Microhabitat selection by small mammals in response to fire
Dylan M. Lees A * , Darcy J. Watchorn A , Don A. Driscoll A and Tim S. Doherty A B
A * , Darcy J. Watchorn A , Don A. Driscoll A and Tim S. Doherty A B
A Centre for Integrative Ecology, School of Life and Environmental Sciences (Burwood Campus), Deakin University, Burwood, Vic. 3125, Australia.
B School of Life and Environmental Sciences, University of Sydney, Sydney, NSW 2006, Australia.
Australian Journal of Zoology 69(3) 67-79 https://doi.org/10.1071/ZO21022
Submitted: 17 June 2021 Accepted: 16 December 2021 Published: 11 February 2022
© 2021 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Understanding how fire influences animal behaviour, such as movement and resource selection, is important for ecosystem management because it can improve our capacity to predict how species will respond. We assessed microhabitat selection by two small mammals, the bush rat (Rattus fuscipes) and agile antechinus (Antechinus agilis), in response to a low intensity prescribed fire. We used spool and line tracking and touch pole vegetation surveys to quantify microhabitat selection along 21 trails for bush rats and 22 for antechinuses before and after fire. In unburnt areas, bush rats showed positive selection for sedges, logs, and habitat complexity, with selection further increasing in burnt areas for sedges, ferns, shrubs, habitat complexity and unburnt patches. Agile antechinuses showed no significant microhabitat selection in unburnt or burnt areas and no change in response to fire. Their lack of response to ground fires may be due, partially, to their scansorial behaviour and use of tree hollows as refuge sites. Strong selection by bush rats for small unburnt patches suggests that even low intensity, patchy fires such as planned burns can impact bush rats and that high burn patchiness may help bush rats persist in recently burnt areas. Future fire planning should consider both behavioural and population responses of animals to fire.
Keywords: bushfire, dasyurid, disturbance, fire ecology, habitat use, megafire, planned burn, prescribed burn, resource selection, rodent, wildfire.
Introduction
Fire plays a major role in shaping ecosystems across the world, especially so as fire regimes are disrupted by human influences (He et al. 2019). With human-induced climate change and land-use practices (e.g. Lindenmayer et al. 2020) leading to a heightened risk of wildfire globally (Pastro et al. 2011; Jolly et al. 2015), prescribed burning is increasingly used as a hazard reduction tool to protect ecological values, human lives, and infrastructure (Moritz et al. 2014). Whilst prescribed burning is now a common management tool across the globe (Fernandes and Botelho 2003; Attiwill and Adams 2013; Clarke et al. 2019), the practice may contribute to inappropriate local fire regimes (Driscoll et al. 2010) and increase the potential for negative impacts on animal species. Immediate and short-term effects of fire may include increased mortality, altered habitat suitability, reduced resource availability, increased predation or competition, or disruption of demographic processes often leading to reduced fitness and survival (Sutherland and Dickman 1999; Whelan et al. 2002; Conlisk et al. 2015; Nimmo et al. 2019).
Fire impacts vegetation composition and microhabitat availability by reducing cover and structural complexity, which in turn can alter the habitat available for animals, leading to changes in abundance and species richness (Griffiths and Brook 2014; Nimmo et al. 2019). Such effects have been recorded for a range of different taxa, including birds, reptiles and small mammals, amongst others (Woinarski et al. 2004; Smucker et al. 2005; Green and Sanecki 2006; Fontaine et al. 2009; Nimmo et al. 2014). For example, in Australia, lizards were found to alter their movement patterns in search of shelter after fire (Driscoll et al. 2012), and the occurrence of feral cats (Felis catus) and foxes (Vulpes vulpes) increased after a prescribed burn as they took advantage of more favourable hunting conditions (Hradsky et al. 2017). A related study also found decreased body condition of bush rats (Rattus fuscipes) in burnt compared to unburnt areas, possibly related to reduced access to resources after fire (Fordyce et al. 2016).
Small mammals and their responses to fire have been the subject of extensive research, but the mechanisms driving changes in behaviour in burnt and unburnt habitat (e.g. predation, immigration and competition) are still relatively poorly understood (Sutherland and Dickman 1999; Griffiths and Brook 2014). Depending on their life histories and habitat requirements, small mammals exhibit a range of behavioural strategies to persist in fire-prone landscapes. Several species change their habitat use and movements to avoid or survive fires. For example, the short-snouted elephant shrew in South Africa (Elephantulus brachyrhynchus) shifted its habitat use from grasslands pre-fire to shrub thickets post-fire (Yarnell et al. 2008). In Australia, the brown antechinus (Antechinus stuartii) and yellow-footed antechinus (Antechinus flavipes) increased periods of torpor and decreased foraging times in recently burnt areas (Stawski et al. 2015; Matthews et al. 2017), while bush rats exhibited sharper turning angles in small unburnt patches, suggesting they prefer to avoid burnt areas (Fordyce et al. 2016). For some species though, fire is important for ongoing persistence. The eastern chestnut mouse (Pseudomys gracilicaudatus) is a post-fire specialist and disperses willingly through burned vegetation (Pereoglou et al. 2013). These examples highlight the value of understanding species’ habitat use for fire management and wildlife conservation by, for example, highlighting when patchy burns or habitat-specific burns may aid persistence.
Fire is common in south-eastern Australia and this region recently experienced its worst bushfire season on record (Boer et al. 2020), with fire regimes expected to continue intensifying under both a warming and drying climate (King et al. 2013; Bradstock et al. 2014) and increased rates of prescribed burning (Gazzard et al. 2020; Russell-Smith et al. 2020). Our study aimed to assess how a low intensity, prescribed fire affected microhabitat selection by two small mammal species in south-eastern Australia: the agile antechinus (Antechinus agilis) and the bush rat. While microhabitat use by these two species is relatively well studied (see below paragraph), little attention has been paid to the effects of fire on microhabitat use (but see Fordyce et al. 2016; Banks et al. 2017). We measured microhabitat use before and after a prescribed burn in the eastern Otway Ranges of Victoria to address three key questions:
-
Does each species exhibit microhabitat selection in unburnt areas?
-
Does each species exhibit microhabitat selection in burnt areas?
-
Does microhabitat selection by the agile antechinus change in response to fire?
The third question was restricted to the antechinus due to sample size limitations for the bush rat (see Methods). Based on previous research, we hypothesised that the bush rat would preferentially select for habitat attributes associated with dense understorey cover in unburnt areas, and that the preference for these attributes would increase at recently burnt sites (Catling 1991; Fordyce et al. 2016). We also hypothesised that the agile antechinus would display positive selection for certain microhabitat attributes (e.g. logs, complex vegetation) in both pre- and post-fire environments (Sutherland and Predavec 1999; Johnstone et al. 2011; Swan et al. 2016).
Materials and methods
Study area
This study was carried out between February and August 2019 in the Great Otway National Park, Vic., south-eastern Australia, approximately 5 km north of Aireys Inlet (38.415°S, 144.094°E; Fig. 1). The area has a temperate climate with a mean maximum temperature of 18.4°C and mean annual rainfall of 628 mm (Bureau of Meteorology 2019). The vegetation in the study area is primarily comprised of heathy woodland and open forest. The dominant plant species include messmate (Eucalyptus obliqua) and brown stringybark (Eucalyptus baxteri) in the canopy, with the mid-storey and ground cover dominated by prickly tea tree (Leptospermum continentale), twisted bearded heath (Leucopogon glacialis), myrtle wattle (Acacia myrtifolia), thatch saw sedge (Gahnia radula), austral grass tree (Xanthorrhoea australis), bracken fern (Pteridium esculentum), and grey tussock-grass (Poa sieberiana hirtella). The study area is subjected to prescribed burning for both hazard reduction and ecological values by creating a mosaic of different fire classes, and the last major bushfire in the area was the 1983 Ash Wednesday fire (Gazzard et al. 2020).
