Cloning, characterisation and expression profile of kisspeptin1 and the kisspeptin1 receptor in the hypothalamic–pituitary–ovarian axis of Chinese alligator Alligator sinensis during the reproductive cycle
Ruidong Zhang A B * , Haitao Nie A * , Shulong Duan A , Peng Yan A , Ali Izaz A , Renping Wang C , Yongkang Zhou C and Xiaobing Wu
A * , Shulong Duan A , Peng Yan A , Ali Izaz A , Renping Wang C , Yongkang Zhou C and Xiaobing Wu  A D
A D
A Key Laboratory for Conservation and Use of Important Biological Resources of Anhui Province, College of Life Sciences, Anhui Normal University, Wuhu, Anhui 241000, China.
B College of Life Sciences, Inner Mongolia Normal University, Hohhot, Inner Mongolia 010022, China.
C Alligator Research Center of Anhui Province, Xuanzhou 242000, China.
D Corresponding author. Email:wuxb@mail.ahnu.edu
Reproduction, Fertility and Development 32(8) 792-804 https://doi.org/10.1071/RD19332
Submitted: 27 August 2019 Accepted: 22 January 2020 Published: 20 April 2020
Abstract
Kisspeptin1 (Kiss1), a product of the Kiss1 gene, plays an important role in the regulation of reproduction in vertebrates by activating the Kiss1 receptor (Kiss1R) and its coexpression with gonadotrophin-releasing hormone (GnRH) in GnRH neurons. The purpose of this study was to clone the Kiss1 and Kiss1R genes found in the brain of Alligator sinensis and to explore their relationship with reproduction. The full-length cDNA of Kiss1 is 816 bp, the open reading frame (ORF) is 417 bp and the gene encodes a 138-amino acid precursor protein. The full-length cDNA of Kiss1R is 2348 bp, the ORF is 1086 bp and the gene encodes a 361-amino acid protein. Quantitative polymerase chain reaction showed that, except for Kiss1R expression in the hypothalamus, the expression of Kiss1 and Kiss1Rduring the reproductive period of A. sinensis was higher than that in the hypothalamus, pituitary gland and ovary during the hibernation period. The changes in GnRH2 mRNA in the hypothalamus were similar to those of GnRH1 and peaked during the reproductive period. This study confirms the existence of Kiss1 and Kiss1R in A. sinensis and the findings strongly suggest that Kiss1 and Kiss1R may participate in the regulation of GnRH secretion in the hypothalamus of alligators during the reproductive period. Furthermore, this is the first report of the full-length cDNA sequences of Kiss1 and Kiss1R in reptiles.
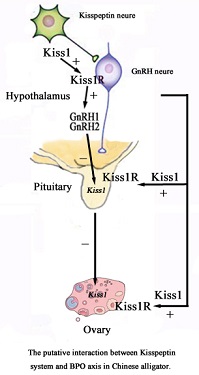
Additional keywords: hypothalamus, neuropeptide, ovary, reproduction.
References
Alvarado, M. V., Carrillo, M., and Felip, A. (2013). Expression of kisspeptins and their receptors, gnrh-1/gnrhr-II-1a and gonadotropin genes in the brain of adult male and female European sea bass during different gonadal stages. Gen. Comp. Endocrinol. 187, 104–116.| Expression of kisspeptins and their receptors, gnrh-1/gnrhr-II-1a and gonadotropin genes in the brain of adult male and female European sea bass during different gonadal stages.Crossref | GoogleScholarGoogle Scholar | 23583767PubMed |
Bhattacharya, M., and Babwah, A. V. (2015). Kisspeptin: beyond the brain. Endocrinology 156, 1218–1227.
| Kisspeptin: beyond the brain.Crossref | GoogleScholarGoogle Scholar | 25590245PubMed |
Cejudo-Román, A., Pinto, F. M., Dorta, I., Almeida, T. A., Hernández, M., Illanes, M., Tenasempere, M., and Candenas, L. (2012). Analysis of the expression of neurokinin B, kisspeptin, and their cognate receptors NK3R and KISS1R in the human female genital tract. Fertil. Steril. 97, 1213–1219.
