Nitrogen utilisation-efficient oilseed rape (Brassica napus) genotypes exhibit stronger growth attributes from flowering stage onwards
Xiao Guo A B , Bao-Luo Ma B , Neil B. McLaughlin B , Xiaoming Wu C , Biyun Chen C and Yajun Gao
A B , Bao-Luo Ma B , Neil B. McLaughlin B , Xiaoming Wu C , Biyun Chen C and Yajun Gao  A D E
A D E
A College of Natural Resource and Environment, Northwest A and F University, 712100 Yangling, Shaanxi, China.
B Ottawa Research and Development Centre, Agriculture and Agri-Food Canada, K1A 0C6 Ottawa, Ontario, Canada.
C Institute of Oil Crop Research, Chinese Academy of Agricultural Sciences, 430062 Wuhan, Hubei, China.
D Key Laboratory of Plant Nutrition and the Agri-environment in Northwest China, Ministry of Agriculture, 712100 Yangling, Shaanxi, China.
E Corresponding author. Email: yajungao@nwsuaf.edu.cn
Functional Plant Biology 48(8) 755-765 https://doi.org/10.1071/FP20263
Submitted: 26 August 2020 Accepted: 11 February 2021 Published: 15 March 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC-ND © Crown Copyright of Canada and The Authors 2021
Abstract
Preliminary studies observed a lower growth activity during the vegetative stage with higher growth attributes at the pod-filling stage among the high nitrogen (N) utilisation efficiency (NUtE) oilseed rape (Brassica napus L.) genotypes, compared with the low NUtE genotypes. Therefore, we hypothesised that there would exist a critical growth stage when distinctive phenotypic traits are exhibited to regulate yield formation and NUE. A field experiment and a hydroponic culture were conducted to characterise the differences in shoot and root physiological indicators of the high and low NUtE oilseed rape genotypes at seedling, bud, bolting, flowering and pod-filling stages. We found that flowering was the critical period when the reverse growth habit occurred between high and low NUtE genotypes. The high NUtE genotypes displayed larger values of root traits, stronger N uptake kinetics parameters, higher activity of leaf glutamine synthetase (GS) and glutamate synthetase (GOGAT), larger SPAD values and net photosynthetic rate, ultimately leading to higher seed yield and NUE. Our results indicate that flowering is the critical growth stage to distinguish the high from low NUtE oilseed rape genotypes, and plant breeders may focus on selecting root and shoot phenotypic traits from flowering stage onwards to achieve both high yields and NUE for oilseed rape genotypes.
Keywords: nitrogen utilisation efficiency, growth attributes, flowering stage, root function, Brassica napus L., oilseed rape.
Introduction
With the world population being expected to reach near 10 billion by 2050, agricultural production must increase crop yields dramatically to meet future food demands (Raza et al. 2019; Stevens 2019). Owing to the limited arable land, yield improvements per unit area are of primary importance (Stahl et al. 2019). Nitrogen (N) is the most important nutrient required for plant growth, and appropriate use of N fertiliser plays a key role in increasing food crop productivity (Liu et al. 2016; Stahl et al. 2019) in a sustainable way (Oldroyd and Dixon 2014). To increase crop productivity, N fertilisers are applied at rates that exceed crop demand in many regions around the world, resulting in both higher production costs and a greater risk of environmental pollution (Zhang et al. 2015; Albornoz 2016). To continue increasing crop productivity, improving N use efficiency (NUE), defined as the yield produced per unit of soil available N or practically fertiliser N application (Moll et al. 1982), is an essential way to meet the need for environmentally friendly farming agriculture (Lammerts van Bueren and Struik 2017; Yu et al. 2019). Moll et al. (1982) dissected NUE into two components: (1) grain yield produced per unit of plant N uptake, denoted as N utilisation efficiency (NUtE) and (2) the amount of plant N uptake as a ratio of soil available N, named as N uptake efficiency (NUpE).
Oilseed rape (Brassica napus L.) is an important crop, which is grown worldwide to produce vegetable oil for human consumption, animal feed, and biodiesel (Ma et al. 2017; Wagner et al. 2018). Compared with small grain cereal crops, all the three major ecogeographical forms of oilseed rape (winter, spring and semi-winter types) require relatively high amounts of N, but an N surplus remains in the soil at maturity (Rathke et al. 2006; Bouchet et al. 2016; Stahl et al. 2019). This is mainly due to the low N uptake after flowering and incomplete N remobilisation from the source organs to the seeds, leading to low NUE (Ulas et al. 2012). According to Sylvester-Bradley and Kindred (2009), who compared the NUE of 21 main arable crops, NUtE rather than NUpE had the main impact on NUE in oilseed rape genotypes. Furthermore, Stahl et al. (2019) also reported a stronger correlation of NUE with NUtE than with NUpE in oilseed rape varieties under the low N supply condition. Therefore, understanding the physiological characteristics of high NUtE oilseed rape genotypes would help improve the overall NUE, reduce N input and mitigate environment pressure.
In a preliminary study (Guo et al. 2019), we observed that compared with the high NUtE oilseed rape genotypes, the low NUtE genotypes exhibited stronger growth attributes during the vegetative growth stage. In addition, other literature also reported an interesting phenomenon, in which the growth attributes of the high NUtE oilseed rape genotypes were lower than those of the low NUtE genotypes at the vegetative stage, but higher during the pod-filling stage (Svečnjak and Rengel 2006; Ulas et al. 2013). Therefore, there would be a critical period for high NUtE oilseed rape genotypes to exhibit stronger growth attributes than that of the low NUtE genotypes, and plant breeders can make good use of this knowledge to select for both high yield and NUE of oilseed rape genotypes. Kirkegaard et al. (2018) implemented discrete shade treatments to define the critical period and found that flowering is the critical period for yield determination in field-grown canola. Similar findings have highlighted the importance of focusing on the interaction of pre- and post-flowering water and N, and establishing a strong sink capacity to improve seed yield in oilseed rape (Riar et al. 2016, 2017, 2020).
Herein, we hypothesised that there would be a critical growth period throughout the crop’s life cycle, at which the high NUtE oilseed rape genotypes exhibit distinct growth attributes that were responsible for the high seed yield and NUE. To test this hypothesis, a field experiment and a hydroponic culture study were conducted with the objectives of identifying the critical growth stage by examining various shoot and root phenotypic traits at several key stages and determining the traits at the key stage to be used as indicators for selecting high NUtE oilseed rape genotypes. The shoot traits investigated included biomass and N concentration, leaf chlorophyll (SPAD readings) and net photosynthetic rate, leaf glutamine synthetase (GS) and glutamate synthetase (GOGAT) activity, yield and NUE; while the characterised root traits were biomass, N concentration, root length, volume, surface area and root vigour, N uptake kinetics parameters, root GS and GOGAT activity.
Methods and materials
Field experiment
Materials
In a preliminary study, 50 diverse oilseed rape genotypes were tested and categorised into four groups, among which 18 genotypes were used for the present study. They were five Nt-responder (entry no. 1–5), five Nt-nonresponder (entry no. 6–10), four Nt-efficient (entry no. 11–14) and four Nt-inefficient (entry no. 15–18) (Table 1). The classification was based on their NUtE values relative to the overall mean and has been reported previously: Nt-responder referred to genotypes with NUtE values above the mean at high N, while genotypes with NUtE values below the mean were named non-responder. At the low N supply, genotypes displayed NUtE values above the mean were called Nt-efficient, and Nt-inefficient genotypes were those with NUtE values below the mean (Koeslin-Findeklee et al. 2014; He et al. 2017).