Study species
The bush rat is a small (40–225 g) nocturnal species, with an omnivorous diet of seeds, fungi and invertebrates (Carron et al. 1990). It is primarily found in coastal and temperate areas of southern and eastern Australia and prefers dense understory vegetation (Dickman and Woodside 1983). The agile antechinus is a small (16–40 g) cathemeral dasyurid marsupial, with a diet of invertebrates, small vertebrates, and sometimes plant material (Goldingay 2000; Parrott et al. 2007). It is primarily found in wet forest ecosystems of south-eastern Australia (Van Dyck et al. 2013). It is semi-arboreal, with a semelparous breeding strategy whereby most males die shortly after breeding (Parrott et al. 2007; Swan et al. 2016).
Experimental design
The prescribed burn took place in mid-May 2019 to varying degrees of severity, resulting in a mosaic of unburnt and burnt patches throughout the study site (Figs 1 and 2). We collected pre-fire data at five sites between February and April 2019 and post-fire data at seven sites (four burnt, three unburnt) between June and August 2019. We initially intended to implement a full before–after, control–impact design, but due to the patchy nature of the fire, only two of the five sites surveyed pre-fire were adequately burnt, so an additional two burnt sites were added post-fire. Sites were 1 ha in size and each separated by 0.5–1.3 km. To maximise our chances of catching sufficient numbers of the target species, sites with a moderate slope and densely vegetated gullies were chosen (Bennett 1993; Sutherland and Predavec 1999; Claridge et al. 2008).
|
|
Animal trapping and handling
All procedures involving animals were approved by the Deakin University Animal Ethics Committee (project B26-2018). Animals were caught using Type A Elliott traps (9 × 10 × 33 cm, Elliott Scientific, Upwey, Australia). Each site comprised 30 trap stations positioned in three parallel lines running downslope into the gully, with each line separated by 30 m and traps within lines separated by 15 m. Traps were baited with a mixture of rolled oats, peanut butter and honey. Two to four sites were operated on any given night, each with 20–30 traps active. We operated control and impact sites concurrently to reduce any bias in data collection. Traps were opened each afternoon before 1700 hours and subsequently checked between 2200 and 0300 hours each night. We captured and tracked animals within this period since this is when they are most active (Van Dyck et al. 2013). We recorded the sex, weight and pes length of all animals and marked antechinuses with a unique ear punch and bush rats with a metal ear tag.
Spool-and-line tracking
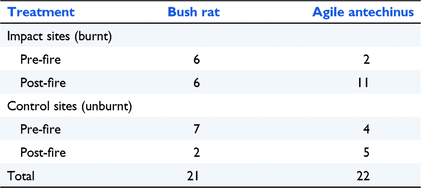
The spools used to track the animals consisted of ∼5 g of nylon thread sourced from Danfield Ltd in Lancashire, UK (thread size number metric 120/2, size 10 cocoon bobbin). To ensure that spools weighed less than 5% of animal body weight, we reduced spool lengths by removing excess thread. We enclosed spools in heat shrink and final package weights were 1–1.5 g for antechinus and 2.5–4.5 g for bush rats. We attached spools to the lower back of animals using a small amount of cyanoacrylate glue, holding it in place for approximately 30 s. We then tied the loose end of the thread to the base of a shrub at the point of capture. Spools were attached to the animals’ fur and spool packages were expected to detach upon reaching the end of the thread or naturally shed soon thereafter. We then released the animal, allowing it to resume normal activities while leaving a trail indicating the microhabitat it used. We followed each spool line the next day and recorded microhabitat attributes along the lines. We recorded data from 43 animal trails (Table 1), with three individuals being spooled twice post-fire, but in different sessions (two antechinus and one bush rat). Some glue and minor fur loss was visible on animals we retrapped in the same session. No animals were spooled more than once in the same session, with a ‘session’ indicating a 1-to-2-week period of trapping with approximately 3–4 weeks between each session.
|
Measuring used and available habitat
We followed each spool line to its end. The length of the lines varied between 20 and 100 m, with bush rat trails typically longer than those of agile antechinuses. Similar to previous studies (e.g. Molyneux et al. 2017), we established one random straight line of the same length for each corresponding animal trail. The random lines began at the same starting point, but the direction was randomised using a mobile phone app (Spinner App). Habitat was quantified along both used and available (random) lines every 4 m for bush rats and 2 m for antechinuses. We chose a shorter interval for antechinus because their spools were shorter than those of bush rats. The initial 10 m of each line for both species was excluded from measurement to avoid any potential bias from unnatural movements due to a flight response (Kearney et al. 2007; Fordyce et al. 2016). On several occasions, the spool detached within the first 10 m, therefore precluding any measurements. All lines longer than 10 m and long enough for the above-mentioned sampling distances irrespective of distance were recorded.
Vegetation density was measured vertically at each point along the line using the touch pole method (Elzinga et al. 2007), with a 2 m high pole segregated into six height brackets (0–25, 26–50, 51–75, 76–100, 101–150, and 151–200 cm). We recorded the following functional plant groups as present at each height category if they were touching the pole: dead material (e.g. dead branches, shrubs), sedge, fern, spreading grass, tussock grass, grass tree, tree, log, forb, and creeper. At the impact sites post-fire, we also recorded the presence of burnt plant material at each height category, which refers to any plant with only burnt stems and branches remaining. Care was taken to avoid trampling vegetation directly along the lines so as not to affect measurements on the touch pole.
We used the quadrant cover method (Glen et al. 2010) to measure the presence or absence of log cover at each point, with a score of 1 applied to each of the four quadrants within a radius of either 1 m (bush rat) or 50 cm (agile antechinus) providing a score of between 0 (no logs) and 4 (present in all quadrants) (Fordyce et al. 2016). We used a different radius for each species to reflect their differing sizes. As per Fordyce et al. (2016), the minimum measurements for defining a log were 50 cm in length and 10 cm in diameter. To assess litter cover, the same circular radius measurements were used and a scale of 0–4 applied corresponding to percentage cover (0 = 0%, 1 = 1–25%, 2 = 26–50%, 3 = 51–75%, 4 = 76–100%; Fordyce et al. 2016).
Statistical analysis
The final list of habitat attributes was narrowed down to nine variables most appropriate for determining microhabitat selection of the focal species, while those that were rejected contained insufficient data to provide any meaningful additional insight. Seven of the included variables were calculated as the proportion of sampling points on a line where a plant functional group was present within a specific height category. The variables were: dead material 0–50 cm, sedge 0–100 cm, fern 0–100 cm, shrub 0–200 cm, grass tree 0–100 cm, spreading grass 0–50 cm, and log 0–50 cm. These categories encompassed multiple height brackets, as outlined above, in order to best represent the heights that each variable was present at. We also generated a habitat complexity score by taking the average number of functional plant groups at each height category at individual sampling points and then calculating the mean across all points along each line (Fordyce et al. 2016). Finally, because the impact sites contained a mosaic of burnt and unburnt vegetation post-fire, we calculated the proportion of points along each line that were located in unburnt vegetation (‘unburnt patches’). The chosen variables had low auto-correlation (Pearson’s r < ±0.5), except for the habitat complexity score, which had moderate correlation (r = 0.56–0.73) with dead material, sedge and unburnt patches; and unburnt patches with sedge and shrub (r = 0.56–0.59). We take these correlations into consideration when interpreting the results.
