Involvement of peroxiredoxin 2 in cumulus expansion and oocyte maturation in mice
You-Jee Jang A , Jin-Seon Kim B , Pu-Reum Yun B , Young-Woo Seo A , Tae-Hoon Lee C , Jae-Il Park A D and Sang-Young Chun B D
B D
A Animal Facility of Aging Science, Korea Basic Science Institute, Gwangju 61186, Republic of Korea.
B School of Biological Sciences and Biotechnology, Faculty of Life Science, Chonnam National University, Gwangju 61186, Republic of Korea.
C Department of Oral Biochemistry, College of Dentistry, Chonnam National University, Gwangju 61186, Republic of Korea.
D Corresponding authors. Email: jaeil74@kbsi.re.kr; sychun@jnu.ac.kr
Reproduction, Fertility and Development 32(8) 783-791 https://doi.org/10.1071/RD19310
Submitted: 3 April 2019 Accepted: 30 November 2019 Published: 7 May 2020
Journal Compilation © CSIRO 2020 Open Access CC BY-NC-ND
Abstract
Peroxiredoxin 2 (Prdx2), an antioxidant enzyme, is expressed in the ovary during the ovulatory process. The aim of the present study was to examine the physiological role of Prdx2 during ovulation using Prdx2-knockout mice and mouse cumulus–oocyte complex (COC) from WT mice. Two days of treatment of immature mice (21–23 days old) with equine chorionic gonadotrophin and followed by treatment with human chorionic gonadotrophin greatly impaired cumulus expansion and oocyte maturation in Prdx2-knockout but not wild-type mice. Treatment of COCs in culture with conoidin A (50 µM), a 2-cys Prdx inhibitor, abolished epiregulin (EPI)-induced cumulus expansion. Conoidin A treatment also inhibited EPI-stimulated signal molecules, including signal transducer and activator of transcription-3, AKT and mitogen-activated protein kinase 1/2. Conoidin A treatment also reduced the gene expression of EPI-stimulated expansion-inducing factors (hyaluronan synthase 2 (Has2), pentraxin 3 (Ptx3), TNF-α induced protein 6 (Tnfaip6) and prostaglandin-endoperoxide synthase 2 (Ptgs2)) and oocyte-derived factors (growth differentiation factor 9 (Gdf9) and bone morphogenetic protein 15 (Bmp15)). Furthermore, conoidin A inhibited EPI-induced oocyte maturation and the activity of connexins 43 and 37. Together, these results demonstrate that Prdx2 plays a role in regulating cumulus expansion and oocyte maturation during the ovulatory process in mice, probably by modulating epidermal growth factor receptor signalling.
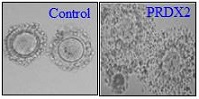
Additional keywords: cumulus cell, Graafian follicle, ovary, ovulation.
References
Cao, J., Schulte, J., Knight, A., Leslie, N. R., Zagozdzon, A., Bronson, R., Manevich, Y., Beeson, C., and Neumann, C. A. (2009). Prdx1 inhibits tumorigenesis via regulating PTEN/AKT activity. EMBO J. 28, 1505–1517.| Prdx1 inhibits tumorigenesis via regulating PTEN/AKT activity.Crossref | GoogleScholarGoogle Scholar | 19369943PubMed |
Conti, M., Hsieh, M., Zamah, A. M., and Oh, J. S. (2012). Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol. Cell. Endocrinol. 356, 65–73.
| Novel signaling mechanisms in the ovary during oocyte maturation and ovulation.Crossref | GoogleScholarGoogle Scholar | 22101318PubMed |
Dagnell, M., Pace, P. E., Cheng, Q., Frijhoff, J., Ostman, A., Arner, E. S. J., Hampton, M. B., and Winterbourn, C. C. (2017). Thioredoxin reductase 1 and NADPH directly protect protein tyrosine phosphatase 1B from inactivation during H2O2 exposure. J. Biol. Chem. 292, 14371–14380.
| Thioredoxin reductase 1 and NADPH directly protect protein tyrosine phosphatase 1B from inactivation during H2O2 exposure.Crossref | GoogleScholarGoogle Scholar | 28684416PubMed |
DeYulia, G. J., and Carcamo, J. M. (2005). EGF receptor–ligand interaction generates extracellular hydrogen peroxide that inhibits EGFR-associated protein tyrosine phosphatases. Biochem. Biophys. Res. Commun. 334, 38–42.
