Microbiome engineering to combat antimicrobial resistance and upsurge productivity of food animals: a systematic review
Al-Reem A. Johar A , Lubna I. Abu-Rub B , Hassan Al Mana B , Hadi M. Yassine B and Nahla O. Eltai B *
B *
A Research and Development Department, Barzan Holdings, Doha 7178, Qatar.
B Microbiology Department, Biomedical Research Centre, Qatar University, Doha 2713, Qatar.
Animal Production Science 63(2) 101-112 https://doi.org/10.1071/AN22233
Submitted: 24 June 2022 Accepted: 10 August 2022 Published: 12 September 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Extensive antimicrobial usage in animal farming plays a prominent role in the antimicrobial resistance (AMR) crisis and is repeatedly highlighted as an area needing development under the ‘One Health’ approach. Alternative therapies such as microbiome products can be used as prophylaxis to help avoid infectious disease. However, a limited number of studies have focused on AMR-targeted microbiome products. We conducted this systematic review by using PRISMA guidelines to screen for literature that have evaluated food animals’ health when administrated with microbiome products targeting antimicrobial resistance (AMR) or antibiotic-resistant genes (ARGs). We searched and examined studies from SCOPUS, Web of Science, Embase, and Science direct databases for studies published up to November 2021, restricted to the English language. The findings of this review showed that microbiome products have a promising capability to tackle specific AMR/ARGs coupled with animal’s health and productivity improvement. Furthermore, our study showed that probiotics were the most favourable tested microbiome products, with the most targeted resistance being to tetracycline, macrolides, and beta-lactams. While microbiome products are promising alternatives to antibiotic prophylactics, there is a dearth of studies investigating their efficacy in targeting AMR. Thus, it is highly recommended to further investigate, develop, and improve the microbiome, to better understand their utility and circumvent their limitations.
Keywords: AMR, antimicrobials, ARG, bacteria, food animals, microbiome, microbiome products, probiotics.
Introduction
Antimicrobials are used extensively as treatments, prophylactics, and growth promoters in large-scale animal-farming systems (Kimera et al. 2020). According to the United Nations Report (2019), the world population is estimated to be 9.7 billion by 2050 (United Nations PD 2019). This increased population will proliferate the demand for food-producing animals and their products. To fulfil this demand, several countries are shifting to intensive livestock production systems that use antimicrobial (AM) treatments to maintain animal health and improve growth and productivity (Kober et al. 2022). Accordingly, the global consumption of AMs used for food animals is predicted to increase by up to 67%, from 63 151 Mg in 2010 to 105 596 Mg in 2030 (Xiong et al. 2018). This dependence on antimicrobials has contributed to the increase in AM resistance (AMR), which was predicted by Sir Alexander Fleming, who introduced the first antibiotic, during his Nobel prize speech in 1945 (Diarra et al. 2021). AMR is a natural process and a common defence mechanism in bacteria. It can lead to difficulties in treatment and increases healthcare costs, especially in the case of multidrug resistance. In 2018, it was estimated that the numbers of deaths from AMR were about 700 000 per year (Seong et al. 2021). An additional consequence of the long-lasting practice of subtherapeutic antibiotic doses in food animals is the selection of antibiotic-resistant bacteria (ARB), which in some cases may increase the mutation rate (Revitt-Mills and Robinson 2020; Zalewska et al. 2021). ARB can transfer antibiotic-resistance genes (ARGs) to other enteric bacteria in the host’s intestinal tract (Zalewska et al. 2021). For instance, the transfer of ciprofloxacin, azithromycin, or tigecycline resistance has been detected in Pseudomonas aeruginosa and Enterococcus faecalis (Brooks et al. 2021). Food-producing animal farms are ARB hotspots (Alhababi et al. 2020; Eltai et al. 2020a, 2020b; Guo et al. 2021). There have been many studies on the extensive use of antibiotics in livestock and aquaculture industries and the potential risks posed to animal and public health through the spread of AMR (Baquero 2012; Kimera et al. 2020; Kim et al. 2021; Yun et al. 2021; Lin et al. 2021; Pissetti et al. 2021).
Several efforts have been made to minimise excessive AM usage in food-producing animal farms. The European Union banned their use as growth promoters and prophylactics, and the United States significantly reduced their usage in food animals (Kogut 2017; World Health Organization 2017). Nevertheless, AMs remain necessary for limiting disease in food animals and maintaining production levels to meet global demand. An important aspect of AM stewardship is identifying alternatives to continue treating animals but limiting AMR spread (Ricker et al. 2020). One promising approach is microbiome engineering (Foo et al. 2017; Kogut 2017; Cullen et al. 2020; Bae et al. 2021; Diarra et al. 2021). Studies have shown that manipulation of domesticated animal microbiomes can be a powerful tool to reduce morbidity and fight infectious diseases. The most studied animal microbiome engineering products are probiotics and prebiotics; however, there are reports of the use of postbiotics and combinations of the three (synbiotics; Jin Song et al. 2019; Kober et al. 2022).
Prebiotics are non-digestible food ingredients that stimulate the growth and/or activity of beneficial gut microbiota (Mountzouris 2022). These authors demonstrated an improvement in the abundance of beneficial bacteria such as Bifidobacterium and/or Lactobacillus spp., which help in digestion, defence against pathogens, constipation relief, and shift the microbial populations reducing pathogen numbers (Cullen et al. 2020). Probiotics are viable ingestible microorganisms obtained from a healthy donor. They are used to restore or enhance gut microbiota. The best-studied probiotics include members of the genera Bacillus, Lactobacillus, Bifidobacterium, Enterococcus, Lactococcus, Megasphaera, Pediococcus, and Propionibacterium (Wideman et al. 2015; Jin Song et al. 2019). These bacteria aid in fibre fermentation, regulate inflammatory responses, help amino acid and vitamin production, and support the maintenance of gut–brain axis (Yan and Polk 2020).
Additionally, these organisms play a crucial role in improving host immunity against pathogens by preventing colonisation or proliferation through competition, releasing antimicrobial molecules, and improving the intestinal barrier function and immunomodulation (Wan et al. 2019; Cullen et al. 2020). Most notably, probiotics can fight infectious diseases in animals, thus reducing the pressure on antibiotic use (Jin Song et al. 2019). The most frequently used probiotics in livestock are the lactic acid bacteria and Bifidobacterium strains (Kober et al. 2022). Postbiotics, are products or metabolic by-products secreted by bacteria or released after bacterial lysis (Aguilar-Toalá et al. 2018; Wan et al. 2019). An example of postbiotics are short-chain fatty acids (SCFAs), enzymes, peptides, and organic acids. Notably, organic acids were found to have an AM effect against ARB (Roth et al. 2017).
Several studies have investigated microbiome products as substitutes for AMs for improving food-producing animal production and animal health (Han et al. 2017; Ayala et al. 2019; González-Ortiz et al. 2020; Helmy et al. 2020; Bae et al. 2021; Zhe et al. 2021; Pham et al. 2022). Yet, data on their effects on AMR are limited. Therefore, reviewing major literature databases and selecting original studies from a set of criteria may help identify the missing gap in the impact of microbiome products in fighting AMR in food animals. Herein, we present a systematic review that may unify the assessment of microbiome products in combating antimicrobial resistance in food animals. The main objectives extended to investigate the common type and composition of the products used for food animals and evaluate their effects on animals’ state of health and productivity.
Materials and methods
Database searches
This systematic review was performed following the PRISMA checklist for standards for systematic reviews (Moher et al. 2009). Three databases were screened on 28 November 2021, no date limits were applied. The filtered databases were ScienceDirect, SCOPUS, and Web of Science. The search was updated on 14 March 2022, by adding a fourth database (Embase), with date restrictions for the articles published before 28 November 2021. Additional articles were identified by searching the references of the included studies and the first 100 results of Google Scholar.