We used logistic regression models (logit link function) with Firth’s bias reduction method to assess microhabitat selection, implemented in the logistf R package (Heinze et al. 2018). The response variable indicated whether the microhabitat variables were from used (1) or available (0) trails. We used Firth’s bias reduction to counter the effects of complete or quasi-complete separation which can result in infinite or very large parameter estimates and confidence intervals that are not informative (Albert and Anderson 1984; Firth 1993). It was not possible to include random effects in the bias-corrected models, which in this case may have been appropriate given the paired nature of the data (i.e. paired used and available trails). However, we initially fitted generalised linear mixed models that included a random effect of trail identity, and the variance component was 0 in every case, which indicates that the inclusion of a random effect is not necessary. We did not consider it necessary or feasible to include a random effect representing individual animals because only three individuals were tracked more than once.
Ideally the data would have been analysed using two crossed factors of time and treatment. However, this was not possible due to low numbers of animals tracked for some treatment combinations (Table 1). We instead analysed the pre-fire and post-fire data separately for both species. We first analysed the pre-fire data (pooled for impact and control sites) to answer Question 1: Does each species exhibit microhabitat selection in unburnt areas? We then analysed the data from only the burnt sites post-fire to answer Question 2: Does each species exhibit microhabitat selection in burnt areas? The predictor variables in each case were the nine microhabitat variables described above. We only included one variable per model because the small dataset precluded fitting more complex models. In the context of this study, positive parameter estimates from the logistic regression models indicate that average values of a microhabitat variable were higher on used compared to available trails, and the opposite for negative estimates. When 95% confidence intervals did not overlap zero, we inferred selection for or against individual variables and present plots of the modelled relationships.
For the agile antechinus, we were also able to pool the pre-fire data for the control and impact sites (to create an adequate sample size), such that there were three treatments: unburnt pre-fire, burnt post-fire and unburnt post-fire. This allowed us to answer Question 3: Does agile antechinus microhabitat selection change in response to fire? By including unburnt post-fire sites, we control for any possible temporal changes independent of fire. This was not possible for the bush rat (due to there only being two unburnt trails sampled post-fire), however, we are confident that our results accurately reflect the effects of fire because they strongly align with previous research (e.g. Banks et al. 2011; Fordyce et al. 2016). The models for question 3 were fitted in the same way as for questions 1 and 2 (see above), except that an interaction between the microhabitat variable and treatment was included. We specified ‘burnt post-fire’ as the reference level in the model, which allows us to determine whether selection/avoidance of a microhabitat variable at unburnt sites pre- and post-fire differs from that at burnt sites. We present parameter estimates and 95% confidence intervals to make these inferences.
Results
Across all sites and treatments, we measured 21 trails for the bush rat and 22 for the agile antechinus, with average trail length being 57.81 m (5.32 s.e.) and 43.36 m (3.32 s.e.), respectively. Both pre- and post-fire, bush rats predominantly passed through dense understory vegetation and along and under logs within a well-established network of tunnels created by a thick matting of sedges, ferns, and to a lesser extent, grass trees. The tunnels and pathways appeared to be used by more than one individual both within and between species. Agile antechinuses, on the other hand, frequented areas where the understory was more open and quite often spool lines traversed logs or scaled trees.
Question 1: Microhabitat selection in unburnt areas
Bush rats exhibited significant selection for three of the eight microhabitat variables analysed: sedges, logs, and habitat complexity (Table 2, Fig. 3). Positive trends were also observed for spreading grass, dead material, ferns and negative trends for shrubs and grass trees, although the confidence intervals overlapped zero (Table 2, Fig. 3). Agile antechinuses displayed no significant preferential selection for any of the habitat attributes, although there were non-significant positive trends for logs and habitat complexity (Table 2, Fig. 3).

|
Question 2: Post-fire microhabitat selection in burnt areas
Bush rats exhibited selection for a higher number of variables post-fire. There was a significant positive selection for sedges, ferns, shrubs, habitat complexity, and unburnt patches (Table 2, Fig. 4). Although there was no evidence of significant preferential selection by agile antechinuses in burnt areas, dead material, sedges, and unburnt patches had confidence intervals that only marginally included zero (−0.85, 8.63; −0.96, 5.41; −0.74, 7.50, respectively), thus suggesting a possible weak positive association with those variables (Table 2, Fig. 4).
Question 3: Changes in antechinus habitat selection in response to fire
Similar to the separate pre- and post-fire analyses for the agile antechinus, there were no significant relationships when comparing microhabitat selection in burnt areas to either unburnt areas pre-fire or unburnt areas post-fire (Table 3). All variables had wide confidence intervals that included zero (Table 3).

|
Discussion
Fire can have strong impacts on vegetation structure by reducing understorey and ground cover, but not all mammal species find this change disadvantageous (Monamy and Fox 2000; Letnic 2003; Torre and Díaz 2004; Lindenmayer et al. 2008; Kelly et al. 2010; Zwolak et al. 2012; Doherty et al. 2015; Sharp Bowman et al. 2017). We found that bush rats, as predicted, demonstrated clear selection for dense understorey vegetation, with stronger habitat selection exhibited after fire. We found no evidence for selection of specific microhabitat features by agile antechinuses, nor changes in habitat use in response to fire.
Pre-fire microhabitat use
In unburnt areas pre-fire, bush rats displayed non-random movement, with preferences for sedges, habitat complexity and logs (acknowledging that the first two were moderately correlated with each other). This is consistent with an earlier study that found positive selection for logs, rushes and complex habitat (Fordyce et al. 2016). Other studies have also made similar findings, with Spencer et al. (2005) highlighting the importance of complex and dense vegetation, and Strauß et al. (2008) and Maitz and Dickman (2001) identifying logs and rushes as important habitat components, respectively. These have been suggested as key habitat features used by the bush rat for foraging, nesting, and predator avoidance (Strauß et al. 2008; Fordyce et al. 2016). Many other small mammals depend on similar features, including the swamp rat (Rattus lutreolus; Kearney et al. 2007), common vole (Microtus arvalis; Jacob and Brown 2000) and oldfield mouse (Peromyscus polionotus; Orrock et al. 2004).
In contrast to the bush rat, there was no evidence of selection for specific habitat features by the agile antechinus in unburnt areas, although there were positive trends for logs and complex habitat. Previous studies on agile antechinus microhabitat have revealed conflicting results, in particular regarding vegetation density. Some have pointed to positive selection for dense low cover (Bennett 1993; Sutherland and Predavec 1999), or association with sparse but structurally complex vegetation (Moro 1991), whilst others found that microhabitat use was proportional to availability (Wilson et al. 1986). These differing results across studies may be attributable to site specific differences in vegetation composition and structure, variable behaviour by the agile antechinus across its range, or differences in methodology. Previous studies were based on trapping data (Moro 1991; Bennett 1993; Wilson et al. 1986), or both trapping data and fluorescent pigment tracking (Sutherland and Predavec 1999). We may not have detected selection for specific microhabitat components by the agile antechinus if the trails were too short to adequately capture foraging and sheltering behaviour, with mean trail length being 43 m. However, fluorescent pigment tracking does not seem to be a suitable alternative given that pigment trails are of a similar length or shorter than the average spool length in our study. For example, the average pigment trail length was 26 m for Sminthopsis youngsoni (Haythornthwaite 2005), 32 m for A. flavipes and 46 m for S. murina (Stokes et al. 2004), which are of broadly similar size to A. agilis. Automated high frequency radio-tracking (e.g. Wallace et al. 2021) may be the only suitable alternative for high resolution tracking of this species over longer periods until miniature GPS tags (∼1 g) become widely available and more affordable.
