The evolution of abscisic acid (ABA) and ABA function in lower plants, fungi and lichen
Wolfram HartungA Lehrstuhl Botanik I, der Universität Würzburg, Julius von Sachsplatz 2, D 97082 Würzburg, Germany. Email: hartung@botanik.uni-wuerzburg.de
This paper is part of an ongoing series: ‘The Evolution of Plant Functions’.
Functional Plant Biology 37(9) 806-812 https://doi.org/10.1071/FP10058
Submitted: 16 March 2010 Accepted: 27 May 2010 Published: 24 August 2010
Abstract
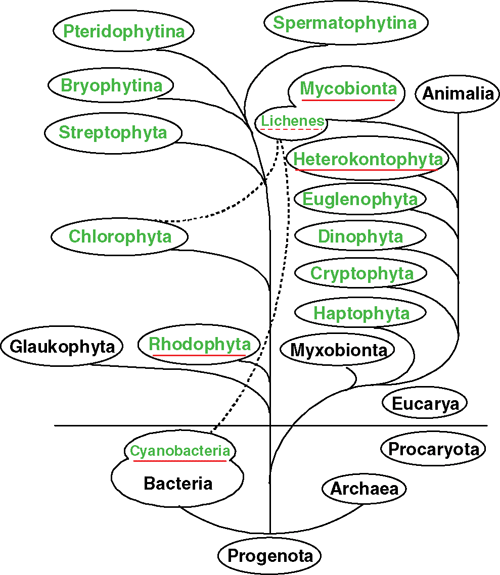
Abscisic acid (ABA) – the universal stress hormone of cormophytes – was detected in very low concentrations in almost all organisms tested from a range of cyanobacteria, algae, bryophytes, fungi and lichens and higher plants (Fig. 1). There are a few reports only on stress-induced ABA biosynthesis in cyanobacteria and algae. This extra ABA is released to the external medium. Application of external ABA has been shown to produce weak and contradicting effects on development and metabolism of algae. In most studies, extremely high concentrations of external ABA have been applied, those being far beyond any physiological concentration range. It is, therefore, extremely difficult to discuss those data satisfactorily. When organisms start to colonise terrestrial habitats (e.g. aquatic liverworts, mosses), endogenous ABA is increased even under mild drought stress, then desiccation protecting mechanisms are stimulated and the formation of terrestrial organs is induced. The same can be observed in water ferns (Marsilea) and in a range of heterophyllous angiosperms. Sporophytes of hornwort and mosses that bear true stomata, have particularly high ABA levels and their stomata respond to ABA as is the case in cormophytes, although a significant regulatory function of these stomata does not exist. Fungi produce large amounts of ABA that are released into the external medium and do not seem to have a function for the fungus. Fungal ABA, however, may be significant in associations of fungi with cyanophytes and algae (lichens), in mycorrhizal associations and in the rhizosphere of higher plants.
Additional keywords: algae, bacteria, cyanophytes, desiccation, landform, rhizosphere, soil, stomata, stress.
Introduction
In 1880, Charles Darwin postulated that endogenous substances of plants (‘matter’) were formed in the tips of the coleoptiles of Phalaris canarienensis in response to external influences and were then transported to a target tissue where they induce tropistic movements1 (Darwin 1880). From this, research into one of the most exciting fields of botanical sciences – the physiology of plant hormones – was initiated. Starting from Darwin’s ‘matter’, the five classical groups of phytohormones, auxins, gibberellins, cytokinins, abscisic acid and ethylene have been detected and investigated. Later on abscisic acid was shown to be such a ‘matter’ in tropistic movements of root tips.
Darwin was not greatly interested in stress effects on plants, or in external stresses that disturb the water balance of plants, otherwise he may have also postulated the existence of a stress hormone. In cormophytes, the phytohormone abscisisc acid has been proved to be the universal stress hormone, with a stress dependent biosynthesis, transport to target cells (predominantly stomata) and an action that enables the plant to cope better with the stress situation (e.g. reduction of water loss, induction of desiccation tolerance). Such can be seen in the relevant chapter in any textbook of botany and plant sciences.
In lower plants and prokaryotes the situation is more complex and contradictory. Although ABA and ABA-like substances, detected by bioassays, have been found since the late 1960s in cyanophytes and algae, no clear physiological functions of ABA could be demonstrated. Without a function, however, such substances only can be regarded as marginal compounds of the secondary metabolism without any significance for the development and metabolism in the organism.
In the following, I intend to show the evolution of ABA from a compound of secondary metabolism without a physiological function to a true stress hormone, the biosynthesis of which is stimulated under stress with beneficial effect on the organism, which enables it to survive the stress situation.
ABA in bacteria
Until recently it was well accepted that bacteria do not synthesise ABA. Hirsch et al. (1989) and Müller et al. (1989) investigated 22 bacteria species, many of them soil bacteria, including Azospirillum brasiliense, using extremely sensitive immunological methods. They could not find ABA. Recently, however, Forchetti et al. (2007) detected ABA in isolated strains of endophytic bacteria in roots of Helianthus annuus and Cohen et al. (2009) found ABA in Azospirillum brasiliense. When the rhizosphere of Zea mays was enriched with this bacterium, ABA was taken up by the roots and contributed there to an increased ABA.
Nothing is known about the biochemistry of ABA (biosynthesis and metabolism) or about a possible function of ABA for bacteria. The situation for bacteria is entirely unclear – considerable research is required in this field.
ABA in cyanobacteria
ABA was detected in 11 species of cyanobacteria (Hirsch et al. 1989; Zahradnickova et al. 1991; Marsalek et al. 1992a; Manickavelu et al. 2006). Four of them showed an increase of ABA biosynthesis under salt stress. (Trichormis variabilis; Zahradnickova et al. 1991; Nostoc muscorum, Synechococcus leopoliensis; Marsalek et al. 1992a; and Plectonema sp. from paddy fields, Manickavelu et al. 2006). In all these cases the extra ABA was released into the external medium, consequently, we do not have to expect significant stress-dependent increases of internal ABA.
A physiological role remains obscure. In response to external ABA application, Ahmad et al. (1978) observed an ABA induced growth of Anacystis nidulans, Huang (1991) a reduction of plasmamembrane permeability and Marsalek et al. a stimulation of nitrogenase activity (Marsalek and Simek 1992; Trichormus variabilis; Marsalek et al. 1992c; Nostoc muscorum; Simek and Marsalek 1992, 1993; Trichormus variabilis). In all cases, very high concentrations (>10−4 M) have been applied: concentrations that are well above physiological concentration ranges.
