Sperm-borne sncRNAs: potential biomarkers for semen fertility?
Eli Sellem A * , Hélène Jammes B C and Laurent Schibler AA R&D Department, ALLICE, 149 rue de Bercy, 75012 Paris, France.
B Université Paris Saclay, UVSQ, INRAE, BREED, 78350 Jouy en Josas, France.
C Ecole Nationale Vétérinaire d’Alfort, BREED, 94700 Maisons–Alfort, France.
Reproduction, Fertility and Development 34(2) 160-173 https://doi.org/10.1071/RD21276
Published online: 15 October 2021
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the IETS. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Semen infertility or sub-fertility, whether in humans or livestock species, remains a major concern for clinicians and technicians involved in reproduction. Indeed, they can cause tragedies in human relationships or have a dramatic overall negative impact on the sustainability of livestock breeding. Understanding and predicting semen fertility issues is therefore crucial and quality control procedures as well as biomarkers have been proposed to ensure sperm fertility. However, their predictive values appeared to be too limited and additional relevant biomarkers are still required to diagnose sub-fertility efficiently. During the last decade, the study of molecular mechanisms involved in spermatogenesis and sperm maturation highlighted the regulatory role of a variety of small non-coding RNAs (sncRNAs) and led to the discovery that sperm sncRNAs comprise both remnants from spermatogenesis and post-testicular sncRNAs acquired through interactions with extracellular vesicles along epididymis. This has led to the hypothesis that sncRNAs may be a source of relevant biomarkers, associated either with sperm functionality or embryo development. This review aims at providing a synthetic overview of the current state of knowledge regarding implication of sncRNA in spermatogenesis defects and their putative roles in sperm maturation and embryo development, as well as exploring their use as fertility biomarkers.
Keywords: epididymosomes, epigenetic, fertility, fertility prediction, non-genetic sperm legacy, semen, sncRNAs, sperm-born sncRNAs.
Introduction
A fully mature sperm is the result of multiple processes, starting before birth in the testis, followed by several maturation steps during the epididymis transit or following interactions with seminal plasma and ending with final modifications in the female tract. Multiple hormones and intermediates are required to achieve all these steps, leading to a sperm capable of fertilising the oocyte and potentially driving the embryo early development. Disruption of any of these processes can lead to sperm abnormalities and potentially reduce semen fertility. In humans, about 50% of infertility cases are related to men issues, with no clear aetiology for 30–50% of these cases. (Hamada et al. 2012). Identification of the disturbance is the first step towards understanding the biological mechanisms involved and potential treatment.
In livestock farming, since sub-fertile males or ejaculates decrease the breeding efficiency and induce economic losses, several semen quality control procedures have been proposed to ensure semen fertility (Vincent et al. 2014) and biomarkers have been searched for to establish effective fertility predictors. For instance, sperm characteristics, functionality and physiology have been assessed using computer analysis semen assessment (CASA) (Amann and Waberski 2014) or flow cytometry (e.g. membrane integrity, mitochondrial potential, oxidation sensitivity, acrosome statue, DNA compaction and fragmentation).
Due to the multifactorial nature of semen fertility, however, individual biomarkers remain insufficient to achieve reliable predictions. Even the combination of these biomarkers by ‘stepwise’ or ‘logistic’ regression approaches have failed to explain more than 40% of the variability in fertility (Sellem et al. 2015). This has led to the hypothesis that additional biomarkers are required to reach good fertility predictions, which should be unrelated to biological processes already included in routine semen quality assessment protocols. In this respect, the study of molecular mechanisms involved in spermatogenesis and sperm maturation have highlighted the role of a variety of sncRNAs (de Mateo and Sassone-Corsi 2014). In addition, the discovery that mature sperm carry thousands of sncRNAs, which comprise both remnants from spermatogenesis and post-testicular sncRNAs acquired through interactions with extracellular vesicles along epididymis, has raised many questions about their putative role. Though their functional significance is still a matter of debate, growing evidence suggests that sperm RNAs are thus delivered to the oocyte at fertilisation (Sendler et al. 2013), providing resources for embryo development (Shi et al. 2020), and being involved in paternal epigenetic transgenerational inheritance (Sharma 2019; Le Blevec et al. 2020). Given their suggested roles both in spermatogenesis and embryo development, sncRNAs have gained interest as relevant biomarkers of semen fertility.
This review aims at providing a synthetic overview of the current state of knowledge regarding implication of sncRNA in spermatogenesis defects and their putative roles in sperm maturation and embryo development, as well as exploring their use as fertility biomarkers.
Discovery, biogenesis and function of the main sncRNA classes
SncRNAs are a broad and heterogeneous family of 18–200 nucleotides-long RNAs, having mainly a regulatory function through either RNA interference, RNA modification or spliceosome regulation. Next-generation sequencing (NGS) has led to the discovery and quantification of a diversity of sncRNA classes (Hombach and Kretz 2016; Wei et al. 2017), including microRNAs (miRNAs), small nuclear RNAs (snRNAs), piwi-interacting RNAs (piRNAs), rRNA-derived small RNAs (rsRNAs) and tRNA-derived small RNAs (tsRNAs). Overall, regulatory sncRNAs are expressed at specific development stages or under particular stimuli and are involved in a growing number of important biological processes. sncRNAs which repress expression of messenger RNA (mRNA) at the post-transcriptional level provide a rapid and adaptive mechanism to modulate gene expression. Other sncRNAs such as piRNAs can modulate gene expression at the epigenetic level, being likely involved in establishing and maintaining long-term patterns of RNA expression. Furthermore, snRNAs can influence alternative splicing, guide specific proteins to apply RNA modifications at a given location and may be involved in translational regulation due to their essential role for the spliceosome and the ribosome function (Karijolich and Yu 2010; Jia et al. 2012).
Advances in high-throughput sequencing technologies (NGS), which facilitated genome-wide determination and comparison of DNA and RNA sequences, have led to the discovery of a wealth of sncRNA variants produced by RNA editing. RNA editing is a core co- or post-transcriptional enzymatic modification process, through which a primary RNA sequence is altered by single-nucleotide substitutions, insertions, or deletions (Gott and Emeson 2000). In humans, the adenosine-to-inosine (A-to-I) RNA editing, mediated by adenosine deaminase acting on RNA (ADAR) family of enzymes, and the cytosine-to-uracil (C-to-U) RNA editing mediated by Apolipoprotein B mRNA Editing Complexes (APOBECs) are considered the canonical types, with the A-to-I type being the most prevalent form, accounting for millions of edits in both coding and noncoding transcripts (Zinshteyn and Nishikura 2009; Savva et al. 2012; Picardi et al. 2016). sncRNAs have been recognised as major targets for RNA editing enzymes and single-nucleotide changes through editing are thought to impact their biogenesis as well as their target specificity (Paris et al. 2012; Yang et al. 2015; Penzo et al. 2016; Zheng et al. 2016; Li et al. 2018a), but the large number of identified editing sites may also merely represent, to some extent, neutral transcriptional noise (Gommans et al. 2009).
miRNAs
miRNAs are a class of non-coding RNAs with an average of 22 nucleotides in length, discovered in the 1990s through the interaction of Lin-4 and Lin-14 in Caenorhabditis elegans and then the discovery of Let-7 in multiple species (Wightman et al. 1993). Most miRNAs are transcribed by RNA polymerase II in long precursors called pri-miRNA (Hammond 2015). In human or bull, almost half of miRNA coding sequences are intergenic, while the others are mainly intronic or located inside UTR regions (Galatenko et al. 2018; Sellem et al. 2020). They are separated from each other or organised within ‘miRNA clusters’ (ex: miR-323, -758, -329b and -329a on bovine chromosome 21). These sequences are then processed by DROSHA coupled to DGCR8, resulting in shorter sequences called pre-miRNA (Lee et al. 2003). The pre-miRNA are exported from nucleus to cytoplasm by Exportin 5 and processed by DICER to cleave the secondary structure (stem loop) and form mature miRNAs (Hutvágner et al. 2001) (Fig. 1).
The mature 5′ stand interacts with an Argonaut protein (Ago2) to form the RISC complex (RNA-induced silencing complex). The second strand is cleaved or can also interact with Ago2.
In most cases, the RISC complex interact with the 3′ untranslated region (3′ UTR) of target mRNAs to suppress expression through mRNA degradation or translational repression (O’Brien et al. 2018). However, interaction of miRNAs with 5′ UTR of coding sequences have also been reported. Advances in high-throughput sequencing technologies have led to the discovery of isomiRs, which are miRNA variants produced by editing, including substitutions (Li et al. 2018a), 3′ or 5′ additions (Katoh et al. 2009; Tan et al. 2014) and deletions (Lee et al. 2019). These isomiRs target distinct sets of mRNA, have been described in multiple species and are expressed constitutively in several tissues in a tissue-specific manner (Fernandez-Valverde et al. 2010; Telonis et al. 2017) as well as according to sex and health status (Loher et al. 2014; Telonis et al. 2015b), suggesting distinct functional roles (Tan et al. 2014; Tan and Dibb 2015).
Since they regulate almost 60% of genes (Friedman et al. 2009), miRNAs are involved in a variety of biological processes and are critical for normal animal development, as exemplified by the constitutive or tissue-specific DICER knock-out in mice, leading to embryogenic lethality (Bernstein et al. 2003) or impairment of several tissues such as skin, heart, lungs, muscle (Andl et al. 2006; Krill et al. 2013). Likewise, aberrant expression of miRNAs is associated with many human diseases and miRNAs have been reported as potential biomarkers for a variety of diseases (Paul et al. 2018).
piRNAs
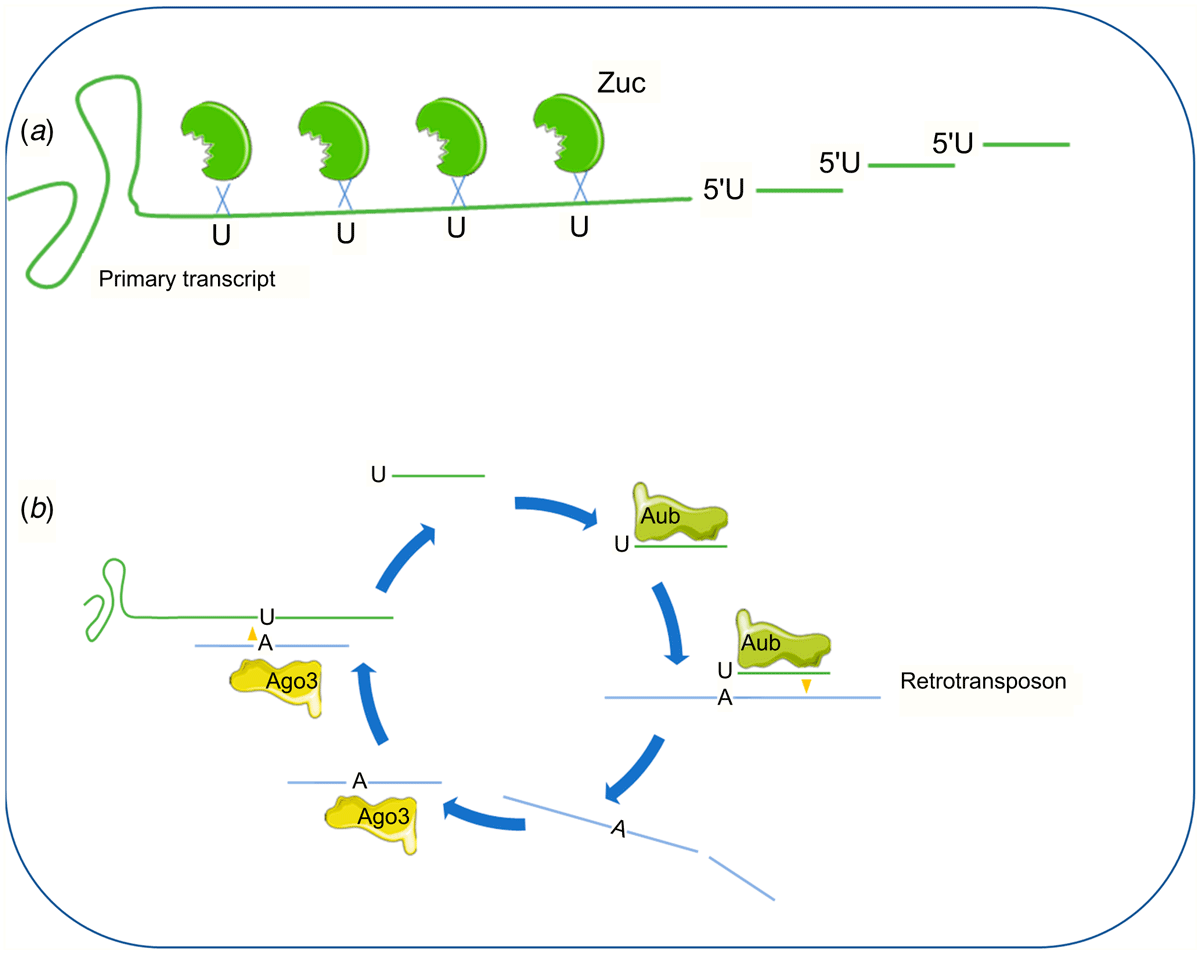
PIWI proteins, a subfamily of Argonaut proteins, were discovered in the late 1990s in Drosophila melanogaster and then in other species (Weick and Miska 2014; Russell et al. 2016). They associate with a particular class of 26–33 nt sncRNAs called piRNAs. piRNAs are produced from long precursor transcripts, which are exported into the cytoplasm and cut every 25–35 nucleotides at an uracil by the endonuclease ZUC (in association with cofactors such as VRET, MINO or GASZ). This biogenesis pathway generates piRNAs having a 5′1U structure, called primary piRNAs’ (Czech et al. 2018). Primary piRNA can enter the ‘ping-pong cycle’, that is initiated after interaction with argonaut protein AUB. The piRNA-AUB complexes cut active transposon transcripts at the nucleotide that pairs with the 10th nucleotide of piRNA, producing ‘secondary piRNA’ in sense orientation to transposons and having no 1U bias but Adenosine at position 10. Secondary piRNA are loaded into Ago3 and Ago3-piRNA complexes in turn recognise and cleave precursor transcripts to generate more primary piRNAs and start yet another cycle (Czech and Hannon 2016) (Fig. 2).
