Inferring vascular architecture of the wheat spikelet based on resource allocation in the branched headt (bht-A1) near isogenic lines
Gizaw M. Wolde A B D and Thorsten Schnurbusch
A B D and Thorsten Schnurbusch  A C D
A C D
A Independent HEISENBERG-Research Group Plant Architecture, Leibniz Institute of Plant Genetics and Crop Plant Research (IPK), Corrensstr. 3, OT Gatersleben, D-06466 Seeland, Germany.
B Present address: Department of Plant Sciences, University of California, Davis, CA 95616, USA.
C Faculty of Natural Sciences III, Institute of Agricultural and Nutritional Sciences, Martin Luther University Halle-Wittenberg, 06120 Halle, Germany.
D Corresponding authors. Emails: wolde@ipk-gatersleben.de; thor@ipk-gatersleben.de
Functional Plant Biology 46(11) 1023-1035 https://doi.org/10.1071/FP19041
Submitted: 12 June 2018 Accepted: 21 June 2019 Published: 23 September 2019
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
Substantial genetic and physiological efforts were made to understand the causal factors of floral abortion and grain filling problem in wheat. However, the vascular architecture during wheat spikelet development is surprisingly under-researched. We used the branched headt near-isogenic lines, FL-bht-A1-NILs, to visualise the dynamics of spikelet fertility and dry matter accumulation in spikelets sharing the same rachis node (henceforth Primary Spikelet, PSt, and Secondary Spikelet, SSt). The experiment was conducted after grouping FL-bht-A1-NILs into two groups, where tillers were consistently removed from one group. Our results show differential spikelet fertility and dry matter accumulation between the PSt and SSt, but also showed a concomitant improvement after de-tillering. This suggests a tight regulation of assimilate supply and dry matter accumulation in wheat spikelets. Since PSt and SSt share the same rachis node, the main vascular bundle in the rachis/rachilla is expected to bifurcate to connect each spikelet/floret to the vascular system. We postulate that the vascular structure in the wheat spikelet might even follow Murray’s law, where the wide conduits assigned at the base of the spikelet feed the narrower conduits of the distal florets. We discuss our results based on the two modalities of the vascular network systems in plants.
Additional keywords: assimilate partitioning, bifurcation, modeling, aorta, primary spikelet, secondary spikelet, spikelet fertility, Triticum durum, vascular bundle.
Introduction
Wheat (Triticum spp.) is the most widely grown crop in the world as a direct source for human calorie consumption (Shiferaw et al. 2013; Shewry and Hey 2015). The source of the calories – the grain – is produced on a sessile specialised branch, called the spikelet, which contains a collection of grain-producing florets that are arranged on the main axis of the spikelet called the rachilla. Grain number in wheat is therefore strongly influenced by the number and arrangement of spikelets (Boden et al. 2015; Dobrovolskaya et al. 2015; Poursarebani et al. 2015; Dixon et al. 2018; Wolde et al. 2019a), as well as the fertility of the spikelet (Guo et al. 2017, 2018; Prieto et al. 2018; Sakuma et al. 2019). Hence, the simultaneous increase in spikelet number and spikelet fertility is important for increasing grain yield in wheat. Despite the fact that more floret primordium are developed indeterminately in the wheat spikelet (a maximum of 8–12 floret primordia initials from each mid-central spikelet), less than half of these are fertile at anthesis due to floret abortion and/or insufficient development (Kirby 1974, 1988; Whingwiri and Stern 1982; Kirby and Appleyard 1984; McMaster 1997; Guo et al. 2017). In this regard, three hypotheses were previously suggested as a probable cause of floret abortion in wheat (Whingwiri and Stern 1982; McMaster 1997): (i) inadequate supply of mineral nutrients, water, and carbohydrates; (ii) hormonal imbalances; and (iii) a problem related to vascular development.
Since degeneration of floral primordia in wheat proceeds in a basipetal order, i.e. starts from the apex and proceeds to the base of the rachilla, only few of those florets at the base of the rachilla reach anthesis and set grains. In line with this, Hanif and Langer (1972) suggested that further development of the distal floral primordia is dependent on the development and anatomical structure of the vascular system. This suggests that a detailed understanding of the vascular bundle development and connectivity is essential to better understand floret abortion in wheat. Surprisingly, very few studies have attempted to explore and formulate the vascular structure of the wheat spikelet.
Whingwiri and Stern studied the vascular system in the rachis of the wheat spike and observed a 1 : 1 relation between spikelet number and the number of central vascular bundles at the base of the rachis, suggesting that a single vascular bundle is assigned to each spikelet (Whingwiri et al. 1981). The authors further observed branching of the main vascular bundle in the rachis and suggested that the vascular bundle connection between the rachis and the floret occurs after the initiation of the terminal spikelet (Whingwiri et al. 1981; Whingwiri and Stern 1982). Further, these researchers have also observed the decline in the number and size of vascular bundles acropetally, i.e. from the base to the apex of the rachis. However, apart from the presence of more vascular bundles in the more basal sections of the spike, it is not yet clear whether basal spikelets do benefit sufficiently from the seemingly available assimilate supply route.
Kirby also studied the vascular system of the barley spike and observed that the differentiation of phloem and xylem elements first starts in the lower mid part of the spike and then proceeds in a proximo-distal manner along the spike (Kirby and Rymer 1974). This suggests that the vascular bundles of spikelets in central spike positions are established first compared with those in the apical and basal spikelets. Thus, despite the presence of more vascular bundles in the basal section of the spike, and the proximity of the basal spikelets to the main assimilate route in the peduncle, connection of the basal spikelets to vascular system might occur later as the connection of the spikelets to the vascular system proceeds from the centre to the base (Kirby and Rymer 1974). This might also explain the 1 : 1 correlation between spikelet number and central vascular bundles at the base of the wheat spike observed by Whingwiri et al. (1981).
Before the observations of Whingwiri et al. (1981), Hanif and Langer (1972) also suggested that florets closer to the rachis node, i.e. the basal three florets in the spikelets, were directly supplied by the principal vascular bundles of the rachilla, while the distal florets lack a direct connection to the vascular bundle (Hanif and Langer 1972). This suggests that the distal florets might be subject to some mechanical modifications in the vascular connectivity and assimilate translocation. Therefore, distal florets might not have an equal chance of accessing assimilates from the source.
In line with the fact that only those few florets close to the main vascular system of the rachis are fertile and set grains, Bremner and Rawson (1978) also suggested the physical distance of the distal floret from the rachis as a cause for abortion. By changing the dynamics of assimilate supply through de-tillering, Guo and Schnurbusch (2015) also found a significant delay in the degeneration of the floral primordia resulting in increased floret fertility. Hence, such a sequential pattern of floret abortion in a wheat spikelet (González-Navarro et al. 2015; Guo and Schnurbusch 2015) suggests certain regulation of assimilate loading to the florets.
Although Kirby and Rymer (1974) and Whingwiri et al. (1981) independently suggested branching of the main vascular bundles in barley as well as wheat spike rachis (Kirby and Rymer 1974; Whingwiri et al. 1981), it is not yet clear as to how the assimilates are allocated to each of the branch units, i.e. the spikelets and florets. Hence, knowledge of the development of the vascular tissues, especially the main conducting elements i.e. the sieve tube elements, and their architectural configurations in wheat spikelet/floret is very essential. Nevertheless, our knowledge and even theoretical working models in this regard is very limiting in wheat.
Based on a literature review, two contrasting models – the ‘pipe’ (Shinozaki et al. 1964) and ‘aorta’ models (McCulloh et al. 2003) – were suggested as working models for the architectural configuration of the vascular network system in plants. The main differences between the aorta and the pipe model is that the aorta transport networks are composed of several branching tube networks; while the pipe model is composed of multiple parallel or diverging vessels or tubes (McCulloh et al. 2003; McCulloh and Sperry 2006; Seki et al. 2015). Hence, unlike the pipe model, bifurcation of the mother tube into daughter tubes is a typical characteristic of the aorta model (Murray 1926; McCulloh et al. 2003; Seki et al. 2015). Thus, the aorta model states that across branching generations, the sum of the conduit radii (r) cubed at every level (Σr3) is proportional to the volume flow rate at any point (Murray 1926; McCulloh et al. 2004; McCulloh and Sperry 2005). This principle is also independent of the vessel length and branching pattern. Hence, Σr3 is conserved at any cross-section in the system (McCulloh et al. 2004). In contrast, each tube in the pipe model is considered to be independent and thus, no exchange of solutions among the pipes i.e. neither of the pipes bifurcates nor tapers at any location (Shinozaki et al. 1964; West et al. 1997; West et al. 1999; Seki et al. 2015).