| Analysis of the expression of neurokinin B, kisspeptin, and their cognate receptors NK3R and KISS1R in the human female genital tract.Crossref | GoogleScholarGoogle Scholar | 22424618PubMed |
Chianese, R., Ciaramella, V., Fasano, S., Pierantoni, R., and Meccariello, R. (2013). Kisspeptin receptor, gpr54, as a candidate for the regulation of testicular activity in the frog Rana esculenta. Biol. Reprod. 88, 73.
| Kisspeptin receptor, gpr54, as a candidate for the regulation of testicular activity in the frog Rana esculenta.Crossref | GoogleScholarGoogle Scholar | 23365413PubMed |
Chianese, R., Colledge, W. H., Fasano, S., and Meccariello, R. (2018). Editorial: the multiple facets of kisspeptin activity in biological systems. Front. Endocrinol. 9, 727.
| Editorial: the multiple facets of kisspeptin activity in biological systems.Crossref | GoogleScholarGoogle Scholar |
Cobellis, G., Vallarino, M., Meccariello, R., Pierantoni, R., Masini, M. A., Mathieu, M., Pernas-Alonso, R., Chieffi, P., and Fasano, S. (1999). Fos localization in cytosolic and nuclear compartments in neurones of the frog, Rana esculenta, brain: an analysis carried out in parallel with GnRH molecular forms. J. Neuroendocrinol. 11, 725–735.
| Fos localization in cytosolic and nuclear compartments in neurones of the frog, Rana esculenta, brain: an analysis carried out in parallel with GnRH molecular forms.Crossref | GoogleScholarGoogle Scholar | 10447811PubMed |
D’Anglemont, T. X., and Colledge, W. H. (2010). The role of kisspeptinsignaling in reproduction. Physiology (Bethesda) 25, 207–217.
| The role of kisspeptinsignaling in reproduction.Crossref | GoogleScholarGoogle Scholar |
Dunham, L. A., Lutterschmidt, D. I., and Wilczynski, W. (2009). Kisspeptin-like immunoreactive neuron distribution in the green anole (Anolis carolinensis). Brain Behav. Evol. 73, 129–137.
| Kisspeptin-like immunoreactive neuron distribution in the green anole (Anolis carolinensis).Crossref | GoogleScholarGoogle Scholar | 19420914PubMed |
Fairgrieve, M. R., Shibata, Y., Smith, E. K., Hayman, E. S., and Luckenbach, J. A. (2016). Molecular characterization of the gonadal kisspeptin system: cloning, tissue distribution, gene expression analysis and localization in sablefish (Anoplopoma fimbria). Gen. Comp. Endocrinol. 225, 212–223.
| Molecular characterization of the gonadal kisspeptin system: cloning, tissue distribution, gene expression analysis and localization in sablefish (Anoplopoma fimbria).Crossref | GoogleScholarGoogle Scholar | 26386183PubMed |
Felip, A., Zanuy, S., Pineda, R., Pinilla, L., Carrillo, M., Tena-Sempere, M., and Gómez, A. (2009). Evidence for two distinct KiSS genes in non-placental vertebrates that encode kisspeptins with different gonadotropin-releasing activities in fish and mammals. Mol. Cell. Endocrinol. 312, 61–71.
| Evidence for two distinct KiSS genes in non-placental vertebrates that encode kisspeptins with different gonadotropin-releasing activities in fish and mammals.Crossref | GoogleScholarGoogle Scholar | 19084576PubMed |
Funes, S., Hedrick, J. G., Markowitz, L., Abbondanzo, S., Golovko, A., Yang, S., Monsma, F. J., and Gustafson, E. L. (2003). The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem. Biophys. Res. Commun. 312, 1357–1363.
