Does reducing grazing pressure or predation conserve kowaris? A case study at Diamantina National Park
John Augusteyn A * , Maree Rich B , Chris Mitchell B , Eridani Mulder C , Barry Nolan D , Leong Lim E and Rhonda Melzer A
A * , Maree Rich B , Chris Mitchell B , Eridani Mulder C , Barry Nolan D , Leong Lim E and Rhonda Melzer A
A Queensland Parks and Wildlife Service and Partnerships, PO Box 3130, Red Hill, Qld 4701, Australia.
B Queensland Parks and Wildlife Service and Partnerships, PO Box 202, Longreach, Qld 4730, Australia.
C Australian Wildlife Conservancy, Wongalara Wildlife Sanctuary, PMB 162, Katherine, NT 0852, Australia.
D Queensland Parks and Wildlife Service and Partnerships, PO Box 5332, Airlie Beach, Qld 4802, Australia.
E Queensland National Parks and Wildlife Service, PO Box 281, Brooklyn, NSW 2083, Australia.
Australian Journal of Zoology 70(2) 56-73 https://doi.org/10.1071/ZO22027
Submitted: 11 May 2022 Accepted: 15 September 2022 Published: 8 December 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Livestock contributes to the decline of many species in Australia. However, they may have less impact in arid environments, where annual plant species dominate. Kowaris (Dasyuroides byrnei), a small carnivorous marsupial, living on Diamantina National Park were monitored to assess the success of ecosystem recovery following a reduction in cattle. Kowaris were found at 10 locations within the study area: five where they had been recorded prior to the area becoming a national park and five ‘new’ locations. No kowaris were found at one of the historical sites. The density was estimated to range from 1 to 2.5 kowaris per square kilometre from 2007 to 2009. The results suggest that the population likely increased following a reduction in grazing pressure. However, a boom in rodents and predators occurred during the study with a corresponding decline in kowari detections. Kowaris have not been detected at any of the study sites since 2012. These results suggest that management of top-down factors as well as bottom-up factors are required to conserve kowaris. The work further highlights the need for replicated, long-term studies if the interactions between complex ecological processes, at a landscape scale, are to be understood so that threatened species, like the kowari, can be managed effectively.
Keywords: arid zone, cat, cattle grazing, Dasyuroides byrnei, dingo, monitoring, predation, Rattus villosissimus, threatened species, top-down and bottom-up effects.
Introduction
Understanding the role that bottom-up (food and habitat limited) and top-down (predation limited) drivers have on threatened mammal species populations is critical to their management. However, undertaking robust grazing and predator removal experiments can be problematic in unfenced arid landscapes and, as a result, the impacts of grazing and predation are not well understood, particularly for some small mammal communities (Read and Cunningham 2010). Arid systems are prone to boom-and-bust events where rainfall, or the lack of it, and introduced predator populations can confound data and alter the balance between drivers (Letnic and Dickman 2006). The stocking rates on neighbouring properties can also vary considerably across the landscape and over time, which can confound data and make ascribing changes to a particular management regime difficult.
Cattle grazing is known to contribute to the loss of species diversity and the destruction/simplification of habitats (Friedel et al. 1990; Morton 1990; Bastin et al. 1993; Abensperg-Traun et al. 1996; Read and Cunningham 2010; Fensham et al. 2019; Silcock et al. 2019; Neilly et al. 2021). The impact of grazing on vegetation is better studied in comparison to mammals, but the dynamics of the semi-arid and arid systems are complex, and some studies suggest that removing stock does not always result in improvements to the vegetation (Read 1999; Page 2001; Fensham et al. 2010; Silcock and Fensham 2013, 2019). In part, this is because distinguishing between the effects of rainfall and the effects of cattle grazing on species richness can be difficult. The impact of grazing in the arid zone of Australia may also be less severe because the habitats are dominated by annual and ephemeral plant species, which naturally senesce and break down each year (Fensham et al. 2010; Silcock and Fensham 2013, 2019). The impact of cattle on fauna communities is considered to be principally through their effect on vegetation and how this affects the ability of native fauna to find shelter and food (Friedel 1990, 1997; Read and Cunningham 2010; Frank et al. 2013; Neilly et al. 2016; Neilly and Schwarzkopf 2018).
Kowaris are a small (up to 175 g), carnivorous marsupial that live on the ironstone plains in western Queensland, and South Australia (Lim 2008). They are usually solitary, and males and females have a mean home range size of between 9.0 and 10.3 km2, respectively (Lim 2008). Kowaris are listed as ‘Vulnerable’ under the Queensland Nature Conservation Act 1992 and the Environment Protection and Biodiversity Conservation Act 1999, ‘Endangered’ under the South Australian Endangered Species Protection Act 1992 and ‘Extinct’ under the Northern Territory’s Territory Parks and Wildlife Conservation Act 2000.
Kowari populations are known to increase episodically across their range in response to rainfall and prey populations – in particular, insects (grasshoppers) (Greenville et al. 2018) and juvenile long-haired rats (Rattus villosissimus) (Lim 1992), which can plague following above average rainfall (Greenville et al. 2013). In addition to grazing impacts, predation is considered a threat to kowaris (Palmer 1999). Predators include owls, dingoes (treated here as including dingoes (Canis familiaris), feral dogs (C. familiaris) and their hybrids), feral cats (Felis catus), and large reptiles (snakes and goannas) (Lim 1992, 1998; Palmer 1999; Greenville et al. 2018; Woolley et al. 2019). However, the nature of how these top-down (predation) and bottom-up (prey) drivers interact and affect kowaris, and small mammal populations in general, is largely unknown. Lim (1992), Maxwell et al. (1996) and Brandle et al. (2002) have suggested that there is likely to be a link between cattle grazing intensity and kowari population density, mainly because of the effect cattle have on altering vegetation communities. Cattle have been implicated in the disappearance of kowaris from Sandringham Station (Maxwell et al. 1996).
In 2006, a project was initiated on Diamantina National Park (hereafter Diamantina or the Park) to investigate ecosystem recovery as well as the conservation of the threatened kowari following efforts to substantially reduce stray stock on the Park. At Diamantina, cattle grazed the vegetated drainage lines (in addition to the landscape more broadly) and disturbed mammal burrows (McRae 2004; Woinarski et al. 2014). The vegetated drainage lines provided important refuges for invertebrates and small vertebrates, which in turn provided food for kowaris (Lim 1992). The aim of this study was to use long-term monitoring data to determine if the size and distribution of the kowari population on Diamantina changed following a reduction in grazing pressure and in the process, compare the relative influence of other drivers, including predation. We present 15 years of survey data, compare them to historical records collected from Diamantina and to more recent records obtained from the nearby Astrebla Downs National Park (Astrebla) and compare vegetation structure and composition data between on- and off-park sites.
