Marine invasive species: establishing pathways, their presence and potential threats in the Galapagos Marine Reserve
Inti Keith A B E , Terence P. Dawson A , Ken J. Collins C and Marnie L. Campbell DA Centre for Environmental Change and Human Resilience, University of Dundee, Dundee, DD1 4HN, UK.
B Charles Darwin Foundation, Marine Science Department, Santa Cruz Island, Galapagos, Ecuador.
C Ocean and Earth Science, University of Southampton, National Oceanography Centre, Southampton SO14 3ZH, UK.
D Environmental Research Institute, University of Waikato, Hamilton, New Zealand.
E Corresponding author. Email: inti.keith@fcdarwin.org.ec
Pacific Conservation Biology 22(4) 377-385 https://doi.org/10.1071/PC15020
Submitted: 25 June 2015 Accepted: 10 June 2016 Published: 17 August 2016
Abstract
Worldwide, marine biological invasions of non-native species have increased significantly in recent years due to a rapid rise in global trade, transport and tourism. Invasions occur when non-native species are transported from one region to another and establish, often resulting in competition displacing native species and changing ecosystems. Historic literature searches were conducted along with dive surveys of the main ports and in sites around the archipelago in order to produce a baseline of which non-native species are present in the Galapagos Marine Reserve at this time. Confounding processes of anthropogenic and natural activities are increasing the potential spread of marine invasive species in the Eastern Tropical Pacific and the Galapagos Marine Reserve. We discuss the potential vectors facilitating marine invasions with the suggestion that marine traffic could be the most influential vector in the transport of marine non-natives to the Galapagos Marine Reserve. The challenge for marine park authorities is to identify those species that are likely to cause negative impacts on native biodiversity and ecosystems before they establish in the Galapagos, and to develop pre-emptive strategies that would likely include prevention as well as risk-based management strategies to remove them or to mitigate their harmful effects.
Additional keywords: bioinvasions, GMR, non-native.
Introduction
The Galapagos archipelago is located 1000 km off the coast of Ecuador in the Eastern Tropical Pacific (ETP). The archipelago is a volcanic hotspot that consists of 13 large islands and over 100 smaller islands, islets, and rocks (Sachs and Ladd 2010). This oceanic archipelago is home to two important Natural Heritage Sites, the Galapagos National Park (GNP), created in 1959, and the Galapagos Marine Reserve (GMR), created in 1998 with the Special Law for the Conservation and Sustainable Development of the Galapagos Province (LOREG 1998). The GMR extends to a distance of 40 nautical miles out from the coastal baseline that surrounds the archipelago, creating a protected area of ~138 000 km2 (Danulat and Edgar 2002).
The Galapagos Islands are renowned for their unique biological diversity, high levels of endemism, and the unique currents and oceanographic features that allow a variety of habitats to exist (Hickman 2009). The archipelago is influenced by several major surface and submarine current systems and is characterised by a diverse wildlife compared with other islands, with representatives from the Indo-Pacific, Panama, and Peru regions of the Pacific (Muromtsev 1963; Banks 2002; Hickman 2009). Studies have shown, however, that marine ecosystems in the Galapagos are sensitive to climate change and not well adapted to extreme thermal impacts (Edgar et al. 2010).
The introduction of non-native species has been identified as the second most important reason for biodiversity loss worldwide after habitat destruction (Jäger et al. 2007; IUCN 2011). The rate of biological invasions has increased during the last decades, mostly due to the accelerated spread of species due to growing global trade, transport, and tourism overcoming natural barriers to marine migration, such as currents, land masses, and temperature gradients that once limited the movement of species (Carlton 1996; Seebens et al. 2013). Marine bioinvasions are currently recognised as a problem throughout the world’s oceans, with humans having moved species beyond their native ranges for many years, whether deliberately or not, and some of these species have managed to establish and proliferate, causing significant ecological, economic and health impacts (Vitousek et al. 1997; Campbell and Hewitt 2013). Many marine organisms need assistance in order to move from one region to another, through anthropogenic or natural vectors (Hewitt and Hayes 2002). Here we define a vector as the physical means, agent or mechanism, that facilitates the transfer of organisms or their propagules from one place to another (Carlton 1996; Hewitt and Hayes 2002; Hilliard 2004; Campbell and Hewitt 2013).
Several anthropogenic vector categories exist: a prime example is marine traffic, where shipping vessels can act as biological islands for species that live in harbours around the world (Wonham et al. 2001). For example, as ships transit or anchor in these areas, some species colonise their subsurface areas and ‘hitch a ride’. Maritime traffic includes ballast water and biofouling as well as numerous other mechanisms (e.g. anchor lockers). These vessels provide places for the settlement of associated species, provide protected spaces where both sessile and mobile fauna can settle, and enclosed spaces that hold water in which a wide range of organisms from plankton to fish can travel in (Wonham et al. 2001; Godwin 2003). Biofouling of maritime traffic plays a key role in the spread of species due to the fact that many of these organisms can be moved between regions by commercial vessels and recreational vessels (Kolar and Lodge 2002; Hulme 2009). Other vectors exist that can disperse marine organisms throughout the world: some examples include current systems, climate variations, migrating species, and natural phenomena, such a major storm events. However, another vector that has been identified in recent years is marine debris. The possibility has been explored that marine species can adhere themselves to floating waste and can be transported thousands of miles to different bioregions (Chan 2012).
The geographic isolation of the Galapagos Islands has limited natural immigration of new species, historically enabling those few species that did arrive to evolve in the absence of competitors and predators. For this reason, oceanic islands are more prone to invasion by non-native species because of the paucity of natural competitors and predators that control populations in their native ecosystem. Islands often have ecological niches that have not been filled because of the distance from colonising populations, increasing the probability of successful invasion (Loope et al. 1988).
The impacts of terrestrial invasive species have been studied extensively in the Galapagos Islands, with the consequence that there are now strict control and quarantine protocols to prevent the entry of terrestrial introduced species (Zapata 2006). The Agencia de Regulación y Control de la Bioseguridad y Cuarentena para Galápagos (ABG) is the Galapagos Biosecurity Agency created in 2012. This agency is in charge of controlling, regulating, preventing and reducing the risk of the introduction, movement and dispersal of non-native organisms that might threaten human health, the terrestrial and marine ecosystems, the integrity of the islands and the conservation of biodiversity of the Galapagos Province (ABG 2015). While research on terrestrial invasive species such as mammals, birds, plants and insects is well established, research conducted on marine invasive species and the impacts to the Galapagos Marine Reserve is sparse. The management of marine invasive species presents more challenges than terrestrial invasive species due to the high degree of natural connectivity that exists between the islands within the GMR and the logistics required to work in the marine environment.
