Determination of the optimal priming interval of rumen fluids used as inocula for the in vitro digestibility trials through radial enzyme diffusion method
M. Simoni A C , E. Tsiplakou B , R. Pitino A , A. Quarantelli A and F. Righi A
A C , E. Tsiplakou B , R. Pitino A , A. Quarantelli A and F. Righi A
A University of Parma, Department of Veterinary Science, Via del Taglio, 10, 43126, Parma, Italy.
B Agricultural University of Athens, Department of Nutritional Physiology and Feeding, Iera odos 75, GR-11855, Athens, Greece.
C Corresponding author. Email: marica.simoni@unipr.it
Animal Production Science 61(5) 525-531 https://doi.org/10.1071/AN20197
Submitted: 27 March 2020 Accepted: 23 October 2020 Published: 20 November 2020
Journal Compilation © CSIRO 2021 Open Access CC BY
Abstract
Context: Determination of the neutral detergent fibre digestibility is one of the important parameters to consider when formulating diets. However, the in vitro determination shows low repeatability because of the source of rumen-fluid inoculum. Priming of the rumen fluid inocula, obtained through an oesophageal probe, has been proposed to overcome this issue.
Aim: The objective of the study was to investigate the evolution of the microbial enzymatic activities of different rumen fluids during a priming procedure, to establish the fermentation interval that minimises the differences among rumen-fluid degradative potentials.
Methods: Three farms for each type of diet were involved in the study. Rumen fluids were obtained from dry and lactating cows fed the following four diet types: 100% hay or a diet with 80 : 20 forage : concentrate ratio (F : C) as dry-cow diets, and ad libitum hay and concentrate, or a total mixed ration (both at 60 : 40 F : C) as lactating-cow diets. On each farm, rumen fluid was collected from three Holstein cows by using an oesophageal probe, and mixed. Two aliquots of each rumen fluid mix were added to the medium containing the same priming substrate in an in vitro batch-fermentation system. During the incubation, the fermentation fluids were sampled in duplicate at 0-, 1-, 2-, 4-, 8-, 24- and 48-h intervals. Enzymatic activities of amylase, cellulase and xylanase were determined by radial enzyme diffusion method.
Key results: Initial enzymatic activities were quite variable and increased with an increasing incubation time. By 24 h, amylase showed similar values among high-concentrate diet fermentation fluids, and a lower data dispersion in comparison to the other intervals; cellulase was characterised by similar values in all the fermentation fluids derived from diets including concentrates, and xylanase showed similar activity in the fermentation fluids derived from high-concentrate diets. Development of the enzymatic activity of the fermentation fluids derived from the 100% hay diet differed from the others.
Conclusions: A 24-h priming procedure was needed to stabilise and equalise the enzymatic activity of the rumen fluid from cows fed high-concentrate diets. This was not observed in rumen fluid from cows fed hay-based diets.
Implications: The 24-h-primed rumen fluid can be used to increase the repeatability of neutral detergent fibre digestibility determination.
Keywords: dairy cows, in vitro digestibility, nutrient analysis.
Introduction
Determination of the neutral detergent fibre digestibility (NDFD) is one of the important parameters for the evaluation of both feed and diet nutritional values. However, this technique has no standardised procedure, showing low repeatability (17% of variability of the NDFD at 30 h of fermentation) partially dependent on rumen-fluid consistency (Hall and Mertens 2012). The rumen-fluid activity can, in fact, be affected by several individual and dietary factors (Chaudhry 2008; Kim et al. 2019). Additionally, rumen fluid collected with an oesophageal probe contains variable amounts of saliva, which affects microbial dilution and content. To reduce the impact of the rumen-fluid variability on the NDFD assay, Goeser and Combs (2009) proposed priming (pre-incubation) of the rumen-fluid inoculum by using cellulose as substrate (priming substrate), demonstrating that this practice improves the repeatability of the in vitro NDFD assay; however, they found a depression in fibre digestibility. A further experiment was conducted by Goeser et al. (2009), where rumen fluid was primed with a carbohydrate–urea mixture and fermented until a specific amount of gas was produced. They found a higher repeatability of the analysis, avoiding, in this case, the NDFD depression, probably as a result of a more complete priming diet. However, the incubation time in the latter experiment was unknown and the criteria used to standardise the rumen fluid was unrelated to a time-point and, consequently, difficult to apply in routine analysis. Moreover, the parameter tested was the cumulative gas production, which is, by definition, time-dependent and it is not a direct expression of the degradative potential of the rumen fluid. The latter is better described by the enzymatic activity (EA) of the rumen fluid, which, in turn, can be considered as a quantitative–qualitative reflection of rumen microbiota (Raghuvansi et al. 2007) and, on the basis of data from Dadvar et al. (2018) and Morgavi et al. (2000), a potential measure of its fermentative capacity.
It has been demonstrated that diet characteristics significantly affect the rumen microbiota and, consequently, the rumen enzymatic profile. Indeed, rumen-fluid carboxymethyl cellulase and xylanase activities have been shown to be lower, whereas the amylase expression is improved in dry cows and heifers fed a high-grain compared with a high-forage diet (Hristov et al. 1999). Moreover, an enhancement of the rumen carboxymethyl cellulase and xylanase activities of buffalo rumen fluid has been observed after increasing the concentration of roughages in their diets (Kamra et al. 2003).
