Novel microsatellites and investigation of faecal DNA as a non-invasive population monitoring tool for the banded hare-wallaby (Lagostrophus fasciatus)
Saul Cowen A B * , Michael Smith B C , Shelley McArthur A , Kelly Rayner A , Chantelle Jackson C , Georgina Anderson C and Kym Ottewell
A B * , Michael Smith B C , Shelley McArthur A , Kelly Rayner A , Chantelle Jackson C , Georgina Anderson C and Kym Ottewell  A D
A D
A Department of Biodiversity, Conservation and Attractions, Biodiversity and Conservation Science, Locked Bag 104, Bentley, WA 6983, Australia.
B School of Biological Sciences, University of Western Australia, 35 Stirling Highway, Crawley, WA 6009, Australia.
C Australian Wildlife Conservancy, PO Box 8070, Subiaco East, Perth, WA 6008, Australia.
D Environmental and Conservation Sciences, Murdoch University, 90 South Street, Murdoch, WA 6150, Australia.
Australian Journal of Zoology 69(2) 55-66 https://doi.org/10.1071/ZO21015
Submitted: 7 May 2021 Accepted: 16 December 2021 Published: 8 February 2022
© 2021 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Monitoring programs for populations of small or medium-sized animals often use live-capture or photo-monitoring trapping methods to estimate population size. The banded hare-wallaby (Lagostrophus fasciatus), a small macropodiform marsupial, does not readily enter traps or have individually unique distinguishing physical features and is consequently difficult to monitor using these methods. Isolating DNA from faecal material to obtain individual genotypes is a promising monitoring technique and may present an alternative approach for this species. We developed novel species-specific microsatellite markers and undertook trials to assess faecal DNA degradation in ambient environmental conditions at two locations where this species has been translocated. The quality of DNA yielded from faecal pellets was evaluated through amplification failure and genotyping error rates of microsatellite markers. Error rates were compared for different treatments and exposure duration across multiple individuals. DNA was successfully obtained from all samples and error rates increased with exposure duration, peaking after 14–30 days depending on the site and treatment. The level of solar exposure was the most significant factor affecting degradation rate but both this and exposure duration had significant effects on amplification failure. Analysing DNA obtained from faecal pellets may represent a practical non-invasive method of deriving population estimates for this species and warrants further development.
Keywords: conservation, faeces, hare-wallaby, Lagostrophus, minimally invasive, molecular genetics, monitoring, threatened species, wildlife management.
Introduction
When it comes to managing threatened species, population size is a fundamental metric (Williams et al. 2002; Lindenmayer et al. 2012). Appropriate monitoring methods will vary according to the ecology and behaviour of the target species, as well as consideration of detection and cost-efficiencies (Garden et al. 2007). By monitoring the size of a population, conservation managers can assess the species’ status relative to specific targets and management goals. Popular and robust methods of obtaining population estimates are capture–recapture models (Nichols 1992; Chao 2001; Efford and Fewster 2013). These methods rely on the ability to identify individual animals in a population across survey periods, after physical or non-physical (i.e. photographs from camera traps) capture (Jones et al. 1996; Chao 2001). For example, in Australia, live-capture surveys have been an effective method of monitoring a suite of taxa, including reptiles, frogs and small and medium-sized mammals and are identified as such in survey guidelines for threatened taxa drawn up by the Australian government (DEWHA 2010; DSEWPaC 2011a, 2011b). However, some species may not be readily lured to live-capture or remote camera traps and live-capture can sometimes pose serious risk to captured animals (Cole et al. 1994; Soulsbury et al. 2020).
Whilst camera traps may offer a practical, non-invasive alternative for some taxa (De Bondi et al. 2010), they have inherent limitations (Ballard et al. 2014; Meek et al. 2015) and may not be appropriate for obtaining estimates of population size or density due to the inability to identify individual animals. Sticky hair-tubes or other capture devices (e.g. barbed wire on scratching trees (Mowat and Strobeck 2000)) represent another form of minimally-invasive monitoring that can be used to gather information on presence/absence (Mortelliti and Boitani 2008) or occupancy (Pocock and Bell 2011). These methods also have the advantage that DNA analysis of sampled hair can provide individual identification for capture–recapture analysis (Mowat and Strobeck 2000; Sloane et al. 2000; Banks et al. 2003). However, to obtain sufficient samples for analysis, they still rely on animals responding to a lure or positioning of devices to exploit predictable animal behaviours (Ruibal et al. 2010; Chiron et al. 2018) such as the use of scratching trees (Mowat and Strobeck 2000) or when entering or exiting burrows (Banks et al. 2003).
Faecal DNA analysis is a non-invasive sampling approach that holds promise for species that do not respond to lures and for which other non-invasive (e.g. hair) sampling is unsuitable. Faecal DNA, i.e. DNA derived from epithelial cells on the surface of faecal pellets (scats) excreted by the target species (Waits and Paetkau 2005), can be of sufficient quality and quantity to be used to identify individuals, representing a non-invasive form of capture–recapture survey (Mills et al. 2000; Lukacs and Burnham 2005). This method has already been used successfully for a range of mammal species (Piggott et al. 2006; Goode et al. 2014; Fuller et al. 2016; Morin et al. 2016; Woodruff et al. 2016; Dziminski et al. 2021).
An important consideration for incorporating faecal DNA genotyping into a monitoring program is the rate at which the DNA degrades in the environment (Piggott 2004; Panasci, Ballard et al. 2011; Woodruff et al. 2015; Carpenter and Dziminski 2017). Environmental variables such as moisture (Harestad and Bunnell 1987; Murphy et al. 2007; Brinkman et al. 2010), ultraviolet light (UV) (Ravanat et al. 2001) and temperature (Murphy et al. 2007; DeMay et al. 2013) are known to influence the rate of degradation of faecal DNA. King et al. (2018) found that arid climates may be advantageous for the preservation of faecal DNA in the environment, notwithstanding the high UV exposure and temperatures these areas may receive. The use of faecal DNA is also prone to genotyping errors, such as allelic dropout or identification of false alleles, that may become more frequent with increasing DNA degradation. Allelic dropout is where only one allele of a heterozygous individual is obtained (‘false homozygote’), whereas false alleles are when an artefact of amplification is incorrectly assigned as an allele leading to a ‘false heterozygote’. Allelic dropout may occur if only one allele is aliquoted from a sample for polymerase chain reaction (PCR); false alleles result from low rates of amplification of the target locus, which makes it difficult to differentiate from artefactual amplification (Pompanon et al. 2005). In addition to genotyping errors, amplification failure may occur when quantities of nuclear DNA containing the target loci are simply too low or fragmented. Each of these errors can result from low or highly fragmented quantities of DNA (Taberlet et al. 1999), which may occur with prolonged exposure to environmental variables. Furthermore, genotyping errors can lead to false individual assignments that can severely bias capture–recapture and population estimates (Taberlet et al. 1999; Wright et al. 2009).
The banded hare-wallaby (Lagostrophus fasciatus) is a small species of macropodiform marsupial. While related to true kangaroos, the banded hare-wallaby is placed in the monotypic subfamily Lagostrophinae. The species is listed as Vulnerable under international (Burbidge and Woinarski 2016) and Australian criteria (Department of the Environment 2019). Since this species became extinct on the Australian mainland, the only natural populations remain on Bernier and Dorre Islands on the west coast of Western Australia (WA). Due to its currently restricted distribution, the species has been the subject of several reintroduction attempts (White et al. 2020), most recently to Dirk Hartog Island National Park (DHI) and a feral predator-proof fenced area at Mount (Mt) Gibson Wildlife Sanctuary (Australian Wildlife Conservancy 2018a; Cowen et al. 2018).