The GMR is under threat from possible marine non-native species arrivals, given the connectivity that exists with the ETP, the increase in tourism and associated marine traffic and the effect of extreme climatic events such as the El Niño Southern Oscillation (ENSO). This type of event brings unusually warm water across the central and east-central equatorial Pacific, giving opportunistic non-native species a window of opportunity to move into new ecosystems and outcompete native and endemic species.
The Charles Darwin Foundation (CDF) has been working with the ABG and the Galapagos National Park Directorate (GNPD), both part of the Ecuadorian Ministry of the Environment, the Ecuadorian Navy, and the Navy’s Oceanographic Institute to establish a baseline of non-native marine species that are already established in the GMR, and to develop control measures and a management plan to prevent the arrival of new non-native marine species into the GMR. This paper illustrates the initial work that has been undertaken by the CDF and local government institutions since the start of the Darwin Initiative–funded project ‘Marine Invasive Species Project: Prevention, Detection and Management’ that began in 2012. The objectives of this paper are to present a baseline list of marine non-native species in the GMR, discuss the possible vectors by which these species could enter the GMR and how they could be managed.
Methods
A compilation of historical literature was gathered for non-native species reported in the Galapagos, with some of these records dating back to the Allan Hancock Pacific Expeditions conducted in the early 1930s (Taylor 1945). In addition, monitoring surveys were undertaken in the main ports of the archipelago, in selected sites around the GMR, and in protected bays and mangrove areas to assess the presence of non-native species in the GMR at the present time.
The species reported in the literature were then investigated further, looking at (1) their current native and introduced distribution, (2) their invasive capacity and whether the species has demonstrated invasive behaviour in other parts of the world, (3) whether the ecological conditions are suitable in the GMR for the species to proliferate, and (4) whether the species could have been transported by one of the dispersal vectors affecting the GMR. The global distributions of these species were determined using the Global Invasive Species Database (ISSG 2015), the World Register of Introduced Marine Species (Pagad et al. 2015), the World Register of Marine Species (WoRMS Editorial Board 2015) and Algaebase (Guiry and Guiry 2015). Records of these species’ presence were also checked on the CDF marine database that holds records of all species reported in the GMR and their distribution (Bungartz et al. 2009).
Subtidal monitoring
There are ~380 sites that have been monitored as part of the GMR baseline and these are documented in the CDF marine database (Bungartz et al. 2009). In 2004 the GNPD led a process to signal all the coastal subzones in the GMR for management purposes; during this time the design of an annual subtidal monitoring program run by CDF was finalised. This program was based on the repetition of monitoring more than 64 sites around the GMR; each site had three zones marked tourism, fishing and protection. (Banks et al. 2014). The sites chosen for this study were based on the sites monitored in the past in the GMR in order to have a reference of the species recorded previously.
In total, 115 sites were surveyed using a proven standardised methodology developed by the CDF for long-term evaluation of subtidal communities in the GMR; this methodology is also applied in other marine protected areas in the ETP (Banks et al. 2014). This methodology focused primarily on recording the diversity, abundance and size of the species present in three major groups of macrofauna: fish, macroinvertebrates and sessile organisms. Each sample consisted of divers moving along a 50-m transect parallel to the coast where visual censuses were conducted for the three taxonomic groups; this was done at depths of 15 m and 6 m.
The fish monitoring consisted of identifying the levels of species richness, measuring the population density, determining the size structure of each species and conducting a visual inspection for non-native species. An area of 500 m2 was monitored by a diver who swam parallel to the transect in a corridor 5 m wide × 5 m high × 50 m long.
The mobile macroinvertebrate monitoring focuses on simultaneously measuring the density and abundance of several species at a time, including commercial, non-commercial and non-native species. An area of 100 m2 was monitored along the same 50-m transect: the diver swam along in 5-m segments considering a 1-m strip at either side of the transect and recording the number of invertebrates larger than 2 cm.
Sessile organisms are an important component of marine communities. Due to their sedentary lifestyle, sessile organisms are good indicators of local conditions, long-term physical changes, biological changes and any effects that can be produced by natural phenomena or human-caused disturbances. Their presence or absence is a good indicator of prevailing biological and abiotic processes, such as competition, interactions with predators or prey or large-scale effects such as current circulation patterns, recruitment events, temperature, or marine invasions. An area of 2.5 m2 was monitored using a PVC quadrat of 0.5 × 0.5 m (0.25 m2). Each quadrat had a grid of 5 × 5 cm constructed with polypropylene twine with 81 intersection points to determine the abundance of each species. Quadrats were placed systematically every 5 m along the same 50-m transect. In each quadrat all species that fell on the 81 intersections were counted and recorded; species that did not fall on the intersections were recorded as present (Banks et al. 2014). Various samples were collected for later identification, or were sent to taxonomic experts to confirm identification or to conduct DNA studies.
Port monitoring
There are five populated islands in the archipelago, each with a main dock and several smaller docks: (1) Puerto Baquerizo Moreno, on the Island of San Cristobal, (2) Puerto Ayora, on the Island of Santa Cruz, (3) Puerto Villamil, on the Island of Isabela, (4) Puerto Velasco Ibarra, on the Island of Floreana, and (5) Puerto de Seymour, on the Island of Baltra. There are several different components in the port-monitoring methodology. Each port has a different layout and each has a different number of docks that require inspecting. Permission to inspect has to be obtained from the port authority as the ports are heavily visited by marine traffic, and health and safety protocols need to be followed. The monitoring of docks consisted of conducting a visual inspection and recording the species present, taking scrapings from the dock walls or pylons for later identification in the laboratory, and recording a video transect for comparative analysis.
Two divers conducted the visual inspection: one recorded all fish and macroinvertebrates in the surrounding dock area and the other diver recorded the percentage cover of sessile organisms. The area surveyed was the total area around the dock starting at the shallowest depth possible to divers. The area covered varied on each dock, as the size of each dock was different. Sessile organisms were recorded in a PVC quadrat of 0.5 × 0.5 m (0.25 m2) (Banks et al. 2014) and records were taken at three depths (e.g. 0.5 m, –3 m, and –7 m). In addition, scrapings were collected at the same three depths as the sessile survey for later identification in the laboratory. The video transect recorded all areas surveyed by the divers including the areas where scrapings were taken. Photographs of potential non-native species that were present around the docks were also recorded to facilitate later species identification. During port monitoring, mooring buoys and/or navigation buoys were also inspected. The buoys consisted of different parts: the marker buoy floating on the surface of the water, the chain and cement block on the sea floor. Visual inspections of all these areas were conducted, recording all species present. Scrapings of the base of the buoy were taken for later identification and a video recording of the marker buoy, the chain and the cement block was recorded. The area surrounding the cement block was also inspected for non-native species.