The EAs can be evaluated by using different methods. Among them, the radial enzyme diffusion (RED) has been applied to quantify the exogenous cellulase, xylanase, amylase and protease in supplemental animal feedstuffs (Walsh et al. 2005). Furthermore, this method has been applied to the measurement of non-starch polysaccharide-degrading EAs in the rumen fluid of cows fed corn silage of different particle sizes (Zebeli et al. 2008). On the basis of the literature, it appears that RED could be a quick and reliable method for the evaluation of rumen-fluid EAs, allowing for the screening of the degradative capacity of the primed rumen fluids.
With this assumption, and considering the effect exerted by the diet on rumen-microbiota enzyme synthesis, we hypothesise that in vitro incubation of different rumen fluids on a common complete priming substrate can reduce the differences among their degradative potentials. Starting from this hypothesis, the present study was designed to investigate the evolution of the microbial EAs of different rumen fluids, obtained with an oesophageal probe, during a priming procedure, so as to establish the fermentation interval that minimises the differences among rumen-fluid degradative potentials.
Materials and methods
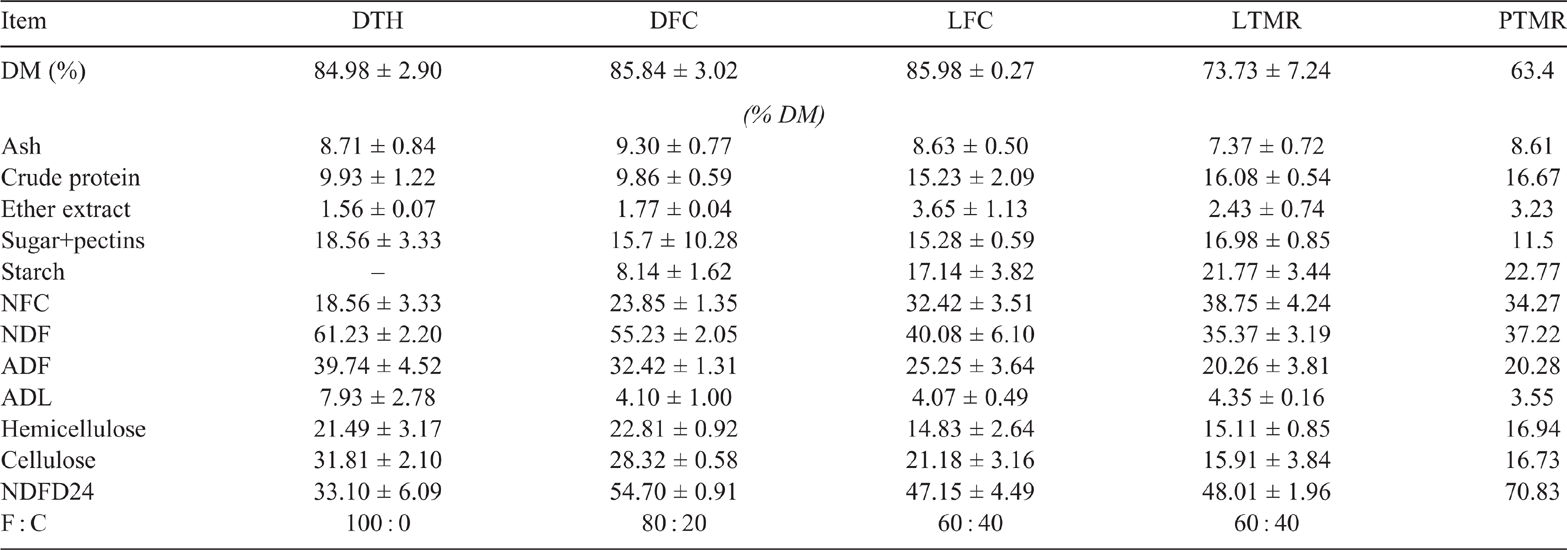
The animal procedures were conducted in accordance with the EU Directive 2010/63/EU (European Parliament and Council of the European Union 2010) for animal experiments. The trial involved three farms for each diet type. The rumen fluids were obtained from intact cows fed four type of diets, including two dry-cow diets and two lactating-cow diets (Table 1).
The dry-cow diets were a 100% forage diet (DTH) or a diet with a forage: concentrate ratio (F : C) of 80 : 20 (DFC). The lactating-cow diets were ad libitum hay administered separately from a concentrate distributed by an automatic feeder at an estimated F : C of 60 : 40 (LFC), or a total mixed ration (LTMR) at F : C of 60 : 40. On each farm, individual rumen fluids were collected from a total of three Holstein cows on each diet type, by using an oesophageal probe as described by Cinotti (1987), 3 h after the morning feed delivery. The individual rumen fluids maintained at 39°C were mixed in the laboratory in anaerobic conditions described by Simoni et al. (2020). Each rumen-fluid mix was blended, filtered through four layers of cheesecloth and divided into two aliquots. From each of these, a volume of 100 mL of rumen fluid was added at a ratio of 1 : 4 to a medium in a 1000-mL flask containing 5 g of TMR, ground to pass a 1-mm screen, used as a unique substrate (priming substrate). The TMR was a typical hay-based diet formulated for lactating cows employed in northern Italy, similar to those described by Righi et al. (2007, 2009) and Comino et al. (2015), the composition and formula of which are reported in Table 1.