Banded hare-wallabies are considered to be ‘trap-shy’, i.e. not readily trapped using conventional live-capture methods (Richards et al. 2001; Woinarski et al. 2014). Passive techniques such as camera traps have not yet proved an effective monitoring method both in recently translocated and well-established populations (Australian Wildlife Conservancy 2018b; Cowen et al. 2018). Monitoring of the populations on Bernier and Dorre Islands has involved distance sampling analysis from observations during spotlight surveys conducted on foot along transects (Chapman et al. 2015). However, this method relies on a minimum number of observations which may not always be achievable and is highly labour-intensive. As such, a non-invasive method of estimating abundance using genetic analysis of DNA extracted from scats could be a highly useful monitoring tool for this species.
Here, we undertook a pilot study to investigate the feasibility of using DNA derived from banded hare-wallaby faecal pellets to monitor population abundance and density. Such studies are recommended for developing an effective monitoring methodology using these techniques (Waits and Paetkau 2005; Valière et al. 2006; Luikart et al. 2010).
The aims of this study were: (1) develop an array of short-tandem repeat (microsatellite) markers for use in assigning individual genotypes in banded hare-wallabies; (2) evaluate the rate of degradation of DNA on the surface of banded hare-wallaby scats over time, by assessing rates of amplification success and genotyping error; and (3) assess the effect of solar exposure on the rate of degradation of DNA.
In order to achieve the latter two aims, we replicated a faecal exposure experiment in two target environments into which banded hare-wallabies have been translocated (Dirk Hartog Island, Mt Gibson Wildlife Sanctuary) and that differ primarily in ambient moisture/humidity. To genotype individuals, we developed a novel, species-specific set of microsatellite markers for the banded hare-wallaby. The specificity of the markers is critical since rufous hare-wallabies (Lagorchestes hirsutus) have also been translocated to Dirk Hartog Island and their faecal pellets are similar in size and shape to banded hare-wallabies.
We predicted that increased DNA fragmentation, increased genotyping error rates and decreased likelihood of successful amplification by PCR would result from increased time in the environment. We also predicted that the humid coastal environment of Dirk Hartog Island would result in more rapid DNA degradation than the mainland Mt Gibson site which is ≥250 km from the coast. Other environmental variables (e.g. mean solar exposure) were predicted to be similar at both locations, but we expected to see high rates of amplification failure and genotyping errors in samples with higher levels of solar exposure.
Materials and methods
Study sites
Dirk Hartog Island National Park (DHI) is in the Shire of Shark Bay, 750 km NNW of Perth, and is managed by the WA Department of Biodiversity, Conservation and Attractions. The island lies at the western edge of the Shark Bay UNESCO World Heritage Area (Fig. 1) with an approximate location of −26°S, 113°E. The climate is semi-arid with a mean annual rainfall of 200–300 mm (winter dominant), a mean maximum temperature of 27–30°C and an average daily solar exposure of 21–24 MJ/m2 (Bureau of Meteorology 2021). Due to its proximity to the Indian Ocean, the island is relatively humid and can experience heavy dews.
Mt Gibson Wildlife Sanctuary is located in the Shire of Yalgoo, ≥250 km from the Indian Ocean at an approximate location of −29°S, 117°E (Fig. 1). The sanctuary is managed by the not-for-profit organisation, the Australian Wildlife Conservancy (Smith et al. 2020). The climate is semi-arid with an average rainfall of 322 mm (winter dominant) but with substantial inter-annual variation. The property experiences a mean maximum temperature of 30°C and an average daily solar exposure of 21–24 MJ/m2 (Bureau of Meteorology 2021).
Specimen collection
Scat collections were made during the translocation of banded hare-wallabies from Bernier and Dorre Islands (Fig. 1) to DHI on 16 September 2018 and from Bernier Island and Faure Island Wildlife Sanctuary to Mt Gibson on 18, 19 and 27 October 2018. Fresh scat samples were collected from cotton handling bags used to hold wallabies during the translocation and as such were obtained from known individuals. Pellets were handled with sterile latex gloves or sterilised tweezers, which were replaced/sterilised between sample collections to avoid cross-contamination between individual animals.
In-situ degradation trial
As per Carpenter and Dziminski (2017), pellets were placed on 5 cm × 5 cm ceramic dishes on clean, dry sand. Dishes were kept in three individual enclosures 30 cm (W) × 70 cm (L) × 30 cm (H) made from 3 mm wire-mesh. Each enclosure was covered with two 17 mm pieces of UV transmitting ‘Plexiglas’ (Evonik Industries, Essen, Germany) in an ‘A’ formation. This material ensured protection of pellet treatments from excessive precipitation and wind which could render the experiment null, without excluding UV rays. Enclosures were placed either in full sun, semi-shade or full shade, to simulate different solar exposure environments. The full sampling schedule is outlined diagrammatically in Supplementary Fig. S1.
For DHI, samples from three individuals per site were selected for the trial based on a sufficient number of intact pellets for a control (0 days exposure) and six different exposure times: 1, 7, 14, 21, 30 and 60 days. Two pellets per individual per treatment were used, so a total of 42 pellets per individual were required.
For Mt Gibson, samples from four individuals were selected for the trial based on a sufficient number of intact pellets for six different exposure times: 1, 7, 14, 21, 30 and 60 days. Two pellets per individual per treatment were used, so a total of 36 pellets per individual were required.
On the completion of each exposure period, pellets were transferred to 50 mL vials with silica gel beads filling the bottom third of the tube and a ball of cotton wool separating scats from the gel beads. These were frozen at -20°C until they could be transported from the field to the laboratory for DNA extraction.
Ex-situ degradation trial
Samples were collected from a further three animals from DHI to investigate the rate of DNA degradation in storage. Again, two pellets were used per sample with one pair acting as a control (stored at room temperature) and two being stored immediately after sampling at −20°C and −80°C for 90 days prior to extraction. Pellets were collected, handled and transferred as above.
Meteorological data
Temperature data were obtained from the weather station at DHI and the nearest weather station to Mt Gibson (Paynes Find), although the DHI station had a hardware malfunction 43 days into the trial and failed to record after this date. Midday shade temperature (°C) was used as a proxy for maximum temperature each day. Measurements for solar exposure (as a proxy for UV) and relative humidity (RH) were obtained through the Bureau of Meteorology (BOM) (http://www.bom.gov.au) from the nearest weather stations recording this information. For DHI, this was Steep Point for solar exposure and Denham for relative humidity (Fig. 1). For Mt Gibson, all climatic data were obtained from the Paynes Find BOM weather station. Single-station climate data do not account for any potential micro-site variation but were still useful for comparing broader climatic variation between DHI and Mt Gibson.
Microsatellite development
Tissue DNA extraction
Genomic DNA was extracted from an ear biopsy sample from a single individual (DEC02715) for microsatellite library development using a standard ‘salting out’ procedure (Sunnucks and Hales 1996) modified with the addition of 3 μL of 10 mg/mL ribonuclease (RNase) to the TNES extraction buffer. Genomic DNA was also extracted from additional L. fasciatus and L. hirsutus ear biopsy samples for microsatellite screening.