The search strategy included terms on the topics of animal, microbiome, antimicrobial resistance, and antibiotics, consistent with the eligibility criteria. One example of the exact search string was (‘(animal)’ AND ‘(microbiome)’ AND ‘(antimicrobial resistance)’ AND ‘(antibiotics)’). Only studies published in English were included.
Study screening
All studies were imported into Zotero, and duplicates were removed using the built-in ‘Find Duplicates’ feature. The titles and abstracts were independently screened by two independent reviewers (LA and A-RJ). The following three questions were used to determine whether the study met the eligibility criteria:
-
Does the paper describe a primary research study?
-
Does the paper describe the use of microbiome products (prebiotics, probiotics, postbiotics, or synbiotics) and have they been tested on food animals?
-
Does the paper include the outcome of using the microbiome products on the antimicrobial-resistant bacteria and animals’ productivity?
Full-text review using the same criteria was performed for (1) studies that met all of the inclusion criteria and (2) studies for which this could not be conclusively determined. Studies that did not meet all eligibility criteria were excluded. Full-text review was similarly performed by two independent reviewers (LA and A-RJ). A third independent reviewer (HA) resolved disputes between the two reviewers during the title/abstract screening and full-text review stages. The reviewers screened the references in the included papers after completing data extraction. The titles and abstracts of the references were screened by the reviewer (LA), following the same criteria as in the original search and then double-checked by the reviewer (A-R J). The full texts of these articles were reviewed following the same process as above.
Data extraction
The author (LA) reviewed all full-text studies meeting the initial criteria and extracted data from included papers using a data-collection form. The following information was recorded for each manuscript where applicable: the author, publication year, country, food animal and the number of animals, type of microbiome products (prebiotics, probiotics, postbiotics, or synbiotics), proposed mode of administration, targeted ARB, effect on or ARG abundance, outcome measures evaluated (effect on animal health/production), and the authors’ conclusions. The data were then reviewed by the author (A-R J) for final inclusion in the review, duplicate screening, eligibility, and quality assurance. Any disagreements were resolved by consensus.
Data synthesis
The primary outcome was the effect of the product on the abundance of ARB or the ARGs. We also assessed the studies according to the frequently studied food animals and the most investigated AM-resistant bacteria or genes. Likewise, we examined the the effects of the product on animals’ health. We stratified the microbiome products according to their components (prebiotics, probiotics, postbiotics, or synbiotics) to find the rate of its effect on the AMR/ARGs in animals. In each study, ARB or gene results were then sorted as showing an increase, decrease, or no change in resistance.
All study results were compared in regard to the efficiency of the applied products on AMR abundance and the outcomes.
Results
The search and selection processes are shown in Fig. 1. In total, 925 records were identified from the databases and manual searches. Removing duplicates resulted in 755 records for the initial title and abstract screening. From which, only 49 papers were retained. After reviewing the full texts, eight studies were included from the search, and two were abstracts only (Hofacre et al. 2002; Sommer-Lassa et al. 2019). In total, 402 references were retrieved from the eight included studies. Their titles and abstracts were screened, and only three studies met the inclusion criteria. The full texts of these were assessed, and all were included.
|
|
Information on the year of publication, country of the study, animal species studied, microbiome studied, investigated resistance, targeted bacteria, and other general characteristics are described in Tables 1–3. The earliest article was published in 2002, while all the remaining 10 papers were published from 2014 onward, with 63.6% of them published between 2014 and 2019. Only 27.3% were published in the last 2 years (i.e. 2020–2021). The United States was the most represented country (36.4%), followed by the Netherlands (18.2%). The five remaining studies were conducted in the United Kingdom, Ireland, Austria, Spain, and Colombia. It is worth mentioning that the study location in three of the included studies was not specified but was inferred from the first author’s primary affiliation (Hofacre et al. 2002; Delgado et al. 2014; Ceccarelli et al. 2017).

|
|

|
The most investigated food animals were chickens (63.6%), followed by bovines (e.g. steers, bulls, and heifers, at 36.4%). Pigs were investigated only in one study (9.1%). The average age of the food animals studied ranged from 1 day to 218.6 days. Most of the studied animals were young; all chickens investigated were chicks, and most of the studied bovines and pigs were weaned. Only two studies used adult animals for their investigation (e.g. bulls and heifers).
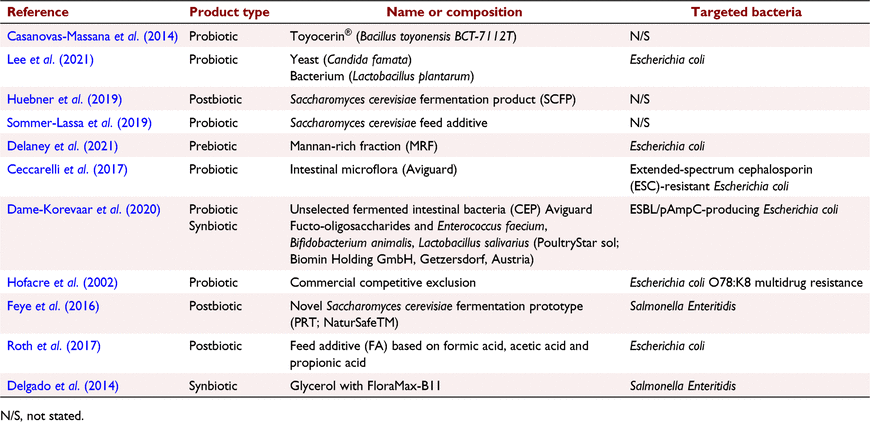
Information about the microbiome products and the targeted bacteria addressed in the included studies are summarised in Table 2. In general, most of the included studies investigated the effect of probiotics on AMR (54.5%). Postbiotics were tested in three studies, synbiotics in two studies, and prebiotics were evaluated only in one study. Many probiotic products contained Lactobacillaceae species or the products of Saccharomycetaceae species. Multiple routes of dietary product administration were used in all the studies, except one study in which a direct administration challenge was employed. Escherichia coli was the most frequently targeted bacteria in the included studies (45.5%), followed by Salmonella Enteritidis (18.2%). The targeted bacteria were not specified in four of the included studies.
The abundance of different AMR or ARGs in food animals was investigated in the included studies, to evaluate the efficacy of microbiome products in tackling AMR. Briefly, three included studies analysed resistance in both AMR and ARG, five studies targeted AMR only, and three studies targeted ARGs only. Only one study did not specify the AMR or ARGs of the investigated resistance. Interestingly, tetracycline resistance was the most frequently investigated in the included studies, followed by beta-lactam and macrolide resistance. Other investigated AMR and ARGs are shown in Table 3. Overall, 90.9% of the samples were from the gastrointestinal tract (GIT), including rumen, colon, small intestine, large intestine, whole intestine, duodenal and faecal swabs (Table 3). Alternatively, 9.1% of the collected samples were taken from organs other than the GIT, such as the yolk sac.
The effectiveness of the microbiome products on animal health and on AMR/ARG abundance was also evaluated. Generally, product effectiveness varied according to the type and composition of the microbiome product. In the case of probiotics, 50% of the studies described an increase in animal health, 25% reported no increase, and 25% did not specify the product effectiveness. All other types of microbiome products included in the review (prebiotic, postbiotic, and synbiotic) showed increased animal health and productivity. In terms of the effectiveness of the tested product on AMR and ARGs, varying results were observed in the included studies. A significant decrease in the AMR or ARG abundance was reported in 62.5% of the studies that examined probiotics, whereas the remaining 37.5% showed no effect. Likewise, precise results varying between an increase and a decrease and no impact on the AMR or ARGs abundance were reported while using the prebiotic product. The remaining products (postbiotic and synbiotic) reported a reduction in the number of the AMR or ARGs.