Each flask containing the rumen-fluid inoculum, the medium and the priming substrate was incubated for 48 h by using an in vitro batch-fermentation system similar to that described by Goering and Van Soest (1970). Two 5-mL aliquots of the liquid phase (fermentation fluid) were sampled from each flask at 0, 1, 2, 4, 8, 24 and 48 h after inoculation and centrifuged at 5000g for 15 min at room temperature (around 20 to 24°C). The supernatant was collected, filtered through a 0.45-µm porosity PVDF (polyvinylidene fluoridone) syringe filter, and a volume of 0.125 µL of protease inhibitor (Protease Inhibitor Cocktail powder, code P2714-1BTL, Sigma Aldrich, Milano, Italy) was added to prevent enzyme degradation by protease, and the sample was then stored at –20°C for subsequent analysis.
Amylase (Amy), cellulase (Cell) and xylanase (Xyl) activities, as the main representatives of carbohydrate-degrading agents, were tested with the RED method, as described by Walsh et al. (2005). The method adopted was subjected to an internal variability evaluation, giving average coefficients of variation of 5.84%, 4.69% and 6.80% for Amy, Cell and Xyl respectively.
The Petri dishes used for the assay contained 0.5%, 0.5% or 0.1% (w/v) of cellulose (cellulose powder from cotton linter, code 22183, Fluka BioChemika, Buchs, Switzerland), starch (soluble starch, code 417585, Farmitalia Carlo Erba S.p.a, Milano, Italy) or xylan (AZCL-Arabinoxylan from wheat, cod. I-AZWAX, Megaxyme, Wicklow, Ireland), as substrates. Each enzyme substrate was solubilised with 1.5% (w/v) agar (Agar N°1, code LP001, Oxoid, Milan, Italy) in the proper buffer, represented by 100 mM Na-acetate, pH 5.0 for cellulase; 100 mM Na-acetate, pH 4.8 for α-amylase; and 100 mM Na-citrate, pH 5.3 for xylanase. Gelation was obtained by heating at 100°C for 12 min.
Four aluminium cylinders had been previously placed in each Petri dish, to create the wells for the fermentation-fluid inoculation. The gel was cooled to 50°C and a volume of 20 mL was poured into each Petri dish (90-mm diameter) while vigorously stirring, yielding a gel depth of 3 mm. Circular wells (diameter 10 mm) were obtained by removal of the aluminium cylinders from the solidified agar.
Cellulase and Amy analyses were repeated in quadruplicate for each interval sample in different Petri plates, whereas Xyl was measured in duplicate. After the substrate and the Petri dish preparation, the frozen supernatant aliquots previously obtained were thawed at room temperature and a volume of 300 µL was poured in each well of the agar. The plates were then incubated for 16 h at 50°C for Cell and Amy measurements, and at 37°C for Xyl determination. Cell and Amy hydrolyses were shown by staining. Plates were flooded with 0.2% (w/v) I2 in 2.0% KI staining solution for 15–20 min for Cell detection, and 1 : 40 diluted Lugol solution was put in contact with the plate for 5 s, so as to detect Amy; both procedures were followed by multiple rinses with water. No staining was needed for Xyl hydrolysis halo detection, since halos were already evident after incubation.
The halo of hydrolysis generated by the enzymes in the supernatant was measured through photographic digitalisation of the plate surface, followed by image measurement performed through the MeazureTM 2.0 software (C Thing Software, Sunnyvale, CA, USA). The well area was then subtracted from the total area of the halo circle, and the result was corrected accounting for the dry matter of the rumen fluid after filtration through the syringe filter. Results are, thus, expressed as the corrected area of the surface of the halo (mm2).
All statistical analyses were performed using the SPSS for Windows software package (ver. 25.0; SPSS Inc., Chicago, IL, USA). The differences in Cell, Amy and Xyl EAs in the rumen fluid among the diet types were tested separately by using the repeated-measure procedure of the general linear model, with diet type as a fixed factor and priming intervals as repeated measures. Farm, which represented the experimental unit, was considered a random effect. Differences were declared significant at P ≤ 0.05. Results are reported as least-square means.
Results
The EAs of the different fermentation fluids were quite variable at the beginning of the priming process (Table 2).