Microsatellite library and screening
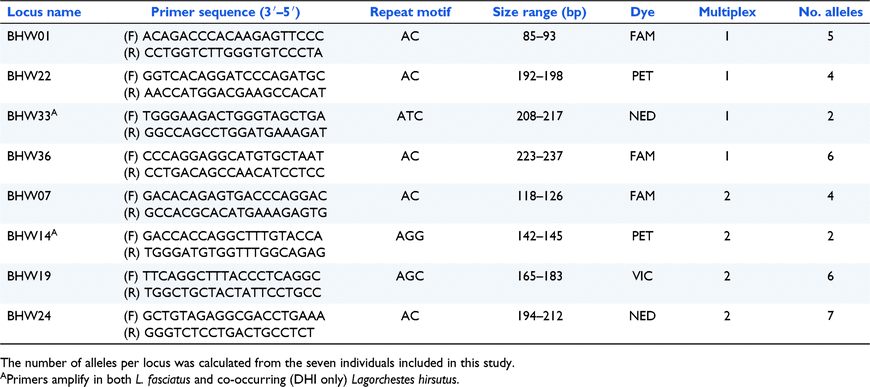
Library preparation and partial sequencing using the Illumina MiSeq platform was undertaken through a commercial service at the Monash University Malaysia Genome Facility. QDD software (Meglécz et al. 2010) was used to identify novel microsatellite sequences from the short-read data and undertake primer design using default parameters. Microsatellite loci identified by QDD were further filtered to include only loci with pure microsatellite repeats, target length greater than seven repeat units and excluding (AT) repeats, with 48 microsatellite primer pairs chosen for further screening. To confirm microsatellite loci were specific to L. fasciatus, we screened primer pairs across tissue samples from three L. fasciatus individuals and three individuals of the co-occurring L. hirsutus. After further screening on a panel of eight L. fasciatus samples (representing two samples each from four populations) to assess amplification across populations, ease of scoring and reliability, successful microsatellite primers were fluorescently labelled using ABI dyes (FAM, NED, VIC and PET; Applied Biosystems, Waltham, MA, USA) and arranged in multiplexes for PCR amplification. To determine if potential amplification failures in future field-based surveys would be caused by erroneous use of rufous hare-wallaby scat (as opposed to a systemic failure of reagents or equipment), we also sought to develop a primer-set that amplified both rufous and banded hare-wallaby DNA to be used as a positive control.
Scat DNA extraction, amplification and fragment analysis
All scat DNA extractions were undertaken at the end of the experiment, with field collected samples stored at −20°C until this time. To maximise the amount of DNA from epithelial cells of the target species, banded hare-wallaby scats were prepared for DNA extraction by scraping the outer surface of each pellet with a razor blade before proceeding to DNA extraction. DNA was extracted using the Qiagen QIAamp Fast DNA Stool Mini Kit (Qiagen Inc, Germany) following the manufacturer’s instructions with two modifications: a second centrifugation and transferal step following the addition of InhibitEX buffer to the scraped faecal material was included to ensure no carryover of insoluble material to the subsequent steps and DNA was eluted from the spin column at the final step using 100 μL ATE Buffer. Due to the potential for DNA from dietary items to be co-extracted with our target species DNA, we were unable to specifically quantify changes in target species DNA with time.
Faecal DNA samples were PCR amplified using the Qiagen Multiplex PCR Plus kit (Qiagen Inc). Each multiplex reaction contained 4 μL Qiagen mastermix, 1 μL primer mastermix and 4 μL DNA and were run on an Eppendorf Mastercycler (Eppendorf, Germany) using cycling conditions recommended by the manufacturer with an annealing temperature of 60°C, with 35 cycles. 2 μL of PCR product was mixed with 10 μL highly deionised (Hi-Di) formamide for fragment analysis. Fragment analysis was conducted on an Applied Biosystems 3100 capillary sequencer using a commercial service (State Agricultural Biotechnology Centre, Western Australia) and GENEMAPPER 4.0 software (Applied Biosystems, Foster City, CA) was used to score microsatellite alleles with reference to an internal size standard (LIZ500, Applied Biosystems). Three replicate PCRs were performed for each DNA sample to assess genotyping errors. To assess genotyping error rates for scats, we used the control samples (0 days exposure) as the reference genotypes, and these were also replicated three times to obtain a consensus, although the genotypes obtained were consistent for all replicates, validating their use as reference genotypes.
Probability of identity, i.e. the estimated probability that two samples from different individuals will have identical genotypes with the given marker array, was calculated in GenAlEx 6.503 (Peakall and Smouse 2006; Peakall and Smouse 2012).
Genotyping error and amplification failure calculation
Errors were categorised either as genotyping errors (allelic dropout or false alleles) or amplification failure. Error rates were quantified by calculating the total number of each type across all eight loci for each of the three PCR replicates against the genotype obtained from the control samples. This was done for each of the three solar exposure treatment types individually. Mean and s.d. of each error type were calculated across the three replicate scat genotypes. For comparison of solar exposure treatments, we combined all three types of error to obtain a total error estimate, since any error would render a replicate unusable for subsequent analysis at that locus. This was done by calculating the percentage of amplifications with any errors across all three replicates for all individuals combined but for separate treatments. To assess the relative importance of overall solar exposure treatment and length of exposure on amplification rate, a repeated measures mixed-model ANOVA was used in R (R Development Core Team 2019) with the package nlme (Pinheiro et al. 2020).
Results
Microsatellite panel development
Of the 48 microsatellite primer pairs tested, eight failed to amplify with DNA from either L. fasciatus or L. hirsutus, 26 amplified in both species and 14 amplified only in L. fasciatus with four of these monomorphic. Upon further testing, we selected six L. fasciatus-specific microsatellite markers and two that amplified in both L. fasciatus and L. hirsutus to serve as identification controls (one per multiplex). Details of the eight loci and multiplex information are in Table 1. Across the seven individuals from which scats were collected in this study a total of 36 alleles were detected ranging from two to seven alleles per locus. Probability of identity values by locus ranged from 6.9 × 10−1 to 7.5 × 10−2. Overall, the probability of identity for all loci was 2.0 × 10−6.
|
Climatic data
Midday shade temperature (°C) readings were initially higher for DHI than Mt Gibson but by Day 35, readings were roughly equivalent and readings for Mt Gibson continued to increase after the DHI weather-station malfunctioned on Day 43 (Supplementary Fig. S2). Relative humidity (RH) was generally higher for DHI (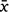 = 72.36, s.e. 1.60) than Mt Gibson (
= 72.36, s.e. 1.60) than Mt Gibson (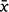 = 39.34, s.e. 2.37) across the period of the trial (Fig. S2). There was a notable spike in RH around Days 26 and 60 at Mt Gibson but generally values remained below 50%, the former coinciding with a 52 mm rainfall event. At DHI, RH was consistently between 60 and 90% except for 3 days when it fell to around 40%. Solar exposure was consistently higher for Mt Gibson than DHI across the period of the trial (Fig. S2) but Mt Gibson showed larger fluctuations, falling to c.11 MJ/m2 around Days 24 and 25 (coinciding with heavy rainfall and the associated spike in RH; Fig. S2). In contrast solar exposure for DHI was more consistent with no obvious troughs or peaks, except for around Day 56 when values fell from c. 27 to c. 17 MJ/m2.
= 39.34, s.e. 2.37) across the period of the trial (Fig. S2). There was a notable spike in RH around Days 26 and 60 at Mt Gibson but generally values remained below 50%, the former coinciding with a 52 mm rainfall event. At DHI, RH was consistently between 60 and 90% except for 3 days when it fell to around 40%. Solar exposure was consistently higher for Mt Gibson than DHI across the period of the trial (Fig. S2) but Mt Gibson showed larger fluctuations, falling to c.11 MJ/m2 around Days 24 and 25 (coinciding with heavy rainfall and the associated spike in RH; Fig. S2). In contrast solar exposure for DHI was more consistent with no obvious troughs or peaks, except for around Day 56 when values fell from c. 27 to c. 17 MJ/m2.