Materials and methods
Study area
Diamantina is located 306 km south-west of Winton in central Queensland. The 507 000 ha area was made a national park in 1992 to conserve ‘the high number of landscape types, land zones, land systems and associated vegetation types typical of the northern Channel Country and Mitchell Grass Downs Bioregions – a mix not achieved elsewhere’ (Wilson and Mitchell 1992; Sattler 2014). Prior to becoming Diamantina in the 1990s, the property was managed as a cattle station and at times had over 12 000 head of cattle (Barry 2007). The Park has largely been destocked but large cattle grazing enterprises surround Diamantina, with the exception being Pullen Pullen – a Special Wildlife Reserve to the north, and stray stock continue to graze (up to approximately 1000 head: C. Mitchell, QPWS, pers. comm., 2020) the Park, albeit at a much-reduced scale. Ranger staff constructed and repaired boundary fences, restricted access to water points and organised cattle musters. The Park includes several ‘of concern’ regional ecosystems and provides habitat for many threatened flora and fauna species, and internationally listed species including the iconic greater bilby (Macrotis lagotis), kowari, night parrot (Pezoporus occidentalis), plains wanderer (Pedionomus torquatus), plains mouse (Pseudomys australis) and dusky hopping-mouse (Notomys fuscus). This study was conducted in the south-east section of Diamantina – an area that was approximately 54 000 ha in size. The study area was dominated by large sections of ironstone downs – an open rocky landscape that often provides habitat for kowaris. Adjacent to the ironstone downs are often large ashy clay plains, sand dunes, low hills and claypans. Several creeks flow through the area and the Diamantina River runs north to south along the western edge of the study area, which includes a large blue bush swamp.
The mean annual rainfall at Diamantina is approximately 260 mm (Australian Government Bureau of Meteorolgy (BOM) 2021). Most of this rain falls between December and March but there is considerable year-to-year variation in timing. The survey work was conducted when temperatures were mild (15–32°C). Daily summer temperatures often exceed 35°C and winters can be cool with a mean minimum temperature of 7°C.
Vegetation
Vegetation was measured within or adjacent to the kowari trapping sites, including six ironstone downs sites on-park (low grazing), and two ironstone downs sites on the neighbouring property – Davenport Downs (highly grazed). Fifty metre transects, running north–south, were used to record the vegetation twice a year in 2007, 2008, 2009, 2011 and 2012 and annually in 2010 and 2013. The foliage projective cover (FPC) of the understorey; understorey species composition, frequency and dominance; soil disturbance and biomass were recorded at each site. A detailed description of the methodology along with the site location details are provided in Appendix 1. We used a paired t-test to look for differences in the on- and off-park ironstone downs vegetation sites. We also undertook a basic regression analysis in Excel comparing six monthly rainfall totals, recorded at the Diamantina River Gauging Station (data supplied by the Department of Regional Development, Manufacturing and Water) with the average amount of cattle-related soil disturbance, and the percentage cover of forbs, and perennial and annual grasses.
Kowari predator/prey assessment
Repeated, vehicle-based, line transect surveys using two 100 W spotlights were conducted, both on- and off-road, to search for kowaris and their predators and prey twice a year in 2007, 2008, 2009 and 2011 and annually in 2010, 2013 and 2017. After it was found that thermal cameras (FLIR MD625) could be used to improve the detection of small mammals by up to five times compared with spotlights (Augusteyn et al. 2020a), they were used instead of spotlights. They were used annually from 2014 to 2021, except in 2016 and 2019 when there was nil survey effort. The vehicle was driven at 10–15 km/h with two observers standing in the tray, one on each side of the vehicle. In 2020 and 2021, three thermal cameras (Two Flir MD625 and a Tau2) were used, each with their own observer. All surveys were conducted between 2000 hours and 0100 hours. All fauna observed along the transects, including prey and predators of kowaris, were recorded. The details of these surveys have been divided into two groups – those conducted in suitable kowari habitat (ironstone dominated) and those conducted adjacent to the suitable habitat (ashy clay soils and drainage lines adjacent to the ironstone dominated plains). Between 23 and 54 km of transect were surveyed within suitable ironstone habitat and between 32 and 98 km were surveyed that included both suitable kowari habitat and habitat adjacent to the suitable kowari habitat. The habitat adjacent to the kowari habitat was included to survey for predators. The length of the transects conducted within the kowari habitat is provided in Appendix 2. The details for the transects adjacent to the kowari habitat are provided in Appendix 3. Survey transects conducted through the trapping grids (Appendix 4) were completed either before or after the trapping commenced at a site so that the spotlighting did not affect the trapping. In addition to the spotlight/TIC surveys, all kowaris observed incidentally while staff were carrying out other activities were recorded.
Kowari distribution
Historical records
The Department’s wildlife record system – WildNet (WN) and the Atlas of Living Australia (ALA) (Atlas of Living Australia website at http://www.ala.org.au, accessed 28 June 2005) were searched for records of kowaris to help identify suitable kowari habitat on Diamantina. Researchers who had previously conducted small mammal surveys/monitoring on the Park were also contacted to determine whether there were locations not captured in the aforementioned databases.
Trapping and nocturnal searches
Elliott traps were initially used to locate kowaris in April and September 2007 (Fig. 1). These presence/absence surveys were conducted in areas where kowaris had been trapped previously or where the habitat looked similar to that of known kowari capture sites. The location details of these presence/absence sites are provided in Appendix 5.
|
|
The Elliott traps were set for three nights and were placed 100 m apart and baited with either a bolus of peanut butter, rolled oats, sardines, sesame seed oil and cat biscuit or dog biscuit soaked in fish oil or mixed with sardines. The bait type used was changed daily, with the type being selected randomly, to reduce the chance of any caught kowaris developing a negative association with the bait. The traps were checked early in the morning and all animals caught were released at the site of capture if it was prior to sunrise. Animals found in traps after sunrise were kept in calico bags and stored indoors in a cool dark cupboard during the day and released at the place of capture after sunset. This was done to reduce the chance of a captured animal being preyed upon after they were released – before they had a chance to find and retreat to their burrow. The traps were closed during the day and reopened each afternoon. A peg fashioned out of a piece of reinforced steel mesh was used to secure the trap in place and reduce the chance of it blowing away during strong wind events/dust storms (Fig. 2).
|
|
Camera survey
In April 2018, 85 Reconyx PC850 white flash remote cameras were deployed at four of the trapping sites (20–23 per site) where kowaris had been trapped previously (see below in Kowari Density) (Sites 2, 8, 9, and 11) to determine their presence/absence. The cameras were placed in a grid, approximately 200 m apart – generally at every second Elliot trap location used between 2007 and 2012. The cameras were attached to a 600 mm long wooden garden stake and left in place for 11 nights. Cameras were baited with a bolus of peanut butter, rolled oats and sardines placed in a canister pegged to the ground 1 m in front of the camera. The cameras were set to take still images 24 h per day, with five images per trigger and no quiet period between triggers.