Protected bays and mangrove monitoring
The Galapagos Islands have many protected bays, with most located on the western islands of Isabela and Fernandina. A separate monitoring technique was developed for these areas, as these bays are small in size, shallow, and have very low wave exposure, hence diving is not necessary. The monitoring of these bays was undertaken through directed searches for non-native species using snorkelling apparatus. A list of potential non-natives used for the identification of species during the directed searches was compiled from literature collected on marine invasive species worldwide. Photographs and samples of specimens were collected for later identification in the laboratory. The many bays of the archipelago support several mangrove habitats, where visual inspections of the intertidal zone of the mangroves were conducted in order to evaluate the presences of non-native species.
Results
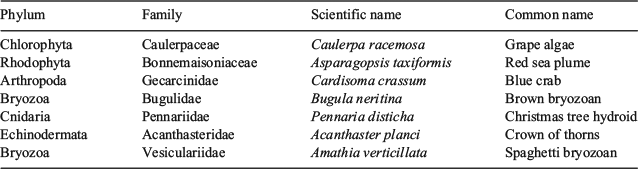
The literature search produced seven potentially non-native species reported in the GMR. The first record found was for Caulerpa racemosa, with this species registered in Galapagos by Farlow in 1899 on the Island of Isabela. It was registered again by Allan Hancock during the Pacific Expeditions in the 1930s (Farlow 1902; Taylor 1945; Eldredge and Smith 2001; Molnar et al. 2008; Ruiz and Ziemmeck 2011). Asparagopsis taxiformis was first registered in the Galapagos by Dawson in 1963 (Taylor 1945; Dawson 1963; Chualáin et al. 2004; Ruiz and Ziemmeck 2011). According to Hickman (1997), the blue crab Cardisoma crassum was an introduction to the Galapagos Islands although the evidence is uncertain. It was thought it was originally introduced when some live crabs escaped after being taken to a hotel in the town of Puerto Ayora on the Island of Santa Cruz. However, in a publication on land crabs of Costa Rica, Bright (1966) reports the presence of the blue crab in the Galapagos Islands. On the other hand, Garth (1991) cites this species as absent and with undetermined invasiveness. Bugula neritina and Pennaria disticha were first registered during the Allan Hancock Pacific Expeditions (Taylor 1945; Eldredge and Smith 2001; Danulat and Edgar 2002; Hickman 2008; Molnar et al. 2008; Ryland et al. 2011; Vieira et al. 2012). Acanthaster planci was first reported in the Galapagos by Hickman; it is found only at Darwin Island in the north of the Archipelago (Cohen-Rengifo et al. 2009). A small colony of Schizoporella unicornis was reported by Osborn (Taylor 1945) on the island of Santiago between 1932 and 1949 by the Allan Hancock Pacific Expeditions. In his report Osborn cites that this species had not been found previously in the eastern Pacific and further suggests that it could have been a recent introduction as it was found along with oysters from the Atlantic coast (Taylor 1945; Banta and Redden 1990).
In contrast, diving expeditions conducted since 2012 produced a list containing six of the seven previously reported species in the literature and one new record for Galapagos (Table 1). S. unicornis is classed as introduced and naturalised by the Charles Darwin Foundation Checklist (Bungartz et al. 2009) but there has been no record of this species since Osborn reported it as present in the 1930s (Taylor 1945). This species was not found during the yearly ecological monitoring surveys carried out by CDF since 2002 or by searches conducted in this research. For this reason, it has not been put on the list of non-natives present in the GMR at this time. A new record that this research produced was Amathia verticillatum, commonly known as the spaghetti bryozoans (McCann et al. 2015).
|
The historic records of Caulerpa racemosa might influence people to think that this species is native due to the fact it has been present in the GMR for so long. CDRS has been been running marine monitoring programs since 1997 (Bustamante et al. 2000; Danulat and Edgar 2002) and there are records of C. racemosa that date back to the 1970s. In this paper, it is suggested that C. racemosa is non-native due to the more recent findings of this species being found in sites where it had never been reported previously and the observation that this species’ distribution can expand or contract due to water temperature changes, suggesting that previous ENSO events could have influenced this species’ presence and distribution. Similarly, historical records for A. taxiformis list this species as present since the 1960s, but recent dive surveys have discovered new areas where this species was never recorded and has expanded rapidly, an example being the Mariela Islands off the island of Isabela.
Discussion and Conclusion
This research shows the presence of seven non-native species reported in the GMR at the current time. The historical literature and recent dive surveys support these findings but it is difficult to demonstrate whether anthropogenic vectors resulted in the introduction of these species or if they arrived naturally. An excellent example of an anthropogenic vector that could aid in the transport of non-native species from different regions to the GMR is marine traffic; however, natural dispersal could also facilitate the transport of non-natives through oceanic currents. Other vectors include climate change and marine debris but these vectors raise the question of how to categorise non-native species transported by them as they are both natural processes that have been influenced by anthropogenic activity. The authors suggest that these species could have arrived at the islands through marine traffic, current systems and climate variations. Six of the seven non-native species from Table 1 are also found in continental Ecuador and in other regions in the ETP. A. planci has not yet been recorded in continental Ecuador but has been recorded on the island of Cocos in Costa Rica, and in Panama.
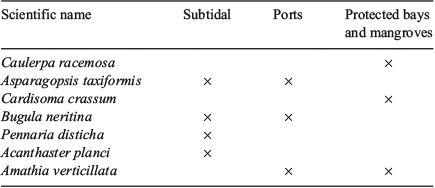
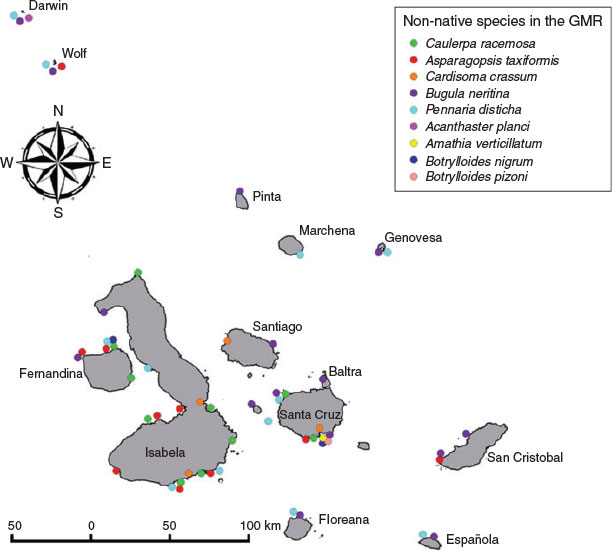
The different methods used to search for non-native species has enabled the coverage of a wider range of habitats than if only one method had been utilised, likely resulting in more species now being identified (Table 2). The subtidal monitoring was essential because this method allowed us to search for species at different depths. The monitoring of the main ports in the region is of great importance and considered high priority, as these are the most likely areas where possible invaders can arrive due to the marine traffic from abroad and from continental Ecuador. The protected bays provide excellent habitats for certain species to establish, reproduce and compete with native species due to particular environmental conditions (such as water temperature, depth, visibility and low wave exposure) that favour certain categories of non-native species. With the information recorded we were able to map out the distribution of the seven species identified as non-native in the GMR currently (Fig. 1).
|
|
The marine species in the GMR have evolved in relative isolation and include a large number of endemic species. The exposure of oceanic islands to marine non-natives has often been discussed in reviews of invasion biology (e.g. Elton 1958; Simberloff 1995; Inglis et al. 2006). For a non-native species to establish in a new environment there must be suitable environmental conditions, lack of predators and the availability of resources for the species to proliferate, and these can be dynamic and highly variable in marine ecosystems. It has been suggested that island ecosystems often have accessible ecological niches that can be filled by opportunistic non-native species arriving from other regions and species that are associated with anthropogenic vectors are often more successful in filling these niches (Wonham et al. 2000; Inglis et al. 2006).
