dried. All samples were subsequently pulverised (2 min) by puck mill (Labtechnics pulverising mill, Model LM1, Adelaide Australia) to a powder of no more than 50 μm particle size. The samples were then analysed for total C and N using a mass spectrometer dry combustion analyser (IRMS; Europa Scientific Model 20-20, Crewe, UK). In addition, the fine material that was dislodged from gravel samples during physical disruption was also collected and analysed for C and N.
Soil gravel and gravel-associated organic matter
Extensive sampling was conducted at the site in 2015 to assess the amount of gravel throughout the soil profile (to a depth of 180 cm) and to determine the amount and composition of associated organic matter (OM) in both the FEF and gravel (CMF > 2 mm) fractions. Two treatments as reported by Kirkby et al. (2016) were sampled where the previous application of supplementary nutrients to incorporated crop residue had significantly changed the level of FEF-C down the soil profile. These treatments were designated Residue Incorporate + Nutrients (RI + N) and Residue Incorporate − Nutrients (RI − N) and had total C stocks to 160 cm depth of 63.5 and 54.8 t C ha−1 in the FEF, respectively (Kirkby et al. 2016).
Three soil cores (43 mm diameter) were taken to a depth of 180 cm from across the plots in four replicates from each of the RI + N and RI − N treatments (i.e. total of 24 cores from eight plots) using a tractor-mounted hydraulic corer. Each core was divided into eight soil depth increments, 0–10, 10–20, 20–30, 30–60, 60–90, 90–120, 120–150 and 150–180 cm and the soil from the individual depths from each plot (three cores) was bulked for each replicate. The composite soil samples were air dried and sieved to separate the CMF and FEF fractions as outlined by procedure 1 in Table 2, and the weight of each mass fraction was determined. Separate 100 g sub-samples of each fraction were then pulverised in a puck mill and analysed for C and N as outlined above. The concentrations of C and N per soil fraction mass were determined and C and N stocks (t ha−1) for each soil layer were calculated using averaged soil bulk densities (Table 1) and expressed both as stocks per 10 cm of soil layer and for the entire soil profile to 180 cm depth.
Statistical analysis
Soil data (%C and %N, C:N ratios, C and N stocks) were analysed and presented separately at each sampling depth using two-way ANOVA (Genstat V20 and SigmaPlot 14; Supplementary Tables S1 and S2) to test the main effect and interactions for components (gravel and FEF) and nutrient treatments (+/−). Where significant differences occurred (P < 0.05), the treatment means were compared using l.s.d. Analysis was also carried out on whole of profile C and N stocks by analysing the total C and N in gravel and FEF added across all depths. In order to analyse the data for the proportion of C and N on gravel (i.e. percentages), the analysis was carried out on ArcSin-transformed data, while the back-transformed data were presented graphically.
Results
Preliminary assessment of gravel-associated OM
The gravel sieved from the soil was mostly 2–5 mm in size (with occasional peds up to 10 mm; see Fig. 1) and was generally spherical to irregular shaped. Analysis by XRF indicated it was mostly quartz-based with some of the larger pieces (representing less than ∼5% of the total) being iron stone (data not presented). After routine sieving, the gravel had a distinct brownish colour and examination under a low power microscope suggested an ‘organic-like’ coating (Fig. 1a). The gravel subjected to physical disruption had a lighter brown to yellow colour (Fig. 1b). Samples washed with mild detergent were cleaner but were more variable in colour (Fig. 1c). After washing with hydrogen peroxide the gravel was highly bleached with apparent removal of the external coating (Fig. 1d).
Analysis of the gravel following the different physical and chemical treatments indicated that the ‘coating’ was mostly organic in nature (Table 3). Vigorous shaking in a puck mill (without the puck) removed 11% of the C and with no detectable change in N. Analysis of the fine material dislodged by vigorous shaking verified it was organic in nature based on its composition being similar to that of previously analysed FEF-C and -N, with 12.9% and 1.2% C and N, respectively (Table 3; Kirkby et al. 2016). Washing the gravel in mild detergent removed a greater amount of the coating with 53.3% and 20.9% decrease in C and N, respectively. The peroxide treatment removed 70–72% of the C and N on the originally sieved gravel, providing further evidence that coating of the gravel was organic.