Post-fire microhabitat use
Following the prescribed burn, bush rats exhibited significant selection for an additional three microhabitat features compared to pre-fire: ferns, shrubs and unburnt patches (the latter being moderately correlated with sedges and shrubs). Fordyce et al. (2016) also noted a strengthening of bush rat microhabitat selection in post-fire environments, although they also observed increased selection for spreading grass, which was not common at our study sites. Reasons for these observed increases could include loss of food and shelter due to a reduction in understory vegetation from the burn and a resultant increase in perceived or actual predation risk. Indeed, it has been noted that small mammals succumb primarily to predation or starvation rather than fire itself (Thompson et al. 1989; Hale et al. 2021), as evidenced in studies on hispid cotton rats (Sigmodon hispidus) in North America (Conner et al. 2011; Morris et al. 2011). In northern Australia, predation rates on rodents were greater in burnt compared to unburnt habitat (Leahy et al. 2016) and feral cats were attracted to recently burnt areas (McGregor et al. 2016), likely due to the increased hunting success they experience in simplified vegetation (McGregor et al. 2014). However, the relative importance of changes in food availability, shelter and predation post-fire is poorly resolved and ideally would be assessed using further manipulative experiments in a range of environments.
Of particular note in this study was the strong selection for unburnt patches post-fire, coupled with an increase in the number of attributes compared to pre-fire. As a result, we suggest that the additional microhabitat features used by bush rats post-fire in our study may be a method of seeking refuge to decrease the risk of predation. No bush rats were recorded moving in burnt patches, despite their ready availability at impact sites. Other studies have noted this preferential selection for unburnt patches in other small mammals, including the yellow-footed antechinus A. flavipes in the jarrah forest of Western Australia, which preferentially used unburnt grass trees (Swinburn et al. 2007), the eastern chestnut mouse in New South Wales, which selected for tall and dense vegetation (Pereoglou et al. 2011), and populations of small mammals more generally (e.g. Lunney et al. 2008). In this regard, unburnt patches can allow bush rat populations to persist within a burnt landscape and re-establish over time (Lunney et al. 2008; Robinson et al. 2013; Banks et al. 2017).
In a similar trend to its pre-fire microhabitat use, agile antechinuses showed no significant preference for any of the habitat attributes analysed. There were, however, positive increases across all variables tested, except for shrubs. This could indicate a weak preference for unburnt patches within burnt locations, similar to that found for the yellow-footed antechinus (Swinburn et al. 2007). In terms of changes in response to fire, selection for dead material and sedges increased, although the confidence intervals included zero. Overall, these findings are consistent with previous studies suggesting the agile antechinus is more resilient to low intensity fires than bush rats, which they may achieve by seeking refuge in tree hollows (Banks et al. 2011; Swan et al. 2016). Additionally, studies on the brown antechinus, A. stuartii, reported increased periods of torpor and reduced foraging activity for those in burnt patches compared to unburnt habitat (Stawski et al. 2016). This further suggests that the agile antechinus is able to employ alternative strategies to cope with fire and hence be less reliant on specific microhabitat features after fire, in contrast to the bush rat (although bush rats may occasionally go into torpor, Nowack et al. 2020).
Our results are consistent with previous studies suggesting bush rats have more specific microhabitat requirements than agile antechinuses and therefore could be more susceptible to the immediate and short-term effects of low-severity fire including planned burns. For example, Banks et al. (2011) reported a higher occurrence of agile antechinuses compared to bush rats in very recently burnt areas, most likely due to higher rates of in situ survival by antechinuses. Similarly, Swan et al. (2016), found that the ability of agile antechinuses to disperse and their flexibility in the use of available habitat better equipped them to survive the immediate impacts of a low intensity prescribed burn, compared to the bush rat’s greater reliance on dense understorey vegetation.
Additionally, body size could be an influential factor. A systematic review by Griffiths and Brook (2014) reported increased sensitivity to fire for mammals between 101 and 1000 g and with higher affinity with dense ground cover. This is pertinent since bush rats weigh up to 225 g and prefer dense ground vegetation, where agile antechinuses reach up to 45 g, prefer more open vegetation and are semi-arboreal. The agile antechinus also primarily feeds on invertebrates (Moro 1991; Lunney et al. 2001), which may be less limited after fire compared to plant matter and fungi, which are important to bush rats (Cheal 1987; Carron et al. 1990). Agile antechinuses also shelter in tree hollows, which could further mitigate predation risk in the short-term following fire, although high intensity burns may destroy some hollows. Loyn et al. (1986) found that sooty owls (Tyto tenebricosa) preyed solely on bush rats post-fire, even when arboreal mammals were available, presumably due to reduced cover and increased exposure of bush rats, whereas arboreal mammals likely sought shelter in tree hollows and benefitted from persistent canopy cover.
Conservation implications and future research
This study has provided further understanding of multi-species microhabitat use in response to fire. Future research could look to build on this by increasing sample sizes, potentially using more sophisticated tracking methods, and importantly, by considering a wider range of fire conditions. Although logistically challenging, this would further add to the body of knowledge regarding the effects of varying fire regimes on animal movement and behaviour, particularly higher severity fires. Furthermore, this study only took into account animal responses immediately following the fire (0–3 months). Examining successional changes over a longer period would enable the duration of impacts to be defined more precisely. For example, bush rat abundance would be expected to increase along with time-since-fire as understorey vegetation gradually recovers (Lunney and Ashby 1987), and this may also be reflected in changes in their microhabitat use.
Improved knowledge of small mammal responses to fire is particularly important as fire frequency and intensity is forecasted to increase in warming and drying climates (King et al. 2013; Bradstock et al. 2014). Small mammals respond to fire in varying ways as evidenced in this study, and in other ecosystems worldwide (Torre and Díaz 2004; Horn et al. 2012; Doherty et al. 2015; Sharp Bowman et al. 2017). Strong selection by bush rats for small unburnt patches within burnt areas shows that even low intensity, patchy fires such as planned burns, can substantially constrain their use of the landscape, and suggests that more complete or severe fires would be extremely detrimental. In contrast, low intensity planned burns apparently have comparatively little impact on the habitat use of the small, semi-arboreal agile antechinus, though higher severity fires are more likely to impact on the availability of tree hollows where they seek shelter. Unburnt refuges will therefore become increasingly important for many small mammal species to persist in fire-prone landscapes (Robinson et al. 2013). Future work should take into account multi-species responses and identify key habitat features for at-risk species to avoid implementing inappropriate fire regimes.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This study was funded by the Hermon Slade Foundation and Deakin University. T.S.D. was supported by an Alfred Deakin Post-doctoral Research Fellowship (Deakin University) and a Discovery Early Career Researcher Award (Australian Research Council, DE200100157).
Acknowledgements
We acknowledge the Wadawurrung people as the Traditional Owners of the land on which this research was conducted. We thank Parks Victoria for facilitating access to the study site, Barbara Wilson and Mark Garkaklis for their local knowledge, and volunteers for help with fieldwork. We thank three anonymous reviewers for their comments on an earlier version of the manuscript.