Anthropogenic and natural vectors in the GMR
Marine traffic
Since the accidental discovery of the Galapagos Islands in 1535 (McBride 1918) and during the seventeenth and eighteenth centuries, the islands became a haven for pirates and whalers and the first introductions of domestic animals and invertebrates occurred. In the nineteenth century, whalers were attracted by the high productivity of the seas surrounding the islands and made this region their hunting grounds. Hence, it is thought that various marine species could have been introduced to the islands at this time with the amount of maritime traffic that existed. Following this, industrial fishing boats entered the territorial waters during the 1940s and 1950s (Causton et al. 2008), and in 1942 during the Second World War, the USA constructed a Naval Base on the island of Baltra, which increased the number of vessels in the area.
As described, the history of the maritime traffic in the GMR is extensive, which makes it more difficult to know with certainty if some species existed naturally or if they were introduced in the past. An example is B. neritina, a brown bryozoan that has a worldwide distribution: it is thought that this species could have been transported on wooden hulls around the world (Eldredge and Smith 2001; Molnar et al. 2008; Ryland et al. 2011; Vieira et al. 2012). Currently, the Galapagos Islands receive a large amount of marine traffic, and there are several categories of vessels: tourism, transport, cargo, fishing, private, scientific, patrol boats and oil tankers as well as the illegal fishing boats that enter the GMR (Campbell and Hewitt 2007; Campbell et al. 2015). This movement of different vessels increases the threat of non-native species entering and spreading within the GMR.
Tourism is the principal economy of the Galapagos Islands (Piu and Muñoz 2008), where 61% of the tourists who visit do so from boats. There are several different tourist itineraries that are administered by the Galapagos National Park. Cargo boats operate on a weekly basis and bring supplies to the main ports from the port of Guayaquil on mainland Ecuador. The number of boats traveling between the populated islands fluctuates significantly according to demand. During the first half of 2007, ~1900 trips were made between the populated islands (Causton et al. 2008). However, a study conducted between February and November 2012, a period of only 10 months, showed 8685 departures and arrivals of interisland vessels registered in the Isla Santa Cruz by the Navy of Ecuador (Parra et al. 2013). This shows an increase in marine traffic between the populated islands. Fishing boats, private, scientific and patrol boats are more difficult to record, since these do not have itineraries or fixed routes. Private yachts enter the GMR every year, with most of them arriving between December and June. These yachts arrive from all over the world but most of the captains report Panama as their last port of call (Keith and Martinez 2014). According to records from the ABG, in 2013 there were 273 private boats that entered the GMR (ABG 2014).
Oceanic currents
Oceanic currents heavily influence transoceanic dispersal, making it possible for species to be dispersed between widely separated areas, especially species capable of long-distance larval transport (Hickman 2009). The islands are no longer considered an isolated place due to the dynamic convergence of different oceanic regimes that provides incredible connectivity, which is partly responsible for the island’s unique biodiversity (Hickman 2009). It is widely recognised that four main currents influence the Galapagos archipelago (Banks 2002) and these currents show a marked seasonality in their intensity and direction (Chavez and Brusca 1991) and provide connectivity between the Galapagos Islands groups. For most marine organisms with sessile, benthic or sedentary adult phases, movement is often limited to their larval phase and dispersal. However, these early life-history stages are never entirely passive and represent a unique opportunity for individuals to be transported between geographically separated populations using the oceanic currents (Pineda et al. 2007; Paris et al. 2013).
Climate variability
The ocean is well known to play a dominant role in the climate system because it can initiate and amplify climate change on many different time scales. The best known examples are the interannual variability of ENSO events and the potential modification of the major patterns for oceanic heat transport as a result of increasing greenhouse gases (Semtner 1995). The Galapagos Islands are regularly subjected to extreme climate variability through ENSO events. These strong climatic events cause increases in temperature, changes in current circulation and changes in precipitation. During 1982–83 and 1997–98, two strong El Niño events were marked with widespread damage caused to the marine ecosystem of the Galapagos Islands, largely due to trophic cascades and food shortages. During ENSO events, prolonged increases in sea temperature are induced as the warm surface waters of the western Pacific band migrate to the coast of South America (Banks 2002). During such events when extreme conditions occur, the geographic range of some warm-water species can expand, moving them to different regions. In the GMR populations of the green sea urchin (Lytechinus semituberculatus) decreased during the last strong ENSO event, whereas, in contrast, the white sea urchin (Tripneustes depressus) showed high rates of recruitment after the El Niño event (Brandt and Guarderas 2002; Danulat and Edgar 2002).
Natural processes enhanced by anthropogenic activity
Global warming
The earth’s climate has been changing throughout history through natural periodic cycles, but it is now thought that, due to the amount of greenhouse gases in the atmosphere resulting from human activity, global warming is expected to have a significant impact on our future climate (IPCC 2007), resulting in potential major impacts on species and ecosystems (Rahel 2002; Hare and Whitfield 2003). When a habitat has been changed, for example, through climate change, invasive species can take advantage of the disturbed environment to establish and spread more effectively than if the system was stable and could resist the invasion (Emerton and Howard 2008). Biodiversity is being affected by climate change, with changing temperature and rainfall patterns (Dawson et al. 2011). Whilst native species struggle to adapt to new conditions, many invasive species, being generalists, can more easily adapt, establish and spread (Emerton and Howard 2008). There are cases recorded where long-term changes in ocean temperatures have influenced the distribution of fish species, resulting in a poleward expansion from their historical native range (Hare and Whitfield 2003; Perry et al. 2005). How non-native marine species are reacting to these changes is yet to be fully examined or understood (Hewitt and Campbell 2013). The change in global climate could affect the ecosystems in the GMR, allowing marine non-natives to take advantage and proliferate.
