Depredation of eggs of threatened freshwater turtles by the short-beaked echidna (Tachyglossus aculeatus (Shaw, 1792))
Kymberly J. Robinson A * , Duncan J. Limpus B , Brad Crosbie C , Colin J. Limpus B and Larelle D. Fabbro
A * , Duncan J. Limpus B , Brad Crosbie C , Colin J. Limpus B and Larelle D. Fabbro  A
A
A
B
C
Abstract
The echidna (Tachyglossus aculeatus) is documented as a new native predator of freshwater turtle eggs, particularly those of the vulnerable Fitzroy River turtle (Rheodytes leukops), and the critically endangered white-throated snapping turtle (Elseya albagula). This depredation has been identified in the Fitzroy, Burnett and Mary Catchments with echidnas recorded at traditional turtle nesting banks using direct observation of depredation of eggs, remote camera footage and identification of tracks. Echidnas were observed at traditional nesting banks for E. albagula and R. leukops nesting across eight months of the year. The presence of echidnas was more frequent during the R. leukops nesting season in spring. R. leukops is endemic to the Fitzroy Catchment and the depredation rate of eggs was significantly higher than for other species and catchments. The mean top egg depth of R. leukops nests was found to be the same depth as the echidna’s beak length. There was a significant increase in depredation during the five-year study period, with 47.4% of R. leukops clutches depredated by echidna in the 2022 season. This substantial loss of eggs and recruitment of hatchling turtles poses a significant threat to the populations of this threatened species.
Keywords: Burnett River, critically endangered, depredation, echidna, eggs, Elseya albagula, Fitzroy River, freshwater, Mary River, nest, Rheodytes leukops, turtle, vulnerable.
Introduction
Australia’s freshwater turtle populations are under continuous negative pressure due to environmental threats, changes to catchment flow and structure with construction of dams and weirs (Hamann et al. 2008; Limpus et al. 2011) and depredation of eggs by both native and introduced animals (Chessman 2021). The depredation of freshwater turtle eggs has been documented as a significant threatening process (Limpus et al. 2011; Campbell et al. 2020). A high proportion of freshwater turtle clutches succumb to depredation before they hatch (Chessman 2021).
Documented predators of freshwater turtle eggs in Australia include rakali (Hydromys chrysogaster) (Thompson 1983), goannas (Varanus spp.) (Hamann et al. 2008), dingo (Canis lupus) (Thompson 1983) and introduced predators including the European red fox (Vulpes vulpes) (Thompson 1983; Dawson et al. 2016) and pigs (Sus scrofa) (Chessman 2021). Clutches are also lost to livestock trampling, primarily by cattle (Flakus 2002).
The Fitzroy, Burnett and Mary Catchments are located in central and southern Queensland, Australia. The critically endangered white-throated snapping turtle (Elseya albagula) (Thomson et al. 2006) is found in all three catchments and the vulnerable Fitzroy River turtle (Rheodytes leukops) (Legler and Cann 1980) is endemic to the Fitzroy Catchment. Both species nest at traditional nesting banks in aggregations (Limpus et al. 2011). E. albagula lays one clutch each season with an average of 14 eggs (Thomson et al. 2006) and R. leukops lays up to two clutches each season with an average of 18 eggs per clutch (Legler and Cann 1980).
This paper describes egg depredation by the echidna (Tachyglossus aculeatus) in the Fitzroy, Burnett and Mary Catchments. Although multiple species have been recorded depredating nests, this paper will exclusively review the data directly related to the echidna collected from each catchment between 2018 and 2022. The percentage of clutches destroyed by echidna will be analysed for each catchment on an annual basis. This research forms part of an ongoing study of freshwater turtles in Queensland, Australia.
Methods
Study area
The lower Fitzroy, Burnett and Mary Catchments were surveyed annually during freshwater turtle nesting monitoring activities. Seasonal surveys were undertaken in the Burnett and Mary Catchments during May–July to monitor the nesting of E. albagula, and during May–December in the Fitzroy Catchment to monitor the nesting of R. leukops and E. albagula. Each individual predator species was identified and recorded on the nesting banks through tracks and depredation of eggs. The presence of echidna as a predator was identified during the surveys through tracks and clutch depredation. The echidna tracks were verified using Triggs (2004).