|
Distribution of gravel and gravel-associated C and N to 1.8 m soil depth
The mean gravel content across the site increased from around 5% mass fraction in the surface 0–10 cm layer to ∼45% in the deepest 150–180 cm soil layer (Fig. 2). There was no difference in the proportion of gravel across the four experimental blocks or between the RI + N and RI − N treatments.

|
There was a decline in C concentration with depth for both the FEF-C and gravel-associated C fractions, with a more evident reduction of FEF-C in the surface layers (Fig. 3a). FEF-C concentrations were, however, significantly higher than the gravel-C at all depths. As previously reported (Kirkby et al. 2016), the addition of nutrients to the returned crop residues (RI + N treatment) increased the C levels in the FEF through the entire soil profile. However, as shown here, this effect was primarily associated with the FEF only in the surface layers (above 30 cm), and the significant interaction of soil fraction by nutrient addition in the surface was not maintained throughout the entire soil profile (Fig. 3a).
Nitrogen concentrations for the FEF and gravel fractions similarly declined with depth and significantly differed between the two fractions at all depths. The addition of nutrients to the RI + N treatment increased the soil N concentration with a more significant effect in the FEF above 30 cm, but with generally no effect at depths below this (Fig. 3b).
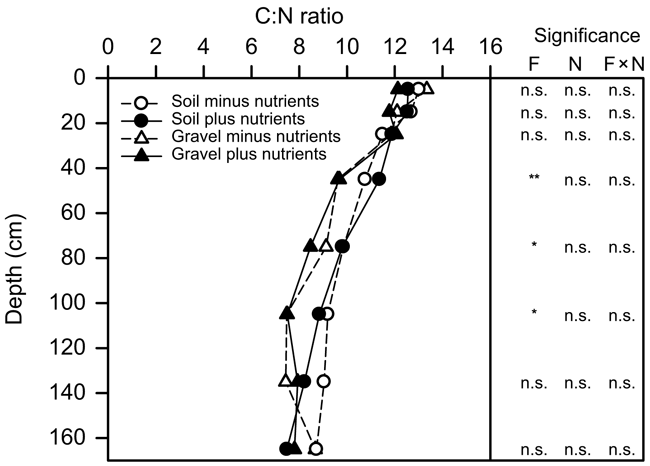
The C:N ratio of both the FEF and gravel declined steadily from an average of ∼13 in the surface soil to ∼8 in the subsoil (Fig. 4). There was no effect of nutrient addition in any soil layer and no interaction between the soil fraction and nutrient additions. The FEF and gravel components had similar C:N ratios in the surface layers and at depth, but in the 40–100 cm layer the gravel had a consistently lower C:N ratio than the FEF (Fig. 4).
Stocks of gravel-associated C and N and whole-soil profile stocks
At all soil depths, there were greater C and N stocks in the FEF than in the gravel fraction (Fig. 5). This was most obvious in the surface layers but declined with depth as the amount of gravel increased (Fig. 2) and as the difference in C and N concentration between the components diminished (Fig. 3). Interestingly, while there was a clear decrease in FEF-C and -N stocks with depth, the gravel-C and -N stocks increased slightly with depth primarily due to the large increase in gravel content below 30 cm (Fig. 2). The addition of nutrients generally resulted in higher FEF-C and -N stocks throughout the profile, although this varied with depth and was most evident for C and N in the top 20 or 30 cm layers of the soil, respectively, where a significant interaction between the soil fraction and the effect of nutrient addition occurred. By contrast, there was little impact of the nutrient treatment on C or N stocks in the gravel fraction at any depth (Fig. 5).
The contribution of the gravel-associated C and N to the total C and N stocks (i.e. gravel-C and -N as a percentage of gravel plus FEF-C and -N) increased markedly down the soil profile from around 5% in the top 20 cm to more than 30–40% below 1 m, and was similar for both C and N (Fig. 6). There was no impact of nutrient treatment on the proportion of the C and N stocks contributed by the gravel across the various soil depths.