| EGF receptor–ligand interaction generates extracellular hydrogen peroxide that inhibits EGFR-associated protein tyrosine phosphatases.Crossref | GoogleScholarGoogle Scholar | 15982634PubMed |
Elvin, J. A., Clark, A. T., Wang, P., Wolfman, N. M., and Matzuk, M. M. (1999). Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol. Endocrinol. 13, 1035–1048.
| Paracrine actions of growth differentiation factor-9 in the mammalian ovary.Crossref | GoogleScholarGoogle Scholar | 10379900PubMed |
Fan, H. Y., Liu, Z., Mullany, L. K., and Richards, J. S. (2012). Consequences of RAS and MAPK activation in the ovary: the good, the bad and the ugly. Mol. Cell. Endocrinol. 356, 74–79.
| Consequences of RAS and MAPK activation in the ovary: the good, the bad and the ugly.Crossref | GoogleScholarGoogle Scholar | 22197887PubMed |
Fujii, J., Iuchi, Y., and Okada, F. (2005). Fundamental roles of reactive oxygen species and protective mechanisms in the female reproductive system. Reprod. Biol. Endocrinol. 3, 43–52.
| Fundamental roles of reactive oxygen species and protective mechanisms in the female reproductive system.Crossref | GoogleScholarGoogle Scholar | 16137335PubMed |
Gershon, E., Plaks, V., and Dekel, N. (2007). Gap junctions in the ovary: expression, localization and function. Mol. Cell. Endocrinol. 282, 18–25.
| Gap junctions in the ovary: expression, localization and function.Crossref | GoogleScholarGoogle Scholar | 18162286PubMed |
Guarnaccia, M. M., Takami, M., Jones, E. E., Preston, S. L., and Behrman, H. R. (2000). Luteinizing hormone depletes ascorbic acid in preovulatory follicles. Fertil. Steril. 74, 959–963.
| Luteinizing hormone depletes ascorbic acid in preovulatory follicles.Crossref | GoogleScholarGoogle Scholar | 11056240PubMed |
Haraldsen, J. D., Liu, G., Botting, C. H., Walton, J. G., Storm, J., Phalen, T. J., Kwok, L. Y., Soldati-Favre, D., Heintz, N. H., Müller, S., Westwood, N. J., and Ward, G. E. (2009). Identification of conoidin A as a covalent inhibitor of peroxiredoxin II. Org. Biomol. Chem. 7, 3040–3048.
| Identification of conoidin A as a covalent inhibitor of peroxiredoxin II.Crossref | GoogleScholarGoogle Scholar | 21359112PubMed |
Ho, Y. S., Gargano, M., Cao, J., Bronson, R. T., Heimler, I., and Hutz, R. J. (1998). Reduced fertility in female mice lacking copper–zinc superoxide dismutase. J. Biol. Chem. 273, 7765–7769.
| Reduced fertility in female mice lacking copper–zinc superoxide dismutase.Crossref | GoogleScholarGoogle Scholar | 9516486PubMed |
Jang, Y. J., Park, J. I., Moon, W. J., Dam, P. T., Cho, M. K., and Chun, S. Y. (2015). Cumulus cell-expressed type I interferons induce cumulus expansion in mice. Biol. Reprod. 92, 20–27.
| Cumulus cell-expressed type I interferons induce cumulus expansion in mice.Crossref | GoogleScholarGoogle Scholar | 25429090PubMed |
Jeon, H. J., Park, Y. S., Cho, D. H., Kim, J. S., Kim, E., Chae, H. Z., Chun, S. Y., and Oh, J. S. (2017). Peroxiredoxins are required for spindle assembly, chromosome organization, and polarization in mouse oocytes. Biochem. Biophys. Res. Commun. 489, 193–199.
| Peroxiredoxins are required for spindle assembly, chromosome organization, and polarization in mouse oocytes.Crossref | GoogleScholarGoogle Scholar | 28552528PubMed |
Johnson, K. J., Ward, P. A., Kunkel, R. G., and Wilson, B. S. (1986). Mediation of IgA induced lung injury in the rat. Role of macrophages and reactive oxygen products. Lab. Invest. 54, 499–506.