Discussion
To our knowledge, this study is the first systematic review providing an overview of the different microbiome products targeting antimicrobial resistance in food animals. Unfortunately, only a few studies have been concerned with fighting a specific AMR. Through the systematic search in four screened databases, only 11 studies fit our eligibility criteria. A possible reason is the established inclusion/exclusion criteria of this review. Many of the previously published studies were aimed to find alternatives for the AMs without specifying a certain AMR or focusing on a targeted ARG (Foo et al. 2017; Kogut 2017; Cullen et al. 2020; Bae et al. 2021; Diarra et al. 2021). Also, some investigated products fighting certain AMR in bacteria isolated from food animals but were tested in vitro, not on food animals. In addition, the English language restriction had been a debatable topic in terms of affecting the search strategies while conducting systematic reviews. Dobrescu et al. (2021) reported that English language restriction can slightly affect the estimations and conclusions for most medical topics.
Additionally, there are inconsistent definitions of the term ‘food-producing animals’. The WHO defined this term as ‘all terrestrial and aquatic animals (that is, includes aquaculture) used to produce food’ (World Health Organization 2017). Yet, distinct definitions are used by different countries or regions. For instance, cats, dogs, rats and other wild animals were considered food animals in China before the pandemic of COVID-19 (CMOA 2020). Therefore, more animal species were expected to be in the searching process.
The majority of the included studies were published from 2014 onward. This outcome emphasises how an investigation of the effect of microbiome products on fighting AMR of ARGs is still at a preliminary stage. Therefore, studying the impact of different microbiome products on the AMR or ARGs found in food animals needs further exploration. The United Nations Food and Agriculture Organization (FAO) classified the United States as one of the top food-animal producers (FAO 2021). As such, the findings of this review meet the expectation since most of the included studies were conducted in the USA (36.4%). Moreover, chickens were the most investigated animals in the included studies. Indeed, poultry production is among the widespread industries worldwide (Ma et al. 2021a). Chicken is one of the most commonly farmed species, with over 90 billion Mg of chicken meat produced yearly (Agyare et al. 2018). According to the FAO, more than 10 billion chickens were farmed by China in 2018 alone, and poultry meat production reached more than 100 million chickens globally (FAOSTAT 2018). Thus, special attention has been dedicated by researchers to studying chicken as a food animal.
Interestingly, nearly all the microbiome products of the included studies were tested on animals of a young age. Jackson et al. (2017) reported some limitations of using older animals, such as cost and maintaining historical-data comparability. Hence, young animals helped the researcher establish a baseline or control for the experiment, since young aged animals have less microbiome mixture (Jackson et al. 2017). Thus, they have less contact with the surrounding environment or other animals and no sexual activities (Xu and Zhang 2021); these factors could affect the microbiome mixture and its levels. As well, younger animals may have less possible antibiotic residues in their bodies (Basulira et al. 2019), which can affect the activity of the proposed product tested.
It is worth noting that food animals play a significant role in spreading resistant microorganisms into the environment through their manure and to the final consumer (human) either through their products such as milk or meat or by direct contact with farmworkers (Kumar et al. 2021). The prevention of AMR is associated with the One Health concept, which states that human health is related to the health of animals and the environment (Mackenzie and Jeggo 2019).
Xu et al. (2022) investigated the most prevalent ARB and ARGs in the farm animals considered a tremendous ARB and ARGs reservoir. They found that most resistance is to β-lactams (bla), aminoglycosides (aac), tetracyclines (tet), sulfonamides (sul), macrolide–lincosamide–streptogramin B (MLSB; erm), FCA (fluoroquinolone, quinolone, florfenicol, chloramphenicol, and amphenicol; fca), vancomycin (van), colistin (mcr), and multidrugs (mdr) (Xu et al. 2022). Interestingly, the most commonly used antibiotics in poultry-intensive production are tetracyclines (Mehdi et al. 2018). Skarżyńska et al. (2020) assessed the AMR epidemiology in different animal species, including farm and wild animals, and found that tetracycline resistance was occurring in almost all tested animals, followed by the resistance to macrolides, aminoglycosides, and β-lactams. Not surprisingly, our findings are comparable with the findings of the previous studies that tetracycline resistance is most targeted, followed by macrolide and beta-lactam resistance in different food animals (Mehdi et al. 2018; Skarżyńska et al. 2020; Ma et al. 2021a, 2021b; Xu et al. 2022). This is in agreement with the most reported antimicrobials used in food-animal production systems, namely, tetracycline, sulfonamides, β-lactams aminoglycoside, and penicillin (Kimera et al. 2020; Ma et al. 2021b). Tetracyclines were the most widely used antimicrobial class in veterinary medicine for decades (Skarżyńska et al. 2020). They represent more than two-thirds of antimicrobials administered in poultry production in the USA (Ma et al. 2021b).
Escherichia coli and Salmonella are the major causes of infections in poultry and other food animals such as cattle (Barrow et al. 2012; FDA 2020). For example, Salmonella is an important pathogen in chickens, causing septicaemic diseases such as fowl typhoid (FT) and pullorum disease (PD; Shivaprasad 2000). It has been found that E. coli and Salmonella have several antibiotic-resistant genes isolated from different animal meats and play a major role in AMR dissemination (Feye et al. 2016; Lee et al. 2021). Additionally, previous studies have shown that cattle and pigs are carriers of pathogenic E. coli, such as Shiga toxin-producing E. coli (STEC), and are considered pathways for introducing STEC into the environment (FDA 2020). Furthermore, these organisms have been known to acquire resistance through horizontal gene transfer (Frazão et al. 2019) With the emergence of AMR, the effectiveness of the AM has decreased, posing a risk to the consumer and a threat to public health (Chaudhary et al. 2014; Johar et al. 2021).
The bacteria most widely used as probiotics are Bacillus spp., Lactobacillus spp., Enterococcus spp. Bifidobacterium spp., and Streptococcus spp. (Abd El-Hack et al. 2020; Bhogoju and Nahashon 2022). In recent years, probiotic development has evolved away from bacteria and toward other species such as yeasts, such as Saccharomyces spp. (Elghandour et al. 2020; Ahiwe et al. 2021) and Candida spp. (Mokhber-Dezfouli et al. 2007). Our findings illustrated that Lactobacillus had been extensively used in the composition of the microbiome products. Dowarah et al. 2017 provided a review on the use of Lactobacillus as an alternative to antibiotic growth promoters in pigs. Their main outcomes supported the use of different species of Lactobacillus as an effective and safe alternative to antibiotics for swine production due to their high stability in vivo (Czerucka et al. 2007; Palma et al. 2015). Moreover, the present review has demonstrated that Saccharomyces cerevisiae, as a probiotic, is commonly used alongside the probiotic bacteria (Table 2). Its common usage is likely to be due to its natural presence in the environment, low cost and natural resistance to many antibiotics. Furthermore, its fermentation products reduced the AMR and food-safety pathogens detected in farm animals’ faeces (Huebner et al. 2019). Another important fact is that S. cerevisiae may not acquire genes as the bacterial probiotics do. Bacterial probiotics are capable of acquiring genes that confer resistance to antibiotics from other bacteria in the host, and pass them on to the bacterial pathogen (Temmerman et al. 2003; Mathur and Singh 2005). Hence, S. cerevisiae usage might reduce the spread of AMR or ARGs.
