Platforms to accelerate biomanufacturing of enzyme and probiotic animal feed supplements: discovery considerations and manufacturing implications
Robert E. Speight A B E , Laura Navone A B , Leigh K. Gebbie A , Jo-Anne L. Blinco C and Wayne L. Bryden
A B E , Laura Navone A B , Leigh K. Gebbie A , Jo-Anne L. Blinco C and Wayne L. Bryden  D
D
A School of Biology and Environmental Science, Faculty of Science, Queensland University of Technology, 2 George Street, Brisbane, Qld 4000, Australia.
B Centre of Excellence in Synthetic Biology, Queensland University of Technology, 2 George Street, Brisbane, Qld 4000, Australia.
C Biorefining Research Facility, Queensland University of Technology, 2 George Street, Brisbane, Qld 4000, Australia.
D School of Agriculture and Food Sciences, The University of Queensland, Gatton Campus, Gatton, Qld 4343, Australia.
E Corresponding author. Email: robert.speight@qut.edu.au
Animal Production Science 62(12) 1113-1128 https://doi.org/10.1071/AN21342
Submitted: 25 June 2021 Accepted: 15 November 2021 Published: 25 January 2022
Journal compilation © CSIRO 2022 Open Access CC BY-NC-ND
Abstract
Probiotics and enzymes are important components of the global livestock feed supplement market, which is expected to be approximately US$56 billion by 2027. They make essential contributions to animal health and productivity and are very important for on-farm economics, as well as feed supplement and bulk feed businesses. Despite the variety of on-market products, there remains a strong drive to develop new function or more effective enzymes (e.g. more active or stable) and probiotics (e.g. for specific health or nutrition requirements) that can be produced economically and commercialised to gain market share. Various large and established supplement development, manufacture and supply companies with highly refined, efficient and vertically integrated processes dominate the market. In contrast, many challenges exist for less established players, such as feed companies, large farming corporations, start-up companies and the research community, to develop and commercialise improved feed supplements. These less established players may have niche markets or needs or may have identified highly novel candidate products through basic or collaborative academia-industry applied research. In these situations, the path from discovery and development to a commercial product is unclear and likely to be very challenging. However, the risk of not progressing is that the value of research investments is not realised, or the needs of specific niche markets are not met. For these situations, new pathways to market based on rapid discovery, production (at various scales), and testing feedback loops, along with appropriate intellectual property management and clear regulatory strategies need to be established. To deliver these new pathways, it is essential to define key performance, production and economic criteria, have a rapid route from laboratory to pilot-scale manufacture and livestock feeding trials, and include all the necessary participants in the value chain from research development, manufacturing, distribution, and regulatory management to the end user. These issues are discussed with reference to the current state-of-the-art and our development of new pathways for a specific enzyme and probiotic based on efficient laboratory-to-market platforms. Although new supplements have been brought closer to market, challenges remain regarding scaling to commercial manufacture for new products without an established market.
Keywords: livestock, commercialisation, fermentation, bioprocess.
Introduction
Animal feed supplements deliver nutrition and health benefits to livestock, leading to important productivity gains, and are an essential part of the diet. They augment the energy and nutrients already available in feedstuffs or provide specific functions such as improved feed digestibility or protection from disease or toxins. A 2020 market report suggested that the animal feed supplement business is a growth industry (FiorMarkets 2020). The report divided supplements into the following categories: antibiotics, minerals, binders, vitamins, feed enzymes, feed acidifiers, antioxidants and amino acids that are fed to aquatic animals, ruminants, poultry and pigs globally. The report suggested that this market will ‘grow from US$35.01 billion in 2019 to US$56.22 billion by 2027, at a compound annual growth rate (CAGR) of 6.1%’ during this timeframe.
Reports for individual countries are also available and the Australian market is predicted to grow at 4.3% CAGR over a similar time period (MordorIntelligence 2020). Although the market is quite segmented, eubiotic supplements were dominant with a 20% market share by revenue.
The report defined eubiotics as probiotics, prebiotics and organic acids, with probiotics being the most used of the three followed by prebiotics. It is generally understood that eubiotics are supplements that can modulate the composition, distribution or activity of gastrointestinal microflora leading to improved outcomes for livestock. Eubiotic supplements have increased in importance during the search for functional replacements for antibiotic growth promoters (AGPs) that have been banned or restricted in various jurisdictions (Brüssow 2017). A meta-analysis of publications that compared poultry diets with and without AGPs suggested that the economic benefit of using AGPs (and therefore economic loss if they are not used) was approximately US$0.03 per bird (Cardinal et al. 2019). This potential loss equated to an estimated US$183 million loss in Brazil on the basis on the annual slaughter of 5.84 billion chickens in 2017. Australia processes a much lower number of broilers annually (~665 million) and it would amount to a loss approaching US$20 million. Inspection of the Food and Agriculture Organisation of the United Nations data (http://www.fao.org/faostat/en/#data/QL) on global poultry slaughtered in 2019 showed that just over 83 billion birds were processed, with a potential global impact of not using AGPs in the order of US$2.5 billion per year. As well as the functional replacement of AGPs, the additional animal health and productivity benefits of using eubiotics (such as reduced gut inflammation or increased feed conversion efficiency) lead to a significant economic opportunity for improved supplements.
Likewise, enzymes have had a major impact on livestock performance as they either improve nutrient digestibility or degrade anti-nutritional factors present in the diet. In 2015, livestock feed enzymes comprised ~20% of the approximate US$5 billion global industrial enzyme market (Guerrand 2018). The major enzymes added to animal feeds are phytases, xylanases, cellulases, glucanases, other enzymes for the hydrolysis of non-starch polysaccharides, and proteases.
Feed supplement development and manufacture is performed by large international feed supplement companies, as outlined in the next section. Despite the dominance, expertise and efficiencies of these major market players, this paper argues that other players, such as universities and smaller companies in diverse geographical areas, could and should play an important role in the development of future feed supplements. There could be specific niche applications, markets, customers and geographies that are not well served by the major players. The research community working in close collaboration with small companies and producers has much to offer regarding the innovation of new feed supplement products based on academic research or on-farm experience and geographically specific market expertise. However, compared with the established companies, there are major challenges for less established, minor or nascent players to bring new supplements to market. These other players will rely on collaboration and a fragmented, and likely incomplete, discovery to distribution pipeline. This fragmentation leads to issues related to communication, intellectual property management and ownership, value capture, leadership/responsibility, and translation of the technologies among multiple stakeholders across the value chain. This value chain may include universities, government agencies and research and development funders, investors, regulatory consultants, manufacturers, feed supply companies, farming companies and farmers. Although individual stakeholders may hold specific expertise, this lack of cohesion compared with major players often leads to roadblocks that can slow down or prevent the realisation of the technology.
A particular key issue for smaller players and consortia is the lack of access to suitable manufacturing expertise and facilities at the required scales. A successful pipeline requires multiple production scales from small scale for development, pilot scale for process validation, livestock feeding trial supply and seeding the market, and large scale for full commercialisation. The established companies typically have laboratory to commercial-scale manufacturing and multiple products, leading to full manufacturing plant equipment utilisation across the year, and therefore significant economies of scale that are not available to other smaller players and companies with new-to-market products.
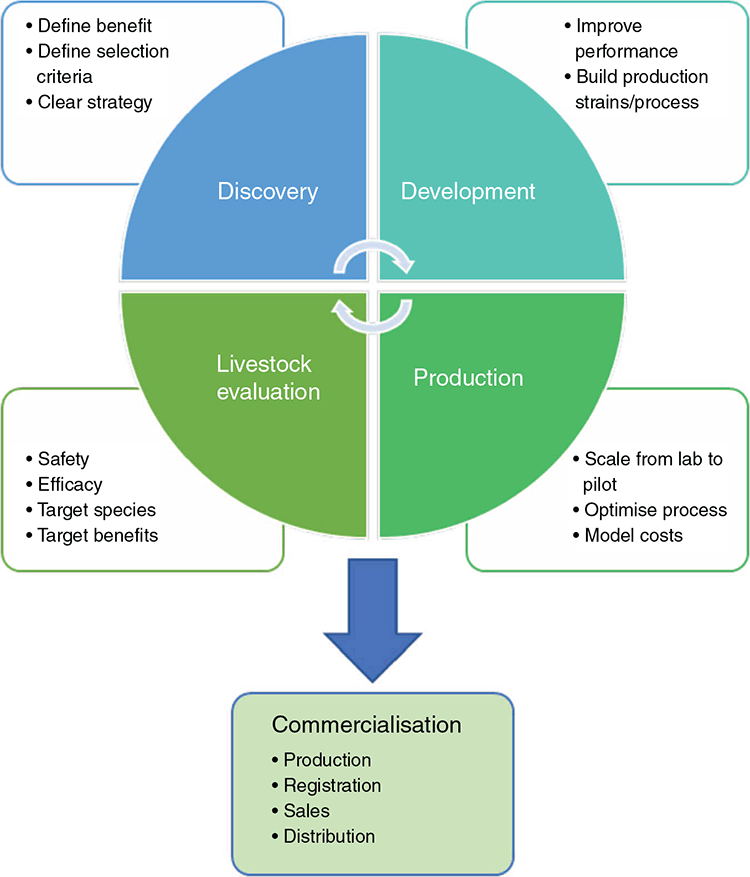
Focusing on enzyme and probiotic discovery, production and initial animal product testing, this paper outlines a model for non-established and non-vertically integrated players and consortia to more efficiently develop and commercialise livestock feed supplements through a rapid and iterative ‘laboratory-to-market’ pipeline (Fig. 1). The pipeline consists of a series of integrated platforms that take a new or novel product through the stages of discovery, laboratory and animal testing, and scaling up of manufacturing for commercialisation. Such a pipeline was proposed, built and tested through a recently completed Advanced Queensland government-funded project involving two universities and three companies. This paper will outline the outcomes of that project and suggest areas for further improvement and refinement.
Feed enzymes and probiotics
In describing the development of platforms that make-up a pipeline for feed supplement discovery and production, we have used feed enzymes and probiotics as examples of how such a pipeline may be utilised. Before describing the platforms, a brief overview of feed enzymes and probiotics is given, along with the major suppliers and markets for these feed supplements.