| 3009967PubMed |
Kang, S. W., Rhee, S. G., Chang, T. S., Jeong, W., and Choi, M. H. (2005). 2-Cys peroxiredoxin function in intracellular signal transduction: therapeutic implications. Trends Mol. Med. 11, 571–578.
| 2-Cys peroxiredoxin function in intracellular signal transduction: therapeutic implications.Crossref | GoogleScholarGoogle Scholar | 16290020PubMed |
Kennedy, C. R., Zhang, Y., Brandon, S., Guan, Y., Coffee, K., Funk, C. D., Magnuson, M. A., Oates, J. A., Breyer, M. D., and Breyer, R. M. (1999). Salt-sensitive hypertension and reduced fertility in mice lacking the prostaglandin EP2 receptor. Nat. Med. 5, 217–220.
| Salt-sensitive hypertension and reduced fertility in mice lacking the prostaglandin EP2 receptor.Crossref | GoogleScholarGoogle Scholar | 9930871PubMed |
Kim, J. H., Park, S. J., Chae, U., Seong, J., Lee, H. S., Lee, S. R., Lee, S., and Lee, D. S. (2018). Peroxiredoxin 2 mediates insulin sensitivity of skeletal muscles through regulation of protein tyrosine phosphatase oxidation. Int. J. Biochem. Cell Biol. 99, 80–90.
| Peroxiredoxin 2 mediates insulin sensitivity of skeletal muscles through regulation of protein tyrosine phosphatase oxidation.Crossref | GoogleScholarGoogle Scholar | 29605633PubMed |
Kiyosu, C., Tsuji, T., Yamada, K., Kajita, S., and Kunieda, T. (2012). NPPC/NPR2 signaling is essential for oocyte meiotic arrest and cumulus oophorus formation during follicular development in the mouse ovary. Reproduction 144, 187–193.
| NPPC/NPR2 signaling is essential for oocyte meiotic arrest and cumulus oophorus formation during follicular development in the mouse ovary.Crossref | GoogleScholarGoogle Scholar | 22696190PubMed |
Komatsu, K., and Masubuchi, S. (2018). Mouse oocytes connect with granulosa cells by fusing with cell membranes and form a large complex during follicle development. Biol. Reprod. 99, 527–535.
| Mouse oocytes connect with granulosa cells by fusing with cell membranes and form a large complex during follicle development.Crossref | GoogleScholarGoogle Scholar | 29590310PubMed |
Laloraya, M., Pradeep, K. G., and Laloraya, M. M. (1988). Changes in the levels of superoxide anion radical and superoxide dismutase during the estrous cycle of Rattus norvegicus and induction of superoxide dismutase in rat ovary by lutropin. Biochem. Biophys. Res. Commun. 157, 146–153.
| Changes in the levels of superoxide anion radical and superoxide dismutase during the estrous cycle of Rattus norvegicus and induction of superoxide dismutase in rat ovary by lutropin.Crossref | GoogleScholarGoogle Scholar | 2848516PubMed |
Latimer, H. R., and Veal, E. A. (2016). Peroxiredoxins in regulation of MAPK signalling pathways; sensors and barriers to signal transduction. Mol. Cells 39, 40–45.
| Peroxiredoxins in regulation of MAPK signalling pathways; sensors and barriers to signal transduction.Crossref | GoogleScholarGoogle Scholar | 26813660PubMed |
Lee, T. H., Kim, S. U., Yu, S. L., Kim, S. H., Park, D. S., Moon, H. B., Dho, S. H., Kwon, K. S., Kwon, H. J., Han, Y. H., Jeong, S., Kang, S. W., Shin, H. S., Lee, K. K., Rhee, S. G., and Yu, D. Y. (2003). Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice. Blood 101, 5033–5038.
| Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice.Crossref | GoogleScholarGoogle Scholar | 12586629PubMed |
Leyens, G., Donnay, I., and Knoops, B. (2003). Cloning of bovine peroxiredoxins – gene expression in bovine tissues and amino acid sequence comparison with rat, mouse and primate peroxiredoxins. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 136, 943–955.
| Cloning of bovine peroxiredoxins – gene expression in bovine tissues and amino acid sequence comparison with rat, mouse and primate peroxiredoxins.Crossref | GoogleScholarGoogle Scholar | 14662316PubMed |
Liu, Z., de Matos, D. G., Fan, H. Y., Shimada, M., Palmer, S., and Richards, J. S. (2009). Interleukin-6: an autocrine regulator of the mouse cumulus cell–oocyte complex expansion process. Endocrinology 150, 3360–3368.