Marine debris
Oceanic currents can also transport marine debris that can have species attached. Examples of these include lost fishing nets and abandoned fish aggregating devices. These can potentially harbour invasive species and can be carried by currents to different locations (Hilliard 2004). The marine debris provides another example of a potential vector for introduced species (Vegter et al. 2014) – a prime example of this was the Japanese tsunami in 2011. A year after the devastating earthquake and subsequent tsunami, a floating dock appeared on the coast of Oregon in the United States with several invasive species attached to it. Some examples were: Undaria pinnatifida (‘wakame’) also known as Asian kelp, Hemigraspus sanguineus, commonly known as the Japanese shore crab, and Asterias amurensis, known as the northern Pacific sea star (Chan 2012). This demonstrates how invasive species can be transported across a large body of water by currents and winds attached to floating debris. Marine debris is human-created waste that enters a natural environment where natural processes spread the debris.
Possible vectors for the present non-native species list of the GMR
Marine traffic is thought to be the most important anthropogenic vector for the transport of non-natives to the GMR. B. neritina and A. verticillatum are both well known fouling organisms that have been transported around the world for centuries. A. verticillatum continues to appear in new regions around the world, which resulted in the new record in the GMR (McCann et al. 2015). Due to the increase in traffic over the years and vessels arriving from around the world combined with the fact this species has been recorded on vessel hulls around the world (McCann et al. 2015), it is likely that this non-native arrival resulted from marine traffic. The non-native species C. racemosa, A. taxiformis and P. disticha could have been transported by marine traffic as well as through natural dispersal, whereas A. planci could have arrived at Darwin by means of oceanic currents or it could have migrated due to sea temperature changes due to climate change or ENSO events. This is thought to be the case as it was reported after the 1997–98 El Niño event (Hickman 1998). It has been suggested that the lack of genetic research conducted on A. planci has shown the lack of understanding of different populations in different regions around the world (Vogler et al. 2008). This discovery could lead to new findings of this species’ distribution. The crab C. crassum could have arrived naturally through transoceanic dispersal or, as Hickman (1997) proposes, was unintentionally introduced to the Galapagos Islands when some individuals were brought from continental Ecuador as food.
Biological invasions have been reported on the coasts of Chile in recent years as well as on the coasts of Peru, with some introductions taking place due to aquaculture and some species undergoing range expansion (Castilla et al. 2005). Surveys have also been conducted in Panama on both the Pacific and Atlantic sides of the Panama Canal and species from Peru have been observed on Pacific Panama (Schlöder et al. 2013). These are just some examples of how the connectivity in the ETP and the Southern Hemisphere should be taken into account when looking at possible invasions occurring in the GMR.
Management of marine non-native species
The possible invasion of marine non-native species into the GMR, given the rapid expansion of marine traffic, the connectivity through oceanic currents and the climatic events that occur in the region is a reality that should not be ignored. The introduction of species and their subsequent proliferation in the archipelago have been identified for well over a decade as the principal threat to the conservation of Galapagos (CDF and WWF 2002). The number of vessels arriving in the Galapagos from different parts of the world due to the connectivity has increased in recent years (ABG 2014), escalating the possibility of an invasion. As more tourism and commerce grows in the islands the higher the risk of an invasion by marine non-native species. An efficient policy to support conservation and social sustainability must act on the connections between Galapagos, continental Ecuador, and the rest of the world, to reduce the flows of non-native species that enter (and leave) the archipelago (Grenier 2010). The management of incoming vessels and adequate quarantine protocols need to be put in place. The ABG and the GNPD have commenced hull inspections to all boats entering the GMR, which is a starting point for the control of non-native species entering the GMR. However, more work has to be done to prevent species arriving. The inspection protocols have to be extended beyond the GMR, to the last port of call or beyond – all boats should arrive at the Galapagos with clean hulls and be reinspected upon arrival.
It is uncertain how these species might respond to climate change or climate variability, which is why these species have been placed on a priority ‘watch list’. The Charles Darwin Foundation, the GNPD and the Ecuadorian Biosecurity Agency (ABG), have established monitoring programs in order to keep an eye on these species spreading or causing further impacts to the GMR. Currently, the species mentioned here have been observed in competition with native species for space, the most apparent example being the spread of C. racemosa in some of the protected bays around the archipelago. An increase in sea temperature could favour this species and allow it to expand further and proliferate.
There are several potential high-risk species that could damage the marine ecosystems of the Galapagos Islands. Some of these species have been identified by Campbell and Hewitt (2007) and more investigation is being undertaken currently. Species like the white coral Carijoa riisei have already been reported in continental Ecuador and in the island of Malpelo, Colombia (Sánchez et al. 2011), located 500 km west of continental Colombia and ~1200 km north-west from the island of Darwin. This species is a well known fouling organism (Eldredge and Smith 2001) that could hitch a ride on boat hulls or be transported by currents. It is a priority to establish what the high-risk species are for the GMR in order to improve management protocols for marine invasive species. Prevention, early detection and rapid response protocols have to be put in place along with risk assessments and management strategies.
Acknowledgements
We thank Darwin Initiative UK, Galapagos Conservancy, The Galapagos National Park and The Galapagos Biosecurity Agency for their support in this research.
References
Agencia de Regulación y Control de la Bioseguridad y Cuarentena para Galápagos (ABG) (2014). Marine traffic database. ABG, Ministerio del Ambiente, Puerto Ayora, Galapagos, Ecuador.Agencia de Regulación y Control de la Bioseguridad y Cuarentena para Galápagos (ABG) (2015). Agency for Regulation and Control of Biosecurity and Quarantine for Galapagos. Available at: http://bioseguridadgalapagos.gob.ec [accessed 1 April 2015].
Banks, S. (2002). Ambiente físico. In ‘Reserva Marina de Galápagos. Línea Base de la Biodiversidad’. (Eds E. Danulat, and G. J. Edgar.) pp. 29–42. (Fundación Charles Darwin/Servicio Parque Nacional Galápagos: Santa Cruz, Galápagos, Ecuador.)
Banks, S. A., Acuña, D., Calderón, R., Garske-Garcia, L., Edgar, G. E., Keith, I., Kuhn, A., Pépolas, R., Ruiz, D., Suarez, J., Tirado-Sánchez, N., Vera, M., Vinueza, L., and Wakefield, E. (2014). Manual de Monitoreo Submareal Ecológico para la RMG. Informe Técnico. Fundación Charles Darwin, Puerto Ayora, Galápagos, Ecuador.
Banta, W. C., and Redden, J. C. (1990). A checklist of the Bryozoa of the Galapagos Proceedings of the Biological Society of Washington 103, 789–802.
Brandt, M., and Guarderas, P. (2002). Erizos de Mar. In ‘Reserva Marina de Galápagos. Línea Base de la Biodiversidad’. (Eds E. Danulat, and G. J. Edgar.) pp. 403–425. (Fundación Charles Darwin/Servicio Parque Nacional Galápagos: Santa Cruz, Galápagos, Ecuador.)