|
Experimental design
The field experiment was conducted in the 2016–2017 growing season at Yangling city (34°26′N, 108°03′E), Shaanxi province, China. The red loess soil (Earth-cumuli-Orthic Anthrosols; 0–20 cm layer) of the experimental field contained 13.7 g kg–1 organic matter, 1.19 g kg–1 of total N, 24.7 mg kg–1 of available N, 15.7 mg kg–1 of available P and 76.9 mg kg–1 of available K.
In this experiment, the 10 high NUtE genotypes (five Nt-responder and five Nt-nonresponder) were planted with high N level (150 kg N ha–1), while the eight low NUtE genotypes (four Nt-efficient and four Nt-inefficient) were planted with low N level (0 kg N ha–1). The experiment was arranged in a randomised complete block design with four replications. A commercial oilseed rape hybrid variety was planted as the border surrounding the experimental plots in the same day. Each plot consisted of four rows of oilseed rape, with row spacing of 50 cm, row length of 2 m and 40 plants per row, for a total of 160 plants. Once the plant stands were fully established, sampling locations with appropriate bordering within each plot were identified and tagged. There were five sampling dates, and 30 representative plants in each plot were marked for measuring the shoot traits. The field received 135 kg ha–1 of P2O5 and 150 kg ha–1 of K2O as base fertiliser on 3 October 2016 and followed the Province-recommended management practices.
Determination of shoot indices
At seedling (BBCH 15, 5 leaf unfold), bud (BBCH 35, 5 visible extended internodes), bolting (BBCH 50, inflorescence emergence (flower buds present, still enclosed by leaves), flowering (BBCH 65, full flowering (50% flowers on main raceme open, older petals falling)) and pod-filling (BBCH 75, 50% of pods have reached final size) stages, leaf chlorophyll content was determined with a chlorophyll meter (SPAD-502, Konika Minolta Sensing Inc., Japan), on the top fully expanded leaves of six pre-marked plants in each plot. At each growth stage, leaf net photosynthetic rate was measured on the same plants as for chlorophyll measurements, with a Li-6400 portable photosynthesis system (LI-COR, Lincoln, NE, USA) between 1030 hours and 1130 hours.
After the determination of photosynthetic parameters, two fully unfolded leaves from each of the six pre-marked plants were removed, immediately put in liquid nitrogen and brought to a laboratory for analysing the activity of N metabolism enzymes. At each sampling of the five growth stages, the activities of two enzymes, GS and GOGAT, were assayed according to the method by Shah et al. (2017).
Finally, the same six pre-marked plants in each plot were cut at the ground level and oven-dried for the determination of shoot biomass. All the samples were then ground and used for the determination of N concentration (Kjeldahl method). At maturity stage (BBCH 89, fully ripe (nearly all pods ripe, seeds dark and hard)), the average number of seeds per silique was determined (Shi et al. 2015) and seed yield per plant was measured as the average dry weight of seeds of four randomly selected plants from each genotype (Shi et al. 2011). According to He et al. (2017), N utilisation efficiency (NUtE) and N use efficiency (NUE) were estimated with the following equations:
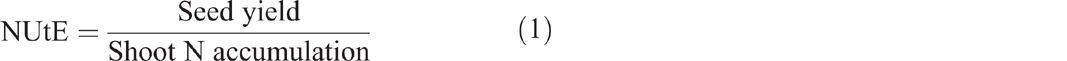

Hydroponic culture
Materials
In the hydroponic experiment, four representative genotypes from the 18 genotypes tested in the field experiment were chosen for phenotyping root traits: Zheyou 18 (Nt-responder), H 49 (Nt-nonresponder), Guiyou 3 (Nt-efficient) and Sollux (Nt-inefficient).
Experimental design
The seeds of uniform size were cleaned with 1% Clorox solution for 10 min, rinsed four times with distilled water, then germinated on a moistened filter paper in Petri dishes. After 7 days, uniform seedlings were transplanted into black plastic boxes (L 35 cm × W 25 cm × H 15 cm) with four plants per box. The plants were divided into two groups, of which different N treatments were given (Zheyou 18 and H 49 for high N; Guiyou 3 and Sollux for low N). The seedlings were cultured in distilled water in the first week, and then in 1/4 strength of modified Hoagland solution in the second week. After that, the culture solution was switched to 1/2 strength for the third week. Finally, all the plants received full strength of nutrient solution until pod-filling stage. The modified Hoagland solution had the following compositions and was adjusted to pH 5.8: 10 (high N) or 1 (low N) mM KNO3, 0.04 mM KH2PO4, 0.015 mM K2HPO4, 0.63 mM KCl, 1 mM K2SO4, 0.5 mM MgSO4, 3 mM CaCl2, 0.2 mM Fe-Na EDTA, 14 µM H3BO3, 3 µM ZnSO4, 5 µM MnSO4, 0.7 µM CuSO4, 0.7 µM (NH4)6Mo7O24 and 0.1 µM CoCl2 (Guo et al. 2019). The nutrient solution was replaced each week. The black boxes on the bench were rotated once a week to avoid positional effect. The plants were managed under the following environmental conditions: day/night mean temperature of 25/15°C, relative humidity of 65% and light intensity of 400 μmol m–2s–1, with a 12 h photoperiod.
At each sampling stage, 14 plants were randomly selected from each replicate and genotype and divided into three groups: (1) four plants were used for measuring root indices as group 1; (2) four plants for assessing the root enzyme activity were referred as to group 2; and (3) six plants as group 3 were used for the determination of N uptake kinetics parameters.
Determination of root indices
Group 1. After cleaning, the plants were partitioned into shoot and root, and the root samples were measured for length, volume and surface area with using the root image analysis scanner and WinRHIZO Pro software (Regent Instruments Inc., Quebec, QC, Canada). After that, the root biomass and N concentration were determined.
Group 2. The partitioned roots were immediately ground in liquid nitrogen and assayed for the root vigour (an indicator of activity for the plant root system, and closely related to N uptake) (Guo et al. 2019) and the activity of two root N metabolism enzymes (GS and GOGAT).
Group 3. The roots of the chosen six plants were incubated for 24 h in a 500 mL beaker with N-free growth medium for NO3- uptake. Then, by adding an aliquot of the N stock solution (KNO3) to the beaker, each plant was provided with a nitrate solution (0, 0.25, 0.50, 0.75, 1.00 and 1.25 mM) for 24 h. After each spiking, NO3– uptake by the plant was estimated from the N depletion in the solution (Hajari et al. 2014). The Vmax and Km were estimated by fitting the data to a modified Michaelis–Menten model:

where V is rate of uptake, Vmax is maximum rate of ion uptake, C is ion concentration, Km is the Michaelis-Menten constant (defined as the concentration required to reach 50% of Vmax).