Over the whole soil profile to 1.8 m depth, gravel-associated C and N accounted for an average of 10 and 1.2 t ha−1 of C and N, respectively (Table 4). The gravel thus constituted 19% of the total C stock within the soil profile and N constituted 23% of the total soil N. (Table 4). In addition, there was more C associated with both the FEF and the gravel fractions in the RI + N treatment, but only more FEF-N in the RI + N treatment with no difference for the gravel-associated N.
Discussion
Carbon and N associated with the gravel fraction in this annually cropped agricultural soil made a significant contribution to the total stocks of soil C and N. This is consistent with other studies previously reported for a number of forest soils (Cromack et al. 1999; Corti et al. 2002; Homann et al. 2004). Approximately 19% of the total soil C and 23% of the total N was associated with the gravel fraction to a soil depth of 180 cm (Table 4). Given that many agricultural soils may contain a significant proportion of gravel, and that most routine procedures for estimation of C stocks specifically promote the removal and exclusion of the gravel component, it is evident that disregarding the gravel fraction will result in a significant underestimation of total soil C and N. This has major implications for the accounting of soil C in agricultural soils used for crops and pastures and for the implementation of management strategies in agricultural systems that specifically aim to sequester soil C.
The proportion of gravel in the soil we studied increased with depth from approximately 5% by mass at the surface to approximately 45% at 180 cm depth, which is a common feature of many soils globally and across Australia (Ugolini et al. 1996; Corti et al. 2002; Whitney and Zabowski 2004). Consequently, the amount of total soil C associated with gravel in the soil we studied ranged markedly, from <5% in surface soil layers to 30–40% in soil layers below 1 m depth. Although Kirkby et al. (2016) previously measured C in the FEF (non-gravel component) of this soil only, they found that approximately 25% of the FEF-C was below 90 cm. Without consideration of the gravel component this represents a substantial underestimate of the actual soil C content. In addition, because the C:N ratio of the FEF decreased with depth from ∼12 at the surface to ∼6 at 1.6 m depth, some 65% of the FEF-N was also below 90 cm. This clearly highlights the need to sample to sufficient depth to obtain an accurate measure of total C and N stocks. The tendency for gravel to increase with depth in many soils further compounds the need to sample to depth and, more importantly, to consider changes in the amount of gravel with depth and its associated contribution to C and N stocks. Although disregard of the gravel component might be relatively insignificant for estimates of C and N in surface layers (i.e. top 10 cm), it can become quite significant for estimates over wider soil profile depths where gravel often becomes more predominant.
A pronounced decline in the FEC-C and -N concentration with depth was most evident in the top 30 cm of soil as compared with smaller declines in gravel-associated C and N concentration in deeper layers (Fig. 3). In contrast, Cromack et al. (1999) found that both FEF-C and -N and gravel-C and -N decreased similarly with depth. Ugolini et al. (1996) and Corti et al. (2002) investigated multiple profiles and also found considerable variation in gravel-C and -N concentrations with depth. Such variation and differences across studies will depend on soil type and be associated with soil textural differences. For example, the texture of the FEF on the Chromosol soil at the site we studied had an appreciable amount of clay that varied with depth, which is typical of these ‘duplex’ soils that have strong textural differences between horizons (Isbell 2002). Such characteristics presumably would influence the potential for binding of OM with depth. This was supported by the increase in FEF-C and -N reported by Kirkby et al. (2016) especially when crop residues were incorporated with extra nutrients. In these soils, the quartz-based gravel also increased appreciably with depth (Fig. 2) and importantly appeared to have a coating of organic-like material (Fig. 1a). Examination of the gravel under a low-power microscope suggested that the OM may be associated with a coating of FEF-C, rather than the OM being directly associated with the gravel itself. This coating containing OM was removed to various extents with a range of mechanical and progressively stronger washing procedures (Table 3). The high C and N concentration of the material that was shaken off the gravel further supports the organic nature of this material. The total mass of the coating material on the gravel is obviously much less than the mass of the total soil FEF. Nonetheless, and on the basis of the similar gravel-C and -N concentrations throughout much of the soil profile, we suggest that the gravel was uniformly coated with the FEF soil, while the FEF throughout the profile was presumably able to differentially accumulate OM (largely depending on clay content) as was observed in the differences in nutrient treatments with depth reported by Kirkby et al. (2016).