Similar concentrations of Amy were initially found in the DTH and DFC fermentation fluids; however, they were significantly (P ≤ 0.001) lower than those in the LFC and LTMR fermentation fluids, whose Amy concentrations were, in turn, similar. All fermentation fluids completely differed for this EA after 1 h of fermentation with the priming diet. The LFC and LTMR fermentation fluids showed the peak of Amy activity between 2 and 4 h of incubation, while DTH and DFC fermentation fluids expressed the maximal Amy EA after 8 h from the starting point. By 24 h, a significant decline was observed, followed by a significant increase at 48 h of fermentation. Similarity in the maximal Amy EA among the fermentation fluids was observed after 8 h of incubation, when the concentration in the fermentation fluid from DTH was similar to that from the LFC, and that from the DFC fermentation fluid appeared similar to that from LTMR, and all values fell within a narrow range (from 233.37 to 272.11 mm2). A 24-h priming procedure was needed to equalise the fermentation fluids from lactating-cow diets (LFC and LTMR), while it failed to equalise the other two types of fermentation fluids (P ≤ 0.001). At 48 h, all fermentation fluids showed different Amy concentrations and both DTH- and DFC-derived fermentation fluids showed a higher Amy concentration than was the initial value, whereas LFC and LTMR fermentation fluids showed a lower Amy concentration than in the initial inoculum (P ≤ 0.001).
Initial values of Cell activity differed among rumen fluids, with values increasing from DTH to LTMR (P ≤ 0.001). A peak of Cell activity was generally found after 2 h of incubation, and a further rise was observed at 8 h for the DFC diet. A subsequent general decrease from the initial values was observed in DFC and LFC fermentation fluids. An opposite trend was found for Cell activity of the fermentation fluid from the DTH diet, which increased over time and was similar to the value of the fermentation fluid from the LTMR by the end of the treatment. The initial differences among the rumen fluids were maintained up to a 2-h interval, while starting from the 4 h, at least two fermentation fluids per interval were similar for Cell activity. At 24 h of fermentation, the Cell concentration appeared similar for the DFC, LFC and LTMR diets, which all differed from that of the DTH (P ≤ 0.001), which showed the highest value. This difference was maintained at 48 h, when also the reduction in Cell EA became significant for the fermentation fluid from the LTMR-fed cows.
The Xyl EA of DTH and DFC fermentation fluids was initially similar, but significantly (P ≤ 0.001) lower than that of the LFC and LTMR fermentation fluids, which also differed from each other (P ≤ 0.001). The general trend was for an increase of the activity of Xyl during the experiment; so, at the end of the trial, Xyl concentrations of all fermentation fluids were higher (P ≤ 0.001). A peak of Xyl activity was found between 1 and 4 h of fermentation in all cases. Of interest, from 24 h onward, the rumen fluid from DTH-fed cows showed a higher production of Xyl than did the other fermentation fluids (P ≤ 0.001). The fermentation fluids from the two lactating-cow diets were equal at 24 h. At 48 h of priming, DFC-, LFC- and LTMR-derived fermentation fluids were almost identical for Xyl activities, with values of ~190 mm2. A higher level of EA of the DTH fermentation fluid was observed with Cell as well, at the same intervals.
Discussion
The present work is focused on establishing a procedure to standardise rumen fluid for use in forage-digestion studies, especially rumen fluid obtained through the oesophagus. This procedure becomes necessary where no information is provided about both the donor-cow diet and the animals themselves, or when an approved donor cow is not available as a result of the legal constraints on animal welfare (Directive 2010/63/UE).
The priming procedure has been demonstrated to be promising, but several aspects still need to be clarified. Among them, the optimal priming time needs to be estimated. The time period evaluated in the present study was based on the measurement of the degradative potential of the rumen fluid as a preliminary step for further studies involving the determination of the NDFD repeatability.
Consistent with literature, in the present trial, rumen fluids derived from the different diets were characterised by different initial EAs, and underwent subsequent modifications due to the adaptation to the priming diet. Similar results have been found in dry cows and heifers (Hristov et al. 1999), wethers (Wolff et al. 2017) and buffaloes (Kamra et al. 2003; Agarwal et al. 2004). In contrast, no significant observations were found in rumen-fluid EAs by Sinha et al. (2017), who changed the ratio of the concentrate mixture to wheat straw and green maize from 60 : 20: 20 to 40 : 30 : 30 and to 20 : 40 : 40 in the diets of buffalo and cross-bred cattle respectively. However, it should be noted that the EAs were tested in the latter study after a long adaptation period to a new diet, which could have allowed for the re-equilibration of the ruminal microbiota and, consequently, of the EAs.
The initial higher Amy concentration found in fermentation fluids from cows fed concentrate diets is consistent with the literature, since higher Amy concentrations have been found in dairy cattle fed high-grain than in those fed high-roughage diets (Huhtanen and Khalili 1992; Martin and Michalet-Doreau 1995; Hristov et al. 1999). The same concept applies longitudinally during the fermentation period, with a general increase in Amy EA in the initial phases of fermentation being related to a higher starch content of the priming diet than that of the diets fed to the donor cows. The rise in the Amy activity was more pronounced and delayed in DTH- and DFC-derived fermentation fluids, probably because of the initial lower abundance of amylolytic bacteria. In addition, the change in the activity of Amy was less pronounced in the fermentation fluids from LFC- and LTMR-fed cows, and this is consistent with a possible higher abundance of amylolytic bacteria in the initial inoculum. The subsequent dynamic of Amy can be attributable to the gradual decrease in the amount of rapidly degradable starch in the system, followed, at 48 h of fermentation, by the degradation of the most crystallised forms of starch (Trináctý et al. 2016). On the basis of the least-square mean values, it seems that the maximum similarity was at 8 h, while the lower variability was measured at 24 h.