| The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system.Crossref | GoogleScholarGoogle Scholar | 14652023PubMed |
Gaytán, F., Gaytán, M., Castellano, J. M., Romero, M., Roa, J., Aparicio, B., Garrido, N., Sánchez-Criado, J. E., Millar, R. P., Pellicer, A., Fraser, H. M., and Tena-Sempere, M. (2009). KiSS-1 in the mammalian ovary: distribution of kisspeptin in human and marmoset and alterations in KiSS-1 mRNA levels in a rat model of ovulatory dysfunction. Am. J. Physiol. Endocrinol. Metab. 296, E520–E531.
| KiSS-1 in the mammalian ovary: distribution of kisspeptin in human and marmoset and alterations in KiSS-1 mRNA levels in a rat model of ovulatory dysfunction.Crossref | GoogleScholarGoogle Scholar | 19141682PubMed |
Gaytan, F., Garcia-Galiano, D., Dorfman, M. D., Manfredi-Lozano, M., Castellano, J. M., Dissen, G. A., Ojeda, S. R., and Tena-Sempere, M. (2014). Kisspeptin receptor haplo-insufficiency causes premature ovarian failure despite preserved gonadotropin secretion. Endocrinology 155, 3088–3097.
| Kisspeptin receptor haplo-insufficiency causes premature ovarian failure despite preserved gonadotropin secretion.Crossref | GoogleScholarGoogle Scholar | 24885574PubMed |
Grone, B. P., Maruska, K. P., Korzan, W. J., and Fernald, R. D. (2010). Social status regulates kisspeptin receptor mRNA in the brain of Astatotilapiaburtoni. Gen. Comp. Endocrinol. 169, 98–107.
| Social status regulates kisspeptin receptor mRNA in the brain of Astatotilapiaburtoni.Crossref | GoogleScholarGoogle Scholar | 20688063PubMed |
Han, S. K., Gottsch, M. L., Lee, K. J., Popa, S. M., Smith, J. T., Jakawich, S. K., Clifton, D. K., Steiner, R. A., and Herbison, A. E. (2005). Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J. Neurosci. 25, 11349–11356.
| Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty.Crossref | GoogleScholarGoogle Scholar | 16339030PubMed |
Herbison, A. E., D’Anglemont de Tassigny, X., Doran, J., and Colledge, W. H. (2010). Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology 151, 312–321.
| Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons.Crossref | GoogleScholarGoogle Scholar | 19966188PubMed |
Higo, S., Honda, S., Iijima, N., and Ozawa, H. (2016). Mapping of kisspeptin receptor mRNA in the whole rat brain and its co-localisation with oxytocin in the paraventricular nucleus. J. Neuroendocrinol. 28, E520–E531.
| Mapping of kisspeptin receptor mRNA in the whole rat brain and its co-localisation with oxytocin in the paraventricular nucleus.Crossref | GoogleScholarGoogle Scholar |
Imamura, S., Hur, S. P., Takeuchi, Y., Bouchekioua, S., and Takemura, A. (2017). Molecular cloning of kisspeptin receptor genes (gpr54-1 and gpr54-2) and their expression profiles in the brain of a tropical damselfish during different gonadal stages. Comp. Biochem. Physiol., Part A Mol. Integr. Physiol. 203, 9–16.
| Molecular cloning of kisspeptin receptor genes (gpr54-1 and gpr54-2) and their expression profiles in the brain of a tropical damselfish during different gonadal stages.Crossref | GoogleScholarGoogle Scholar | 27475299PubMed |
Kanda, S., Akazome, Y., Matsunaga, T., Yamamoto, N., Yamada, S., Tsukamura, H., Maeda, K., and Oka, Y. (2008). Identification of KiSS-1 product kisspeptin and steroid-sensitive sexually dimorphic kisspeptin neurons in medaka (Oryziaslatipes). Endocrinology 149, 2467–2476.
| Identification of KiSS-1 product kisspeptin and steroid-sensitive sexually dimorphic kisspeptin neurons in medaka (Oryziaslatipes).Crossref | GoogleScholarGoogle Scholar | 18202129PubMed |
Kanda, S., Karigo, T., and Oka, Y. (2012). Steroid sensitive kiss2 of side-blotched in the goldfish: evolutionary insights into the duplicate kisspeptin gene-expressing neurones. J. Neuroendocrinol. 24, 897–906.