It is worth mentioning that bacteriophages have great potential to act as an alternative for antimicrobials. Laird et al. (2022) synergised bacteriophages with AMR-free commensal bacteria. They found that this mixture is capable of reducing and possibly eliminating drug-resistant bacteria in vitro (Laird et al. 2022). This study was included with the full-text screening but failed to meet the inclusion criteria as the product should be tested on food animals (in vivo studies). Another similar work that has been recently published demonstrated the effectiveness of using bacteriophages to reduce drug-resistant Salmonella colonisation in pigs (Thanki et al. 2022).
The potential advantages of feeding microbiome products to food animals is to improve their state of health are of growing interest. On the basis of our findings, it has been demonstrated that an intake of specific microbiome products increases the effectiveness of animals’ health. For example, a study conducted by Delaney et al. (2021) found that administering mannan-rich fraction (MRF), a prebiotic, to 16 broiler chickens, starting at birth and continuing to approximately 5 weeks of age, altered the microbiota balance. As a positive consequence, the treated broilers showed improvement in growth performance, indicating weight gain and a higher European production efficiency factor. These results are consistent with those of other similar studies (Feye et al. 2016; Ceccarelli et al. 2017; Roth et al. 2017). They may influence higher effectiveness in animal health achieved through a shift in the functional capability of the microbiota during the administration of microbiome products. This agrees with the findings of Al-Shawi et al. (2020) and Anee et al. (2021). Evidence of improved growth and feed efficiency, reduced mortality, and enhanced health was clearly shown after using probiotics. In their review, Al-Shawi et al. (2020) reported several studies that had shown an increase in the growth and production of animals, consequently improving health states. At the same time, Anee et al. (2021) discussed the general role of probiotics in poultry and ruminants. Their review showed the positive impact of probiotics in improving growth performance, reducing infection and diseases, and inducing beneficial immune response in poultry. As well as improving body weight and milk production, along with lowering infection and diarrhoea in ruminants. Another advantage of using microbiome products is that they do not leave residues, as antibiotics do, so that they can be a better choice as long-term prophylactics and growth enhancers. The continuous administering of probiotics can result in a maintained high state of the stimulated immune system (Lee et al. 2021).
Several studies have confirmed the capability of probiotics to improve animal health and inhibit pathogens. Even though investigations have shown that probiotic effectiveness is uncertain and can be affected by conditions (i.e. environment, sickness, diet), strain-dependent, and transient (Cameron and McAllister 2019). Likewise, probiotics can develop antimicrobial resistance, which can be taken by gene mutations or by horizontal gene transfer (Li et al. 2020) gained from the GIT, since it acts as a reservoir for ARGs (Daniali et al. 2020). Another issue that has been discussed is the poor quality of some commercial probiotic formulations that contain contamination with other microbes (Jackson et al. 2019; Anokyewaa et al. 2021). Also, the absence of standardised protocols for in vitro and in vivo investigations limits the evaluation of the potential of new species and strains, leading to an unclear correlation between the outcomes of both methods (Vinderola et al. 2017).
Conclusions
In conclusion, there is a global agreement that people and animal health are at high risk due to antibiotic resistance. Alternative therapies have been developed to reduce the dependence on AM in intensive animal farming. The present review illustrated that using probiotic-containing Lactobacillus and S. cerevisiae targeting specific AMR/ARGs is promising. Also, we have noticed the apparent gap in the efficacy of microbiome products to fight AMR/ARGs, since data on this topic are limited. Several investigations have targeted food-animal pathogens, but few have battled a specific AMR. Thus, further advanced studies on the effect of microbiome products for combating AMR/ARGs in food animals are needed. Experts from different fields should collaborate to improve the commercial microbiome products and develop novel therapeutics to tackle the ARB problem. Moreover, farmers should decrease or avoid the unnecessary use of antibiotics as a growth promoter in food animals to limit the spread of AMR.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Conflicts of interest
The authors declare no conflicts of interest. The funding bodies had no role in the study design, in the collection, analyses, or interpretation of data, in the writing of the paper, or in the decision to publish the results.
Declaration of funding
This research received no external funding.
Author contributions
Conceptualisation: NOE, and HMY; methodology: LA; validation: A-RJ, LA, HA, NOE, and HMY; formal analysis: A-RJ, LA and HA; data curation: LA, HA, A-RJ; writing – original draft preparation: A-RJ and LA; writing – review and editing: HA and NOE; supervision: NOE and HMY; project administration: NOE, and HMY. ‘All authors have read and agreed to the published version of the paper’.
Acknowledgements
The authors thank Barzan holdings for the financial support.
References
Abd El-Hack ME, El-Saadony MT, Shafi ME, Qattan SYA, Batiha GE, Khafaga AF, Abdel-Moneim AME, Alagawany M (2020) Probiotics in poultry feed: a comprehensive review. Journal of Animal Physiology and Animal Nutrition 104, 1835–1850.| Probiotics in poultry feed: a comprehensive review.Crossref | GoogleScholarGoogle Scholar |
Aguilar-Toalá JE, Garcia-Varela R, Garcia HS, Mata-Haro V, González-Córdova AF, Vallejo-Cordoba B, Hernández-Mendoza A (2018) Postbiotics: an evolving term within the functional foods field. Trends in Food Science & Technology 75, 105–114.
| Postbiotics: an evolving term within the functional foods field.Crossref | GoogleScholarGoogle Scholar |
Agyare C, Boamah VE, Zumbi CN, Osei FB (2018) Antibiotic use in poultry production and its effects on bacterial resistance. In ‘Antimicrobial resistance – a global threat’. (Ed. Y Kumar) pp. 33–51. (IntechOpen)
Ahiwe EU, Tedeschi Dos Santos TT, Graham H, Iji PA (2021) Can probiotic or prebiotic yeast (Saccharomyces cerevisiae) serve as alternatives to in-feed antibiotics for healthy or disease-challenged broiler chickens? A review. Journal of Applied Poultry Research 30, 100164
| Can probiotic or prebiotic yeast (Saccharomyces cerevisiae) serve as alternatives to in-feed antibiotics for healthy or disease-challenged broiler chickens? A review.Crossref | GoogleScholarGoogle Scholar |
Alhababi DA, Eltai NO, Nasrallah GK, Farg EA, Al Thani AA, Yassine HM (2020) Antimicrobial resistance of commensal Escherichia coli isolated from food animals in Qatar. Microbial Drug Resistance 26, 420–427.
| Antimicrobial resistance of commensal Escherichia coli isolated from food animals in Qatar.Crossref | GoogleScholarGoogle Scholar |
Al-Shawi SG, Dang DS, Yousif AY, Al-Younis ZK, Najm TA, Matarneh SK (2020) The potential use of probiotics to improve animal health, efficiency, and meat quality: a review. Agriculture 10, 452
| The potential use of probiotics to improve animal health, efficiency, and meat quality: a review.Crossref | GoogleScholarGoogle Scholar |
Anee IJ, Alam S, Begum RA, Shahjahan RM, Khandaker AM (2021) The role of probiotics on animal health and nutrition. The Journal of Basic and Applied Zoology 82, 52
| The role of probiotics on animal health and nutrition.Crossref | GoogleScholarGoogle Scholar |
Anokyewaa MA, Amoah K, Li Y, Lu Y, Kuebutornye FKA, Asiedu B, Seidu I (2021) Prevalence of virulence genes and antibiotic susceptibility of Bacillus used in commercial aquaculture probiotics in China. Aquaculture Reports 21, 100784
| Prevalence of virulence genes and antibiotic susceptibility of Bacillus used in commercial aquaculture probiotics in China.Crossref | GoogleScholarGoogle Scholar |
Ayala DI, Cook PW, Franco JG, Bugarel M, Kottapalli KR, Loneragan GH, Brashears MM, Nightingale KK (2019) A systematic approach to identify and characterize the effectiveness and safety of novel probiotic strains to control foodborne pathogens. Frontiers in Microbiology 10, 1108
| A systematic approach to identify and characterize the effectiveness and safety of novel probiotic strains to control foodborne pathogens.Crossref | GoogleScholarGoogle Scholar |
Bae D, Lee J-W, Chae J-P, Kim J-W, Eun J-S, Lee K-W, Seo K-H (2021) Characterization of a novel bacteriophage φCJ22 and its prophylactic and inhibitory effects on necrotic enteritis and Clostridium perfringens in broilers. Poultry Science 100, 302–313.