Statistical analysis
Analysis of variance (ANOVA) was performed on all data by using SPSS software version 17.0 (SPSS, Chicago, IL, USA). When the analysis showed significant treatment effects, mean comparisons were made according to the least-significant difference test at P ≤ 0.05 (l.s.d.0.05). Principal component analysis was performed to estimate the contribution of the measured phenotypic traits to NUtE by using Canoco 5.0 software. All figures were created using the Origin 9.0 software and Microsoft Excel 2016.
Results
Field experiment
Yield and NUE
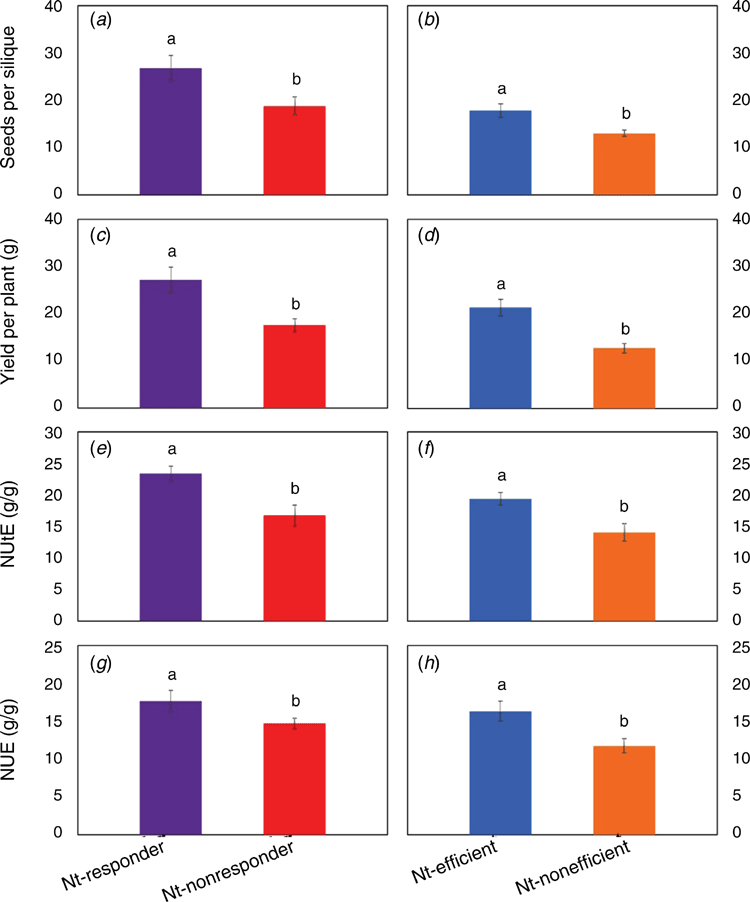
On average, the number of seeds per silique was 34.9% higher for the high NUtE (Nt-responder and Nt-efficient) genotypes than for the low NUtE (Nt-nonresponder and Nt-inefficient) genotypes (Fig. 1a, b; see Table S1), resulting in 75.7% higher yield for the high NUtE genotypes (Fig. 1c, d; see Table S1). The greater productivity of the high NUtE genotypes was associated with 38.8% larger NUtE and 33.2% greater NUE, compared with the low NUtE genotypes (Fig. 1e–h; see Table S1).
Shoot indices
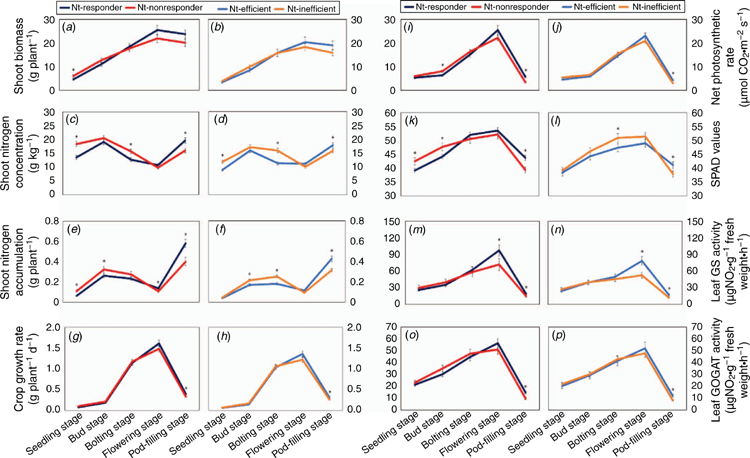
Compared with the high NUtE genotypes, the low NUtE genotypes displayed higher shoot indices from seedling to bolting stage. However, a critical transition phase occurred at the flowering stage when most of the measured phenotypic traits displayed a reverse growth habit, with higher shoot growth indices for the high NUtE than for the low NUtE genotypes. For example, the leaf net photosynthetic rate was 12.4% higher (Fig. 2e, f; see Table S6), associated with 41.3% higher activity for GS (Fig. 2i, j; see Table S8). As a result, the high NUtE genotypes produced 14.0% more shoot biomass (Fig. 2a, b; see Table S2) than the low NUtE genotypes. Clearly, this opposite growth habit occurred from flowering stage onwards and was maintained until pod-filling stage (Fig. 2).
Overall, it was inferred that the shoot biomass, SPAD values, net photosynthetic rate, and leaf GS and GOGAT activity of the 18 genotypes reached a maximum by the beginning of flowering, and then declined from flowering to pod-filling stage (Fig. 2a, b, g–p), while the shoot N concentration of all the genotypes was lower at the seedling stage, and then increased in bud stage, followed by a decline from bud to flowering stage, and finally an increase from flowering to pod-filling stage (Fig. 2c–f; see Tables S3 and S4).