References
Albert, A, and Anderson, JA (1984). On the existence of maximum likelihood estimates in logistic regression models. Biometrika 71, 1–10.| On the existence of maximum likelihood estimates in logistic regression models.Crossref | GoogleScholarGoogle Scholar |
Attiwill, PM, and Adams, MA (2013). Mega-fires, inquiries and politics in the eucalypt forests of Victoria, south-eastern Australia. Forest Ecology and Management 294, 45–53.
| Mega-fires, inquiries and politics in the eucalypt forests of Victoria, south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Banks, SC, Dujardin, M, McBurney, L, Blair, D, Barker, M, and Lindenmayer, DB (2011). Starting points for small mammal population recovery after wildfire: recolonisation or residual populations? Oikos 120, 26–37.
| Starting points for small mammal population recovery after wildfire: recolonisation or residual populations?Crossref | GoogleScholarGoogle Scholar |
Banks, SC, McBurney, L, Blair, D, Davies, ID, and Lindenmayer, DB (2017). Where do animals come from during post-fire population recovery? Implications for ecological and genetic patterns in post-fire landscapes. Ecography 40, 1325–1338.
| Where do animals come from during post-fire population recovery? Implications for ecological and genetic patterns in post-fire landscapes.Crossref | GoogleScholarGoogle Scholar |
Bennett, AF (1993). Microhabitat use by the long-nosed potoroo, Potorous tridactylus, and other small mammals in remnant forest vegetation of south-western Victoria. Wildlife Research 20, 267–285.
| Microhabitat use by the long-nosed potoroo, Potorous tridactylus, and other small mammals in remnant forest vegetation of south-western Victoria.Crossref | GoogleScholarGoogle Scholar |
Boer, MM, Resco de Dios, V, and Bradstock, RA (2020). Unprecedented burn area of Australian mega forest fires. Nature Climate Change 10, 171–172.
| Unprecedented burn area of Australian mega forest fires.Crossref | GoogleScholarGoogle Scholar |
Bradstock, R, Penman, T, Boer, M, Price, O, and Clarke, H (2014). Divergent responses of fire to recent warming and drying across south-eastern Australia. Global Change Biology 20, 1412–1428.
| Divergent responses of fire to recent warming and drying across south-eastern Australia.Crossref | GoogleScholarGoogle Scholar | 24151212PubMed |
Bureau of Meteorology (2019) Climate data online. Available at: http://www.bom.gov.au/climate/data.
Carron, PL, Happold, DCD, and Bubela, TM (1990). Diet of 2 sympatric Australian sub-alpine rodents, Mastacomys fuscus and Rattus fuscipes. Wildlife Research 17, 479–489.
| Diet of 2 sympatric Australian sub-alpine rodents, Mastacomys fuscus and Rattus fuscipes.Crossref | GoogleScholarGoogle Scholar |
Catling PC (1991) Ecological effects of prescribed burning practices on the mammals of south-eastern Australia. In ‘Conservation of Australia’s Forest Fauna’. (Ed. D Lunney) pp. 353–363. (Royal Zoological Society of New South Wales: Sydney, NSW, Australia)
Cheal, DC (1987). The diets and dietary preferences of Rattus fuscipes and Rattus lutreolus at Walkerville in Victoria. Wildlife Research 14, 35–44.
| The diets and dietary preferences of Rattus fuscipes and Rattus lutreolus at Walkerville in Victoria.Crossref | GoogleScholarGoogle Scholar |
Claridge, AW, Tennant, P, Chick, R, and Barry, SC (2008). Factors influencing the occurrence of small ground-dwelling mammals in southeastern mainland Australia. Journal of Mammalogy 89, 916–923.
| Factors influencing the occurrence of small ground-dwelling mammals in southeastern mainland Australia.Crossref | GoogleScholarGoogle Scholar |
Clarke, H, Tran, B, Boer, MM, Price, O, Kenny, B, and Bradstock, R (2019). Climate change effects on the frequency, seasonality and interannual variability of suitable prescribed burning weather conditions in south-eastern Australia. Agricultural and Forest Meteorology 271, 148–157.
| Climate change effects on the frequency, seasonality and interannual variability of suitable prescribed burning weather conditions in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Conlisk, E, Syphard, AD, Franklin, J, and Regan, HM (2015). Predicting the impact of fire on a vulnerable multi-species community using a dynamic vegetation model. Ecological Modelling 301, 27–39.
| Predicting the impact of fire on a vulnerable multi-species community using a dynamic vegetation model.Crossref | GoogleScholarGoogle Scholar |
Conner, LM, Castleberry, SB, and Derrick, AM (2011). Effects of mesopredators and prescribed fire on hispid cotton rat survival and cause-specific mortality. The Journal of Wildlife Management 75, 938–944.
| Effects of mesopredators and prescribed fire on hispid cotton rat survival and cause-specific mortality.Crossref | GoogleScholarGoogle Scholar |
Dickman, CR, and Woodside, DP (1983). A test of a competition model with reference to three species of small mammals in south-eastern Australia. Oecologia 60, 127–134.
| A test of a competition model with reference to three species of small mammals in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar | 28310546PubMed |
Doherty, TS, Davis, RA, Van Etten, EJB, Collier, N, and Krawiec, J (2015). Response of a shrubland mammal and reptile community to a history of landscape-scale wildfire. International Journal of Wildland Fire 24, 534–543.
| Response of a shrubland mammal and reptile community to a history of landscape-scale wildfire.Crossref | GoogleScholarGoogle Scholar |
Driscoll, DA, Lindenmayer, DB, Bennett, AF, Bode, M, Bradstock, RA, Cary, GJ, Clarke, MF, Dexter, N, Fensham, R, Friend, G, Gill, M, James, S, Kay, G, Keith, DA, MacGregor, C, Russell-Smith, J, Salt, D, Watson, JEM, Williams, RJ, and York, A (2010). Fire management for biodiversity conservation: key research questions and our capacity to answer them. Biological Conservation 143, 1928–1939.
| Fire management for biodiversity conservation: key research questions and our capacity to answer them.Crossref | GoogleScholarGoogle Scholar |
Driscoll, DA, Smith, AL, Blight, S, and Maindonald, J (2012). Reptile responses to fire and the risk of post-disturbance sampling bias. Biodiversity and Conservation 21, 1607–1625.
| Reptile responses to fire and the risk of post-disturbance sampling bias.Crossref | GoogleScholarGoogle Scholar |
Elzinga CL, Salzer DW, Willoughby JW (2007) ‘Measuring and Monitoring Plant Populations.’ (Bureau of Land Management: Denver, CO, USA)
Fernandes, PM, and Botelho, HS (2003). A review of prescribed burning effectiveness in fire hazard reduction. International Journal of Wildland Fire 12, 117–128.
| A review of prescribed burning effectiveness in fire hazard reduction.Crossref | GoogleScholarGoogle Scholar |
Firth, D (1993). Bias reduction of maximum likelihood estimates. Biometrika 80, 27–38.
| Bias reduction of maximum likelihood estimates.Crossref | GoogleScholarGoogle Scholar |
Fontaine, JB, Donato, DC, Robinson, WD, Law, BE, and Kauffman, JB (2009). Bird communities following high-severity fire: response to single and repeat fires in a mixed-evergreen forest, Oregon, USA. Forest Ecology and Management 257, 1496–1504.
| Bird communities following high-severity fire: response to single and repeat fires in a mixed-evergreen forest, Oregon, USA.Crossref | GoogleScholarGoogle Scholar |
Fordyce, A, Hradsky, BA, Ritchie, EG, and Di Stefano, J (2016). Fire affects microhabitat selection, movement patterns, and body condition of an Australian rodent (Rattus fuscipes). Journal of Mammalogy 97, 102–111.
| Fire affects microhabitat selection, movement patterns, and body condition of an Australian rodent (Rattus fuscipes).Crossref | GoogleScholarGoogle Scholar |
Gazzard, T, Walshe, T, Galvin, P, Salkin, O, Baker, M, Cross, B, and Ashton, P (2020). What is the ‘appropriate’ fuel management regime for the Otway Ranges, Victoria, Australia? Developing a long-term fuel management strategy using the structured decision-making framework. International Journal of Wildland Fire 29, 354–370.