A remote camera (Swift Enduro 4G) was used to monitor the presence of wildlife at a significant traditional nesting bank (ACJ-14) for both E. albagula and R. leukops in the lower Fitzroy Catchment. The remote camera was in place from May to December 2022 and was positioned in the middle section of nesting bank ACJ-14 where the main nesting activity occurred. Foraging behaviours of the echidna were derived using the remote camera footage.
Results
The echidna is here documented as a new native predator of freshwater turtle eggs, particularly R. leukops and E. albagula. The depredation of freshwater turtle eggs by echidna has been observed and recorded in the lower reaches of the Fitzroy, Burnett and Mary Catchments at traditional nesting banks that were routinely monitored each nesting period. There were less frequent records of egg depredation of the listed least concern freshwater turtle taxa, Krefft’s river turtle (Emydura macquarii krefftii (Gray, 1871)) and broad-shelled turtle (Chelodina expansa (Gray, 1857)), that nested on these monitored sections of riverbanks.
Direct observation of depredation
On the evening of 6 July 2019, an echidna was encountered partially buried into a known traditional freshwater turtle nesting bank (CC-10) within the lower Burnett Catchment (Fig. 1). The echidna was located at the end of a nesting turtle track and appeared to be feeding. The echidna was removed by rolling it sideways out from within its partially buried position in the loam substrate. A clutch of E. albagula eggs (BC2019110) was exposed inside the egg chamber directly below from where the echidna was removed. A clutch of 13 eggs was removed from the egg chamber and all were freshly punctured by the echidna’s beak and the contents consumed. There was only one puncture into each egg where the contents had been eaten. The echidna’s beak was still moist with the contents of the eggs.
Foraging behaviour on nesting bank
Echidna foraging behaviour was interpreted from images recorded by a remote camera monitoring the presence of wildlife at the traditional nesting bank ACJ-14 frequented by E. albagula and R. leukops in the lower Fitzroy Catchment. The echidna appeared to wander in no fixed pattern over the sand/loam, moving its head from side to side with the beak probing the substrate (Fig. 2). When the echidna detected a potential food source, it would stop and push its beak vertically into the substrate to a depth of its beak length and anterior head, up to eye level. It would at times push its beak and head further into the sand/loam to beyond the eye position until it encountered freshwater turtle eggs. The echidna remained at the location of the nest until all the contents of each egg had been consumed. If the echidna found nothing at the site probed, it would continue this behaviour pattern in search of food as it wandered over the nesting bank. The echidna behaviour remained consistent each night it was documented, while it was foraging on the nesting bank.
Occurrence of echidnas on the Fitzroy River nesting bank ACJ-14
Echidnas have been observed both day and night on the traditional nesting areas along the riverbank, although the depredation was recorded at night. The remote camera recorded the foraging behaviour of echidnas at varying times between dusk and dawn.
During the E. albagula nesting season, which occurs during winter, echidnas were observed on 6 of 24 consecutive nights within the lower Fitzroy Catchment at nesting bank ACJ-14. During the R. leukops nesting season, which occurs in spring, echidnas increased activity and were observed on 19 of 32 consecutive nights at nesting bank ACJ-14.
At nesting bank ACJ-14 and other traditional nesting banks, the echidna traffic increased during peak spring nesting for R. leukops, with echidna tracks encompassing the entire extent of the nesting bank, preventing identification of tracks from nesting turtles and other species of animal on the following morning. The exact timeframe of foraging each night is not known, nor is it known whether it is dependent on the number of turtles nesting on that night and providing a food source.
Description of egg depredation
The depredated clutches were not always obvious, with few signs of a nest or visible eggshell at the surface after the echidna had visited the nest location. Each depredated egg within the egg chamber had a single puncture hole through the shell wall and the contents eaten. The clutches were destroyed from the substrate surface without the echidna digging down to the eggs within the nesting bank (Fig. 3). When a depredated nest was discovered, all eggs within the clutch had been consumed.
Photographs of nests showing (a) depredated R. leukops clutch by echidna in damp sand, (b) close up of R. leukops depredated eggs in an egg chamber.