Even though the aorta model was initially proposed based on the mammalian system describing the functional relationship between vessel radii and the volumetric flow during the branching of vasculature of the mammalian circulatory system (Murray 1926; Sherman 1981), both models, i.e. the aorta and pipe models, were also tested in plants (West et al. 1999; McCulloh et al. 2003, 2004; McCulloh and Sperry 2005; Savage et al. 2010; Price et al. 2013) including the critics on the model (Kozłowski and Konarzewski 2004, 2005). So far, the transport of water in plants, i.e. in the xylem and the branching order in the leaf vein geometries, follow the aorta model or Murray’s law (McCulloh et al. 2003; Price et al. 2013). Different studies have also suggested that the optimal supply and resource delivery in the external branching network might have also coevolved with internal vascular network system (West et al. 1997; West et al. 1999; Savage et al. 2010).
Unlike the mammalian aorta model, where Σr3 is conserved at any cross-section, diminishing of Σr3 has been reported in a plant system to the decline in volume flow rate (McCulloh et al. 2004; McCulloh and Sperry 2005). Also, the law still proved to be true even under such diminishing Σr3, provided that the reduction is in a direct proportion to the decline in volume flow rate (McCulloh et al. 2004; McCulloh and Sperry 2005). Furthermore, Murray’s law takes into account the cost of building, maintaining and transporting fluid, as well as the maximisation of the hydraulic conductance of the blood vessels across the branching points that also fit to the xylem system of seed plants (McCulloh et al. 2003; McCulloh and Sperry 2006; Price et al. 2013). Recently, Seki et al. (2015) mathematically predicted that phloem sucrose transport in the rice panicle is also likely to obey the aorta model (Seki et al. 2015).
Regardless of its importance, little is known about how sieve tube elements are distributed in wheat spikelets and how a single tube bifurcates at each junction of the branches. Therefore, developing the appropriate genetic material and the mechanistic working model are important to further study the phloem anatomy and structure in wheat spikelets. Previously, we developed FL-bht-A1-NILs, which are typically characterised by the formation of supernumerary secondary spikelets (SSt) sharing the same rachis node with the primary spikelets (PSt) (Wolde et al. 2019a). The unique spikelet arrangement in FL-bht-A1-NILs, i.e. PSt and SSt, which are connected to a similar assimilate outlet (the rachis node) provides a compelling opportunity to further study source–sink relationship, spikelet fertility, and the dynamics of assimilate partitioning and dry matter accumulation between wheat spikelets. In this study, we have systematically manipulated the dynamics of assimilate supply through de-tillering and investigated spikelet fertility and assimilate partitioning and dry matter accumulation between the PSt and SSt. Even though the PSt and SSt share the same rachis node, the differential spikelet fertility index and dry matter accumulation between PSt and SSt suggested a tighter regulation of assimilate supply to the individual spikelets in wheat. This possibly indicates that there might be an underlying mechanism controlling assimilates allocation and distribution between wheat spikelets, especially limiting distal florets from sufficiently accessing the assimilate route. We will further discuss our results based on the suggested modalities on the architectural configuration of the vascular network system in plants.
Materials and methods
We used cv. Floradur Near Isogeneic Lines (FL-bht-A1-NILs) carrying alleles of the branched headt (bht-A1) locus derived from tetraploid wheat mutant accession, TRI 19165 or ‘Miracle Wheat’ (Poursarebani et al. 2015; Wolde et al. 2019a). Floradur is a German commercial elite spring durum wheat (Triticum durum L.) variety with a standard spike. Five different plant families of FL-bht-A1-NILs (at BC2F3, BC3F2 and BC3F3 generations) were used in this study. Generally, besides carrying the primary standard spikelets (PSt), FL-bht-A1-NILs are characterised by carrying secondary spikelets (SSt) sharing the same rachis node with that of the PSt (Wolde et al. 2019a). First, the five homozygous BC3F2 family plants (n = 25) were evaluated for spike morphology under the field conditions at the Leibniz Institute of Plant Genetics and Crop Plant Research, Gatersleben, Germany (51°49′23″N, 11°17′13″E, altitude 112 m above sea level) during the 2015 growing season. Grains were first sown in 96 well trays under controlled long day conditions i.e. 16/8 h day/night and 19/17°C day/night temperatures for 15 days. Then, plants were vernalised for about four weeks at 4°C. After 1 week of hardening at 15/12°C day/night temperature, seedlings were transplanted in the field characterised by silty loam soil. The distance between plants and rows was ~10 and 20 cm respectively. All spikes from each plant were harvested and the PSt and SSt were processed separately from the remaining spikelets. Spikelet fertility (grain number per spikelet), grain weight (thousand kernel weight) and grain morphometric traits: grain length, grain width, and grain area were analysed using Marvin digital seed analyser (GAT Sensoric GmbH). An additional experiment was set up in the greenhouse under controlled conditions based on the five plant families (BC3F3 generation). Seven grains per family were germinated on 96 well trays under controlled long day conditions: 16/8 h day/night and 19/17°C day/night temperatures for 15 days. After 15 days of seedling establishment, plants were vernalised for about four weeks at 4°C. After 1 week of hardening at 15/12°C day/night temperature, each seedling was transferred to a pot size 0.5 L filled with substrate 2 (Klasmann-Deilmann GmbH, 49744 Geeste), compost and sand on a proportion of 2 : 2 : 1 respectively. Each plant received 10 g of NPK fertiliser called Plantacote Depot 4M (Wilhelm Haug GmbH and Co.) 1 week after potting. Plants were grouped into two major groups in three biological replicates for each group. About 35 plants per replicate were used in each group. Each pot was randomly arranged within the group and each plant received all the standard treatments (light, water, fertilizer, disease and pest controls) uniformly. Tiller removal experiment was conducted to group 1 plants (DT plants) while those in group 2 were allowed to freely tiller (FT plants). Tillers were always checked and removed every three days until maturity. Traits such as tiller number (TN), heading date (HD), plant height (PH), peduncle length (PdL), spike length (SL), spike dry weight at harvest (SDWH), node number per spike (NPS), node density (ND), number of secondary spikelets per spike (SSt), total spikelet per spike (sum of PSt and SSt), grain number per spike (GNS), grain number per spikelet (GPS), and thousand kernel weight (TKW) from the main culm were analysed. All grain related traits from PSt and SSt sharing the same rachis node were analysed separately to investigate the dynamics of spikelet fertility and dry matter accumulation in the grains. Mean phenotypic data from the three replications were subjected to unpaired two-tailed Student’s t-test.
Results
Spike architecture of the FL-bht-A1-NILs (BC3F3)
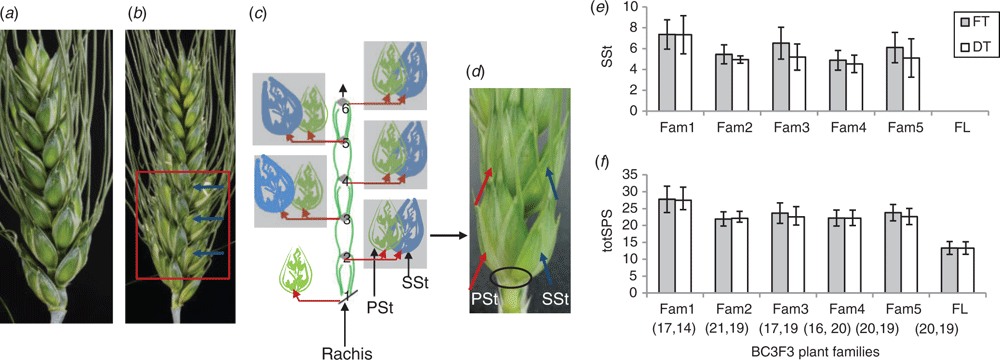
Canonical spikelets in wheat are distichously arranged on the main spike axis (rachis) and each rachis node usually bears only a single spikelet. Due to the introgressed branched headt or bht-A1 allele from ‘miracle wheat’, FL-bh-A1t-NILs carry SSt that share the same rachis node with the PSt in a ventral-to-ventral orientation, more or less facing each other (Fig. 1b–d). The ‘paired spikelet’ phenotype, however, seen in many other forms, for example in (Boden et al. 2015; Dixon et al. 2018) is distinct in the way that spikelets, i.e. PSt and SSt, share one rachis node in a clearly dorsal-to-ventral fashion, rather resembling a piggy-back situation. Although there are clear anatomical differences among different non-canonical spikelet types, collectively, all of these ‘additional spikelets’ are often subsumed as ‘supernumerary spikelets’.
Here, it is important to note that spike-branching in ‘miracle wheat’ or TRI 19165, the donor of the bht-A1 allele on chromosome 2A, is a true type or genuine spike-branching accession, whereupon ‘mini spike-like branches’ appear from the distichously arranged rachis nodes by replacing the spikelets (Poursarebani et al. 2015; Wolde et al. 2019b). However, FL-bh-A1t-NILs predominantly show supernumerary spikelets without genuine spike-branching (Wolde et al. 2019a). This suggests the presence of other spike-branching modifiers suppressing genuine spike-branching in the recurrent parent, Floradur, background.