| Characterization of a novel bacteriophage φCJ22 and its prophylactic and inhibitory effects on necrotic enteritis and Clostridium perfringens in broilers.Crossref | GoogleScholarGoogle Scholar |
Baquero F (2012) Metagenomic epidemiology: a public health need for the control of antimicrobial resistance. Clinical Microbiology and Infection 18, 67–73.
| Metagenomic epidemiology: a public health need for the control of antimicrobial resistance.Crossref | GoogleScholarGoogle Scholar |
Barrow PA, Jones MA, Smith AL, Wigley P (2012) The long view: Salmonella – the last forty years. Avian Pathology 41, 413–420.
| The long view: Salmonella – the last forty years.Crossref | GoogleScholarGoogle Scholar |
Basulira Y, Olet SA, Alele PE (2019) Inappropriate usage of selected antimicrobials: comparative residue proportions in rural and urban beef in Uganda. PLoS ONE 14, e0209006
| Inappropriate usage of selected antimicrobials: comparative residue proportions in rural and urban beef in Uganda.Crossref | GoogleScholarGoogle Scholar |
Bhogoju S, Nahashon S (2022) Recent advances in probiotic application in animal health and nutrition: a review. Agriculture 12, 304
| Recent advances in probiotic application in animal health and nutrition: a review.Crossref | GoogleScholarGoogle Scholar |
Brooks JP, Durso LM, Ibekwe AM (2021) Editorial: exposure, risks, and drivers of the mobile antimicrobial resistance genes in the environment – a global perspective. Frontiers in Microbiology 12, 803282
| Editorial: exposure, risks, and drivers of the mobile antimicrobial resistance genes in the environment – a global perspective.Crossref | GoogleScholarGoogle Scholar |
Cameron A, McAllister TA (2019) Could probiotics be the panacea alternative to the use of antimicrobials in livestock diets? Beneficial Microbes 10, 773–799.
| Could probiotics be the panacea alternative to the use of antimicrobials in livestock diets?Crossref | GoogleScholarGoogle Scholar |
Casanovas-Massana A, Sala-Comorera L, Blanch AR (2014) Quantification of tetracycline and chloramphenicol resistance in digestive tracts of bulls and piglets fed with Toyocerin®, a feed additive containing Bacillus toyonensis spores. Veterinary Microbiology 173, 59–65.
| Quantification of tetracycline and chloramphenicol resistance in digestive tracts of bulls and piglets fed with Toyocerin®, a feed additive containing Bacillus toyonensis spores.Crossref | GoogleScholarGoogle Scholar |
Ceccarelli D, van Essen-Zandbergen A, Smid B, Veldman KT, Boender GJ, Fischer EAJ, Mevius DJ, van der Goot JA (2017) Competitive exclusion reduces transmission and excretion of extended-spectrum-β-lactamase-producing Escherichia coli in broilers. Applied and Environmental Microbiology 83, e03439-16
| Competitive exclusion reduces transmission and excretion of extended-spectrum-β-lactamase-producing Escherichia coli in broilers.Crossref | GoogleScholarGoogle Scholar |
Chaudhary S, Khurana SK, Mane BG (2014) Escherichia coli: animal foods and public health-review. Journal of Microbiology, Immunology and Biotechnology 1, 31–46.
CMOA (2020) List of farmed animals. (Ministry of Agriculture and Rural Affairs of China). Available at http://www.moa.gov.cn/hd/zqyj/202004/t20200408_6341067.htm
Cullen CM, Aneja KK, Beyhan S, Cho CE, Woloszynek S, Convertino M, McCoy SJ, Zhang Y, Anderson MZ, Alvarez-Ponce D, Smirnova E, Karstens L, Dorrestein PC, Li H, Sen Gupta A, Cheung K, Powers JG, Zhao Z, Rosen GL (2020) Emerging priorities for microbiome research. Frontiers in Microbiology 11, 136
| Emerging priorities for microbiome research.Crossref | GoogleScholarGoogle Scholar |
Czerucka D, Piche T, Rampal P (2007) Review article: yeast as probiotics – Saccharomyces boulardii. Alimentary Pharmacology & Therapeutics 26, 767–778.
| Review article: yeast as probiotics – Saccharomyces boulardii.Crossref | GoogleScholarGoogle Scholar |
Dame-Korevaar A, Fischer EAJ, van der Goot J, Velkers F, Ceccarelli D, Mevius D, Stegeman A (2020) Early life supply of competitive exclusion products reduces colonization of extended spectrum beta-lactamase-producing Escherichia coli in broilers. Poultry Science 99, 4052–4064.
| Early life supply of competitive exclusion products reduces colonization of extended spectrum beta-lactamase-producing Escherichia coli in broilers.Crossref | GoogleScholarGoogle Scholar |
Daniali M, Nikfar S, Abdollahi M (2020) Antibiotic resistance propagation through probiotics. Expert Opinion on Drug Metabolism & Toxicology 16, 1207–1215.
| Antibiotic resistance propagation through probiotics.Crossref | GoogleScholarGoogle Scholar |
Delaney S, Do TT, Corrigan A, Murphy R, Walsh F (2021) Investigation into the effect of mannan-rich fraction supplementation on the metagenome of broiler chickens. Microbial Genomics 7, 000602
| Investigation into the effect of mannan-rich fraction supplementation on the metagenome of broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Delgado R, Latorre JD, Vicuña E, Hernandez-Velasco X, Vicente JL, Menconi A, Kallapura G, Layton S, Hargis BM, Téllez G (2014) Glycerol supplementation enhances the protective effect of dietary FloraMax-B11 against Salmonella enteritidis colonization in neonate broiler chickens. Poultry Science 93, 2363–2369.
| Glycerol supplementation enhances the protective effect of dietary FloraMax-B11 against Salmonella enteritidis colonization in neonate broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Diarra MS, Zhao X, Butaye P (2021) Editorial: Antimicrobial use, antimicrobial resistance, and the microbiome in food animals. Frontiers in Veterinary Science 7, 638781
| Editorial: Antimicrobial use, antimicrobial resistance, and the microbiome in food animals.Crossref | GoogleScholarGoogle Scholar |
Dobrescu AI, Nussbaumer-Streit B, Klerings I, Wagner G, Persad E, Sommer I, Herkner H, Gartlehner G (2021) Restricting evidence syntheses of interventions to English-language publications is a viable methodological shortcut for most medical topics: a systematic review. Journal of Clinical Epidemiology 137, 209–217.
| Restricting evidence syntheses of interventions to English-language publications is a viable methodological shortcut for most medical topics: a systematic review.Crossref | GoogleScholarGoogle Scholar |
Dowarah R, Verma AK, Agarwal N (2017) The use of Lactobacillus as an alternative of antibiotic growth promoters in pigs: a review. Animal Nutrition 3, 1–6.
| The use of Lactobacillus as an alternative of antibiotic growth promoters in pigs: a review.Crossref | GoogleScholarGoogle Scholar |
Elghandour MMY, Tan ZL, Abu Hafsa SH, Adegbeye MJ, Greiner R, Ugbogu EA, Cedillo Monroy J, Salem AZM (2020) Saccharomyces cerevisiae as a probiotic feed additive to non and pseudo-ruminant feeding: a review. Journal of Applied Microbiology 128, 658–674.