Interestingly the C:N ratio of both FEF and gravel both declined with depth, from ∼13 at the soil surface to ∼8 at 180 cm depth, which was evident despite a substantial increase in gravel with depth. This decline in C:N ratio of both the FEF and gravel has also been reported by others (Ugolini et al. 1996; Corti et al. 2002). The consistency in decline of C:N ratio for both the FEF and gravel (Fig. 4) suggests that the mechanisms for the formation of the gravel-associated OM and FEF-OM might be common for both fractions across the same depths, but for both fractions different across soil depths. In a recent review of the literature, Coonan et al. (2020) and Liang et al. (2019) reported that a significant proportion of SOM derives from microbial turnover, where microbial detritus accounts for on average 59% and 64% of the total soil FEF-C in arable agricultural and grassland systems, respectively, with fungal detritus contributing ∼70% of this pool in both systems (Coonan et al. 2020). The variation in C:N with depth may thus be associated with variation in the ratio of fungal to bacteria with depth whereby surface soils are more fungal dominant and microbial cycling at depth is mediated more by bacterial processes. This is consistent with the narrower (i.e. lower) C:N ratio that was observed here at depth and the more narrow C:N ratio of bacterial biomass compared to fungal biomass (Richardson et al. 2014).
Our results highlight that discarding gravel from soil samples during processing along with sampling to inadequate depths, may lead to significant underestimates of total C and N stocks. In the annually cropped agricultural soil reported here, the C and N stocks were thus previously underestimated by approximately 20% as reported for this soil by Kirkby et al. (2016). Interestingly though, while the FEF-C and -N stocks for the RI + N and RI − N treatments reported in Kirkby et al. (2016) differed by 8.7 t C ha−1 and 0.77 t N ha−1, respectively (in 2012), they differed here in 2015 by 8.6 t C ha−1 and 0.85 t N ha−1, such that the differences in C and N stocks in response to nutrient supplementation were fairly consistent over time. By contrast if the gravel-associated C and N was also included, the difference between the two treatments would equate to a total of 9.9 t ha−1 for C and 1.03 t ha−1 for N to 180 cm of soil depth. Thus, although omission of the gravel-associated C and N changed the absolute magnitude of the C and N stocks in the soil, the relative difference associated with the FEF between the RI + N and RI − N treatments reported by Kirkby et al. (2016) were maintained. This indicates that the contribution of the gravel fraction to C and N stocks in the soil was essentially similar in both the RI + N and RI − N treatments and that the observed effect of nutrient treatments in building C stocks in this soil reported by Kirkby et al. (2016) remain valid.
Despite a modest decline in the gravel-associated C:N ratio over the soil profile studied here, the proportion of total C and N on the gravel approximately followed the amount of gravel in the profile. Thus, as a simple correction to obtain a more accurate measure of total soil C and N stocks across different soil treatments based on analysis of the FEF only (i.e. where it might be necessary to remove gravel during processing), the C and N could be assessed on a single composite and representative gravel sample from within the profile. Analysis of this for C and N concentration could then be used to correct for total soil C and N within the FEF based on the proportions of gravel found as a mass fraction across soil depths. Alternatively, analysis of the whole soil could be undertaken (i.e. with inclusion of the gravel fraction) to provide a more accurate estimate of total soil C and N stocks, as these stocks may otherwise be underestimated. This also requires careful consideration of the presence of larger stones and coarse OM and how this is either treated or removed with respect to determination of soil C pools. Further investigations to explore the proportion of total C contributed by gravel on a wider range of agricultural soils are thus warranted.