Bright, D. (1966). The land crabs of Costa Rica. Biotropica 14, 183–203.
Bungartz, F., Herrera, H. W., Jaramillo, P., Tirado, N., Jiménez-Uzcátegui, G., Ruiz, D., Guézou, A., and Ziemmeck, F. (Eds.) (2009). Charles Darwin Foundation Galapagos Species Checklist – Lista de Especies de Galápagos de la Fundación Charles Darwin. Charles Darwin Foundation, Puerto Ayora, Galápagos. Available at: http://www.darwinfoundation.org/datazone/checklists/ [accessed13 Apr 2011].
Bustamante, R., Collins, K. J., and Bensted-Smith, R. (2000). Biodiversity conservation in the Galápagos Marine Reserve. In ‘Proccedings of the Symposium, Science for Conservation in Galápagos, 15th April 1998’. Institut Royal des Science Naturelles de Belgique, Brussels Bulletin de l’Institut Royal des Science Naturelles de Belgique 70(supplement), 31–38.
Campbell, M. L., and Hewitt, C. L. (2007). Preliminary assessment of marine biosecurity risks to the Galapagos Islands. National Centre for Marine & Coastal Conservation.
Campbell, M. L., and Hewitt, C. L. (2013). Protecting high-value areas from introduced marine species. Management of Biological Invasions 4, 171–189.
| Protecting high-value areas from introduced marine species.Crossref | GoogleScholarGoogle Scholar |
Campbell, M. L., Keith, I., Hewitt, C. L., Dawson, T. P., and Collins, K. (2015). Evolving marine biosecurity in the Galapagos Islands. Management of Biological Invasions 6, 227–230.
| Evolving marine biosecurity in the Galapagos Islands.Crossref | GoogleScholarGoogle Scholar |
Carlton, J. T. (1996). Biological invasions and cryptogenic species. Ecology 77, 1653–1655.
| Biological invasions and cryptogenic species.Crossref | GoogleScholarGoogle Scholar |
Castilla, J. C., Uribe, M., Bahamonde, N., Clarke, M., Desqueyroux-Faúndez, R., Kong, I., Moyano, H., Rozbaczylo, N., Santelices, B., Valdovinos, C., and Zavala, P. (2005). Down under the Southeastern Pacific: marine non-indigenous species in Chile. Biological Invasions 7, 213–232.
| Down under the Southeastern Pacific: marine non-indigenous species in Chile.Crossref | GoogleScholarGoogle Scholar |
Causton, C., Campbell, M., Hewitt, C., and Boada, R. (2008). Riesgos Asociados con las Rutas Marítimas Hacia y Entre Galápagos. Fundación Charles Darwin, Puerto Ayora, Galápagos, Ecuador.
Chan, S. (2012). Japanese tsunami debris and invasive species: lessons being learned in Oregon and applications for the region. Oregon State University, Oregon Sea Grant, National Sea Grant and NOAA.
Charles Darwin Foundation (CDF) and World Wildlife Fund (WWF) (2002). A biodiversity vision for the Galapagos Islands: based on international workshop of conservation biologists in Galapagos in May 1999. World Wildlife Fund.
Chavez, F. P., and Brusca, R. C. (1991). , .
| Crossref | GoogleScholarGoogle Scholar |
Chualáin, F. N., Maggs, C. A., Saunders, G. W., and Guiry, M. D. (2004). The invasive genus Asparagopsis (Bonnemaisoniaceae, Rhodophyta): molecular systematics, morphology, and ecophysiology of Falkenbergia isolates. Journal of Phycology 40, 1112–1126.
| The invasive genus Asparagopsis (Bonnemaisoniaceae, Rhodophyta): molecular systematics, morphology, and ecophysiology of Falkenbergia isolates.Crossref | GoogleScholarGoogle Scholar |
Cohen-Rengifo, M., Bessudo, S., and Soler, G. (2009). Echinoderms, Malpelo fauna and flora sanctuary, Colombian Pacific: new reports and distributional issues. Check List 5, 702–711.
Danulat, E., and Edgar, G. J. (2002). Reserva Marina de Galápagos. Línea Base de la Biodiversidad. Fundación Charles Darwin/Servicio Parque Nacional Galápagos, Santa Cruz, Galápagos, Ecuador.
Dawson, E. Y. (1963). New records of marine algae from the Galapagos Islands. Pacific Naturalist 4, 1–23.
Dawson, T. P., Jackson, S. T., House, J. I., Prentice, I. C., and Mace, G. M. (2011). Beyond predictions: biodiversity conservation in a changing climate. Science 332, 53–58.
| Beyond predictions: biodiversity conservation in a changing climate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjvVyisbk%3D&md5=9d94b68035a0fd53b8ac4100c70e1555CAS | 21454781PubMed |
Edgar, G. J., Banks, S. A., Brandt, M., Bustamante, R. H., Chiriboga, A., Earle, S. A., Garske, L. E., and Wellington, G. M. (2010). El Niño, grazers and fisheries interact to greatly elevate extinction risk for Galapagos marine species. Global Change Biology 16, 2876–2890.
| El Niño, grazers and fisheries interact to greatly elevate extinction risk for Galapagos marine species.Crossref | GoogleScholarGoogle Scholar |
Eldredge, L. G., and Smith, C. M. (2001). A guidebook of introduced marine species in Hawai’’i. Bishop Museum Technical Report 21. Bishop Museum, Honolulu. A1–A54; B1–B60 p.
Elton, C. S. (1958). ‘The Ecology of Invasions by Animals and Plants.’ (Methuen: London.)
Emerton, L., and Howard, G. (2008). A toolkit for the economic analysis of invasive species. Available at: http://www.issg.org/pdf/publications/GISP/Guidelines_Toolkits_BestPractice/Emerton&Howard_2008_EN.pdf [accessed 15 September 2014].
Farlow, W. G. (1902). Algae. In ‘Flora of the Galapagos Islands’. (Ed. B. L. Robinson.) Proceedings of the American Academy of Arts and Sciences 38, 89–99.
Garth, J. S. (1991). Taxonomy, distribution and ecology of Galapagos Brachyura. In ‘Galapagos Marine Invertebrates’. (Ed. M. J. James.) pp. 123–145. (Springer: New York.)
Godwin, L. S. (2003). Hull fouling of maritime vessels as a pathway for marine species invasions to the Hawaiian Islands. Biofouling 19, 123–131.
| Hull fouling of maritime vessels as a pathway for marine species invasions to the Hawaiian Islands.Crossref | GoogleScholarGoogle Scholar | 14618713PubMed |
Grenier, C. (2010). La apertura geográfica de Galápagos. In ‘Informe Galápagos 2009–2010’. pp. 123–131. (Puerto Ayora, Galápagos, Ecuador.)