Kowari density
To track changes in the size of the kowari population, up to six trapping grids (4 km2) were monitored twice a year in 2007, 2008, 2009 and 2011 and annually in 2010 and 2012. The grids were placed in areas containing suitable ironstone habitat and in areas where the species had previously been recorded. These grids were separate from the trapping transects used to initially locate kowaris. The density of kowaris was initially assessed at four grids (4 km2) (Sites 5, 8, 9, and 11) with 80 Elliott traps placed every 100 m along four 2 km long parallel transects (20 traps per transect) spaced 660–680 m apart (Appendix 5). In April 2008, two more grids (Sites 2, 3, 4), were surveyed with the same arrangement and number of traps used previously. In April 2009 and in subsequent surveys, sampling effort was reduced and only 40 traps were used per site. Their placement along a linear transect was selected based on landscape features and kowari captures that occurred in 2007 and 2008. Prior to 2009, traps remained opened for three nights. In 2009 and thereafter, traps were opened for four nights. The traps were baited with the same bait types described earlier for Elliott trapping.
Each captured kowari was measured (hind foot length, head–body length, tail length), DNA sampled (hair follicle), weighed and microchipped (Trovan ID100 (1.25 mm × 7 mm) Nanotransponder). The Schnabel method (Schnabel 1938), for a closed population, was used to calculate density. Abundance was estimated by multiplying the density by the survey area (4 km2). Abundance and density were not able to be calculated for most grids or years due to the low number of individuals caught and the low number of recaptures.
Results
Vegetation
The mean species richness for each plant group (forbs, perennial and annual grasses) varied across years but was generally low (<5) except for forbs in autumn 2010 and 2011 and spring 2012, when species richness increased (>7) (Fig. 3). Species richness for all plant groups weakly correlated positively with six monthly rainfall totals, particularly summer–autumn rainfall (forbs y = 23.297x + 85.63 (R2 = 0.5259), annual grass y = 57.909x + 46.747 (R2 = 0.7767), and perennial grass y = 87.414x + 75.577 (R2 = 0.6855)). Forbs were present at most of the vegetation sites throughout the year, both on- (low grazing) and off-park (higher grazing), with a large increase in the mean percentage cover at all sites occurring after 2011. Even though the percentage cover of forbs off-park was higher than on-park in most years, the difference was not significant. However, there was significantly more grass cover (t10 = 1.812, P = 0.0001) and more biomass (t11 = 1.795, P = 0.03) on-park than off-park and there was significantly less (t11 = 1.795, P = 0.009) soil disturbance by stock on-park than off-park. The number of grass species on-park was also significantly greater than off-park (t10 = 1.812, P = 0.0001).
|
|
Kowari predator/prey assessment
The number of kowari predators (e.g. feral cats and dingoes) observed on spotlight transects was relatively low until 2010, after which their numbers, in particular those of feral cats, spiked (Fig. 4). We recorded more kowari prey species after 2009, except kultarrs (Antechinomys laniger) whose populations appeared to be more abundant before 2009 (Fig. 5).
Kowari distribution (presence/absence)
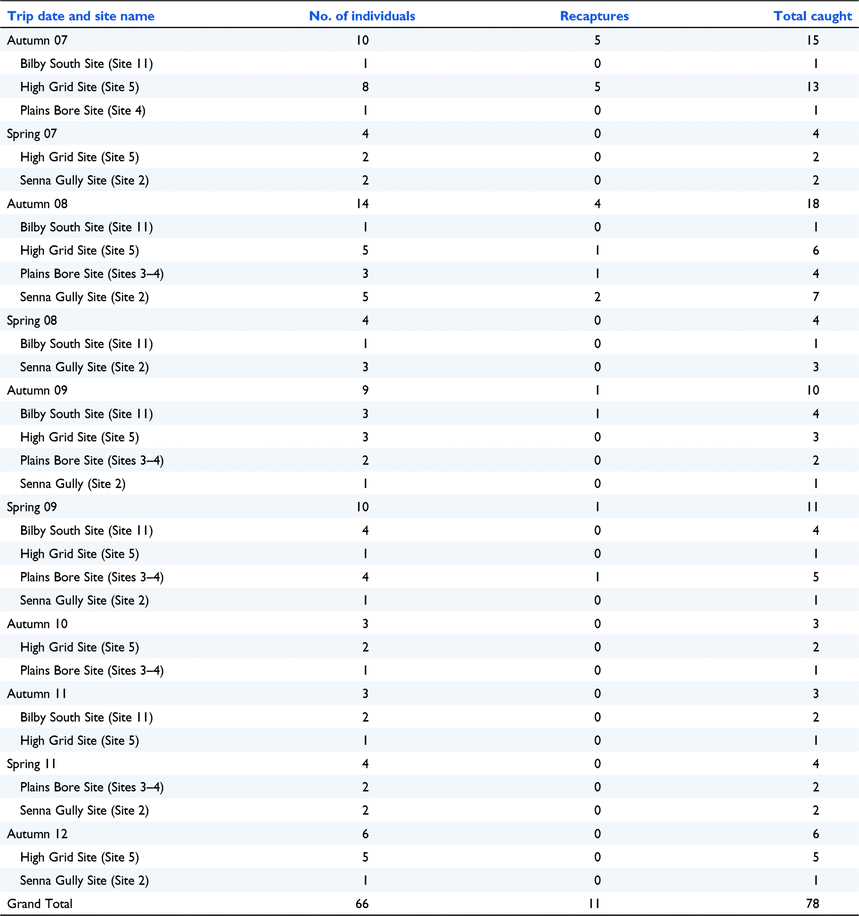
Prior to this study, WN and the ALA contained a total of six historic kowari areas (an area was determined to be 9 km2 – the kowari’s mean home range: Lim 2008) from 10 sightings on Diamantina. Of these six historic areas, kowaris were detected at five during the current study – Sites 2, 6, 7, 9, and 11 (Fig. 6). Since 2007, kowaris have been recorded in 10 sites within the study area including five that are new areas – Sites 1, 3, 4, 5, and 10. Ten kowaris were caught in Elliott traps during the line transect trapping in 2007 as part of the initial search for kowaris and then a further 24 kowaris were seen across all spotlight surveys. In total, 78 kowaris (including recaptures) were caught in Elliott traps set as part of the density surveys during 2007–2012. The numbers of individual kowaris caught and observed are shown in Table 1. These numbers may include individuals that were both caught in a trap and spotlighted during a survey. After 2012, kowaris were not detected at any of the study sites.