| What is the ‘appropriate’ fuel management regime for the Otway Ranges, Victoria, Australia? Developing a long-term fuel management strategy using the structured decision-making framework.Crossref | GoogleScholarGoogle Scholar |
Glen, AS, Sutherland, DR, and Cruz, J (2010). An improved method of microhabitat assessment relevant to predation risk. Ecological Research 25, 311–314.
| An improved method of microhabitat assessment relevant to predation risk.Crossref | GoogleScholarGoogle Scholar |
Goldingay, RL (2000). Small dasyurid marsupials – are they effective pollinators? Australian Journal of Zoology 48, 597–606.
| Small dasyurid marsupials – are they effective pollinators?Crossref | GoogleScholarGoogle Scholar |
Green, K, and Sanecki, G (2006). Immediate and short-term responses of bird and mammal assemblages to a subalpine wildfire in the Snowy Mountains, Australia. Austral Ecology 31, 673–681.
| Immediate and short-term responses of bird and mammal assemblages to a subalpine wildfire in the Snowy Mountains, Australia.Crossref | GoogleScholarGoogle Scholar |
Griffiths, AD, and Brook, BW (2014). Effect of fire on small mammals: a systematic review. International Journal of Wildland Fire 23, 1034–1043.
| Effect of fire on small mammals: a systematic review.Crossref | GoogleScholarGoogle Scholar |
Hale, S, Mendoza, L, Yeatman, T, Cooke, R, Doherty, T, Nimmo, D, and White, JG (2021). Evidence that post-fire recovery of small mammals occurs primarily via in situ survival. Diversity and Distributions , 1–13.
| Evidence that post-fire recovery of small mammals occurs primarily via in situ survival.Crossref | GoogleScholarGoogle Scholar |
Haythornthwaite, AS (2005). Microhabitat use and foraging behaviour of Sminthopsis youngsoni (Marsupialia: Dasyuridae) in arid central Australia. Wildlife Research 32, 609–615.
| Microhabitat use and foraging behaviour of Sminthopsis youngsoni (Marsupialia: Dasyuridae) in arid central Australia.Crossref | GoogleScholarGoogle Scholar |
He, T, Lamont, BB, and Pausas, JG (2019). Fire as a key driver of earth’s biodiversity. Biological Reviews 94, 1983–2010.
| Fire as a key driver of earth’s biodiversity.Crossref | GoogleScholarGoogle Scholar | 31298472PubMed |
Heinze G, Ploner M, Jiricka L (2018) logistf: firth’s bias-reduced logistic regression. R package version 1.23. Available at https://CRAN.R-project.org/package=logistf
Horn, KJ, McMillan, BR, and St. Clair, SB (2012). Expansive fire in Mojave Desert shrubland reduces abundance and species diversity of small mammals. Journal of Arid Environments 77, 54–58.
| Expansive fire in Mojave Desert shrubland reduces abundance and species diversity of small mammals.Crossref | GoogleScholarGoogle Scholar |
Hradsky, BA, Mildwaters, C, Ritchie, EG, Christie, F, and Di Stefano, J (2017). Responses of invasive predators and native prey to a prescribed forest fire. Journal of Mammalogy 98, 835–847.
| Responses of invasive predators and native prey to a prescribed forest fire.Crossref | GoogleScholarGoogle Scholar |
Jacob, J, and Brown, JS (2000). Microhabitat use, giving-up densities and temporal activity as short- and long-term anti-predator behaviors in common voles. Oikos 91, 131–138.
| Microhabitat use, giving-up densities and temporal activity as short- and long-term anti-predator behaviors in common voles.Crossref | GoogleScholarGoogle Scholar |
Johnstone, CP, Lill, A, and Reina, RD (2011). Response of the agile antechinus to habitat edge, configuration and condition in fragmented forest. PLoS ONE 6, e27158.
| Response of the agile antechinus to habitat edge, configuration and condition in fragmented forest.Crossref | GoogleScholarGoogle Scholar | 22076129PubMed |
Jolly, WM, Cochrane, MA, Freeborn, PH, Holden, ZA, Brown, TJ, Williamson, GJ, and Bowman, DMJS (2015). Climate-induced variations in global wildfire danger from 1979 to 2013. Nature Communications 6, 7537.
| Climate-induced variations in global wildfire danger from 1979 to 2013.Crossref | GoogleScholarGoogle Scholar | 26172867PubMed |
Kearney, N, Handasyde, K, Ward, S, and Kearney, M (2007). Fine-scale microhabitat selection for dense vegetation in a heathland rodent, Rattus lutreolus: insights from intraspecific and temporal patterns. Austral Ecology 32, 315–325.
| Fine-scale microhabitat selection for dense vegetation in a heathland rodent, Rattus lutreolus: insights from intraspecific and temporal patterns.Crossref | GoogleScholarGoogle Scholar |
Kelly, LT, Nimmo, DG, Spence-Bailey, LM, Clarke, MF, and Bennett, AF (2010). The short-term responses of small mammals to wildfire in semiarid mallee shrubland, Australia. Wildlife Research 37, 293–300.
| The short-term responses of small mammals to wildfire in semiarid mallee shrubland, Australia.Crossref | GoogleScholarGoogle Scholar |
King, KJ, Cary, GJ, Bradstock, RA, and Marsden-Smedley, JB (2013). Contrasting fire responses to climate and management: insights from two Australian ecosystems. Global Change Biology 19, 1223–1235.
| Contrasting fire responses to climate and management: insights from two Australian ecosystems.Crossref | GoogleScholarGoogle Scholar | 23504898PubMed |
Leahy, L, Legge, SM, Tuft, K, McGregor, HW, Barmuta, LA, Jones, ME, and Johnson, CN (2016). Amplified predation after fire suppresses rodent populations in Australia’s tropical savannas. Wildlife Research 42, 705–716.
| Amplified predation after fire suppresses rodent populations in Australia’s tropical savannas.Crossref | GoogleScholarGoogle Scholar |
Letnic, M (2003). The effects of experimental patch burning and rainfall on small mammals in the Simpson Desert, Queensland. Wildlife Research 30, 547–563.
| The effects of experimental patch burning and rainfall on small mammals in the Simpson Desert, Queensland.Crossref | GoogleScholarGoogle Scholar |
Lindenmayer, DB, MacGregor, C, Welsh, A, Donnelly, C, Crane, M, Michael, D, Montague-Drake, R, Cunningham, RB, Brown, D, Fortescue, M, Dexter, N, Hudson, M, and Gill, AM (2008). Contrasting mammal responses to vegetation type and fire. Wildlife Research 35, 395–408.
| Contrasting mammal responses to vegetation type and fire.Crossref | GoogleScholarGoogle Scholar |
Lindenmayer, DB, Kooyman, RM, Taylor, C, Ward, M, and Watson, JEM (2020). Recent Australian wildfires made worse by logging and associated forest management. Nature Ecology & Evolution 4, 898–900.
| Recent Australian wildfires made worse by logging and associated forest management.Crossref | GoogleScholarGoogle Scholar |
Loyn, RH, Traill, BJ, and Triggs, BE (1986). Prey of sooty owls in East Gippsland before and after fire. Victorian Naturalist 103, 147–149.