Hydroponic culture
Root morphological indices
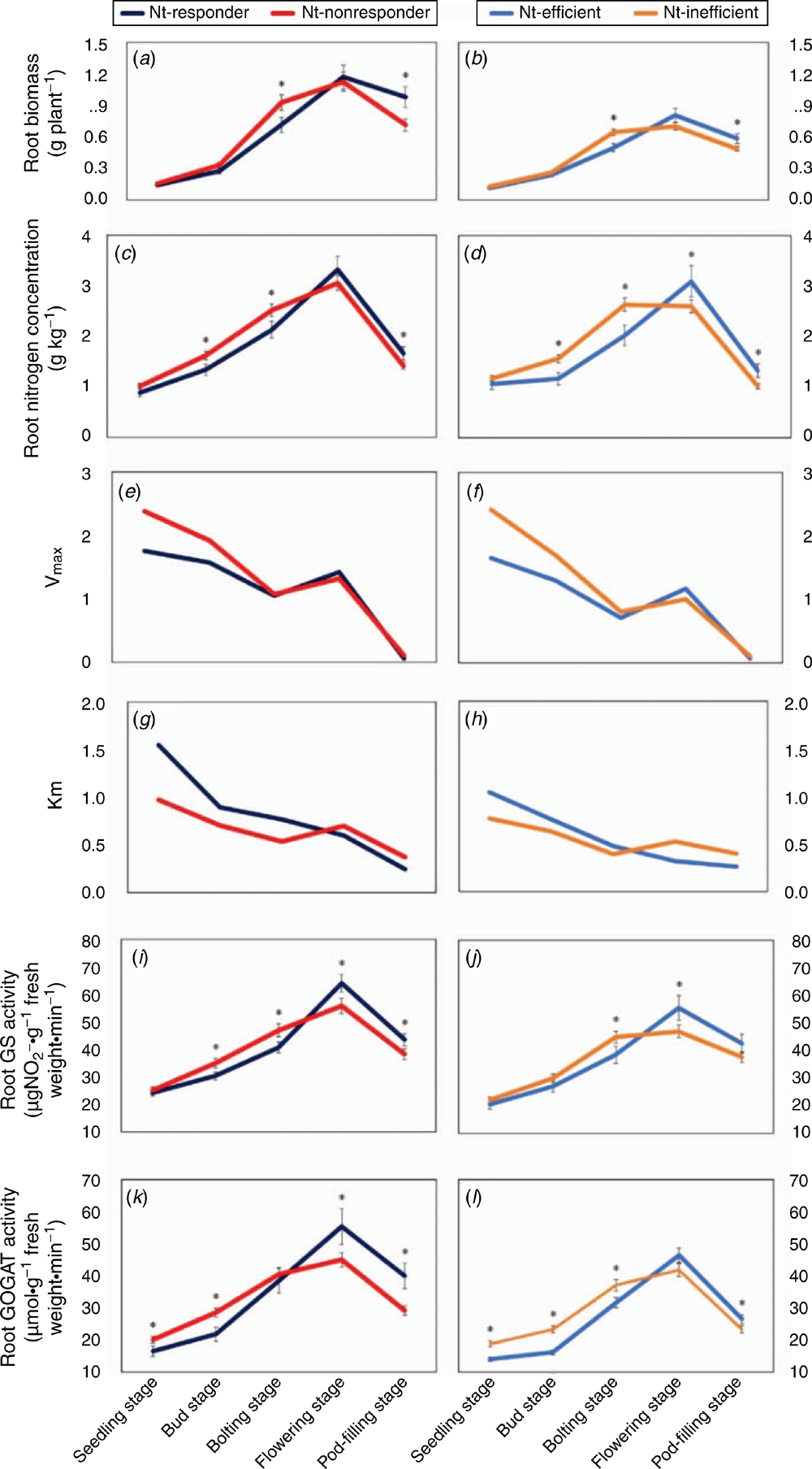
The genotypes with contrasting NUtE differed largely in morphological indices during the different growth stages (Fig. 3). The root morphological characteristics were higher for the low NUtE genotypes than for the high NUtE genotypes from the seedling to bolting stage. However, stronger root morphological indices of the high NUtE genotypes were exhibited at the flowering stage with 10.5% longer total root length and 25.7% larger root volume, and 15.0% greater root surface area for the high NUtE genotypes than for the low NUtE genotypes. Thereafter, these root morphological indices for the high NUtE genotypes remained significantly greater than for the low NUtE genotypes until the pod-filling stage.
In general, the root morphological traits of both the high and low NUtE genotypes increased in size rapidly from seedling stage and reached peak values at the flowering stage (254 cm root length, 2.53 cm3 of root volume, and 94.7 cm2 of root surface area per plant with 0.72 mg g–1 h–1 of root vigour). Following flowering, all the measured root characteristics showed a declining trend, with faster reduction in the low NUtE genotypes than in the high NUtE genotypes, except for root volume, which continued to increase but at a slower rate from the flowering to pod-filling stage (Fig. 3).
Root indices
The low NUtE genotypes under the hydroponic culture conditions displayed higher root indices than the high NUtE genotypes, up to bolting stage. At the flowering stage, the high NUtE genotypes exhibited 12.4% greater root biomass and 13.5% larger root N concentration, associated with the greater activity of GS (16.4%) and GOGAT (17.3%), higher Vmax and lower Km than the low NUtE genotypes. Our data indicated that flowering was the critical phase at which a reversal of trends was observed in the phenotypic traits between the NUtE genotypes, and these differences in the root indices were maintained from the flowering to pod-filling stage (Fig. 4).
As the plants progressed to the advanced stages, root biomass and N concentration, root GS and GOGAT activity of the contrasting NUtE genotypes increased rapidly from bolting and peaked at the flowering stage. Then all the growth parameters were declined through pod-filling (Fig. 4a–d, i–l). However, Vmax and Km displayed lower values from seedling to bolting stage, followed by a rising trend from bolting to flowering (except for Km in Nt-responder and Nt-efficient) and declining trend thereafter (Fig. 4e–h).
Correlations between NUtE and phenotypic traits
Based on the correlation matrix, we performed the principal component analysis (Fig. 5) and found that the two principal components explained over 90% of the total variation (92.02% and 91.56% in the high and low N groups, respectively). The first principal component (x-axis) is mainly spanned by Km on the positive side, balanced by root morphological traits, root biomass, root N concentration, root enzyme activity, shoot biomass, photosynthetic characteristics, leaf enzyme activity, seeds per silique, yield per plant and NUE. The smallest angles of NUtE with the root morphological traits, root biomass, root N concentration, Vmax, root enzyme activity, and shoot biomass, photosynthetic characteristics, leaf enzyme activity, seeds per silique, yield per plant and NUE indicated a positive relationship of NUtE with these traits, while the Km were negatively correlated with NUtE.
Discussion
In this study, we documented the reversal of growth habit that happened from flowering stage onwards between the high and low NUtE genotypes, with significant growth increases in both the root and shoot traits in the high NUtE genotypes. Specifically, at the flowering stage, compared with the low NUtE genotypes, the high NUtE genotypes exhibited greater root characteristics (12.9% greater root biomass and 13.6% higher root N concentration), accompanied by higher root GS and GOGAT activity and larger N uptake kinetic parameters, which led to a significantly higher potential rate of nitrate uptake to support shoot growth and yield formation. Above the ground, the plant shoots of the high NUtE genotypes also exhibited stronger growth performance, evidenced by an increased net photosynthetic rate by 12.4%, accompanied by 25.4% higher leaf N metabolism enzyme activity, 14.0% larger shoot biomass and 10.1% higher shoot N concentration (Fig. 2) at flowering and thereafter. This opposite growth habit was responsible for the higher carbohydrate supply to the developing pods, leading to ultimately higher yield and NUE in the high NUtE genotypes (Fig. 1).