The piRNA pathway provides a powerful system to prevent transposon expression and propagation, through both direct slicing and transcriptional silencing via interaction with two other epigenetic mechanisms: piRNAs can recruit DNMT3L, 3A and 3B, to locally induce DNA methylation and block their activity (LINE or LTR; Aravin et al. 2008; Pillai and Chuma 2012; Weick and Miska 2014) or interact with factors leading to post translational repressive modifications on histones aiming to locally close the chromatin, resulting in retrotransposons silencing (Czech et al. 2018). Moreover, analysis of pachytene piRNAs in elongating spermatids showed a catalytic activity, leading to the concerted degradation of the bulk of cellular mRNAs, illustrating a role in gene expression regulation (Gou et al. 2014).
tRNAs derived fragments (tsRNAs)
Transfer RNAs are a conserved and highly abundant RNA class with a well-defined role in protein translation. They are transcribed by RNA Pol III as a premature tRNA transcript, which undergoes processing by two endonucleases, namely RNase P (5′ trimming) and RNaze Z (3′ trimming). High-throughput sequencing has led to the discovery of a variety of abundant RNA fragments related to tRNAs, with a diversity of size suggesting the existence of multiple biogenesis mechanisms (Keam and Hutvagner 2015; Chen et al. 2021). Indeed, tsRNAs can be broadly classified into two main groups: tRNA-halves (5′ and 3′ tRHs) and tRNA-derived fragments (tRF5s, i-tRFs and tRF3s). tRNA-halves are 35–45 nt RNA species produced by ribonucleolytic cleavage of mature tRNAs by Angiogenin. They are referred to 5′-halves or 3′-halves according to their mapping on the tRNA sequence: 5′-tRHs usually start at the first nucleotide of the tRNAs and typically terminate around the anticodon loop of the tRNA, while 3′-tRHs usually encompass the anticodon region and they may extend into the CCA region of the mature tRNA (Fig. 3a). tRFs ranging from 18 to 35 nt can be processed from either the 5′ or the 3′ arm of the mature tRNA, resulting from a cleavage by DICER, RNase Z or other RNAses in the TψC-arm of mature tRNA or a cleavage in the D-arm, respectively. Internal tRFs (i-tRFs) are produced from a combination of cleavages in the anticodon loop and either D-arm or TψC-arm (Fig. 3a).
Until recently, tsRNA were considered as aberrant degradation products of endonuclease activity and their functional potential remained overlooked until recently. Intriguingly, a growing amount of evidence suggests that tRF may harbour unanticipated biological activity and dynamically impact genome expression (Guzzi and Bellodi 2020). For instance, the processing of mature tRNA into tRFs appears to be site-specific and restricted to specific isotypes of tRNAs, generating tRFs with specific lengths among different cell types. In addition, no correlation was observed between tRFs expression and abundance of their precursor tRNAs (Telonis et al. 2015a). Multiple studies have shown that tRFs can repress the expression of endogenous targets and have a role in regulating the simplest to complex biological processes. In particular, tRF5s and tRF3s have been shown to target the 3′UTR of specific mRNAs and regulate gene expression post-transcriptionally (Maute et al. 2013; Deng et al. 2015). This regulation seems to be Dicer-independent and Argonaute-dependent (Kuscu et al. 2018; Chen et al. 2021). tRF5s and tRF3s interact with AGO1, AGO3 and AGO4 to form AGO complexes (Fig. 3b) which regulate target RNA expression based on seed sequences complementary to target mRNAs (Kumar et al. 2016). Accumulation of tRNA-halves has been associated with oxidative and metabolic stress (Emara et al. 2010), leading to translational repression (Ivanov et al. 2011). Abnormal tRFs expression levels in disease conditions have been observed, including cancer and neurological disorders, suggesting that tRFs could be relevant biomarkers (Raina and Ibba 2014; Krishna et al. 2021). Moreover, in mice embryos, tRF3 are involved in retrotransposons repression during the epigenetic reprogramming (Schorn et al. 2017).
rRNAs derived fragments (rsRNAs)
rRNAs are the most abundant RNA molecules in eukaryotic cells, including four rRNAs (5S, 5.8S, 18S, and 28S) being encoded by the nuclear genome and two (12S and 16S) by the mitochondrial genome. Similar to tsRNAs, short fragments derived from rRNAs have been discovered in several species, which are produced non-randomly by at least four biogenesis pathways (Lambert et al. 2019). All six rRNAs produce rRFs with unique features, from the 5′-end (rRF5s), the interior (i-rRFs), and the 3′-end (rRF3s) of the parental rRNA. Expression of rRFs appears to be influenced by multiple factors, including tissue, health status and sex (Chu et al. 2017; Locati et al. 2018; Cherlin et al. 2020). Similar to tRF, several rRFs have been shown to interact with AGO proteins suggesting a role in gene silencing (Guan and Grigoriev 2020). For instance, change in expression of several key metabolic enzymes was observed in mouse hepatoma cells after overexpression or inhibition of several rRFs, showing that rRFs can regulate metabolic processes in a similar manner to miRNAs (Wei et al. 2013). rRFs have also been shown to act as precursors for the production of miRNAs or piRNAs (Chak et al. 2015; Locati et al. 2018).
Ejaculated sperm contains abundant sncRNAs
Several sncRNAs classes have been identified in ejaculated sperm, including miRNAs, piRNAs, tsRNAs, rsRNAs, snoRNAs or mtRNAs, with differences in terms of sequence diversity and expression levels according to species, sequencing technology and coverage, bioinformatic pipeline etc. Of note, even if most studies include technical steps to remove somatic cells (e.g. Percoll gradient or incubation in hypotonic somatic cell lysis buffer and washing steps), somatic cell contamination can’t be ruled out and may at least in part explain differences observed between studies.
According to studies in humans (Hua et al. 2019; Nätt et al. 2019; Xu et al. 2020), bull (Fagerlind et al. 2015; Capra et al. 2017; Sellem et al. 2020; Sellem et al. 2021), swine (Li et al. 2018b; Gòdia et al. 2019; Alvarez-Rodriguez et al. 2020) and other species, about 85% of reads produced after NGS sequencing of ejaculated sperm small RNAs are annotated as sncRNAs, mainly piRNAs, rRFs, miRNAs and tRFs. For instance, 701 known miRNAs and about 2000 putative miRNA sequences have been identified in bovine, producing about 200 000 isomiRs, with 70 isomiRs per miRNA on average. (Sellem et al. 2020).
However, most publications agree on main sncRNAs classes and similarities were observed across species. For instance, based on SpermBase (www.spermbase.org), about 42% of human sperm miRNAs have been also identified in mouse. About 50% of rat sperm miRNAs were also described in humans and this inter species coverage reached 72% between rabbit and human sperm (Fig. 4).

|
Focusing on bovine, about 45% of sperm miRNAs were also described in mouse, including several key miRNAs involved in cell differentiation, proliferation, spermatogenesis, or embryo development (miR-10, miR-29, miR-34, miR-100, miR-148, miR-191) (Nixon et al. 2015; Chu et al. 2019). Likewise, human and bovine ejaculated sperm were shown to share a subset of their 20 most expressed miRNAs (miR-100, miR-34c, miR-191 and miR-30d) (Fagerlind et al. 2015; Capra et al. 2017; Hua et al. 2019; Sellem et al. 2020; Xu et al. 2020). Likewise, tsRNAs0 originating from glycine and glutamate isoacceptors are the most expressed tsRNAs in both human and bovine ejaculated sperm, accounting altogether for about 70% of tsRNAs expression (Hua et al. 2019; Sellem et al. 2020). Interestingly, while i-tRFs and tRF5s account altogether for about 75% of tRFs diversity in both species, they exhibit low expression levels. As a result, 5′-tRHs which represent only 10% of sequence diversity, account for a vast majority of tRFs expression in both species (52 and 82%, in bovine and human sperm, respectively).
These studies revealed another striking feature of sperm sncRNAome, namely the impressive diversity of isoforms (isomiRs, isopiRs, variants of tsRNAs and rsRNAs) produced by RNA editing, as for bovine where some miRNAs were composed by several thousand of isomiRs (Sellem et al. 2020).
Since ejaculated sperm are transcriptionally silent, the sperm sncRNAome was initially considered as a legacy of spermatogenesis. However, several studies in mice (Sharma et al. 2016; Sharma et al. 2018; Chu et al. 2019) and bulls (Sellem et al. 2021) have shown a remarkable level of sncRNAome plasticity resulting from the combination of multiple factors, including interaction with epididymosomes. Indeed, beyond spermatogenesis, spermatozoa undergo several functional maturation steps to acquire their fertilising capacity. In particular, during epididymal transit, the sperm membrane is remodelled, with sequential attachment and shedding of various molecules provided by the epididymal lumen fluid (Zhou et al. 2018) and extracellular vesicles such as epididymosomes (Rejraji et al. 2006; Sullivan et al. 2007; Girouard et al. 2011; Rowlison et al. 2018; Nixon et al. 2019b). Interestingly, sncRNA cargo are delivered to sperm by epididymosomes (Belleannee et al. 2013; Vojtech et al. 2014; Reilly et al. 2016; Sharma et al. 2018), whose contents vary along the epididymis whether in mice, humans or bulls (Nixon et al. 2015; Russell et al. 2016; Chu et al. 2019; Nixon et al. 2019a; Trigg et al. 2019; Sellem et al. 2021). As a result, the payload of sperm sncRNAs is dramatically remodelled as sperm mature along the epididymis from caput to the cauda.
One common finding across species is the observed enrichment of piRNAs within testis parenchyma relative to the sperm fraction isolated from epididymis or ejaculated sperm. Conversely, an increase in miRNA, tsRNA and rsRNA content is observed from testis to ejaculated sperm, with distinct trends according to species. For instance, expression of rsRNA was shown to increase continuously in mouse (Chu et al. 2019), whereas a particular expression profile peaking at epididymis corpus was observed in bovine sperm (Sellem et al. 2021). As a result, rsRNA are the most expressed sncRNA species in mouse cauda sperm, whereas miRNA and tsRNA are the most prevalent sncRNA species in bovine cauda sperm.
Unfortunately, data unavailability, methodological differences as well as the plethora of isoforms produced through RNA editing mechanisms preclude a detailed comparison between species. Whether observed differences represent species specificities or technical artefacts warrants further study.
It can be assumed that sncRNA acquired during epididymal transit or later may be related to sperm functionality, fertilising capacity and embryo development, while the others can be considered as remnant from spermatogenesis, maybe related for instance to sperm concentration or morphology. In this respect, sncRNA may represent relevant biomarkers associated with semen fertility.