| Interleukin-6: an autocrine regulator of the mouse cumulus cell–oocyte complex expansion process.Crossref | GoogleScholarGoogle Scholar | 19299453PubMed |
Masciarelli, S., Horner, K., Liu, C., Park, S. H., Hinckley, M., Hockman, S., Nedachi, T., Jin, C., Conti, M., and Manganiello, V. (2004). Cyclic nucleotide phosphodiesterase 3A-deficient mice as a model of female infertility. J. Clin. Invest. 114, 196–205.
| Cyclic nucleotide phosphodiesterase 3A-deficient mice as a model of female infertility.Crossref | GoogleScholarGoogle Scholar | 15254586PubMed |
Matzuk, M. M., Dionne, L., Guo, Q., Kumar, T. R., and Lebovitz, R. M. (1998). Ovarian function in superoxide dismutase 1 and 2 knockout mice. Endocrinology 139, 4008–4011.
| Ovarian function in superoxide dismutase 1 and 2 knockout mice.Crossref | GoogleScholarGoogle Scholar | 9724058PubMed |
National Research Council (2011). ‘National Institutes of Health Guide for the Care and Use of Laboratory Animals.’ (National Academy Press: Washington DC, USA.)
Nicolussi, A., D’Inzeo, S., Capalbo, C., Giannini, G., and Coppa, A. (2017). The role of peroxiredoxins in cancer. Mol. Clin. Oncol. 6, 139–153.
| The role of peroxiredoxins in cancer.Crossref | GoogleScholarGoogle Scholar | 28357082PubMed |
Noda, Y., Ota, K., Shirasawa, T., and Shimizu, T. (2012). Copper/zinc superoxide dismutase insufficiency impairs progesterone secretion and fertility in female mice. Biol. Reprod. 86, 1–8.
| Copper/zinc superoxide dismutase insufficiency impairs progesterone secretion and fertility in female mice.Crossref | GoogleScholarGoogle Scholar | 21900685PubMed |
Nomura, T., Sasaki, J., Mori, H., Sato, E. F., Watanabe, S., Kanda, S., Matsuura, J., Watanabe, H., and Inoue, M. (1996). Expression of manganese superoxide dismutase mRNA in reproductive organs during the ovulatory process and the estrous cycle of the rat. Histochem. Cell Biol. 105, 1–6.
| Expression of manganese superoxide dismutase mRNA in reproductive organs during the ovulatory process and the estrous cycle of the rat.Crossref | GoogleScholarGoogle Scholar | 8824900PubMed |
Norris, R. P., Freudzon, M., Nikolaev, V. O., and Jaffe, L. A. (2010). Epidermal growth factor receptor kinase activity is required for gap junction closure and for part of the decrease in ovarian follicle cGMP in response to LH. Reproduction 140, 655–662.
| Epidermal growth factor receptor kinase activity is required for gap junction closure and for part of the decrease in ovarian follicle cGMP in response to LH.Crossref | GoogleScholarGoogle Scholar | 20826538PubMed |
Park, J. I., Jeon, H. J., Jung, N. K., Jang, Y. J., Kim, J. S., Seo, Y. W., Jeong, M., Chae, H. Z., and Chun, S. Y. (2012). Periovulatory expression of hydrogen peroxide-induced sulfiredoxin and peroxiredoxin 2 in the rat ovary: gonadotropin regulation and potential modification. Endocrinology 153, 5512–5521.
| Periovulatory expression of hydrogen peroxide-induced sulfiredoxin and peroxiredoxin 2 in the rat ovary: gonadotropin regulation and potential modification.Crossref | GoogleScholarGoogle Scholar | 22989627PubMed |
Rhee, S. G. (2016). Overview on peroxiredoxin. Mol. Cells 39, 1–5.
| Overview on peroxiredoxin.Crossref | GoogleScholarGoogle Scholar | 26831451PubMed |
Richard, S., and Baltz, J. M. (2014). Prophase I arrest of mouse oocytes mediated by natriuretic peptide precursor C requires GJA1 (connexin-43) and GJA4 (connexin-37) gap junctions in the antral follicle and cumulus–oocyte complex. Biol. Reprod. 90, 137–146.