Lunney, D, and Ashby, E (1987). Population-changes in Sminthopsis leucopus (gray) (Marsupialia, Dasyuridae), and other small mammal species, in forest regenerating from logging and fire near Bega, New South Wales. Wildlife Research 14, 275–284.
| Population-changes in Sminthopsis leucopus (gray) (Marsupialia, Dasyuridae), and other small mammal species, in forest regenerating from logging and fire near Bega, New South Wales.Crossref | GoogleScholarGoogle Scholar |
Lunney, D, Matthews, A, and Grigg, J (2001). The diet of Antechinus agilis and A. swainsonii in unlogged and regenerating sites in Mumbulla State Forest, south-eastern New South Wales. Wildlife Research 28, 459–464.
| The diet of Antechinus agilis and A. swainsonii in unlogged and regenerating sites in Mumbulla State Forest, south-eastern New South Wales.Crossref | GoogleScholarGoogle Scholar |
Lunney, D, Lunney, HWM, and Recher, HF (2008). Bushfire and the Malthusian guillotine: survival of small mammals in a refuge in Nadgee Nature Reserve, south-eastern New South Wales. Pacific Conservation Biology 14, 263–278.
| Bushfire and the Malthusian guillotine: survival of small mammals in a refuge in Nadgee Nature Reserve, south-eastern New South Wales.Crossref | GoogleScholarGoogle Scholar |
Maitz, WE, and Dickman, CR (2001). Competition and habitat use in native Australian Rattus: is competition intense, or important? Oecologia 128, 526–538.
| Competition and habitat use in native Australian Rattus: is competition intense, or important?Crossref | GoogleScholarGoogle Scholar | 28547398PubMed |
Matthews, JK, Stawski, C, Körtner, G, Parker, CA, and Geiser, F (2017). Torpor and basking after a severe wildfire: mammalian survival strategies in a scorched landscape. Journal of Comparative Physiology B 187, 385–393.
| Torpor and basking after a severe wildfire: mammalian survival strategies in a scorched landscape.Crossref | GoogleScholarGoogle Scholar |
McGregor, HW, Legge, S, Jones, ME, and Johnson, CN (2014). Landscape management of fire and grazing regimes alters the fine-scale habitat utilisation by feral cats. PLoS ONE 9, e109097.
| Landscape management of fire and grazing regimes alters the fine-scale habitat utilisation by feral cats.Crossref | GoogleScholarGoogle Scholar | 25329902PubMed |
McGregor, HW, Legge, S, Jones, ME, and Johnson, CN (2016). Extraterritorial hunting expeditions to intense fire scars by feral cats. Scientific Reports 6, 22559.
| Extraterritorial hunting expeditions to intense fire scars by feral cats.Crossref | GoogleScholarGoogle Scholar | 26932268PubMed |
Molyneux, J, Pavey, CR, James, AI, and Carthew, SM (2017). Habitat use by the brush-tailed mulgara (Dasycercus blythi). Australian Journal of Zoology 65, 335–345.
| Habitat use by the brush-tailed mulgara (Dasycercus blythi).Crossref | GoogleScholarGoogle Scholar |
Monamy, V, and Fox, BJ (2000). Small mammal succession is determined by vegetation density rather than time elapsed since disturbance. Austral Ecology 25, 580–587.
| Small mammal succession is determined by vegetation density rather than time elapsed since disturbance.Crossref | GoogleScholarGoogle Scholar |
Moritz, MA, Batllori, E, Bradstock, RA, Gill, AM, Handmer, J, Hessburg, PF, Leonard, J, McCaffrey, S, Odion, DC, Schoennagel, T, and Syphard, AD (2014). Learning to coexist with wildfire. Nature 515, 58–66.
| Learning to coexist with wildfire.Crossref | GoogleScholarGoogle Scholar | 25373675PubMed |
Moro, D (1991). The distribution of small mammal species in relation to heath vegetation near Cape Otway, Victoria. Wildlife Research 18, 605–618.
| The distribution of small mammal species in relation to heath vegetation near Cape Otway, Victoria.Crossref | GoogleScholarGoogle Scholar |
Morris, G, Hostetler, JA, Mike Conner, L, and Oli, MK (2011). Effects of prescribed fire, supplemental feeding, and mammalian predator exclusion on hispid cotton rat populations. Oecologia 167, 1005–1016.
| Effects of prescribed fire, supplemental feeding, and mammalian predator exclusion on hispid cotton rat populations.Crossref | GoogleScholarGoogle Scholar | 21706331PubMed |
Nimmo, DG, Kelly, LT, Farnsworth, LM, Watson, SJ, and Bennett, AF (2014). Why do some species have geographically varying responses to fire history? Ecography 37, 805–813.
| Why do some species have geographically varying responses to fire history?Crossref | GoogleScholarGoogle Scholar |
Nimmo, DG, Avitabile, S, Banks, SC, Bliege Bird, R, Callister, K, Clarke, MF, Dickman, CR, Doherty, TS, Driscoll, DA, Greenville, AC, Haslem, A, Kelly, LT, Kenny, SA, Lahoz-Monfort, JJ, Lee, C, Leonard, S, Moore, H, Newsome, TM, Parr, CL, Ritchie, EG, Schneider, K, Turner, JM, Watson, S, Westbrooke, M, Wouters, M, White, M, and Bennett, AF (2019). Animal movements in fire-prone landscapes. Biological Reviews 94, 981–998.
| Animal movements in fire-prone landscapes.Crossref | GoogleScholarGoogle Scholar | 30565370PubMed |
Nowack, J, Levesque, DL, Reher, S, and Dausmann, KH (2020). Variable climates lead to varying phenotypes: “weird” mammalian torpor and lessons from non-holarctic species. Frontiers in Ecology and Evolution 8, 60.
| Variable climates lead to varying phenotypes: “weird” mammalian torpor and lessons from non-holarctic species.Crossref | GoogleScholarGoogle Scholar |
Orrock, JL, Danielson, BJ, and Brinkerhoff, RJ (2004). Rodent foraging is affected by indirect, but not by direct, cues of predation risk. Behavioral Ecology 15, 433–437.
| Rodent foraging is affected by indirect, but not by direct, cues of predation risk.Crossref | GoogleScholarGoogle Scholar |
Parrott, ML, Ward, SJ, Temple-Smith, PD, and Selwood, L (2007). Effects of drought on weight, survival and breeding success of agile antechinus (Antechinus agilis), dusky antechinus (A. swainsonii) and bush rats (Rattus fuscipes). Wildlife Research 34, 437–442.
| Effects of drought on weight, survival and breeding success of agile antechinus (Antechinus agilis), dusky antechinus (A. swainsonii) and bush rats (Rattus fuscipes).Crossref | GoogleScholarGoogle Scholar |
Pastro, LA, Dickman, CR, and Letnic, M (2011). Burning for biodiversity or burning biodiversity? Prescribed burn vs. wildfire impacts on plants, lizards, and mammals. Ecological Applications 21, 3238–3253.
| Burning for biodiversity or burning biodiversity? Prescribed burn vs. wildfire impacts on plants, lizards, and mammals.Crossref | GoogleScholarGoogle Scholar |
Pereoglou, F, Macgregor, C, Banks, SC, Ford, F, Wood, J, and Lindenmayer, DB (2011). Refuge site selection by the eastern chestnut mouse in recently burnt heath. Wildlife Research 38, 290–298.
| Refuge site selection by the eastern chestnut mouse in recently burnt heath.Crossref | GoogleScholarGoogle Scholar |
Pereoglou, F, Lindenmayer, DB, MacGregor, C, Ford, F, Wood, J, and Banks, SC (2013). Landscape genetics of an early successional specialist in a disturbance-prone environment. Molecular Ecology 22, 1267–1281.
| Landscape genetics of an early successional specialist in a disturbance-prone environment.Crossref | GoogleScholarGoogle Scholar | 23379886PubMed |
Robinson, NM, Leonard, SWJ, Ritchie, EG, Bassett, M, Chia, EK, Buckingham, S, Gibb, H, Bennett, AF, and Clarke, MF (2013). Review: refuges for fauna in fire-prone landscapes: their ecological function and importance. Journal of Applied Ecology 50, 1321–1329.