Deregulation of sncRNAs related to spermatogenesis impairs fertility
Spermatogenesis is a tightly regulated stepwise process involving thousands of genes (Chalmel and Rolland 2015) as well as sncRNAs, especially microRNAs (miRNAs) and piwi-interacting RNAs (piRNAs) as illustrated by the high concentration of DICER, AGO proteins and miRNAs in the chromatoid body in male germ cells (Kotaja et al. 2006; de Mateo and Sassone-Corsi 2014). Direct evidence for the involvement of sncRNA in spermatogenesis regulation has been provided in mice by several selective knock-out of genes involved in the sncRNA biogenesis pathway. For instance, the selective knock-out of Dicer1 in the male germ cell leads to a decrease in testis volume and sperm number as well as multiple cumulative defects at the spermatogenesis and spermiogenesis stages, leading to morphological abnormalities and infertility (Korhonen et al. 2011; Romero et al. 2011). Likewise, piRNAs are known to play roles in spermatogenesis, as evidenced by the mitosis, meiosis, chromatin compaction, flagella elongation and fertility defects in mutants lacking Piwi (Weick and Miska 2014). Knock-out of other key genes belonging to the piRNA pathway (Miwi, Mili, Tdrd1, Tdrd9, Mvh, Maelstrom, Pld6 or SPOCD1) results also in major spermatogenesis defects during meiosis or spermiogenesis (Aravin et al. 2008; Pillai and Chuma 2012; Chuma and Nakano 2013; de Mateo and Sassone-Corsi 2014; Russell et al. 2016) leading to sterility.
Many studies in mouse have now identified key sncRNAs regulating spermatogenesis. For instance, miR-20, miR-21, miR-100, miR-106b, miR-146a, miR-182, miR-183, miR-222 and miR-383 have been shown to be involved in the ‘differentiation vs proliferation’ balance in spermatogonial stem cells: miR-20, miR-100 and miR-106a are critical for spermatogonial stem cell proliferation through their interactions with Stat3 and Ccnd1 (He et al. 2013; Huang et al. 2017); regulation of retinoic acid by miR-146a (Huszar and Payne 2013) and cKIT by miR-222 (Yang et al. 2013) maintain the undifferentiated status of spermatogonial cells; inhibition of miR-21, which accounts for 11% of total miRNA expression in spermatogonia, increases apoptosis leading to a loss of cells (Niu et al. 2011). Meiosis is also regulated by miRNAs, as exemplified by miR-34c, miR-29 and miR-214 which target Nanos2, favouring the meiosis process (Yu et al. 2014; Hilz et al. 2017). Double knock out mice for miR-34b/c and miR-449 are infertile, due to a drastic reduction in sperm number, a motility loss and an increased proportion of decapitated sperm (oligoasthenoteratozoospermia) (Comazzetto et al. 2014; Yuan et al. 2015).
In bovine, enriched GO terms and Kegg pathways associated with predicted targets of miRNA preferentially expressed in testicular parenchyma sperm highlighted biological processes such as cell proliferation as well as response to stress and protein ubiquitination (Sellem et al. 2021). Interestingly, ER stress and UPR signalling cascades are involved in spermatogenesis, (Karna et al. 2020). Likewise, the ubiquitin–proteasome pathway plays a key role at several stages of spermatogenesis (meiosis, histone–protamine transition, acrosome biogenesis, and spermatozoa maturation) to remove many proteins and organelles and help the formation of condensed sperm. Knock-out mice lacking ubiquitylation enzymes such as UBE2J1 suffer from male sterility associated with flagella and acrosomal defects (Koenig et al. 2014). Interestingly, piRNAs are also involved in this pathway and have been shown to induce ubiquitination of MIWI, a process essential for maturation from late spermatid to sperm (Zhao et al. 2013). Of note, while silencing of retrotransposons in the germline during genome demethylation is the unifying function of piRNAs in mammals, they have also been shown to be crucial for spermatogenesis by regulating meiotically expressed protein-coding genes. Indeed, as spermatocytes reach the pachytene stage of meiosis, a second wave of piRNA transcription starts, producing a particular class of piRNAs termed pachytene piRNAs (Girard et al. 2006). These pachytene piRNAs have been shown to form a silencing complex (pi-RISC) containing MIWI and deadenylase CAF1 to induce deadenylation and subsequent decay of spermatogenic mRNAs in elongating spermatids, via a mechanism similar to, but distinct from, the classical miRNA/siRNA machinery (Gou et al. 2014; Goh et al. 2015). Recently, CRISPR/Cas9 was used to knock-out the expression of a pachytene piRNA precursor locus on mouse chromosome 18, leading to male sterility due to sperm head dysmorphology, acrosome dysgenesis, poor motility and defects in interactions with zona pellucida impairing penetration (Choi et al. 2021).
Post testicular acquired sncRNAs: a role in embryo development?
Since sperm RNAs can be delivered to the oocyte during fertilisation and remain stable until the onset of embryonic genome activation (Ostermeier et al. 2004), it has long been hypothesised that they may impact early embryo development. Evidence of such a role has recently emerged, when Guo et al treated mouse mature spermatozoa with lysolecithin, pronase and RNases (RNase A and RNase H) to deplete sperm from RNAs and used treated sperm to perform intracytoplasmic sperm injection (ICSI). Reduced blastocyst formation rate and live birth rate of the embryos, as well as lower body weight of F1 mice were observed (Guo et al. 2017), suggesting that sperm RNAs, including sncRNAs, play critical roles in embryonic development and may act as an additional source of paternal hereditary information.
Recently, sperm sncRNAs gained at epididymis cauda were shown to be crucial for normal embryonic development after the blastocyst stage in mice (Conine et al. 2018). Indeed, blastocysts produced by ICSI with spermatozoa obtained from epididymis caput showed a significant overexpression of multiple genes encoding RNA-binding proteins and chromatin-associated factors during the preimplantation period, including overexpression of SMARCA5, a key protein involved in chromatin remodelling. These caput-derived embryos then failed to efficiently implant and develop. These preimplantation molecular defects as well as the post-implantation lethality could be rescued by microinjection of purified cauda-specific sncRNAs into caput-derived embryos, providing evidence that cauda sperm are epigenetically immature. Interestingly, microinjection of separate gel-purified sncRNA population was performed, using either 18–26 nt sncRNA fraction enriched with microRNAs or 26–40 nt sncRNA faction primarily comprising tRFs. Gene expression was globally restored only for the 18–26 nt fraction, suggesting that miRNA gained by sperm as they further transit the epididymis to cauda are required to support normal preimplantation gene regulation. In this respect, the dramatic increase in miR-100 expression observed from caput to cauda, at least in bovine sperm, may explain the observed SMARCA5 overexpression in caput-derived embryos compared to cauda-derived embryos, SMARCA5 being a target gene of miR-100. Of note, miR-100, is also highly expressed in bovine ejaculated sperm and has been proposed as one of the main factors associated with the initiation of pluripotency (Morikawa and Cserjesi 2004; Fei et al. 2010). Likewise, miRNA whose expression peak at epididymis cauda or ejaculated sperm in cattle were found to be associated with GO terms related to embryo development such as developmental process, embryonic morphogenesis as well as cell proliferation, differentiation, and migration (Sellem et al. 2021). In addition, miR-34c and miR-191, two highly expressed miRNA in bovine ejaculated sperm (Sellem et al. 2020) have been associated with murine embryo early development (Liu et al. 2012; Donkin and Barrès 2018; Le Blevec et al. 2020) and fertilisation rate and embryo quality in humans (Xu et al. 2020), respectively. Of note, sncRNA in the range 18–26 nt also contain short piRNAs, rsRNAs and tsRNA, which may also be involved in the observed phenotype. In this respect, some tsRNA peaking at epididymis cauda or ejaculated sperm in cattle have also been shown to contribute to early cleavage of porcine preimplantation embryos (Chen et al. 2020) and injection of tRNA-Gly-GCC fragments into zygotes results in slowdown of embryo development (Sharma et al. 2016).
Beyond their role in embryonic development, sncRNAs, especially tsRNAs, may have a role in trans-generational inheritance. A growing body of evidence published recently suggest that sperm sncRNA-encoded information is decoded in early embryos to control offspring phenotypes (Zhang et al. 2019). For instance, changes in sncRNA content was observed in sperm of F0 males subjected to a high-fat diet both in mouse (Grandjean et al. 2016) and rat (de Castro Barbosa et al. 2016), including differentially expressed miRNAs, tsRNAs and piRNAs. These changes were associated in F1 and F2 newborn offspring with reduced body weight and pancreatic beta-cell mass, as well as glucose intolerance, insulin resistance and type II diabetes. Sperm from F1 rat offspring also showed a modified sncRNAs expression profile. In particular, altered expression of sperm miRNA let-7c was observed in F0 rats and was transmitted to offspring, leading to a transcriptomic shift of let-7c targets, including AKT2, IGF2R and UCP2 which are involved in glucose metabolism and insulin-related biological processes.
Molecular mechanisms underlying this trans-generational inheritance have yet to be ascertained and may probably result from the combination or cooperation of several sncRNA species, including at least miRNAs et tsRNAs. Indeed miR-19b was identified as overexpressed in male mice fed a high-fat diet and its microinjection into naive zygotes leads to metabolic alterations in the resulting progeny (Grandjean et al. 2016). Similarly, a high-fat diet was shown to disturb the expression of about 11% of mouse sperm tsRNAs. Injection of mouse sperm tsRNAs from males subjected to a high-fat diet was shown to alter metabolic pathways in early embryos and to induce metabolic disorders in the F1 offspring (Chen et al. 2016).
Taken together, these results demonstrate that the dynamic remodelling of sperm sncRNA payload occurring during post testicular maturation has dramatic functional consequences on embryos preimplantation development, may downregulate a set of genes encoding RNA-binding proteins and chromatin-associated factors, thus modifying the epigenetic program and have a global impact on offspring development.
Sperm-borne sncRNAs as fertility biomarkers?
Diagnosis of idiopathic diseases, as well as prediction of the disease course or monitoring the response to therapeutic approaches is a common challenge in the clinical field. In this respect, diagnostic, prognostic and predictive biomarkers have gained major interest. Proteins have long been suggested as biomarkers, however low sensitivity and specificity of detection, as well as the struggle in developing efficient detection methods, often limit their usefulness.
Next to proteins, circulating miRNAs were previously shown to be relevant biomarkers in several pathologies such as cancer or cardiovascular disease. In the last decade, the search for miRNAs biomarkers related to various conditions was an impressive research field, including in sperm fertility assessment. Of note, various fertility definitions were used according to studies and species, based on either spermogram parameters (sperm quantity, motility and absence of morphological abnormalities) or fertilisation/pregnancy rates after artificial insemination (especially for cattle) or other assisted reproductive technologies.
In humans, sperm miRNAs expression profiles were used to detect infertile patients, with or without morpho-functional sperm alterations (Alves et al. 2020; Momeni et al. 2020; Vashisht and Gahlay 2020). For instance, 50 and 42 miRNAs including miR-15, miR-16a, miR-19a, miR-34b, miR-34c-5p, miR-122, miR-449 and miR-1973 were shown to be upregulated while 27 and 44 miRNA were found to be downregulated in asthenozoospermia and oligoasthenozoospermia, respectively (Abu-Halima et al. 2013). Reduced expression of miR-10b and miR-135b was observed in semen of asthenospermic patients (Tian et al. 2017). Likewise, decreased expression of miR-25, miR-34b, miR-34c-5p, miR-122, miR-152, miR-192, miR-335, miR-449a was observed in oligozoospermic patients, while 12 miRNAs were significantly more abundant: Let-7b, let-7c, let-7g, miR-21, miR22, miR-30a, miR-148a, miR-221, miR-320a, miR-375, miR-423-3p and miR-423-5p (Muñoz et al. 2015). Six miRNAs (miR-125a-3p, miR-132-5p miR-151-5p, miR-195-5p, miR-320 and miR-935) were shown to be downregulated in case of teratozoospermia (Salas-Huetos et al. 2015). However, usefulness of these biomarkers is unclear, as fast and inexpensive technologies such as spermograms are already used to efficiently detect these abnormalities in infertile patients. As a biomarker, miRNA would provide an added value in order to diagnose idiopathic infertility or, for instance, refine current diagnosis and differentiate between obstructive and nonobstructive azoospermia without requirement of testicular biopsy. In this respect, miR-34b, miR-34c-5p, miR-429 and miR-122 were suggested to improve diagnosis of patients with nonobstructive azoospermia along with traditional techniques (Abu-Halima et al. 2014). In addition, 57 miRNAs were identified as differentially expressed between two groups of normozoospermic men having contrasting fertility levels, highlighting biological processes such as embryonic morphogenesis and chromatin modification, and suggesting that subfertility of these patients may be related to the dysregulation in early embryo of key genes targeted by these sperm miRNAs (Salas-Huetos et al. 2016). Likewise, expression of sperm miRNA miR-101-3p, miR-132-3p and miR-191-5p was shown to be associated with either fertilisation rate, blastocyst rate or high-quality embryo rate after in vitro fertilisation (Hua et al. 2019; Xu et al. 2020). Recently, 48 pairs of miRNAs, whose expression is strongly correlated in fertile men but disrupted in infertile patients, were identified (Corral-Vazquez et al. 2019). The most suitable diagnostic miRNA pairs were determined for asthenozoospermia (miR-942-5p/miR-1208), teratozoospermia (miR-296-5p/miR-328-3p), normozoospermic infertility (miR-34b-3p/miR-93-3p) and oligozoospermia (miR-139-5p/-miR-1260a).