Flowering is an indicator of the transition from the vegetative growth to the reproductive growth stage and is the critical phase of growth and development in the life cycle of a plant (Cai et al. 2018; Kirkegaard et al. 2018). Our results demonstrated stronger growth attributes of the high NUtE genotypes from flowering stage onwards, and the regulatory mechanisms can be explained in two aspects. From the root perspective, the greater activity of N metabolism enzymes for the high NUtE genotypes than for the low NUtE genotypes at post-flowering (Fig. 4i–l) indicated greater functions of the high NUtE genotypes in the N absorption and assimilation process to support plant growth (York et al. 2016; Guo and York 2019; Santiago-Arenas et al. 2019). The uptake kinetic parameters have been recognised as useful indices of the absorption of nutrients by the plant, and the high uptake rate of N may be closely related to its high maximal velocity (Vmax) and low affinity constant (Km) (Cao et al. 2015; Hao et al. 2015; York et al. 2016). Larger root uptake kinetic parameters observed in this study suggested that the high NUtE genotypes had a greater potential of N uptake and capability of metabolising the absorbed N to support the root and shoot growth. Another important contributor to N acquisition efficiency is the root system architecture, which has a great influence on dry matter accumulation and translocation during the whole growing season (Liu et al. 2018; Guo and York 2019; Santiago-Arenas et al. 2019). Our results revealed that higher root traits for the high NUtE genotypes than for the low NUtE genotypes (Figs 3, 4) were conducive to the better N uptake process of the high NUtE genotypes, thereby accumulating more N for plant growth during the pod-filling stage (York et al. 2016; Riar et al. 2020). Oilseed rape is a sink-limited crop, and its yield is best defined by the number of seeds per plant (Berry and Spink 2006). The significantly enhanced growth habit for the high NUtE genotypes compared with those for the low NUtE genotypes at flowering is beneficial to produce/maintain more viable seeds. In the present study, we also observed that at flowering and thereafter, the SPAD values and net photosynthetic rates were significantly greater in the high NUtE genotypes than in the low NUtE genotypes. This resulted in larger plants with a longer leaf area duration, providing greater capacity for photosynthesis to fill the seeds (Norton 2016), which ultimately improved yield and NUE (Fig. 1). In another study, the leaf GS and GOGAT activities of Triticum aestivum L. (wheat) were shown to be positively correlated with grain yield and NUE, which is of great importance for developing new crop cultivars with the potential to improve NUE and maintaining yield potential (Swarbreck et al. 2011). In this study, we found higher activity of the leaf GS and GOGAT in the high NUtE genotypes than in the low NUtE genotypes during the post-flowering stage, which provided capacity for their improved N assimilation and higher grain yield (Fig. 1).
Plants (both higher and lower plants) have usually evolved their own survival strategies to drive growth and development in complex environments where conditions change in space and time (Anderson et al. 2014; Dolferus 2014). Not surprisingly, adverse environmental phenomenon, such as mineral nutrition (e.g. deficiency, salinity), temperature (cold or hot stress), light (crowding, senescence, stay-green) and water availability (drought, flooding) presented challenges for the plant to achieve high yields (Abid et al. 2016; Ghate et al. 2017; Gu et al. 2017; Byun et al. 2018; Wang et al. 2019). Over time, crop plants have evolved adaptive strategies by maintaining adequate plasticity to take the advantage of various resource availability under environmental conditions (Ehrlén 2015; van Loon 2016). Our results indicate that flowering was the stage at which the contrasting NUtE genotypes showed opposite growth characteristics, and the high NUtE genotypes exhibited stronger growth attributes to better adapt to the environment and utilise resources to obtain greater yields and higher NUE. Yield formation is the result of cooperation between both the total shoot dry matter (source) and translocation of the photosynthates to the harvestable seeds (sink), as well as feedback from the sink strength to the source activity (production of assimilates) through an exerted requirement (Smith et al. 2018). Our present study demonstrated that high NUtE genotypes not only had a higher NUE but also produced greater seed yield, compared with the low NUtE genotypes (Fig. 1). Thus, the greater sink for high NUtE genotypes may be due to a stronger uptake of soil N in the post-flowering stage, thus driving a higher source strength (i.e. shoot biomass and leaf photosynthesis) (Figs 2a, b, 4e–h). From a long history of evolution, plant competition or size-asymmetric competition, which is defined as the ability of individuals to usurp resources or otherwise suppress their neighbours to adapt, happens when the resources required for plant growth are limited, and the plants that can access the resources will grow faster to produce seeds for further propagation (Aschehoug et al. 2016; Damgaard and Weiner 2017). An earlier study allowed us to divide the 50 tested accessions into high and low NUtE genotypes (He et al. 2017), and certain identified high NUtE genotypes could be considered as the ideotypes. In this study, we revealed an interesting strategy for the high NUtE genotypes as a true ideotype that can win the competition: lower growth attributes at the early vegetative stage but significantly higher growth activity at and after flowering (Figs 2–4). Wang et al. (2011) reported that the key stage for seed set is the beginning of flowering, and the survival of young pods is the result of competition for assimilates and nutrients. It appears that to adapt to the external environment and win the competition, the high NUtE genotypes have developed a strategy to invest in growth after flowering, rather than before flowering, thereby allowing sufficient assimilate production and nutrient uptake to fill the developing seeds.
Developing shoots and roots are the main N sinks in the vegetative stage, while developing seeds are the major N assimilate-importing sinks during the post-flowering stage (Tegeder and Masclaux-Daubresse 2018). Grain N originated from both the remobilised N from the vegetative tissues and current N uptake from the soil at post-flowering (Juraniec et al. 2017). Interestingly, significant negative correlations between the contributions of these two sources to seed N have been reported, which may be explained by the presence of a limited amount of available N that is available to be absorbed either before or after flowering (Gaju et al. 2014). Uptake of N by roots at pre-flowering is temporally stored in the vegetative organs (root, stem and leaf). During seed filling and plant senescence process, the preserved vegetative N is remobilised into the seeds (Gaju et al. 2014). Based on this, seed N is largely influenced by both the amount of N taken up post-flowering and remobilisation of N originating from the pre-flowering N uptake. Our previous research found that the N uptake post-flowering rather than N remobilisation was responsible for genotypic variation in seed N accumulation, resulting in the different NUtE among the contrasting NUtE genotypes (data not shown), although the post-flowering N uptake by roots represented only a small portion of the final seed N (Ulas et al. 2013; Taulemesse et al. 2015). In the current study, the greater root indices for the high NUtE genotypes than for the low NUtE genotypes at post-flowering stage indicated that the high NUtE genotypes with better root systems will absorb more N from external environment for growth of shoot parts, supporting for higher seed yield and NUtE (Fig. 1). Correspondingly, the high NUtE genotypes displayed an advantage in higher post-flowering uptake of N, with higher SPAD values and higher net photosynthetic rate than the low NUtE genotypes (Fig. 2e–h). This supported delaying leaf senescence process or better maintaining stay-green trait, thereby better retaining capability for nutrient absorption and thus maintaining a balance between supply and demand after flowering (Jordan et al. 2012; Lammerts van Bueren and Struik 2017). This phenomenon is consistent with the results of shoot growth potential as the driving force for N uptake by maize, indicating that the stay-green phenotype is beneficial to N-uptake capacity, and was positively related to NUtE (Peng et al. 2010). Therefore, it can be considered that the high NUtE genotypes could absorb more N during post-flowering stage, maintain higher leaf photosynthesis which would provide more carbohydrates for seed growth and development.
Conclusion
Our study discovered that flowering was the critical stage in distinguishing high from low NUtE oilseed rape genotypes. In order to promote efficient utilisation of N to improve the sustainability of agroecosystem without hindering production levels, it is important to assess genotypes that are more suitable for their natural environment and have stronger growth attributes at flowering and post-flowering. The higher post-flowering leaf photosynthetic parameters and the larger root volume and surface area are simple and reliable indicators in selection of higher NUtE oilseed rape plants. Therefore, as determined in this study, it is recommended that crop physiologists and plant breeders focus their selection on root and shoot phenotypic traits from flowering stage onwards to improve yield with high NUE of oilseed rape varieties.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This study was supported by grants from the National Key R&D Program of China (2018YFD0200907), the grants from the Special Fund for Agro-scientific Research in the Public Interest (201503124), the Innovative Research Team Plan of the Agriculture Ministry. The senior author was sponsored by the MOE-AAFC PhD Training Program. This is a joint contribution of North-west A and F University and Agriculture and Agri-Food Canada (AAFC). AAFC-ORDC contribution no. 20-111.