| Saccharomyces cerevisiae as a probiotic feed additive to non and pseudo-ruminant feeding: a review.Crossref | GoogleScholarGoogle Scholar |
Eltai N, Al Thani AA, Al-Hadidi SH, Abdfarag EA, Al-Romaihi H, Mahmoud MH, Alawad OK, Yassine HM (2020a) Antibiotic resistance profile of commensal Escherichia coli isolated from healthy sheep in Qatar. The Journal of Infection in Developing Countries 14, 138–145.
| Antibiotic resistance profile of commensal Escherichia coli isolated from healthy sheep in Qatar.Crossref | GoogleScholarGoogle Scholar |
Eltai NO, Yassine HM, El-Obeid T, Al-Hadidi SH, Al Thani AA, Alali WQ (2020b) Prevalence of antibiotic-resistant Escherichia coli isolates from local and imported retail chicken carcasses. Journal of Food Protection 83, 2200–2208.
| Prevalence of antibiotic-resistant Escherichia coli isolates from local and imported retail chicken carcasses.Crossref | GoogleScholarGoogle Scholar |
FAO (2021) ‘World food and agriculture: statistical yearbook 2021.’ (Food and Agriculture Organization of the United Nations: Rome, Italy) https://doi.org/10.4060/cb4477en-fig27
FAOSTAT (2018) Crops and livestock products statistics. Available at https://www.fao.org/faostat/en/#data/QCL
FDA (2020) E. coli and foodborne illness information for the public, FDA actions, and recommendations. Available at https://www.fda.gov/news-events/public-health-focus/e-coli-and-foodborne-illness#:∼:text=Escherichia%20coli%20%28E.%20coli%29%20are%20mostly%20harmless%20bacteria,coli%20can%20cause%20mild%20to%20severe%20gastrointestinal%20illness
Feye KM, Anderson KL, Scott MF, Henry DL, Dorton KL, Depenbusch BE, Carlson SA (2016) Abrogation of Salmonella and E. coli O157: H7 in feedlot cattle fed a proprietary Saccharomyces cerevisiae fermentation prototype. Journal of Veterinary Science & Technology 7, 350
| Abrogation of Salmonella and E. coli O157: H7 in feedlot cattle fed a proprietary Saccharomyces cerevisiae fermentation prototype.Crossref | GoogleScholarGoogle Scholar |
Foo JL, Ling H, Lee YS, Chang MW (2017) Microbiome engineering: Current applications and its future. Biotechnology Journal 12, 1600099
| Microbiome engineering: Current applications and its future.Crossref | GoogleScholarGoogle Scholar |
Frazão N, Sousa A, Lässig M, Gordo I (2019) Horizontal gene transfer overrides mutation in Escherichia coli colonizing the mammalian gut. Proceedings of the National Academy of Sciences 116, 17906–17915.
González-Ortiz G, Callegari MA, Wilcock P, Melo-Duran D, Bedford MR, Oliveira HRV, da Silva MAA, Pierozan CR, da Silva CA (2020) Dietary xylanase and live yeast supplementation influence intestinal bacterial populations and growth performance of piglets fed a sorghum-based diet. Animal Nutrition 6, 457–466.
| Dietary xylanase and live yeast supplementation influence intestinal bacterial populations and growth performance of piglets fed a sorghum-based diet.Crossref | GoogleScholarGoogle Scholar |
Guo X, Akram S, Stedtfeld R, Johnson M, Chabrelie A, Yin D, Mitchell J (2021) Distribution of antimicrobial resistance across the overall environment of dairy farms – a case study. Science of The Total Environment 788, 147489
| Distribution of antimicrobial resistance across the overall environment of dairy farms – a case study.Crossref | GoogleScholarGoogle Scholar |
Han X-Y, Yan F-Y, Nie X-Z, Xia W, Chen S, Zhang X-X, Qian L-C (2017) Effect of replacing antibiotics using multi-enzyme preparations on production performance and antioxidant activity in piglets. Journal of Integrative Agriculture 16, 640–647.
| Effect of replacing antibiotics using multi-enzyme preparations on production performance and antioxidant activity in piglets.Crossref | GoogleScholarGoogle Scholar |
Helmy YA, Kathayat D, Ghanem M, Jung K, Closs G, Deblais L, Srivastava V, El-Gazzar M, Rajashekara G (2020) Identification and characterization of novel small molecule inhibitors to control Mycoplasma gallisepticum infection in chickens. Veterinary Microbiology 247, 108799
| Identification and characterization of novel small molecule inhibitors to control Mycoplasma gallisepticum infection in chickens.Crossref | GoogleScholarGoogle Scholar |
Hofacre CL, Johnson AC, Kelly BJ, Froyman R (2002) Effect of a commercial competitive exclusion culture on reduction of colonization of an antibiotic-resistant pathogenic Escherichia coli in day-old broiler chickens. Avian Diseases 46, 198–202.
| Effect of a commercial competitive exclusion culture on reduction of colonization of an antibiotic-resistant pathogenic Escherichia coli in day-old broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Huebner KL, Martin JN, Weissend CJ, Holzer KL, Parker JK, Lakin SM, Doster E, Weinroth MD, Abdo Z, Woerner DR, Metcalf JL, Geornaras I, Bryant TC, Morley PS, Belk KE (2019) Effects of a Saccharomyces cerevisiae fermentation product on liver abscesses, fecal microbiome, and resistome in feedlot cattle raised without antibiotics. Scientific Reports 9, 2559
| Effects of a Saccharomyces cerevisiae fermentation product on liver abscesses, fecal microbiome, and resistome in feedlot cattle raised without antibiotics.Crossref | GoogleScholarGoogle Scholar |
Jackson SJ, Andrews N, Ball D, Bellantuono I, Gray J, Hachoumi L, Holmes A, Latcham J, Petrie A, Potter P, Rice A, Ritchie A, Stewart M, Strepka C, Yeoman M, Chapman K (2017) Does age matter? The impact of rodent age on study outcomes. Laboratory Animals 51, 160–169.
| Does age matter? The impact of rodent age on study outcomes.Crossref | GoogleScholarGoogle Scholar |
Jackson SA, Schoeni JL, Vegge C, Pane M, Stahl B, Bradley M, Goldman VS, Burguière P, Atwater JB, Sanders ME (2019) Improving end-user trust in the quality of commercial probiotic products. Frontiers in Microbiology 10, 739
| Improving end-user trust in the quality of commercial probiotic products.Crossref | GoogleScholarGoogle Scholar |
Jin Song S, Woodhams DC, Martino C, Allaband C, Mu A, Javorschi-Miller-Montgomery S, Suchodolski JS, Knight R (2019) Engineering the microbiome for animal health and conservation. Experimental Biology and Medicine 244, 494–504.