| Prophase I arrest of mouse oocytes mediated by natriuretic peptide precursor C requires GJA1 (connexin-43) and GJA4 (connexin-37) gap junctions in the antral follicle and cumulus–oocyte complex.Crossref | GoogleScholarGoogle Scholar | 24804968PubMed |
Richards, J. S., and Ascoli, M. (2018). Endocrine, paracrine, and autocrine signaling pathways that regulate ovulation. Trends Endocrinol. Metab. 29, 313–325.
| Endocrine, paracrine, and autocrine signaling pathways that regulate ovulation.Crossref | GoogleScholarGoogle Scholar | 29602523PubMed |
Richards, J. S., Russell, D. L., Ochsner, S., Hsieh, M., Doyle, K. H., Falender, A. E., Lo, Y. K., and Sharma, S. C. (2002a). Novel signaling pathways that control ovarian follicular development, ovulation, and luteinization. Recent Prog. Horm. Res. 57, 195–220.
| Novel signaling pathways that control ovarian follicular development, ovulation, and luteinization.Crossref | GoogleScholarGoogle Scholar | 12017544PubMed |
Richards, J. S., Russell, D. L., Ochsner, S., and Espey, L. L. (2002b). Ovulation: new dimensions and new regulators of the inflammatory-like response. Annu. Rev. Physiol. 64, 69–92.
| Ovulation: new dimensions and new regulators of the inflammatory-like response.Crossref | GoogleScholarGoogle Scholar | 11826264PubMed |
Sasaki, J., Sato, E. F., Nomura, T., Mori, H., Watanabe, S., Kanda, S., Watanabe, H., Utsumi, K., and Inoue, M. (1994). Detection of manganese superoxide dismutase mRNA in the theca interna cells of rat ovary during the ovulatory process by in situ hybridization. Histochemistry 102, 173–176.
| Detection of manganese superoxide dismutase mRNA in the theca interna cells of rat ovary during the ovulatory process by in situ hybridization.Crossref | GoogleScholarGoogle Scholar | 7868359PubMed |
Shkolnik, K., Tadmor, A., Ben-Dor, S., Nevo, N., Galiani, D., and Dekel, N. (2011). Reactive oxygen species are indispensable in ovulation. Proc. Natl Acad. Sci. USA 108, 1462–1467.
| Reactive oxygen species are indispensable in ovulation.Crossref | GoogleScholarGoogle Scholar | 21220312PubMed |
Sobotta, M. C., Liou, W., Stocker, S., Talwar, D., Oehler, M., Ruppert, T., Scharf, A. N., and Dick, T. P. (2015). Peroxiredoxin-2 and STAT3 form a redox relay for H2O2 signaling. Nat. Chem. Biol. 11, 64–70.
| Peroxiredoxin-2 and STAT3 form a redox relay for H2O2 signaling.Crossref | GoogleScholarGoogle Scholar | 25402766PubMed |
Sugino, N. (2005). Reactive oxygen species in ovarian physiology. Reprod. Med. Biol. 4, 31–44.
| 29699208PubMed |
Tilly, J. L., and Tilly, K. I. (1995). Inhibitors of oxidative stress mimic the ability of follicle-stimulating hormone to suppress apoptosis in cultured rat ovarian follicles. Endocrinology 136, 242–252.
| Inhibitors of oxidative stress mimic the ability of follicle-stimulating hormone to suppress apoptosis in cultured rat ovarian follicles.Crossref | GoogleScholarGoogle Scholar | 7828537PubMed |
Tsai-Turton, M., and Luderer, U. (2006). Opposing effects of glutathione depletion and follicle-stimulating hormone on reactive oxygen species and apoptosis in cultured preovulatory rat follicles. Endocrinology 147, 1224–1236.
| Opposing effects of glutathione depletion and follicle-stimulating hormone on reactive oxygen species and apoptosis in cultured preovulatory rat follicles.Crossref | GoogleScholarGoogle Scholar | 16339198PubMed |
Tsai-Turton, M., Nakamura, B. N., and Luderer, U. (2007). Induction of apoptosis by 9,10-dimethyl-1,2-benzanthracene in cultured preovulatory rat follicles is preceded by a rise in reactive oxygen species and is prevented by glutathione. Biol. Reprod. 77, 442–451.