In this paper we report that the gravel fraction of a soil used for continuous cropping in Eastern Australia accounted for 19% and 23% of the total C and N stocks, respectively, to a depth of 180 cm. In the Red Chromosol soil we studied, the gravel content of the soil ranged from ∼5% mass fraction in the surface layers to ∼45% at depth and as such accounted for ∼5% of the total C and N in the upper layers (0–30 cm) of the soil profile and up to 40% of the total soil C and N below 100 cm. The gravel-associated OM occurred as a surface coating of FEF-C that was removed to varying extents (up to 70%) by mechanical disruption, washing or peroxide treatment. Gravel-associated C and N was evident in two crop management systems with contrasting nutrient management histories that had previously been shown to have different stocks of C and N in the FEF. Across these two treatments, gravel-associated OM accounted for up to 10.6 t C ha−1 and 1.32 t N ha−1. In conclusion, our work highlights the significance of gravel in contributing to SOM and the importance of sampling to depth to obtain more reliable estimates of total stocks of C and N in soil. Importantly, and as highlighted in the study, a disregard of the gravel fraction during soil processing may result in a significant underestimation of total soil C and N stocks. This has consequences for the accounting of soil C in agricultural soils and for the development and implementation of practices that specifically aim to sequester C. Furthermore, it has major implications for potential payment systems that are directed at C sequestration and for C accounting systems in agricultural soils subject to different management strategies.
Supplementary material
Supplementary material is available online.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest in the publication of this paper. AE Richardson was a Guest Editor for Soil Research special issue (2021, vol. 59, issue 6), Soil Organic Matter in a Stressed World.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
The authors gratefully acknowledge the O’Connor family, Oxton Park West, for provision of the land for the long-term experiment and assistance in managing the site which commenced in 1990. We also thank technical and farm staff from CSIRO Ginninderra Research Station for assisting in trial management, and Dr Julianne Lilley CSIRO for expert assistance in the graphics.
References
Bauhus J, Khanna PK, Hopmans P, Weston C (2002) Is soil carbon a useful indicator of sustainable forestsoil management? – a case study from native eucalypt forests of south-eastern Australia. Forest Ecology and Management 171, 59–74.| Is soil carbon a useful indicator of sustainable forestsoil management? – a case study from native eucalypt forests of south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Coonan EC, Kirkby CA, Kirkegaard JA, Amidy MR, Strong C, Richardson AE (2020) Microorganisms and nutrient stoichiometry as mediators of soil organic matter dynamics. Nutrient Cycling in AgroEcosystems 117, 273–298.
| Microorganisms and nutrient stoichiometry as mediators of soil organic matter dynamics.Crossref | GoogleScholarGoogle Scholar |
Corti G, Ugolini FC, Agnelli A, Certini G, Cuniglio R, Berna F, Fernandez MJ (2002) The soil skeleton, a forgotten pool of carbon and nitrogen in soil. European Journal of Soil Science 53, 283–298.
| The soil skeleton, a forgotten pool of carbon and nitrogen in soil.Crossref | GoogleScholarGoogle Scholar |
Cromack K, Miller RE, Helgerson OT, Smith RB, Anderson HW (1999) Soil carbon and nutrients in a coastal Oregon douglas-fir plantation with red alder. Soil Science Society America Journal 63, 232–239.
| Soil carbon and nutrients in a coastal Oregon douglas-fir plantation with red alder.Crossref | GoogleScholarGoogle Scholar |
Department of the Environment (2014) Carbon farming initiative, soil sampling and analysis, methods and guidelines. Department of the Environment, Australian Government. Available at https://www.scu.edu.au/media/scueduau/eal/documents/Carbon-farming-initiative-soil-sampling-and-analysis-method-guidelines.pdf [accessed December 2020]
FAO (2020) ‘A protocol for measurement, monitoring, reporting and verification of soil organic carbon in agricultural landscapes, GSOC-MRV Protocol’. (FAO: Rome).