The response of spike development after source–sink manipulation (de-tillering)
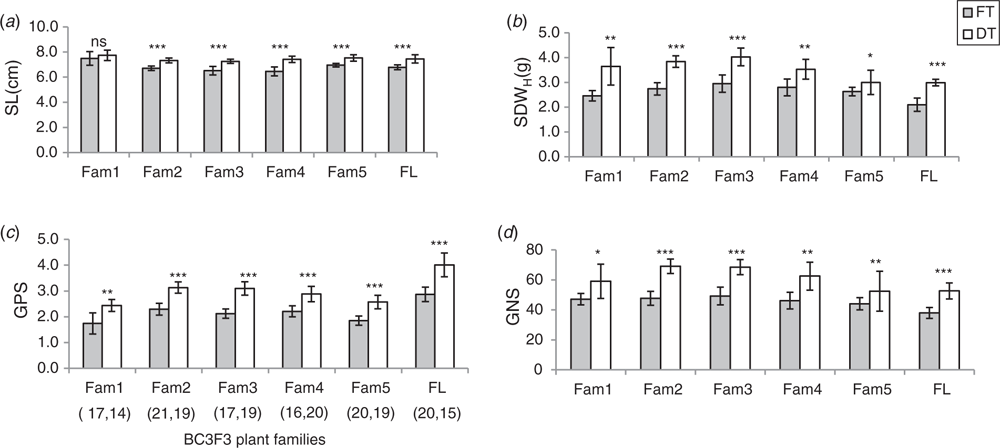
Apart from the increase in SL of DT plants, no change in spikelet number between FT and DT plants was observed (Fig. 1e, f; Table S1, available as Supplementary Material to this paper). Hence, ND (number of nodes per unit length of the spike) in DT plants was lower than FT plants, indicating that DT plants somehow showed an elongated rachis internode. This might be one of the reasons for higher chaff weight in the DT plants (Table S1). Despite the increased SDWH of the DT plants (Fig. 2b), no significant difference for spike harvest index (SHI) between FT and DT plants was found (Table S1), suggesting an inefficient conversion of biomass to the final grain weight. However, compared with the FT plants, the DT plants showed increased spikelet fertility (GPS) both in the PSt and SSt which led to increased grain number per spike (Fig. 2d). Improved spikelet fertility after de-tillering (Fig. 2c) suggested source linked problems which might be linked with the translocation and/or distribution of assimilates.
Source–sink manipulation and the dynamics of spikelet fertility in the PSt and SSt
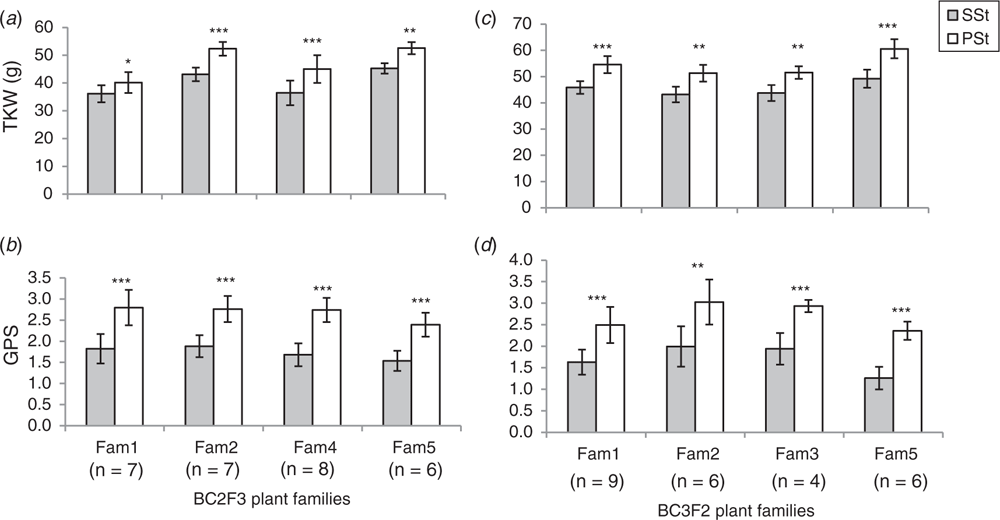
Before we set up source-sink manipulation under greenhouse conditions, we studied spikelet fertility in the PSt and SSt from plants grown under field conditions. The study was conducted using homozygous FL-bht-A1-NILs at BC2F3 and BC3F2 generations comprising of 28 and 25 individual plants respectively. Spikelet fertility between the PSt and SSt was measured in terms of GPS from each of these spikelets. Results showed that spikelet fertility and dry matter accumulation in the SSt was always significantly lower than in the PSt (Fig. 3a, b). Even though PSt and SSt share the same rachis node (i.e. potentially connected to similar assimilate sources) (Fig. 1b–d), the differential spikelet fertility and dry matter accumulation suggest a distinctive, yet unknown factor controlling assimilate partitioning and dry matter accumulation in wheat spikelets.
Although spikelet primordia in spike-branching or ‘miracle wheat’ mutants appear indeterminately (Shitsukawa et al. 2009; Poursarebani et al. 2015), SSt might also be developmentally delayed compared with the PSt. Although we have not studied the developmental differences that might exist between the PSt and SSt, we reasoned that the observed difference in spikelet fertility and dry matter accumulation between PSt and SSt might also be due to the differences in ‘sink strength’ and hence, competition effects. Therefore, we conducted a greenhouse experiment to study the dynamics of assimilate partitioning and dry matter accumulation in the PSt and SSt by diverting more resources to the main culm through de-tillering (see ‘Materials and methods’ for details).
Results showed a concomitant improvement of spikelet fertility both in the PSt and SSt even after de-tillering (Fig. 4). We noted that unlike the SSt in FT plants, SSt in the DT plants showed up to 65% improvements in spikelet fertility, suggesting that the lowered spikelet fertility of the SSt in the FT plants was due, partly, to assimilate supply and/or competition effect. However, the SSt always tends to have lowered spikelet fertility compared with the corresponding PSt even after de-tillering, clearly indicating that more assimilates were translocated to the PSt compared with SSt in all conditions. Nevertheless, the concomitant improvement of assimilate partitioning to the PSt and SSt in the DT plants further suggested the tighter regulation of assimilate supply to the individual spikelets in wheat. Thus, to better understand the regulation of assimilate partitioning to the individual wheat spikelets, especially from the perspectives of the conducting sieve tube elements, it is very important to dissect the temporal and spatial mechanics of vascular bundle development between the PSt and SSt. As ovary (carpel) size in wheat was also shown to be one of the factors in floret and grain survival in wheat (Xie et al. 2015; Guo et al. 2016), it would also be worthwhile to conduct further studies on the floral development in the PSt and SSt.
Source-sink manipulation and the dynamics of dry matter accumulation in the PSt and SSt
Thousand kernel weight (TKW) and the grain morphometric traits, i.e. grain length, grain width and grain area, were used to analyse grain development and dry matter accumulation in the PSt and SSt (Fig. 5). De-tillering did not significantly affect the TKW of the grains in the PSt or SSt (Fig. 5b). Nevertheless, the differences in TKW of grains between the PSt and SSt both in the FT and DT plants was significant (Fig. 5a). This suggests that besides the variation in spikelet fertility between the PSt and SSt (Fig. 4), there was a clear variation in dry matter accumulation and partitioning in the PSt and SSt even though both are connected to the same rachis node. We further analysed grain morphometric traits in the PSt and SSt (Fig. 6). Results showed that the grains in the PSt depicted enhanced grain length, grain width, and grain area compared with the grains in the SSt. These results show that besides the variation in spikelet fertility, there is also variation in grain development and dry matter accumulation in the PSt and SSt.
Discussion
FL-bht-A1-NILs represent novel spike morphology for use in wheat breeding
The genetic gains in wheat yield due to the introduction of semi-dwarf wheat varieties are associated with the partitioning of more photosynthate to the developing spikelets (Siddique et al. 1989; Miralles et al. 1998; Pearce et al. 2011; Thomas 2017). Nevertheless, the change in spikelet number per spike has been minimal (Royo et al. 2007; Álvaro et al. 2008; Philipp et al. 2018). As the modern wheat cultivars are also gradually approaching the theoretical upper limit for harvest index (Austin et al. 1980; Gaju et al. 2014), wheat breeding for new spike types is becoming imminent. Previously, the ‘ideal wheat plant ideotype’ characterised as mono-culm, lodging resistant with larger spike or sink size was proposed by Donald (1968). Such concept of a ‘communal wheat ideotype’, which defines a model of the idealised theoretical crop plant architecture under the assumption of minimised intra-specific growth competition, has never been tested experimentally in wheat. In the present study we phenocopied the mono-culm characteristics through de-tillering (DT plants) and a genetically controlled spike or sink size, i.e. FL-bht-A1-NILs, which carry more spikelets per spike (Fig. 1b, e) (Wolde et al. 2019a).