Genotyping error and amplification failure rates
Across all replicates and loci, a total of 1872 sample genotypes were analysed for DHI and 3408 were analysed for Mt Gibson. Overall, errors were marginally more frequent at Mt Gibson, with 33% of all samples at this site reporting some type of error, compared to 29% for DHI. The most frequent errors were amplification failures, which accounted for 62% and 87% of all errors for DHI and Mt Gibson respectively. Allelic dropout frequency was highest on DHI where it accounted for 33% of all errors, compared to Mt Gibson where it accounted for just 11%. False alleles were less frequent at both sites, accounting for 4% and 2% of errors at DHI and Mt Gibson respectively.
Error rates for specific loci appeared to be random, with few clear patterns emerging for either DHI or Mt Gibson sample replicates for allelic dropout or false alleles. Allelic dropout was evenly distributed between 0 and 1.5% of all replicates at seven loci for DHI and between 0 and 1% at five loci for Mt Gibson (Supplementary Fig. S3). False alleles were identified at six loci for DHI, of which three also had false alleles identified for Mt Gibson. The rate of amplification failure for locus BHW33 was the highest of any error at any locus [23.9% at DHI and 67% for Mt Gibson (Fig. S3)] and significantly higher than most other loci at both sites (P < 0.05, Kruskal–Wallis test). Locus BHW24 also had relatively high amplification failure rates (19% for DHI and 33% for Mt Gibson) but these were comparable to other loci at each site.
Error rates with exposure time
Successful amplification rates decreased with increasing exposure time, from means of 98.1% and 94.1% for DHI and Mt Gibson respectively at Day 1 to 49.1% and 44.4% by Day 28/30 (Table 2). However, while mean amplification rates continued to decrease to 60 days at Mt Gibson (30.1%), the mean rate increased at DHI to 73.1%. The pattern for genotyping errors was less apparent (Table 2). False alleles were not recorded before Day 7 and remained uncommon. Values at DHI were consistently between 1.4 and 1.9%, while means at Mt Gibson increased from 0.3% at 7 days to 1.8% at Day 60. Allelic dropout rates again increased over exposure time at both sites, although values peaked much higher at DHI at 47.2% on Day 30, compared to a peak of 17.2% on Day 60 at Mt Gibson. Control values for all three metrics at Day 0 (DHI only) were close to identical to the Day 1 treatment (Table 2). Amplification rates were high after storage at room temperature, −20°C and −80°C for 90 days, with values of 82.5%, 100% and 94.4% respectively. Genotyping errors for all ex-situ storage treatments were correspondingly low.
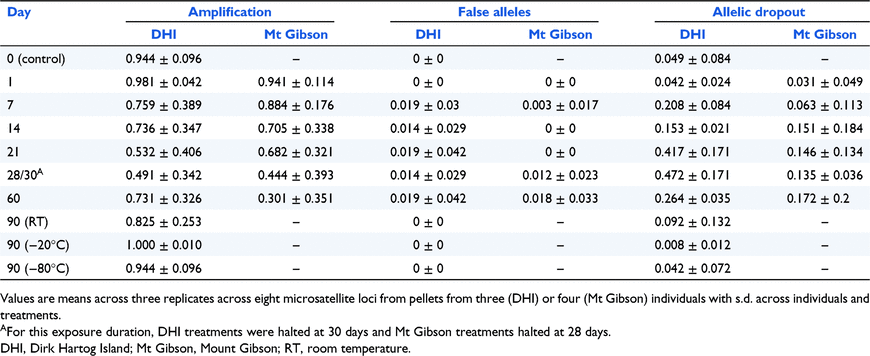
|
Error rates with treatment
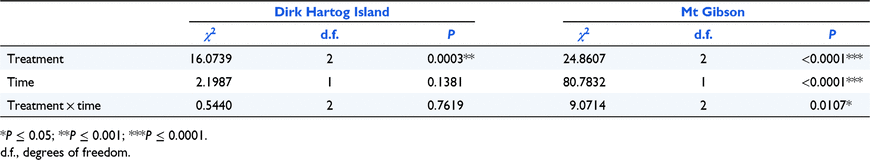
Combined (total) error rates generally increased with increasing time in all three solar exposure treatments for both sites (Fig. 2). Error rates were highest in the sun treatment and lowest in the shade treatment for every exposure time, with the exception of Day 14 at Mt Gibson, where the sun treatment samples had lower error rates than both the part-shade and shade treatments. Error rates in the shade treatment were almost universally the lowest of the three treatments. As previously discussed, error rates for DHI were actually lower at 60 days than at 28 days and this was consistent for all three treatments.
The error bars in Fig. 2 indicate a high degree of variation between individuals within treatments, except genotyping error rates at Mt Gibson which were consistently low. Variation between individuals was highest for the sun treatment for DHI but the shade treatment at Mt Gibson displayed more variation, particularly after 14 days exposure. Results of the repeated-measures mixed-model ANOVA (Table 3) showed that, at DHI, there was a significant difference (P < 0.001) in amplification failure resulting from treatment types. At Mt Gibson, this effect was highly significant (P < 0.0001), but similarly, time of exposure was also highly significant (P < 0.0001). The interaction between treatment and time was also significant at Mt Gibson, but less so (P < 0.05).
Discussion
Capture–recapture approaches to estimating population size using non-invasive sampling methods, such as individual genotypes obtained from faecal DNA, have been used for estimating abundance in a range of mammals (Lukacs and Burnham 2005), including some Australian species such as the greater bilby (Macrotis lagotis) (Dziminski and Carpenter 2018) and brush-tailed rock-wallaby (Petrogale penicillata) (Piggott et al. 2006). Some studies that have engaged the use of faecal DNA in a capture–recapture modelling framework have obtained improved population estimates (Goode et al. 2014; Fuller et al. 2016; Woodruff et al. 2016) and found them superior to traditional live-capture approaches (Rodgers and Janečka 2013; Sabino-Marques et al. 2018; Dziminski et al. 2021). Pilot studies to quantify the rate of degradation of DNA (through genotyping and amplification error rates) in environmental conditions are recommended for developing robust survey methodologies (Taberlet et al. 1999; Valière et al. 2006; Luikart et al. 2010).
To undertake such a pilot study for L. fasciatus, we developed an array of eight novel, polymorphic microsatellite markers, which were successfully used to genotype individuals from faecal DNA and may be more broadly useful in population monitoring of other sites in addition to those included here. Markers were arrayed in two PCR multiplexes to enable rapid and cost-effective genotyping. Additionally, six markers were designed to be species-specific for banded hare-wallaby and two were included to amplify both banded and rufous hare-wallaby. While not directly relevant to this trial, this is a useful development for future use in the field to identify when amplification failure of species-specific markers is caused by mistaken sampling of the latter species, rather than poor quality DNA. The markers used in this trial were able to discriminate between individual genotypes (with a low probability of identity for all loci in combination), despite the relatively small number of individual animals (three to four) used in this study.
We undertook in-situ trials to quantify genotyping error and amplification failure rates in banded hare-wallaby faecal DNA samples when exposed to ambient conditions at two locations where this species has been translocated, and ex-situ trials to assess the effect of long-term storage of pellets. We also sought to evaluate the use of faecal DNA as a population monitoring tool, as previous sampling approaches for the banded hare-wallaby have proved difficult (live-capture; Richards et al. 2001) or have been time- and labour-intensive (e.g. distance sampling; Chapman et al. 2015). While this study was limited in terms of our ability to discriminate the specific effects of various interacting variables at different locations, it provided useful insight into the feasibility of using faecal DNA to discriminate individual genotypes in this species at locations where active monitoring is required.