ABA in algae
Until now ~100 species of algae have been tested for ABA, 96% of which contained ABA (e.g. Hirsch et al. 1989; Jameson 1993; Hartung and Gimmler 1994).
The studies included the following algal divisions: Rhodophyta, Heterokontophyta, Haptophyta, Cryptophyta, Dinophyta, Euglenophyta, Chlorophyta and Streptophyta (Fig. 1). The the rare cases in which ABA could not be detected, the negative result is likely due to insufficiencies in the analytical methods applied (Enteromorpha compressa, Niemann and Dörffling 1980; Caulerpa paspaloides, Jacobs 1985; Fritschiella tuberosa, Tietz and Kasprik 1986; Porphyra, Zhang et al. 1993). For positive reports on ABA in Rhodophyta, including Porphyra, see Hirsch et al. (1989) and Yokoya et al. (2009) (12 Brazilian species).
|
ABA contents in algal cells vary between 7 and 34 nmol ABA kg–1 FW. These numbers are similar as those of liverwort growing under water (Hartung and Gimmler 1994) but significantly lower than those of unstressed terrestrial plants.
Only limited data are available about stress effects on algal ABA concentrations. Mostly salt stress has been applied to Dunaliella species (Hirsch et al. 1989; Cowan and Rose 1991; Cowan et al. 1992) and Chlorella vulgaris (Marsalek et al. 1992b). In all cases, salt stress increased ABA formation. ABA production was stimulated by nitrogen deficiency in Dunaliella sp. (Tominaga et al. 1993), heat stress in Chlorella vulgaris (Bajguz 2009) drought and acid stress in Stichococcus bacillaris and Chlorella vulgaris (Marsalek et al. 1992b), oxidative stress in Haematococcus pluvialis (Kobayashi et al. 1997, 1998), light stress in Chlorella minutissima (Stirk et al. 2005) and alkaline shock in Dunaliella acidophila (Hirsch et al. 1989). Compared with cormophytes (10–20-fold increase), the stress-dependent increase of ABA biosynthesis of cyanophytes and algae is much weaker (2–5-fold increase). In all cases, stress ABA appears to be released across the plasmamembrane to the surrounding medium and often it has been analysed only there. Because of the strong stress-dependent ABA efflux, cellular ABA may not be increased significantly. An effect of ABA that has been released from the algae to the surrounding media on aquatic organisms in the neighbourhood, as suggested by Hussain and Boney (1973), seems to be extremely unlikely, since it is extremely diluted.
A stress physiological function may exist in aerial terrestrial algae, such as Trentepohlia, Apatococcus and Desmococcus, especially with respect to drought stress. However, such investigations are absent.
ABA biosynthesis and metabolism in algae
Bopp-Buhler et al. (1991) and Cowan and Rose (1991) have observed incorporation of mevalonolacton into ABA of Dunaliella parva. This incorporation was increased by salt stress. They have also performed inhibitor experiments with fluridone, norflurazon and AMO 1618; compounds that clearly inhibit the formation of those carotenoids that are believed to be important precursors of ABA. From a relatively weak reduction of ABA biosynthesis, these authors conclude that at least a significant part of ABA is not formed from carotenoids.
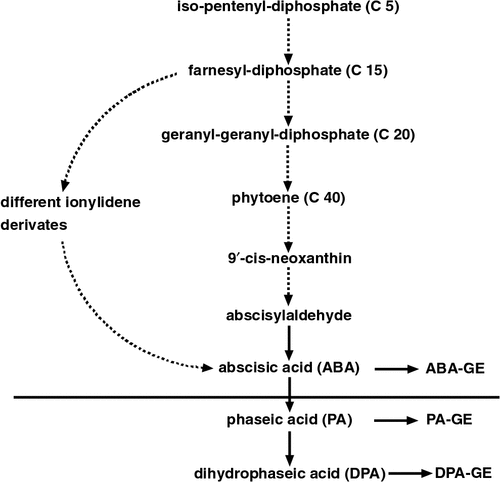
In contrast, 9′-cis-neoxanthin, a suitable substrate for ABA synthesis, was found in green algae that contain chlorophyll a and b. It was not found in other algal divisions such as Heterokontophyta, Rhodophyta and photoautotrophic prokaryotes. However, all these organisms form ABA. Therefore, different from cormophytes, the latter may synthesise ABA on the direct pathway via farnesyl-diphosphate (Figs 1, 2).
|
ABA effects on algae
There have been several attempts to elucidate the physiological function of ABA in algae by treatment of the cells with external ABA. Most of these experiments have been performed in a situation of extremely poor knowledge of the physiology of ABA in algae (e.g. occurrence, internal concentration, uptake and metabolism, permeabilities of membranes for ABA). Further, in most cases, extremely high external ABA concentration have been applied; these greatly exceeding any physiological situation and, therefore, producing pharmacological effects of only marginal significance. From this, contradicting data have been observed and they do not give a consistent picture (see reviews by Jameson 1993; Hartung and Gimmler 1994). For example, it has been shown that permeability of membranes to different solutes was either stimulated (Chara, Wanless et al. 1973; Ord et al. 1977) or reduced (Dunaliella, Hirsch et al. 1989). Ion uptake was stimulated (Wanless et al. 1973, Na+, K+, Cl–; Ullrich and Kunz 1984, nitrate) and inhibited, respectively (Hirsch et al. 1989, K+). Photosynthesis was not affected in most cases. Concerning respiration, Ullrich and Kunz (1984) observed stimulation, Huang (1991), however, inhibition.
Algal differentiation has been shown to be affected (gametogenesis in Chlamydomonas, Ishiura 1976; abscission of oospores in Chara, Vanden Driessche et al. 1997). ABA promotes growth in the cyanobacteria Nostoc and Anacystis, (Ahmad et al. 1978) and inhibits growth in Coscinodiscus (Kentzer and Mazur 1991). Hirsch et al. (1989) speculated that within algae, a positive correlation may exist between ABA content and the organisation level.
Tanaka et al. (2004) have shown that halotolerant Chlamydomonas strains express LEA genes (designated cw80 Lea3), which could be induced by salt and cold stresses, but not by ABA. This indicates that although ABA is present and may be stimulated under stress, a clear physiological function (e.g. in gene expression regulation) could not be established until now.
Recently it has been suggested several times that ABA may protect algal cells against photoinhibition in Chlamydomonas reinhardtii (Saradhi et al. 2000). Yoshida (2005) and Yoshida et al. (2003, 2004) found, in the same species, a protective effect of ABA against oxidative damage caused by salt and osmotic stress. Catalase and ascorbate peroxidase activities were shown to be significantly higher in ABA treated cells (Yoshida et al. 2003). Kobayashi et al. (1997, 1998) suggest that ABA protects Haematococcus pluvialis against oxidative stress. ABA may be important in mitigation of oxidative damage in stressed algae. Again, more research is required in this field.