Feed enzymes
Enzymes have had a major impact on livestock performance, with their commercial use beginning some 30 years ago (Campbell and Bedford 1992). The dominant feed enzyme is phytase with up to 40% market share, with the other major enzymes being xylanases, cellulases, glucanases and proteases. These enzymes are generally supplemented to aid feed digestion and to remove anti-nutritional compounds (Ravindran 2013). The use of phytase allows livestock such as poultry and pigs to obtain phosphate from the diet, thereby reducing or eliminating the need for dietary inclusion of inorganic phosphate and pollution through phosphorus excretion in manure (Ravindran et al. 1995; Li et al. 2016). Phytate is also an anti-nutritional factor (Bryden et al. 2007) that binds proteins and metal ion minerals, leading to increased gut viscosity and nutrient depletion; so, its removal has multiple benefits (Selle et al. 2000; Selle and Ravindran 2007, 2008; Samtiya et al. 2020). The use of non-starch polysaccharide degrading enzymes alone or as part of an enzyme cocktail in a variety of diets may enhance breakdown of fibre to metabolisable sugars and reduce gut viscosity to improve feed conversion efficiency (Aftab and Bedford 2018; Bedford 2018).
Desirable enzyme supplement improvements include increased thermal stability for survival during feed pellet formation, while maintaining high activity at gut temperatures, new substrate specificities, higher production levels in the microbial production strain, and higher specific enzyme activities (which also reduces production costs; Rebello et al. 2017). As with probiotics, clear and well defined performance criteria need to be defined to successfully develop new enzymes as discussed below.
Feed supplement companies
Given the large market size and penetration of livestock feed enzymes, there are several established multinational companies successfully developing, manufacturing and selling a range of different products. Such companies include BASF, AB Vista, Novozymes (with feed enzymes marketed through DSM), Adisseo and Dupont. The market is dominated by large companies, with Novozymes/DSM, AB Vista and Dupont collectively having ~80% market share (Guerrand 2018). Although probiotics are more nascent than enzymes, their increasing market penetration, especially in poultry, has led to major companies also being at the forefront of product development and commercialisation. Examples of these major players include the following: Danisco Animal Nutrition (part of DuPont Industrial Biosciences) and their product Enviva PRO; Evonik who sell Ecobiol (which was previously owned by the Spanish company Norel) and GutCare; Chr. Hansen who manufacture a range of probiotic species that are then sold through global distributors, with brands such as GalliPro and BioPlus; and Kemin who sell CLOSTAT for protection of poultry against Clostridium perfringens, the causative agent of necrotic enteritis. Novozymes and DSM, who are traditionally more known for enzyme production, have entered the market with the product Balancius for the improved digestion of bacterial cell wall debris for improved feed efficiency and nutrient absorption. Novozymes has also collaborated with Adisseo to make Alterion, with claims around improved gut health as an alternative to AGPs. Several of these companies have commenced marketing combinations of feed supplements, especially probiotics in combination with feed enzymes.
These large companies have long histories of livestock feed supplement development and established pipelines for discovery, manufacture, testing, product registration, marketing and distribution. In many cases, these companies are vertically integrated across the entire pipeline (discovery to distribution), bringing efficiencies and reliability to the delivery of new supplements. There is very little information in the public domain about the specific processes and systems that these companies use to efficiently develop new feed supplements.
Feed supplement discovery
It is apparent from the discussion above that the commercial supply of feed supplements is a very competitive global industry. It is therefore essential for those wishing to enter the industry that they have a defined market niche and defined performance criteria for the new product. A further complication is that probiotics often contain multiple species or are combined with other supplements such as feed enzymes. The challenges presented by this diversity suggest a need for standardisation as well as very clearly defined selection criteria for new feed supplements.
The need to define selection and assessment criteria
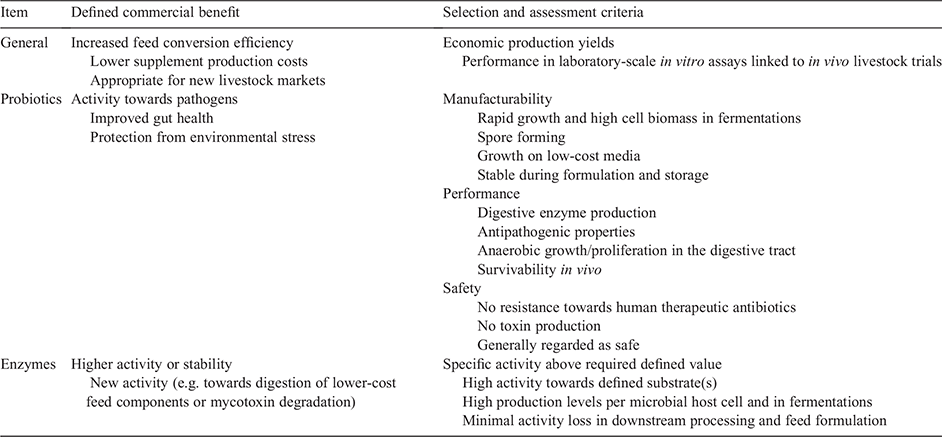
To avoid unfocussed and inefficient enzyme or probiotic discovery, it is crucial to carefully define assessment criteria for laboratory-based discovery and evaluation that anticipate performance and regulatory requirements when the supplement is commercialised (Table 1). These criteria also define when candidates are suitable to move from the laboratory to production development and initial animal evaluation. If criteria are not defined, then the discovery and development phase may be unnecessarily extended while optimising non-critical parameters or trying to improve beyond what is actually required. Worse, the supplements could be developed in a way that they fail in efficacy trials, are unsafe to the animal, or fail regulatory approval.
As might be expected, human probiotics are more widely researched than are livestock probiotics (over 19 000 articles on PubMed versus 371 for ‘livestock probiotic’ and 1200 for ‘poultry probiotic’ in May 2021) and there are many papers addressing probiotic selection criteria that are also relevant to livestock probiotic selection. A recent review categorised the criteria according to WHO and FAO Guidelines into Stress Tolerance (survivability and viability in vivo, e.g. towards digestive enzymes or low pH in the stomach), Adhesion Ability, Antipathogenic Activity, Safety and Clinical Trials (to prove safety and efficacy; de Melo Pereira et al. 2018). The authors also proposed additional criteria based on functional properties related to benefit to the host (e.g. cholesterol reduction or the secretion of functional molecules), industrial requirements (e.g. cell viability after food processing or storage), and the availability of detailed molecular characterisations (e.g. full genome sequences or proteomic profiles). Many of these criteria also apply to livestock, with another recent review on the role of probiotics in animal nutrition categorising criteria into Safety (e.g. precise species identification, absence of mobile antibiotic resistance encoding genes), Functionality (including antipathogenic activity and strain survivability/viability in vivo) and Technological Usability (e.g. facile biomass production for manufacturing, stability during feed formulation and storage; Markowiak and Śliżewska 2018). Several commercial livestock probiotics are based on Bacillus species and another recent review further refined livestock probiotic selection criteria as well as the process for selection and testing for these species (Mingmongkolchai and Panbangred 2018).
Enzyme selection criteria should be based on the catalytic activity of the enzyme (high specific activity towards the required substrate(s)), enzyme stability (during steam pelleting and storage, and in the animal digestive tract) and ease/economics of manufacturing. Enzymes must be powerful catalysts towards the feed component substrate (e.g. phytate), retain high levels of activity to the site of action in the animal, and be able to be synthesised in high volumetric productivities in (usually) recombinant microbial fermentation systems. All these factors contribute directly to the economics of enzyme use, with the cost of the enzyme and its benefit to livestock (e.g. increased feed conversion efficiency or the reduced need for inorganic phosphate in feed in the case of phytase) needing to overall deliver increased on-farm profits. A clear economic understanding therefore should drive defined numerical selection criteria around the number of units of enzyme activity (in physiological conditions) per gram of enzyme protein (the catalytic efficiency) and per litre of fermentation culture per day in manufacturing (the volumetric productivity), as well as the degree of storage and thermal stability (e.g. % loss per minute at temperatures experienced during pelleting).
On the basis of the literature and our own experience, we observed that it is essential to consider both performance criteria and manufacturability/regulatory criteria from the start. For non-established players wishing to enter the market or attract partnerships with established companies, it is necessary to focus on performance criteria that are novel or where performance could significantly exceed what is currently on the market.
The need for a discovery and development strategy
Although there are many similarities in the way that both probiotics and enzymes are selected and assessed, the differences make it important that discovery and development strategies be developed for each new product.
Probiotics
While it has been common to try to isolate potential probiotics from the microbiomes of healthy or high-performing livestock, it has been somewhat imprecise, necessitating significant candidate screening. Only recently, with the establishment of protocols and reduced sequencing cost of high-throughput and high-accuracy metagenomics (Parks et al. 2017), has determining a more accurate a priori association between specific microbial species and livestock performance become a possibility. In the future, it may be possible to link phenotypic (diet, health, growth performance, biomarkers, environment) studies on thousands of livestock with microbiome analyses to identify effective probiotics, with such approaches now emerging in human studies (Veiga et al. 2020; Almeida et al. 2021; Parks et al. 2021). These advanced sequencing techniques may identify novel probiotics but many microorganisms remain unculturable and therefore unable to meet the manufacturing selection criteria; only 30% of the species in the human gut microbiome have cultured equivalents (Almeida et al. 2021). Although research towards culturing these ‘unculturable’ microorganisms is making progress (Browne et al. 2016), there remains a significant opportunity to tap into more of nature’s vast microbial diversity for probiotics.
While targeting the gut of healthy or high-performing livestock has advantages from a safety perspective, this approach is somewhat limited in scope and there may be microbes in other environments that could provide a step-change in performance or functional novelty. For example, to find potential probiotics that would synergise and enhance lower-value high-fibre feeds based on sugarcane bagasse, we analysed and characterised the microbial community within a bagasse pile that had been undisturbed for an extended period of time (Gebbie et al. 2020). Our hypothesis was that microbes degrading and growing on the fibre in the pile should produce relevant non-starch polysaccharide-degrading enzymes and might therefore be probiotic candidates. Following metagenomic analyses, the development strategy involved culture and analysis of the microbes for their enzyme potential as well as testing towards the probiotic selection criteria described above, as well as full genome sequencing, fermentation production at laboratory and 800 L scale, and feed testing in poultry and pigs (unpubl. data).