Amplification failure was particularly high in one locus (BHW33) at both trial sites, indicating this marker is more prone to failure than the others in the array and replacement with an alternative marker that is more reliable may be worthwhile. Given the rapid increase in accessibility of genomic markers in recent years, the development of single-nucleotide polymorphism (SNP) markers to replace or complement the current microsatellite array may be worth investigating if faecal DNA monitoring is to be adopted as a formal monitoring tool. SNPs are less prone to false alleles or allelic dropout and are consequently less ambiguous, can be used for high-throughput genotyping (Fabbri et al. 2012; Carroll et al. 2018) and have been used successfully in faecal DNA studies (Blåhed, et al. 2019; Bourgeois et al. 2019). While SNP markers may incur higher developmental costs, these may be offset in the long term by providing a more efficient method of obtaining individual genotypes.
Regardless of genetic methodology, our in-situ exposure trials indicated that DNA can be successfully extracted and amplified from banded hare-wallaby faecal pellets at least 60 days after deposition. However, genotyping quality declined rapidly after 14 days exposure with a high degree of variation in error rates between individuals evident. Although we were unable to discriminate target species DNA from dietary items in our DNA eluates, we found that DNA concentration typically declined below detectable limits (∼1 ng/μL) after 14 days (data not shown) which likely contributed to the increasing variation in genotyping quality beyond this time point.
Despite high s.d. across treatment types, three replicates per sample was sufficient to ensure a mean successful amplification rate of >50%. Amplification success in the 90-day ex-situ trial was high and comparable to results from 1-day duration samples, showing the reduction in amplification rates in the treatments was mainly due to exposure to environmental variables and not solely time since deposition. It also provides confidence that faecal pellets can be stored for at least a short period of time if laboratory analyses cannot be undertaken immediately.
Error rates were most strongly influenced by the type of exposure treatment, with error rates for shade and part-shade treatment generally lower than for the full-sun treatment. Duration of exposure was also significant for the Mt Gibson trial but was not significant for DHI, and amplification success was actually higher at 60 days at this site than at 21 and 30 days. Potentially this could relate to uncontrolled factors (e.g. bacteria) that may inhibit amplification decreasing with exposure duration, although this is purely speculative. These results highlight the unpredictability of rates of DNA degradation and how unknown and uncontrolled factors may play a role. After amplification failure, the most common genotyping error was allelic dropout, which corresponds with previous findings in similar studies (Piggott 2004; Carpenter and Dziminski 2017). However, this rate was consistently lower at Mt Gibson than DHI across the duration of the study. Since allelic dropout rates will tend to increase with declining DNA quantities (Morin et al. 2016), this possibly indicates that DNA quantities and quality generally remained higher for longer in the Mt Gibson study than for DHI. Genotyping errors (particularly allelic dropout) can be problematic for capture–recapture studies (Taberlet et al. 1999), but this can be partially mitigated through the use of appropriately robust modelling methods that allow for genotype uncertainty (Wright et al. 2009).
The reason for the variation between locations is not clear but could relate to differences in ambient environmental conditions. As per our predictions, relative humidity was higher at DHI and, for the first half of the trial at least, it was generally warmer on DHI as well. However, solar exposure was proportionally higher at Mt Gibson, which was not expected. We suggest that, while higher temperature and moisture may influence the overall decline in DNA quantity on DHI [as observed by King et al. (2018)], by Day 60, the effect of increased solar exposure at Mt Gibson may ultimately have resulted in lower rates of amplification success at this site. Both DHI and Mt Gibson receive most of their annual rainfall in winter (Bureau of Meteorology 2021), so early summer may be the optimal period for faecal DNA monitoring, when moisture levels are decreasing but before temperatures and solar exposure peak in mid- to late-summer (DeMay et al. 2013).
In an operational monitoring context, the discrimination of fresh scats from those that have received more environmental exposure would be useful to avoid collecting samples that may not be usable for subsequent analyses or could reduce their accuracy. The appearance of faecal pellets may be influenced by other factors such as diet and individual variation, making it hard to develop standardised collection criteria. Here, we observed that freshly deposited scats (less than 7 days old) retained a glossy surface that was gradually lost with increased exposure duration. This feature of freshly deposited pellets may assist with their identification as such and should be further tested.
In summary, this trial found that sampling of faecal DNA shows promise for population monitoring of banded hare-wallabies, although improvements to the methodology could be made. Even though a small number of individuals were used in this study, the eight microsatellite markers developed were able to successfully discriminate individual genotypes. Quantifying the effect of exposure duration on genotyping error and amplification failure rates is key to developing a robust survey design and we found that error rates did increase with duration of exposure, but this was highly variable amongst individuals and locations. Treatment type was found to have a large influence on amplification success with shade and part-shade treatments having lower error rates than full-sun treatments. As banded hare-wallabies show a preference for shady dense Acacia-shrubland communities (Short et al. 1992, 1998) this may help to facilitate amplification success. Continued refinement of this method should include undertaking trials on scats collected from the wild and the identification of SNP loci which may increase the discriminatory power of the current array of microsatellite markers.
Supplementary material
Supplementary material is available online.
Ethical approval
The translocations of banded hare-wallabies to Mt Gibson and Dirk Hartog Island were carried out under Department of Biodiversity, Conservation and Attractions Animal Ethics Committee approvals 2017-25 and 2018-14A respectively.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
The Dirk Hartog Island National Park Ecological Restoration Project is funded by the Gorgon-Barrow Island Net Conservation Benefits Fund. This research did not receive any specific funding.
Acknowledgements
The authors acknowledge the Malgana and Badimaya peoples as the Traditional Owners of the land in the Shark Bay (Gutharraguda) and Mt Gibson regions respectively. The authors would like to thank Fiona Carpenter for her advice on design and material requirements for this study. We also thank those involved in set-up and running of the DNA degradation trials on Dirk Hartog Island (Aline Gibson Vega, Gary Pekel, Colleen Sims and Lucie Scheelen) and Mt Gibson (Adele Thomasz, Carly Moir, Georgia Volck, Raquel Parker, Rebecca O’Rourke). Argosee Greenhouse Technology supported this study by supplying Plexiglas at a significant discount.
References
Australian Wildlife Conservancy (2018a) ‘Movement and Habitat use of Banded Hare-wallabies Posttranslocation: Faure Island (2013) and Mt Gibson (2017).’ (Australian Wildlife Conservancy: Perth, Australia)Australian Wildlife Conservancy (2018b) ‘Mt Gibson Mammal Translocation Summary May 2018.’ (Australian Wildlife Conservancy: Perth, Australia)
Ballard G, Meek PD, Doak S, Fleming PJS, Sparkes J (2014) Camera traps, sand plots and known events: what do camera traps miss? In ‘Camera Trapping: Wildlife Management and Research’. (Eds PM P Fleming, P Banks, G Ballard, A Claridge, J Sanderson, D Swann) pp. 189–202. (CSIRO Publishing: Melbourne, Vic., Australia)
Banks, SC, Hoyle, SD, Horsup, A, Sunnucks, P, and Taylor, AC (2003). Demographic monitoring of an entire species (the northern hairy-nosed wombat, Lasiorhinus krefftii) by genetic analysis of non-invasively collected material. Animal Conservation 6, 101–107.
| Demographic monitoring of an entire species (the northern hairy-nosed wombat, Lasiorhinus krefftii) by genetic analysis of non-invasively collected material.Crossref | GoogleScholarGoogle Scholar |
Blåhed, I-M, Ericsson, G, and Spong, G (2019). Noninvasive population assessment of moose (Alces alces) by SNP genotyping of fecal pellets. European Journal of Wildlife Research 65, 96.