| Steroid sensitive kiss2 of side-blotched in the goldfish: evolutionary insights into the duplicate kisspeptin gene-expressing neurones.Crossref | GoogleScholarGoogle Scholar | 22340198PubMed |
Kotani, M., Detheux, M., Vandenbogaerde, A., Communi, D., Vanderwinden, J., Le-Poul, E., Brezillon, S., Tyldesley, R., Suarez-Huerta, N., and Vandeput, F. (2001). The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J. Biol. Chem. 276, 34631–34636.
| The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54.Crossref | GoogleScholarGoogle Scholar | 11457843PubMed |
Lee, J. H., Miele, M. E., Hicks, D. J., Phillips, K. K., Trent, J. M., Weissman, B. E., and Welch, D. R. (1996). KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J. Natl Cancer Inst. 88, 1731–1737.
| KiSS-1, a novel human malignant melanoma metastasis-suppressor gene.Crossref | GoogleScholarGoogle Scholar | 8944003PubMed |
Lee, Y. R., Tsunekawa, K., Moon, M. J., Um, H. N., Hwang, J. I., Osugi, T., Otaki, N., Sunakawa, Y., Kim, K., Vaudry, H., Kwon, H. B., Seong, J. Y., and Tsutsui, K. (2009). Molecular evolution of multiple forms of kisspeptins and GPR54 receptors in vertebrates. Endocrinology 150, 2837–2846.
| Molecular evolution of multiple forms of kisspeptins and GPR54 receptors in vertebrates.Crossref | GoogleScholarGoogle Scholar | 19164475PubMed |
Liu, X., and Herbison, A. E. (2016). Kisspeptinregulation of neuronal activity throughout the central nervous system. Endocrinol. Metab. 31, 193–205.
| Kisspeptinregulation of neuronal activity throughout the central nervous system.Crossref | GoogleScholarGoogle Scholar |
Luque, R. M., José, C. C., Gahete, M. D., Navarro, V. M., Manuel, T. S., Kineman, R. D., and Casta, O. J. P. (2011). Kisspeptin regulates gonadotroph and somatotroph function in nonhuman primate pituitary via common and distinct signaling mechanisms. Endocrinology 152, 957–966.
| Kisspeptin regulates gonadotroph and somatotroph function in nonhuman primate pituitary via common and distinct signaling mechanisms.Crossref | GoogleScholarGoogle Scholar | 21209013PubMed |
Magee, C., Foradori, C. D., and Bruemmer, J. E. (2009). Biological and anatomical evidence for kisspeptin regulation of the hypothalamic–pituitary–gonadal axis of estrous horse mares. Endocrinology 150, 2813–2821.
| Biological and anatomical evidence for kisspeptin regulation of the hypothalamic–pituitary–gonadal axis of estrous horse mares.Crossref | GoogleScholarGoogle Scholar | 19228887PubMed |
Meccariello, R., Mathieu, M., Cobellis, G., Vallarino, M., Bruzzone, F., Fienga, G., Pierantoni, R., and Fasano, S. (2004). Jun localization in cytosolic and nuclear compartments in brain-pituitary system of the frog, Rana esculenta: an analysis carried out in parallel with GnRH molecular forms during the annual reproductive cycle. Gen. Comp. Endocrinol. 135, 310–323.
| Jun localization in cytosolic and nuclear compartments in brain-pituitary system of the frog, Rana esculenta: an analysis carried out in parallel with GnRH molecular forms during the annual reproductive cycle.Crossref | GoogleScholarGoogle Scholar | 14723883PubMed |
Migaud, H., Ismail, R., Cowan, M., and Davie, A. (2012). Kisspeptin and seasonal control of reproduction in male European sea bass (Dicentrarchuslabrax). Gen. Comp. Endocrinol. 179, 384–399.