ABA in bryophytes
ABA has been detected in Hepaticae, especially in the Marchantiopsida (Hartung and Gimmler 1994), in a few species of Anthocerotopsida (Hartung et al. 1987) and in a few mosses (Ergün et al. 2002, nine moss species) including the gametophytes of Funaria (Werner et al. 1991). In the Marchantiopsida, a large variability could be observed, ranging from 30 nmol g–1 FW in the extremely desiccation tolerant Exormotheca species to 1–10 pmol g–1 FW in the submerged living thallus of Riccia fluitans (Hartung and Gimmler 1994).
ABA biosynthesis and metabolism bryophytes
Very few, partly indirect, data are available regarding biosynthesis and metabolism of ABA. According to Takaichi and Mimuro (1998) the 9′-cis-neoxanthin is present in bryophytes, showing that an important precursor of the indirect biosynthetic pathway exists in bryophytes. Hellwege and Hartung (1997) detected phaseic acid, dihydrophaseic acid and the glucose ester of ABA in Riccia fluitans, suggesting that degradation of ABA occurs by similar reactions as known for cormophytes (Fig. 2).
ABA functions in bryophytes
The induction of land form characteristics
Some members of Marchantiopsida live submerged (Riccia fluitans) or on the surface of the water (Ricciocarpus natans) and experience developmental changes (e.g. increased lateral growth, formation of rhizoids and pores) when the thallus becomes terrestrial – as happens when a pond is drying out. During this period ABA increases 20–30-fold. Treatment of the submerged thallus with external ABA also induces the formation of landform characteristics, even when the thallus is forced to stay under water (Hellwege et al. 1992). This process is accompanied by changes of the pattern of proteins, including dehydrins and LEA proteins, which are believed to have an important function in the induction of desiccation tolerance (Hellwege et al. 1996). In submerged liverworts, ABA enables the organism to occupy the terrestrial environment. This resembles the induction of terrestrial leaves in the heterophyllous water fern (Marsilea quadrifolia, Lin et al. 2005) and angiosperms (Proserpinaca intermedia, Kane and Albert 1982; Ranunculus flabellaris, Young et al. 1987; Potamogeton nodosus, Gee and Anderson 1998; Hippuris vulgaris, Goliber 1989; Ludwigia arcuta, Kuwabara et al. 2003) that occurs under the control of ABA.
Other developmental changes induced by external ABA include inhibition of callus and protonema growth and gemmae formation (for references see Hartung and Gimmler 1994).
Desiccation tolerance
Those tissues of bryophytes (thallus of Exormotheca species, tubers of Anthoceros dichotomus) that have the highest endogenous ABA concentrations, also when fully hydrated, belong to those organisms or organs with the highest desiccation tolerance. ABA-treatment of mesophytic thalli of different species that are not desiccation tolerant (protonemata of Funaria hygrometrica, Olaf Werner et al. 1991; Marchantia polymorpha, Riccia fluitans, Hellwege et al. 1994, 1996; Pallavicina lyellii, Pence et al. 2005) and of non-hardened thalli of Exormotheca (Hellwege et al. 1994) increases desiccation tolerance significantly.
Stoma-bearing bryophytes
The sporophyte of Anthocerotopsida bears highly differentiated stomata of the Amaryllis type, whereas gametophyte thallus is extremely primitive. Hartung et al. (1987) found particularly high ABA concentrations in the sporophyte, which could be further increased under stress. The stomata reacted normally to increases in ABA (closing) and to fusicoccin (opening). The physiological function of ABA-responding stomata in Anthoceros is unclear. They cannot have a function comparable to those of cormophytes, because the Anthoceros-sporophyte is a only few mm high with stomata that mostly do not have a substomatal intercellalar space and that are not connected to a xylem like water transport system. Thus, a role in gas exchange and water transport can be only negligible. Hence, a stress physiological system including stomata that respond to ABA was developed at very early stage of evolution of land plants.
Garner and Paolillo (1973) have shown that stomata of the sporogone of Funaria respond in a similar way to ABA.
ABA in fungi
Assante et al. (1977) showed that Cercospora rosicola produces large amounts of ABA. Since then ABA has been detected in many species of ascomycetes, fungi imperfecti and basidiomycetes. A review on phytohormones in fungi including ABA has been published by Tudzynski and Sharon (2002) including a list of ABA producing fungi.
The fungal biosynthesis of ABA has been investigated in detail in different Cercospora species (C. rosicola, C. cruenta, C. pini-densiflori). All these investigations showed that ABA is formed directly via farnesyl-diphosphate, different ionylidene derivates and deoxyABA (Fig. 2; for references see Tudzynski and Sharon 2002). Kettner (1991), Kettner and Dörffling (1987) and Hartung and Eggeling (1984) were unable to find conversion of ABA to phaseic acid and dihydrophaseic acid, as was the case in autotrophic organisms including algae and liverworts (Fig. 2). The endogenous content is very likely regulated by release of ABA to the external medium.
Hartung and Eggeling (1984) have performed pH-dependent uptake experiments with radioactive ABA and C. cruenta and in contrast with cells from higher plants, observed high uptake rates at pH 8.0 when ABA is present predominantly as ABA-anion, thus showing that fungal cells do not function as an anion trap. They have also determined ABA efflux rates from the mycelium of C. cruenta and found them to be at least 10 times higher than in root tissues of Hordeum vulgare L. (barley). They concluded that the fungal plasma membrane exhibits a high permeability for ABA–, which causes a continuous release of newly synthesised ABA.
ABA biosynthesis in fungi