References
Abid M, Tian ZW, Ata-Ul-Karim ST, Cui YK, Liu Y, Zahoor R, Jiang D, Dai TB (2016) Nitrogen nutrition improves the potential of wheat (Triticum aestivum L.) to alleviate the effects of drought stress during vegetative growth periods. Frontiers in Plant Science 7, 981| Nitrogen nutrition improves the potential of wheat (Triticum aestivum L.) to alleviate the effects of drought stress during vegetative growth periods.Crossref | GoogleScholarGoogle Scholar | 27446197PubMed |
Albornoz F (2016) Crop responses to nitrogen overfertilization: A review. Scientia Horticulturae 205, 79–83.
| Crop responses to nitrogen overfertilization: A review.Crossref | GoogleScholarGoogle Scholar |
Anderson JT, Wagner MR, Rushworth CA, Prasad KV, Mitchell-Olds T (2014) The evolution of quantitative traits in complex environments. Heredity 112, 4–12.
| The evolution of quantitative traits in complex environments.Crossref | GoogleScholarGoogle Scholar | 23612691PubMed |
Aschehoug ET, Brooker R, Atwater DZ, Maron JL, Callaway RM (2016) The mechanisms and consequences of interspecific competition among plants. Annual Review of Ecology, Evolution, and Systematics 47, 263–281.
| The mechanisms and consequences of interspecific competition among plants.Crossref | GoogleScholarGoogle Scholar |
Berry PM, Spink JH (2006) A physiological analysis of oilseed rape yields: Past and future. The Journal of Agricultural Science 144, 381
| A physiological analysis of oilseed rape yields: Past and future.Crossref | GoogleScholarGoogle Scholar |
Bouchet AS, Laperche A, Bissuel-Belaygue C, Snowdon R, Nesi N, Stahl A (2016) Nitrogen use efficiency in rapeseed. A review. Agronomy for Sustainable Development 36, 38
| Nitrogen use efficiency in rapeseed. A review.Crossref | GoogleScholarGoogle Scholar |
Byun MY, Cui LH, Lee J, Park H, Lee A, Kim WT, Lee H (2018) Identification of rice genes associated with enhanced cold tolerance by comparative transcriptome analysis with two transgenic rice plants overexpressing DaCBF4 or DaCBF7, isolated from antarctic flowering plant Deschampsia antarctica. Frontiers in Plant Science 9, 601
| Identification of rice genes associated with enhanced cold tolerance by comparative transcriptome analysis with two transgenic rice plants overexpressing DaCBF4 or DaCBF7, isolated from antarctic flowering plant Deschampsia antarctica.Crossref | GoogleScholarGoogle Scholar | 29774046PubMed |
Cai Y, Chen L, Liu X, Guo C, Sun S, Wu C, Jiang B, Han T, Hou W (2018) CRISPR/Cas9-mediated targeted mutagenesis of GmFT2a delays flowering time in soya bean. Plant Biotechnology Journal 16, 176–185.
| CRISPR/Cas9-mediated targeted mutagenesis of GmFT2a delays flowering time in soya bean.Crossref | GoogleScholarGoogle Scholar | 28509421PubMed |
Cao JY, Zheng XX, Nie Y (2015) Research on the design of CORDIC vector magnitude calculator by using model-based design. International Journal of Signal Processing, Image Processing and Pattern Recognition 8, 247–258.
| Research on the design of CORDIC vector magnitude calculator by using model-based design.Crossref | GoogleScholarGoogle Scholar |
Damgaard C, Weiner J (2017) It’s about time: a critique of macroecological inferences concerning plant competition. Trends in Ecology & Evolution 32, 86–87.
| It’s about time: a critique of macroecological inferences concerning plant competition.Crossref | GoogleScholarGoogle Scholar |
Dolferus R (2014) To grow or not to grow: a stressful decision for plants. Plant Science 229, 247–261.
| To grow or not to grow: a stressful decision for plants.Crossref | GoogleScholarGoogle Scholar | 25443851PubMed |
Ehrlén J (2015) Selection on flowering time in a life-cycle context. Oikos 124, 92–101.
| Selection on flowering time in a life-cycle context.Crossref | GoogleScholarGoogle Scholar |
Gaju O, Allard V, Martre P, Le Gouis J, Moreau D, Bogard M, Hubbart S, Foulkes MJ (2014) Nitrogen partitioning and remobilization in relation to leaf senescence, grain yield and grain nitrogen concentration in wheat cultivars. Field Crops Research 155, 213–223.
| Nitrogen partitioning and remobilization in relation to leaf senescence, grain yield and grain nitrogen concentration in wheat cultivars.Crossref | GoogleScholarGoogle Scholar |
Ghate T, Deshpande S, Bhargava S (2017) Accumulation of stem sugar and its remobilisation in response to drought stress in a sweet sorghum genotype and its near-isogenic lines carrying different stay-green loci. Plant Biology 19, 396–405.
| Accumulation of stem sugar and its remobilisation in response to drought stress in a sweet sorghum genotype and its near-isogenic lines carrying different stay-green loci.Crossref | GoogleScholarGoogle Scholar | 28032438PubMed |
Gu JF, Chen Y, Zhang H, Li ZK, Zhou Q, Yu C, Kong XS, Liu LJ, Wang ZQ, Yang JC (2017) Canopy light and nitrogen distributions are related to grain yield and nitrogen use efficiency in rice. Field Crops Research 206, 74–85.
| Canopy light and nitrogen distributions are related to grain yield and nitrogen use efficiency in rice.Crossref | GoogleScholarGoogle Scholar |
Guo H, York LM (2019) Maize with fewer nodal roots allocates mass to more lateral and deep roots that improve nitrogen uptake and shoot growth. Journal of Experimental Botany 70, 5299–5309.
| Maize with fewer nodal roots allocates mass to more lateral and deep roots that improve nitrogen uptake and shoot growth.Crossref | GoogleScholarGoogle Scholar | 31145788PubMed |
Guo X, He HY, An R, Zhang YY, Yang R, Cao LQ, Wu XM, Chen BY, Tian H, Gao YJ (2019) Nitrogen use-inefficient oilseed rape genotypes exhibit stronger growth potency during the vegetative growth stage. Acta Physiologiae Plantarum 41, 175
| Nitrogen use-inefficient oilseed rape genotypes exhibit stronger growth potency during the vegetative growth stage.Crossref | GoogleScholarGoogle Scholar |
Hajari E, Snyman SJ, Watt MP (2014) Inorganic nitrogen uptake kinetics of sugarcane (Saccharum spp.) varieties under in vitro conditions with varying N supply. Plant Cell, Tissue and Organ Culture 117, 361–371.
| Inorganic nitrogen uptake kinetics of sugarcane (Saccharum spp.) varieties under in vitro conditions with varying N supply.Crossref | GoogleScholarGoogle Scholar |
Hao YS, Lei J, Wang QL, Wu LS, Jiang CC (2015) Two typical K-efficiency cotton genotypes differ in potassium absorption kinetic parameters and patterns. Acta Agriculturæ Scandinavica. Section B, Soil and Plant Science 65, 45–53.