|
All the sites supporting kowaris contained ironstone downs habitat or were near the edge where the ironstone and surrounding clayey plain met. Drainage lines intersected most sites that contained kowaris. These drainage lines often contained a mixture of grass and shrub species.
Kowari density
The density ranged from 1 to 2.75 kowaris per square kilometre. The density and abundance estimates and 95% CI for all sites where there were sufficient recaptures, are presented in Table 2. The number of individuals and the recaptures caught in the grids is provided in Appendix 6. The low number of recaptures meant that we were only able to produce meaningful estimates on six occasions (five grids and four seasons). Environmental factors, such as rain and windstorms which sometimes blew traps away (traps did not contain kowaris at the time), and corvids who opened traps and removed bait, made trapping difficult at times. Long-haired rats were detectable during 2008–2012 but only occurred in large numbers in two trips in 2011, when they interfered with traps and reduced our ability to sample the kowari population. During the plague of long-haired rats in 2011, the Elliott traps often contained multiple rats, with one trap containing four long-haired rats.

|
At Diamantina, kowaris appear to have a reasonably large home range. Several animals that were caught and released were recaught several hundreds of metres from where they were released. One individual travelled more than 2 km between Elliott traps in a single night.
Discussion
Reducing livestock grazing initially appeared to benefit the vegetation, kowaris and small mammals on Diamantina. However, any possible benefit of reduced grazing on kowari prey and the kowari population appears to have been over-ridden by an increase in predators. No kowaris were detected between 2013 and 2021 within the study area, suggesting that the kowari population didn’t recover following a boom of resources and predators in 2011/12. The study highlights that controlling grazing alone is not enough to conserve threatened mammal species like the kowari and it is important to control predators as well. This finding is similar to that of Neilly et al. (2021), who found that destocking pastoral properties is rarely sufficient on its own to lead to ecosystem recovery and land managers must deal with a complex legacy of impacts when converting pastoral land to conservation reserve. Similarly, Read and Cunningham (2010) found that reducing stocking rates alone was insufficient to facilitate the restoration of an arid zone mammal community and predator control was critical to conserve mammals.
The role of vegetation, kowari predators and prey in determining kowari population size
There were significant differences in the amount of cattle related soil disturbance, and the percentage cover of grass, between sites on- and off-park – the former being greater off-park and the latter greater on-park. According to Mitchell (2022), the species composition on Diamantina has slowly changed after gazettal from a landscape dominated by Sclerolaena species to one with more grass and forbs other than Sclerolaena species. Most of this change occurred prior to the commencement of our study in 2007. The percentage cover of forbs, including Sclerolaena species, was higher off-park (heavily grazed) than on-park (lightly grazed) for most years. This may be because cattle preferentially graze on grass and soil disturbance is known to stimulate the growth of some forbs, including Sclerolaena species. Frank et al. (2013) found that neither vegetation nor small vertebrates responded immediately to the removal of livestock, but that rainfall events and cumulative grazing history were the key determinants of flora and fauna health in arid grasslands. They found the reproductive output of vegetation was greater in areas ungrazed for two years or more compared to areas still being grazed. Page (2001) found that a reduction of grazing pressure improved vegetation composition in the Mulga Land systems but, like Frank et al. (2013), found that rainfall had by far the greatest impact on plant biomass. These examples and the results from this study highlight the difficulties of monitoring ecosystem change in arid landscapes where large changes in biomass can occur regardless of other drivers, particularly in the absence of paired controlled sites.
Above average rain in 2009/10 resulted in a plague of long-haired rats in 2011 and an associated boom in predators (raptors and owls, monitor lizards, snakes, feral cats and dingoes), a phenomenon that also occurred at nearby Astrebla (Rich et al. 2014). This was the first major boom event to occur in the area since Diamantina became a national park. Despite the apparent increase in resources available to kowaris, their numbers unexpectedly declined. This contrasted with Lim (1998), who recorded an increase in kowaris, during and immediately after a rat plague and a decline during a plague of locusts. He suggested that the lack of rainfall at the time, and its effect on habitat quality had a greater effect on kowaris than the number of locusts. However, this does not accord with the results of Greenville et al. (2018) at Clifton Hills pastoral lease, who found kowari numbers to be higher when locusts were abundant and lower after the rat plague. Greenville et al. (2018) found that kowaris declined on Clifton Hills regardless of climatic conditions and suggested they were under stress from extrinsic factors such as predation. This was similar to what was observed on Astrebla where the kowari population did recover (refer to Appendix 7), despite experiencing a rainfall pattern similar to that at Diamantina and being destocked (Orr 1986). Not only was the predator control effort on the two parks vastly different but Astrebla is further away from the Diamantina River, an area that is thought to provide refuge to predators during drought (Palmer 1999). Over 3000 feral cats and at least 66 dingoes/wild dogs were shot/baited at Astrebla between 2012 and 2021 (Rich et al. 2014; Augusteyn et al. 2020b) whereas fewer than 100 cats were shot or trapped and no dingoes were removed from Diamantina between 2006 and 2021 (unpublished QPWS data). Predator control by spotlight shooting on Diamantina is more difficult than at Astrebla because of the greater number of thickly vegetated drainage lines. The permanent presence of rabbits at Diamantina compared to Astrebla might be another factor that hampered recovery at Diamantina. Rabbits are known to support larger populations of predators than is otherwise possible (Cruz et al. 2013; Pedler et al. 2016) and this is particularly problematic during drought when rabbit numbers decline, and the predators are forced to seek alternative prey (Lim 1998).