| Crossref |
Harrison RB, Adams AB, Licata C, Flaming B, Wagoner GL, Carpenter P, Vance ED (2003) Quantifying deep-soil and coarse-soil fractions: avoiding sampling bias. Soil Science Society America Journal 67, 1602–1606.
| Quantifying deep-soil and coarse-soil fractions: avoiding sampling bias.Crossref | GoogleScholarGoogle Scholar |
Harrison RB, Footen PW, Strahm BD (2011) Deep soil horizons: contribution and importance to soil carbon pools and in assessing whole-ecosystem response to management and global change. Forest Science 57, 67–76.
| Deep soil horizons: contribution and importance to soil carbon pools and in assessing whole-ecosystem response to management and global change.Crossref | GoogleScholarGoogle Scholar |
Homann PS, Remillard SM, Harmon ME, Bormann BT (2004) Carbon storage in coarse and fine fractions of pacific northwest old-growth forest soils. Soil Science Society America Journal 68, 2023–2030.
| Carbon storage in coarse and fine fractions of pacific northwest old-growth forest soils.Crossref | GoogleScholarGoogle Scholar |
ISRIC (2002) Procedures for soil analysis – technical paper 09 (6th edition). ISRIC (International Soil Reference and Information Centre), Wageningen, The Netherlands.
Isbell RF (2002) ‘The Australian soil classification’. Australian soil and land survey handbook, Series 4. (CSIRO Publishing: Melbourne, Australia).
| Crossref |
James JN, Devine WD, Harrison RB, Terry TA (2014) Deep soil carbon: quantification and modeling in subsurface layers. Soil Science Society of America Journal 78, S1–S10.
| Deep soil carbon: quantification and modeling in subsurface layers.Crossref | GoogleScholarGoogle Scholar |
Kirkby CA, Kirkegaard JA, Richardson AE, Wade LJ, Blanchard C, Batten G (2011) Stable soil organic matter: a comparison of C:N:P:S ratios in Australian and other world soils. Geoderma 163, 197–208.
| Stable soil organic matter: a comparison of C:N:P:S ratios in Australian and other world soils.Crossref | GoogleScholarGoogle Scholar |
Kirkby CA, Richardson AE, Wade LJ, Conyers M, Kirkegaard JA (2016) Inorganic nutrients increase humification efficiency and C-sequestration in an annually cropped soil. PLoS ONE 11, e0153698
| Inorganic nutrients increase humification efficiency and C-sequestration in an annually cropped soil.Crossref | GoogleScholarGoogle Scholar | 27144282PubMed |
Kirkegaard JA, Angus JF, Gardner PA, Muller W (1994) Reduced growth and yield of wheat with conservation cropping. I. Field studies in the first year of the cropping phase. Australian Journal of Agricultural Research 45, 511–528.
| Reduced growth and yield of wheat with conservation cropping. I. Field studies in the first year of the cropping phase.Crossref | GoogleScholarGoogle Scholar |
Lal R (2004) Soil carbon sequestration to mitigate climate change. Geoderma 123, 1–22.
| Soil carbon sequestration to mitigate climate change.Crossref | GoogleScholarGoogle Scholar |
Lal R (2006) Enhancing crop yields in the developing countries through restoration of the soil organic carbon pool in agricultural lands. Land Degradation & Development 17, 197–209.
| Enhancing crop yields in the developing countries through restoration of the soil organic carbon pool in agricultural lands.Crossref | GoogleScholarGoogle Scholar |
Liang C, Amelung W, Lehmann J, Kästner M (2019) Quantitative assessment of microbial necromass contribution to soil organic matter. Global Change Biology 25, 3578–3590.
| Quantitative assessment of microbial necromass contribution to soil organic matter.Crossref | GoogleScholarGoogle Scholar | 31365780PubMed |
Liu S, Wei Y, Post WM, Cook RB, Schaefer K, Thornton MM (2013) The unified North American soil map and its implication on the soil organic carbon stock in North America. Biogeosciences 10, 2915–2930.