Although FL-bht-A1-NILs are free tillering, and noting the fact that fertile tillers contribute to yield in wheat, our results clearly highlighted the benefit of having a single (mono) culm for improved spikelet fertility (Fig. 4). Previously, studies have also linked tiller abortion with the onset of stem elongation and anthesis suggesting that tiller death (abortion) is due, partly to resource competition (Mohamed and Marshall 1979; Kemp and Whingwiri 1980; Fraser et al. 1982; Davidson and Chevalier 1990; Berry et al. 2003; Xie et al. 2016). This suggests a benefit of resource diversion to the main culm for improved spikelet fertility that is otherwise spent on tiller development and maintenance, the fate of which is usually uncertain. We note that reports on the benefit of tiller inhibition, tin, genes and their positive effects in improving the harvest index have also started to emerge (Duggan et al. 2005; Kebrom et al. 2012; Gaju et al. 2014; Hendriks et al. 2016). For example, Hendriks et al. (2016) indicated the influence of the tin gene on root–shoot carbon partitioning. Even though the effect of introgressing the tiller inhibition gene into FL-bht-A1-NIL is to be seen in the future, the DT plants suggest the benefit of having mono-culm ideotype for resource maximisation in wheat for improving the harvest index, and possibly the application of mono-culm wheat ideotype in wheat breeding by combining bht-A1 and tin genes. Our data based on FL-bht-A1-NILs already suggested a breeding opportunity by moderately modifying spikelet number without significantly compromising the grain weight (Wolde et al. 2019a). We note that FL-bht-A1-NILs are also more lodging resistant due to the presence of the Rht-B1 semi-dwarfing allele paving ways towards the creation of a wheat plant ideotype as suggested by Donald (1968).
FL-bht-A1-NILs are suitable genetic materials to understand the dynamics of resource allocation in wheat spikelets
The wheat spike shows a differential spikelet fertility and dry matter accumulation along with the spike. For example, spikelets in the centre of the spike are more advanced, and hence possess higher grain number per spikelet than those spikelets at the apex and base (Bonnett 1936; Whingwiri et al. 1981; Whingwiri and Stern 1982; González-Navarro et al. 2015; Guo et al. 2015; Li et al. 2016; Philipp et al. 2018). This indicates that central spikelets have an advanced sink activity compared with those spikelets at a basal and apical section of the spike. Therefore, it is likely that the increased spikelet fertility of central spikelets is due, partly, to a better sink activity (Fig. 7b).

|
Often, SSt in FL-bht-A1-NILs appears in the bottom half of the spike (Fig. 1b). Although the time of initiation of the SSt is important in this regard, their unique structural arrangement, i.e. sharing same rachis node on the spike, is also quite important to elucidate on how resources are partitioned between wheat spikelets. Therefore, besides the differential spikelet fertility and dry matter accumulation along the spike, FL-bht-A1-NILs also have typical spikelet arrangement, i.e. PSt and SSt, showing a differential spikelet fertility (Fig. 4) and dry matter accumulation (Fig. 5), which suggests a tight regulation of resource allocation and dry matter accumulation among the wheat spikelets.
Besides sink activity, the sink size is also one of the most yield-limiting factors in wheat (Borrás et al. 2004; Miralles and Slafer 2007; Reynolds et al. 2007; Foulkes et al. 2011). Because of the increased spikelet number per spike followed by their unique arrangement along the spike, FL-bht-A1-NILs are important for the genetic and physiological studies of the source–sink relationship in wheat to better understand the mechanism by which resources are partitioned among wheat spikelets to design future wheat spike ideotypes.
The discovery of the Grain Number Increase 1 (GNI1) alleles also indicated that wheat plants can actively shutdown their yield potential by specifically aborting the most apical florets through apical rachilla degeneration (Sakuma et al. 2019). A disintegrated rachilla most likely works as an obstruction or constriction, depriving all more apically located florets and/or grains from any nutrients by simply starving them to death. This sort of ‘self-restriction’ is perhaps a useful evolutionary limitation in natural habitats, but is unwanted in attempts to maximise agricultural productivity. Therefore, besides the efforts to understand the genetic and physiological cause of floral abortion in wheat, a deeper understanding of the structural anatomy and allocation of resources in wheat spikelets and/or florets is essential.
Grain development and dry matter accumulation in FL-bht-A1-NILs
In addition to grain number, i.e. spikelet fertility, grain weight is also a major component for the determination of grain yield in wheat (Feng et al. 2018). Potentially, more grains can be set in each wheat spikelet, including in FL-bht-A1-NILs, but the relative contribution of each of these grains to TKW, and hence to yield, is variable due to uneven grain development and dry matter accumulation in the spikelet. For example, the relative contribution of distal grains (those that are farther from the base of the spikelet) to TKW is less than with those proximal to the rachis, i.e. at the base of the spikelet (Feng et al. 2018). FL-bht-A1-NILs show not only uneven grain development and dry matter accumulation within spikelets, but also uneven grain development and dry matter accumulation between spikelets sharing same rachis node on the spike, i.e. PSt and SSt. Recently, it has been demonstrated that suppression of distal florets increases the weight of proximal grains by altering assimilate distribution (Golan et al. 2019), most likely as an effect of rachilla degeneration. Millet (1986) also reported a positive correlation between floret cavity size and the weight of the matching grain in wheat (Millet 1986). In this regard, differences in floral development between PSt and SSt, if any, might also partly contribute to the differences in grain weight between PSt and SSt. It has also been shown that carpel size, grain filling rate, and morphology are also determinant factors of grain weight in wheat (Xie et al. 2015).
TKW is also affected by grain shape and size (Su et al. 2018). These characteristics are determined by the temporal and spatial regulation of cell division, cell expansion, and the duration and rate of grain filling (Wheeler et al. 1996; Drea et al. 2005; Pielot et al. 2015; Li and Li 2016), which are dependent on the availability and acquisition of essential resources (Weber et al. 1998; Yang et al. 2004; Lopes et al. 2006; Egle et al. 2015; Weichert et al. 2017). We note that grain development in wheat spikelets follows an acropetal order (Kirby 1974; Whingwiri and Stern 1982), showing a decrease in grain weight or dry matter accumulation acropetally within the spikelets (Rawson and Evans 1970; Kirby 1974; Whingwiri et al. 1981; Guo et al. 2015). Since assimilate supply into the developing grain regulates the number and growth of endosperm cells, thereby affecting the dry matter accumulation (Brocklehurst 1977; Thorne 1985; Morita et al. 2005; Li and Li 2016), the acropetal reduction of grain weight within each spikelet might well be linked with efficiency of assimilate supply to the distal grains along the axis of the spikelet, i.e. the rachilla. Consistent with this, Borrás et al. (2004) and Jenner et al. (1991) have reported the influence of assimilate availability on grain dry matter accumulation (Jenner et al. 1991; Borrás et al. 2004).
Grain weight is also under the influence of the rate and duration of grain filling (Duguid and Brûlé-Babel 1994; Wang et al. 2008; Baillot et al. 2018). Since the main component of the wheat grain is an endosperm, genes regulating starch synthesis are essential for appropriate grain filling (Martínez-Barajas et al. 2011). This suggests that incomplete grain filling is due, in part, to defects in starch synthesis. Sucrose is the source for starch synthesis, and is transported from the source (leaves) into sink organs such as grains, so understanding sucrose delivery from the source to the developing grains is essential for designing novel approaches for improving wheat yield. To this end, the unique spikelet arrangement in FL-bht-A1-NILs is important to better understand the role of phloem architecture and the dynamics of sucrose delivery between and within the wheat spikelets.
Inferring the vascular architecture of the wheat spikelet
The application of a branched internal network system that was originally discovered based on the mammalian circulatory system, i.e. aorta model (Murray 1926), has been discovered in the plant system (McCulloh et al. 2003; Price et al. 2013; Seki et al. 2015). In this model, the mother tube bifurcates into daughter tubes, where the radius of the mother tube cubed will equal the sum of the cubes of the daughter radii (Murray 1926; LaBarbera 1990; McCulloh et al. 2003; Seki et al. 2015). However, with increased branching, the reduction in tube size (West et al. 1997; Seki et al. 2015) attracted our attention, so we were tempted to hypothesise on its application in relation to the general trend of floral abortion in wheat. Although our knowledge on the architectural configuration of the vascular bundle in the wheat spikelet is still incomplete, the acropetal decrease in spikelet fertility and dry matter accumulation within the wheat spikelet might be linked with a proportionate decrease in tube radii of the conducting tube elements in the spikelet. The schematic depiction of our hypothesis of the aorta model in wheat spikelet is shown in Fig. 8. Theoretically, floret abortion in wheat can also be explained, at least partially, by assuming that branching of the main vascular bundle follows the aorta model. Since the aorta model is independent of the length of the tube but affects the radius of the tube (McCulloh and Sperry 2006), further branching of the sieve tube elements within the wheat spikelet might limit assimilate supply to the distal florets (Fig. 8V). This could, in turn, cease cell division, and result in the interruption of the normal floral development in the distal florets.