Regarding livestock species, several studies have been performed on bull and boar and specific sperm-borne miRNA expression profiles have been associated with semen functional parameters, in vitro embryo development or field fertility. For instance miR-93, miR-106b, miR-100, miR-122, miR-184, miR-486-5p and miR-2285n have been associated with sperm motility in cattle (Capra et al. 2017) and let-7d/e in porcine (Curry et al. 2011). Many miRNAs differentially expressed between fertile and sub-fertile bulls have also been identified, such as bta-miR-9-5p, bta-miR-10a-5p, bta-miR-19b-3p, bta-miR-27a-5p, bta-miR-34c, bta-miR-98, bta-miR126-5p, bta-miR-142-5p, bta-miR-148b-3p, bta-miR-182, bta-miR-320a, bta-miR-329a, bta-miR-449a, bta-miR-502-5p, bta-miR-1249-3p, bta-miR-1839, bta-miR-2284y (Fagerlind et al. 2015; Perkins et al. 2020). The expression level of nine miRNAs (miR-9-5p, miR-34c, miR-423-5p, miR-449a, miR-5193-5p, miR-1246, miR-2483-5p, miR-92a, miR-21-5p) were significantly correlated to non-return rate after insemination with sexed semen (Keles et al. 2021). Differential expression of 11 miRNAs was observed between high and low fertility bulls, including miR-33b, miR-126-5p, miR-205, miR-505, miR-532, miR-500 and miR-542-5p which were overexpressed in high fertility bulls and miR-15a, miR-29, miR-216b and miR-339a which were downregulated in high fertility bulls (Alves et al. 2019; Menezes et al. 2020). In a recent study, two miRNAs (miR-221 and mir-621) were found to be down- and up-regulated in high-fertility relative to low-fertility boars, respectively (Alvarez-Rodriguez et al. 2020).
More recently, evidence has been provided regarding the value of other sncRNA classes as fertility biomarkers. For instance, two groups of sperm samples, producing either high or low rates of good quality embryos, were compared by sncRNA-Seq. Ten differentially expressed tsRNAs were identified, including three 5′-tRNA halves, two 3′-tRNA halves, four were 5′-tRFs and one i-tRF. Among them, five were downregulated in the low-quality semen group (two GlyGCC derived tsRNAs, two ThrTGT and one GluTTC) and five were upregulated (two ProAGG, one ProTGG, one AsnATT and one ArgCCG). In addition, six differentially expressed 28S rsRNAs and one 18S rsRNAs were also identified between the two sperm quality groups (Hua et al. 2019). Likewise, sperm tsRNAGln-TTG has been suggested as a potential diagnostic biomarker based on its role in the first cleavage of a porcine embryo as well as development of human embryos (Chen et al. 2021). A more exhaustive list of sncRNAs related to semen quality, in vitro embryo development, fertility and human infertility (spermogram abnormalities) is provided in Supplementary Table S1.
As already noticed, many biomarker studies were based on a small number of extreme samples due to the cost of NGS technology, leading likely to numerous false positives. Further studies, based on a larger set of samples, accounting for the diversity of the general population, will undoubtedly be required to identify robust and relevant fertility biomarkers.
Similar to previous work done to improve the prediction using sperm functional assessment (Sellem et al. 2015), a combination of several sncRNAs biomarkers will undoubtedly be required to reach a good predictive value. Novel statistical approaches, based on deep learning, have also to be implemented to deal with hundreds of sncRNA biomarkers and to combine biomarkers with other functional parameters in order to improve diagnosis as well as fertility prediction.
Conclusions
Ejaculated sperm carry thousands of sncRNAs, including miRNAs, piRNAs, rsRNAs and tsRNAs, whose functions remain to be elucidated. Some are a legacy of spermatogenesis and may be indicative of normal semen production. Some are gained along sperm maturation and may promote acquisition of sperm motility and fertilising capacity or may have a role in embryo development or paternal epigenetic inheritance. In line with these hypotheses, numerous studies in humans and livestock species have established a link between sperm-borne sncRNAs and spermatogenesis defects, embryo development in ART strategy and fertility. However, biological knowledge is still lacking, and further work is still needed to identify robust lists of biomarkers and combine the most relevant ones to improve diagnosis and treatment.
Supplementary material
Supplementary material is available online.
Data availability
All the fastq files focusing on bovine sperm and published by our team have been stored at ENA, European Nucleotide Archive, under the primary accession numbers PRJEB33940 and PRJEB41989.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
References
Abu-Halima, M, Hammadeh, M, Schmitt, J, Leidinger, P, Keller, A, Meese, E, and Backes, C (2013). Altered microRNA expression profiles of human spermatozoa in patients with different spermatogenic impairments. Fertility and Sterility 99, 1249–1255.e16.| Altered microRNA expression profiles of human spermatozoa in patients with different spermatogenic impairments.Crossref | GoogleScholarGoogle Scholar | 23312218PubMed |
Abu-Halima, M, Hammadeh, M, Backes, C, Fischer, U, Leidinger, P, Lubbad, AM, Keller, A, and Meese, E (2014). Panel of five microRNAs as potential biomarkers for the diagnosis and assessment of male infertility. Fertility and Sterility 102, 989–997.e1.
| Panel of five microRNAs as potential biomarkers for the diagnosis and assessment of male infertility.Crossref | GoogleScholarGoogle Scholar | 25108464PubMed |
Alvarez-Rodriguez, M, Martinez, C, Wright, D, Barranco, I, Roca, J, and Rodriguez-Martinez, H (2020). The transcriptome of pig spermatozoa, and its role in fertility. International Journal of Molecular Sciences 21, 1571.
| The transcriptome of pig spermatozoa, and its role in fertility.Crossref | GoogleScholarGoogle Scholar |
Alves, MBR, de Arruda, RP, De Bem, THC, Florez-Rodriguez, SA, de Sá Filho, MF, Belleannée, C, Meirelles, FV, da Silveira, JC, Perecin, F, and Celeghini, ECC (2019). Sperm-borne miR-216b modulates cell proliferation during early embryo development via K-RAS. Scientific Reports 9, 1–14.
| Sperm-borne miR-216b modulates cell proliferation during early embryo development via K-RAS.Crossref | GoogleScholarGoogle Scholar |
Alves, MBR, Celeghini, ECC, and Belleannée, C (2020). From sperm motility to sperm-borne microRNA signatures: new approaches to predict male fertility potential. Frontiers in Cell and Developmental Biology 8, 791.
| From sperm motility to sperm-borne microRNA signatures: new approaches to predict male fertility potential.Crossref | GoogleScholarGoogle Scholar | 32974342PubMed |
Amann, RP, and Waberski, D (2014). Computer-assisted sperm analysis (CASA): capabilities and potential developments. Theriogenology 81, 5–17. e1-3.
| Computer-assisted sperm analysis (CASA): capabilities and potential developments.Crossref | GoogleScholarGoogle Scholar | 24274405PubMed |
Andl, T, Murchison, EP, Liu, F, Zhang, Y, Yunta-Gonzalez, M, Tobias, JW, Andl, CD, Seykora, JT, Hannon, GJ, and Millar, SE (2006). The miRNA-processing enzyme dicer is essential for the morphogenesis and maintenance of hair follicles. Current Biology 16, 1041–1049.
| The miRNA-processing enzyme dicer is essential for the morphogenesis and maintenance of hair follicles.Crossref | GoogleScholarGoogle Scholar | 16682203PubMed |
Aravin, AA, Sachidanandam, R, Bourc’his, D, Schaefer, C, Pezic, D, Toth, KF, Bestor, T, and Hannon, GJ (2008). A piRNA pathway primed by individual transposons is linked to de novo DNA methylation in mice. Molecular Cell 31, 785–799.
| A piRNA pathway primed by individual transposons is linked to de novo DNA methylation in mice.Crossref | GoogleScholarGoogle Scholar | 18922463PubMed |
Belleannee, C, Calvo, E, Caballero, J, and Sullivan, R (2013). Epididymosomes convey different repertoires of microRNAs throughout the bovine epididymis. Biology of Reproduction 89, 30.
| Epididymosomes convey different repertoires of microRNAs throughout the bovine epididymis.Crossref | GoogleScholarGoogle Scholar | 23803555PubMed |
Bernstein, E, Kim, SY, Carmell, MA, Murchison, EP, Alcorn, H, Li, MZ, Mills, AA, Elledge, SJ, Anderson, KV, and Hannon, GJ (2003). Dicer is essential for mouse development. Nature Genetics 35, 215–217.
| Dicer is essential for mouse development.Crossref | GoogleScholarGoogle Scholar | 14528307PubMed |
Capra, E, Turri, F, Lazzari, B, Cremonesi, P, Gliozzi, TM, Fojadelli, I, Stella, A, and Pizzi, F (2017). Small RNA sequencing of cryopreserved semen from single bull revealed altered miRNAs and piRNAs expression between High- and Low-motile sperm populations. BMC Genomics 18, 14.
| Small RNA sequencing of cryopreserved semen from single bull revealed altered miRNAs and piRNAs expression between High- and Low-motile sperm populations.Crossref | GoogleScholarGoogle Scholar | 28052756PubMed |
Chak, L-L, Mohammed, J, Lai, EC, Tucker-Kellogg, G, and Okamura, K (2015). A deeply conserved, noncanonical miRNA hosted by ribosomal DNA. RNA 21, 375–384.
| A deeply conserved, noncanonical miRNA hosted by ribosomal DNA.Crossref | GoogleScholarGoogle Scholar | 25605965PubMed |
Chalmel, F, and Rolland, AD (2015). Linking transcriptomics and proteomics in spermatogenesis. Reproduction 150, R149–R157.
| Linking transcriptomics and proteomics in spermatogenesis.Crossref | GoogleScholarGoogle Scholar | 26416010PubMed |
Chen, Q, Yan, M, Cao, Z, Li, X, Zhang, Y, Shi, J, Feng, GH, Peng, H, Zhang, X, Zhang, Y, Qian, J, Duan, E, Zhai, Q, and Zhou, Q (2016). Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 351, 397–400.
| Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder.Crossref | GoogleScholarGoogle Scholar | 26721680PubMed |
Chen, X, Zheng, Y, Lei, A, Zhang, H, Niu, H, Li, X, Zhang, P, Liao, M, Lv, Y, Zhu, Z, Pan, C, Dong, W, Chen, H, Wu, D, Liu, W, Hamer, G, Zeng, S, and Zeng, W (2020). Early cleavage of preimplantation embryos is regulated by tRNAGln-TTG-derived small RNAs present in mature spermatozoa. Journal of Biological Chemistry 295, 10885–10900.
| Early cleavage of preimplantation embryos is regulated by tRNAGln-TTG-derived small RNAs present in mature spermatozoa.Crossref | GoogleScholarGoogle Scholar |
Chen, X, Sun, Q, Zheng, Y, Liu, Z, Meng, X, Zeng, W, and Lu, H (2021). Human sperm tsRNA as potential biomarker and therapy target for male fertility. Reproduction 161, 111–122.
| Human sperm tsRNA as potential biomarker and therapy target for male fertility.Crossref | GoogleScholarGoogle Scholar | 33434159PubMed |
Cherlin, T, Magee, R, Jing, Y, Pliatsika, V, Loher, P, and Rigoutsos, I (2020). Ribosomal RNA fragmentation into short RNAs (rRFs) is modulated in a sex- and population of origin-specific manner. BMC Biology 18, 38.