The initial Cell EA showed an increase from the DTH to the LTMR diet, indicating a relationship between the Cell EA and the increased amount of dietary non-fibre carbohydrates and concentrates fed to the donor cows. This is in agreement with findings from Ding et al. (2014) who observed that incrementally increasing the proportion of concentrates in steer diets (from 30% to 90%) induced a linear increase in both carboxymethyl cellulase and xylanase activities in the steer rumen fluids. Furthermore, a tendency for higher activities in both carboxymethyl cellulase and xylanase enzymes was observed in the rumen of the steers fed the TMR diet, when compared with those fed a diet where roughages and concentrates were fed separately (Ding et al. 2014). This probably relates to the fact that the TMR system improves ruminal environment with higher activities of fibrolytic (Cell and Xyl) enzymes (Li et al. 2003). Cellulase and hemicellulase are released in the rumen from fungi, protozoa and bacteria, which have generation times of 24–32 h, 8–36 h and ~20 min respectively (Hobson and Stewart 1997). On the basis of this information and the observations from Wang and McAllister (2002), the first peak of Cell activity can be attributed to bacterial enzymatic production, while the second can be connected to the proliferation and activity of protozoa and other slow-growing microorganisms in the rumen, generating the two waves of Cell activity clearly evidenced with the DFC diet. Moreover, on the basis of Belanche et al. (2012), consumption of high-fibre diets increases the bacterial and fungal number and diversity, with a consequent high concentration of cellulolytic microorganisms. This is probably the case of the DTH-derived fermentation fluids which, incubated in the buffered environment of the fermenters with a highly digestible complete diet as substrate, could have found the better conditions to release a higher amount of Cell and Xyl, as evidenced by the concentrations of these enzymes at 24 and 48 h. This is also supported by the study from Baba et al. (2017), who showed that the relative abundance of Ruminococcus albus and flavefaciens, known as cellulolytic, cello-oligosaccharolytic and xylanolytic bacteria, increased, improving the cellulose and hemicellulose degradation rates.
Surprisingly, the high-forage diets (DTH and DFC) induced a lower initial Xyl activity than did the diets including more concentrates in their formula (LFC and LTMR). These results are in contrast with data from Martin and Michalet-Doreau (1995), who found significantly higher ruminal carboxymethyl cellulase and xylanase activities in cows fed Dactylis glomerata hay than in those receiving 65% hay and 35% pelleted ground barley. Moreover, Xyl activity in ruminal content from cattle fed a high-forage diet was 2.5 times higher than that in the rumen content from cattle fed a high-grain diet also in the study from Hristov et al. (1999). During the priming fermentation, an increase in Xyl activity was observed in all cases. Of interest was the higher activity level of both Xyl and Cell found in the DTH-derived fermentation fluid after 24 h of incubation. This can be related to the potential prevalence of fibrolytic bacteria in the initial phase of fermentation, given the complete absence of concentrates in the DTH diet, and to the increase in the NDFD, to which the rumen fluid was exposed moving from the DTH to the priming diet. The lack of non-fibre carbohydrate bacteria could have induced a faster growth of the fibrolytic bacteria on the priming diet that likely generated higher activity levels of Cell and Xyl. Similarly, higher carboxymethyl cellulase and xylanase activities have been observed in vitro by Agarwal et al. (2004), when maize was used as an alternative substrate to lucerne, probably due to its higher digestible-fibre content. In reference to the latter study, both carboxymethyl cellulase and xylanase activities in sheep rumen fluid increased significantly when forages transitioned from alfalfa hay to corn stover (Xie et al. 2018). Moreover, consistent with our in vitro observations from the DTH diet, both carboxymethyl cellulase and xylanase activities were greater 23 h after the meal in a study of Martin and Michalet-Doreau (1995). Effective degradation of plant cell-wall polysaccharides occurs at the later stages of the post-prandial period (Argyle and Hespell 1987), after the soluble carbohydrate utilisation and the build-up of the fibrolytic population. The DFC, LFC and LTMR fermentation fluids showed similar activities for Xyl after 48 h of incubation, while those derived from the DTH diet showed a completely different activity for the Xyl, which increased continuously during the fermentation period.