Norman et al. (1981) investigated the effects of the composition of external media on fungal ABA production. Compounds that can be used as carbon or nitrogen sources stimulate both growth and ABA production. The same is true for polyols such as sorbitol (Hartung and Eggeling 1984) and mannitol (Kitagawa et al. 1995), which are often used to create osmotic stress. In fungal cultures, polyols can be taken up by the mycelium and used as a carbon source. Consequently, polyols are not suitable as osmotica for fungi or for investigation of stress effects on fungal ABA formation. It remains unclear whether fungi exhibit a stress dependent ABA biosynthesis.
ABA functions in fungi
Only few published data are available on the effects of external ABA on fungi. There are two examples of weak stimulations on mycelium growth by external ABA (Stopinska and Michniewicz 1988). Growth of C. rosicola and C. cruenta was not affected by ABA (W. Hartung and S. Eggeling, unpubl. data).
Although ABA does not seem to have any function in fungi, fungal ABA maybe of some importance for plants infected with ABA-producing phytopathogenic fungi, in mycorrhizal associations and in lichens, respectively.
Leaves, infected by pathogenic fungi, often have increased ABA levels, which seem to be partly due to fungal ABA (Kettner 1991; Schmidt et al. 2008). Also, mycorrhizal fungi appear to be able to release ABA to roots (Murakami-Mizukami et al. 1991; Danneberg et al. 1992). In these cases, increases in ABA in plant tissues are mostly too weak (compared with stress-dependent increases) to trigger a stress physiological response. It also should be mentioned that the glomeromycote fungi involved in arbuscular mycorrhizas cannot be cultured in the absence of the host, which makes the investigation of the ABA relation of such a fungus very difficult.
ABA in lichens
Only a few studies have been published on ABA in lichens. Dietz and Hartung (1997, 1998) found ABA in 26 lichen species, Schroeter et al. (2000) detected ABA in 10 Antarctic species, Ott et al. (2000) in five and Ergün et al. (2002) in nine species.
In contrast with higher plants and liverworts, the ABA levels of lichens were increased transiently when dry thalli was moistened, but decreased significantly afterwards (Hypogymnia physoides, Leptogium sp., Parmelia sulcata, Peltigera praetextata, Usnea filipendula; Dietz and Hartung 1998). Biosynthesis of ABA in freshly hydrated thalli might be essential before the next desiccation because of the often short periods of thallus water saturation. The fungal partner is, most likely, the major site of ABA synthesis. Fungal ABA may be important to induce the formation of protective factors in the algae, to improve their desiccation tolerance.
Xanthoria parietina and Peltigera praetextata suffer less from excess of water when pre-treated with ABA or sprayed with ABA solutions (Dietz and Hartung 1999).
Conclusions
At present, we are a long way from understanding the function of ABA in bacteria, cyanophytes and algae. ABA seems to be ubiquitously distributed in cyanobacteria and algae. In many groups, where 9′-cis-neoxanthin is missing, ABA is likely to be synthesised directly via farnesyldiphosphate. Here, comparison of genome sequences, if available, with that from flowering plants might be useful in determining the biosynthetic pathways of ABA in cyanobacteria and algae that lack 9′-cis-neoxanthin. Degradation occurs similarly as in higher plants. In a few examples, salt stress was shown to induce ABA biosynthesis. However, this extra stress-ABA is released into the medium, resulting in a slight increase of internal ABA only. In bacteria the situation is even more obscure. Two positive reports conflict with 20 negative attempts to find ABA in bacteria. Nothing is known about ABA biosynthesis, degradation and function in bacteria.
No clear stress physiological functions of ABA in algae have been determined, although several recent reports suggest that algal ABA may have protective role against oxidative stress.
It cannot be excluded that early in evolution the complete biochemical equipment to form and metabolise ABA was invented in bacteria, cyanophytes and algae, although it may not have been essential for fitness of the organism. With the progress of evolution, when organisms such as submerged living liverworts started to occupy terrestrial habitats, ABA gained an important function as a stress hormone. In liverworts, ABA induces developmental changes resulting in terrestrial organs and tissues and it induces desiccation tolerance by forming protective proteins such as LEA proteins or dehydrins. Early in evolution, ABA proved to be effective in closing stomata of sporophytes of hornworts, although stomata seem to have no stress physiological function there that is comparable to that of cormophytes. In cormophytes, stomata proved to be the most important target cells of the stress hormone ABA in short-term regulation of water relations.
The situation for fungal ABA is similar. There is no clear significant function for the fungus itself. Fungal ABA that is synthesised in high amounts is released easily into the surrounding medium. It may positively affect stress physiological processes of plant partners such as algae in lichens or plant roots, which are surrounded by ABA-containing soil solution or associated to mycorrhizal fungi.
ABA released from soil microorganisms (bacteria, cyanobacteria, algae, fungi) to the soil solution and the rhizosphere has been shown to be important for plant ABA relations (Hartung et al. 1996) and very likely also for stress physiology of roots (Jiang and Hartung 2008).
In the case of algae and cyanobacteria it would be worth investigating aerial algae such as Trentepohlia or cyanobacteria on temporarily moistened and dry habitats such as rocks on land or seashores where desiccation stress is combined with salt stress.
Acknowledgements
The constructive comments of Professor Hermann Heilmeier (Technical University, Freiberg, Germany) and the help of Dr Jiang Fan (Beijing Normal University, Beijing) with the figures are gratefully acknowledged.
Ahmad MR,
Saxena PM,
Amla DV, Shyam R
(1978) Effect of abscisic acid on the blue green algae Anacystis nidulans and Nostoc muscorum. Beiträge zur Biologie der Pflanzen 54, 33–40.