Stobo-Wilson et al. (2020) highlighted that, in northern Australia, complex productive habitats enhanced the capacity of native mammals to cope with cat and dog predation whereas reduced habitat complexity and productivity increased top-down pressures and caused declines across much of the native mammal assemblage. The results of our study indicate that the enhanced productivity and improved habitat complexity observed on Diamantina, following a reduction in grazing, did not override the top-down pressures of predation following a spike in predator numbers. Like the small mammals, the predator populations also likely benefitted from the increased resources following a reduction in grazing which has then put pressure on the small mammal community. The ephemeral nature of the vegetation at Diamantina may mean that the benefits of reduced grazing are only temporary and that a spike in predators when the vegetation is senescing or has been consumed by rodents, i.e. at the end of the boom/start of the bust, is sufficient to remove most of the protective cover and favour the predators. For some species, like the yellow-footed rock-wallaby (Petrogale xanthopus), populations tend to follow the ebb and flow of resources (Sharp and McCallum 2010). However, at Diamantina the kowari population did not return to their pre-boom population size once conditions improved but decreased to levels that were well below it. This suggests that predation pressures play a greater role than habitat condition and prey availability in determining the survival of kowaris living at Diamantina.
The role of dingoes/wild dogs
Wijas and Letnic (2021) suggested that for some prey species the top-down effects of the dingo can have primacy over bottom-up effects on population dynamics. Other ecologists have suggested that dingoes may protect smaller prey from feral cats and foxes (Vulpes vulpes) (the latter are rarely seen in the area) (McRae 2004; Murphy et al. 2018), in habitats that have little cover, through mesopredator release (Moseby et al. 2019). Consistent with the mesopredator release theory, Wallach and O’Neill (2009) predicted that the retention of an uncontrolled dingo population in the landscape would facilitate the conservation of small mammals like the kowari. However, at Diamantina at the end of the boom when resources were running out, feral cats were observed in open habitats well away from the protective creek lines despite the presence of dingoes. Dingoes are also known to prey on kowaris at this time (Palmer 1999; authors’ obs.). Therefore, the period following the boom is likely to be the time when populations of native fauna are most at risk from native and feral predators, particularly if the boom has elevated predator populations and the bust is severe, causing predators to be less risk averse. During this study, we did not find any evidence to suggest that dingoes on Diamantina helped conserve kowaris and their predation at the end of the boom likely contributed to the kowari’s decline.
Kowari habitat and distribution
We found that the kowari’s preferred habitat at Diamantina was the ironstone dominated plains with well vegetated drainage lines. Lim (1998) found that they used the drainage lines to hunt for food, for burrowing and for traversing the landscape. R. Brandle, P. D. Canty, L. Lim, unpubl. data, found that kowaris on Clifton Hills pastoral lease in South Australia preferred stony plains with more than 30% cover of pebbles, that were less than 50 mm in diameter, on a slope of less than 4°. In contrast, kowaris at Astrebla and in other parts of their range were regularly observed living on treeless clayey plains, with little to no vegetation cover, and with 20% or less stone cover (Lim 1992; authors’ obs.).
Kowaris had a limited distribution on Diamantina relative to the abundance of ironstone and clayey/ashy downs/plains habitat. We found kowaris at 10 sites (9 km2 mean home range), five of which were new and five of which were historical (prior to gazettal) (Lim 1992; C. Mitchell, QPWS, pers. comm., 2020). We did not find kowaris at one of the historical sites where the ironstone pebbles had been extracted for roadworks prior to it becoming a national park (C. Mitchell, QPWS, pers. comm., 2020). The new kowari sites suggest that the species has expanded its distribution since the national park was gazetted, but it may just be that the historical surveys were limited and opportunistic and the kowaris were missed. Despite our efforts to contact previous researchers and to search relevant databases, we are unable to determine the precise extent of the historical surveys, which included a combination of trapping and observational records (Lim 1993, 1998; C. Mitchell, QPWS, pers. comm.). Both the current and historical studies included dry and wet periods. The similar climatic conditions of the previous and current study confirms that the changes in the kowari distribution that were observed after gazettal are unlikely to be due to rainfall.
Kowari density
The highest densities of kowaris were found at Sites 2 and 5 in 2007 and 2008 (2–2.75 kowaris per km2) which indicated that the population was doing well on Diamantina at the commencement of the study. The densities at Sites 4 and 11 were lower (1.0 kowaris per km2). The latter density estimate was similar to those obtained from Clifton Hills Pastoral Lease in South Australia (1–1.6 kowaris per km2) (Brandle et al. 2002). The low number of recaptures during our study meant that we were unable to estimate abundance and density for all sites. This was also a problem for Lim (1998) at one of his study sites. However, catching an adequate number of kowaris to estimate density at all sites would have required an enormous trap effort and no long-haired rat plague. Even with a vast increase in the number of traps or trap nights, the density of kowaris may be so low at some sites that it is not possible to robustly estimate density across all sites.
The value of long-term datasets and control sites
The combined results from our and Mitchell’s (2022) long-term studies were valuable in assessing changes in the vegetation and they suggest that kowaris increased following a reduction in grazing pressure. However, despite the long-term dataset (15 years), the results are correlative. The occurrence of an unprecedentedly large boom followed by a severe bust over-rode any possible benefit of reduced grazing pressure. In this case, having a long-term dataset, which may in fact not be long at all in these arid systems, did not seem to compensate for the lack of a control site. Several authors have recommended that long-term monitoring, through a range of seasonal conditions be undertaken to improve our understanding of ecosystem change (Read 2002; Kraaij and Milton 2006; Pedler et al. 2016; Moseby et al. 2021; Piazza et al. 2021). Given that stray stock continue to graze the Park, correlative analysis is limited, and that further years of study will only reduce the uncertainty slowly, it would be more useful to establish a control site on a neighbouring cattle station to better evaluate trends in habitat condition and the kowari population. Finding a suitable control site is, however, very difficult in western Queensland due to the low density and distribution of the kowaris, the patchy rainfall, and the unpredictable boom– bust nature of the ecosystem.
The status of kowaris at Diamantina
The lack of any recent kowari sightings within the study area on Diamantina is cause for concern. Two other endangered species, the plains mouse and the dusky hopping-mouse, have also not been detected on the Park since 2000 (authors’ obs.). Lim (1998) did not detect kowaris towards the end of his study, but animals subsequently recolonised the sites two years later. Given the large area impacted by the plague of long-haired rats in 2011 in and around Diamantina, the widespread abundance of predators, and that kowaris have not been detected within the study area in the last decade, it is likely that the declines have been widespread in western Queensland.