| Induction of apoptosis by 9,10-dimethyl-1,2-benzanthracene in cultured preovulatory rat follicles is preceded by a rise in reactive oxygen species and is prevented by glutathione.Crossref | GoogleScholarGoogle Scholar | 17554082PubMed |
Vanderhyden, B. C., Caron, P. J., Buccione, R., and Eppig, J. J. (1990). Developmental pattern of the secretion of cumulus expansion-enabling factor by mouse oocytes and the role of oocytes in promoting granulosa cell differentiation. Dev. Biol. 140, 307–317.
| Developmental pattern of the secretion of cumulus expansion-enabling factor by mouse oocytes and the role of oocytes in promoting granulosa cell differentiation.Crossref | GoogleScholarGoogle Scholar | 2115479PubMed |
Verrastro, I., Tveen-Jensen, K., Woscholski, R., Spickett, C. M., and Pitt, A. R. (2016). Reversible oxidation of phosphatase and tensin homolog (PTEN) alters its interactions with signaling and regulatory proteins. Free Radic. Biol. Med. 90, 24–34.
| Reversible oxidation of phosphatase and tensin homolog (PTEN) alters its interactions with signaling and regulatory proteins.Crossref | GoogleScholarGoogle Scholar | 26561776PubMed |
Wayne, C. M., Fan, H. Y., Cheng, X., and Richards, J. S. (2007). Follicle-stimulating hormone induces multiple signaling cascades: evidence that activation of Rous sarcoma oncogene, RAS, and the epidermal growth factor receptor are critical for granulosa cell differentiation. Mol. Endocrinol. 21, 1940–1957.
| Follicle-stimulating hormone induces multiple signaling cascades: evidence that activation of Rous sarcoma oncogene, RAS, and the epidermal growth factor receptor are critical for granulosa cell differentiation.Crossref | GoogleScholarGoogle Scholar | 17536007PubMed |
Wood, Z. A., Schroder, E., Robin Harris, J., and Poole, L. B. (2003). Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 28, 32–40.
| Structure, mechanism and regulation of peroxiredoxins.Crossref | GoogleScholarGoogle Scholar | 12517450PubMed |
Yang, S., Luo, A., Hao, X., Lai, Z., Ding, T., Ma, X., Mayinuer, M., Shen, W., Wang, X., Lu, Y., Ma, D., and Wang, S. (2011). Peroxiredoxin 2 inhibits granulosa cell apoptosis during follicle atresia through the NFKB pathway in mice. Biol. Reprod. 84, 1182–1189.
| Peroxiredoxin 2 inhibits granulosa cell apoptosis during follicle atresia through the NFKB pathway in mice.Crossref | GoogleScholarGoogle Scholar | 21248284PubMed |
Yoshino, O., McMahon, H. E., Sharma, S., and Shimasaki, S. (2006). A unique preovulatory expression pattern plays a key role in the physiological functions of BMP-15 in the mouse. Proc. Natl Acad. Sci. USA 103, 10678–10683.
| A unique preovulatory expression pattern plays a key role in the physiological functions of BMP-15 in the mouse.Crossref | GoogleScholarGoogle Scholar | 16818886PubMed |
Zhang, M., Su, Y. Q., Sugiura, K., Xia, G., and Eppig, J. J. (2010). Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science 330, 366–369.
| Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes.Crossref | GoogleScholarGoogle Scholar | 20947764PubMed |
Zhuo, L., and Kimata, K. (2001). Cumulus oophorus extracellular matrix: its construction and regulation. Cell Struct. Funct. 26, 189–196.
| Cumulus oophorus extracellular matrix: its construction and regulation.Crossref | GoogleScholarGoogle Scholar | 11699635PubMed |
Zhuo, L., Yoneda, M., Zhao, M., Yingsung, W., Yoshida, N., Kitagawa, Y., Kawamura, K., Suzuki, T., and Kimata, K. (2001). Defect in SHAP-hyaluronan complex causes severe female infertility. A study by inactivation of the bikunin gene in mice. J. Biol. Chem. 276, 7693–7696.
| Defect in SHAP-hyaluronan complex causes severe female infertility. A study by inactivation of the bikunin gene in mice.Crossref | GoogleScholarGoogle Scholar | 11145954PubMed |