| Ribosomal RNA fragmentation into short RNAs (rRFs) is modulated in a sex- and population of origin-specific manner.Crossref | GoogleScholarGoogle Scholar | 32279660PubMed |
Choi, H, Wang, Z, and Dean, J (2021). Sperm acrosome overgrowth and infertility in mice lacking chromosome 18 pachytene piRNA. PLOS Genetics 17, e1009485.
| Sperm acrosome overgrowth and infertility in mice lacking chromosome 18 pachytene piRNA.Crossref | GoogleScholarGoogle Scholar | 33831001PubMed |
Chu, C, Yu, L, Wu, B, Ma, L, Gou, LT, He, M, Guo, Y, Li, ZT, Gao, W, Shi, H, Liu, MF, Wang, H, Chen, CD, Drevet, JR, Zhou, Y, and Zhang, Y (2017). A sequence of 28S rRNA-derived small RNAs is enriched in mature sperm and various somatic tissues and possibly associates with inflammation. Journal of Molecular Cell Biology 9, 256–259.
| A sequence of 28S rRNA-derived small RNAs is enriched in mature sperm and various somatic tissues and possibly associates with inflammation.Crossref | GoogleScholarGoogle Scholar | 28486659PubMed |
Chu, C, Zhang, YL, Yu, L, Sharma, S, Fei, ZL, and Drevet, JR (2019). Epididymal small non-coding RNA studies: progress over the past decade. Andrology 7, 681–689.
| Epididymal small non-coding RNA studies: progress over the past decade.Crossref | GoogleScholarGoogle Scholar | 31044548PubMed |
Chuma, S, and Nakano, T (2013). piRNA and spermatogenesis in mice. Philosophical Transactions of the Royal Society B: Biological Sciences 368, 20110338.
| piRNA and spermatogenesis in mice.Crossref | GoogleScholarGoogle Scholar |
Comazzetto, S, Di Giacomo, M, Rasmussen, KD, Much, C, Azzi, C, Perlas, E, Morgan, M, and O’Carroll, D (2014). Oligoasthenoteratozoospermia and infertility in mice deficient for miR-34b/c and miR-449 loci. PLoS Genetics 10, e1004597.
| Oligoasthenoteratozoospermia and infertility in mice deficient for miR-34b/c and miR-449 loci.Crossref | GoogleScholarGoogle Scholar | 25329700PubMed |
Conine, CC, Sun, F, Song, L, Rivera-Perez, JA, and Rando, OJ (2018). Small RNAs gained during epididymal transit of sperm are essential for embryonic development in mice. Developmental Cell 46, 470–480.e3.
| Small RNAs gained during epididymal transit of sperm are essential for embryonic development in mice.Crossref | GoogleScholarGoogle Scholar | 30057276PubMed |
Corral-Vazquez, C, Salas-Huetos, A, Blanco, J, Vidal, F, Sarrate, Z, and Anton, E (2019). Sperm microRNA pairs: new perspectives in the search for male fertility biomarkers. Fertility and Sterility 112, 831–841.
| Sperm microRNA pairs: new perspectives in the search for male fertility biomarkers.Crossref | GoogleScholarGoogle Scholar | 31587805PubMed |
Curry, E, Safranski, TJ, and Pratt, SL (2011). Differential expression of porcine sperm microRNAs and their association with sperm morphology and motility. Theriogenology 76, 1532–1539.
| Differential expression of porcine sperm microRNAs and their association with sperm morphology and motility.Crossref | GoogleScholarGoogle Scholar | 21872314PubMed |
Czech, B, and Hannon, GJ (2016). One loop to rule them all: the ping-pong cycle and piRNA-guided silencing. Trends in Biochemical Sciences 41, 324–337.
| One loop to rule them all: the ping-pong cycle and piRNA-guided silencing.Crossref | GoogleScholarGoogle Scholar | 26810602PubMed |
Czech, B, Munafò, M, Ciabrelli, F, Eastwood, EL, Fabry, MH, Kneuss, E, and Hannon, GJ (2018). piRNA-guided genome defense: from biogenesis to silencing. Annual Review of Genetics 52, 131–157.
| piRNA-guided genome defense: from biogenesis to silencing.Crossref | GoogleScholarGoogle Scholar | 30476449PubMed |
de Castro Barbosa, T, Ingerslev, LR, Alm, PS, Versteyhe, S, Massart, J, Rasmussen, M, Donkin, I, Sjö gren, R, Mudry, JM, Vetterli, L, Gupta, S, Krook, A, Zierath, JR, and Barrès, R (2016). High-fat diet reprograms the epigenome of rat spermatozoa and transgenerationally affects metabolism of the offspring. Molecular Metabolism 5, 184–197.
| High-fat diet reprograms the epigenome of rat spermatozoa and transgenerationally affects metabolism of the offspring.Crossref | GoogleScholarGoogle Scholar | 26977389PubMed |
de Mateo, S, and Sassone-Corsi, P (2014). Regulation of spermatogenesis by small non-coding RNAs: role of the germ granule. Seminars in Cell & Developmental Biology 29, 84–92.
| Regulation of spermatogenesis by small non-coding RNAs: role of the germ granule.Crossref | GoogleScholarGoogle Scholar |
Deng, J, Ptashkin, RN, Chen, Y, Cheng, Z, Liu, G, Phan, T, Deng, X, Zhou, J, Lee, I, Lee, YS, and Bao, X (2015). Respiratory Syncytial Virus Utilizes a tRNA Fragment to Suppress Antiviral Responses Through a Novel Targeting Mechanism. Molecular Therapy 23, 1622–1629.
| Respiratory Syncytial Virus Utilizes a tRNA Fragment to Suppress Antiviral Responses Through a Novel Targeting Mechanism.Crossref | GoogleScholarGoogle Scholar | 26156244PubMed |
Donkin, I, and Barrès, R (2018). Sperm epigenetics and influence of environmental factors. Molecular Metabolism 14, 1–11.
| Sperm epigenetics and influence of environmental factors.Crossref | GoogleScholarGoogle Scholar | 29525406PubMed |
Emara, MM, Ivanov, P, Hickman, T, Dawra, N, Tisdale, S, Kedersha, N, Hu, GF, and Anderson, P (2010). Angiogenin-induced tRNA-derived stress-induced RNAs promote stress-induced stress granule assembly. Journal of Biological Chemistry 285, 10959–10968.
| Angiogenin-induced tRNA-derived stress-induced RNAs promote stress-induced stress granule assembly.Crossref | GoogleScholarGoogle Scholar |
Fagerlind, M, Stålhammar, H, Olsson, B, and Klinga-Levan, K (2015). Expression of miRNAs in bull spermatozoa correlates with fertility rates. Reproduction in Domestic Animals 50, 587–594.
| Expression of miRNAs in bull spermatozoa correlates with fertility rates.Crossref | GoogleScholarGoogle Scholar | 25998690PubMed |
Fei, T, Zhu, S, Xia, K, Zhang, J, Li, Z, Han, JD, and Chen, YG (2010). Smad2 mediates Activin/Nodal signaling in mesendoderm differentiation of mouse embryonic stem cells. Cell Research 20, 1306–1318.
| Smad2 mediates Activin/Nodal signaling in mesendoderm differentiation of mouse embryonic stem cells.Crossref | GoogleScholarGoogle Scholar | 21079647PubMed |
Fernandez-Valverde, SL, Taft, RJ, and Mattick, JS (2010). Dynamic isomiR regulation in Drosophila development. RNA 16, 1881–1888.
| Dynamic isomiR regulation in Drosophila development.Crossref | GoogleScholarGoogle Scholar | 20805289PubMed |
Friedman, RC, Farh, KK-H, Burge, CB, and Bartel, DP (2009). Most mammalian mRNAs are conserved targets of microRNAs. Genome Research 19, 92–105.
| Most mammalian mRNAs are conserved targets of microRNAs.Crossref | GoogleScholarGoogle Scholar | 18955434PubMed |
Galatenko, VV, Galatenko, AV, Samatov, TR, Turchinovich, AA, Shkurnikov, MY, Makarova, JA, and Tonevitsky, AG (2018). Comprehensive network of miRNA-induced intergenic interactions and a biological role of its core in cancer. Scientific Reports 8, 2418.
| Comprehensive network of miRNA-induced intergenic interactions and a biological role of its core in cancer.Crossref | GoogleScholarGoogle Scholar | 29402894PubMed |
Girard, A, Sachidanandam, R, Hannon, GJ, and Carmell, MA (2006). A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature 442, 199–202.
| A germline-specific class of small RNAs binds mammalian Piwi proteins.Crossref | GoogleScholarGoogle Scholar | 16751776PubMed |
Girouard, J, Frenette, G, and Sullivan, R (2011). Comparative proteome and lipid profiles of bovine epididymosomes collected in the intraluminal compartment of the caput and cauda epididymidis. International Journal of Andrology 34, e475–e486.
| Comparative proteome and lipid profiles of bovine epididymosomes collected in the intraluminal compartment of the caput and cauda epididymidis.Crossref | GoogleScholarGoogle Scholar | 21875428PubMed |
Gòdia, M, Estill, M, Castelló, A, Balasch, S, Rodríguez-Gil, JE, Krawetz, SA, Sánchez, A, and Clop, A (2019). A RNA-Seq analysis to describe the boar sperm transcriptome and its seasonal changes. Frontiers in Genetics 10, 299.
| A RNA-Seq analysis to describe the boar sperm transcriptome and its seasonal changes.Crossref | GoogleScholarGoogle Scholar | 31040860PubMed |
Goh, WSS, Falciatori, I, Tam, OH, Burgess, R, Meikar, O, Kotaja, N, Hammell, M, and Hannon, GJ (2015). piRNA-directed cleavage of meiotic transcripts regulates spermatogenesis. Genes & Development 29, 1032–1044.
| piRNA-directed cleavage of meiotic transcripts regulates spermatogenesis.Crossref | GoogleScholarGoogle Scholar |
Gommans, WM, Mullen, SP, and Maas, S (2009). RNA editing: a driving force for adaptive evolution? BioEssays 31, 1137–1145.
| RNA editing: a driving force for adaptive evolution?Crossref | GoogleScholarGoogle Scholar | 19708020PubMed |
Gott, JM, and Emeson, RB (2000). Functions and mechanisms of RNA editing. Annual Review of Genetics 34, 499–531.
| Functions and mechanisms of RNA editing.Crossref | GoogleScholarGoogle Scholar | 11092837PubMed |
Gou, LT, Dai, P, Yang, JH, Xue, Y, Hu, YP, Zhou, Y, Kang, JY, Wang, X, Li, H, Hua, MM, Zhao, S, Hu, SD, Wu, LG, Shi, HJ, Li, Y, Fu, XD, Qu, LH, Wang, ED, and Liu, MF (2014). Pachytene piRNAs instruct massive mRNA elimination during late spermiogenesis. Cell Research 24, 680–700.
| Pachytene piRNAs instruct massive mRNA elimination during late spermiogenesis.Crossref | GoogleScholarGoogle Scholar | 24787618PubMed |
Grandjean, V, Fourre, S, De Abreu, DA, Derieppe, M-A, Remy, JJ, and Rassoulzadegan, M (2016). RNA-mediated paternal heredity of diet-induced obesity and metabolic disorders. Scientific Reports 5, 18193.
| RNA-mediated paternal heredity of diet-induced obesity and metabolic disorders.Crossref | GoogleScholarGoogle Scholar |
Guan, L, and Grigoriev, A (2020). Age-related argonaute loading of ribosomal RNA fragments. MicroRNA 9, 142–152.
| Age-related argonaute loading of ribosomal RNA fragments.Crossref | GoogleScholarGoogle Scholar | 31538909PubMed |
Guo, L, Chao, S-B, Xiao, L, Wang, Z-B, Meng, T-G, Li, Y-Y, Han, Z-M, Ouyang, Y-C, Hou, Y, Sun, Q-Y, and Ou, X-H (2017). Sperm-carried RNAs play critical roles in mouse embryonic development. Oncotarget 8, 67394–67405.
| Sperm-carried RNAs play critical roles in mouse embryonic development.Crossref | GoogleScholarGoogle Scholar | 28978041PubMed |
Guzzi, N, and Bellodi, C (2020). Novel insights into the emerging roles of tRNA-derived fragments in mammalian development. RNA Biology 17, 1214–1222.
| Novel insights into the emerging roles of tRNA-derived fragments in mammalian development.Crossref | GoogleScholarGoogle Scholar | 32116113PubMed |
Hamada, AJ, Montgomery, B, and Agarwal, A (2012). Male infertility: a critical review of pharmacologic management. Expert Opinion on Pharmacotherapy 13, 2511–2531.