Conclusion
It is recommended that surveys within Diamantina be extended to include suitable habitat not previously assessed, particularly habitat further away from the Diamantina River, to better determine the status of kowaris on the Park. Kowari refugia may be areas with extensive ironstone and claypan pavements where predators are less abundant. Further studies are also required to determine whether off-park populations, particularly at Lim’s (1998) Birdsville study site, were affected by the 2011 boom of resources and associated increased predation. If the off-park populations are similar to those at Diamantina then we strongly recommend that the species status be reassessed, and consideration be given to its eligibility for the ‘endangered’ category. Greenville et al. (2018) predicted a 20% chance of the species becoming extinct within the next 20 years.
Our study indicates that effective predator control will be critical to the recovery and maintenance of a kowari population on Diamantina. The ongoing measures to minimise cattle grazing will also be beneficial but are not as important as predator control. This study highlights the need for long-term, replicated studies with paired control sites if the interactions between complex ecological processes, at a landscape scale, and their effects on threatened species, like kowaris, are to be better understood and better managed.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
The work was entirely funded by the Queensland Department of Environment and Science.
Acknowledgements
The following people are thanked for their assistance with field work: Ian Andreassen, the late John Clemments, Robert Brodribb, Graham Hemson, Andrew Dinwoodie, Wendy Brodribb, Emily Rush, Andrew Howe, Peter Lehmann, the late Russell Best, Alicia Whittington, the late Dave Delahoy, Robert (Shorty) Cupitt, Ronell Frazer, Dave Nelson, Flynn Major, Sophia Levy, Huw Davie, Mark Constable, Beau Butcher, Dan Beard, Nick Smith, Lyndy Marshall, Graeme Armstrong, James Speed, Michael Brennan and Peter Elsworth. Thanks to Coral Rowston, Kerrie Bennison, Richard Seaton and Steve McKillup for either helping with setting up the project, or providing statistical advice or editorial comments. Tom Mumbray is thanked for his support and his illustration. This study was conducted under animal ethics permit EPA/N/07/09.
References
Abensperg-Traun, M, Smith, GT, Arnold, GW, and Steven, DE (1996). The effects of habitat fragmentation and livestock-grazing on animal communities in remnants of gimlet Eucalyptus salubris woodland in the Western Australian wheatbelt. I. Arthropods. Journal of Applied Ecology 33, 1281–1301.| The effects of habitat fragmentation and livestock-grazing on animal communities in remnants of gimlet Eucalyptus salubris woodland in the Western Australian wheatbelt. I. Arthropods.Crossref | GoogleScholarGoogle Scholar |
Augusteyn, J, Pople, A, and Rich, M (2020a). Evaluating the use of thermal imaging cameras to monitor the endangered greater bilby at Astrebla Downs National Park. Australian Mammalogy 42, 329–340.
| Evaluating the use of thermal imaging cameras to monitor the endangered greater bilby at Astrebla Downs National Park.Crossref | GoogleScholarGoogle Scholar |
Augusteyn, J, Rich, M, Story, G, and Nolan, B (2020b). Canids potentially threaten bilbies at Astrebla Downs National Park. Australian Mammalogy 43, 300–310.
| Canids potentially threaten bilbies at Astrebla Downs National Park.Crossref | GoogleScholarGoogle Scholar |
Australian Government Bureau of Meteorology (BOM) (2021) Rainfall data. Available at http://www.bom.gov.au
Barry JI (2007) ‘Calling 8XD: Diamantina.’ (Photovision)
Bastin, GN, Pickup, G, Chewings, VH, and Pearce, G (1993). Land degradation assessment in central Australia using a grazing gradient method. The Rangeland Journal 15, 190–216.
| Land degradation assessment in central Australia using a grazing gradient method.Crossref | GoogleScholarGoogle Scholar |
Brandle R, Canty P, Pillman S, Lang J (2002) Kowari population monitoring report. South Australian Department of Environment and Water, Adelaide, SA, Australia.
Cruz, J, Glen, AS, and Pech, RP (2013). Modelling landscape-level numerical responses of predators to prey: the case of cats and rabbits. PLoS ONE 8, e73544.
| Modelling landscape-level numerical responses of predators to prey: the case of cats and rabbits.Crossref | GoogleScholarGoogle Scholar |
Daubenmire, R (1959). A canopy coverage method of vegetational analysis. Northwest Science 33, 43–64.
Fensham, RJ, Fairfax, RJ, and Dwyer, JM (2010). Vegetation responses to the first 20 years of cattle grazing in an Australian desert. Ecology 91, 681–692.
| Vegetation responses to the first 20 years of cattle grazing in an Australian desert.Crossref | GoogleScholarGoogle Scholar |
Fensham, RJ, Laffineur, B, Rhodes, JR, and Silcock, JL (2019). Rare plant species do not occupy water-remote refuges in arid environments subject to livestock grazing. Ecological Applications 29, e01911.
| Rare plant species do not occupy water-remote refuges in arid environments subject to livestock grazing.Crossref | GoogleScholarGoogle Scholar |
Frank, ASK, Dickman, CR, Wardle, GM, and Greenville, AC (2013). Interactions of grazing history, cattle removal and time since rain drive divergent short-term responses by desert biota. PLoS ONE 8, e68466.
| Interactions of grazing history, cattle removal and time since rain drive divergent short-term responses by desert biota.Crossref | GoogleScholarGoogle Scholar |
Friedel, MH (1990). Some key concepts for monitoring Australia’s arid and semi-arid rangelands. The Rangeland Journal 12, 21–24.
| Some key concepts for monitoring Australia’s arid and semi-arid rangelands.Crossref | GoogleScholarGoogle Scholar |
Friedel, MH (1997). Discontinuous change in arid woodland and grassland vegetation along gradients of cattle grazing in central Australia. Journal of Arid Environments 37, 145–164.
| Discontinuous change in arid woodland and grassland vegetation along gradients of cattle grazing in central Australia.Crossref | GoogleScholarGoogle Scholar |
Friedel, MH, Foran, BD, and Stafford-Smith, DM (1990). Where the creeks run dry or ten feet high: pastoral management in arid Australia. Proceedings of the Ecological Society of Australia 16, 185–194.