Assante G,
Merlini L, Nasini G
(1977) (+)-Abscisic acid, a metabolite in the fungus Cercospora rosicola. Experientia 33, 1556–1557.
| Crossref | GoogleScholarGoogle Scholar |

Bajguz A
(2009) Brassinosteroid enhanced the level of abscisic acid in Chlorella vulgaris subjected to short term heat stress. Journal of Plant Physiology 166, 882–886.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Bopp-Buhler ML,
Wabra P,
Hartung W, Gimmler H
(1991) Evidence for direct ABA synthesis in Dunaliella (Volvocales). Cryptogamic Botany 2/3, 192–200.

Cohen AC,
Travaglia CN,
Bottini R, Piccoli PN
(2009) Participation of abscisic acid and gibberellins produced by endophytic Azospirillum in the alleviation of drought effects in maize. Botany 87, 455–462.
| Crossref | GoogleScholarGoogle Scholar |

Cowan AK, Rose PD
(1991) Abscisic acid metabolism in salt-stressed cells of Dunaliella salina. Plant Physiology 97, 798–803.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Cowan AK,
Rose PD, Horne LG
(1992) Dunaliella salina: a model system for studying the response of plant cells to stress. Journal of Experimental Botany 43, 1535–1547.
| Crossref | GoogleScholarGoogle Scholar |