Sometimes probiotics are also serendipitously discovered. A strain of Bacillus amyloliquefaciens (H57) was initially developed as an additive for hay to prevent mould and spoilage (Brown and Dart 2005); due to the production of antibiotic lipopeptides (Schofield et al. 2016). Initial safety trials showed increased animal performance (Norton et al. 2008) and so probiotic development was initiated with encouraging results in calves and sheep (Le et al. 2017a, 2017b), and with specific effects towards necrotic enteritis in poultry (Shini et al. 2020).
Enzymes
Phytases and NSP-degrading classes of enzymes have established in-feed applications, especially for poultry and pigs (Bedford and Partridge 2010; Nunes and Kumar 2018). Enzymes in these classes have been derived from microorganisms, for example, the phytase found in the well studied organism Escherichia coli (Greiner et al. 1993) that is now the basis of several commercial products (Table 2). In general, the discovery of new and improved feed enzymes has focused on generating variants of existing natural enzymes (e.g. phytase) with improved properties, rather than the discovery of new functionalities. However, one notable exception is the discovery and development of new enzyme functions for mycotoxin degradation (Loi et al. 2017; Moll 2019).
|
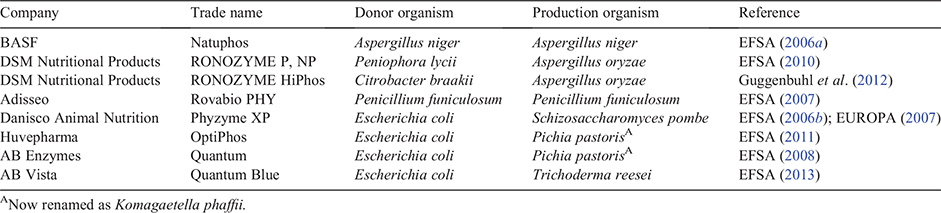
|
The discovery and development strategies to generate improved variant enzymes such as phytases rely on modern biotechnological approaches and engineering of the protein sequence (Speight 2016).
With the vast numbers of sequences in genetic databases, there is ample scope for identifying new phytases. However, having access to large numbers of uncharacterised sequences does not provide a strategy for identifying those that would generate improved feed enzymes. The challenge remains to computationally predict those that address the selection criteria (improved activity and/or stability) and to narrow down the numbers of sequences to those that can be practically and economically tested in the laboratory (Speight 2016). The first step in the development strategy is to synthesise the relevant genes, with the cost of a typical phytase-encoding gene (of around 1000 base pairs) now less than US$100. The second step is to produce the enzyme for testing, and this can still be laborious, although systems involving cell-free protein synthesis have emerged that are rapid and amenable to miniaturisation and robotic liquid handling (Gagoski et al. 2017).
Regardless of the availability of natural enzyme diversity, most commercial phytase development has relied on rational protein engineering and directed evolution techniques (Shivange and Schwaneberg 2017). Mutagenesis of all amino acids in the E. coli phytase sequence was used to identify a variant that, when beneficial mutations were combined, increased the enzyme melting (enzyme unfolding and deactivation) temperature by 12°C, to 75.7°C, with a 3.5-fold increase in stability in simulated gastric fluid (Garrett et al. 2004; Short et al. 2008). This improved protein variant became the successful commercial phytase product Quantum (EFSA 2008).
Another protein-engineering strategy to improve thermostability is the incorporation of additional disulfide bonds to provide additional intramolecular covalent bonds within the protein structure and therefore resistance to unfolding (Sanchez-Romero et al. 2013). The E. coli phytase had three additional disulfide bonds added to the four native ones and this increased the melting temperature by 8.5°C and shifted the optimal temperature for activity to 70–75°C from ~50°C (De Maria et al. 2013). However, this thermostability was coupled to a significant reduction in activity at 30–40°C, which is the temperature at which activity is required in the animal. Overall, these changes mean that more units of enzyme activity will survive storage and steam pelleting, but per mass of enzyme there would be less activity in the animal; a higher dietary enzyme loading (by mass and therefore cost) would therefore be required. With collaborators, we developed and investigated a thermostable E. coli phytase variant with a single additional disulfide bond (Navone et al. 2021b). In contrast to the study above, despite increasing thermostability and survivability considerably at 85°C, we found the temperature and pH activity profiles to be near identical to the native enzyme, with very similar enzyme kinetic values at 37°C. However, the production yields of the thermostable phytase variant in engineered yeast cultures were very low compared with the native enzyme, and impossible to scale-up. Through yeast strain engineering to co-express the enzyme protein disulfide isomerase to aid correct disulfide bond formation and avoid mis-folding and phytase degradation, it was possible to increase production levels to close to those of the native enzyme, thus meeting the selection criteria and allowing progression to scale-up studies.
Overall, while there is the ever-increasing capacity to identify and develop new probiotics and enzymes through advanced genomics and protein engineering coupled to higher throughput laboratory-scale production and screening systems, it remains critical to precisely define assessment criteria to target novel commercially relevant attributes and provide clear stop/go decision points for progressing lead candidates into production development.
Feed supplement production
Once a potential supplement product has been identified, being able to produce the enzyme or probiotic in a sufficient quantity is essential at all stages of the development and commercialisation pipeline. Discovery and testing towards the selection criteria described above relies on the ability to produce the enzyme or probiotic in laboratory conditions that are usually very different from the conditions of larger-scale production (e.g. culture in microtitre plates (<0.2 mL), culture tubes (<50 mL) or shake flasks (<500 mL) rather than fermentation bioreactors (500 mL up to tens or hundreds of thousand litres).
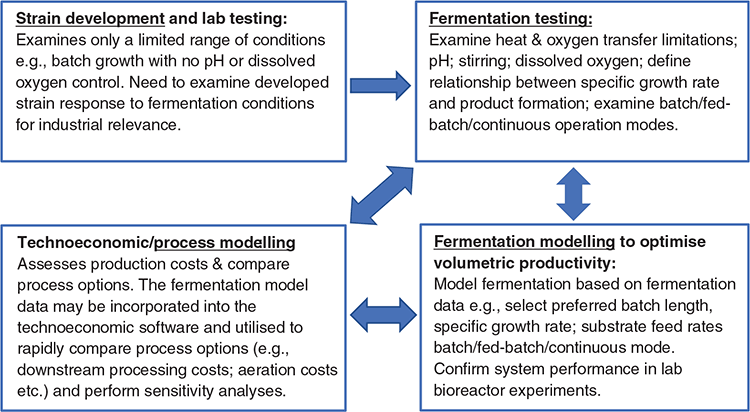
Although the main aim of laboratory-scale production is to generate sufficient material for criteria testing, with important selection criteria also around manufacturability, it is important that the small-scale production systems anticipate what might be appropriate at larger scales (e.g. affordable media components and fast-growing microbial strains). Once lead candidate supplements are identified in the laboratory, the next stage is to produce sufficient volumes for livestock evaluation. Although the required amounts of material vary significantly (e.g. between poultry bioassays to cattle trials), each will require production in fermentation bioreactors of various sizes. Given the importance of manufacturing economics for ultimate commercial success, it is important to perform production process development and modelling in fermenters before and in parallel with the production of material for feeding trials (Fig. 2). It is also important to acknowledge the significant process differences between the different scales of operation from the laboratory to full commercial scale, due to factors such as mixing/stirring and heat and gas transfer. Again, for established players with vertically integrated development platforms, there will be a lot of experience regarding how processes transfer between the various scale-up stages. New or emerging players are less likely to have this knowledge or experience, and this can lead to significant unanticipated manufacturing issues during scale-up.
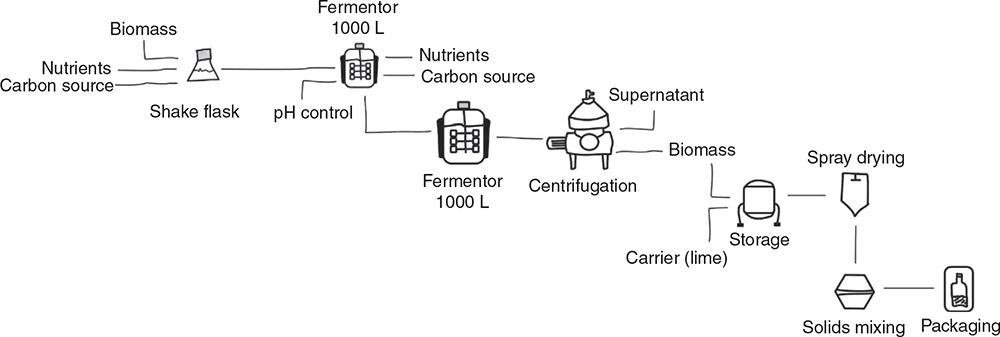
There are many similarities in submerged fermentation processes to produce probiotics (such as Bacillus species for livestock applications) and feed enzymes (Fasim et al. 2021). Both products require growth of a microbial strain that is either the product itself in the case of probiotics or is the agent that synthesises the product in the case of enzymes. Both processes require seed cultures of increasing sizes before the production fermenter, with the associated sterilisation and media preparation as well as steps to remove the cells from the media (e.g. by centrifugation; Fig. 3).
Although both processes require the separation of cells from the growth media, the probiotic is the actual cells whereas the enzyme is generally secreted by the production cells into the media and so is a soluble fraction of the liquid component. For probiotics, the cell paste from centrifugation is generally dried to a powdered product directly. Enzymes need to be precipitated from the media or concentrated using chromatography or membrane systems and either used as a stabilised liquid or dried to a powder (Patel et al. 2017).
The production processes described above are all based on submerged fermentations. Probiotics and enzymes can also be produced using solid state fermentations where the cells are grown on a solid substrate (Vandenberghe et al. 2020). Although less established than submerged fermentations, solid-state processes can have process advantages and be useful when the solid substrate (fibre such as sugarcane bagasse, rice husks, or wheat bran) is also beneficially modified (pre-treated to be more digestible) for inclusion in the final livestock feed (Amini et al. 2021).
Technoeconomic models and evaluation
The engineering design of the manufacturing process is described in a model that captures both technical and economic aspects and estimates capital and operational expenditure. These technoeconomic models include equipment details, process and material flows, energy use, and other factors important to the economics such as personnel time, equipment usage and depreciation.