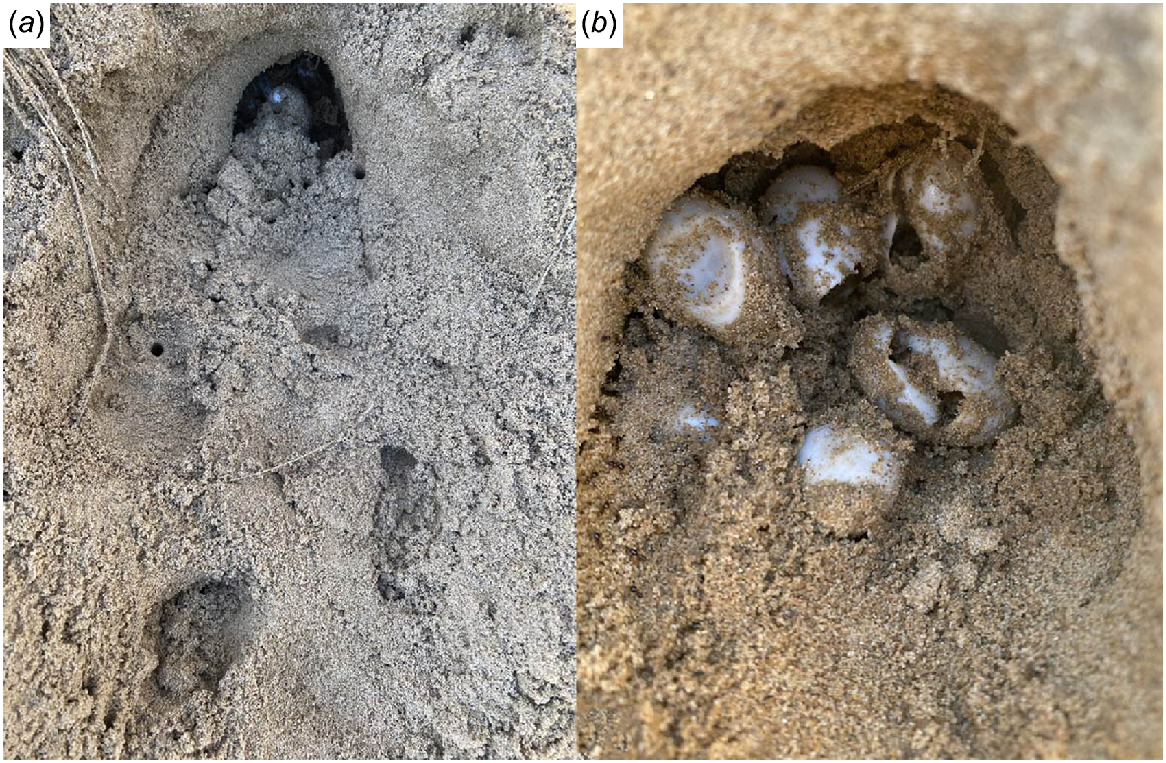
The chambers of previously depredated clutches were observed to be of interest to the echidna and were probed/dug into once more on subsequent nights. It has not been determined if this is due to the sand/loam being soft or if the scent of the eggs was sufficient to attract the echidna back to the same location again.
Comparison of probed depth to nest depth measurements
The individual beak depressions left in the sand/loam were obvious on the riverbank after rainfall and were measured at 14.0 cm in depth from the substrate surface, whereas the probing in dry sand did not leave prominent depressions that could be measured. The depth to which an echidna pushed its beak to eye level into the substrate while foraging was similar to the depth of the top egg measurement for R. leukops and just short of that to the top egg for E. albagula.
The depth to the top egg within the nest chamber from the substrate surface differed between freshwater turtle species. The mean depth was documented from intact nests for both species during the study. The mean top egg depth for E. albagula was 15.5 cm (range 8–23 cm, n = 99) and the mean top egg depth for R. leukops was 14.2 cm (range 4–21 cm, n = 278). Top eggs of R. leukops are located closer to the surface and within the probing range of the echidna’s beak.
