LH upregulates connexin 43 expression in granulosa cells by activating the Wnt/β-catenin signalling pathway
Xiaomin Zheng A B D * , Hua Jing C * , Shanqing Gao A , Changchun Hei A , Xiaofeng Ye A , Yinming Liu A , Yufang Cai A , Bin Kong A , Kai Wu A , Shiwen Jiang B , Chengjun Zhao A D and Qing Chang A D
A D
A Key Laboratory of Fertility Preservation and Maintenance of Ministry of Education, School of Basic Medical Sciences, Department II of Surgical Oncology, Tumor Hospital of General Hospital of Ningxia Medical University, Ningxia Medical University, Shengli Street 1160#, Yinchuan 750004, China.
B Research Institute for Reproductive Medicine and Genetic Diseases, Center for Reproductive Medicine, The Affiliated Wuxi Maternity and Child Health Care Hospital of Nanjing Medical University, Huaishu Lane 48#, Wuxi 214002, China.
C Shanxi Medical College for Continuing Education, Daxue Street 209#, Taiyuan 030600, China.
D Corresponding authors. Email: 20090006@nxmu.edu.cn; 972514523@qq.com; 245542578@qq.com
Reproduction, Fertility and Development - https://doi.org/10.1071/RD20218
Submitted: 20 August 2020 Accepted: 26 November 2020 Published online: 18 January 2021
Abstract
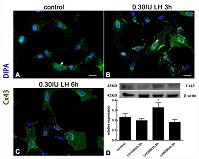
Connexin (Cx) 43 is the most widely expressed gap junction protein in follicle granulosa cells and plays an important role in follicle development and growth. The aims of this study were to investigate the effects of LH on the expression of Cx43 and key proteins in the downstream Wnt-β/catenin signalling pathway and to explore the mechanism underlying the regulation of Cx43 expression in granulosa cells. Primary culture granulosa cells were obtained from 21-day-old Sprague-Dawley rats, and were treated with different concentrations of LH (150, 300 and 600 IU L−1). Cx43 expression in granulosa cells was detected using immunofluorescence. Western blotting was used to detect the expression of Cx43, β-catenin and Axin2 proteins (Axin2 is a protein that in humans is encoded by the AXIN2 gene, which presumably plays an important role in the regulation of the stability of β-catenin in the Wnt signaling pathway) in granulosa cells with and without FH535 treatment (a Wnt/β-catenin signalling pathway inhibitor). Cx43 expression was detected in the cytoplasm and cell membrane of granulosa cells. Treatment with a high concentration of LH (300 IU L−1) increased the expression of β-catenin and Axin2, as well as that of Cx43. FH535 treatment reduced the LH-induced increases in Cx43, β-catenin and Axin2. These results indicate that LH upregulates Cx43 expression in granular cells by activating the Wnt/β-catenin signalling pathway.
Keywords: connexin 43, granulosa cells, immunofluorescence, LH, rat, western blotting.
References
Ackert, C. L., Gittens, J. E., O’Brien, M. J., Eppig, J. J., and Kidder, G. M. (2001). Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse. Dev. Biol. 233, 258–270.| Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse.Crossref | GoogleScholarGoogle Scholar | 11336494PubMed |
Chen, H., Zhao, L., Chu, G., Kito, G., Yamauchi, N., Shigeyoshi, Y., Hashimoto, S., and Hattori, M. A. (2013). FSH induces the development of circadian clockwork in rat granulosa cells via a gap junction protein Cx43-dependent pathway. Am. J. Physiol. Endocrinol. Metab. 304, E566–E575.
| FSH induces the development of circadian clockwork in rat granulosa cells via a gap junction protein Cx43-dependent pathway.Crossref | GoogleScholarGoogle Scholar | 23299500PubMed |
Choi, J. H., Chen, C. L., Poon, S. L., Wang, H. S., and Leung, P. C. (2009). Gonadotropin-stimulated epidermal growth factor receptor expression in human ovarian surface epithelial cells: involvement of cyclic AMP-dependent exchange protein activated by cAMP pathway. Endocr. Relat. Cancer 16, 179–188.