Computer simulations to model and predict costs of production have been used for many industrial processes at different stages of development from idea generation through to technology transfer, introduction of new technologies and process optimisation (Archacka et al. 2020; Czinkóczky and Németh 2020). In the initial process-development phase of a project, it is valuable to model a reliable base-case scenario to pinpoint the cost-sensitive areas of a complex process. The model can be used to estimate of the effect of increasing costs of raw materials or utilities, variations in material composition, as well as changes in the process configuration and the incorporation of new technologies. This information can be used for the identification and optimisation of key process steps which have high capital or operating costs or low yield and production throughput. The ability to perform virtual experiments and process analyses in silico reduces the amount of costly and time-consuming laboratory and pilot plant experiments by focusing research and development on potential process improvements that are likely to have the greatest overall economic impact.
After a process model has been developed, software such as SuperPro Designer may be used to ask and answer ‘what if?’ questions and conduct sensitivity analysis with respect to key design variables. The objective of these studies is to evaluate the impact of critical parameters on various key performance criteria, such as production cost, cycle times and plant throughput. This information may be incorporated into the base case model to improve process performance and reduce constraints of the current process design. Some of the design improvement options to improve performance include increasing the batch size to increase production throughput, improved economies of scale for fermenter equipment and increased utilisation of downstream processing equipment; and adding more fermenters so that production can be staggered to be more efficient with respect to set-up and cleaning time, and to up- and downstream processes. Typically, external economic factors such as the selling price generally have a strong effect on overall economics, whereas raw material costs have a relatively low impact, given the relatively low cost of materials compared with other costs. Usually, one of the most important parameters is the volumetric productivity of the process, represented by the number of grams of enzyme produced per litre of fermentation volume per day, or by the mass or number of spores/cells of probiotic per litre per day. This volumetric productivity parameter is usually the focus of initial laboratory- and pilot-scale process-optimisation experiments.
Process optimisation
For enzyme production in engineered microorganisms, the volumetric productivity is determined by both the cell biomass growth/productivity and the amount of enzyme each cell can produce. Efficient enzyme production fermentation processes are therefore optimised for high cell density using highly productive strains. Such processes typically involve a cell growth-batch phase to achieve high cell densities before the induction of enzyme synthesis in a fed-batch phase (Liu et al. 2019).
A variety of strain engineering strategies can be used to increase enzyme yield per cell. Such strategies include encoding gene sequence codon optimisation, gene copy number modulation (in plasmid-based systems or when chromosomally integrated), promoter selection and induction strategy, and the co-expression of helper proteins such as folding catalysts. In parallel with the phytase disulfide bond engineering described in the previous section, we used yeast strain engineering approaches to improve the production of the E. coli phytase in Pichia pastoris by 2.9-fold compared with the standard established system (Navone et al. 2021a). This improvement was achieved through exploring synergistic optimisation of bidirectional genetic promoters to co-express phytase with disulfide bond isomerases, trafficking proteins, and a cytosolic redox metabolism protein. This near three-fold improvement had a strong effect on the modelled manufacturing economics. For example, three-fold fewer runs would need to be performed or manufacturing facilities could be three-times smaller.
For both enzyme and probiotic production, development of the overall fermentation process is used to optimise volumetric productivities, with the main parameters being the components of the growth media and the process operational design (including temperature, stirring/mixing, aeration, pH, and feeding strategy in fed-batch mode). While the media components may not be a dominant cost in themselves, they often have a strong bearing on the volumetric productivity of the system, with different carbon sources (e.g. specific sugars), nitrogen sources, vitamins and mineral salts being specifically optimal for different microorganisms.
As well as the fermentation volumetric productivity, downstream processing can have a major impact on final yields of active enzyme or viable probiotic and therefore process costs. Enzymes and probiotics are both sensitive to heat and other stressors that can be experienced during drying and formulation, and so these processes should also be optimised (Chávez and Ledeboer 2007; Huang et al. 2017). The inherent robustness of the probiotic strain or enzyme, operating parameters of the dryer (e.g. spray dryer or fluidised bed dryer) such as flow rate or temperature and chosen carrier or protective agents (such as maltodextrin, milk powder, starch, or calcium carbonate) can all influence survivability, as well as the ongoing stability of the product. In the case of probiotics, it can be advantageous to induce sporulation during production as spores are inherently more robust than vegetative cells, with the Bacillus species forming many commercial livestock probiotic products being particularly amenable to spore formation (Cutting 2011; Elisashvili et al. 2019). In the case of enzymes, the drying and formulation method can be used to generate either powders or granules that may enhance enzyme survival during subsequent steam pelleting of feed or in the low stomach pH of livestock. However, granules may be slower to dissolve and solubilise than powders, thereby limiting their availability in the animal. In some cases, the product can also be formulated as a concentrated liquid to avoid the drying step, provided the product is stable in this form.
The challenges of enzyme and probiotic manufacturing development
As can be seen above, the development, optimisation and scale-up of enzyme and probiotic production processes are complex and require significant experience, infrastructure and skills, all of which vary considerably among research/laboratory, pilot and manufacturing scales. For the non-established players and consortia, efficiently moving through manufacturing scales to a profitable commercial process is challenging. Often, consortia of academic groups and livestock feed companies who develop a new enzyme or probiotic may lack the infrastructure and expertise to manufacture beyond laboratory scale and are unable to effectively perform manufacturing process development or produce the required material for livestock evaluation in house. Established players also have an advantage at commercial scale compared with new players. They typically have access to larger fermentation vessels and can achieve higher levels of equipment utilisation by efficient scheduling and by using multiple vessels to make a range of products.
To address some of these challenges, some government initiatives, research organisations and universities seek to bridge the gap between research and commercialisation through the establishment of pilot-scale fermentation facilities. The need for such facilities has been reviewed in the UK (https://bbsrc.ukri.org/documents/1503-ib-process-plant-report/) and select facilities that focus on fermentation and industrial bioproducts are highlighted in Table 3. The European Union has developed an asset register to pilot facilities relevant to the bioeconomy (https://biopilots4u.eu/). The major European pilot facilities have also come together in the SmartPilots scheme to optimise and enable access to facilities and to reduce the barriers to scale up for new projects and thereby increase the likelihood of impact (https://www.interregeurope.eu/smartpilots/). This optimisation is not possible in areas with limited facilities, such as Australia.
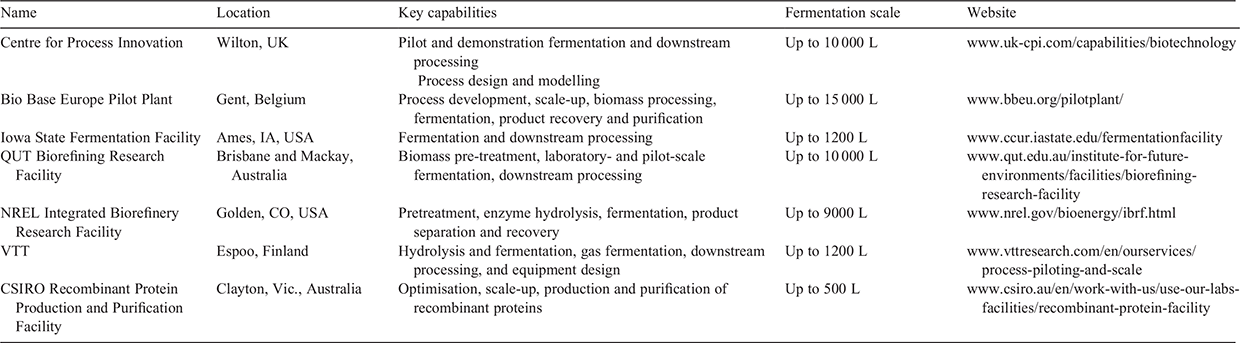
|
Despite the pilot-scale facility examples provided herein, access to appropriate facilities in different parts of the world can be challenging, with many of these facilities still being remote from the researchers discovering new probiotics and enzymes and the companies wishing to commercialise them. Europe has made significant progress in developing and integrating facilities to a level not commonly seen elsewhere. We endeavoured to achieve this deep integration in Queensland by utilising the skills of university and industry partners and collaborators.
Animal feed supplement evaluation
Although laboratory testing can provide information about the performance of an enzyme or probiotic that may suggest how a supplement might perform in vivo, the complexity of animal systems and the very different environment in an animal compared with a laboratory means that feeding trials are required. There are several books that detail the design and conduct of animal experimentation to test or evaluate diets, feedstuffs and feed supplements for both ruminants and non-ruminants (Schneider and Flatt 1975; McLean and Tobin 1987; Wiseman and Cole 1990; Whittemore 1990; Fuller 1991; D’Mello 2000; Lewis and Southern 2001; McNab and Boorman 2002; Bedford et al. 2016; Moughan and Hendriks 2018).
Trials with animals should be conducted as early as possible, once candidate supplements meet the laboratory criteria. The trials should also be designed to test hypotheses on the basis of the development criteria. For example, if a probiotic candidate was developed to address C. perfringens infections, then it should be tested in a disease challenge model (Jayaraman et al. 2013). If probiotic selection criteria were focused on the production of fibre-degrading enzymes, then it should be tested using high-fibre diets that would otherwise be a challenge to the animal. If a phytase was developed to meet improved thermostability criteria, then it should be formulated using high-temperature steam pelleting before feeding. The first livestock trials following laboratory development should be as rapid and small scale as possible. Smaller trials will be cheaper, allow more candidate supplements to be tested in more treatment conditions, and will screen out under-performing candidates early, while informing revisions of specific development criteria in laboratory discovery and development. In initial trials, it is also an advantage to require only small amounts of candidate supplement that can be readily and cheaply produced in a laboratory-scale fermentation and downstream formulation. These smaller amounts of material can also be produced before the production processes have been optimised, meaning that the trials can happen earlier in the overall development timeline.