|
The alternative model – the pipe model – seems to be less likely in wheat spikes because the assumption of the pipe model is that each conducting (sieve tube) element neither bifurcates nor tapers at any site (Seki et al. 2015) (Fig. 8S). Thus, conducting sieve tube elements are assumed to connect each spikelet/floret to the source (i.e. leaf); which means that each spikelet/floret have their own pipe that connects them directly to the source. Because competition for assimilates in the pipe model is believed to be distance-dependent (Seki et al. 2015), and the fact that the PSt and SSt share the same rachis node (Fig. 1d), they should have benefited equally, i.e. should have a similar spikelet fertility and dry matter accumulation index. However, the SSt has lower spikelet fertility and dry matter accumulation than the PSt both in FT and DT plants (Fig. 4). This difference might be attributed to the differential assimilate partitioning to each of these spikelets which might be linked with the varying tube radii of the vascular architecture, especially the conducting sieve tube elements in the PSt and SSt. If this is true, it is more compelling to hypothesise and model the vascular architecture from the basis of the principle and assumptions of the aorta model.
The second reason why the pipe model is less likely for the wheat spikelet is the very nature of the most basal wheat spikelets, which are often less fertile or even sterile (Fraser 1950; Kirby 1974; Guo et al. 2015; Li et al. 2016; Philipp et al. 2018). These spikelets have more vascular bundles and are closer to the source (leaf) than those from the centre or apical part of the spike (Fig. 7b), so according to the pipe model they should have benefited more than those in the central and/or apical portion of the spike simply because they are closer to the source (leaf). However, this is generally not the case in wheat. This suggests that the pipe model is again a less likely model for the wheat spikelet. Therefore, based on (i) the diminishing characteristics of Σr3 with further branching in the modified version of the aorta model in plants combined with the inferred trend of distal floret degeneration and the acropetal decrease of the dry matter accumulation in each spikelet; and (ii) the inferred differential spikelet fertility index along the wheat spike, as well as spikelets sharing the same rachis node, i.e. PSt and SSt, it is likely that the aorta model is an imminent working model for assimilate transport network in wheat spikelet/floret.
Since the conducting tubes are not continuous in plants, i.e. divided into conduits that run in parallel (McCulloh et al. 2003), as shown in Fig. 8S and Fig. 8T, several of these conduits are approximated into one large tube per vascular bundle (Seki et al. 2015). Hence, after the split of the vascular bundle in the rachis/rachilla, one branch goes to the immediate spikelet/floret and the other branches to rachis/rachilla internode (Fig. 8V).
Regardless of the fact that more vascular bundles are found at the base of the spike (Whingwiri et al. 1981) (Fig. 7b), it seems that the basal spikelets might not be directly connected to the main vascular bundle of the rachis. This could highlight that the branching of the main vascular bundle might start from the centre of the spike and progresses in a proximo-distal fashion following the direction of spikelet differentiation (Bonnett 1936) where the reduction in the size of the main conducting elements might have contributed to the reduced spikelet/floret fertility in the distal spikelets/florets (Fig. 8).
Due to increased demand for carbohydrates by the developing spike, a sucrose feeding experiment through the flag leaf rescued more florets that otherwise aborted (Ghiglione et al. 2008). Consistent with this, enhanced spikelet fertility of SSt after de-tillering (Fig. 4) also suggests that more florets are rescued from being aborted due, partly, to the diverted assimilate before it drops-off to the level that cannot sustain floret development anymore (Fig. 8). The sieve tube elements also transport amino acids and hormones (Ham and Lucas 2014). Thus, depletion of hormones and amino acids from the distal florets could also lead to similar effects.
Conclusion
A more detailed understanding of sink strength and source activity is fundamental for enhancing crop productivity. By developing FL-bht-A1-NILs, with increased sink size and spikelet arrangement, and by artificially manipulating the dynamics of the source–sink balance (through de-tillering), we observed differential spikelet fertility and dry matter accumulation patterns between sister spikelets, i.e. the PSt and SSt, sharing the rachis node, suggesting the action of important, but as yet unknown mechanisms controlling the process. Hence, a detailed understanding of the developmental and anatomical differences between the PSt and SSt will illustrate how resources are partitioned between similar sink organs, and lead towards a better understanding of floret and even grain abortion in wheat spikelets.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We thank Corinna Trautewig and Mechthild Pürschel for their excellent technical assistance. This work was supported by the Wissenschaftsgemeinschaft Gottfried Wilhelm Leibniz (WGL) and the Leibniz-Graduate School Gatersleben under ‘Yield formation in cereals – overcoming yield limiting factors’ (Grant number FKZ 401410). During parts of this study, TS received financial support from the HEISENBERG Program of the German Research Foundation (DFG), grant no. SCHN 768/8–1, and IPK core budget.
References
Álvaro F, Isidro J, Villegas D, del Moral LFG, Royo C (2008) Old and modern durum wheat varieties from Italy and Spain differ in main spike components. Field Crops Research 106, 86–93.| Old and modern durum wheat varieties from Italy and Spain differ in main spike components.Crossref | GoogleScholarGoogle Scholar |
Austin RB, Bingham J, Blackwell RD, Evans LT, Ford MA, Morgan CL, Taylor M (1980) Genetic improvements in winter-wheat yields since 1900 and associated physiological-changes. The Journal of Agricultural Science 94, 675–689.
| Genetic improvements in winter-wheat yields since 1900 and associated physiological-changes.Crossref | GoogleScholarGoogle Scholar |
Baillot N, Girousse C, Allard V, Piquet-Pissaloux A, Le Gouis J (2018) Different grain-filling rates explain grain-weight differences along the wheat ear. PLoS One 13, e0209597
| Different grain-filling rates explain grain-weight differences along the wheat ear.Crossref | GoogleScholarGoogle Scholar | 30596702PubMed |
Berry PM, Spink JH, Foulkes MJ, Wade A (2003) Quantifying the contributions and losses of dry matter from non-surviving shoots in four cultivars of winter wheat. Field Crops Research 80, 111–121.
| Quantifying the contributions and losses of dry matter from non-surviving shoots in four cultivars of winter wheat.Crossref | GoogleScholarGoogle Scholar |
Boden SA, Cavanagh C, Cullis BR, Ramm K, Greenwood J, Finnegan EJ, Trevaskis B, Swain SM (2015) Ppd-1 is a key regulator of inflorescence architecture and paired spikelet development in wheat. Nature Plants 1, 14016
| Ppd-1 is a key regulator of inflorescence architecture and paired spikelet development in wheat.Crossref | GoogleScholarGoogle Scholar | 27246757PubMed |
Bonnett O (1936) The development of the wheat spike. Agricultural Research 53, 445–451.
Borrás L, Slafer GA, Otegui ME (2004) Seed dry weight response to source-sink manipulations in wheat, maize and soybean: a quantitative reappraisal. Field Crops Research 86, 131–146.
| Seed dry weight response to source-sink manipulations in wheat, maize and soybean: a quantitative reappraisal.Crossref | GoogleScholarGoogle Scholar |
Bremner P, Rawson H (1978) The weights of individual grains of the wheat ear in relation to their growth potential, the supply of assimilate and interaction between grains. Functional Plant Biology 5, 61–72.
| The weights of individual grains of the wheat ear in relation to their growth potential, the supply of assimilate and interaction between grains.Crossref | GoogleScholarGoogle Scholar |
Brocklehurst PA (1977) Factors controlling grain weight in wheat. Nature 266, 348–349.
| Factors controlling grain weight in wheat.Crossref | GoogleScholarGoogle Scholar |
Davidson DJ, Chevalier PM (1990) Preanthesis tiller mortality in spring wheat. Crop Science 30, 832–836.
| Preanthesis tiller mortality in spring wheat.Crossref | GoogleScholarGoogle Scholar |
Dixon LE, Greenwood JR, Bencivenga S, Zhang P, Cockram J, Mellers G, Ramm K, Cavanagh C, Swain SM, Boden SA (2018) TEOSINTE BRANCHED1 regulates inflorescence architecture and development in bread wheat (Triticum aestivum). The Plant Cell 30, 563–581.
| TEOSINTE BRANCHED1 regulates inflorescence architecture and development in bread wheat (Triticum aestivum).Crossref | GoogleScholarGoogle Scholar | 29444813PubMed |
Dobrovolskaya O, Pont C, Sibout R, Martinek P, Badaeva E, Murat F, Chosson A, Watanabe N, Prat E, Gautier N, Gautier V, Poncet C, Orlov YL, Krasnikov AA, Berges H, Salina E, Laikova L, Salse J (2015) FRIZZY PANICLE drives supernumerary spikelets in bread wheat. Plant Physiology 167, 189–199.
| FRIZZY PANICLE drives supernumerary spikelets in bread wheat.Crossref | GoogleScholarGoogle Scholar | 25398545PubMed |
Donald CM (1968) The breeding of crop ideotypes. Euphytica 17, 385–403.
| The breeding of crop ideotypes.Crossref | GoogleScholarGoogle Scholar |
Drea S, Leader DJ, Arnold BC, Shaw P, Dolan L, Doonan JH (2005) Systematic spatial analysis of gene expression during wheat caryopsis development. The Plant Cell 17, 2172–2185.
| Systematic spatial analysis of gene expression during wheat caryopsis development.Crossref | GoogleScholarGoogle Scholar | 16006577PubMed |
Duggan BL, Richards RA, van Herwaarden AF (2005) Agronomic evaluation of a tiller inhibition gene (tin) in wheat. II. Growth and partitioning of assimilate. Australian Journal of Agricultural Research 56, 179–186.