| Engineering the microbiome for animal health and conservation.Crossref | GoogleScholarGoogle Scholar |
Johar A, Al-Thani N, Al-Hadidi SH, Dlissi E, Mahmoud MH, Eltai NO (2021) Antibiotic resistance and virulence gene patterns associated with avian pathogenic Escherichia coli (APEC) from broiler chickens in Qatar. Antibiotics 10, 564
| Antibiotic resistance and virulence gene patterns associated with avian pathogenic Escherichia coli (APEC) from broiler chickens in Qatar.Crossref | GoogleScholarGoogle Scholar |
Kim WS, Morishita TY, Dong F (2021) Antimicrobial resistance in Escherichia coli between conventional and organic broiler flocks. Journal of Applied Poultry Research 30, 100158
| Antimicrobial resistance in Escherichia coli between conventional and organic broiler flocks.Crossref | GoogleScholarGoogle Scholar |
Kimera ZI, Mshana SE, Rweyemamu MM, Mboera LEG, Matee MIN (2020) Antimicrobial use and resistance in food-producing animals and the environment: an African perspective. Antimicrobial Resistance & Infection Control 9, 37
| Antimicrobial use and resistance in food-producing animals and the environment: an African perspective.Crossref | GoogleScholarGoogle Scholar |
Kober AKMH, Riaz Rajoka MS, Mehwish HM, Villena J, Kitazawa H (2022) Immunomodulation potential of probiotics: a novel strategy for improving livestock health, immunity, and productivity. Microorganisms 10, 388
| Immunomodulation potential of probiotics: a novel strategy for improving livestock health, immunity, and productivity.Crossref | GoogleScholarGoogle Scholar |
Kogut MH (2017) Issues and consequences of using nutrition to modulate the avian immune response. Journal of Applied Poultry Research 26, 605–612.
| Issues and consequences of using nutrition to modulate the avian immune response.Crossref | GoogleScholarGoogle Scholar |
Kumar M, Sarma DK, Shubham S, Kumawat M, Verma V, Nina PB, Jp D, Kumar S, Singh B, Tiwari RR (2021) Futuristic non-antibiotic therapies to combat antibiotic resistance: a review. Frontiers in Microbiology 12, 609459
| Futuristic non-antibiotic therapies to combat antibiotic resistance: a review.Crossref | GoogleScholarGoogle Scholar |
Laird T, Abraham R, Sahibzada S, Abraham S, O’Dea M (2022) In Vitro demonstration of targeted phage therapy and competitive exclusion as a novel strategy for decolonization of extended-spectrum-cephalosporin-resistant Escherichia coli. Applied and Environmental Microbiology 88, e02276-21
| In Vitro demonstration of targeted phage therapy and competitive exclusion as a novel strategy for decolonization of extended-spectrum-cephalosporin-resistant Escherichia coli.Crossref | GoogleScholarGoogle Scholar |
Lee A, Aldeieg M, Woodward MJ, Juniper DT, Rymer C (2021) The effect of Candida famata and Lactobacillus plantarum on the number of coliforms and the antibiotic resistance and virulence of Escherichia coli in the gut of broilers. Animal 15, 100310
| The effect of Candida famata and Lactobacillus plantarum on the number of coliforms and the antibiotic resistance and virulence of Escherichia coli in the gut of broilers.Crossref | GoogleScholarGoogle Scholar |
Li T, Teng D, Mao R, Hao Y, Wang X, Wang J (2020) A critical review of antibiotic resistance in probiotic bacteria. Food Research International 136, 109571
| A critical review of antibiotic resistance in probiotic bacteria.Crossref | GoogleScholarGoogle Scholar |
Lin L, Huang X, Yang H, He Y, He X, Huang J, Li S, Wang X, Tang S, Liu G, Pan Z (2021) Molecular epidemiology, antimicrobial activity, and virulence gene clustering of Streptococcus agalactiae isolated from dairy cattle with mastitis in China. Journal of Dairy Science 104, 4893–4903.
| Molecular epidemiology, antimicrobial activity, and virulence gene clustering of Streptococcus agalactiae isolated from dairy cattle with mastitis in China.Crossref | GoogleScholarGoogle Scholar |
Ma T, McAllister TA, Guan LL (2021a) A review of the resistome within the digestive tract of livestock. Journal of Animal Science and Biotechnology 12, 121
| A review of the resistome within the digestive tract of livestock.Crossref | GoogleScholarGoogle Scholar |
Ma F, Xu S, Tang Z, Li Z, Zhang L (2021b) Use of antimicrobials in food animals and impact of transmission of antimicrobial resistance on humans. Biosafety and Health 3, 32–38.
| Use of antimicrobials in food animals and impact of transmission of antimicrobial resistance on humans.Crossref | GoogleScholarGoogle Scholar |
Mackenzie JS, Jeggo M (2019) The one health approach – why is it so important? Tropical Medicine and Infectious Disease 4, 88
| The one health approach – why is it so important?Crossref | GoogleScholarGoogle Scholar |
Mathur S, Singh R (2005) Antibiotic resistance in food lactic acid bacteria – a review. International Journal of Food Microbiology 105, 281–295.
| Antibiotic resistance in food lactic acid bacteria – a review.Crossref | GoogleScholarGoogle Scholar |
Mehdi Y, Létourneau-Montminy M-P, Gaucher M-L, Chorfi Y, Suresh G, Rouissi T, Brar SK, Côté C, Ramirez AA, Godbout S (2018) Use of antibiotics in broiler production: global impacts and alternatives. Animal Nutrition 4, 170–178.
| Use of antibiotics in broiler production: global impacts and alternatives.Crossref | GoogleScholarGoogle Scholar |
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 6, e1000097
| Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement.Crossref | GoogleScholarGoogle Scholar |
Mokhber-Dezfouli MR, Tajik P, Bolourchi M, Mahmoudzadeh H (2007) Effects of probiotics supplementation in daily milk intake of newborn calves on body weight gain, body height, diarrhea occurrence and health condition. Pakistan Journal of Biological Sciences: PJBS 10, 3136–3140.
| Effects of probiotics supplementation in daily milk intake of newborn calves on body weight gain, body height, diarrhea occurrence and health condition.Crossref | GoogleScholarGoogle Scholar |
Mountzouris KC (2022) Prebiotics: types. In ‘Encyclopedia of dairy sciences’. 3rd edn. (Eds PLH McSweeney, JP McNamara) pp. 352–358. (Academic Press)
Palma ML, Zamith-Miranda D, Martins FS, Bozza FA, Nimrichter L, Montero-Lomeli M, Marques ETA, Douradinha B (2015) Probiotic Saccharomyces cerevisiae strains as biotherapeutic tools: is there room for improvement? Applied Microbiology and Biotechnology 99, 6563–6570.
| Probiotic Saccharomyces cerevisiae strains as biotherapeutic tools: is there room for improvement?Crossref | GoogleScholarGoogle Scholar |
Pham VH, Abbas W, Huang J, He Q, Zhen W, Guo Y, Wang Z (2022) Effect of blending encapsulated essential oils and organic acids as an antibiotic growth promoter alternative on growth performance and intestinal health in broilers with necrotic enteritis. Poultry Science 101, 101563
| Effect of blending encapsulated essential oils and organic acids as an antibiotic growth promoter alternative on growth performance and intestinal health in broilers with necrotic enteritis.Crossref | GoogleScholarGoogle Scholar |
Pissetti C, Kich JD, Allen HK, Navarrete C, de Freitas Costa E, Morés N, Cardoso M (2021) Antimicrobial resistance in commensal Escherichia coli and Enterococcus spp. isolated from pigs subjected to different antimicrobial administration protocols. Research in Veterinary Science 137, 174–185.
| Antimicrobial resistance in commensal Escherichia coli and Enterococcus spp. isolated from pigs subjected to different antimicrobial administration protocols.Crossref | GoogleScholarGoogle Scholar |
Revitt-Mills SA, Robinson A (2020) Antibiotic-induced mutagenesis: under the microscope. Frontiers in Microbiology 11, 585175
| Antibiotic-induced mutagenesis: under the microscope.Crossref | GoogleScholarGoogle Scholar |
Ricker N, Trachsel J, Colgan P, Jones J, Choi J, Lee J, Coetzee JF, Howe A, Brockmeier SL, Loving CL, Allen HK (2020) Toward antibiotic stewardship: route of antibiotic administration impacts the microbiota and resistance gene diversity in swine feces. Frontiers in Veterinary Science 7, 255
| Toward antibiotic stewardship: route of antibiotic administration impacts the microbiota and resistance gene diversity in swine feces.Crossref | GoogleScholarGoogle Scholar |
Roth N, Mayrhofer S, Gierus M, Weingut C, Schwarz C, Doupovec B, Berrios R, Domig KJ (2017) Effect of an organic acids based feed additive and enrofloxacin on the prevalence of antibiotic-resistant E. coli in cecum of broilers. Poultry Science 96, 4053–4060.