Rapid poultry bioassays focused on specific criteria, such as apparent metabolisable energy (Mollah et al. 1983) or amino acid availability estimated as digestibility (Ravindran et al. 2005), are established. As important commercial feed parameters, the use of these techniques has been extensively reviewed for their application to poultry and pigs (Sibbald 1982, 1987; Farrell 1999; Ravindran and Bryden 1999; Stein 2017). Where digestibility measurements are made, it is critical that an appropriate inert digestibility marker, both for the diet and the species being fed, is included in the diet (Kotb and Luckey 1972; Faichney 1975; Warner 1981). Digestibility bioassays may be conducted only for a short period (e.g. 1 week) with a small number of birds or even individual birds to further limit the volume of candidate supplement required and reduce costs, while increasing the number of candidates and treatment conditions that can be tested. The discussion so far has concentrated on digestibility assays that provide important feedback for product development. Growth bioassays where rapidly growing broilers are fed the new supplement for periods ranging from 7 days to 5 weeks can provide valuable information on product safety and bird performance, especially growth, feed utilisation and bioavailability. Growth bioassays were perfected by the late David Baker and his group at the University of Illinois (Robbins et al. 1977; Anderson et al. 1978; Robbins et al. 1979; Baker 1984, 1986). With careful planning, growth and digestibility assays can be run sequentially and, in some instances, it may be appropriate to use in vitro methods (Fuller 1991; Moughan 1999). Poultry are often used in initial testing due to the economic importance of the poultry market, low cost of birds, short growth period, and low dietary intake limiting the amount of supplement required.
Other species such as cattle, pigs and sheep are more costly to test and require more feed and feed supplements. In these cases, development teams may choose to perform a simulated in vitro trial using gut contents in laboratory reactors (Ellis et al. 2016; Lu et al. 2021) or in situ methods when cannulated cattle, sheep or pigs are available (White and Ashes 1999; Hristov et al. 2019). These studies can be faster and cheaper than live in vivo animal studies and can be used to further refine the candidate supplements for in vivo testing. However, such systems are only a model for performance in the animal, so feeding trials are still required (Hogan and Flinn 1999). In the case of larger livestock such as cattle and pigs, initial trials can still be designed for low numbers of animals and over short timeframes, to reduce costs and provide rapid results to inform further supplement development.
Once initial small-scale trials have demonstrated safety and efficacy of a lead candidate supplement, production can be scaled up (e.g. to pilot scale at several hundred litre fermentations) to produce material for more extensive trials with more animals, that will deliver data required for regulatory approval and business case development.
Future challenges and opportunities
For new players and industry–academia projects to develop enzyme and probiotic feed supplements efficiently needs extensive and effective collaboration with all the required skills, infrastructure and equipment, and the right partners across the value chain from discovery to commercialisation. It is essential that clear commercial targets, desired product characteristics, and activities are set from the start so that discovery and development is targeted towards products that can make an impact and gain market share. Once a desired product is defined, it is then vital that selection criteria are articulated that include both performance and manufacturing, so that discovery and development are focused and efficient. It will then be clear when criteria are met, and potential products can move into pilot-scale manufacture and the production of material for livestock feeding trials. The pilot-scale manufacture should inform detailed techno-economic models and assessments for establishing practical and economic viability as well as providing material for trials that is representative of how a future commercial product might be formulated. The livestock evaluation trials should be rapid and low-cost in the first instance, and be designed in line with the desired product characteristics and the selection criteria used in discovery and development.
A potential challenge when up-scaling for commercialisation is the supply and cost of ingredients for fermentation and product formulation. Depending on the location of intended production, it can also be advantageous to consider local supply (e.g. sucrose in sugarcane growing regions, or hydrolysed wheat starch or corn steep liquor in other areas). Waste or co-product streams from local agricultural and livestock processing industries can also be considered for the production of feeds and feed supplements (Ramirez et al. 2021), along with human food waste (Torok et al. 2021). Such approaches are often driven by the generators of the material as they seek to reduce waste disposal costs, reduce environmental footprint and regulatory constraints, and move towards more circular business models. With many media component parameters to optimise, many of which will interact with each other, the most efficient approach is often to use design of experiments where multiple factors can be efficiently and simultaneously evaluated using a statistical approach (Mandenius and Brundin 2008).
Systems and protocols need to be developed so that all these steps occur as rapidly as possible. In so doing, both pilot manufacture and feeding trial data inform iterative rounds of product discovery and development. Employing selection criteria and a rapid laboratory-to-testing platform also allows potential products to ‘fail fast’ if they are destined to fail, rather than being researched in the laboratory for extended periods only to fail manufacturing and performance targets after years of research effort.
To address the significant opportunity as well as the challenges, we sought to enhance enzyme and probiotic discovery and pilot-scale manufacture by closely integrating people and infrastructure among laboratory-scale enzyme and microbial strain engineering and fermentation, downstream processing (e.g. centrifugation and spray drying) and product formulation at laboratory and pilot scales. This integration involved parallel use of both university and company laboratories, as well as pilot-scale and production-scale facilities. Both the earlier-stage research and later-stage larger pilot studies were performed between industry and academia rather than there being a point of project transfer and lack of integration. Overall, this integration of teams and facilities was the key part of achieving the development and production platform that was able to deliver a new thermostable phytase and a Bacillus probiotic in sufficient quantities and quality to enable a variety of livestock feeding trials. Further, embedding technoeconomic analyses at each stage of fermentation and downstream processing development focused the research towards areas of largest economic impact, leading to processes that are modelled to be economically viable at a commercial scale. The developed platforms are also amenable to other feed additives and products. In this regard, the platforms have been instrumental in developing a molasses lick block for delivering enzymes to grazing livestock (Ainscough et al. 2019) and an enzyme product for removing dags from cattle before slaughter (Navone and Speight 2019).
Despite the pilot scale manufacture (e.g. up to 1000 L fermentation for the probiotic) being sufficient for several large-scale feeding trials, and, potentially, even early-stage commercialisation, the lack of full vertical integration from research to commercial-scale manufacturing presents challenges for the next stage. Production of the new phytase in engineered yeast strains also requires specialised fermentation facilities with appropriate and certified containment to prevent unintentional release of the production organism. While local (Australian) manufacturing options can be explored (e.g. investment in modifying existing or building new manufacturing facilities), the current lack of full vertical integration means the manufacturing associated economic returns from the initial investments by government and the partners may be overseas. Manufacture overseas can also lead to communication/technical transfer and logistics issues. It is evident that where established development and manufacturing ecosystems exist that have a critical mass of research and fermentation facilities along with the companies that will drive commercialisation, the economic benefits are more likely to accrue locally. Regions wishing to realise economic benefits need to establish the same ecosystems for research and manufacturing through appropriate policies, incentives and investments. In the absence of these ecosystems, new players and consortia rely on government project funding to decrease the risk and enable product commercialisation, with governments supplying this funding to stimulate local manufacturing along with potential future local employment and economic activity.
There are many future opportunities as extensive microbiome and genome sequencing is accelerating the discovery of potential probiotics and feed enzymes and providing a wealth of new targets. At the same time, advances in synthetic biology and the engineering of biology are providing ever-increasing capacity for developing new enzymes and moving beyond probiotics to live microbial therapeutics (O’Toole et al. 2017; Charbonneau et al. 2020). With these enhanced opportunities for the discovery of improved enzymes and probiotics, it will be even more important to bring together excellent collaborative teams with rapid laboratory-to-market platforms to realise these opportunities through on-market products.
Data availability
There are no data associated with this article.
Conflicts of interest
WLB is a member of the Editorial Board of Animal Production Science but was not involved in the review and editorial process for this paper. The authors have no further conflicts of interest to declare.
Declaration of funding
The themes and inspirations for the content are derived from our experience in funded projects on livestock feed supplement development, production, and testing. We acknowledge Advance Queensland Innovation Partnership program funding from the Queensland State Government, along with co-funding from the collaborating industry partners Ridley AgriProducts Pty Ltd, Bioproton Pty Ltd and Kennedy Creek Lime Pty Ltd (Project AQIP01515-16RD1). Also, the Biorefineries for Profit project from Sugar Research Australia, with funding from the Australian Government Department of Agriculture as part of its Rural R&D for Profit program, the Queensland Government and project partners including Cotton Research and Development Corporation, Forest & Wood Products Australia, Australian Pork Ltd, Southern Oil Refining, NSW Department of Primary Industries and Queensland University of Technology (Project RnD4Profit 14-01-044 and RnD4Profit 18-04-016).
Acknowledgements
The authors gratefully thank Mr Juhani von Hellens from Bioproton Pty Ltd and Mr Matthew Callaghan from Ridley Agriproducts Pty Ltd for their collaboration and valuable insights into commercial animal feed supplement development and manufacture that have helped inform this article.
References
Aftab U, Bedford MR (2018) The use of NSP enzymes in poultry nutrition: myths and realities. World’s Poultry Science Journal 74, 277–286.| The use of NSP enzymes in poultry nutrition: myths and realities.Crossref | GoogleScholarGoogle Scholar |
Ainscough RJ, McGree JM, Callaghan MJ, Speight RE (2019) Effective incorporation of xylanase and phytase in lick blocks for grazing livestock. Animal Production Science 59, 1762–1768.
| Effective incorporation of xylanase and phytase in lick blocks for grazing livestock.Crossref | GoogleScholarGoogle Scholar |
Almeida A, Nayfach S, Boland M, Strozzi F, Beracochea M, Shi ZJ, Pollard KS, Sakharova E, Parks DH, Hugenholtz P (2021) A unified catalog of 204,938 reference genomes from the human gut microbiome. Nature Biotechnology 39, 105–114.
| A unified catalog of 204,938 reference genomes from the human gut microbiome.Crossref | GoogleScholarGoogle Scholar | 32690973PubMed |
Amini Z, Self R, Strong J, Speight R, O’Hara I, Harrison MD (2021) Valorization of sugarcane biorefinery residues using fungal biocatalysis. Biomass Conversion and Biorefinery
| Valorization of sugarcane biorefinery residues using fungal biocatalysis.Crossref | GoogleScholarGoogle Scholar |
Anderson PA, Baker DH, Mistry SP (1978) Bioassay determination of the biotin content of corn, barley, sorghum and wheat. Journal of Animal Science 47, 654–659.
| Bioassay determination of the biotin content of corn, barley, sorghum and wheat.Crossref | GoogleScholarGoogle Scholar |
Archacka M, Celińska E, Białas W (2020) Techno-economic analysis for probiotics preparation production using optimized corn flour medium and spray-drying protective blends. Food and Bioproducts Processing 123, 354–366.
| Techno-economic analysis for probiotics preparation production using optimized corn flour medium and spray-drying protective blends.Crossref | GoogleScholarGoogle Scholar |
Bajagai YS, Klieve AV, Dart PJ, Bryden WL (2016) ‘Probiotics in animal nutrition: production, impact and regulation. FAO Animal Production and Health paper no. 179.’ (Ed. HPS Makkar) (FAO: Rome, Italy)
Baker DH (1984) Equalized versus ad libitum feeding. Nutrition Reviews 42, 269–273.