| Agronomic evaluation of a tiller inhibition gene (tin) in wheat. II. Growth and partitioning of assimilate.Crossref | GoogleScholarGoogle Scholar |
Duguid SD, Brûlé-Babel AL (1994) Rate and duration of grain filling in five spring wheat (Triticum aestivum L.) genotypes. Canadian Journal of Plant Science 74, 681–686.
| Rate and duration of grain filling in five spring wheat (Triticum aestivum L.) genotypes.Crossref | GoogleScholarGoogle Scholar |
Egle K, Beschow H, Merbach W (2015) Nitrogen allocation in barley: relationships between amino acid transport and storage protein synthesis during grain filling. Canadian Journal of Plant Science 95, 451–459.
| Nitrogen allocation in barley: relationships between amino acid transport and storage protein synthesis during grain filling.Crossref | GoogleScholarGoogle Scholar |
Feng F, Han Y, Wang S, Yin S, Peng Z, Zhou M, Gao W, Wen X, Qin X, Siddique KHM (2018) The effect of grain position on genetic improvement of grain number and thousand grain weight in winter wheat in North China. Frontiersinf Plant Science 9, 129
| The effect of grain position on genetic improvement of grain number and thousand grain weight in winter wheat in North China.Crossref | GoogleScholarGoogle Scholar |
Foulkes MJ, Slafer GA, Davies WJ, Berry PM, Sylvester-Bradley R, Martre P, Calderini DF, Griffiths S, Reynolds MP (2011) Raising yield potential of wheat. III. Optimizing partitioning to grain while maintaining lodging resistance. Journal of Experimental Botany 62, 469–486.
| Raising yield potential of wheat. III. Optimizing partitioning to grain while maintaining lodging resistance.Crossref | GoogleScholarGoogle Scholar | 20952627PubMed |
Fraser AS (1950) Basal sterility in wheat. Nature 165, 653
| Basal sterility in wheat.Crossref | GoogleScholarGoogle Scholar | 15416758PubMed |
Fraser J, Dougherty CT, Langer RHM (1982) Dynamics of tiller populations of standard height and semi-dwarf wheats. New Zealand Journal of Agricultural Research 25, 321–328.
| Dynamics of tiller populations of standard height and semi-dwarf wheats.Crossref | GoogleScholarGoogle Scholar |
Gaju O, Reynolds MP, Sparkes DL, Mayes S, Ribas-Vargas G, Crossa J, Foulkes MJ (2014) Relationships between physiological traits, grain number and yield potential in a wheat DH population of large spike phenotype. Field Crops Research 164, 126–135.
| Relationships between physiological traits, grain number and yield potential in a wheat DH population of large spike phenotype.Crossref | GoogleScholarGoogle Scholar |
Ghiglione HO, Gonzalez FG, Serrago R, Maldonado SB, Chilcott C, Cura JA, Miralles DJ, Zhu T, Casal JJ (2008) Autophagy regulated by day length determines the number of fertile florets in wheat. The Plant Journal 55, 1010–1024.
| Autophagy regulated by day length determines the number of fertile florets in wheat.Crossref | GoogleScholarGoogle Scholar | 18547393PubMed |
Golan G, Ayalon I, Perry A, Zimran G, Ade-Ajayi T, Mosquna A, Distelfeld A, Peleg Z (2019) GNI-A1 mediates trade-off between grain number and grain weight in tetraploid wheat. Theoretical and Applied Genetics
| GNI-A1 mediates trade-off between grain number and grain weight in tetraploid wheat.Crossref | GoogleScholarGoogle Scholar | 31079164PubMed |
González-Navarro OE, Griffiths S, Molero G, Reynolds MP, Slafer GA (2015) Dynamics of floret development determining differences in spike fertility in an elite population of wheat. Field Crops Research 172, 21–31.
| Dynamics of floret development determining differences in spike fertility in an elite population of wheat.Crossref | GoogleScholarGoogle Scholar |
Guo Z, Schnurbusch T (2015) Variation of floret fertility in hexaploid wheat revealed by tiller removal. Journal of Experimental Botany 66, 5945–5958.
| Variation of floret fertility in hexaploid wheat revealed by tiller removal.Crossref | GoogleScholarGoogle Scholar | 26157170PubMed |
Guo J, Zhang Y, Shi W, Zhang B, Zhang J, Xu Y, Cheng X, Cheng K, Zhang X, Hao C, Cheng S (2015) Association analysis of grain-setting rates in apical and basal spikelets in bread wheat (Triticum aestivum L.). Frontiers in Plant Science 6, 1029
| Association analysis of grain-setting rates in apical and basal spikelets in bread wheat (Triticum aestivum L.).Crossref | GoogleScholarGoogle Scholar | 26635852PubMed |
Guo Z, Slafer GA, Schnurbusch T (2016) Genotypic variation in spike fertility traits and ovary size as determinants of floret and grain survival rate in wheat. Journal of Experimental Botany 67, 4221–4230.
| Genotypic variation in spike fertility traits and ovary size as determinants of floret and grain survival rate in wheat.Crossref | GoogleScholarGoogle Scholar | 27279276PubMed |
Guo Z, Chen D, Alqudah AM, Röder MS, Ganal MW, Schnurbusch T (2017) Genome-wide association analyses of 54 traits identified multiple loci for the determination of floret fertility in wheat. New Phytologist 214, 257–270.
| Genome-wide association analyses of 54 traits identified multiple loci for the determination of floret fertility in wheat.Crossref | GoogleScholarGoogle Scholar | 27918076PubMed |
Guo Z, Chen D, Schnurbusch T (2018) Plant and floret growth at distinct developmental stages during the stem elongation phase in wheat. Frontiers in Plant Science 9, 330
| Plant and floret growth at distinct developmental stages during the stem elongation phase in wheat.Crossref | GoogleScholarGoogle Scholar | 29599792PubMed |
Ham BK, Lucas WJ (2014) The angiosperm phloem sieve tube system: a role in mediating traits important to modern agriculture. Journal of Experimental Botany 65, 1799–1816.
| The angiosperm phloem sieve tube system: a role in mediating traits important to modern agriculture.Crossref | GoogleScholarGoogle Scholar | 24368503PubMed |
Hanif M, Langer RHM (1972) The vascular system of the spikelet in wheat (Triticum aestivum). Annals of Botany 36, 721–727.
| The vascular system of the spikelet in wheat (Triticum aestivum).Crossref | GoogleScholarGoogle Scholar |
Hendriks PW, Kirkegaard JA, Lilley JM, Gregory PJ, Rebetzke GJ (2016) A tillering inhibition gene influences root-shoot carbon partitioning and pattern of water use to improve wheat productivity in rainfed environments. Journal of Experimental Botany 67, 327–340.
| A tillering inhibition gene influences root-shoot carbon partitioning and pattern of water use to improve wheat productivity in rainfed environments.Crossref | GoogleScholarGoogle Scholar | 26494729PubMed |
Jenner C, Ugalde T, Aspinall D (1991) The physiology of starch and protein deposition in the endosperm of wheat. Functional Plant Biology 18, 211–226.
| The physiology of starch and protein deposition in the endosperm of wheat.Crossref | GoogleScholarGoogle Scholar |
Kebrom TH, Chandler PM, Swain SM, King RW, Richards RA, Spielmeyer W (2012) Inhibition of tiller bud outgrowth in the tin mutant of wheat is associated with precocious internode development. Plant Physiology 160, 308–318.
| Inhibition of tiller bud outgrowth in the tin mutant of wheat is associated with precocious internode development.Crossref | GoogleScholarGoogle Scholar | 22791303PubMed |
Kemp DR, Whingwiri EE (1980) Effect of tiller removal and shading on spikelet development and yield components of the main shoot of wheat and on the sugar concentration of the ear and flag leaf. Australian Journal of Plant Physiology 7, 501–510.
| Effect of tiller removal and shading on spikelet development and yield components of the main shoot of wheat and on the sugar concentration of the ear and flag leaf.Crossref | GoogleScholarGoogle Scholar |
Kirby EJM (1974) Ear development in spring wheat. Agricultural Science 82, 437–447.
| Ear development in spring wheat.Crossref | GoogleScholarGoogle Scholar |
Kirby EJM (1988) Analysis of leaf, stem and ear growth in wheat from terminal spikelet stage to anthesis. Field Crops Research 18, 127–140.
| Analysis of leaf, stem and ear growth in wheat from terminal spikelet stage to anthesis.Crossref | GoogleScholarGoogle Scholar |
Kirby EM, Appleyard M (1984) ‘Cereal development guide.’ (NAC Cereal Unit: Stoneleigh, UK)
Kirby EJM, Rymer JL (1974) Development of the vascular system in the ear of barley. Annals of Botany 38, 565–573.
| Development of the vascular system in the ear of barley.Crossref | GoogleScholarGoogle Scholar |
Kozłowski J, Konarzewski M (2004) Is West, Brown and Enquist’s model of allometric scaling mathematically correct and biologically relevant? Functional Ecology 18, 283–289.