Greenville, AC, Wardle, GM, and Dickman, CR (2013). Extreme rainfall events predict irruptions of rat plagues in central Australia. Austral Ecology 38, 754–764.
| Extreme rainfall events predict irruptions of rat plagues in central Australia.Crossref | GoogleScholarGoogle Scholar |
Greenville, AC, Brandle, R, Canty, P, and Dickman, CR (2018). Dynamics, habitat use and extinction risk of a carnivorous desert marsupial. Journal of Zoology 306, 258–267.
| Dynamics, habitat use and extinction risk of a carnivorous desert marsupial.Crossref | GoogleScholarGoogle Scholar |
Kraaij, T, and Milton, SJ (2006). Vegetation changes (1995–2004) in semi-arid Karoo shrubland, South Africa: effects of rainfall, wild herbivores and change in land use. Journal of Arid Environments 64, 174–192.
| Vegetation changes (1995–2004) in semi-arid Karoo shrubland, South Africa: effects of rainfall, wild herbivores and change in land use.Crossref | GoogleScholarGoogle Scholar |
Letnic, M, and Dickman, CR (2006). Boom means bust: interactions between the El Niño/Southern Oscillation (ENSO), rainfall and the processes threatening mammal species in arid Australia. Biodiversity & Conservation 15, 3847–3880.
| Boom means bust: interactions between the El Niño/Southern Oscillation (ENSO), rainfall and the processes threatening mammal species in arid Australia.Crossref | GoogleScholarGoogle Scholar |
Lim L (1992) Recovery Plan for the Kowari Dasyuroides byrnei Spencer, 1896 (Marsupialia: Dasyuridae). a report submitted to the Australian National Parks and Wildlife Service Endangered Species Programme Cremorne, NSW.
Lim L (1993) Kowari project 1993/4 methodologies and data bases. Unpub. 1st progress report. (DEH: Brisbane, Qld, Australia)
Lim L (1998) Kowari – technical background information. Final report research phase, Project No. 186. Department of Environment and Heritage, Brisbane, Qld, Australia.
Lim L (2008) Kowari Dasyuroides byrnei Spencer, 1896. In ‘The Mammals of Australia’. 3rd edn. (Eds S Van Dyck, R Strahan) p. 52. (Reed Books: Chatswood, NSW, Australia)
Maxwell S, Burbidge A, Morris K (1996) (Eds) ‘The 1996 action plan for Australian marsupials and monotremes’. (Wildlife Australia: Canberra, ACT, Australia)
McRae PD (2004) Aspects of the ecology of the greater bilby, Macrotis lagotis, in Queensland. Master’s thesis, University of Sydney, Sydney, NSW, Australia.
Mitchell C (2022) Long-term vegetation data collected from Diamantina National Park before and after gazettal. (Queensland Department of Environment and Science: Longreach, Qld, Australia)
Morton, SR (1990). The impact of European settlement on the vertebrate animals of arid Australia: a conceptual model. Proceedings of the Ecological Society of Australia 16, 201–213.
Moseby, KE, Crowther, MS, and Letnic, M (2019). Ecological role of an apex predator revealed by a reintroduction experiment and Bayesian statistics. Ecosystems 22, 283–295.
| Ecological role of an apex predator revealed by a reintroduction experiment and Bayesian statistics.Crossref | GoogleScholarGoogle Scholar |
Moseby, K, Hodgens, P, Bannister, H, Mooney, P, Brandle, R, Lynch, C, Young, C, Jansen, J, and Jensen, M (2021). The ecological costs and benefits of a feral cat poison-baiting programme for protection of reintroduced populations of the western quoll and brushtail possum. Austral Ecology 46, 1366–1382.
| The ecological costs and benefits of a feral cat poison-baiting programme for protection of reintroduced populations of the western quoll and brushtail possum.Crossref | GoogleScholarGoogle Scholar |
Murphy, SA, Paltridge, R, Silcock, J, Murphy, R, Kutt, AS, and Read, J (2018). Understanding and managing the threats to night parrots in south-western Queensland. Emu - Austral Ornithology 118, 135–145.
| Understanding and managing the threats to night parrots in south-western Queensland.Crossref | GoogleScholarGoogle Scholar |
Neilly, H, and Schwarzkopf, L (2018). Heavy livestock grazing negatively impacts a marsupial ecosystem engineer. Journal of Zoology 305, 35–42.
| Heavy livestock grazing negatively impacts a marsupial ecosystem engineer.Crossref | GoogleScholarGoogle Scholar |
Neilly, H, Vanderwal, J, and Schwarzkopf, L (2016). Balancing biodiversity and food production: a better understanding of wildlife response to grazing will inform off-reserve conservation on rangelands. Rangeland Ecology & Management 69, 430–436.
| Balancing biodiversity and food production: a better understanding of wildlife response to grazing will inform off-reserve conservation on rangelands.Crossref | GoogleScholarGoogle Scholar |
Neilly, H, Ward, M, and Cale, P (2021). Converting rangelands to reserves: small mammal and reptile responses 24 years after domestic livestock grazing removal. Austral Ecology 46, 1112–1124.
| Converting rangelands to reserves: small mammal and reptile responses 24 years after domestic livestock grazing removal.Crossref | GoogleScholarGoogle Scholar |
Orr DM (1986) Factors affecting the vegetation dynamics of Astrebla grasslands. Ph.D. Thesis, University of Queensland, Qld, Australia.
Page MJ (2001) Dynamics and management of vegetation within the Mulga Lands biogeographic region of south-western Queensland. (School of Natural and Rural Systems Management, University of Queensland Gatton, Qld, Australia)
Palmer R (1999) The ecology of feral cats in the mid-reaches of the Diamantina River in far western Queensland. (University of Queensland)
Pedler, RD, Brandle, R, Read, JL, Southgate, R, Bird, P, and Moseby, KE (2016). Rabbit biocontrol and landscape-scale recovery of threatened desert mammals. Conservation Biology 30, 774–782.
| Rabbit biocontrol and landscape-scale recovery of threatened desert mammals.Crossref | GoogleScholarGoogle Scholar |
Piazza, M-V, Oñatibia, GR, Aguiar, MR, and Chaneton, EJ (2021). Long-term impact of domestic ungulates versus the local controls of the litter decomposition process in arid steppes. Plant and Soil 467, 483–497.
| Long-term impact of domestic ungulates versus the local controls of the litter decomposition process in arid steppes.Crossref | GoogleScholarGoogle Scholar |
Read, JL (1999). The initial response of a chenopod shrubland plant and invertebrate community to two pulses of intensive cattle grazing. The Rangeland Journal 21, 169–193.
| The initial response of a chenopod shrubland plant and invertebrate community to two pulses of intensive cattle grazing.Crossref | GoogleScholarGoogle Scholar |
Read, JL (2002). Experimental trial of Australian arid zone reptiles as early warning indicators of overgrazing by cattle. Austral Ecology 27, 55–66.
| Experimental trial of Australian arid zone reptiles as early warning indicators of overgrazing by cattle.Crossref | GoogleScholarGoogle Scholar |
Read, JL, and Cunningham, R (2010). Relative impacts of cattle grazing and feral animals on an Australian arid zone reptile and small mammal assemblage. Austral Ecology 35, 314–324.
| Relative impacts of cattle grazing and feral animals on an Australian arid zone reptile and small mammal assemblage.Crossref | GoogleScholarGoogle Scholar |
Rich M, Nolan B, Gentle M, Speed J (2014) Lessons in feral cat control. Can adaptive management provide the solution? A case study from Astrebla Downs National Park, western Queensland. In ‘16th Australasian Vertebrate Pest Conference’. (Ed. M Gentle) p. 43. (Queensland Department of Agriculture and Fisheries: Brisbane, Qld, Australia)
Sattler, PS (2014). Five million hectares: an historical account of the expansion of Queensland’s national parks, 1975-2000. The Proceedings of the Royal Society of Queensland 119, 53–62.