| Two typical K-efficiency cotton genotypes differ in potassium absorption kinetic parameters and patterns.Crossref | GoogleScholarGoogle Scholar |
He H, Yang R, Li Y, Ma A, Cao L, Wu X, Chen B, Tian H, Gao Y (2017) Genotypic variation in nitrogen utilization efficiency of oilseed rape (Brassica napus) under contrasting N supply in pot and field experiments. Frontiers in Plant Science 8, 1825
| Genotypic variation in nitrogen utilization efficiency of oilseed rape (Brassica napus) under contrasting N supply in pot and field experiments.Crossref | GoogleScholarGoogle Scholar | 29163565PubMed |
Jordan DR, Hunt CH, Cruickshank AW, Borrell AK, Henzell RG (2012) The relationship between the stay-green trait and grain yield in elite sorghum hybrids grown in a range of environments. Crop Science 52, 1153–1161.
| The relationship between the stay-green trait and grain yield in elite sorghum hybrids grown in a range of environments.Crossref | GoogleScholarGoogle Scholar |
Juraniec M, Hermans C, Salis P, Geelen D, Verbruggen N (2017) Impact of post-flowering nitrate availability on nitrogen remobilization in hydroponically grown durum wheat. Journal of Plant Nutrition and Soil Science 180, 273–278.
| Impact of post-flowering nitrate availability on nitrogen remobilization in hydroponically grown durum wheat.Crossref | GoogleScholarGoogle Scholar |
Kirkegaard JA, Lilley JM, Brill RD, Ware AH, Walela CK (2018) The critical period for yield and quality determination in canola (Brassica napus L.). Field Crops Research 222, 180–188.
| The critical period for yield and quality determination in canola (Brassica napus L.).Crossref | GoogleScholarGoogle Scholar |
Koeslin-Findeklee F, Meyer A, Girke A, Beckmann K, Horst WJ (2014) The superior nitrogen efficiency of winter oilseed rape (Brassica napus L.) hybrids is not related to delayed nitrogen starvation-induced leaf senescence. Plant and Soil 384, 347–362.
| The superior nitrogen efficiency of winter oilseed rape (Brassica napus L.) hybrids is not related to delayed nitrogen starvation-induced leaf senescence.Crossref | GoogleScholarGoogle Scholar |
Lammerts van Bueren ET, Struik PC (2017) Diverse concepts of breeding for nitrogen use efficiency. A review. Agronomy for Sustainable Development 37, 50
| Diverse concepts of breeding for nitrogen use efficiency. A review.Crossref | GoogleScholarGoogle Scholar |
Liu X, Vitousek P, Chang Y, Zhang W, Matson P, Zhang F (2016) Evidence for a historic change occurring in China. Environmental Science & Technology 50, 505–506.
| Evidence for a historic change occurring in China.Crossref | GoogleScholarGoogle Scholar |
Liu H, Wang W, He A, Nie L (2018) Correlation of leaf and root senescence during ripening in dry seeded and transplanted rice. Rice Science 25, 279–285.
| Correlation of leaf and root senescence during ripening in dry seeded and transplanted rice.Crossref | GoogleScholarGoogle Scholar |
Ma BL, Zheng ZM, Morrison MJ (2017) Does increasing plant population density alter sugar yield in high stalk-sugar maize hybrids? Crop and Pasture Science 68, 1
| Does increasing plant population density alter sugar yield in high stalk-sugar maize hybrids?Crossref | GoogleScholarGoogle Scholar |
Moll RH, Kamprath EJ, Jackson WA (1982) Analysis and interpretation of factors which contribute to efficiency of nitrogen utilization. Agronomy Journal 74, 562–564.
| Analysis and interpretation of factors which contribute to efficiency of nitrogen utilization.Crossref | GoogleScholarGoogle Scholar |
Norton RM (2016) Nitrogen management to optimise canola production in Australia. Crop and Pasture Science 67, 419
| Nitrogen management to optimise canola production in Australia.Crossref | GoogleScholarGoogle Scholar |
Oldroyd GE, Dixon R (2014) Biotechnological solutions to the nitrogen problem. Current Opinion in Biotechnology 26, 19–24.
| Biotechnological solutions to the nitrogen problem.Crossref | GoogleScholarGoogle Scholar | 24679253PubMed |
Peng YF, Niu JF, Peng ZP, Zhang FS, Li CJ (2010) Shoot growth potential drives N uptake in maize plants and correlates with root growth in the soil. Field Crops Research 115, 85–93.
| Shoot growth potential drives N uptake in maize plants and correlates with root growth in the soil.Crossref | GoogleScholarGoogle Scholar |
Rathke G, Behrens T, Diepenbrock W (2006) Integrated nitrogen management strategies to improve seed yield, oil content and nitrogen efficiency of winter oilseed rape (Brassica napus L.): A review. Agriculture, Ecosystems & Environment 117, 80–108.
| Integrated nitrogen management strategies to improve seed yield, oil content and nitrogen efficiency of winter oilseed rape (Brassica napus L.): A review.Crossref | GoogleScholarGoogle Scholar |
Raza MA, Feng LY, Khalid MHB, Iqbal A, Meraj TA, Hassan MJ, Hassan S, Chen YK, Feng Y, Yang WY (2019) Optimum leaf excision increases the biomass accumulation and seed yield of maize plants under different planting patterns. Annals of Applied Biology 175, 54–68.
| Optimum leaf excision increases the biomass accumulation and seed yield of maize plants under different planting patterns.Crossref | GoogleScholarGoogle Scholar |
Riar A, Gill G, McDonald G (2016) Effect of post-sowing nitrogen management on co-limitation of nitrogen and water in canola and mustard. Field Crops Research 198, 23–31.
| Effect of post-sowing nitrogen management on co-limitation of nitrogen and water in canola and mustard.Crossref | GoogleScholarGoogle Scholar |
Riar A, Gill G, McDonald G (2017) Effect of post-sowing nitrogen management on canola and mustard: I. yield responses. Agronomy Journal 109, 2266–2277.
| Effect of post-sowing nitrogen management on canola and mustard: I. yield responses.Crossref | GoogleScholarGoogle Scholar |
Riar A, Gill G, McDonald G (2020) Different post-sowing nitrogen management approaches required to improve nitrogen and water use efficiency of canola and mustard. Frontiers in Plant Science 11, 1111
| Different post-sowing nitrogen management approaches required to improve nitrogen and water use efficiency of canola and mustard.Crossref | GoogleScholarGoogle Scholar | 32793266PubMed |
Santiago-Arenas R, Hadi SN, Fanshuri BA, Ullah H, Datta A (2019) Effect of nitrogen fertiliser and cultivation method on root systems of rice subjected to alternate wetting and drying irrigation. Annals of Applied Biology 175, 388–399.
| Effect of nitrogen fertiliser and cultivation method on root systems of rice subjected to alternate wetting and drying irrigation.Crossref | GoogleScholarGoogle Scholar |
Shah JM, Bukhari SAH, Zeng JB, Quan XY, Ali E, Muhammad N, Zhang GP (2017) Nitrogen (N) metabolism related enzyme activities, cell ultrastructure and nutrient contents as affected by N level and barley genotype. Journal of Integrative Agriculture 16, 190–198.