| Review: refuges for fauna in fire-prone landscapes: their ecological function and importance.Crossref | GoogleScholarGoogle Scholar |
Russell-Smith, J, McCaw, L, and Leavesley, A (2020). Adaptive prescribed burning in Australia for the early 21st Century – context, status, challenges. International Journal of Wildland Fire 29, 305–313.
| Adaptive prescribed burning in Australia for the early 21st Century – context, status, challenges.Crossref | GoogleScholarGoogle Scholar |
Sharp Bowman, TR, McMillan, BR, and St. Clair, SB (2017). A comparison of the effects of fire on rodent abundance and diversity in the Great Basin and Mojave Deserts. PLoS ONE 12, e0187740.
| A comparison of the effects of fire on rodent abundance and diversity in the Great Basin and Mojave Deserts.Crossref | GoogleScholarGoogle Scholar | 29182632PubMed |
Smucker, KM, Hutto, RL, and Steele, BM (2005). Changes in bird abundance after wildfire: importance of fire severity and time since fire. Ecological Applications 15, 1535–1549.
| Changes in bird abundance after wildfire: importance of fire severity and time since fire.Crossref | GoogleScholarGoogle Scholar |
Spencer, R-J, Cavanough, VC, Baxter, GS, and Kennedy, MS (2005). Adult free zones in small mammal populations: response of Australian native rodents to reduced cover. Austral Ecology 30, 868–876.
| Adult free zones in small mammal populations: response of Australian native rodents to reduced cover.Crossref | GoogleScholarGoogle Scholar |
Stawski, C, Körtner, G, Nowack, J, and Geiser, F (2015). The importance of mammalian torpor for survival in a post-fire landscape. Biology Letters 11, 20150134–6.
| The importance of mammalian torpor for survival in a post-fire landscape.Crossref | GoogleScholarGoogle Scholar | 26063748PubMed |
Stawski, C, Körtner, G, Nowack, J, and Geiser, F (2016). Phenotypic plasticity of post-fire activity and thermal biology of a free-ranging small mammal. Physiology & Behavior 159, 104–111.
| Phenotypic plasticity of post-fire activity and thermal biology of a free-ranging small mammal.Crossref | GoogleScholarGoogle Scholar |
Stokes, VL, Pech, RP, Banks, PB, and Arthur, AD (2004). Foraging behaviour and habitat use by Antechinus flavipes and Sminthopsis murina (Marsupialia: Dasyuridae) in response to predation risk in eucalypt woodland. Biological Conservation 117, 331–342.
| Foraging behaviour and habitat use by Antechinus flavipes and Sminthopsis murina (Marsupialia: Dasyuridae) in response to predation risk in eucalypt woodland.Crossref | GoogleScholarGoogle Scholar |
Strauß, A, Solmsdorff, KY, Pech, R, and Jacob, J (2008). Rats on the run: removal of alien terrestrial predators affects bush rat behaviour. Behavioral Ecology and Sociobiology 62, 1551–1558.
| Rats on the run: removal of alien terrestrial predators affects bush rat behaviour.Crossref | GoogleScholarGoogle Scholar |
Sutherland, EF, and Dickman, CR (1999). Mechanisms of recovery after fire by rodents in the Australian environment: a review. Wildlife Research 26, 405–419.
| Mechanisms of recovery after fire by rodents in the Australian environment: a review.Crossref | GoogleScholarGoogle Scholar |
Sutherland, DR, and Predavec, M (1999). The effects of moonlight on microhabitat use by Antechinus agilis (Marsupialia: Dasyuridae). Australian Journal of Zoology 47, 1–17.
| The effects of moonlight on microhabitat use by Antechinus agilis (Marsupialia: Dasyuridae).Crossref | GoogleScholarGoogle Scholar |
Swan, M, Galindez-Silva, C, Christie, F, York, A, and Di Stefano, J (2016). Contrasting responses of small mammals to fire and topographic refugia. Austral Ecology 41, 437–445.
| Contrasting responses of small mammals to fire and topographic refugia.Crossref | GoogleScholarGoogle Scholar |
Swinburn, ML, Fleming, PA, Craig, MD, Grigg, AH, Garkaklis, MJ, Hobbs, RJ, and Hardy, GESJ (2007). The importance of grasstrees (Xanthorrhoea preissii) as habitat for mardo (Antechinus flavipes leucogaster) during post-fire recovery. Wildlife Research 34, 640–651.
| The importance of grasstrees (Xanthorrhoea preissii) as habitat for mardo (Antechinus flavipes leucogaster) during post-fire recovery.Crossref | GoogleScholarGoogle Scholar |
Thompson, MB, Medlin, G, Hutchinson, R, and West, N (1989). Short-term effects of fuel reduction burning on populations of small terrestrial mammals. Wildlife Research 16, 117–129.
| Short-term effects of fuel reduction burning on populations of small terrestrial mammals.Crossref | GoogleScholarGoogle Scholar |
Torre, I, and Díaz, M (2004). Small mammal abundance in Mediterranean post-fire habitats: a role for predators? Acta Oecologica 25, 137–142.
| Small mammal abundance in Mediterranean post-fire habitats: a role for predators?Crossref | GoogleScholarGoogle Scholar |
Van Dyck S, Gynther I, Baker A (Eds) (2013) ‘Field Companion to the Mammals of Australia.’ (New Holland Publishers: Sydney, NSW, Australia)
Wallace, G, Elden, M, Boucher, R, and Phelps, S (2021). An automated radio-telemetry system (ARTS) for monitoring small mammals. Methods in Ecology and Evolution , in press.
| An automated radio-telemetry system (ARTS) for monitoring small mammals.Crossref | GoogleScholarGoogle Scholar |
Whelan RJ, Rodgerson L, Dickman CR, Sutherland EF (2002) Critical life cycles of plants and animals: developing a process-based understanding of population changes in fire-prone landscapes. In ‘Flammable Australia: the Fire Regimes and Biodiversity of a Continent’. (Eds RA Bradstock, JE Williams, MA Gill) pp. 284–304. (Cambridge University Press: Cambridge, UK)
Wilson, BA, Bourne, AR, and Jessop, RE (1986). Ecology of small mammals in coastal heathland at Anglesea, Victoria. Wildlife Research 13, 397–406.
| Ecology of small mammals in coastal heathland at Anglesea, Victoria.Crossref | GoogleScholarGoogle Scholar |
Woinarski, JCZ, Risler, J, and Kean, L (2004). Response of vegetation and vertebrate fauna to 23 years of fire exclusion in a tropical Eucalyptus open forest, Northern Territory, Australia. Austral Ecology 29, 156–176.
| Response of vegetation and vertebrate fauna to 23 years of fire exclusion in a tropical Eucalyptus open forest, Northern Territory, Australia.Crossref | GoogleScholarGoogle Scholar |
Yarnell, RW, Metcalfe, DJ, Dunstone, N, Burnside, N, and Scott, DM (2008). The impact of fire on habitat use by the short-snouted elephant shrew (Elephantulus brachyrhynchus) in North West Province, South Africa. African Zoology 43, 45–52.
| The impact of fire on habitat use by the short-snouted elephant shrew (Elephantulus brachyrhynchus) in North West Province, South Africa.Crossref | GoogleScholarGoogle Scholar |
Zwolak, R, Pearson, DE, Ortega, YK, and Crone, EE (2012). Mechanisms driving postfire abundance of a generalist mammal. Canadian Journal of Zoology 90, 51–60.
| Mechanisms driving postfire abundance of a generalist mammal.Crossref | GoogleScholarGoogle Scholar |