| Effect of an organic acids based feed additive and enrofloxacin on the prevalence of antibiotic-resistant E. coli in cecum of broilers.Crossref | GoogleScholarGoogle Scholar |
Seong HJ, Kim JJ, Kim T, Ahn SJ, Rho M, Sul WJ (2021) A case study on the distribution of the environmental resistome in Korean shrimp farms. Ecotoxicology and Environmental Safety 227, 112858
| A case study on the distribution of the environmental resistome in Korean shrimp farms.Crossref | GoogleScholarGoogle Scholar |
Shivaprasad HL (2000) Fowl typhoid and pullorum disease. Revue Scientifique et Technique (International Office of Epizootics) 19, 405–424.
| Fowl typhoid and pullorum disease.Crossref | GoogleScholarGoogle Scholar |
Skarżyńska M, Leekitcharoenphon P, Hendriksen RS, Aarestrup FM, Wasyl D (2020) A metagenomic glimpse into the gut of wild and domestic animals: quantification of antimicrobial resistance and more. PLoS ONE 15, e0242987
| A metagenomic glimpse into the gut of wild and domestic animals: quantification of antimicrobial resistance and more.Crossref | GoogleScholarGoogle Scholar |
Sommer-Lassa MM, Reecy J, Severin A, Somwarpet-Seetharam A, Mayes MS (2019) 69 The effects of feeding a novel saccharomyces cerevisiae feed additive on the prevalence and abundance of antimicrobial resistance genes in the microbiome(s) of receiving beef calves. Journal of Animal Science 97, 40–41.
| 69 The effects of feeding a novel saccharomyces cerevisiae feed additive on the prevalence and abundance of antimicrobial resistance genes in the microbiome(s) of receiving beef calves.Crossref | GoogleScholarGoogle Scholar |
Temmerman R, Pot B, Huys G, Swings J (2003) Identification and antibiotic susceptibility of bacterial isolates from probiotic products. International Journal of Food Microbiology 81, 1–10.
| Identification and antibiotic susceptibility of bacterial isolates from probiotic products.Crossref | GoogleScholarGoogle Scholar |
Thanki AM, Mignard G, Atterbury RJ, Barrow P, Millard AD, Clokie MRJ (2022) Prophylactic delivery of a bacteriophage cocktail in feed significantly reduces Salmonella colonization in pigs. Microbiology Spectrum 10, e00422-22
| Prophylactic delivery of a bacteriophage cocktail in feed significantly reduces Salmonella colonization in pigs.Crossref | GoogleScholarGoogle Scholar |
United Nations PD (2019) World population prospects: the 2019 revision, key findings and advance tables. (Department of Economic and Social Affairs) Available at https://www.un.org/development/desa/publications/world-population-prospects-2019-highlights.html
Vinderola G, Gueimonde M, Gomez-Gallego C, Delfederico L, Salminen S (2017) Correlation between in vitro and in vivo assays in selection of probiotics from traditional species of bacteria. Trends in Food Science & Technology 68, 83–90.
| Correlation between in vitro and in vivo assays in selection of probiotics from traditional species of bacteria.Crossref | GoogleScholarGoogle Scholar |
Wan MLY, Forsythe SJ, El-Nezami H (2019) Probiotics interaction with foodborne pathogens: a potential alternative to antibiotics and future challenges. Critical Reviews in Food Science and Nutrition 59, 3320–3333.
| Probiotics interaction with foodborne pathogens: a potential alternative to antibiotics and future challenges.Crossref | GoogleScholarGoogle Scholar |
Wideman RF, Al-Rubaye A, Kwon YM, Blankenship J, Lester H, Mitchell KN, Pevzner IY, Lohrmann T, Schleifer J (2015) Prophylactic administration of a combined prebiotic and probiotic, or therapeutic administration of enrofloxacin, to reduce the incidence of bacterial chondronecrosis with osteomyelitis in broilers. Poultry Science 94, 25–36.
| Prophylactic administration of a combined prebiotic and probiotic, or therapeutic administration of enrofloxacin, to reduce the incidence of bacterial chondronecrosis with osteomyelitis in broilers.Crossref | GoogleScholarGoogle Scholar |
World Health Organization (2017) ‘WHO guidelines on use of medically important antimicrobials in food-producing animals: web annex A: evidence base (No. WHO/NMH/FOS/FZD/17.2).’ (World Health Organization)
Xiong W, Wang Y, Sun Y, Ma L, Zeng Q, Jiang X, Li A, Zeng Z, Zhang T (2018) Antibiotic-mediated changes in the fecal microbiome of broiler chickens define the incidence of antibiotic resistance genes. Microbiome 6, 34
| Antibiotic-mediated changes in the fecal microbiome of broiler chickens define the incidence of antibiotic resistance genes.Crossref | GoogleScholarGoogle Scholar |
Xu X, Zhang Z (2021) Sex- and age-specific variation of gut microbiota in Brandt’s voles. PeerJ 9, e11434
| Sex- and age-specific variation of gut microbiota in Brandt’s voles.Crossref | GoogleScholarGoogle Scholar |
Xu C, Kong L, Gao H, Cheng X, Wang X (2022) A review of current bacterial resistance to antibiotics in food animals. Frontiers in Microbiology 13, 822689
| A review of current bacterial resistance to antibiotics in food animals.Crossref | GoogleScholarGoogle Scholar |
Yan F, Polk DB (2020) Probiotics and probiotic-derived functional factors – mechanistic insights into applications for intestinal homeostasis. Frontiers in Immunology 11, 1428
| Probiotics and probiotic-derived functional factors – mechanistic insights into applications for intestinal homeostasis.Crossref | GoogleScholarGoogle Scholar |
Yun J, Muurinen J, Nykäsenoja S, Seppä-Lassila L, Sali V, Suomi J, Tuominen P, Joutsen S, Hämäläinen M, Olkkola S, Myllyniemi A-L, Peltoniemi O, Heinonen M (2021) Antimicrobial use, biosecurity, herd characteristics, and antimicrobial resistance in indicator Escherichia coli in ten Finnish pig farms. Preventive Veterinary Medicine 193, 105408
| Antimicrobial use, biosecurity, herd characteristics, and antimicrobial resistance in indicator Escherichia coli in ten Finnish pig farms.Crossref | GoogleScholarGoogle Scholar |
Zalewska M, Błażejewska A, Czapko A, Popowska M (2021) Antibiotics and antibiotic resistance genes in animal manure – consequences of its application in agriculture. Frontiers in Microbiology 12, 610656
| Antibiotics and antibiotic resistance genes in animal manure – consequences of its application in agriculture.Crossref | GoogleScholarGoogle Scholar |
Zhe L, Yang L, Lin S, Chen F, Wang P, Heres L, Zhuo Y, Tang J, Lin Y, Xu S, Zhang X, Jiang X, Huang L, Zhang R, Che L, Tian G, Feng B, Wu D, Fang Z (2021) Differential responses of weaned piglets to supplemental porcine or chicken plasma in diets without inclusion of antibiotics and zinc oxide. Animal Nutrition 7, 1173–1181.
| Differential responses of weaned piglets to supplemental porcine or chicken plasma in diets without inclusion of antibiotics and zinc oxide.Crossref | GoogleScholarGoogle Scholar |