Depredation rates and species impacted within the Fitzroy, Burnett and Mary Catchments
Depredation of eggs by echidna has been identified in four turtle species: R. leukops, E. albagula, C. expansa and E. m. krefftii (Table 1). The depredated clutches of E. m. krefftii and C. expansa were located at traditional R. leukops, and E. albagula nesting banks that were annually monitored.
| R. leukops | E. albagula | E. m. kreffti | C. expansa | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TN | DN | TN | DN | TN | DN | TN | DN | ||
| Fitzroy catchment | |||||||||
| 2018 | 178 | 5 | *35 | 2 | 18 | 3 | 0 | 0 | |
| 2019 | 98 | 13 | 199 | 4 | 11 | 1 | 2 | 0 | |
| 2020 | 156 | 27 | 32 | 1 | 4 | 0 | 0 | 0 | |
| 2021 | 139 | 21 | 49 | 4 | 2 | 0 | 0 | 0 | |
| 2022 | 133 | 63 | 41 | 5 | 1 | 0 | 1 | 1 | |
| Burnett catchment | |||||||||
| 2018 | *0 | 0 | *15 | 0 | *0 | 0 | |||
| 2019 | 232 | 2 | 0 | 0 | 0 | 0 | |||
| 2020 | 87 | 0 | 33 | 0 | 1 | 0 | |||
| 2021 | 154 | 1 | 5 | 0 | 0 | 0 | |||
| 2022 | 55 | 0 | 0 | 0 | 2 | 0 | |||
| Mary catchment | |||||||||
| 2018 | *1 | 1 | *0 | 0 | *0 | 0 | |||
| 2019 | 73 | 1 | 0 | 0 | 0 | 0 | |||
| 2020 | *16 | 0 | *1 | 0 | *0 | 0 | |||
| 2021 | 141 | 4 | 0 | 0 | 0 | 0 | |||
| 2022 | 20 | 0 | 0 | 0 | 0 | 0 | |||
TN, total nests; DN, destroyed nests; * indicates incomplete survey.
As noted above, there was a consistently higher presence of echidnas on the traditional nesting banks in the lower Fitzroy Catchment. Echidnas were present during the peak of the nesting season for both E. albagula and R. leukops. As nesting increased, the echidna activity increased. Once frequency of nesting decreased late in the season, the presence of echidnas rapidly decreased.
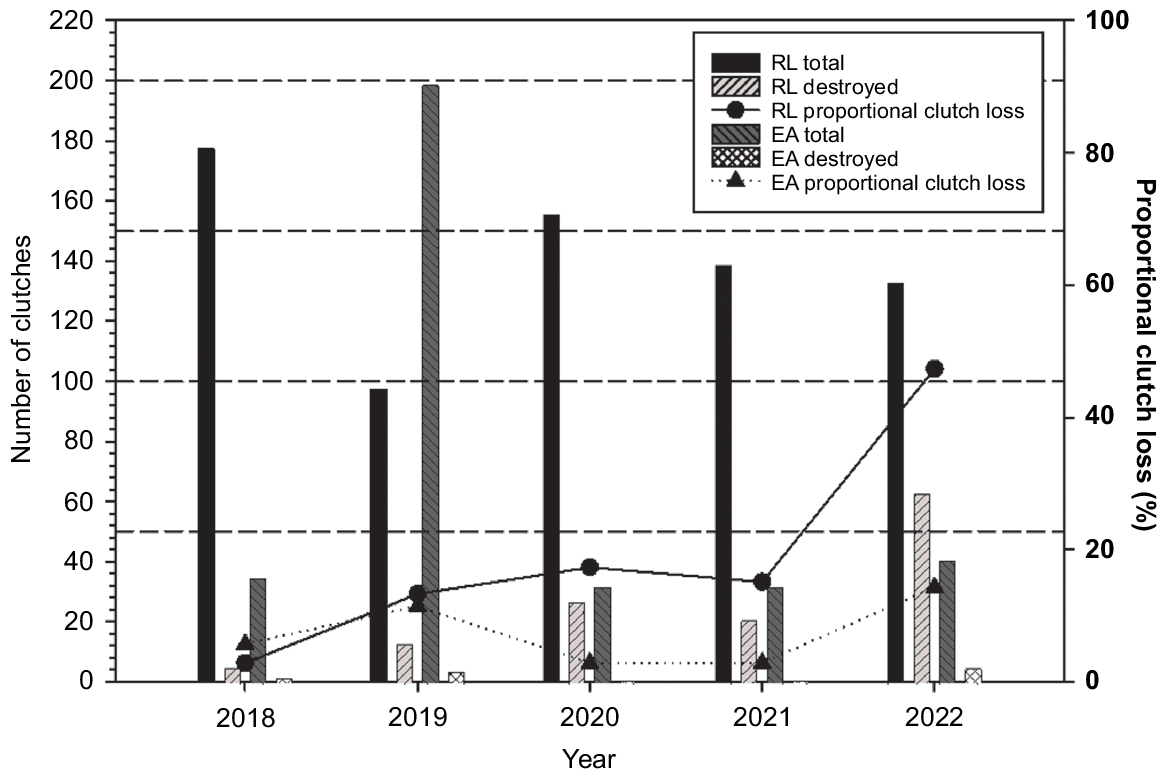
R. leukops clutches were subjected to a higher rate of depredation by echidna than E. albagula clutches laid on the same banks in the lower Fitzroy Catchment (Fig. 4). For both species across the five years, there was a trend for an increasing proportion of clutches laid being depredated by echidna. The annual depredation of R. leukops clutches increased from 2.8% in 2018 to 47.4% in 2022 (Fig. 4).
Discussion
The diet of the short beaked echidna is reported to be insectivorous, consisting largely of ants, termites and scarab beetle larvae, with the diet varying in proportion depending on the locality (Sprent and Nicol 2012; Nicol 2022). No previously published records of echidna diet mention freshwater turtle eggs. Captive echidnas typically are fed a mixture of proteins including egg due to the limitations in providing them with enough ants and termites (Stannard et al. 2017). Nicol (2022) determined