| Male infertility: a critical review of pharmacologic management.Crossref | GoogleScholarGoogle Scholar | 23121497PubMed |
Hammond, SM (2015). An overview of microRNAs. Advanced Drug Delivery Reviews 87, 3–14.
| An overview of microRNAs.Crossref | GoogleScholarGoogle Scholar | 25979468PubMed |
He, Z, Jiang, J, Kokkinaki, M, Tang, L, Zeng, W, Gallicano, I, Dobrinski, I, and Dym, M (2013). MiRNA-20 and mirna-106a regulate spermatogonial stem cell renewal at the post-transcriptional level via targeting STAT3 and Ccnd1. Stem Cells 31, 2205–2217.
| MiRNA-20 and mirna-106a regulate spermatogonial stem cell renewal at the post-transcriptional level via targeting STAT3 and Ccnd1.Crossref | GoogleScholarGoogle Scholar | 23836497PubMed |
Hilz, S, Fogarty, EA, Modzelewski, AJ, Cohen, PE, and Grimson, A (2017). Transcriptome profiling of the developing male germ line identifies the miR-29 family as a global regulator during meiosis. RNA Biology 14, 219–235.
| Transcriptome profiling of the developing male germ line identifies the miR-29 family as a global regulator during meiosis.Crossref | GoogleScholarGoogle Scholar | 27981880PubMed |
Hombach, S, and Kretz, M (2016). Non-coding RNAs: classification, biology and functioning. Advances in Experimental Medicine and Biology 937, 3–17.
| Non-coding RNAs: classification, biology and functioning.Crossref | GoogleScholarGoogle Scholar | 27573892PubMed |
Hua, M, Liu, W, Chen, Y, Zhang, F, Xu, B, Liu, S, Chen, G, Shi, H, and Wu, L (2019). Identification of small non-coding RNAs as sperm quality biomarkers for in vitro fertilization. Cell Discovery 5, 20.
| Identification of small non-coding RNAs as sperm quality biomarkers for in vitro fertilization.Crossref | GoogleScholarGoogle Scholar | 30992999PubMed |
Huang, Y-L, Huang, G-Y, Lv, J, Pan, L-N, Luo, X, and Shen, J (2017). miR-100 promotes the proliferation of spermatogonial stem cells via regulating Stat3. Molecular Reproduction and Development 84, 693–701.
| miR-100 promotes the proliferation of spermatogonial stem cells via regulating Stat3.Crossref | GoogleScholarGoogle Scholar | 28569396PubMed |
Huszar, JM, and Payne, CJ (2013). MicroRNA 146 (Mir146) modulates spermatogonial differentiation by retinoic acid in mice. Biology of Reproduction 88, 15.
| MicroRNA 146 (Mir146) modulates spermatogonial differentiation by retinoic acid in mice.Crossref | GoogleScholarGoogle Scholar | 23221399PubMed |
Hutvágner, G, McLachlan, J, Pasquinelli, AE, Bálint, É, Tuschl, T, and Zamore, PD (2001). A cellular function for the RNA-interference enzyme dicer in the maturation of the let-7 small temporal RNA. Science 293, 834–838.
| A cellular function for the RNA-interference enzyme dicer in the maturation of the let-7 small temporal RNA.Crossref | GoogleScholarGoogle Scholar | 11452083PubMed |
Ivanov, P, Emara, MM, Villen, J, Gygi, SP, and Anderson, P (2011). Angiogenin-induced tRNA fragments inhibit translation initiation. Molecular Cell 43, 613–623.
| Angiogenin-induced tRNA fragments inhibit translation initiation.Crossref | GoogleScholarGoogle Scholar | 21855800PubMed |
Jia, Y, Mu, JC, and Ackerman, SL (2012). Mutation of a U2 snRNA gene causes global disruption of alternative splicing and neurodegeneration. Cell 148, 296–308.
| Mutation of a U2 snRNA gene causes global disruption of alternative splicing and neurodegeneration.Crossref | GoogleScholarGoogle Scholar | 22265417PubMed |
Karijolich, J, and Yu, YT (2010). Spliceosomal snRNA modifications and their function. RNA Biology 7, 192–204.
| Spliceosomal snRNA modifications and their function.Crossref | GoogleScholarGoogle Scholar | 20215871PubMed |
Karna, KK, Shin, YS, Choi, BR, Kim, HK, and Park, JK (2020). The role of endoplasmic reticulum stress response in male reproductive physiology and pathology: a review. The World Journal of Men’s Health 38, 484–494.
| The role of endoplasmic reticulum stress response in male reproductive physiology and pathology: a review.Crossref | GoogleScholarGoogle Scholar | 31385474PubMed |
Katoh, T, Sakaguchi, Y, Miyauchi, K, Suzuki, T, Kashiwabara, S-I, Baba, T, and Suzuki, T (2009). Selective stabilization of mammalian microRNAs by 3′ adenylation mediated by the cytoplasmic poly(A) polymerase GLD-2. Genes & Development 23, 433–438.
| Selective stabilization of mammalian microRNAs by 3′ adenylation mediated by the cytoplasmic poly(A) polymerase GLD-2.Crossref | GoogleScholarGoogle Scholar |
Keam, SP, and Hutvagner, G (2015). tRNA-Derived Fragments (tRFs): emerging new roles for an ancient RNA in the regulation of gene expression. Life 5, 1638–1651.
| tRNA-Derived Fragments (tRFs): emerging new roles for an ancient RNA in the regulation of gene expression.Crossref | GoogleScholarGoogle Scholar | 26703738PubMed |
Keles, E, Malama, E, Bozukova, S, Siuda, M, Wyck, S, Witschi, U, Bauersachs, S, and Bollwein, H (2021). The micro-RNA content of unsorted cryopreserved bovine sperm and its relation to the fertility of sperm after sex-sorting. BMC Genomics 22, 30.
| The micro-RNA content of unsorted cryopreserved bovine sperm and its relation to the fertility of sperm after sex-sorting.Crossref | GoogleScholarGoogle Scholar | 33413071PubMed |
Koenig, P-A, Nicholls, PK, Schmidt, FI, Hagiwara, M, Maruyama, T, Frydman, GH, Watson, N, Page, DC, and Ploegh, HL (2014). The E2 ubiquitin-conjugating enzyme UBE2J1 is required for spermiogenesis in mice. Journal of Biological Chemistry 289, 34490–34502.
| The E2 ubiquitin-conjugating enzyme UBE2J1 is required for spermiogenesis in mice.Crossref | GoogleScholarGoogle Scholar |
Korhonen, HM, Meikar, O, Yadav, RP, Papaioannou, MD, Romero, Y, Da Ros, M, Herrera, PL, Toppari, J, Nef, S, and Kotaja, N (2011). Dicer is required for haploid male germ cell differentiation in mice. PLoS One 6, e24821.
| Dicer is required for haploid male germ cell differentiation in mice.Crossref | GoogleScholarGoogle Scholar | 21949761PubMed |
Kotaja, N, Bhattacharyya, SN, Jaskiewicz, L, Kimmins, S, Parvinen, M, Filipowicz, W, and Sassone-Corsi, P (2006). The chromatoid body of male germ cells: similarity with processing bodies and presence of Dicer and microRNA pathway components. Proceedings of the National Academy of Sciences 103, 2647–2652.
| The chromatoid body of male germ cells: similarity with processing bodies and presence of Dicer and microRNA pathway components.Crossref | GoogleScholarGoogle Scholar |
Krill, KT, Gurdziel, K, Heaton, JH, Simon, DP, and Hammer, GD (2013). Dicer deficiency reveals microRNAs predicted to control gene expression in the developing adrenal cortex. Molecular Endocrinology 27, 754–768.
| Dicer deficiency reveals microRNAs predicted to control gene expression in the developing adrenal cortex.Crossref | GoogleScholarGoogle Scholar | 23518926PubMed |
Krishna, S, Raghavan, S, DasGupta, R, and Palakodeti, D (2021). tRNA-derived fragments (tRFs): establishing their turf in post-transcriptional gene regulation. Cellular and Molecular Life Sciences 78, 2607–2619.
| tRNA-derived fragments (tRFs): establishing their turf in post-transcriptional gene regulation.Crossref | GoogleScholarGoogle Scholar | 33388834PubMed |
Kumar, P, Kuscu, C, and Dutta, A (2016). Biogenesis and function of transfer RNA-Related Fragments (tRFs). Trends in Biochemical Sciences 41, 679–689.
| Biogenesis and function of transfer RNA-Related Fragments (tRFs).Crossref | GoogleScholarGoogle Scholar | 27263052PubMed |
Kuscu, C, Kumar, P, Kiran, M, Su, Z, Malik, A, and Dutta, A (2018). tRNA fragments (tRFs) guide Ago to regulate gene expression post-transcriptionally in a Dicer-independent manner. RNA 24, 1093–1105.
| tRNA fragments (tRFs) guide Ago to regulate gene expression post-transcriptionally in a Dicer-independent manner.Crossref | GoogleScholarGoogle Scholar | 29844106PubMed |
Lambert, M, Benmoussa, A, and Provost, P (2019). Small non-coding RNAs derived from eukaryotic ribosomal RNA. Non-coding RNA 5, 16.
| Small non-coding RNAs derived from eukaryotic ribosomal RNA.Crossref | GoogleScholarGoogle Scholar |
Le Blevec, E, Muronova, J, Ray, PF, and Arnoult, C (2020). Paternal epigenetics: Mammalian sperm provide much more than DNA at fertilization. Mol Cell Endocrinol 518, 110964.
| Paternal epigenetics: Mammalian sperm provide much more than DNA at fertilization.Crossref | GoogleScholarGoogle Scholar | 32738444PubMed |
Lee, Y, Ahn, C, Han, J, Choi, H, Kim, J, Yim, J, Lee, J, Provost, P, Radmark, O, Kim, S, and Kim, VN (2003). The nuclear RNase III Drosha initiates microRNA processing. Nature 425, 415–419.
| The nuclear RNase III Drosha initiates microRNA processing.Crossref | GoogleScholarGoogle Scholar | 14508493PubMed |
Lee, D, Park, D, Park, JH, Kim, JH, and Shin, C (2019). Poly(A)-specific ribonuclease sculpts the 3′ ends of microRNAs. RNA 25, 388–405.
| Poly(A)-specific ribonuclease sculpts the 3′ ends of microRNAs.Crossref | GoogleScholarGoogle Scholar | 30591540PubMed |
Li, L, Song, Y, Shi, X, Liu, J, Xiong, S, Chen, W, Fu, Q, Huang, Z, Gu, N, and Zhang, R (2018a). The landscape of miRNA editing in animals and its impact on miRNA biogenesis and targeting. Genome Research 28, 132–143.
| The landscape of miRNA editing in animals and its impact on miRNA biogenesis and targeting.Crossref | GoogleScholarGoogle Scholar | 29233923PubMed |
Li, Y, Li, R-H, Ran, M-X, Zhang, Y, Liang, K, Ren, Y-N, He, W-C, Zhang, M, Zhou, G-B, Qazi, IH, and Zeng, C-J (2018b). High throughput small RNA and transcriptome sequencing reveal capacitation-related microRNAs and mRNA in boar sperm. BMC Genomics 19, 736.
| High throughput small RNA and transcriptome sequencing reveal capacitation-related microRNAs and mRNA in boar sperm.Crossref | GoogleScholarGoogle Scholar | 30305024PubMed |
Liu, W-M, Pang, RTK, Chiu, PCN, Wong, BPC, Lao, K, Lee, K-F, and Yeung, WSB (2012). Sperm-borne microRNA-34c is required for the first cleavage division in mouse. Proceedings of the National Academy of Sciences 109, 490–494.
| Sperm-borne microRNA-34c is required for the first cleavage division in mouse.Crossref | GoogleScholarGoogle Scholar |
Locati, MD, Pagano, JFB, Abdullah, F, Ensink, WA, van Olst, M, van Leeuwen, S, Nehrdich, U, Spaink, HP, Rauwerda, H, Jonker, MJ, Dekker, RJ, and Breit, TM (2018). Identifying small RNAs derived from maternal- and somatic-type rRNAs in zebrafish development. Genome 61, 371–378.
| Identifying small RNAs derived from maternal- and somatic-type rRNAs in zebrafish development.Crossref | GoogleScholarGoogle Scholar | 29425468PubMed |
Loher, P, Londin, ER, and Rigoutsos, I (2014). IsomiR expression profiles in human lymphoblastoid cell lines exhibit population and gender dependencies. Oncotarget 5, 8790–8802.