Guiry, M. D., and Guiry, G. M. (2015). AlgaeBase. National University of Ireland, Galway. Available at: http://www.algaebase.org [accessed 30 October 2015].
Hare, J. A., and Whitfield, P. E. (2003). An integrated assessment of the introduction of lionfish (Pterois volitans/miles complex) to the western Atlantic Ocean. NOAA Technical Memorandum NOS NCCOS 2, 1–21.
Hewitt, C. L., and Campbell, M. L. (2013). Facilitated dispersal: overwhelming biogeographic boundaries. Paper presented at 8th International Conference on Marine Bioinvasions. Vancouver, Canada.
Hewitt, C. L., and Hayes, K. R. (2002). Risk assessment of marine biological invasions. In ‘Invasive Aquatic Species of Europe. Distribution, Impacts and Management’. (Eds E. Leppäkoski, S. Gollasch, S. Olenin) pp. 456–466. (Springer: Netherlands.)
Hickman, C. P. (1997). ‘Crustaceans of Galápagos: a Field Guide to the Common Barnacles, Shrimp, Lobsters and Crabs of the Galapagos Islands.’ (Lexington, VA.)
Hickman, C. P. (1998). ‘A Field Guide to Sea Stars and Other Echinoderms of Galápagos.’ (Sugar Spring Press: Lexington, VA.)
Hickman, C. P. (2008). ‘A Field Guide to Corals and other Radiates of Galapagos: an Illustrated Guidebook to the Corals, Anemones, Zoanthids, Black Corals, Gorgonians, Sea Pens, and Hydroids of the Galápagos Islands.’ (Sugar Spring Press: Lexington, VA.)
Hickman, C. P. (2009). Evolutionary responses of marine invertebrates to insular isolation in Galapagos. Galapagos Research 66, 32–42.
Hilliard R. (2004). Best practice for the management of introduced marine pests – a review. Global Invasive Species Programme, IUCN.
Hulme, P. E. (2009). Trade, transport and trouble: managing invasive species pathways in an era of globalization. Journal of Applied Ecology 46, 10–18.
| Trade, transport and trouble: managing invasive species pathways in an era of globalization.Crossref | GoogleScholarGoogle Scholar |
Inglis, G. J., Hayden, B. J., and Nelson, W. A. (2006). Are the marine biotas of island ecosystems more vulnerable to invasion? In ‘Ecological Studies. Vol. 186’. (Eds R. B. Allen, and W. G. Lee.) pp. 119–135. (Springer-Verlag: Berlin.)
Invasive Species Specialist Group (ISSG) (2015). The Global Invasive Species Database. Version 2015.1.
IPCC (2007). Summary for policymakers. In ‘Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change’. p. 17. (Cambridge University Press: Cambridge.)
IUCN (2011). IUCN Red List of Threatened Species. Version 2012.2. Available at: http://www.iucnredlist.org [accessed 1 April 2013].
Jäger, H., Tye, A., and Kowarik, I. (2007). Tree invasion in naturally treeless environments: impacts of quinine (Cinchona pubescens) trees on native vegetation in Galápagos. Biological Conservation 140, 297–307.
| Tree invasion in naturally treeless environments: impacts of quinine (Cinchona pubescens) trees on native vegetation in Galápagos.Crossref | GoogleScholarGoogle Scholar |
Keith, I., and Martinez, P. (2014). Especies Invasoras Marinas – Prevención, detección y manejo. Documento Técnico. Fundación Charles Darwin. Puerto Ayora, Galápagos, Ecuador.
Kolar, C. S., and Lodge, D. M. (2002). Ecological predictions and risk assessment for alien fishes in North America. Science 298, 1233–1236.
| Ecological predictions and risk assessment for alien fishes in North America.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XosVerurg%3D&md5=def357236f336061de6e517bc445fc8aCAS | 12424378PubMed |
Loope, L. L., Hamann, O., and Stone, C. P. (1988). Comparative conservation biology of oceanic archipelagoes: Hawaii and the Galapagos. Bioscience 38, 272–282.
| Comparative conservation biology of oceanic archipelagoes: Hawaii and the Galapagos.Crossref | GoogleScholarGoogle Scholar |
LOREG (1998). Ley Orgánica de Régimen Especial para la Conservación y el Desarrollo Sustentable de la Provincia de Galápagos. Congreso Nacional. Registro Oficial No 278 del 18 de marzo de 1998. Ecuador.
McBride, G. M. (1918). The Galapagos Islands. Geographical Review 6, 229–239.
| The Galapagos Islands.Crossref | GoogleScholarGoogle Scholar |
McCann, L., Keith, I., Carlton, J. T., Ruiz, G. M., Dawson, T. P., and Collins, K. J. (2015). First record of the non-native bryozoan Amathia (=Zoobotryon) verticillata (delle Chiaje, 1822) (Ctenostomata) in the Galapagos Islands. Bioinvasions Records 4, 255–260.
| First record of the non-native bryozoan Amathia (=Zoobotryon) verticillata (delle Chiaje, 1822) (Ctenostomata) in the Galapagos Islands.Crossref | GoogleScholarGoogle Scholar |
Molnar, J. L., Gamboa, R. L., Revenga, C., and Spalding, M. D. (2008). Assessing the global threat of invasive species to marine biodiversity. Frontiers in Ecology and the Environment 6, 485–492.
| Assessing the global threat of invasive species to marine biodiversity.Crossref | GoogleScholarGoogle Scholar |
Muromtsev, A. M. (1963). ‘The Principal Hydrology Features of the Pacific Ocean.’ (Jerusalem Post Press.)
Pagad, S., Hayes, K., Katsanevakis, S., and Costello, M. J. (2015). World Register of Introduced Marine Species (WRIMS). Available at: http://www.marinespecies.org/introduced (accessed 30 October 2015.)
Paris, C. B., Helgers, J., Van Sebille, E., and Srinivasan, A. (2013). Connectivity Modeling System: a probabilistic modelling tool for the multi-scale tracking of biotic and abiotic variability in the ocean. Environmental Modelling & Software 42, 47–54.
| Connectivity Modeling System: a probabilistic modelling tool for the multi-scale tracking of biotic and abiotic variability in the ocean.Crossref | GoogleScholarGoogle Scholar |
Parra, D. M., Andrés, M., Jiménez, J., Banks, S., and Muñoz, J. P. (2013). Evaluación de la incidencia de impacto de embarcaciones y distribución de la tortuga verde (Chelonia mydas) en Galápagos. Documento Técnico. Fundación Charles Darwin. Puerto Ayora, Galápagos, Ecuador.