| Kisspeptin and seasonal control of reproduction in male European sea bass (Dicentrarchuslabrax).Crossref | GoogleScholarGoogle Scholar | 23036731PubMed |
Moon, J. S., Lee, Y. R., Oh, D. Y., Hwang, J. I., Lee, J. Y., Kim, J. I., Vaudry, H., Kwon, H. B., and Seong, J. Y. (2009). Molecular cloning of the bullfrog kisspeptin receptor GPR54 with high sensitivity to Xenopuskisspeptin. Peptides 30, 171–179.
| Molecular cloning of the bullfrog kisspeptin receptor GPR54 with high sensitivity to Xenopuskisspeptin.Crossref | GoogleScholarGoogle Scholar | 18550222PubMed |
Neuman-Lee, L., Grieves, T., Hopkins, G. R., and French, S. S. (2017). The role of the kisspeptin system in regulation of the reproductive endocrine axis and territorial behavior in male side-blotched lizards (Uta stansburiana). Horm. Behav. 89, 48–54.
| The role of the kisspeptin system in regulation of the reproductive endocrine axis and territorial behavior in male side-blotched lizards (Uta stansburiana).Crossref | GoogleScholarGoogle Scholar | 28017596PubMed |
Oakley, A. E., Clifton, D. K., and Steiner, R. A. (2009). Kisspeptinsignaling in the brain. Endocr. Rev. 30, 713–743.
| Kisspeptinsignaling in the brain.Crossref | GoogleScholarGoogle Scholar | 19770291PubMed |
Osugi, T., Ohtaki, N., Sunakawa, Y., Son, Y. L., Ohkubo, M., Iigo, M., Amano, M., and Tsutsui, K. (2013). Molecular evolution of kiss2 genes and peptides in vertebrates. Endocrinology 154, 4270–4280.
| Molecular evolution of kiss2 genes and peptides in vertebrates.Crossref | GoogleScholarGoogle Scholar | 23959935PubMed |
Pasquier, J., Lafont, A. G., Rousseau, K., Quérat, B., Chemineau, P., and Dufour, S. (2014). Looking for the bird kiss: evolutionary scenario in sauropsids. BMC Evol. Biol. 14, 30.
| Looking for the bird kiss: evolutionary scenario in sauropsids.Crossref | GoogleScholarGoogle Scholar | 24552453PubMed |
Pineda, R., Aguilar, E., Pinilla, L., and Tena-Sempere, M. (2010). Chapter 5:physiological roles of the kisspeptin/GPR54 system in the neuroendocrine control of reproduction. Prog. Brain Res. 181, 55–77.
| Chapter 5:physiological roles of the kisspeptin/GPR54 system in the neuroendocrine control of reproduction.Crossref | GoogleScholarGoogle Scholar | 20478433PubMed |
Pinilla, L., Aguilar, E., Dieguez, C., Millar, R. P., and Tenasempere, M. (2012). Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol. Rev. 92, 1235–1316.
| Kisspeptins and reproduction: physiological roles and regulatory mechanisms.Crossref | GoogleScholarGoogle Scholar | 22811428PubMed |
Ramaesh, T., Logie, J. J., Roseweir, A. K., Millar, R. P., Walker, B. R., Hadoke, P. W., and Reynolds, R. M. (2010). Kisspeptin-10 inhibits angiogenesis in human placental vessels ex vivo and endothelial cells in vitro. Endocrinology 151, 5927.
| Kisspeptin-10 inhibits angiogenesis in human placental vessels ex vivo and endothelial cells in vitro.Crossref | GoogleScholarGoogle Scholar | 20926586PubMed |
Ramaswamy, S., Gibbs, R. B., and Plant, T. M. (2009). Studies of the localisation of kisspeptin within the pituitary of the rhesus monkey (Macaca mulatta) and the effect of kisspeptin on the release of non-gonadotropic pituitary hormones. J. Neuroendocrinol. 21, 795–804.
| Studies of the localisation of kisspeptin within the pituitary of the rhesus monkey (Macaca mulatta) and the effect of kisspeptin on the release of non-gonadotropic pituitary hormones.Crossref | GoogleScholarGoogle Scholar | 19686451PubMed |
Rather, M. A., Bhat, I. A., Rathor, P. K., Gireesh-Babu, P., Chaudhari, A., Kumar, S. J., and Sharma, R. (2016). In silico analysis and expression studies of kisspeptin gene in C. catla. J. Biomol. Struct. Dyn. 35, 2485–2496.