| IsomiR expression profiles in human lymphoblastoid cell lines exhibit population and gender dependencies.Crossref | GoogleScholarGoogle Scholar | 25229428PubMed |
Maute, RL, Schneider, C, Sumazin, P, Holmes, A, Califano, A, Basso, K, and Dalla-Favera, R (2013). tRNA-derived microRNA modulates proliferation and the DNA damage response and is down-regulated in B cell lymphoma. Proceedings of the National Academy of Sciences 110, 1404–1409.
| tRNA-derived microRNA modulates proliferation and the DNA damage response and is down-regulated in B cell lymphoma.Crossref | GoogleScholarGoogle Scholar |
Menezes, ESB, Badial, PR, El Debaky, H, Husna, AU, Ugur, MR, Kaya, A, Topper, E, Bulla, C, Grant, KE, Bolden-Tiller, O, Moura, AA, and Memili, E (2020). Sperm miR-15a and miR-29b are associated with bull fertility. Andrologia 52, e13412.
| Sperm miR-15a and miR-29b are associated with bull fertility.Crossref | GoogleScholarGoogle Scholar | 31671225PubMed |
Momeni, A, Najafipour, R, Hamta, A, Jahani, S, and Moghbelinejad, S (2020). Expression and methylation pattern of hsa-miR-34 family in sperm samples of infertile men. Reproductive Sciences 27, 301–308.
| Expression and methylation pattern of hsa-miR-34 family in sperm samples of infertile men.Crossref | GoogleScholarGoogle Scholar | 32046388PubMed |
Morikawa, Y, and Cserjesi, P (2004). Extra-embryonic vasculature development is regulated by the transcription factor HAND1. Development 131, 2195–2204.
| Extra-embryonic vasculature development is regulated by the transcription factor HAND1.Crossref | GoogleScholarGoogle Scholar | 15073150PubMed |
Muñoz, X, Mata, A, Bassas, L, and Larriba, S (2015). Altered miRNA signature of developing germ-cells in infertile patients relates to the severity of spermatogenic failure and persists in spermatozoa. Scientific Reports 5, 17991–17991.
| Altered miRNA signature of developing germ-cells in infertile patients relates to the severity of spermatogenic failure and persists in spermatozoa.Crossref | GoogleScholarGoogle Scholar | 26648257PubMed |
Nätt, D, Kugelberg, U, Casas, E, Nedstrand, E, Zalavary, S, Henriksson, P, Nijm, C, Jäderquist, J, Sandborg, J, Flinke, E, Ramesh, R, Örkenby, L, Appelkvist, F, Lingg, T, Guzzi, N, Bellodi, C, Löf, M, Vavouri, T, and Öst, A (2019). Human sperm displays rapid responses to diet. PLoS Biology 17, e3000559.
| Human sperm displays rapid responses to diet.Crossref | GoogleScholarGoogle Scholar | 31877125PubMed |
Niu, Z, Goodyear, SM, Rao, S, Wu, X, Tobias, JW, Avarbock, MR, and Brinster, RL (2011). MicroRNA-21 regulates the self-renewal of mouse spermatogonial stem cells. Proceedings of the National Academy of Sciences 108, 12740–12745.
| MicroRNA-21 regulates the self-renewal of mouse spermatogonial stem cells.Crossref | GoogleScholarGoogle Scholar |
Nixon, B, Stanger, SJ, Mihalas, BP, Reilly, JN, Anderson, AL, Tyagi, S, Holt, JE, and McLaughlin, EA (2015). The MicroRNA signature of mouse spermatozoa is substantially modified during epididymal maturation. Biology of Reproduction 93, 91.
| The MicroRNA signature of mouse spermatozoa is substantially modified during epididymal maturation.Crossref | GoogleScholarGoogle Scholar | 26333995PubMed |
Nixon, B, De Iuliis, GN, Dun, MD, Zhou, W, Trigg, NA, and Eamens, AL (2019a). Profiling of epididymal small non-protein-coding RNAs. Andrology 7, 669–680.
| Profiling of epididymal small non-protein-coding RNAs.Crossref | GoogleScholarGoogle Scholar | 31020794PubMed |
Nixon, B, De Iuliis, GN, Hart, HM, Zhou, W, Mathe, A, Bernstein, IR, Anderson, AL, Stanger, SJ, Skerrett-Byrne, DA, Jamaluddin, MFB, Almazi, JG, Bromfield, EG, Larsen, MR, and Dun, MD (2019b). Proteomic Profiling of Mouse Epididymosomes Reveals their Contributions to Post-testicular Sperm Maturation. Molecular & Cellular Proteomics 18, S91–S108.
| Proteomic Profiling of Mouse Epididymosomes Reveals their Contributions to Post-testicular Sperm Maturation.Crossref | GoogleScholarGoogle Scholar |
O’Brien, J, Hayder, H, Zayed, Y, and Peng, C (2018). Overview of MicroRNA biogenesis, mechanisms of actions, and circulation. Frontiers in Endocrinology 9, 402.
| Overview of MicroRNA biogenesis, mechanisms of actions, and circulation.Crossref | GoogleScholarGoogle Scholar | 30123182PubMed |
Ostermeier, GC, Miller, D, Huntriss, JD, Diamond, MP, and Krawetz, SA (2004). Reproductive biology: delivering spermatozoan RNA to the oocyte. Nature 429, 154.
| Reproductive biology: delivering spermatozoan RNA to the oocyte.Crossref | GoogleScholarGoogle Scholar | 15141202PubMed |
Paris, Z, Fleming, IMC, and Alfonzo, JD (2012). Determinants of tRNA editing and modification: avoiding conundrums, affecting function. Seminars in Cell & Developmental Biology 23, 269–274.
| Determinants of tRNA editing and modification: avoiding conundrums, affecting function.Crossref | GoogleScholarGoogle Scholar |
Paul, P, Chakraborty, A, Sarkar, D, Langthasa, M, Rahman, M, Bari, M, Singha, RS, Malakar, AK, and Chakraborty, S (2018). Interplay between miRNAs and human diseases. Journal of Cellular Physiology 233, 2007–2018.
| Interplay between miRNAs and human diseases.Crossref | GoogleScholarGoogle Scholar | 28181241PubMed |
Penzo, M, Galbiati, A, Treré, D, and Montanaro, L (2016). The importance of being (slightly) modified: the role of rRNA editing on gene expression control and its connections with cancer. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer 1866, 330–338.
| The importance of being (slightly) modified: the role of rRNA editing on gene expression control and its connections with cancer.Crossref | GoogleScholarGoogle Scholar |
Perkins SD, Keel BN, Northrop EJ, McDaneld TG, Cushman RA, Harstine BR, DeJarnette JM, Utt MD, Perry GA (2020) Influence of microRNAs from semen on bovine fertility. In ‘Society for the Study of Reproduction Annual Meeting. Abstract Program’. pp. 181–182. (South Dakota State University: Brookings, SD, USA)
Picardi, E, Manzari, C, Mastropasqua, F, Aiello, I, D’Erchia, AM, and Pesole, G (2016). Corrigendum: Profiling RNA editing in human tissues: towards the inosinome Atlas. Scientific Reports 6, 20755.
| Corrigendum: Profiling RNA editing in human tissues: towards the inosinome Atlas.Crossref | GoogleScholarGoogle Scholar | 26854421PubMed |
Pillai, RS, and Chuma, S (2012). piRNAs and their involvement in male germline development in mice. Development, Growth & Differentiation 54, 78–92.
| piRNAs and their involvement in male germline development in mice.Crossref | GoogleScholarGoogle Scholar |
Raina, M, and Ibba, M (2014). tRNAs as regulators of biological processes. Frontiers in Genetics 5, 171.
| tRNAs as regulators of biological processes.Crossref | GoogleScholarGoogle Scholar | 24966867PubMed |
Reilly, JN, McLaughlin, EA, Stanger, SJ, Anderson, AL, Hutcheon, K, Church, K, Mihalas, BP, Tyagi, S, Holt, JE, Eamens, AL, and Nixon, B (2016). Characterisation of mouse epididymosomes reveals a complex profile of microRNAs and a potential mechanism for modification of the sperm epigenome. Scientific Reports 6, 31794.
| Characterisation of mouse epididymosomes reveals a complex profile of microRNAs and a potential mechanism for modification of the sperm epigenome.Crossref | GoogleScholarGoogle Scholar | 27549865PubMed |
Rejraji, H, Sion, B, Prensier, G, Carreras, M, Motta, C, Frenoux, J-M, Vericel, E, Grizard, G, Vernet, P, and Drevet, JR (2006). Lipid remodeling of murine epididymosomes and spermatozoa during epididymal maturation. Biology of Reproduction 74, 1104–1113.
| Lipid remodeling of murine epididymosomes and spermatozoa during epididymal maturation.Crossref | GoogleScholarGoogle Scholar | 16510839PubMed |
Romero, Y, Meikar, O, Papaioannou, MD, Conne, B, Grey, C, Weier, M, Pralong, F, De Massy, B, Kaessmann, H, Vassalli, J-D, Kotaja, N, and Nef, S (2011). Dicer1 depletion in male germ cells leads to infertility due to cumulative meiotic and spermiogenic defects. PLoS One 6, e25241.
| Dicer1 depletion in male germ cells leads to infertility due to cumulative meiotic and spermiogenic defects.Crossref | GoogleScholarGoogle Scholar | 21998645PubMed |
Rowlison, T, Ottinger, MA, and Comizzoli, P (2018). Key factors enhancing sperm fertilizing ability are transferred from the epididymis to the spermatozoa via epididymosomes in the domestic cat model. Journal of Assisted Reproduction and Genetics 35, 221–228.
| Key factors enhancing sperm fertilizing ability are transferred from the epididymis to the spermatozoa via epididymosomes in the domestic cat model.Crossref | GoogleScholarGoogle Scholar | 29134478PubMed |
Russell, SJ, Stalker, L, Gilchrist, G, Backx, A, Molledo, G, Foster, RA, and LaMarre, J (2016). Identification of PIWIL1 isoforms and their expression in bovine testes, oocytes, and early embryos. Biology of Reproduction 94, 75.
| Identification of PIWIL1 isoforms and their expression in bovine testes, oocytes, and early embryos.Crossref | GoogleScholarGoogle Scholar | 26911426PubMed |
Salas-Huetos, A, Blanco, J, Vidal, F, Godo, A, Grossmann, M, Pons, MC, F-Fernández, S, Garrido, N, and Anton, E (2015). Spermatozoa from patients with seminal alterations exhibit a differential micro-ribonucleic acid profile. Fertility and Sterility 104, 591–601.
| Spermatozoa from patients with seminal alterations exhibit a differential micro-ribonucleic acid profile.Crossref | GoogleScholarGoogle Scholar | 26143365PubMed |
Salas-Huetos, A, Blanco, J, Vidal, F, Grossmann, M, Pons, MC, Garrido, N, and Anton, E (2016). Spermatozoa from normozoospermic fertile and infertile individuals convey a distinct miRNA cargo. Andrology 4, 1028–1036.
| Spermatozoa from normozoospermic fertile and infertile individuals convey a distinct miRNA cargo.Crossref | GoogleScholarGoogle Scholar | 27676136PubMed |
Savva, YA, Rieder, LE, and Reenan, RA (2012). The ADAR protein family. Genome Biology 13, 252.
| The ADAR protein family.Crossref | GoogleScholarGoogle Scholar | 23273215PubMed |
Schorn, AJ, Gutbrod, MJ, LeBlanc, C, and Martienssen, R (2017). LTR-Retrotransposon Control by tRNA-Derived Small RNAs. Cell 170, 61–71.e11.
| LTR-Retrotransposon Control by tRNA-Derived Small RNAs.Crossref | GoogleScholarGoogle Scholar | 28666125PubMed |
Sellem, E, Broekhuijse, MLWJ, Chevrier, L, Camugli, S, Schmitt, E, Schibler, L, and Koenen, EPC (2015). Use of combinations of in vitro quality assessments to predict fertility of bovine semen. Theriogenology 84, 1447–1454.e5.
| Use of combinations of in vitro quality assessments to predict fertility of bovine semen.Crossref | GoogleScholarGoogle Scholar | 26296523PubMed |
Sellem, E, Marthey, S, Rau, A, Jouneau, L, Bonnet, A, Perrier, J-P, Fritz, S, Le Danvic, C, Boussaha, M, Kiefer, H, Jammes, H, and Schibler, L (2020). A comprehensive overview of bull sperm-borne small non-coding RNAs and their diversity across breeds. Epigenetics & Chromatin 13, 19.