| Is West, Brown and Enquist’s model of allometric scaling mathematically correct and biologically relevant?Crossref | GoogleScholarGoogle Scholar |
Kozłowski J, Konarzewski M (2005) West, Brown and Enquist’s model of allometric scaling again: the same questions remain. Functional Ecology 19, 739–743.
| West, Brown and Enquist’s model of allometric scaling again: the same questions remain.Crossref | GoogleScholarGoogle Scholar |
LaBarbera M (1990) Principles of design of fluid transport systems in zoology. Science 249, 992–1000.
| Principles of design of fluid transport systems in zoology.Crossref | GoogleScholarGoogle Scholar | 2396104PubMed |
Li N, Li Y (2016) Signaling pathways of seed size control in plants. Current Opinion in Plant Biology 33, 23–32.
| Signaling pathways of seed size control in plants.Crossref | GoogleScholarGoogle Scholar | 27294659PubMed |
Li Y, Cui Z, Ni Y, Zheng M, Yang D, Jin M, Chen J, Wang Z, Yin Y (2016) Plant density effect on grain number and weight of two winter wheat cultivars at different spikelet and grain positions. PLoS One 11, e0155351
| Plant density effect on grain number and weight of two winter wheat cultivars at different spikelet and grain positions.Crossref | GoogleScholarGoogle Scholar | 28036354PubMed |
Lopes MS, Cortadellas N, Kichey T, Dubois F, Habash DZ, Araus JL (2006) Wheat nitrogen metabolism during grain filling: comparative role of glumes and the flag leaf. Planta 225, 165–181.
| Wheat nitrogen metabolism during grain filling: comparative role of glumes and the flag leaf.Crossref | GoogleScholarGoogle Scholar | 16804706PubMed |
Martínez-Barajas E, Delatte T, Schluepmann H, de Jong GJ, Somsen GW, Nunes C, Primavesi LF, Coello P, Mitchell RA, Paul MJ (2011) Wheat grain development is characterized by remarkable trehalose 6-phosphate accumulation pregrain filling: tissue distribution and relationship to SNF1-related protein kinase1 activity. Plant Physiology 156, 373–381.
| Wheat grain development is characterized by remarkable trehalose 6-phosphate accumulation pregrain filling: tissue distribution and relationship to SNF1-related protein kinase1 activity.Crossref | GoogleScholarGoogle Scholar | 21402798PubMed |
McCulloh KA, Sperry JS (2005) The evaluation of Murray’s law in Psilotum nudum (Psilotaceae), an analogue of ancestral vascular plants. American Journal of Botany 92, 985–989.
| The evaluation of Murray’s law in Psilotum nudum (Psilotaceae), an analogue of ancestral vascular plants.Crossref | GoogleScholarGoogle Scholar | 21652482PubMed |
McCulloh KA, Sperry JS (2006) Murray’s law and the vascular architecture of plants. In ‘Ecology and biomechanics: a mechanical approach to the ecology of animals and plants’. (Eds A Herrel, T Speck, NP Rowe) (CRC Press: Boca Raton, FL, USA)
McCulloh KA, Sperry JS, Adler FR (2003) Water transport in plants obeys Murray’s law. Nature 421, 939–942.
| Water transport in plants obeys Murray’s law.Crossref | GoogleScholarGoogle Scholar | 12607000PubMed |
McCulloh KA, Sperry JS, Adler FR (2004) Murray’s law and the hydraulic vs mechanical functioning of wood. Functional Ecology 18, 931–938.
| Murray’s law and the hydraulic vs mechanical functioning of wood.Crossref | GoogleScholarGoogle Scholar |
McMaster GS (1997) Phenology, development, and growth of the wheat (Tritimm aestivum L.) shoot apex: a review. Advances in agronomy 59, 63–118.
Millet E (1986) Relationships between grain weight and the size of floret cavity in the wheat spike. Annals of Botany 58, 417–423.
| Relationships between grain weight and the size of floret cavity in the wheat spike.Crossref | GoogleScholarGoogle Scholar |
Miralles DJ, Slafer GA (2007) Sink limitations to yield in wheat: how could it be reduced? The Journal of Agricultural Science 145, 139–149.
| Sink limitations to yield in wheat: how could it be reduced?Crossref | GoogleScholarGoogle Scholar |
Miralles DJ, Katz SD, Colloca A, Slafer GA (1998) Floret development in near isogenic wheat lines differing in plant height. Field Crops Research 59, 21–30.
| Floret development in near isogenic wheat lines differing in plant height.Crossref | GoogleScholarGoogle Scholar |
Mohamed GES, Marshall C (1979) Physiological-aspects of tiller removal in spring wheat. The Journal of Agricultural Science 93, 457–463.
| Physiological-aspects of tiller removal in spring wheat.Crossref | GoogleScholarGoogle Scholar |
Morita S, Yonemaru J-I, Takanashi J-I (2005) Grain growth and endosperm cell size under high night temperatures in rice (Oryza sativa L.). Annals of Botany 95, 695–701.
| Grain growth and endosperm cell size under high night temperatures in rice (Oryza sativa L.).Crossref | GoogleScholarGoogle Scholar | 15655104PubMed |
Murray CD (1926) The physiological principle of minimum work: I. The vascular system and the cost of blood volume. Proceedings of the National Academy of Sciences of the United States of America 12, 207–214.
| The physiological principle of minimum work: I. The vascular system and the cost of blood volume.Crossref | GoogleScholarGoogle Scholar | 16576980PubMed |
Pearce S, Saville R, Vaughan SP, Chandler PM, Wilhelm EP, Sparks CA, Al-Kaff N, Korolev A, Boulton MI, Phillips AL, Hedden P, Nicholson P, Thomas SG (2011) Molecular characterization of Rht-1 dwarfing genes in hexaploid wheat. Plant Physiology 157, 1820–1831.
| Molecular characterization of Rht-1 dwarfing genes in hexaploid wheat.Crossref | GoogleScholarGoogle Scholar | 22013218PubMed |
Philipp N, Weichert H, Bohra U, Weschke W, Schulthess AW, Weber H (2018) Grain number and grain yield distribution along the spike remain stable despite breeding for high yield in winter wheat. PLoS One 13, e0205452.
| Grain number and grain yield distribution along the spike remain stable despite breeding for high yield in winter wheat.Crossref | GoogleScholarGoogle Scholar | 30304020PubMed |
Pielot R, Kohl S, Manz B, Rutten T, Weier D, Tarkowska D, Rolcik J, Strnad M, Volke F, Weber H, Weschke W (2015) Hormone-mediated growth dynamics of the barley pericarp as revealed by magnetic resonance imaging and transcript profiling. Journal of Experimental Botany 66, 6927–6943.
| Hormone-mediated growth dynamics of the barley pericarp as revealed by magnetic resonance imaging and transcript profiling.Crossref | GoogleScholarGoogle Scholar | 26276866PubMed |
Poursarebani N, Seidensticker T, Koppolu R, Trautewig C, Gawronski P, Bini F, Govind G, Rutten T, Sakuma S, Tagiri A, et al. (2015) The genetic basis of composite spike form in barley and ‘miracle-wheat’. Genetics 201, 155–165.
| The genetic basis of composite spike form in barley and ‘miracle-wheat’.Crossref | GoogleScholarGoogle Scholar | 26156223PubMed |
Price CA, Knox SJ, Brodribb TJ (2013) The influence of branch order on optimal leaf vein geometries: Murray’s law and area preserving branching. PLoS One 8, e85420.
| The influence of branch order on optimal leaf vein geometries: Murray’s law and area preserving branching.Crossref | GoogleScholarGoogle Scholar | 24392008PubMed |
Prieto P, Ochagavia H, Savin R, Griffiths S, Slafer GA (2018) Dynamics of floret initiation/death determining spike fertility in wheat as affected by Ppd genes under field conditions. Journal of Experimental Botany 69, 2633–2645.
| Dynamics of floret initiation/death determining spike fertility in wheat as affected by Ppd genes under field conditions.Crossref | GoogleScholarGoogle Scholar | 29562264PubMed |
Rawson H, Evans L (1970) The pattern of grain growth within the ear of wheat. Australian Journal of Biological Sciences 23, 753–764.
| The pattern of grain growth within the ear of wheat.Crossref | GoogleScholarGoogle Scholar |
Reynolds M, Calderini D, Condon A, Vargas M (2007) Association of source/sink traits with yield, biomass and radiation use efficiency among random sister lines from three wheat crosses in a high-yield environment. Journal of Agricultural Science 145, 3–16.
| Association of source/sink traits with yield, biomass and radiation use efficiency among random sister lines from three wheat crosses in a high-yield environment.Crossref | GoogleScholarGoogle Scholar |
Royo C, Alvaro F, Martos V, Ramdani A, Isidro J, Villegas D, del Moral LFG (2007) Genetic changes in durum wheat yield components and associated traits in Italian and Spanish varieties during the 20th century. Euphytica 155, 259–270.