Perry, A. L., Low, P. J., Ellis, J. R., and Reynolds, J. D. (2005). Climate change and distribution shifts in marine fishes. Science 308, 1912–1915.
| Climate change and distribution shifts in marine fishes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXlsVWmtbg%3D&md5=188655b8a809aef6b70c1ee10e14863dCAS | 15890845PubMed |
Pineda, J., Hare, J. A., and Sponaungle, S. (2007). Larval transport and dispersal in the coastal ocean and consequences for population connectivity. Oceanography 20, 22–39.
| Larval transport and dispersal in the coastal ocean and consequences for population connectivity.Crossref | GoogleScholarGoogle Scholar |
Piu, M., and Muñoz, E. (2008). General characteristics of the tourist fleet in Galapagos and its compliance with environmental standards. In ‘Galapagos Report 2007–2008’. Puerto Ayora, Galapagos, Ecuador.
Rahel, F. J. (2002). Using current biogeographic limits to predict fish distributions following climate change. In ‘Fisheries in a Changing Climate, American Fisheries Society Symposium 32’. (Ed. Nature A. McGinn) pp. 99–112. (American Fisheries Society.)
Ruiz, D., and Ziemmeck, F. (2011). CDF Checklist of Galápagos Green Algae – FCD Lista de especies de Algas verdes de Galápagos. In ‘Charles Darwin Foundation Galapagos Species Checklist – Lista de Especies de Galápagos de la Fundación Charles Darwin’. (Eds F. Bungartz, H. Herrera, P. Jaramillo, N. Tirado, G. Jímenez-Uzcategui, D. Ruiz, A. Guézou, and F. Ziemmeck.) (Charles Darwin Foundation/ Fundación Charles Darwin, Puerto Ayora, Galápagos.) Available at: http://www.darwinfoundation.org/datazone/checklists/algae/chlorophyta/ [accessed 13 April 2011].
Ryland, J. S., Bishop, J. D. D., De Blauwe, H., El Nagar, E., Minchin, D., Wood, C. A., and Yunnie, A. L. E. (2011). Alien species of Bugula (Bryozoa) along the Atlantic coasts of Europe Aquatic Invasions 6, 17–31.
| Alien species of Bugula (Bryozoa) along the Atlantic coasts of EuropeCrossref | GoogleScholarGoogle Scholar |
Sachs, J., and Ladd, N. (2010). Climate and oceanography of the Galápagos in the 21st Century: expected changes and research needs. Galapagos Research 67, 50–54.
Sánchez, J. A., Gómez, C. E., Escobar, D., and Dueñas, L. F. (2011). Diversidad, abundancia y amenazas de los octocorales de la isla Malpelo, Pacífico Oriental Tropical, Colombia. Boletín Investigaciones Marinas y Costeras 40, 139–154.
Schlöder, C., Canning-Clode, J., Saltonstall, K., Strong, E. E., Ruiz, G. M., and Torchin, M. E. (2013). The Pacific bivalve Anomia peruviana in the Atlantic: a recent invasion across the Panama Canal. Aquatic Invasions 8, 443–448.
| The Pacific bivalve Anomia peruviana in the Atlantic: a recent invasion across the Panama Canal.Crossref | GoogleScholarGoogle Scholar |
Seebens, H., Gastner, M., and Blasius, B. (2013). The risk of marine bioinvasion caused by global shipping. Ecology Letters 16, 782–790.
| The risk of marine bioinvasion caused by global shipping.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3srns1Oruw%3D%3D&md5=4d04858a932a035f84a302cb8672f246CAS | 23611311PubMed |
Semtner, A. J. (1995). Modeling ocean circulation. Science 269, 1379–1384.
| Modeling ocean circulation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXnvFClu7c%3D&md5=1add204bcfe7a0fcfb2c2e854b47637eCAS | 17731147PubMed |
Simberloff, D. (1995). Why do introduced species appear to devastate islands more than mainland areas? Pacific Science 49, 87–97.
Taylor, W. R. (1945). Pacific marine algae of the Allan Hancock Expeditions to the Galápagos Islands. Allan Hancock Pacific Expeditions 12, 1–528.
Vegter, A. C., Barletta, M., Beck, C., Borrero, J., Burton, H., Campbell, M. L., Eriksen, M., Eriksson, C., Estrades, A., Gilardi, K. V. K., Hardesty, B. D., Assuncao, J., do Sul, I., Lavers, J. L., Lazar, B., Lebreton, L., Nichols, W. J., Ribic, C. A., Ryan, P. G., Schuyler, Q. A., Smith, S. D. A., Takada, H., Townsend, K., Wabnitz, C. C. C., Wilcox, C., Young, L. C., and Hamann, M. (2014). Global research priorities to mitigate plastic pollution impacts on marine wildlife. Endangered Species Research 25, 225–247.
| Global research priorities to mitigate plastic pollution impacts on marine wildlife.Crossref | GoogleScholarGoogle Scholar |
Vieira, L. M., Winston, J. E., and Fehlauer-Ale, K. H. (2012). Nine new species of Bugula Oken (Bryozoa: Cheilostomata) in Brazilian shallow waters. PLoS One 7, e40492.
| Nine new species of Bugula Oken (Bryozoa: Cheilostomata) in Brazilian shallow waters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtVOnsLfN&md5=a1fbe60540eaf81da546d5546393423bCAS | 22808173PubMed |
Vitousek, P. M., D’Antonio, C. M., Loope, L. L., Rejmanek, M., and Westbrooks, R. (1997). Introduced species: a significant component of human-caused global change. New Zealand Journal of Ecology 21, 1–16.
Vogler, C., Benzie, J., Lessios, H., Barber, P., and Wörheide, G. (2008). A threat to coral reefs multiplied? Four species of crown-of-thorns starfish. Biology Letters 4, 696–699.
| A threat to coral reefs multiplied? Four species of crown-of-thorns starfish.Crossref | GoogleScholarGoogle Scholar | 18832058PubMed |
Wonham, M. J., Carlton, J. T., Ruiz, G. M., and Smith, L. D. (2000). Fish and ships: relating dispersal frequency to success in biological invasions. Marine Biology 136, 1111–1121.
| Fish and ships: relating dispersal frequency to success in biological invasions.Crossref | GoogleScholarGoogle Scholar |
Wonham, M. J., Walton, W. C., Ruiz, G. M., Frese, A. M., and Galil, B. S. (2001). Going to the source: role of the invasion pathway in determining potential invaders. Marine Ecology Progress Series 215, 1–12.
| Going to the source: role of the invasion pathway in determining potential invaders.Crossref | GoogleScholarGoogle Scholar |
WoRMS Editorial Board (2015). World Register of Marine Species. Available at: http://www.marinespecies.org [accessed 2 November 2015].
Zapata, C. (2006). Evaluación de la eficiencia técnica-operativa del Sistema de Inspección y Cuarentena para Galápagos (SICGAL). Charles Darwin Foundation, Puerto Ayora, Galápagos.