| Equalized versus ad libitum feeding.Crossref | GoogleScholarGoogle Scholar | 6384834PubMed |
Baker DH (1986) Problems and pitfalls in animal experiments designed to establish dietary requirements for essential nutrients. The Journal of Nutrition 116, 2339–2349.
| Problems and pitfalls in animal experiments designed to establish dietary requirements for essential nutrients.Crossref | GoogleScholarGoogle Scholar | 3543259PubMed |
Bedford MR (2018) The evolution and application of enzymes in the animal feed industry: the role of data interpretation. British Poultry Science 59, 486–493.
| The evolution and application of enzymes in the animal feed industry: the role of data interpretation.Crossref | GoogleScholarGoogle Scholar | 29877713PubMed |
Bedford MR, Partridge GG (Eds) (2010) Enzymes in farm animal nutrition. 2nd edn. (CAB International: Wallingford, UK)
Bedford MR, Choct M, O’Neill HM (Eds) (2016) ‘Nutrition experiments in pigs and poultry: a practical guide.’ (CAB International: Wallingford, UK)
Brown S, Dart P (2005) ‘Testing hay treated with mould-inhibiting, biocontrol inoculum.’ (Rural Industries Research and Development Corporation: Canberra, ACT, Australia)
Browne HP, Forster SC, Anonye BO, Kumar N, Neville BA, Stares MD, Goulding D, Lawley TD (2016) Culturing of ‘unculturable’ human microbiota reveals novel taxa and extensive sporulation. Nature 533, 543–546.
| Culturing of ‘unculturable’ human microbiota reveals novel taxa and extensive sporulation.Crossref | GoogleScholarGoogle Scholar | 27144353PubMed |
Brüssow H (2017) Adjuncts and alternatives in the time of antibiotic resistance and in-feed antibiotic bans. Microbial Biotechnology 10, 674–677.
| Adjuncts and alternatives in the time of antibiotic resistance and in-feed antibiotic bans.Crossref | GoogleScholarGoogle Scholar | 28643479PubMed |
Bryden WL, Selle PH, Ravindran V, Acamovic T (2007) Phytate: An anti-nutrient factor in animal diets. In ‘Poisonous plants: global research and solutions’. (Eds KE Panter, TL Wierenga, JA Pfister) pp. 279–284. (CAB International Publishing: Wallingford, UK)
Campbell GL, Bedford MR (1992) Enzyme applications for monogastric feeds: a review. Canadian Journal of Animal Science 72, 449–466.
| Enzyme applications for monogastric feeds: a review.Crossref | GoogleScholarGoogle Scholar |
Cardinal KM, Kipper M, Andretta I, Ribeiro AML (2019) Withdrawal of antibiotic growth promoters from broiler diets: performance indexes and economic impact. Poultry Science 98, 6659–6667.
| Withdrawal of antibiotic growth promoters from broiler diets: performance indexes and economic impact.Crossref | GoogleScholarGoogle Scholar |
Charbonneau MR, Isabella VM, Li N, Kurtz CB (2020) Developing a new class of engineered live bacterial therapeutics to treat human diseases. Nature Communications 11, 1738
| Developing a new class of engineered live bacterial therapeutics to treat human diseases.Crossref | GoogleScholarGoogle Scholar | 32269218PubMed |
Chávez B, Ledeboer A (2007) Drying of probiotics: optimization of formulation and process to enhance storage survival. Drying Technology 25, 1193–1201.
| Drying of probiotics: optimization of formulation and process to enhance storage survival.Crossref | GoogleScholarGoogle Scholar |
Cutting SM (2011) Bacillus probiotics. Food Microbiology 28, 214–220.
| Bacillus probiotics.Crossref | GoogleScholarGoogle Scholar | 21315976PubMed |
Czinkóczky R, Németh Á (2020) Techno-economic assessment of Bacillus fermentation to produce surfactin and lichenysin. Biochemical Engineering Journal 163, 107719
| Techno-economic assessment of Bacillus fermentation to produce surfactin and lichenysin.Crossref | GoogleScholarGoogle Scholar |
D’Mello JPF (Ed.) (2000) ‘Farm animal metabolism and nutrition.’ (CAB International: Wallingford, UK)
De Maria L, Skov LK, Skjoet M (2013) Thermostable Phytase Variants. US 2013/0017185.
de Melo Pereira GV de Melo Pereira GV (2018) How to select a probiotic? A review and update of methods and criteria. Biotechnology Advances 36, 2060–2076.
| How to select a probiotic? A review and update of methods and criteria.Crossref | GoogleScholarGoogle Scholar | 30266342PubMed |
EFSA (2006a) Opinion of the Scientific Panel on Additives and Products or Substances used in Animal Feed and of the Scientific Panel on Genetically Modified Organisms on the safety and efficacy of the enzyme preparation Natuphos® (3-phytase) produced by Aspergillus niger. EFSA Journal 369, 1–19.
EFSA (2006b) Opinion of the Scientific Panel on Additives and Products or Substances used in Animal Feed and the Scientific Panel on Genetically Modified Organisms on the safety and efficacy of the enzymatic preparation Phyzyme XP (6- Phytase) for use as feed additive for chickens for fattening EFSA Journal 350, 1–14.
EFSA (2007) Opinion of the Scientific Panel on Additives and Products or Substances used in Animal Feed and the Scientific Panel on Genetically Modified Organisms on the safety and efficacy of the enzyme preparation Rovabio™ PHY AP/LC (3-phytase) as feed additive for chickens for fattening, laying hens, piglets and pigs for fattening in accordance with Regulation (EC) No 1831/2003. EFSA Journal 471, 1–29.
EFSA (2008) Safety and efficacy of the product Quantum™ Phytase 5000 L and Quantum™ Phytase 2500 D (6-phytase) as a feed additive for chickens for fattening, laying hens, turkeys for fattening, ducks for fattening and piglets (weaned). EFSA Journal 627, 1–27.
EFSA (2010) Scientific Opinion on Ronozyme® P (6-phytase) as feed additive for chickens and turkeys for fattening, laying hens, and piglets (weaned), pigs for fattening and sows (poultry and pigs). EFSA Journal 8, 1862
| Scientific Opinion on Ronozyme® P (6-phytase) as feed additive for chickens and turkeys for fattening, laying hens, and piglets (weaned), pigs for fattening and sows (poultry and pigs).Crossref | GoogleScholarGoogle Scholar |
EFSA (2011) Scientific Opinion on the safety and efficacy of Optiphos® (6-phytase) as a feed additive for chickens and turkeys for fattening, chickens reared for laying, turkeys reared for breeding, laying hens, other birds for fattening and laying, weaned piglets, pigs for fattening and sows. EFSA Journal 9, 2414
EFSA (2013) Scientific Opinion on the safety and efficacy of Quantum® Blue (6-phytase) as a feed additive for laying hens and minor laying poultry species. EFSA Journal 11, 3433
Elisashvili V, Kachlishvili E, Chikindas ML (2019) Recent advances in the physiology of spore formation for Bacillus probiotic production. Probiotics and Antimicrobial Proteins 11, 731–747.
| Recent advances in the physiology of spore formation for Bacillus probiotic production.Crossref | GoogleScholarGoogle Scholar | 30515722PubMed |
Ellis JL, Bannink A, Hindrichsen IK, Kinley RD, Pellikaan WF, Milora N, Dijkstra J (2016) The effect of lactic acid bacteria included as a probiotic or silage inoculant on in vitro rumen digestibility, total gas and methane production. Animal Feed Science and Technology 211, 61–74.
| The effect of lactic acid bacteria included as a probiotic or silage inoculant on in vitro rumen digestibility, total gas and methane production.Crossref | GoogleScholarGoogle Scholar |
EUROPA (2007) Concerning the authorisation of 6-phytase EC 3.1.3.26 (Phyzyme XP 5000G Phyzyme XP 5000L) as a feed additive. Official Journal of the European Union, L 175,
Faichney GJ (1975) The use of markers to partition digestion within the gastro-intestinal tract of ruminants. In ‘Digestion and metabolism in the ruminant’. (Eds IW McDonald, ACI Warner) pp. 277–291. (University of New England Publishing Unit: Armidale, NSW, Australia)
Farrell DJ (1999) In vivo and in vitro techniques for the assessment of the energy content of feed grains for poultry: a review. Australian Journal of Agricultural Research 50, 881–888.
| In vivo and in vitro techniques for the assessment of the energy content of feed grains for poultry: a review.Crossref | GoogleScholarGoogle Scholar |
Fasim A, More VS, More SS (2021) Large-scale production of enzymes for biotechnology uses. Current Opinion in Biotechnology 69, 68–76.
| Large-scale production of enzymes for biotechnology uses.Crossref | GoogleScholarGoogle Scholar | 33388493PubMed |
FiorMarkets (2020) Animal Feed Additives Market by Type (Antibiotics, Minerals, Binders, Vitamins, Feed Enzymes, Feed Acidifiers, Antioxidants, Amino Acids), Livestock (Aquatic Animals, Ruminants, Poultry, Swine), Form (Liquid, Dry), Region, Global Industry Analysis, Market Size, Share, Growth, Trends, and Forecast 2020 to 2027. Available at https://www.fiormarkets.com/report/animal-feed-additives-market-by-type-antibiotics-minerals-418379.html
Fuller MF (Ed.) (1991) ‘In vitro digestion for pigs and poultry.’ (CAB International: Wallingford, UK)
Gagoski DShZ, Nielsen LK, Vickers CE, Mahler S, Speight R, Johnston WA, Alexandrov K (2017) Cell-free pipeline for discovery of thermotolerant xylanases and endo-1, 4-β-glucanases. Journal of Biotechnology 259, 191–198.
| Cell-free pipeline for discovery of thermotolerant xylanases and endo-1, 4-β-glucanases.Crossref | GoogleScholarGoogle Scholar |
Garrett JB, Kretz KA, O’Donoghue E, Kerovuo J, Kim W, Barton NR, Hazlewood GP, Short JM, Robertson DE, Gray KA (2004) Enhancing the thermal tolerance and gastric performance of a microbial phytase for use as a phosphate-mobilizing monogastric-feed supplement. Applied and Environmental Microbiology 70, 3041–3046.
| Enhancing the thermal tolerance and gastric performance of a microbial phytase for use as a phosphate-mobilizing monogastric-feed supplement.Crossref | GoogleScholarGoogle Scholar | 15128565PubMed |
Gebbie L, Dam TT, Ainscough R, Palfreyman R, Cao L, Harrison M, O’Hara I, Speight R (2020) A snapshot of microbial diversity and function in an undisturbed sugarcane bagasse pile. BMC Biotechnology 20, 12
| A snapshot of microbial diversity and function in an undisturbed sugarcane bagasse pile.Crossref | GoogleScholarGoogle Scholar | 32111201PubMed |
Greiner R, Konietzny U, Jany K-D (1993) Purification and characterization of two phytases from Escherichia coli. Archives of Biochemistry and Biophysics 303, 107–113.
| Purification and characterization of two phytases from Escherichia coli.Crossref | GoogleScholarGoogle Scholar | 8387749PubMed |
Guerrand D (2018) Economics of food and feed enzymes: status and prospectives. In ‘Enzymes in human and animal nutrition’. (Eds CS Nunes, FV Kumar) pp. 487–514. (Academic Press: London, UK)
Guggenbuhl P, Torrallardona D, Cechova I, Wache Y, Nunes CS, Fru F, Broz J (2012) The efficacy of a novel microbial 6-phytase expressed in Aspergillus oryzae on the performance and phosphorus utilization in swine. Journal of Animal Science Advances 2, 438–452.