Danneberg G,
Latus C,
Zimmer W,
Hundshagen B,
Schneider-Poetsch JH, Bothe H
(1992) Influence of vesicular arbuscular mycorrhiza on phytohormone balances in maize (Zea mays L.). Journal of Plant Physiology 141, 33–39.

Dietz S, Hartung W
(1997) Regulation of the abscisic acid content in the two lichen species Hypogymnia physoides and Peltigera praetextata. Bibliotheca Lichenologica 20, 119–126.

Dietz S, Hartung W
(1998) Abscisic acid in lichens: variation, water relations and metabolism. New Phytologist 138, 99–106.
| Crossref | GoogleScholarGoogle Scholar |

Dietz S, Hartung W
(1999) The effect of abscisic acid (ABA) on chlorphyll fluorescence in lichens under extreme water regimes. New Phytologist 143, 495–501.
| Crossref | GoogleScholarGoogle Scholar |

Ergün N,
Topcuoglu SF, Yildiz A
(2002) Auxin (indole-3-acetic acid) gibberellic acid (GA3), abscisic acid (ABA) and cytokinin (zeatin) production in some species of mosses and lichens. Turkish Journal of Botany 26, 13–18.

Forchetti G,
Masciarelli O,
Alemano S,
Alvarez D, Abdala G
(2007) Endophytic bacteria in sunflower (Helianthus annuus L.): isolation, characterization, and production of jasmonates and abscisic acid in culture medium. Applied Microbiology and Biotechnology 76, 1145–1152.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Garner LB, Paolillo DJ
(1973) On the function of the stomata in Funaria. The Bryologist 76, 423–427.
| Crossref | GoogleScholarGoogle Scholar |

Gee D, Anderson LWJ
(1998) Influence of leaf age on responsiveness of Potamogeton nodosus to ABA induced heterophylly. Plant Growth Regulation 24, 119–125.
| Crossref | GoogleScholarGoogle Scholar |

Goliber TE
(1989) Endogenous ABA content correlates with photon fluence rate and induced leaf morphology in Hippuris vulgaris. Plant Physiology 89, 732–734.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Hartung W, Gimmler H
(1994) A stress physiological role for abscisic acid (ABA) in lower plants. Progress in Botany 55, 157–173.

Hartung W,
Weiler EW, Volk OH
(1987) Immunochemical evidence that abscisic acid is produced by several species of Anthocerotae and Marchantiales. The Bryologist 90, 393–400.
| Crossref | GoogleScholarGoogle Scholar |

Hartung W,
Sauter A,
Turner NC,
Fillery I, Heilmeier H
(1996) Abscisic acid in soils: what is its function and which factors influence its concentration? Plant and Soil 184, 105–110.
| Crossref | GoogleScholarGoogle Scholar |

Hellwege EM, Hartung W
(1997) Synthesis, metabolism and compartmentation of abscisic acid in Riccia fluitans L. Journal of Plant Physiology 150, 287–291.

Hellwege E,
Volk OH, Hartung W
(1992) A physiological role of abscisic acid in the liverwort Riccia fluitans. Journal of Plant Physiology 140, 553–556.

Hellwege E,
Dietz KJ,
Volk OH, Hartung W
(1994) Abscisic acid and the induction of desiccation resistance in the extremely xerophilic liverwort Exormotheca holstii. Planta 194, 525–531.
| Crossref | GoogleScholarGoogle Scholar |

Hellwege E,
Dietz KJ, Hartung W
(1996) Abscisic acid causes changes in gene expression involved in the induction of the landform in the liverwort Riccia fluitans L. Planta 198, 423–432.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Hirsch R,
Hartung W, Gimmler H
(1989) Abscisic acid content of algae under stress. Botanica Acta 102, 326–334.

Huang CY
(1991) Regulation of ionic fluxes and protein release from Anabaena HA101 by exogenous abscisic acid. Botanical Bulletin Academia Sinica 32, 265–270.

Hussain A, Boney AD
(1973) Hydrophilic growth inhibitors from Laminaria digitata and Ascophyllum nodosum. New Phytologist 72, 403–410.
| Crossref | GoogleScholarGoogle Scholar |

Ishiura M
(1976) Gametogenesis of Chlamydomonas in the dark. Plant & Cell Physiology 17, 1141–1151.