| In silico analysis and expression studies of kisspeptin gene in C. catla.Crossref | GoogleScholarGoogle Scholar | 27687705PubMed |
Richard, N., Galmiche, G. S., Caraty, A., and Kottler, M. (2008). KiSS-1 and GPR54 genes are co-expressed in rat gonadotrophs and differentially regulated in vivo by oestradiol and gonadotrophin-releasing hormone. J. Neuroendocrinol. 20, 381–393.
| KiSS-1 and GPR54 genes are co-expressed in rat gonadotrophs and differentially regulated in vivo by oestradiol and gonadotrophin-releasing hormone.Crossref | GoogleScholarGoogle Scholar | 18208554PubMed |
Roa, J., Aguilar, E., Dieguez, C., Pinilla, L., and Tena-Sempere, M. (2008). New frontiers in kisspeptin/GPR54 physiology as fundamental gatekeepers of reproductive function. Front. Neuroendocrinol. 29, 48–69.
| New frontiers in kisspeptin/GPR54 physiology as fundamental gatekeepers of reproductive function.Crossref | GoogleScholarGoogle Scholar | 17870152PubMed |
Saha, A., Pradhan, A., Sengupta, S., Nayak, M., Samanta, M., Sahoo, L., and Giri, S. S. (2016). Molecular characterization of two kiss genes and their expression in rohu (Labeorohita) during annual reproductive cycle. Comp. Biochem. Physiol. B 191, 135–145.
| Molecular characterization of two kiss genes and their expression in rohu (Labeorohita) during annual reproductive cycle.Crossref | GoogleScholarGoogle Scholar | 26506261PubMed |
Selvaraj, S., Kitano, H., Ohga, H., Yamaguchi, A., and Matsuyama, M. (2015). Expression changes of mRNAs encoding kisspeptins and their receptors and gonadotropin-releasing hormones during early development and gonadal sex differentiation periods in the brain of chub mackerel (Scomber japonicus). Gen. Comp. Endocrinol. 222, 20–32.
| Expression changes of mRNAs encoding kisspeptins and their receptors and gonadotropin-releasing hormones during early development and gonadal sex differentiation periods in the brain of chub mackerel (Scomber japonicus).Crossref | GoogleScholarGoogle Scholar | 25304825PubMed |
Servili, A., Le Page, Y., Leprince, J., Caraty, A., Escobar, S., Parhar, I. S., Seong, J. Y., Vaudry, H., and Kah, O. (2011). Organization of two independent kisspeptin systems derived from evolutionary-ancient kiss genes in the brain of zebrafish. Endocrinology 152, 1527–1540.
| Organization of two independent kisspeptin systems derived from evolutionary-ancient kiss genes in the brain of zebrafish.Crossref | GoogleScholarGoogle Scholar | 21325050PubMed |
Shahi, N., Singh, A. K., Sahoo, M., Mallik, S. K., and Thakuria, D. (2017). Molecular cloning, characterization and expression profile of kisspeptin1 and kisspeptin1 receptor at brain–pituitary–gonad (BPG) axis of golden mahseer, Tor putitora (Hamilton, 1822) during gonadal development. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 205, 13–29.
| Molecular cloning, characterization and expression profile of kisspeptin1 and kisspeptin1 receptor at brain–pituitary–gonad (BPG) axis of golden mahseer, Tor putitora (Hamilton, 1822) during gonadal development.Crossref | GoogleScholarGoogle Scholar | 27914954PubMed |
Song, H., Wang, M., Wang, Z., Yu, H., Wang, Z., and Zhang, Q. (2016). Identification and characterization of kiss2 and kissr2 homologs in Paralichthysolivaceus. Fish Physiol. Biochem. 42, 1073–1092.