| A comprehensive overview of bull sperm-borne small non-coding RNAs and their diversity across breeds.Crossref | GoogleScholarGoogle Scholar |
Sellem, E, Marthey, S, Rau, A, Jouneau, L, Bonnet, A, Le Danvic, C, Guyonnet, B, Kiefer, H, Jammes, H, and Schibler, L (2021). Dynamics of cattle sperm sncRNAs during maturation, from testis to ejaculated sperm. Epigenetics & Chromatin 14, 24.
| Dynamics of cattle sperm sncRNAs during maturation, from testis to ejaculated sperm.Crossref | GoogleScholarGoogle Scholar |
Sendler, E, Johnson, GD, Mao, S, Goodrich, RJ, Diamond, MP, Hauser, R, and Krawetz, SA (2013). Stability, delivery and functions of human sperm RNAs at fertilization. Nucleic Acids Research 41, 4104–4117.
| Stability, delivery and functions of human sperm RNAs at fertilization.Crossref | GoogleScholarGoogle Scholar | 23471003PubMed |
Sharma, U (2019). Paternal contributions to offspring health: role of sperm small RNAs in intergenerational transmission of epigenetic information. Frontiers in Cell and Developmental Biology 7, 215.
| Paternal contributions to offspring health: role of sperm small RNAs in intergenerational transmission of epigenetic information.Crossref | GoogleScholarGoogle Scholar | 31681757PubMed |
Sharma, U, Conine, CC, Shea, JM, Boskovic, A, Derr, AG, Bing, XY, Belleannee, C, Kucukural, A, Serra, RW, Sun, F, Song, L, Carone, BR, Ricci, EP, Li, XZ, Fauquier, L, Moore, MJ, Sullivan, R, Mello, CC, Garber, M, and Rando, OJ (2016). Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 351, 391–396.
| Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals.Crossref | GoogleScholarGoogle Scholar | 26721685PubMed |
Sharma, U, Sun, F, Conine, CC, Reichholf, B, Kukreja, S, Herzog, VA, Ameres, SL, and Rando, OJ (2018). Small RNAs are trafficked from the epididymis to developing Mammalian sperm. Developmental Cell 46, 481–494.e6.
| Small RNAs are trafficked from the epididymis to developing Mammalian sperm.Crossref | GoogleScholarGoogle Scholar | 30057273PubMed |
Shi, S, Shi, Q, and Sun, Y (2020). The effect of sperm miR-34c on human embryonic development kinetics and clinical outcomes. Life Sciences 256, 117895.
| The effect of sperm miR-34c on human embryonic development kinetics and clinical outcomes.Crossref | GoogleScholarGoogle Scholar | 32502545PubMed |
Sullivan, R, Frenette, G, and Girouard, J (2007). Epididymosomes are involved in the acquisition of new sperm proteins during epididymal transit. Asian Journal of Andrology 9, 483–491.
| Epididymosomes are involved in the acquisition of new sperm proteins during epididymal transit.Crossref | GoogleScholarGoogle Scholar | 17589785PubMed |
Tan, GC, and Dibb, N (2015). IsomiRs have functional importance. The Malaysian Journal of Pathology 37, 73–81.
| 26277662PubMed |
Tan, GC, Chan, E, Molnar, A, Sarkar, R, Alexieva, D, Isa, IM, Robinson, S, Zhang, S, Ellis, P, Langford, CF, Guillot, PV, Chandrashekran, A, Fisk, NM, Castellano, L, Meister, G, Winston, RM, Cui, W, Baulcombe, D, and Dibb, NJ (2014). 5′ isomiR variation is of functional and evolutionary importance. Nucleic Acids Research 42, 9424–9435.
| 5′ isomiR variation is of functional and evolutionary importance.Crossref | GoogleScholarGoogle Scholar | 25056318PubMed |
Telonis, AG, Loher, P, Honda, S, Jing, Y, Palazzo, J, Kirino, Y, and Rigoutsos, I (2015a). Dissecting tRNA-derived fragment complexities using personalized transcriptomes reveals novel fragment classes and unexpected dependencies. Oncotarget 6, 24797–24822.
| Dissecting tRNA-derived fragment complexities using personalized transcriptomes reveals novel fragment classes and unexpected dependencies.Crossref | GoogleScholarGoogle Scholar | 26325506PubMed |
Telonis, AG, Loher, P, Jing, Y, Londin, E, and Rigoutsos, I (2015b). Beyond the one-locus-one-miRNA paradigm: microRNA isoforms enable deeper insights into breast cancer heterogeneity. Nucleic Acids Research 43, 9158–9175.
| Beyond the one-locus-one-miRNA paradigm: microRNA isoforms enable deeper insights into breast cancer heterogeneity.Crossref | GoogleScholarGoogle Scholar | 26400174PubMed |
Telonis, AG, Magee, R, Loher, P, Chervoneva, I, Londin, E, and Rigoutsos, I (2017). Knowledge about the presence or absence of miRNA isoforms (isomiRs) can successfully discriminate amongst 32 TCGA cancer types. Nucleic Acids Research 45, 2973–2985.
| Knowledge about the presence or absence of miRNA isoforms (isomiRs) can successfully discriminate amongst 32 TCGA cancer types.Crossref | GoogleScholarGoogle Scholar | 28206648PubMed |
Tian, H, Li, Z, Peng, D, Bai, X, and Liang, W (2017). Expression difference of miR-10b and miR-135b between the fertile and infertile semen samples (p). Forensic Science International: Genetics Supplement Series 6, e257–e259.
| Expression difference of miR-10b and miR-135b between the fertile and infertile semen samples (p).Crossref | GoogleScholarGoogle Scholar |
Trigg, NA, Eamens, AL, and Nixon, B (2019). The contribution of epididymosomes to the sperm small RNA profile. Reproduction 157, R209–R223.
| The contribution of epididymosomes to the sperm small RNA profile.Crossref | GoogleScholarGoogle Scholar | 30780129PubMed |
Vashisht, A, and Gahlay, GK (2020). Using miRNAs as diagnostic biomarkers for male infertility: opportunities and challenges. Molecular Human Reproduction 26, 199–214.
| Using miRNAs as diagnostic biomarkers for male infertility: opportunities and challenges.Crossref | GoogleScholarGoogle Scholar | 32084276PubMed |
Vincent P, Underwood SL, Dolbec C, Bouchard N, Kroetsch T, Blondin P (2014) Bovine semen quality control in artificial insemination centers. In ‘Bovine reproduction’. (Ed. RM Hopper) pp. 685–695. (John Wiley & Sons, Inc: Hoboken, NJ, USA)
Vojtech, L, Woo, S, Hughes, S, Levy, C, Ballweber, L, Sauteraud, RP, Strobl, J, Westerberg, K, Gottardo, R, Tewari, M, and Hladik, F (2014). Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic Acids Research 42, 7290–7304.
| Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions.Crossref | GoogleScholarGoogle Scholar | 24838567PubMed |
Wei, H, Zhou, B, Zhang, F, Tu, Y, Hu, Y, Zhang, B, and Zhai, Q (2013). Profiling and identification of small rDNA-derived RNAs and their potential biological functions. PLoS One 8, e56842.
| Profiling and identification of small rDNA-derived RNAs and their potential biological functions.Crossref | GoogleScholarGoogle Scholar | 23418607PubMed |
Wei, J-W, Huang, K, Yang, C, and Kang, C-S (2017). Non-coding RNAs as regulators in epigenetics (Review). Oncology Reports 37, 3–9.
| Non-coding RNAs as regulators in epigenetics (Review).Crossref | GoogleScholarGoogle Scholar | 27841002PubMed |
Weick, E-M, and Miska, EA (2014). piRNAs: from biogenesis to function. Development 141, 3458–3471.
| piRNAs: from biogenesis to function.Crossref | GoogleScholarGoogle Scholar | 25183868PubMed |
Wightman, B, Ha, I, and Ruvkun, G (1993). Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 75, 855–862.
| Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans.Crossref | GoogleScholarGoogle Scholar | 8252622PubMed |
Xu, H, Wang, X, Wang, Z, Li, J, Xu, Z, Miao, M, Chen, G, Lei, X, Wu, J, Shi, H, Wang, K, Zhang, T, and Sun, X (2020). MicroRNA expression profile analysis in sperm reveals hsa-mir-191 as an auspicious omen of in vitro fertilization. BMC Genomics 21, 165.
| MicroRNA expression profile analysis in sperm reveals hsa-mir-191 as an auspicious omen of in vitro fertilization.Crossref | GoogleScholarGoogle Scholar | 32066367PubMed |
Yang, Q-E, Racicot, KE, Kaucher, AV, Oatley, MJ, and Oatley, JM (2013). MicroRNAs 221 and 222 regulate the undifferentiated state in mammalian male germ cells. Development 140, 280–290.
| MicroRNAs 221 and 222 regulate the undifferentiated state in mammalian male germ cells.Crossref | GoogleScholarGoogle Scholar | 23221369PubMed |
Yang, X-Z, Chen, J-Y, Liu, C-J, Peng, J, Wee, YR, Han, X, Wang, C, Zhong, X, Shen, QS, Liu, H, Cao, H, Chen, X-W, Tan, BC-M, and Li, C-Y (2015). Selectively constrained RNA editing regulation crosstalks with piRNA biogenesis in primates. Molecular Biology and Evolution 32, 3143–3157.
| Selectively constrained RNA editing regulation crosstalks with piRNA biogenesis in primates.Crossref | GoogleScholarGoogle Scholar | 26341297PubMed |
Yu, M, Mu, H, Niu, Z, Chu, Z, Zhu, H, and Hua, J (2014). miR-34c enhances mouse spermatogonial stem cells differentiation by targeting Nanos2. Journal of Cellular Biochemistry 115, 232–242.
| miR-34c enhances mouse spermatogonial stem cells differentiation by targeting Nanos2.Crossref | GoogleScholarGoogle Scholar | 24038201PubMed |
Yuan, S, Tang, C, Zhang, Y, Wu, J, Bao, J, Zheng, H, Xu, C, and Yan, W (2015). mir-34b/c and mir-449a/b/c are required for spermatogenesis, but not for the first cleavage division in mice. Biology Open 4, 212–223.
| mir-34b/c and mir-449a/b/c are required for spermatogenesis, but not for the first cleavage division in mice.Crossref | GoogleScholarGoogle Scholar | 25617420PubMed |
Zhang, Y, Shi, J, Rassoulzadegan, M, Tuorto, F, and Chen, Q (2019). Sperm RNA code programmes the metabolic health of offspring. Nature Reviews Endocrinology 15, 489–498.
| Sperm RNA code programmes the metabolic health of offspring.Crossref | GoogleScholarGoogle Scholar | 31235802PubMed |
Zhao, S, Gou, L-T, Zhang, M, Zu, L-D, Hua, M-M, Hua, Y, Shi, H-J, Li, Y, Li, J, Li, D, Wang, E-D, and Liu, M-F (2013). piRNA-triggered MIWI ubiquitination and removal by APC/C in late spermatogenesis. Developmental Cell 24, 13–25.
| piRNA-triggered MIWI ubiquitination and removal by APC/C in late spermatogenesis.Crossref | GoogleScholarGoogle Scholar | 23328397PubMed |
Zheng, Y, Ji, B, Song, R, Wang, S, Li, T, Zhang, X, Chen, K, Li, T, and Li, J (2016). Accurate detection for a wide range of mutation and editing sites of microRNAs from small RNA high-throughput sequencing profiles. Nucleic Acids Research 44, e123.
| Accurate detection for a wide range of mutation and editing sites of microRNAs from small RNA high-throughput sequencing profiles.Crossref | GoogleScholarGoogle Scholar | 27229138PubMed |
Zhou, W, De Iuliis, GN, Dun, MD, and Nixon, B (2018). Characteristics of the epididymal luminal environment responsible for sperm maturation and storage. Frontiers in Endocrinology 9, 59.
| Characteristics of the epididymal luminal environment responsible for sperm maturation and storage.Crossref | GoogleScholarGoogle Scholar | 29541061PubMed |
Zinshteyn, B, and Nishikura, K (2009). Adenosine-to-inosine RNA editing. Wiley Interdisciplinary Reviews: Systems Biology and Medicine 1, 202–209.
| Adenosine-to-inosine RNA editing.Crossref | GoogleScholarGoogle Scholar | 20835992PubMed |