| Genetic changes in durum wheat yield components and associated traits in Italian and Spanish varieties during the 20th century.Crossref | GoogleScholarGoogle Scholar |
Sakuma S, Golan G, Guo Z, Ogawa T, Tagiri A, Sugimoto K, Bernhardt N, Brassac J, Mascher M, Hensel G, Ohnishi S, Jinno H, Yamashita Y, Ayalon I, Peleg Z, Schnurbusch T, Komatsuda T (2019) Unleashing floret fertility in wheat through the mutation of a homeobox gene. Proceedings of the National Academy of Sciences of the United States of America 116, 5182–5187.
| Unleashing floret fertility in wheat through the mutation of a homeobox gene.Crossref | GoogleScholarGoogle Scholar | 30792353PubMed |
Savage VM, Bentley LP, Enquist BJ, Sperry JS, Smith DD, Reich PB, von Allmen EI (2010) Hydraulic trade-offs and space filling enable better predictions of vascular structure and function in plants. Proceedings of the National Academy of Sciences of the United States of America 107, 22722–22727.
| Hydraulic trade-offs and space filling enable better predictions of vascular structure and function in plants.Crossref | GoogleScholarGoogle Scholar | 21149696PubMed |
Seki M, Feugier FG, Song XJ, Ashikari M, Nakamura H, Ishiyama K, Yamaya T, Inari-Ikeda M, Kitano H, Satake A (2015) A mathematical model of phloem sucrose transport as a new tool for designing rice panicle structure for high grain yield. Plant & Cell Physiology 56, 605–619.
| A mathematical model of phloem sucrose transport as a new tool for designing rice panicle structure for high grain yield.Crossref | GoogleScholarGoogle Scholar |
Sherman TF (1981) On connecting large vessels to small. The meaning of Murray’s law. Journal of General Physiology 78, 431–453.
| On connecting large vessels to small. The meaning of Murray’s law.Crossref | GoogleScholarGoogle Scholar | 7288393PubMed |
Shewry PR, Hey SJ (2015) The contribution of wheat to human diet and health. Food and Energy Security 4, 178–202.
| The contribution of wheat to human diet and health.Crossref | GoogleScholarGoogle Scholar | 27610232PubMed |
Shiferaw B, Smale M, Braun H-J, Duveiller E, Reynolds M, Muricho G (2013) Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Security 5, 291–317.
| Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security.Crossref | GoogleScholarGoogle Scholar |
Shinozaki K, Yoda K, Hozumi K, Kira T (1964) A quantitative analysis of plant form-the pipe model theory: I. Basic analyses. Japanese Journal of Ecology 14, 97–105.
Shitsukawa N, Kinjo H, Takumi S, Murai K (2009) Heterochronic development of the floret meristem determines grain number per spikelet in diploid, tetraploid and hexaploid wheats. Annals of Botany 104, 243–251.
| Heterochronic development of the floret meristem determines grain number per spikelet in diploid, tetraploid and hexaploid wheats.Crossref | GoogleScholarGoogle Scholar | 19491089PubMed |
Siddique KHM, Kirby EJM, Perry MW (1989) Ear stem ratio in old and modern wheat-varieties - relationship with improvement in number of grains per ear and yield. Field Crops Research 21, 59–78.
| Ear stem ratio in old and modern wheat-varieties - relationship with improvement in number of grains per ear and yield.Crossref | GoogleScholarGoogle Scholar |
Su Q, Zhang X, Zhang W, Zhang N, Song L, Liu L, Xue X, Liu G, Liu J, Meng D, Zhi L, Ji J, Zhao X, Yang C, Tong Y, Liu Z, Li J (2018) QTL detection for kernel size and weight in bread wheat (Triticum aestivum L.) using a high-density SNP and SSR-based linkage map. Frontiers in Plant Science 9, 1484.
| QTL detection for kernel size and weight in bread wheat (Triticum aestivum L.) using a high-density SNP and SSR-based linkage map.Crossref | GoogleScholarGoogle Scholar | 30364249PubMed |
Thomas SG (2017) Novel Rht-1 dwarfing genes: tools for wheat breeding and dissecting the function of DELLA proteins. Journal of Experimental Botany 68, 354–358.
| Novel Rht-1 dwarfing genes: tools for wheat breeding and dissecting the function of DELLA proteins.Crossref | GoogleScholarGoogle Scholar | 28201630PubMed |
Thorne JH (1985) Phloem unloading of C and N assimilates in developing seeds. Annual Review of Plant Physiology 36, 317–343.
| Phloem unloading of C and N assimilates in developing seeds.Crossref | GoogleScholarGoogle Scholar |
Wang E, Wang J, Zhu X, Hao W, Wang L, Li Q, Zhang L, He W, Lu B, Lin H, Ma H, Zhang G, He Z (2008) Control of rice grain-filling and yield by a gene with a potential signature of domestication. Nature Genetics 40, 1370–1374.
| Control of rice grain-filling and yield by a gene with a potential signature of domestication.Crossref | GoogleScholarGoogle Scholar | 18820698PubMed |
Weber H, Heim U, Golombek S, Borisjuk L, Wobus U (1998) Assimilate uptake and the regulation of seed development. Seed Science Research 8, 331–346.
| Assimilate uptake and the regulation of seed development.Crossref | GoogleScholarGoogle Scholar |
Weichert H, Hogy P, Mora-Ramirez I, Fuchs J, Eggert K, Koehler P, Weschke W, Fangmeier A, Weber H (2017) Grain yield and quality responses of wheat expressing a barley sucrose transporter to combined climate change factors. Journal of Experimental Botany 68, 5511–5525.
| Grain yield and quality responses of wheat expressing a barley sucrose transporter to combined climate change factors.Crossref | GoogleScholarGoogle Scholar | 29069444PubMed |
West GB, Brown JH, Enquist BJ (1997) A general model for the origin of allometric scaling laws in biology. Science 276, 122–126.
| A general model for the origin of allometric scaling laws in biology.Crossref | GoogleScholarGoogle Scholar | 9082983PubMed |
West GB, Brown JH, Enquist BJ (1999) A general model for the structure and allometry of plant vascular systems. Nature 400, 664–667.
| A general model for the structure and allometry of plant vascular systems.Crossref | GoogleScholarGoogle Scholar |
Wheeler TR, Hong TD, Ellis RH, Batts GR, Morison JIL, Hadley P (1996) The duration and rate of grain growth, and harvest index, of wheat (Triticum aestivum L.) in response to temperature and CO2. Journal of Experimental Botany 47, 623–630.
| The duration and rate of grain growth, and harvest index, of wheat (Triticum aestivum L.) in response to temperature and CO2.Crossref | GoogleScholarGoogle Scholar |
Whingwiri EE, Stern WR (1982) Floret survival in wheat – significance of the time of floret initiation relative to terminal spikelet formation. Agricultural Science 98, 257–268.
| Floret survival in wheat – significance of the time of floret initiation relative to terminal spikelet formation.Crossref | GoogleScholarGoogle Scholar |
Whingwiri EE, Kuo J, Stern WR (1981) The vascular system in the rachis of a wheat ear. Annals of Botany 48, 189–202.
| The vascular system in the rachis of a wheat ear.Crossref | GoogleScholarGoogle Scholar |
Wolde GM, Mascher M, Schnurbusch T (2019a) Genetic modification of spikelet arrangement in wheat increases grain number without significantly affecting grain weight. Molecular Genetics and Genomics 294, 457–468.
| Genetic modification of spikelet arrangement in wheat increases grain number without significantly affecting grain weight.Crossref | GoogleScholarGoogle Scholar | 30591960PubMed |
Wolde GM, Trautewig C, Mascher M, Schnurbusch T (2019b) Genetic insights into morphometric inflorescence traits of wheat. Theoretical and Applied Genetics 132, 1661–1676.
| Genetic insights into morphometric inflorescence traits of wheat.Crossref | GoogleScholarGoogle Scholar | 30762083PubMed |
Xie Q, Mayes S, Sparkes DL (2015) Carpel size, grain filling, and morphology determine individual grain weight in wheat. Journal of Experimental Botany 66, 6715–6730.
| Carpel size, grain filling, and morphology determine individual grain weight in wheat.Crossref | GoogleScholarGoogle Scholar | 26246614PubMed |
Xie Q, Mayes S, Sparkes DL (2016) Optimizing tiller production and survival for grain yield improvement in a bread wheat × spelt mapping population. Annals of Botany 117, 51–66.
| Optimizing tiller production and survival for grain yield improvement in a bread wheat × spelt mapping population.Crossref | GoogleScholarGoogle Scholar | 26424785PubMed |
Yang J, Zhang J, Wang Z, Xu G, Zhu Q (2004) Activities of key enzymes in sucrose-to-starch conversion in wheat grains subjected to water deficit during grain filling. Plant Physiology 135, 1621–1629.
| Activities of key enzymes in sucrose-to-starch conversion in wheat grains subjected to water deficit during grain filling.Crossref | GoogleScholarGoogle Scholar | 15235118PubMed |