| Noninvasive population assessment of moose (Alces alces) by SNP genotyping of fecal pellets.Crossref | GoogleScholarGoogle Scholar |
Bourgeois, S, Kaden, J, Senn, H, Bunnefeld, N, Jeffery, KJ, Akomo-Okoue, EF, Ogden, R, and McEwing, R (2019). Improving cost-efficiency of faecal genotyping: new tools for elephant species. PLoS ONE 14, e0210811.
| Improving cost-efficiency of faecal genotyping: new tools for elephant species.Crossref | GoogleScholarGoogle Scholar | 30699177PubMed |
Brinkman, TJ, Schwartz, MK, Person, DK, Pilgrim, KL, and Hundertmark, KJ (2010). Effects of time and rainfall on PCR success using DNA extracted from deer fecal pellets. Conservation Genetics 11, 1547–1552.
| Effects of time and rainfall on PCR success using DNA extracted from deer fecal pellets.Crossref | GoogleScholarGoogle Scholar |
Burbidge, AA, and Woinarski, J (2016). Lagostrophus fasciatus. The IUCN Red List of Threatened Species 2016 , e.T11171A21955969.
| Lagostrophus fasciatus.Crossref | GoogleScholarGoogle Scholar |
Bureau of Meteorology (2021) Climate classification maps. Available at http://www.bom.gov.au/jsp/ncc/climate_averages/climate-classifications. [Accessed 26 February 2021]
Carpenter, FM, and Dziminski, MA (2017). Breaking down scats: degradation of DNA from greater bilby (Macrotis lagotis) faecal pellets. Australian Mammalogy 39, 197–204.
| Breaking down scats: degradation of DNA from greater bilby (Macrotis lagotis) faecal pellets.Crossref | GoogleScholarGoogle Scholar |
Carroll, EL, Bruford, MW, DeWoody, JA, Leroy, G, Strand, A, Waits, L, and Wang, J (2018). Genetic and genomic monitoring with minimally invasive sampling methods. Evolutionary Applications 11, 1094–1119.
| Genetic and genomic monitoring with minimally invasive sampling methods.Crossref | GoogleScholarGoogle Scholar | 30026800PubMed |
Chao, A (2001). An overview of closed capture–recapture models. Journal of Agricultural, Biological, and Environmental Statistics 6, 158–175.
| An overview of closed capture–recapture models.Crossref | GoogleScholarGoogle Scholar |
Chapman TF, Sims C, Thomas ND, Reinhold L (2015) Assessment of mammal populations on Bernier and Dorre Island 2006–2013. Department of Parks and Wildlife, Perth, Australia.
Chiron, F, Hein, S, Chargé, R, Julliard, R, Martin, L, Roguet, A, and Jacob, J (2018). Validation of hair tubes for small mammal population studies. Journal of Mammalogy 99, 478–485.
| Validation of hair tubes for small mammal population studies.Crossref | GoogleScholarGoogle Scholar |
Cole, JR, Langford, DG, and Gibson, DF (1994). Capture myopathy in Lagorchestes hirsutus (Marsupialia: Macropodidae). Australian Mammalogy 17, 137–138.
| Capture myopathy in Lagorchestes hirsutus (Marsupialia: Macropodidae).Crossref | GoogleScholarGoogle Scholar |
Cowen S, Rayner K, Sims C, Morris K (2018) Dirk Hartog Island National Park Ecological Restoration Project: stage one – trial hare-wallaby translocations and monitoring. Department of Biodiversity, Conservation and Attractions, Perth, Australia.
De Bondi, ND, White, JG, Stevens, M, and Cooke, R (2010). A comparison of the effectiveness of camera trapping and live trapping for sampling terrestrial small-mammal communities. Wildlife Research 37, 456–465.
| A comparison of the effectiveness of camera trapping and live trapping for sampling terrestrial small-mammal communities.Crossref | GoogleScholarGoogle Scholar |
DeMay, SM, Becker, PA, Eidson, CA, Rachlow, JL, Johnson, TR, and Waits, LP (2013). Evaluating DNA degradation rates in faecal pellets of the endangered pygmy rabbit. Molecular Ecology Resources 13, 654–662.
| Evaluating DNA degradation rates in faecal pellets of the endangered pygmy rabbit.Crossref | GoogleScholarGoogle Scholar | 23590236PubMed |
Department of the Environment (2019) Lagostrophus fasciatus fasciatus in species profile and threats database. Commonwealth Department of the Environment, Canberra, Australia.
DEWHA (2010) Survey guidelines for Australia’s threatened frogs: guidelines for detecting frogs listed as threatened under the Environment Protection and Biodiversity Conservation Act 1999. Department of the Environment, Water, Heritage and the Arts, Canberra, Australia.
DSEWPaC (2011a) Survey guidelines for Australia’s threatened mammals: guidelines for detecting mammals listed as threatened under the Environment Protection and Biodiversity Conservation Act 1999. Department of Sustainability, Environment, Water, Population and Communities, Canberra, Australia.
DSEWPaC (2011b) Survey guidelines for Australia’s threatened reptiles: guidelines for detecting reptiles listed as threatened under the Environment Protection and Biodiversity Conservation Act 1999. Department of Sustainability, Environment, Water, Population and Communities, Canberra, Australia.
Dziminski MA, Carpenter F (2018) The conservation and management of the bilby (Macrotis lagotis) in the Pilbara, Annual Report 2017-18. Department of Biodiversity, Conservation and Attractions, Perth, Australia.
Dziminski, MA, Carpenter, FM, and Morris, F (2021). Monitoring the abundance of wild and reintroduced bilby populations. The Journal of Wildlife Management 85, 240–253.
| Monitoring the abundance of wild and reintroduced bilby populations.Crossref | GoogleScholarGoogle Scholar |
Efford, MG, and Fewster, RM (2013). Estimating population size by spatially explicit capture–recapture. Oikos 122, 918–928.
| Estimating population size by spatially explicit capture–recapture.Crossref | GoogleScholarGoogle Scholar |
Fabbri, E, Caniglia, R, Mucci, N, Thomsen, HP, Krag, K, Pertoldi, C, Loeschcke, V, and Randi, E (2012). Comparison of single nucleotide polymorphisms and microsatellites in non-invasive genetic monitoring of a wolf population. Archives of Biological Sciences 64, 321–335.
| Comparison of single nucleotide polymorphisms and microsatellites in non-invasive genetic monitoring of a wolf population.Crossref | GoogleScholarGoogle Scholar |
Fuller, AK, Sutherland, CS, Royle, JA, and Hare, MP (2016). Estimating population density and connectivity of American mink using spatial capture–recapture. Ecological Applications 26, 1125–1135.
| Estimating population density and connectivity of American mink using spatial capture–recapture.Crossref | GoogleScholarGoogle Scholar | 27509753PubMed |
Garden, JG, McAlpine, CA, Possingham, HP, and Jones, DN (2007). Using multiple survey methods to detect terrestrial reptiles and mammals: what are the most successful and cost-efficient combinations? Wildlife Research 34, 218–227.
| Using multiple survey methods to detect terrestrial reptiles and mammals: what are the most successful and cost-efficient combinations?Crossref | GoogleScholarGoogle Scholar |
Goode, MJ, Beaver, JT, Muller, LI, Clark, JD, van Manen, FT, Harper, CA, and Basinger, PS (2014). Capture–recapture of white-tailed deer using DNA from fecal pellet groups. Wildlife Biology 20, 270–278.
| Capture–recapture of white-tailed deer using DNA from fecal pellet groups.Crossref | GoogleScholarGoogle Scholar |
Harestad, AS, and Bunnell, FL (1987). Persistence of black-tailed deer fecal pellets in coastal habitats. The Journal of Wildlife Management 51, 33–37.