| Gonadotropin-stimulated epidermal growth factor receptor expression in human ovarian surface epithelial cells: involvement of cyclic AMP-dependent exchange protein activated by cAMP pathway.Crossref | GoogleScholarGoogle Scholar | 19022848PubMed |
Clevers, H., and Nusse, R. (2012). Wnt/beta-catenin signaling and disease. Cell 149, 1192–1205.
| Wnt/beta-catenin signaling and disease.Crossref | GoogleScholarGoogle Scholar | 22682243PubMed |
Fang, W. L., Lai, S. Y., Lai, W. A., Lee, M. T., Liao, C. F., Ke, F. C., and Hwang, J. J. (2015). CRTC2 and Nedd4 ligase involvement in FSH and TGFbeta1 upregulation of connexin43 gap junction. J. Mol. Endocrinol. 55, 263–275.
| CRTC2 and Nedd4 ligase involvement in FSH and TGFbeta1 upregulation of connexin43 gap junction.Crossref | GoogleScholarGoogle Scholar | 26508620PubMed |
Ganesan, S., Nteeba, J., and Keating, A. F. (2015). Impact of obesity on 7,12-dimethylbenz[a]anthracene-induced altered ovarian connexin gap junction proteins in female mice. Toxicol. Appl. Pharmacol. 282, 1–8.
| Impact of obesity on 7,12-dimethylbenz[a]anthracene-induced altered ovarian connexin gap junction proteins in female mice.Crossref | GoogleScholarGoogle Scholar | 25447408PubMed |
Hernandez Gifford, J. A. (2015). The role of WNT signaling in adult ovarian folliculogenesis. Reproduction 150, R137–R148.
| The role of WNT signaling in adult ovarian folliculogenesis.Crossref | GoogleScholarGoogle Scholar | 26130815PubMed |
Kleopa, K. A., and Sargiannidou, I. (2015). Connexins, gap junctions and peripheral neuropathy. Neurosci. Lett. 596, 27–32.
| Connexins, gap junctions and peripheral neuropathy.Crossref | GoogleScholarGoogle Scholar | 25449862PubMed |
Krysko, D. V., Mussche, S., Leybaert, L., and D’Herde, K. (2004). Gap junctional communication and connexin43 expression in relation to apoptotic cell death and survival of granulosa cells. J. Histochem. Cytochem. 52, 1199–1207.
| Gap junctional communication and connexin43 expression in relation to apoptotic cell death and survival of granulosa cells.Crossref | GoogleScholarGoogle Scholar | 15314087PubMed |
Matzuk, M. M., Burns, K. H., Viveiros, M. M., and Eppig, J. J. (2002). Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science 296, 2178–2180.
| Intercellular communication in the mammalian ovary: oocytes carry the conversation.Crossref | GoogleScholarGoogle Scholar | 12077402PubMed |
Palermo, R. (2007). Differential actions of FSH and LH during folliculogenesis. Reprod. Biomed Online 15, 326–337.
| Differential actions of FSH and LH during folliculogenesis.Crossref | GoogleScholarGoogle Scholar | 17854533PubMed |
Ping, T., Manyi, W., and Xiaol, Y. (2007). Function of connexins Cx43 and Cx37 in follicular development. Prog. Anim. Med. 28, 91–94.
Pogoda, K., and Kameritsch, P. (2019). Molecular regulation of myoendothelial gap junctions. Curr. Opin. Pharmacol. 45, 16–22.
| Molecular regulation of myoendothelial gap junctions.Crossref | GoogleScholarGoogle Scholar | 30999095PubMed |
Sedgwick, A. E., and D’Souza-Schorey, C. (2016). Wnt signaling in cell motility and invasion: drawing parallels between development and cancer. Cancers (Basel) 8, 80.