Concerning the ideal priming fermentation interval, the results showed that especially when the rumen fluids were derived from cows fed diets with at least 40% of concentrate, 24-h interval is needed for a complete equalisation of the EAs. At this time point, Amy activity showed similar values among the fermentation fluids derived from cows fed high-concentrate diets, and a lower data dispersion; Cell was characterised by similar values in all fermentation fluids from cows fed diets including concentrates (DFC, LFC and LTMR) and Xyl showed similar activity in the fermentation fluids derived from cows fed high-concentrate diets, which were comparable to the DFC-derived fermentation fluid (20% of concentrate in the ration). It should be highlighted that there was a clear difference between the EA evolution of the fermentation fluid derived from the cows fed diet comprising only hay (DTH) and that derived from cows fed diets including concentrates. The priming procedure was able to equalise the EAs of rumen fluids originating from diets containing at least 20% concentrate, while it failed to equalise rumen fluids from total hay-based diets. However, so as to comply with rules on animal welfare, several laboratories collect rumen fluids at the slaughterhouses that usually receive cows that are fed diets containing concentrates. High-energy diets are, in fact, needed to satisfy the high nutrient requirements of the high-producing dairy cows; so, it is rare that slaughtered cows would be fed only hay and, thus, our results can have a direct application in the real situation. Moreover, from a practical point of view, the 24 h of fermentation represent also a favourable interval for the laboratory routine, since it allows for a distribution of the work over 2 days when a digestion trial has to be started after priming.
Further studies should be conducted to test the fermentation fluids primed for 24 h as inocula for in vitro digestibility, so as to verify the actual impact of the priming procedure on the NDFD assay in terms of lag time, digestibility at specific intervals and repeatability. Moreover, it will be important to test the real relationship between the primed fermentation fluid EAs and their content of active microbes available for colonising other samples. In the standardised fermentation fluid, microbes could be attached to the priming substrate and, after filtration, the ability of measuring NDFD would rely on the presence of daughter cells to be harvested within the liquid phase. High enzymatic concentration suggests a high and healthy rumen microbe population, but it might not demonstrate the availability of active bacteria able to colonise the samples to be tested for NDFD.
Conclusions
The rumen fluids of different dietary origin can significantly modify their EAs during the in vitro priming procedure. These variations are dependent on the characteristics of the diet from which the rumen fluids originate and the fermentation interval considered. In general, using a complete diet as a priming substrate, the fermentation fluids derived from high-fibre diets improved their EAs during the fermentation, while fermentation fluids derived from cows fed low-fibre diets showed decreasing EAs during the incubation. Xyl was improved by the priming process in all cases.
The priming procedure was able to equalise the EAs of rumen fluids originating from diets containing at least 20% of concentrate, while it failed to equalise rumen fluid from total hay-based diets. Despite some similarities in the degradative potential of the different fermentation fluids, the 24-h priming interval is needed for a complete equalisation of the EAs of the fermentation fluids derived from diets containing concentrates.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors gratefully acknowledge Dr Marco Renzi for his technical assistance. This project has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No. 777974.
References
Agarwal N, Saxena J, Saha S, Chaudhary LC, Kamra DN (2004) Changes in fermentation characteristics, microbial populations and enzyme profile in the rumen of buffalo as affected by roughage level in the diet. Bubalus Bubalis 3, 81–90.Argyle JL, Hespell RB (1987) Digestion of alfalfa hay using washed ruminal bacteria. Journal of Dairy Science 70, 2525–2533.
| Digestion of alfalfa hay using washed ruminal bacteria.Crossref | GoogleScholarGoogle Scholar |
Baba Y, Matsuki Y, Mori Y, Suyama Y, Tada C, Fukuda Y, Saito M, Nakai Y (2017) Pretreatment of lignocellulosic biomass by cattle rumen fluid for methane production: bacterial flora and enzyme activity analysis. Journal of Bioscience and Bioengineering 123, 489–496.
| Pretreatment of lignocellulosic biomass by cattle rumen fluid for methane production: bacterial flora and enzyme activity analysis.Crossref | GoogleScholarGoogle Scholar | 28143676PubMed |
Belanche A, Doreau M, Edwards JE, Moorby JM, Pinloche E, Newbold CJ (2012) Shifts in the rumen microbiota due to the type of carbohydrate and level of protein ingested by dairy cattle are associated with changes in rumen fermentation. The Journal of Nutrition 142, 1684–1692.
| Shifts in the rumen microbiota due to the type of carbohydrate and level of protein ingested by dairy cattle are associated with changes in rumen fermentation.Crossref | GoogleScholarGoogle Scholar | 22833657PubMed |
Chaudhry AS (2008) Slaughtered cattle as a source of rumen fluid to evaluate supplements for in vitro degradation of grass nuts and barley straw. The Open Veterinary Science Journal 2, 16–22.
| Slaughtered cattle as a source of rumen fluid to evaluate supplements for in vitro degradation of grass nuts and barley straw.Crossref | GoogleScholarGoogle Scholar |
Cinotti S (1987) Proposta di una nuova sonda per il prelievo del liquido ruminale. In ‘Atti della Società Italiana di Buiatria, XIX Congresso Nazionale’, Punta Ala (GR), Italy, 8–10 May 1987, 775–782. (Società Italiana di Buiatria: Perugia, Italy).
Comino L, Righi F, Coppa M, Quarantelli A, Tabacco E, Borreani G (2015) Relationships among early lactation milk fat depression, cattle productivity and fatty acid composition on intensive dairy farms in northern Italy. Italian Journal of Animal Science 14, 350–361.
| Relationships among early lactation milk fat depression, cattle productivity and fatty acid composition on intensive dairy farms in northern Italy.Crossref | GoogleScholarGoogle Scholar |
Dadvar P, Mohammadabadi T, Sari M, Fayazi J (2018) In vitro fiber digestibility, gas production and enzyme activity of cellulolytic bacteria of Arabian camels (dromedary) fed cultivable and pasture forage. Iranian Journal of Applied Animal Science 8, 527–538.