beak length and climate as contributing factors to the diet and foraging of each subspecies of short-beaked echidna. Echidnas are opportunistic feeders and use more foraging effort in areas where food source is more concentrated (Abensperg-Traun et al. 1991). The nesting activity of both E. albagula and R. leukops within the lower Fitzroy Catchment provided a relatively consistent food supply at the traditional nesting bank locations for eight months of the year for the echidna. Winter depredation by echidna of E. albagula clutches was observed to be as high as 12.2%, whereas the frequency of depredation of R. leukops clutches during spring was as high as 47.4% in 2022. The rate of depredation for R. leukops is significantly higher than that for the other recorded turtle species. The echidna’s beak allows the eggs to be depredated without removing them from the egg chamber. In contrast, other predators dig the eggs out of the chamber and chew or tear the eggshell to consume the contents. As observed during this study, the European red fox, varanid and rakali depredation typically leave eggshells scattered across the nesting bank with the egg chamber location visible where the contents have been dug out.
This research has established that the echidna, unexpectedly, is a significant native predator of freshwater turtle eggs. The Fitzroy Catchment supported a higher rate of depredation of turtle eggs by echidna than the Burnett and Mary Catchments. The depredation rate of R. leukops clutches by echidna has increased by 44.6% over five years. The increase of depredation was not due to an increase of survey effort as every clutch was documented each year of the study at these banks.
Within the lower Fitzroy Catchment, the depredation of turtle eggs by echidna was identified at traditional nesting banks of E. albagula and R. leukops that have been monitored across the last 20 years by the authors. Although depredation of turtle eggs by echidna had been identified prior to 2018, the data from the earlier years was not collected systematically.
The cause(s) of this increase in clutch loss have not been determined at present. However, there may be multiple factors to consider. Recent changes in adjacent landscape to the riverbank, especially in the Fitzroy Catchment, may have impacted upon the foraging behaviour of the echidna due to areas being cleared of vegetation for bank restoration and agriculture. As a consequence of recent extreme flooding of the riverbanks since 2010, there may have been changes in the abundance of other predators competing with the echidna for the turtle eggs within the Fitzroy Catchment.