| Wnt signaling in cell motility and invasion: drawing parallels between development and cancer.Crossref | GoogleScholarGoogle Scholar |
Sela-Abramovich, S., Chorev, E., Galiani, D., and Dekel, N. (2005). Mitogen-activated protein kinase mediates luteinizing hormone-induced breakdown of communication and oocyte maturation in rat ovarian follicles. Endocrinology 146, 1236–1244.
| Mitogen-activated protein kinase mediates luteinizing hormone-induced breakdown of communication and oocyte maturation in rat ovarian follicles.Crossref | GoogleScholarGoogle Scholar | 15576461PubMed |
Sela-Abramovich, S., Galiani, D., Nevo, N., and Dekel, N. (2008). Inhibition of rat oocyte maturation and ovulation by nitric oxide: mechanism of action. Biol. Reprod. 78, 1111–1118.
| Inhibition of rat oocyte maturation and ovulation by nitric oxide: mechanism of action.Crossref | GoogleScholarGoogle Scholar | 18337515PubMed |
Stapp, A. D., Gomez, B. I., Gifford, C. A., Hallford, D. M., and Hernandez Gifford, J. A. (2014). Canonical WNT signaling inhibits follicle stimulating hormone mediated steroidogenesis in primary cultures of rat granulosa cells. PLoS One 9, e86432.
| Canonical WNT signaling inhibits follicle stimulating hormone mediated steroidogenesis in primary cultures of rat granulosa cells.Crossref | GoogleScholarGoogle Scholar | 24466091PubMed |
Tepekoy, F., and Akkoyunlu, G. (2020). The interaction of Wnt signaling members with growth factors in cultured granulosa cells. Anim. Reprod. 17, e20190106.
| The interaction of Wnt signaling members with growth factors in cultured granulosa cells.Crossref | GoogleScholarGoogle Scholar | 32714449PubMed |
Tepekoy, F., Uysal, F., Acar, N., Ustunel, I., and Akkoyunlu, G. (2019). The efect of GnRH antagonist cetrorelix on Wnt signaling members in pubertal and adult mouse ovaries. Histochem. Cell Biol. 152, 423–437.
| The efect of GnRH antagonist cetrorelix on Wnt signaling members in pubertal and adult mouse ovaries.Crossref | GoogleScholarGoogle Scholar | 31630211PubMed |
Wang, H. X., Gillio-Meina, C., Chen, S., Gong, X. Q., Li, T. Y., Bai, D., and Kidder, G. M. (2013). The canonical WNT2 pathway and FSH interact to regulate gap junction assembly in mouse granulosa cells. Biol. Reprod. 89, 39.
| The canonical WNT2 pathway and FSH interact to regulate gap junction assembly in mouse granulosa cells.Crossref | GoogleScholarGoogle Scholar | 23843235PubMed |
Wright, C. S., Becker, D. L., Lin, J. S., Warner, A. E., and Hardy, K. (2001). Stage-specific and differential expression of gap junctions in the mouse ovary: connexin-specific roles in follicular regulation. Reproduction 121, 77–88.
| Stage-specific and differential expression of gap junctions in the mouse ovary: connexin-specific roles in follicular regulation.Crossref | GoogleScholarGoogle Scholar | 11226030PubMed |
Yamamoto, Y., Luckenbach, J. A., Middleton, M. A., and Swanson, P. (2011). The spatiotemporal expression of multiple coho salmon ovarian connexin genes and their hormonal regulation in vitro during oogenesis. Reprod. Biol. Endocrinol. 9, 52.
| The spatiotemporal expression of multiple coho salmon ovarian connexin genes and their hormonal regulation in vitro during oogenesis.Crossref | GoogleScholarGoogle Scholar | 21501524PubMed |
Yang, M., Li, J., An, Y., and Zhang, S. (2015). Effects of androgen on immunohistochemical localization of androgen receptor and connexin 43 in mouse ovary. Tissue Cell 47, 526–532.
| Effects of androgen on immunohistochemical localization of androgen receptor and connexin 43 in mouse ovary.Crossref | GoogleScholarGoogle Scholar | 26206424PubMed |