| Identification and characterization of kiss2 and kissr2 homologs in Paralichthysolivaceus.Crossref | GoogleScholarGoogle Scholar | 26905261PubMed |
Song, H., Wang, M., Wang, Z., Liu, J., Qi, J., and Zhang, Q. (2017). Characterization of kiss2 and kissr2 genes and the regulation of kisspeptin on the HPG axis in Cynoglossussemilaevis. Fish Physiol. Biochem. 43, 731–753.
| Characterization of kiss2 and kissr2 genes and the regulation of kisspeptin on the HPG axis in Cynoglossussemilaevis.Crossref | GoogleScholarGoogle Scholar | 28120214PubMed |
Stafford, L. J., Xia, C., Ma, W., Cai, Y., and Liu, M. (2002). Identification and characterization of mouse metastasis-suppressor KiSS1 and its G-protein-coupled receptor. Cancer Res. 62, 5399.
| 12359743PubMed |
Taranger, G. L., Carrillo, M., Schulz, R. W., Fontaine, P., Zanuy, S., Felip, A., Weltzien, F. A., Dufour, S., and Karlsen, O. Taranger, G. L., Carrillo, M., Schulz, R. W., Fontaine, P., Zanuy, S., Felip, A., Weltzien, F. A., Dufour, S., and Karlsen, O. (2010). Control of puberty in farmed fish. Gen. Comp. Endocrinol. 165, 483–515.
| Control of puberty in farmed fish.Crossref | GoogleScholarGoogle Scholar | 19442666PubMed |
Tena-Sempere, M., Felip, A., Gómez, A., Zanuy, S., and Carrillo, M. (2012). Comparative insights of the kisspeptin/kisspeptin receptor system: lessons from non-mammalian vertebrates. Gen. Comp. Endocrinol. 175, 234–243.
| Comparative insights of the kisspeptin/kisspeptin receptor system: lessons from non-mammalian vertebrates.Crossref | GoogleScholarGoogle Scholar | 22137912PubMed |
Tomikawa, J., Homma, T., Tajima, S., Shibata, T., Inamoto, Y., Takase, K., Inoue, N., Ohkura, S., Uenoyama, Y., Maeda, K., and Tsukamura, H. (2010). Molecular characterization and estrogen regulation of hypothalamic KISS1 gene in the pig. Biol. Reprod. 82, 313–319.
| Molecular characterization and estrogen regulation of hypothalamic KISS1 gene in the pig.Crossref | GoogleScholarGoogle Scholar | 19828777PubMed |
Um, H. N., Han, J. M., Hwang, J. I., Hong, S. I., Vaudry, H., and Seong, J. Y. (2010). Molecular coevolution of kisspeptins and their receptors from fish to mammals. Ann. N. Y. Acad. Sci. 1200, 67–74.
| Molecular coevolution of kisspeptins and their receptors from fish to mammals.Crossref | GoogleScholarGoogle Scholar | 20633134PubMed |
Wu, X. B., Wang, Y. Q., Zhou, K. Y., Zhu, W. Q., Nie, J. S., and Wang, C. L. (2003). Complete mitochondrial DNA sequence of Chinese alligator, Alligator sinensis, and phylogeny of crocodiles. Chin. Sci. Bull. 48, 2050–2054.
| Complete mitochondrial DNA sequence of Chinese alligator, Alligator sinensis, and phylogeny of crocodiles.Crossref | GoogleScholarGoogle Scholar |
Xu, L., Xue, H., Li, S., Xu, J., and Chen, L. (2016). Seasonal differential expression of KiSS-1/GPR54 in the striped hamsters (Cricetulusbarabensis) among different tissues. Integr. Zool. 12, 260–268.
| Seasonal differential expression of KiSS-1/GPR54 in the striped hamsters (Cricetulusbarabensis) among different tissues.Crossref | GoogleScholarGoogle Scholar |
Yang, B., Jiang, Q., Chan, T., Ko, W. K. W., and Wong, A. O. L. (2009). Goldfish kisspeptin: molecular cloning, tissue distribution of transcript expression, and stimulatory effects on prolactin, growth hormone and luteinizing hormone secretion and gene expression via direct actions at the pituitary level. Gen. Comp. Endocrinol. 165, 60–71.