Jiang F, Hartung W
(2008) Long-distance signalling of abscisic acid (ABA): the factors regulating the intensity of the ABA signal. Journal of Experimental Botany 59, 37–43.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Kane ME, Albert LS
(1982) Environmental and growth regulator effects on heterophylly and growth of Proserpinaca intermedia Haloragaceae. Aquatic Botany 13, 73–85.
| Crossref | GoogleScholarGoogle Scholar |

Kentzer T, Mazur H
(1991) Abscisic acid as endogenous inhibitor of the marine diatom Coscinodiscus granii. Acta Physiologiae Plantarum 13, 153–157.

Kettner J, Dörffling K
(1987) Abscisic acid metabolism in Ceratocystis coerulescens. Physiologia Plantarum 69, 278–282.
| Crossref | GoogleScholarGoogle Scholar |

Kitagawa Y,
Yamamoto H, Oritani T
(1995) Biosynthesis of abscisic acid in the fungus Cercospora cruenta: stimulation of biosynthesis by water stress and isolation of a transgenic mutant with reduced biosynthetic capacity. Plant & Cell Physiology 36, 557–564.

Kobayashi M,
Hirai N,
Kurimura Y,
Ohigashi H, Tsuji Y
(1997) Abscisic acid dependent morphogenesis in the unicellular green alga Haematococcus pluvialis. Plant Growth Regulation 22, 79–85.
| Crossref | GoogleScholarGoogle Scholar |

Kobayashi M,
Todoroki Y,
Hirai N,
Kurimura Y,
Ohigashi H, Tsuji Y
(1998) Biological activities of abscisic acid analogs in the morphological change of the green alga Haematococcus pluvialis. Journal of Fermentation and Bioengineering 85, 529–531.
| Crossref | GoogleScholarGoogle Scholar |

Kuwabara A,
Ikegami K,
Koshiba T, Nagata T
(2003) Effects of ethylene and abscisic acid upon heterophylly in Ludwigia arcuata (Onagraceae). Planta 217, 880–887.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Lin BL,
Wang HJ,
Wang JS,
Zaharia LI, Abrams SR
(2005) Abscisic acid regulation of heterophylly in Marsilea quadrifolia L.: effects of R-(–) an S-(+) isomers. Journal of Experimental Botany 56, 2935–2948.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Manickavelu A,
Nadarajan N,
Ganesh SK,
Ramalingam R,
Raguraman S, Gnanamalar RP
(2006) Organogenesis induction in rice callus by cyanobacterial extracellular product. African Journal of Biotechnology 5, 437–439.

Marsalek B, Simek M
(1992) Abscisic acid and its synthetic analog in relation to growth and nitrogenase activity of Azotobacter chroococcum and Nostoc muscorum. Folia Microbiologica 37, 159–160.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Marsalek B,
Zahradnickova H, Hronkova M
(1992a) Extracellular abscisic acid produced by cyanobacteria under salt stress. Journal of Plant Physiology 139, 506–508.

Marsalek B,
Zahradnickova H, Hronkova M
(1992b) Extracellular production of abscisic acid by soil algae under salt, acid or drought stress. Zeitschrift für Naturforschung 47c, 701–704.

Marsalek B,
Simek M, Lukesova A
(1992c) The effect of abscisic and gibberellic acids on nitrogenase activity and growth of the cyanobacterium Nostoc muscorum AGARDH. Algological Studies 66, 119–127.

Müller M,
Deigele C, Ziegler H
(1989) Hormonal interaction in the rhizosphere of maize (Zea mays L.) and their effects on plant development. Zeitschrift für Pflanzenernährung und Bodenkunde 152, 247–254.
| Crossref | GoogleScholarGoogle Scholar |

Murakami-Mizukami Y,
Yamamoto Y, Yamaki S
(1991) Analyses of IAA and abscisic acid contents in nodules of soy bean plants bearing VA mycorrhizas. Soil Science and Plant Nutrition 37, 291–298.

Niemann DI, Dörffling K
(1980) Growth inhibitors and growth promoters in Enteromorpha compressa (Chlorophyta). Journal of Phycology 16, 383–389.
| Crossref | GoogleScholarGoogle Scholar |

Norman SM,
Maier VP, Echols SLC
(1981) Influence of nitrogen source, thiamine and light on biosynthesis of abscisic acid by Cercospora rosicola. Applied and Environmental Microbiology 41, 981–985.
| PubMed |

Ord GNSG,
Cameron IF, Fensom DS
(1977) The effect of pH and ABA on hydraulic conductivity of Nitella membranes. Canadian Journal of Botany 55, 1–4.
| Crossref | GoogleScholarGoogle Scholar |

Ott S,
Krieg T,
Spanier U, Schieleit P
(2000) Phytohormones in lichens with emphasis on ethylene biosynthesis and functional aspects on lichen symbiosis. Phyton 40, 83–94.

Pence VC,
Dunford SS, Redella S
(2005) Differential effects of abscisic acid on desiccation tolerance and carbohydrates in three species of liverworts. Journal of Plant Physiology 162, 1331–1337.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Saradhi PP,
Suzuki I,
Katoh A,
Sakamoto A,
Sharmila P,
Shi DJ, Murata N
(2000) Protection against the photoinduced inactivation of the photosystem II complex by abscisic acid. Plant, Cell & Environment 23, 711–718.
| Crossref | GoogleScholarGoogle Scholar |

Schmidt K,
Pflugmacher M,
Klages S,
Mäser A,
Mock A, Stahl DJ
(2008) Accumulation of the hormone abscisic acid (ABA) at the infection site of the fungus Cercospora beticola supports the role of ABA as a repressor of plant defence in sugar beet. Molecular Plant Pathology 9, 661–673.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Schroeter B,
Schlensog M, Hartung W
(2000) Abscisic acid in antarctic lichens. Bibliotheca Lichenologica 75, 93–98.