Schnabel, ZE (1938). The estimation of total fish populations of a lake. The American Mathematical Monthly 45, 348–352.
| The estimation of total fish populations of a lake.Crossref | GoogleScholarGoogle Scholar |
Sharp, A, and McCallum, H (2010). The decline of a large yellow-footed rock-wallaby (Petrogale xanthopus) colony following a pulse of resource abundance. Australian Mammalogy 32, 99–107.
| The decline of a large yellow-footed rock-wallaby (Petrogale xanthopus) colony following a pulse of resource abundance.Crossref | GoogleScholarGoogle Scholar |
Silcock, JL, and Fensham, RJ (2013). Arid vegetation in disequilibrium with livestock grazing: evidence from long-term exclosures. Austral Ecology 38, 57–65.
| Arid vegetation in disequilibrium with livestock grazing: evidence from long-term exclosures.Crossref | GoogleScholarGoogle Scholar |
Silcock, JL, and Fensham, RJ (2019). Degraded or just dusty? Examining ecological change in arid lands. BioScience 69, 508–522.
| Degraded or just dusty? Examining ecological change in arid lands.Crossref | GoogleScholarGoogle Scholar |
Silcock, JL, Fairfax, RJ, and Fensham, RJ (2019). Feral fuchsia eating: long-term decline of a palatable shrub in grazed rangelands. Journal of Arid Environments 163, 1–8.
| Feral fuchsia eating: long-term decline of a palatable shrub in grazed rangelands.Crossref | GoogleScholarGoogle Scholar |
Stobo-Wilson, AM, Stokeld, D, Einoder, LD, Davies, HF, Fisher, A, Hill, BM, Mahney, T, Murphy, BP, Scroggie, MP, Stevens, A, Woinarski, JCZ, Bawinanga Rangers Warddeken Rangers Gillespie, GR (2020). Bottom-up and top-down processes influence contemporary patterns of mammal species richness in Australia’s monsoonal tropics. Biological Conservation 247, 108638.
| Bottom-up and top-down processes influence contemporary patterns of mammal species richness in Australia’s monsoonal tropics.Crossref | GoogleScholarGoogle Scholar |
Wallach, AD, and O’Neill, AJ (2009). Threatened species indicate hot-spots of top-down regulation. Animal Biodiversity and Conservation 32, 127–133.
| Threatened species indicate hot-spots of top-down regulation.Crossref | GoogleScholarGoogle Scholar |
Wijas, B, and Letnic, M (2021). Top-down effects have primacy over bottom-up effects on the population dynamics of a flightless desert bird. Journal of Arid Environments 195, 104611.
| Top-down effects have primacy over bottom-up effects on the population dynamics of a flightless desert bird.Crossref | GoogleScholarGoogle Scholar |
Wilson B, Mitchell C (1992) Conservation strategy – channel country biogeographic region. Unpublished report. (Queensland Department of Environment and Heritage: Brisbane, Qld, Australia)
Woinarski JCZ, Burbidge AA, Harrison PL (2014) ‘The Action Plan for Australian Mammals 2012.’ (CSIRO Publishing: Melbourne, Vic., Australia)
Woolley, L-A, Geyle, HM, Murphy, BP, Legge, SM, Palmer, R, Dickman, CR, Augusteyn, J, Comer, S, Doherty, TS, Eager, C, Edwards, G, Harley, DKP, Leiper, I, McDonald, PJ, McGregor, HW, Moseby, KE, Myers, C, Read, JL, Riley, J, Stokeld, D, Turpin, JM, and Woinarski, JCZ (2019). Introduced cats Felis catus eating a continental fauna: inventory and traits of Australian mammal species killed. Mammal Review 49, 354–368.
| Introduced cats Felis catus eating a continental fauna: inventory and traits of Australian mammal species killed.Crossref | GoogleScholarGoogle Scholar |
Appendix 1. The vegetation monitoring
Species composition, frequency and dominance
Ten 1 m × 1 m quadrats placed 5 m apart along the 50 m transect were used to assess understorey species composition. To avoid trampling, the first quadrat was placed at the 5 m point. The cover of each species present in the quadrat was recorded using the technique of Daubenmire (1959). A species was deemed to be present in a quadrat if it was rooted in the quadrat. A species was considered to be part of the understorey if it was herbaceous or was a woody plant no taller than 50 cm. Roots belonging to trees taller than a half a metre were classed as litter.
Foliage projective cover (FPC) of the understorey and soil disturbance
The point intercept technique at half metre intervals along each transect was used to assess FPC. The ‘units’ of cover were bare ground (separated into disturbed or not), rock, cryptogram crust, litter (detached organic material and dead organic material attached to a dead plant) and vegetation (including alive or dead material providing it is still attached to an alive plant).
Biomass
The dry weight of all alive and dead organic matter, up to a diameter of 5 mm, was collected, dried and weighed from five 50 cm × 50 cm quadrats. The location of each quadrat was random within an area of between 5 and 30 m from the transect line. Each sample was dried in the oven for 48 h at 70°C.

|
Appendix 2. Kowari habitat search spotlight or thermal camera transects 2007–2021 (Ironstone habitat only). No surveys were conducted in 2016 or 2019

|
Appendix 3. Kowari predator/prey spotlight transects, 2007–2021. No surveys were conducted in 2016 or 2019

|
Appendix 4. Kowari trapping grid locations and the start and end point for each grid line in April 2007 and 2008

|
Appendix 5. Kowari search trapping sites, April and September 2007

|
Appendix 6. The number of individual Kowaris caught per survey for 2007–2012
|
Appendix 7. Kowari numbers recorded during line transect surveys at the nearby Astrebla Downs National Park 2012–2021 (unpublished QPWS data)

|