Ding G, Chang Y, Zhou Z, Ren L, Meng Q (2014) Effect of Saccharomyces cerevisiae on rumen fermentation characteristics, nutrient degradation and cellulase activity of steers fed diets with different concentrate to forage ratios. World Journal of Agricultural Research 2, 303–308.
| Effect of Saccharomyces cerevisiae on rumen fermentation characteristics, nutrient degradation and cellulase activity of steers fed diets with different concentrate to forage ratios.Crossref | GoogleScholarGoogle Scholar |
European Parliament and Council of the European Union (2010) Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals used for Scientific Purposes. Official Journal of European Union L276, 33–79.
Goering HK, Van Soest PJ (1970) ‘Forage fiber analyses (apparatus, reagent, procedures and some applications)’. Agriculture handbook no. 379, pp. 20 (USDA-ARS: Washington, DC)
Goeser JP, Combs DK (2009) An alternative method to assess 24-h ruminal in vitro neutral detergent fiber digestibility. Journal of Dairy Science 92, 3833–3841.
| An alternative method to assess 24-h ruminal in vitro neutral detergent fiber digestibility.Crossref | GoogleScholarGoogle Scholar | 19620667PubMed |
Goeser JP, Hoffman PC, Combs DK (2009) Modification of a rumen fluid priming technique for measuring in vitro neutral detergent fiber digestibility. Journal of Dairy Science 92, 3842–3848.
| Modification of a rumen fluid priming technique for measuring in vitro neutral detergent fiber digestibility.Crossref | GoogleScholarGoogle Scholar | 19620668PubMed |
Hall MB, Mertens DR (2012) A ring test of in vitro neutral detergent fiber digestibility: analytical variability and sample ranking. Journal of Dairy Science 95, 1992–2003.
| A ring test of in vitro neutral detergent fiber digestibility: analytical variability and sample ranking.Crossref | GoogleScholarGoogle Scholar | 22459845PubMed |
Hobson PN, Stewart CC (1997‘The rumen microbial eco-system.’ Blackie Academic & Professional, London)
Hristov AN, McAllister TA, Cheng K-J (1999) Effect of diet, digesta processing, freezing and extraction procedure on some polysaccharide-degrading activities of ruminal contents. Canadian Journal of Animal Science 79, 73–81.
| Effect of diet, digesta processing, freezing and extraction procedure on some polysaccharide-degrading activities of ruminal contents.Crossref | GoogleScholarGoogle Scholar |
Huhtanen P, Khalili H (1992) The effect of sucrose supplements on particle-associated carboxymethylcellulase (EC 3.2.1.4) and xylanase (EC 3.2.1.8) activities in cattle given grass–silage-based diet. British Journal of Nutrition 67, 245–255.
| The effect of sucrose supplements on particle-associated carboxymethylcellulase (EC 3.2.1.4) and xylanase (EC 3.2.1.8) activities in cattle given grass–silage-based diet.Crossref | GoogleScholarGoogle Scholar | 1317721PubMed |
Kamra DN, Saha S, Bhatt N, Chaudhary LC, Agarwal N (2003) Effect of diet on enzyme profile, biochemical changes and in sacco degradability of feeds in the rumen of buffalo. Asian–Australasian Journal of Animal Sciences 16, 374–379.
| Effect of diet on enzyme profile, biochemical changes and in sacco degradability of feeds in the rumen of buffalo.Crossref | GoogleScholarGoogle Scholar |
Kim JN, Song J, Kim EJ, Chang J, Kim C-H, Seo S, Chang MB, Bae G-S (2019) Effects of short-term fasting on in vivo rumen microbiota and in vitro rumen fermentation characteristics. Asian–Australasian Journal of Animal Science 32, 776–782.
| Effects of short-term fasting on in vivo rumen microbiota and in vitro rumen fermentation characteristics.Crossref | GoogleScholarGoogle Scholar |
Li DY, Lee SS, Choi NJ, Lee SY, Sung HG, Ko JY, Yun SG, Ha JK (2003) Feeding system on rumen fermentation and digestion. Asian–Australian Journal of Animal Science 16, 1482–1486.
Martin C, Michalet-Doreau B (1995) Variations in mass and enzyme activity of rumen microorganisms: effect of barley and buffer supplements. Journal of the Science of Food and Agriculture 67, 407–413.
| Variations in mass and enzyme activity of rumen microorganisms: effect of barley and buffer supplements.Crossref | GoogleScholarGoogle Scholar |
Morgavi DP, Beauchemin KA, Nsereko VL, Rode LM, Iwaasa AD, Yang WZ, McAllister TA, Wang Y (2000) Synergy between ruminal fibrolytic enzymes and enzymes from Trichoderma longibrachiatum. Journal of Dairy Science 83, 1310–1321.