Hogan JP, Flinn PC (1999) An assessment by in vivo methods of grain quality for ruminants. Australian Journal of Agricultural Research 50, 843–854.
| An assessment by in vivo methods of grain quality for ruminants.Crossref | GoogleScholarGoogle Scholar |
Homan VB, Boney JW, Moritz JS (2019) Effects of steam conditioning temperatures on commercial phytases and subsequent broiler performance and tibia mineralization. Applied Animal Science 35, 298–303.
| Effects of steam conditioning temperatures on commercial phytases and subsequent broiler performance and tibia mineralization.Crossref | GoogleScholarGoogle Scholar |
Hristov AN, Bannink A, Crompton LA, Huhtanen P, Kreuzer M, McGee M, Nozière P, Reynolds CK, Bayat AR, Yáñez-Ruiz DR, Dijkstra J, Kebreab E, Schwarm A, Shingfield KJ, Yu Z (2019) Nitrogen in ruminant nutrition: a review of measurement techniques. Journal of Dairy Science 102, 5811–5852.
| Nitrogen in ruminant nutrition: a review of measurement techniques.Crossref | GoogleScholarGoogle Scholar | 31030912PubMed |
Huang S, Vignolles M-L, Chen XD, Le Loir Y, Jan G, Schuck P, Jeantet R (2017) Spray drying of probiotics and other food-grade bacteria: a review. Trends in Food Science & Technology 63, 1–17.
| Spray drying of probiotics and other food-grade bacteria: a review.Crossref | GoogleScholarGoogle Scholar |
Inborr J, Bedford MR (1994) Stability of feed enzymes to steam pelleting during feed processing. Animal Feed Science and Technology 46, 179–196.
| Stability of feed enzymes to steam pelleting during feed processing.Crossref | GoogleScholarGoogle Scholar |
Jayaraman S, Thangavel G, Kurian H, Mani R, Mukkalil R, Chirakkal H (2013) Bacillus subtilis PB6 improves intestinal health of broiler chickens challenged with Clostridium perfringens-induced necrotic enteritis. Poultry Science 92, 370–374.
| Bacillus subtilis PB6 improves intestinal health of broiler chickens challenged with Clostridium perfringens-induced necrotic enteritis.Crossref | GoogleScholarGoogle Scholar | 23300303PubMed |
Kotb AR, Luckey TD (1972) Markers in nutrition. Nutrition Abstracts and Reviews 42, 813–845.
Le OT, Dart PJ, Harper K, Zhang D, Schofield B, Callaghan MJ, Lisle AT, Klieve AV, McNeill DM (2017a) Effect of probiotic Bacillus amyloliquefaciens strain H57on productivity and the incidence of diarrhoea in dairy calves. Animal Production Science 57, 912–919.
| Effect of probiotic Bacillus amyloliquefaciens strain H57on productivity and the incidence of diarrhoea in dairy calves.Crossref | GoogleScholarGoogle Scholar |
Le OT, Schofield B, Dart PJ, Callaghan MJ, Lisle AT, Ouwerkerk D, Klieve AV, McNeill DM (2017b) Production responses of reproducing ewes to a by-product-based diet inoculated with the probiotic Bacillus amyloliquefaciens strain H57. Animal Production Science 57, 1097–1105.
| Production responses of reproducing ewes to a by-product-based diet inoculated with the probiotic Bacillus amyloliquefaciens strain H57.Crossref | GoogleScholarGoogle Scholar |
Lewis AJ, Southern LL (Eds) (2001) ‘Swine nutrition.’ 2nd edn. (CRC Press: Boca Raton, FL, USA)
Li X, Zhang D, Yang TY, Bryden WL (2016) Phosphorus bioavailability: a key aspect for conserving this critical animal feed resource with reference to broiler nutrition. Agriculture 6, 25
| Phosphorus bioavailability: a key aspect for conserving this critical animal feed resource with reference to broiler nutrition.Crossref | GoogleScholarGoogle Scholar |
Liu W-C, Inwood S, Gong T, Sharma A, Yu L-Y, Zhu P (2019) Fed-batch high-cell-density fermentation strategies for Pichia pastoris growth and production. Critical Reviews in Biotechnology 39, 258–271.
| Fed-batch high-cell-density fermentation strategies for Pichia pastoris growth and production.Crossref | GoogleScholarGoogle Scholar | 30599783PubMed |
Loi M, Fanelli F, Liuzzi VC, Logrieco AF, Mulè G (2017) Mycotoxin biotransformation by native and commercial enzymes: present and future perspectives. Toxins 9, 111
| Mycotoxin biotransformation by native and commercial enzymes: present and future perspectives.Crossref | GoogleScholarGoogle Scholar |
Lu S, Mikkelsen D, Yao H, Williams BA, Flanagan BM, Gidley MJ (2021) Wheat cell walls and constituent polysaccharides induce similar microbiota profiles upon in vitro fermentation despite different short chain fatty acid end-product levels. Food & Function 12, 1135–1146.
| Wheat cell walls and constituent polysaccharides induce similar microbiota profiles upon in vitro fermentation despite different short chain fatty acid end-product levels.Crossref | GoogleScholarGoogle Scholar |
Mandenius CF, Brundin A (2008) Bioprocess optimization using design‐of‐experiments methodology. Biotechnology Progress 24, 1191–1203.
| Bioprocess optimization using design‐of‐experiments methodology.Crossref | GoogleScholarGoogle Scholar | 19194932PubMed |
Markowiak P, Śliżewska K (2018) The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathogens 10, 21
| The role of probiotics, prebiotics and synbiotics in animal nutrition.Crossref | GoogleScholarGoogle Scholar | 29930711PubMed |
McLean JA, Tobin G (1987) ‘Animal and human calorimetry.’ (Cambridge University Press: Cambridge, UK)
McNab JM, Boorman KN (Eds) (2002) ‘Poultry feedstuffs: supply, composition and nutritive value.’ (CAB International: Wallingford, UK)
Mingmongkolchai S, Panbangred W (2018) Bacillus probiotics: an alternative to antibiotics for livestock production. Journal of Applied Microbiology 124, 1334–1346.
| Bacillus probiotics: an alternative to antibiotics for livestock production.Crossref | GoogleScholarGoogle Scholar | 29316021PubMed |
Moll D (2019) Enzyme technology for detoxification of mycotoxins in animal feed. In ‘Industrial Enzyme Applications’. (Eds A Vogel, O May) pp. 219–254. (Wiley-VCH Verlag GmbH &Co.: Weinheim, Germany)
Mollah Y, Bryden WL, Wallis IR, Balnave D, Annison EF (1983) Sudies on low metabolisable energy wheats for poultry using conventional and rapid assay procedures and the effects of processing. British Poultry Science 24, 81–89.
| Sudies on low metabolisable energy wheats for poultry using conventional and rapid assay procedures and the effects of processing.Crossref | GoogleScholarGoogle Scholar |
Mordor Intelligence (2020) Australia feed additives market: growth, trends, COVID-19 impact, and forecasts (2021–2026). Available at https://www.mordorintelligence.com/industry-reports/australia-feed-additives-market-industry
Moughan PJ (1999) In vitro techniques for the assessment of the nutritive value of feed grains for pigs: a review. Australian Journal of Agricultural Research 50, 871–879.
| In vitro techniques for the assessment of the nutritive value of feed grains for pigs: a review.Crossref | GoogleScholarGoogle Scholar |
Moughan PJ, Hendriks WH (Eds) (2018) ‘Feed evaluation science.’ (Wageningen Academic Publishers: The Netherlands)
Navone L, Speight RE (2019) Enzyme systems for effective dag removal from cattle hides. Animal Production Science 59, 1387–1398.
| Enzyme systems for effective dag removal from cattle hides.Crossref | GoogleScholarGoogle Scholar |
Navone L, Vogl T, Luangthongkam P, Blinco J-A, Luna-Flores C, Chen X, von Hellens J, Speight R (2021a) Synergistic optimisation of expression, folding, and secretion improves E. coli AppA phytase production in Pichia pastoris. Microbial Cell Factories 20, 8
| Synergistic optimisation of expression, folding, and secretion improves E. coli AppA phytase production in Pichia pastoris.Crossref | GoogleScholarGoogle Scholar | 33494776PubMed |
Navone L, Vogl T, Luangthongkam P, Blinco J-A, Luna-Flores CH, Chen X, von Hellens J, Mahler S, Speight R (2021b) Disulfide bond engineering of AppA phytase for increased thermostability requires co-expression of protein disulfide isomerase in Pichia pastoris. Biotechnology for Biofuels 14, 80
| Disulfide bond engineering of AppA phytase for increased thermostability requires co-expression of protein disulfide isomerase in Pichia pastoris.Crossref | GoogleScholarGoogle Scholar | 33789740PubMed |
Norton BW, Dart PJ, Brown SM (2008) The effects of microbial amendment of rye-grass clover hay at baling on intake and nutritive value for pregnant sheep. Proceedings of the Australian Society for Animal Production 27, 110.