| Persistence of black-tailed deer fecal pellets in coastal habitats.Crossref | GoogleScholarGoogle Scholar |
Jones C, McShea WJ, Conroy MJ, Kunz TH (1996) Capturing mammals. In ‘Measuring and Monitoring Biological Diversity: Standard Methods for Mammals’. (Eds DE Wilson, FR Cole, JD Nichols, R Rudran, MS Foster) pp. 115–156. (Smithsonian Institution Press: Washington, DC, USA)
King, SRB, Schoenecker, KA, Fike, JA, and Oyler-McCance, SJ (2018). Long-term persistence of horse fecal DNA in the environment makes equids particularly good candidates for noninvasive sampling. Ecology and Evolution 8, 4053–4064.
| Long-term persistence of horse fecal DNA in the environment makes equids particularly good candidates for noninvasive sampling.Crossref | GoogleScholarGoogle Scholar | 29721279PubMed |
Lindenmayer, DB, Gibbons, P, Bourke, M, Burgman, M, Dickman, CR, Ferrier, S, Fitzsimons, J, Freudenberger, D, Garnett, ST, Groves, C, Hobbs, RJ, Kingsford, RT, Krebs, C, Legge, S, Lowe, AJ, McLean, R, Montambault, J, Possingham, H, Radford, J, Robinson, D, Smallbone, L, Thomas, D, Varcoe, T, Vardon, M, Wardle, G, Woinarski, J, and Zerger, A (2012). Improving biodiversity monitoring. Austral Ecology 37, 285–294.
| Improving biodiversity monitoring.Crossref | GoogleScholarGoogle Scholar |
Luikart, G, Ryman, N, Tallmon, DA, Schwartz, MK, and Allendorf, FW (2010). Estimation of census and effective population sizes: the increasing usefulness of DNA-based approaches. Conservation Genetics 11, 355–373.
| Estimation of census and effective population sizes: the increasing usefulness of DNA-based approaches.Crossref | GoogleScholarGoogle Scholar |
Lukacs, PM, and Burnham, KP (2005). Review of capture–recapture methods applicable to noninvasive genetic sampling. Molecular Ecology 14, 3909–3919.
| Review of capture–recapture methods applicable to noninvasive genetic sampling.Crossref | GoogleScholarGoogle Scholar | 16262847PubMed |
Meek, PD, Ballard, G-A, and Fleming, PJS (2015). The pitfalls of wildlife camera trapping as a survey tool in Australia. Australian Mammalogy 37, 13–22.
| The pitfalls of wildlife camera trapping as a survey tool in Australia.Crossref | GoogleScholarGoogle Scholar |
Meglécz, E, Costedoat, C, Dubut, V, Gilles, A, Malausa, T, Pech, N, and Martin, J-F (2010). QDD: a user-friendly program to select microsatellite markers and design primers from large sequencing projects. Bioinformatics 26, 403–404.
| QDD: a user-friendly program to select microsatellite markers and design primers from large sequencing projects.Crossref | GoogleScholarGoogle Scholar | 20007741PubMed |
Mills, LS, Citta, JJ, Lair, KP, Schwartz, MK, and Tallmon, DA (2000). Estimating animal abundance using noninvasive DNA sampling: promise and pitfalls. Ecological Applications 10, 283–294.
| Estimating animal abundance using noninvasive DNA sampling: promise and pitfalls.Crossref | GoogleScholarGoogle Scholar |
Morin, DJ, Kelly, MJ, and Waits, LP (2016). Monitoring coyote population dynamics with fecal DNA and spatial capture–recapture. The Journal of Wildlife Management 80, 824–836.
| Monitoring coyote population dynamics with fecal DNA and spatial capture–recapture.Crossref | GoogleScholarGoogle Scholar |
Mortelliti, A, and Boitani, L (2008). Inferring red squirrel (Sciurus vulgaris) absence with hair tubes surveys: a sampling protocol. European Journal of Wildlife Research 54, 353–356.
| Inferring red squirrel (Sciurus vulgaris) absence with hair tubes surveys: a sampling protocol.Crossref | GoogleScholarGoogle Scholar |
Mowat, G, and Strobeck, C (2000). Estimating population size of grizzly bears using hair capture, DNA profiling, and mark–recapture analysis. The Journal of Wildlife Management 64, 183–193.
| Estimating population size of grizzly bears using hair capture, DNA profiling, and mark–recapture analysis.Crossref | GoogleScholarGoogle Scholar |
Murphy, MA, Kendall, KC, Robinson, A, and Waits, LP (2007). The impact of time and field conditions on brown bear (Ursus arctos) faecal DNA amplification. Conservation Genetics 8, 1219–1224.
| The impact of time and field conditions on brown bear (Ursus arctos) faecal DNA amplification.Crossref | GoogleScholarGoogle Scholar |
Nichols, JD (1992). Capture–recapture models. BioScience 42, 94–102.
| Capture–recapture models.Crossref | GoogleScholarGoogle Scholar |
Panasci, M, Ballard, WB, Breck, S, Rodriguez, D, Densmore, LD, Wester, DB, and Baker, RJ (2011). Evaluation of fecal DNA preservation techniques and effects of sample age and diet on genotyping success. The Journal of Wildlife Management 75, 1616–1624.
| Evaluation of fecal DNA preservation techniques and effects of sample age and diet on genotyping success.Crossref | GoogleScholarGoogle Scholar |
Peakall, R, and Smouse, PE (2006). GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Molecular Ecology Resources 6, 288–295.
| GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research.Crossref | GoogleScholarGoogle Scholar |
Peakall, R, and Smouse, PE (2012). GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research – an update. Bioinformatics 28, 2537–2539.
| GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research – an update.Crossref | GoogleScholarGoogle Scholar | 22820204PubMed |
Piggott, MP (2004). Effect of sample age and season of collection on the reliability of microsatellite genotyping of faecal DNA. Wildlife Research 31, 485–493.
| Effect of sample age and season of collection on the reliability of microsatellite genotyping of faecal DNA.Crossref | GoogleScholarGoogle Scholar |
Piggott, MP, Banks, SC, Stone, N, Banffy, C, and Taylor, AC (2006). Estimating population size of endangered brush-tailed rock-wallaby (Petrogale penicillata) colonies using faecal DNA. Molecular Ecology 15, 81–91.
| Estimating population size of endangered brush-tailed rock-wallaby (Petrogale penicillata) colonies using faecal DNA.Crossref | GoogleScholarGoogle Scholar | 16367832PubMed |
Pinheiro J, Bates D, DebRoy S, Sarkar D, Team RC (2020) nlme: linear and nonlinear mixed effects models. R package version 3.1-148. Available at https://CRAN.R-project.org/package=nlme
Pocock, MJO, and Bell, SC (2011). Hair tubes for estimating site occupancy and activity-density of Sorex minutus. Mammalian Biology 76, 445–450.
| Hair tubes for estimating site occupancy and activity-density of Sorex minutus.Crossref | GoogleScholarGoogle Scholar |
Pompanon, F, Bonin, A, Bellemain, E, and Taberlet, P (2005). Genotyping errors: causes, consequences and solutions. Nature Reviews Genetics 6, 847–859.
| Genotyping errors: causes, consequences and solutions.Crossref | GoogleScholarGoogle Scholar | 16304600PubMed |
Ravanat, J-L, Douki, T, and Cadet, J (2001). Direct and indirect effects of UV radiation on DNA and its components. Journal of Photochemistry and Photobiology B: Biology 63, 88–102.
| Direct and indirect effects of UV radiation on DNA and its components.Crossref | GoogleScholarGoogle Scholar |
R Development Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at https://www.R-project.org/.