| The unified North American soil map and its implication on the soil organic carbon stock in North America.Crossref | GoogleScholarGoogle Scholar |
Perruchoud D, Walthert L, Zimmermann S, Lüscher P (2000) Contemporary carbon stocks of mineral forest soils in the Swiss Alps. Biogeochemistry 50, 111–136.
| Contemporary carbon stocks of mineral forest soils in the Swiss Alps.Crossref | GoogleScholarGoogle Scholar |
Poesen J (1990) Erosion process research in relation to soil erodibility and some implications for improving soil quality. In ‘Soil degradation and rehabilitation in Mediterranean environmental conditions’. (Eds J Albaladejo, MA Stocking, E Diaz) pp. 159–170. (C.S.I.C.: Murcia, Spain)
Richardson AE, Kirkby CA, Banerjee S, Kirkegaard JA (2014) The inorganic nutrient cost of building soil carbon. Carbon Management 5, 265–268.
| The inorganic nutrient cost of building soil carbon.Crossref | GoogleScholarGoogle Scholar |
Rumpel C, Kögel-Knabner I (2011) Deep soil organic matter—a key but poorly understood component of terrestrial C cycle. Plant and Soil 338, 143–158.
| Deep soil organic matter—a key but poorly understood component of terrestrial C cycle.Crossref | GoogleScholarGoogle Scholar |
Scharlemann JPW, Tanner EVJ, Hiederer R, Kapos V (2014) Global soil carbon: understanding and managing the largest terrestrial carbon pool. Carbon Management 5, 81–91.
| Global soil carbon: understanding and managing the largest terrestrial carbon pool.Crossref | GoogleScholarGoogle Scholar |
Schlesinger W (1984) Soil organic matter: a source of atmospheric CO2. In ‘The role of terrestrial vegetation in the global carbon cycle: measurements by remote sensing’. (Eds GM Woodwell) pp. 111–127. (SCOPE, John Wiley & Sons)
Smith P, Soussana J, Angers D, Schipper L, Chenu C, Rasse DP, Batjes NH, van Egmond F, McNeill S, Kuhnert M, Arias-Navarro C, Olesen JE, Chirinda N, Fornara D, Wollenberg E, Álvaro-Fuentes J, Sanz-Cobena A, Klumpp K (2020) How to measure, report and verify soil carbon change to realize the potential of soil carbon sequestration for atmospheric greenhouse gas removal. Global Change Biology 26, 219–241.
| How to measure, report and verify soil carbon change to realize the potential of soil carbon sequestration for atmospheric greenhouse gas removal.Crossref | GoogleScholarGoogle Scholar | 31469216PubMed |
Stolbovoy V, Montanarella L, Filippi N, Jones A, Gallego J, Grassi G (2007) ‘Soil sampling protocol to certify the changes of organic carbon stock in mineral soil of the European Union. Version 2’. (Office for Official Publications of the European Communities: Luxembourg)
Ugolini FC, Corti G, Agnelli A, Piccardi F (1996) Mineralogical, physical and chemical properties of rock fragments in soil. Soil Science 161, 521–542.
| Mineralogical, physical and chemical properties of rock fragments in soil.Crossref | GoogleScholarGoogle Scholar |
Wang M, Su Y, Yang X (2014) Spatial distribution of soil organic carbon and its influencing factors in desert grasslands of the Hexi corridor, northwest China. PLoS ONE 9, e94652
| Spatial distribution of soil organic carbon and its influencing factors in desert grasslands of the Hexi corridor, northwest China.Crossref | GoogleScholarGoogle Scholar | 24732375PubMed |
Whitney N, Zabowski D (2004) Total soil nitrogen in the coarse fraction and at depth. Soil Science Society America Journal 68, 612–619.
| Total soil nitrogen in the coarse fraction and at depth.Crossref | GoogleScholarGoogle Scholar |
Zabowski D, Whitney N, Gurung J, Hatten J (2011) Total soil carbon in the coarse fraction and at depth. Forest Science 57, 11–18.
| Total soil carbon in the coarse fraction and at depth.Crossref | GoogleScholarGoogle Scholar |