| Synergy between ruminal fibrolytic enzymes and enzymes from Trichoderma longibrachiatum.Crossref | GoogleScholarGoogle Scholar | 10877396PubMed |
Raghuvansi SKSS, Prasad R, Tripathi MK, Mishra AS, Chaturvedi OH, Misra AK, Saraswat BL, Jakhmola RC (2007) Effect of complete feed blocks or grazing and supplementation of lambs on performance, nutrient utilisation, rumen fermentation and rumen microbial enzymes. Animal 1, 221–226.
| Effect of complete feed blocks or grazing and supplementation of lambs on performance, nutrient utilisation, rumen fermentation and rumen microbial enzymes.Crossref | GoogleScholarGoogle Scholar |
Righi F, Quarantelli A, Tonelli L, Renzi M, Gandolfi B (2007) Use of Penn State particle separator for the evaluation of total mixed rations typical of Parmigiano Reggiano cheese production area. Italian Journal of Animal Science 6, 347–349.
| Use of Penn State particle separator for the evaluation of total mixed rations typical of Parmigiano Reggiano cheese production area.Crossref | GoogleScholarGoogle Scholar |
Righi F, Romanelli S, Renzi M, Quarantelli A (2009) “In vivo” and “in vitro” degradability of diets for Parmigiano Reggiano cheese production. Italian Journal of Animal Science 8, 331–333.
| “In vivo” and “in vitro” degradability of diets for Parmigiano Reggiano cheese production.Crossref | GoogleScholarGoogle Scholar |
Simoni M, Temmar R, Bignamini DA, Foskolos A, Sabbioni A, Ablondi M, Quarantelli A, Righi F (2020) Effects of the combination between selected phytochemicals and the carriers silica and Tween 80 on dry matter and neutral detergent fibre digestibility of common feeds. Italian Journal of Animal Science 19, 723–738.
| Effects of the combination between selected phytochemicals and the carriers silica and Tween 80 on dry matter and neutral detergent fibre digestibility of common feeds.Crossref | GoogleScholarGoogle Scholar |
Sinha SK, Chaturvedi VB, Singh P, Chaudhary LC, Ghosh M, Shivani S (2017) Effect of high and low roughage total mixed ration diets on rumen metabolites and enzymatic profiles in crossbred cattle and buffaloes. Veterinary World 10, 616–622.
| Effect of high and low roughage total mixed ration diets on rumen metabolites and enzymatic profiles in crossbred cattle and buffaloes.Crossref | GoogleScholarGoogle Scholar | 28717312PubMed |
Trináctý J, Nedelník J, Lang J, Loucka R, Kucera J (2016) Effect of maize kernel endosperm type and maturity stage on ruminal in situ degradability and post-ruminal in vitro dry matter and starch digestibility. Czech Journal of Animal Science 61, 351–359.
| Effect of maize kernel endosperm type and maturity stage on ruminal in situ degradability and post-ruminal in vitro dry matter and starch digestibility.Crossref | GoogleScholarGoogle Scholar |
Walsh G, Murphy RA, Killeen GF, Power RF (2005) Quantification of supplemental enzymes in animal feeding stuffs by radial enzyme diffusion. Applied Microbiology and Biotechnology 67, 70–74.
| Quantification of supplemental enzymes in animal feeding stuffs by radial enzyme diffusion.Crossref | GoogleScholarGoogle Scholar | 15580494PubMed |
Wang Y, McAllister TA (2002) Rumen microbes, enzymes and feed digestion. A review. Asian–Australasian Journal Animal Science 15, 1659–1676.
| Rumen microbes, enzymes and feed digestion. A review.Crossref | GoogleScholarGoogle Scholar |
Wolff SM, Ellison MJ, Hao Y, Cockrum RR, Truong H, Baraboo M, Cammack KM, Maurer T, Taxis TM, Burch K, Wolff SM, Patil R, Ravelo A, Lamberson WR, Lee HJ, Conant GC, Austin KJ (2017) Diet shifts provoke complex and variable changes in the metabolic networks of the ruminal microbiome. Microbiome 5, 60
| Diet shifts provoke complex and variable changes in the metabolic networks of the ruminal microbiome.Crossref | GoogleScholarGoogle Scholar | 28595639PubMed |
Xie X, Yang C, Guan LL, Wang J, Xue M, Liu JX (2018) Persistence of cellulolytic bacteria fibrobacter and treponema after short-term corn stover-based dietary intervention reveals the potential to improve rumen fibrolytic function. Frontiers in Microbiology 9, 1363
| Persistence of cellulolytic bacteria fibrobacter and treponema after short-term corn stover-based dietary intervention reveals the potential to improve rumen fibrolytic function.Crossref | GoogleScholarGoogle Scholar | 29997589PubMed |
Zebeli Q, Tafaj M, Junck B, Ölschläger V, Ametaj BN, Drochner W (2008) Evaluation of the response of ruminal fermentation and activities of nonstarch polysaccharide-degrading enzymes to particle length of corn silage in dairy cows. Journal of Dairy Science 91, 2388–2398.
| Evaluation of the response of ruminal fermentation and activities of nonstarch polysaccharide-degrading enzymes to particle length of corn silage in dairy cows.Crossref | GoogleScholarGoogle Scholar | 18487661PubMed |