Richards, JD, Short, J, Prince, RIT, Friend, JA, and Courtenay, JM (2001). The biology of banded (Lagostrophus fasciatus) and rufous (Lagorchestes hirsutus) hare-wallabies (Diprotodontia: Macropodidae) on Dorre and Bernier Islands, Western Australia. Wildlife Research 28, 311–322.
| The biology of banded (Lagostrophus fasciatus) and rufous (Lagorchestes hirsutus) hare-wallabies (Diprotodontia: Macropodidae) on Dorre and Bernier Islands, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Rodgers, TW, and Janečka, JE (2013). Applications and techniques for non-invasive faecal genetics research in felid conservation. European Journal of Wildlife Research 59, 1–16.
| Applications and techniques for non-invasive faecal genetics research in felid conservation.Crossref | GoogleScholarGoogle Scholar |
Ruibal, M, Peakall, R, Claridge, A, Murray, A, and Firestone, K (2010). Advancement to hair-sampling surveys of a medium-sized mammal: DNA-based individual identification and population estimation of a rare Australian marsupial, the spotted-tailed quoll (Dasyurus maculatus). Wildlife Research 37, 27–38.
| Advancement to hair-sampling surveys of a medium-sized mammal: DNA-based individual identification and population estimation of a rare Australian marsupial, the spotted-tailed quoll (Dasyurus maculatus).Crossref | GoogleScholarGoogle Scholar |
Sabino-Marques, H, Ferreira, CM, Paupério, J, Costa, P, Barbosa, S, Encarnação, C, Alpizar-Jara, R, Alves, PC, Searle, JB, Mira, A, Beja, P, and Pita, R (2018). Combining genetic non-invasive sampling with spatially explicit capture–recapture models for density estimation of a patchily distributed small mammal. European Journal of Wildlife Research 64, 44.
| Combining genetic non-invasive sampling with spatially explicit capture–recapture models for density estimation of a patchily distributed small mammal.Crossref | GoogleScholarGoogle Scholar |
Short, J, Bradshaw, SD, Giles, J, Prince, RIT, and Wilson, GR (1992). Reintroduction of macropods (Marsupialia: Macropodoidea) in Australia – a review. Biological Conservation 62, 189–204.
| Reintroduction of macropods (Marsupialia: Macropodoidea) in Australia – a review.Crossref | GoogleScholarGoogle Scholar |
Short, J, Turner, B, Majors, C, and Leone, J (1998). The fluctuating abundance of endangered mammals on Bernier and Dorre Islands, Western Australia – conservation implications. Australian Mammalogy 20, 53–61.
| The fluctuating abundance of endangered mammals on Bernier and Dorre Islands, Western Australia – conservation implications.Crossref | GoogleScholarGoogle Scholar |
Sloane, MA, Sunnucks, P, Alpers, D, Beheregaray, LB, and Taylor, AC (2000). Highly reliable genetic identification of individual northern hairy-nosed wombats from single remotely collected hairs: a feasible censusing method. Molecular Ecology 9, 1233–1240.
| Highly reliable genetic identification of individual northern hairy-nosed wombats from single remotely collected hairs: a feasible censusing method.Crossref | GoogleScholarGoogle Scholar | 10972763PubMed |
Smith, M, Volck, G, Palmer, N, Jackson, C, Moir, C, Parker, R, Palmer, B, and Thomasz, A (2020). Conserving the endangered woylie (Bettongia penicillata ogilbyi): establishing a semi-arid population within a fenced safe haven. Ecological Management & Restoration 21, 108–114.
| Conserving the endangered woylie (Bettongia penicillata ogilbyi): establishing a semi-arid population within a fenced safe haven.Crossref | GoogleScholarGoogle Scholar |
Soulsbury, CD, Gray, HE, Smith, LM, Braithwaite, V, Cotter, SC, Elwood, RW, Wilkinson, A, Collins, LM, and Fisher, D (2020). The welfare and ethics of research involving wild animals: a primer. Methods in Ecology and Evolution 11, 1164–1181.
| The welfare and ethics of research involving wild animals: a primer.Crossref | GoogleScholarGoogle Scholar |
Sunnucks, P, and Hales, DF (1996). Numerous transposed sequences of mitochondrial cytochrome oxidase I–II in aphids of the genus Sitobion (Hemiptera: Aphididae). Molecular Biology and Evolution 13, 510–524.
| Numerous transposed sequences of mitochondrial cytochrome oxidase I–II in aphids of the genus Sitobion (Hemiptera: Aphididae).Crossref | GoogleScholarGoogle Scholar | 8742640PubMed |
Taberlet, P, Waits, LP, and Luikart, G (1999). Noninvasive genetic sampling: look before you leap. Trends in Ecology & Evolution 14, 323–327.
| Noninvasive genetic sampling: look before you leap.Crossref | GoogleScholarGoogle Scholar |
Valière, N, Bonenfant, C, Toïgo, C, Luikart, G, Gaillard, J-M, and Klein, F (2006). Importance of a pilot study for non-invasive genetic sampling: genotyping errors and population size estimation in red deer. Conservation Genetics 8, 69–78.
| Importance of a pilot study for non-invasive genetic sampling: genotyping errors and population size estimation in red deer.Crossref | GoogleScholarGoogle Scholar |
Waits, LP, and Paetkau, D (2005). Noninvasive genetic sampling tools for wildlife biologists: a review of applications and recommendations for accurate data collection. Journal of Wildlife Management 69, 1419–1433.
| Noninvasive genetic sampling tools for wildlife biologists: a review of applications and recommendations for accurate data collection.Crossref | GoogleScholarGoogle Scholar |
White, DJ, Ottewell, K, Spencer, PBS, Smith, M, Short, J, Sims, C, and Mitchell, NJ (2020). Genetic consequences of multiple translocations of the banded hare-wallaby in Western Australia. Diversity 12, .
| Genetic consequences of multiple translocations of the banded hare-wallaby in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Williams BK, Nichols JD, Conroy MJ (2002) ‘Analysis and Management of Animal Populations.’ (Academic Press: San Diego, CA, USA)
Woinarski JCZ, Burbidge AA, Harrison PL (2014) ‘The Action Plan for Australian Mammals 2012.’ (CSIRO Publishing: Melbourne, Vic., Australia)
Woodruff, SP, Johnson, TR, and Waits, LP (2015). Evaluating the interaction of faecal pellet deposition rates and DNA degradation rates to optimize sampling design for DNA-based mark–recapture analysis of Sonoran pronghorn. Molecular Ecology Resources 15, 843–854.
| Evaluating the interaction of faecal pellet deposition rates and DNA degradation rates to optimize sampling design for DNA-based mark–recapture analysis of Sonoran pronghorn.Crossref | GoogleScholarGoogle Scholar | 25522240PubMed |
Woodruff, SP, Lukacs, PM, Christianson, D, and Waits, LP (2016). Estimating Sonoran pronghorn abundance and survival with fecal DNA and capture–recapture methods. Conservation Biology 30, 1102–1111.
| Estimating Sonoran pronghorn abundance and survival with fecal DNA and capture–recapture methods.Crossref | GoogleScholarGoogle Scholar | 26918820PubMed |
Wright, JA, Barker, RJ, Schofield, MR, Frantz, AC, Byrom, AE, and Gleeson, DM (2009). Incorporating genotype uncertainty into mark–recapture-type models for estimating abundance using DNA samples. Biometrics 65, 833–840.
| Incorporating genotype uncertainty into mark–recapture-type models for estimating abundance using DNA samples.Crossref | GoogleScholarGoogle Scholar | 19173702PubMed |