| Nitrogen (N) metabolism related enzyme activities, cell ultrastructure and nutrient contents as affected by N level and barley genotype.Crossref | GoogleScholarGoogle Scholar |
Shi J, Li R, Zou J, Long Y, Meng J (2011) A dynamic and complex network regulates the heterosis of yield-correlated traits in rapeseed (Brassica napus L.). PLoS One 6, e21645
| A dynamic and complex network regulates the heterosis of yield-correlated traits in rapeseed (Brassica napus L.).Crossref | GoogleScholarGoogle Scholar | 22216358PubMed |
Shi J, Zhan J, Yang Y, Ye J, Huang S, Li R, Wang X, Liu G, Wang H (2015) Linkage and regional association analysis reveal two new tightly-linked major-QTLs for pod number and seed number per pod in rapeseed (Brassica napus L.). Scientific Reports 5, 14481
| Linkage and regional association analysis reveal two new tightly-linked major-QTLs for pod number and seed number per pod in rapeseed (Brassica napus L.).Crossref | GoogleScholarGoogle Scholar | 26434411PubMed |
Smith MR, Rao IM, Merchant A (2018) Source-sink relationships in crop plants and their influence on yield development and nutritional quality. Frontiers in Plant Science 9, 1889
| Source-sink relationships in crop plants and their influence on yield development and nutritional quality.Crossref | GoogleScholarGoogle Scholar | 30619435PubMed |
Stahl A, Vollrath P, Samans B, Frisch M, Wittkop B, Snowdon RJ (2019) Effect of breeding on nitrogen use efficiency-associated traits in oilseed rape. Journal of Experimental Botany 70, 1969–1986.
| Effect of breeding on nitrogen use efficiency-associated traits in oilseed rape.Crossref | GoogleScholarGoogle Scholar | 30753580PubMed |
Stevens CJ (2019) Nitrogen in the environment. Science 363, 578–580.
| Nitrogen in the environment.Crossref | GoogleScholarGoogle Scholar | 30733401PubMed |
Svečnjak Z, Rengel Z (2006) Nitrogen utilization efficiency in canola cultivars at grain harvest. Plant and Soil 283, 299–307.
| Nitrogen utilization efficiency in canola cultivars at grain harvest.Crossref | GoogleScholarGoogle Scholar |
Swarbreck SM, Defoin-Platel M, Hindle M, Saqi M, Habash DZ (2011) New perspectives on glutamine synthetase in grasses. Journal of Experimental Botany 62, 1511–1522.
| New perspectives on glutamine synthetase in grasses.Crossref | GoogleScholarGoogle Scholar | 21172814PubMed |
Sylvester-Bradley R, Kindred DR (2009) Analysing nitrogen responses of cereals to prioritize routes to the improvement of nitrogen use efficiency. Journal of Experimental Botany 60, 1939–1951.
| Analysing nitrogen responses of cereals to prioritize routes to the improvement of nitrogen use efficiency.Crossref | GoogleScholarGoogle Scholar | 19395389PubMed |
Taulemesse F, Gouis JL, Gouache D, Gibon Y, Allard V (2015) Post-flowering nitrate uptake in wheat is controlled by N status at flowering, with a putative major role of root nitrate transporter NRT2.1. PLoS One 10, e0120291
| Post-flowering nitrate uptake in wheat is controlled by N status at flowering, with a putative major role of root nitrate transporter NRT2.1.Crossref | GoogleScholarGoogle Scholar | 25798624PubMed |
Tegeder M, Masclaux-Daubresse C (2018) Source and sink mechanisms of nitrogen transport and use. New Phytologist 217, 35–53.
| Source and sink mechanisms of nitrogen transport and use.Crossref | GoogleScholarGoogle Scholar |
Ulas A, Schulte auf’m Erley G, Kamh M, Wiesler F, Horst WJ (2012) Root-growth characteristics contributing to genotypic variation in nitrogen efficiency of oilseed rape. Journal of Plant Nutrition and Soil Science 175, 489–498.
| Root-growth characteristics contributing to genotypic variation in nitrogen efficiency of oilseed rape.Crossref | GoogleScholarGoogle Scholar |
Ulas A, Behrens T, Wiesler F, Horst WJ, Erley GSA (2013) Does genotypic variation in nitrogen remobilisation efficiency contribute to nitrogen efficiency of winter oilseed-rape cultivars (Brassica napus L.)? Plant and Soil 371, 463–471.
| Does genotypic variation in nitrogen remobilisation efficiency contribute to nitrogen efficiency of winter oilseed-rape cultivars (Brassica napus L.)?Crossref | GoogleScholarGoogle Scholar |
van Loon LC (2016) The intelligent behavior of plants. Trends in Plant Science 21, 286–294.
| The intelligent behavior of plants.Crossref | GoogleScholarGoogle Scholar | 26690331PubMed |
Wagner C, Bonte A, Bruhl L, Niehaus K, Bednarz H, Matthaus B (2018) Micro-organisms growing on rapeseed during storage affect the profile of volatile compounds of virgin rapeseed oil. Journal of the Science of Food and Agriculture 98, 2147–2155.
| Micro-organisms growing on rapeseed during storage affect the profile of volatile compounds of virgin rapeseed oil.Crossref | GoogleScholarGoogle Scholar | 28960362PubMed |
Wang XJ, Mathieu A, Cournede PH, Allirand JM, Jullien A, Reffye PD, Zhang BG (2011) Variability and regulation of the number of ovules, seeds and pods according to assimilate availability in winter oilseed rape (Brassica napus L.). Field Crops Research 122, 60–69.
| Variability and regulation of the number of ovules, seeds and pods according to assimilate availability in winter oilseed rape (Brassica napus L.).Crossref | GoogleScholarGoogle Scholar |
Wang JF, Tian P, Christensen MJ, Zhang XX, Li CJ, Nan ZB (2019) Effect of Epichloë gansuensis endophyte on the activity of enzymes of nitrogen metabolism, nitrogen use efficiency and photosynthetic ability of Achnatherum inebrians under various NaCl concentrations. Plant and Soil 435, 57–68.
| Effect of Epichloë gansuensis endophyte on the activity of enzymes of nitrogen metabolism, nitrogen use efficiency and photosynthetic ability of Achnatherum inebrians under various NaCl concentrations.Crossref | GoogleScholarGoogle Scholar |
York LM, Silberbush M, Lynch JP (2016) Spatiotemporal variation of nitrate uptake kinetics within the maize (Zea mays L.) root system is associated with greater nitrate uptake and interactions with architectural phenes. Journal of Experimental Botany 67, 3763–3775.
| Spatiotemporal variation of nitrate uptake kinetics within the maize (Zea mays L.) root system is associated with greater nitrate uptake and interactions with architectural phenes.Crossref | GoogleScholarGoogle Scholar | 27037741PubMed |
Yu J, Zhen X, Li X, Li N, Xu F (2019) Increased autophagy of rice can increase yield and nitrogen use efficiency (NUE). Frontiers in Plant Science 10, 584
| Increased autophagy of rice can increase yield and nitrogen use efficiency (NUE).Crossref | GoogleScholarGoogle Scholar | 31134120PubMed |
Zhang X, Davidson EA, Mauzerall DL, Searchinger TD, Dumas P, Shen Y (2015) Managing nitrogen for sustainable development. Nature 528, 51–59.
| Managing nitrogen for sustainable development.Crossref | GoogleScholarGoogle Scholar | 26595273PubMed |