Nunes CS, Kumar FV (Eds) (2018) ‘Enzymes in human and animal nutrition: principles and perspectives.’ (Academic Press: London, UK)
O’Toole PW, Marchesi JR, Hill C (2017) Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nature Microbiology 2, 17057
| Next-generation probiotics: the spectrum from probiotics to live biotherapeutics.Crossref | GoogleScholarGoogle Scholar | 28440276PubMed |
Parks DH, Rinke C, Chuvochina M, Chaumeil P-A, Woodcroft BJ, Evans PN, Hugenholtz P, Tyson GW (2017) Recovery of nearly 8,000 metagenome-assembled genomes substantially expands the tree of life. Nature Microbiology 2, 1533–1542.
| Recovery of nearly 8,000 metagenome-assembled genomes substantially expands the tree of life.Crossref | GoogleScholarGoogle Scholar | 28894102PubMed |
Parks DH, Rigato F, Vera-Wolf P, Krause L, Hugenholtz P, Tyson GW, Wood DL (2021) Evaluation of the microba community profiler for taxonomic profiling of metagenomic datasets from the human gut microbiome. Frontiers in Microbiology 12, 643682
| Evaluation of the microba community profiler for taxonomic profiling of metagenomic datasets from the human gut microbiome.Crossref | GoogleScholarGoogle Scholar | 33959106PubMed |
Patel AK, Singhania RR, Pandey A (2017) Production, purification, and application of microbial enzymes. In ‘Biotechnology of microbial enzymes’. (Ed. G Brahmachari) pp. 13–41. (Academic Press: London, UK)
Ramirez J, McCabe B, Jensen PD, Speight R, Harrison M, van den Berg L, O’Hara I (2021) Wastes to profit: a circular economy approach to value-addition in livestock industries. Animal Production Science 61, 541–550.
| Wastes to profit: a circular economy approach to value-addition in livestock industries.Crossref | GoogleScholarGoogle Scholar |
Ravindran V (2013) Feed enzymes: the science, practice, and metabolic realities. Journal of Applied Poultry Research 22, 628–636.
| Feed enzymes: the science, practice, and metabolic realities.Crossref | GoogleScholarGoogle Scholar |
Ravindran V, Bryden WL (1999) Amino acid availability in poultry: in vitro and in vivo measurements. Australian Journal of Agricultural Research 50, 889–908.
| Amino acid availability in poultry: in vitro and in vivo measurements.Crossref | GoogleScholarGoogle Scholar |
Ravindran V, Bryden WL, Kornegay ET (1995) Phytates: occurrence, bioavailability and implications in poultry nutrition. Poultry and Avian Biology Reviews 6, 125–143.
Ravindran V, Hew LI, Ravindran G, Bryden WL (2005) Apparent ileal digestibility of amino acids in dietary ingredients for broiler chickens. Animal Science 81, 85–97.
| Apparent ileal digestibility of amino acids in dietary ingredients for broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Rebello S, Jose L, Sindhu R, Aneesh EM (2017) Molecular advancements in the development of thermostable phytases. Applied Microbiology and Biotechnology 101, 2677–2689.
| Molecular advancements in the development of thermostable phytases.Crossref | GoogleScholarGoogle Scholar | 28233043PubMed |
Robbins KR, Baker DH, Norton HW (1977) Histidine status in the chick as measured by growth rate, plasma free histidine and breast muscle carnosine The Journal of Nutrition 107, 2055–2061.
| Histidine status in the chick as measured by growth rate, plasma free histidine and breast muscle carnosineCrossref | GoogleScholarGoogle Scholar | 908963PubMed |
Robbins KR, Norton HW, Baker DH (1979) Estimation of nutrient requirements from growth data. The Journal of Nutrition 109, 1710–1714.
| Estimation of nutrient requirements from growth data.Crossref | GoogleScholarGoogle Scholar | 490209PubMed |
Samtiya M, Aluko RE, Dhewa T (2020) Plant food anti-nutritional factors and their reduction strategies: an overview. Food Production. Processing and Nutrition 2, 6
| Plant food anti-nutritional factors and their reduction strategies: an overview.Crossref | GoogleScholarGoogle Scholar |
Sanchez-Romero I, Ariza A, Wilson KS, Skjot M, Vind J, De Maria L, Skov LK, Sanchez-Ruiz JM (2013) Mechanism of protein kinetic stabilization by engineered disulfide crosslinks. PLoS One 8, e70013
| Mechanism of protein kinetic stabilization by engineered disulfide crosslinks.Crossref | GoogleScholarGoogle Scholar | 23936134PubMed |
Schneider BH, Flatt WP (1975) ‘The evaluation of feeds through digestibility experiments.’ (University of Georgia Press: Athens, GA, USA)
Schofield BJ, Skarshewski A, Lachner N, Ouwerkerk D, Klieve AV, Dart P, Hugenholtz P (2016) Near complete genome sequence of the animal feed probiotic, Bacillus amyloliquefaciens H57. Standards in Genomic Sciences 11, 60
| Near complete genome sequence of the animal feed probiotic, Bacillus amyloliquefaciens H57.Crossref | GoogleScholarGoogle Scholar | 27602182PubMed |
Selle PH, Ravindran V (2007) Microbial phytase in poultry nutrition. Animal Feed Science and Technology 135, 1–41.
| Microbial phytase in poultry nutrition.Crossref | GoogleScholarGoogle Scholar |
Selle PH, Ravindran V (2008) Phytate-degrading enzymes in pig nutrition. Livestock Science 113, 99–122.
| Phytate-degrading enzymes in pig nutrition.Crossref | GoogleScholarGoogle Scholar |
Selle PH, Ravindran V, Caldwell RA, Bryden WL (2000) Phytate and phytase: consequences for protein utilisation. Nutrition Research Reviews 13, 255–278.
| Phytate and phytase: consequences for protein utilisation.Crossref | GoogleScholarGoogle Scholar | 19087442PubMed |
Shini S, Bryden WL (2021) Probiotics and gut health: linking intestinal homeostasis to poultry productivity. Animal Production Science
| Probiotics and gut health: linking intestinal homeostasis to poultry productivity.Crossref | GoogleScholarGoogle Scholar |
Shini S, Zhang D, Aland RC, Li X, Dart PJ, Callaghan MJ, Speight RE, Bryden WL (2020) Probiotic Bacillus amyloliquefaciens H57 ameliorates subclinical necrotic enteritis in broiler chicks by maintaining intestinal mucosal integrity and improving feed efficiency. Poultry Science 99, 4278–4293.
| Probiotic Bacillus amyloliquefaciens H57 ameliorates subclinical necrotic enteritis in broiler chicks by maintaining intestinal mucosal integrity and improving feed efficiency.Crossref | GoogleScholarGoogle Scholar | 32867972PubMed |
Shini S, Aland RC, Bryden WL (2021) Avian intestinal ultrastructure changes provide insight into the pathogenesis of enteric diseases and probiotic mode of action. Scientific Reports 11, 167
| Avian intestinal ultrastructure changes provide insight into the pathogenesis of enteric diseases and probiotic mode of action.Crossref | GoogleScholarGoogle Scholar | 33420315PubMed |
Shivange AV, Schwaneberg U (2017) Recent advances in directed phytase evolution and rational phytase engineering. In ‘Directed Enzyme Evolution: Advances and Applications’. pp. 145–172.
Short JM, Gray KA, Barton NR, Garrett JB, O’Donoghue E, Robertson DE (2008) Phytases and methods for making and using them. US 7432098 B2.
Sibbald IR (1982) Measurement of bioavailable energy in poultry feedingstuffs: a review. Canadian Journal of Animal Science 62, 983–1048.
Sibbald IR (1987) Estimation of bioavailable amino acids in feedingstuffs for poultry and pigs: a review with emphasis on balance experiments. Canadian Journal of Animal Science 67, 221–300.
| Estimation of bioavailable amino acids in feedingstuffs for poultry and pigs: a review with emphasis on balance experiments.Crossref | GoogleScholarGoogle Scholar |
Speight RE2016 Biotechnology in the development of improved phytases.Proceedings of the Australian Poultry Science Symposium27 158165
Stein HH (2017) Procedures for determining digestibility of amino acids, lipids, starch, fibre, phosphorus, and calcium in feed ingredients fed to pigs. Animal Production Science 57, 2317–2324.
| Procedures for determining digestibility of amino acids, lipids, starch, fibre, phosphorus, and calcium in feed ingredients fed to pigs.Crossref | GoogleScholarGoogle Scholar |
Teo AY-L, Tan H-M (2005) Inhibition of Clostridium perfringens by a novel strain of Bacillus subtilis isolated from the gastrointestinal tracts of healthy chickens. Applied and Environmental Microbiology 71, 4185–4190.
| Inhibition of Clostridium perfringens by a novel strain of Bacillus subtilis isolated from the gastrointestinal tracts of healthy chickens.Crossref | GoogleScholarGoogle Scholar |
Torok VA, Luyckx K, Lapidge S (2021) Human food waste to animal feed: opportunities and challenges. Animal Production Science
| Human food waste to animal feed: opportunities and challenges.Crossref | GoogleScholarGoogle Scholar |
Vandenberghe LP, Pandey A, Carvalho JC, Letti LA, Woiciechowski AL, Karp SG, Thomaz-Soccol V, Martínez-Burgos WJ, Penha RO, Herrmann LW (2020) Solid-state fermentation technology and innovation for the production of agricultural and animal feed bioproducts. Systems Microbiology and Biomanufacturing 1–24.
Veiga P, Suez J, Derrien M, Elinav E (2020) Moving from probiotics to precision probiotics. Nature Microbiology 5, 878–880.
| Moving from probiotics to precision probiotics.Crossref | GoogleScholarGoogle Scholar | 32393856PubMed |
Warner ACI (1981) Rate of passage of digesta through the gut of mammals and birds. Nutrition Abstracts and Reviews 51, 789–820.
White CL, Ashes JR (1999) A review of methods for assessing the protein value of grain fed to ruminants. Australian Journal of Agricultural Research 50, 855–869.
| A review of methods for assessing the protein value of grain fed to ruminants.Crossref | GoogleScholarGoogle Scholar |
Whittemore CT (1990) ‘Elements of pig science.’ (Longman Scientific and Technical: Harlow, Essex, UK)
Wiseman J, Cole DJA (Eds) (1990) ‘Feedstuff evaluation.’ (Butterworths: London, UK)