Simek M, Marsalek B
(1992) Evidence for abscisic acid caused enhancement of nitrogenase activity in Trichormus variabilis. Algological Studies 67, 91–102.

Simek M, Marsalek B
(1993) Response of the cyanobacterium Nostoc muscorum to abscisic acid and its analog LAB. Algological Studies 71, 75–79.

Stirk WA,
Drimalova D,
Strnad M,
van Staden J,
Oerdoeg V, Balint P
(2005) Diurnal fluctuations in the phytohormones of Chlorella minutissima. Phycologia 44(Suppl. S), 98–99.

Stopinska J, Michniewicz M
(1988) Control of growth and development of Ceratocystis fimbriata Ell. et Halst. by plant growth regulators. II. Abscisic acid. Bulletin of the Polish Academy of Sciences Biological Sciences 36, 253–258.

Takaichi S, Mimuro M
(1998) Distribution and geometric isomerism of neoxanthin in oxygenic phototrophs: 9′-cis, a sole molecular form. Plant & Cell Physiology 39, 968–977.

Tanaka S,
Ikeda K, Miyasaka H
(2004) Isolation of a new member of group 3 late embryogenesis abundant protein gene from a halotolerant gree alga by a functional expressiom screening with cyanobacterial cells. FEMS Microbiology Letters 236, 41–45.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Tietz A, Kasprik W
(1986) Identification of abscisic acid in green alga. Biochemie und Physiologie der Pflanzen 181, 259–266.

Tominaga N,
Takahata M, Tominaga H
(1993) Effects of NaCl and KNO3
– concentrations on the abscisic acid content of Dunaliella sp. (Chlorophyta). Hydrobiologia 267, 163–168.
| Crossref | GoogleScholarGoogle Scholar |

Ullrich WR, Kunz G
(1984) Effect of abscisic acid on nitrate uptake respiration and photosynthesis in green algae. Plant Science Letters 37, 9–14.
| Crossref | GoogleScholarGoogle Scholar |

Vanden Driessche T,
Petiau-de Vries GM, Guisset JL
(1997) Differentiation, growth and morphogenesis: Acetabularia as a model system. New Phytologist 135, 1–20.
| Crossref | GoogleScholarGoogle Scholar |

Wanless IR,
Bryniak N, Fensom DS
(1973) The effect of some growth regulating compounds upon electroosmotic measurements, transcellular water flow, and Na, K and Cl influx in Nitella flexilis. Canadian Journal of Botany 51, 1055–1070.
| Crossref | GoogleScholarGoogle Scholar |

Werner O,
Ros Espín RM,
Bopp M, Atzorn R
(1991) Abscisic-acid-induced drought tolerance in Funaria hygrometrica Hedw. Planta 186, 99–103.
| Crossref |

Yokoya NS,
Stirk W,
Strnad M, van Staden J
(2009) Endogenous cytokinins, auxin and abscisic acid in Brazilian red algae. Phycologia 48(4, Suppl. S), 147–148.

Yoshida K
(2005) Evolutionary process of stress response systems controlled by abscisic acid in photosynthetic organisms. Yakugaku Zasshi 125, 927–936.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Yoshida K,
Igarashi E,
Mukai M,
Hirata K, Miyamato K
(2003) Induction of tolerance to oxidative stress in green alga, Chlamydomonas reinhardtii, by abscisic acid. Plant, Cell & Environment 26, 451–457.
| Crossref | GoogleScholarGoogle Scholar |

Yoshida K,
Igarashi E,
Wakatsugi E,
Myamoto K, Hirata K
(2004) Mitigation of osmotic and salt stresses by abscisic acid through reduction of stress derived oxidative damage in Chlamydomonas reinhardtii. Plant Science 167, 1335–1341.
| Crossref | GoogleScholarGoogle Scholar |

Young JP,
Dengler NG, Horton RF
(1987) Heterophylly in Ranunculus flabellaris. The effect of abscisic acid on leaf anatomy. Annals of Botany 60, 117–126.

Zahradnickova H,
Marsalek B, Polisenska M
(1991) High performance thin layer chromatographic and high performance liquid chromatographic determination of abscisic acid produced by cyanobacteria. Journal of Chromatography. A 555, 239–245.
| Crossref | GoogleScholarGoogle Scholar |

Zhang W,
Yamane H, Chapman DJ
(1993) The phytohormone profile in the red alga Porphyra perforata. Botanica Marina 36, 257–266.
| Crossref | GoogleScholarGoogle Scholar |

1 1The results seem to imply the presence of ‘some matter’ in the upper part which is acted on by light and which transmits its effect to the lower part.