This study has shown a high depredation rate of the threatened freshwater turtle species that aggregate to nest at traditional nesting areas. Echidna depredation was highest for R. leukops, whose nests were shallower and whose eggs have a thinner shell that is easier to penetrate during the foraging. It is still to be determined if the foraging behaviour observed is a learned behaviour resulting in echidnas learning to utilise areas while there is an abundant/predictable food source. It is unknown if a single individual or multiple individuals are responsible for depredation at each nesting bank. This study identifies the potential for depredation of turtle eggs by echidna to be a significant threat to the survival of populations of at least two threatened freshwater turtle species, E. albagula and R. leukops, in the Fitzroy Catchment and possibly further afield. The high depredation rate, particularly for R. leukops eggs, requires further research to quantify the number of echidnas present at each nesting bank.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Declaration of funding
Funding for this project was provided in part by the Australian Department of Environment and Energy – White-throated Snapping Turtle Recovery Project.
Acknowledgements
We thank Sunwater, Henrik Christiansen (MacField Farms), and David Meyers for providing permission to access private property. We also thank all volunteers associated with Turtles of Central Queensland, WYLD Projects Indigenous Corporation and the Department of Environment and Science, particularly Suzannah Robinson and Kate Beskeen, for their contribution to nesting surveys.
References
Abensperg-Traun M, Dickman CR, Boer ESD (1991) Patch use and prey defence in a mammalian myrmecophage, the echidna (Tachyglossus aculeatus) (Monotremata: Tachyglossidae): a test of foraging efficiency in captive and free-ranging animals. Journal of Zoology 225, 481-493.
| Crossref | Google Scholar |
Campbell MA, Connell MJ, Collett SJ, Udyawer V, Crewe TL, McDougall A, Campbell HA (2020) The efficacy of protecting turtle nests as a conservation strategy to reverse population decline. Biological Conservation 251, 108769.
| Crossref | Google Scholar |
Chessman BC (2021) Introduced red foxes (Vulpes vulpes) driving Australian freshwater turtles to extinction? A critical evaluation of the evidence. Pacific Conservation Biology 28(6), 462-471.
| Crossref | Google Scholar |
Dawson SJ, Crawford HM, Huston RM, Adams PJ, Fleming PA (2016) How to catch red foxes red handed: identifying predation of freshwater turtles and nests. Wildlife Research 43, 615-622.
| Crossref | Google Scholar |
Hamann M, Schäuble CS, Emerick SP, Limpus DJ, Limpus CJ (2008) Freshwater turtle populations in the Burnett River. Memoirs of the Queensland Museum 52(2), 221-232.
| Google Scholar |
Legler JM, Cann J (1980) A new genus and species of chelid turtle from Queensland, Australia. Contributions in Science 324, 1-18.
| Crossref | Google Scholar |
Limpus CJ, Limpus DJ, Parmenter CJ, Hodge J, Forect M, McLachlan J (2011) The biology and management strategies for freshwater turtles in the Fitzroy catchment, with a particular emphasis on Elseya albagula and Rheodytes leukops: a study initiated in response to the proposed construction of Rookwood Weir and the raising of Eden Bann Weir. Report. Department of Environment and Heritage Protection.
Nicol SC (2022) Diet, feeding behaviour and echidna beaks: a review of functional relationships within the tachyglossids. Australian Mammalogy 44(1), 39-50.
| Crossref | Google Scholar |
Sprent J, Nicol SC (2012) Influence of habitat on home-range size in the short-beaked echidna. Australian Journal of Zoology 60, 46-53.
| Crossref | Google Scholar |
Stannard HJ, Bekkers JM, Old JM, McAllan BM, Shaw ME (2017) Digestibility of a new diet for captive short-beaked echidnas (Tachyglossus aculeatus). Zoo Biology 36, 56-61.
| Crossref | Google Scholar | PubMed |
Thompson MB (1983) Populations of the Murray River Tortoise, Emydura (Chelodina): the effect of egg predation by the red fox, Vulpes vulpes. Australian Wildlife Research 10, 363-371.
| Crossref | Google Scholar |
Thomson S, Georges A, Limpus CJ (2006) A new species of freshwater turtle in the genus Elseya (Testudines: Chelidae) from central coastal Queensland, Australia. Chelonian Conservation and Biology 5(1), 74-86.
| Crossref | Google Scholar |