| Goldfish kisspeptin: molecular cloning, tissue distribution of transcript expression, and stimulatory effects on prolactin, growth hormone and luteinizing hormone secretion and gene expression via direct actions at the pituitary level.Crossref | GoogleScholarGoogle Scholar | 19501591PubMed |
Yang, Y., Gao, J., Yuan, C., Zhang, Y., Guan, Y., and Wang, Z. (2016). Molecular identification of Kiss/GPR54 and function analysis with mRNA expression profiles exposure to 17α-ethinylestradiol in rare minnow Gobiocyprisrarus. Mol. Biol. Rep. 43, 737–749.
| Molecular identification of Kiss/GPR54 and function analysis with mRNA expression profiles exposure to 17α-ethinylestradiol in rare minnow Gobiocyprisrarus.Crossref | GoogleScholarGoogle Scholar | 27216535PubMed |
Yip, S. H., Boehm, U., Herbison, A. E., and Campbell, R. E. (2015). Conditional viral tract tracing delineates the projections of the distinct kisspeptin neuron populations to gonadotropin-releasing hormone (GnRH) neurons in the mouse. Endocrinology 156, 2582–2594.
| Conditional viral tract tracing delineates the projections of the distinct kisspeptin neuron populations to gonadotropin-releasing hormone (GnRH) neurons in the mouse.Crossref | GoogleScholarGoogle Scholar | 25856430PubMed |
Zhang, R., Hu, Y., Wang, H., Yan, P., Zhou, Y., Wu, R., and Wu, X. (2016). Molecular cloning, characterization, tissue distribution and mRNA expression changes during the hibernation and reproductive periods of estrogen receptor alpha (esr1) in Chinese alligator, Alligatorsinensis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 200, 28–35.
| Molecular cloning, characterization, tissue distribution and mRNA expression changes during the hibernation and reproductive periods of estrogen receptor alpha (esr1) in Chinese alligator, Alligatorsinensis.Crossref | GoogleScholarGoogle Scholar | 27212643PubMed |
Zhang, R., Yin, Y., Sun, L., Yan, P., Zhou, Y., and Wu, X. (2017). Molecular cloning of esr2 and gene expression analysis of esr1 and esr2 in the pituitary gland of the Chinese alligator (Alligator sinensis) during female reproductive cycle. Gene 623, 15–23.
| Molecular cloning of esr2 and gene expression analysis of esr1 and esr2 in the pituitary gland of the Chinese alligator (Alligator sinensis) during female reproductive cycle.Crossref | GoogleScholarGoogle Scholar | 28433658PubMed |
Zhang, R., Zhang, Y., Wu, M., Yan, P., Izaz, A., Wang, R., Zhu, H., Zhou, Y., and Wu, X. (2018). Molecular cloning of androgen receptor and gene expression of sex steroid hormone receptors in the brain of newborn Chinese alligator (Alligator sinensis). Gene 674, 178–187.
| Molecular cloning of androgen receptor and gene expression of sex steroid hormone receptors in the brain of newborn Chinese alligator (Alligator sinensis).Crossref | GoogleScholarGoogle Scholar | 29958951PubMed |
Zmora, N., Stubblefield, J., Golan, M., Servili, A., Levavi-Sivan, B., and Zohar, Y. (2014). The medio-basal hypothalamus as a dynamic and plastic reproduction-related kisspeptin–GnRH–pituitary center in fish. Endocrinology 155, 1874–1886.
| The medio-basal hypothalamus as a dynamic and plastic reproduction-related kisspeptin–GnRH–pituitary center in fish.Crossref | GoogleScholarGoogle Scholar | 24484170PubMed |
Zohar, Y., Muñoz-Cueto, J. A., Elizur, A., and Kah, O. (2010). Neuroendocrinology of reproduction in teleost fish. Gen. Comp. Endocrinol. 165, 438–455.
| Neuroendocrinology of reproduction in teleost fish.Crossref | GoogleScholarGoogle Scholar | 19393655PubMed |


