Multiple trans-Torres Strait colonisations by tree frogs in the Litoria caerulea group, with the description of a new species from New Guinea
Paul M. Oliver A F , Eric N. Rittmeyer B , Janne Torkkola C , Stephen C. Donnellan D , Chris Dahl E and Stephen J. Richards D
A F , Eric N. Rittmeyer B , Janne Torkkola C , Stephen C. Donnellan D , Chris Dahl E and Stephen J. Richards D
A Centre for Planetary Health and Food Security, Griffith University, 170 Kessels Road, Brisbane, Qld 4121, and Biodiversity and Geosciences Program, Queensland Museum, South Brisbane, Qld 4101, Australia.
B Division of Ecology and Evolution, Research School of Biology, Australian National University, Canberra, ACT 0200, Australia.
C Snake Out Brisbane, 14 Ranger Street, Kenmore, Qld 4069, Australia.
D South Australian Museum, North Terrace, Adelaide, SA 5000, Australia.
E New Guinea Binatang Research Center, PO Box 604, Madang, Papua New Guinea.
F Corresponding author. Email: p.oliver@griffith.edu.au
Australian Journal of Zoology 68(1) 25-39 https://doi.org/10.1071/ZO20071
Submitted: 26 July 2020 Accepted: 2 March 2021 Published: 20 May 2021
Journal Compilation © CSIRO 2020 Open Access CC BY-NC
Abstract
Australia and New Guinea (together referred to as Sahul) were linked by land for much of the late Tertiary and share many biotic elements. However, New Guinea is dominated by rainforest, and northern Australia by savannah. Resolving patterns of biotic interchange between these two regions is critical to understanding the expansion and contraction of both habitat types. The green tree frog (Litoria caerulea) has a vast range across northern and eastern Australia and New Guinea. An assessment of mitochondrial and morphological diversity in this nominal taxon in New Guinea reveals two taxa. True Litoria caerulea occurs in disjunct savannahs of the Trans-Fly, Central Province and across northern Australia, with very low genetic divergence, implying late Pleistocene connectivity. A previously unrecognised taxon is endemic to New Guinea and widespread in lowland swampy rainforest. Date estimates for the divergence of the new species suggest Pliocene connectivity across lowland tropical habitats of northern Australia and New Guinea. In contrast, the new species shows shallow phylogeographic structuring across the central mountains of New Guinea, implying recent dispersal between the northern and southern lowlands. These results emphasise that the extent and connectivity of lowland rainforest and savannah environments across northern Australia and southern New Guinea have undergone profound shifts since the late Pliocene.
http://zoobank.org/urn:lsid:zoobank.org:pub:A577A415-0B71-4663-B4C1-7271B97298CD
Keywords: Australian Monsoonal Tropics, biotic exchange, Litoria caerulea, Litoria mira sp. nov., New Guinea, rainforest, Sahul, savannah, Trans-Fly, tree frog.
Introduction
Australia and New Guinea (together referred to as Sahul) have a linked geological history, and many shared biotic elements (Schodde and Calaby 1972). However, the dominant climates of these two regions contrast notably, Australia being comparatively much more xeric (Nix 1982). So, while environmental differences may have limited terrestrial biotic dispersal over recent geological times, changing sea levels through the Plio-Pleistocene and formation of land bridges in the region that is now Torres Strait may have periodically aided it (Jones and Torgersen 1988; Reeves et al. 2008). Examination of the divergence history of lineages that occur across Australia and New Guinea offers opportunities to understand how past environmental changes have shaped dispersal and diversification across northern Sahul (Fisher et al. 2013; Norman et al. 2018; Joseph et al. 2019; Tallowin et al. 2020).
Patterns of distribution and divergence for many Australopapuan radiations and species complexes have been examined – especially among reptiles (Wüster et al. 2005; Todd et al. 2014), mammals (Rowe et al. 2008; Macqueen et al. 2010; Mitchell et al. 2014), and birds (Schweizer et al. 2015; Irestedt et al. 2016; Peñalba et al. 2019). At the level of species or species complexes there are generally two extremes in distribution patterns. Some taxa are widespread in New Guinea, but restricted to rainforests in eastern Australia, especially Cape York Peninsula (Irestedt et al. 2016; Natusch et al. 2020). Conversely, some widespread Australian taxa are confined to limited patches of savannah woodlands in southern and eastern New Guinea – the Trans-Fly in central New Guinea, monsoon forest in Central Province in eastern Papua New Guinea, and occasionally through other areas of the Papuan Peninsula (Wüster et al. 2005; Williams et al. 2008; Joseph et al. 2019).
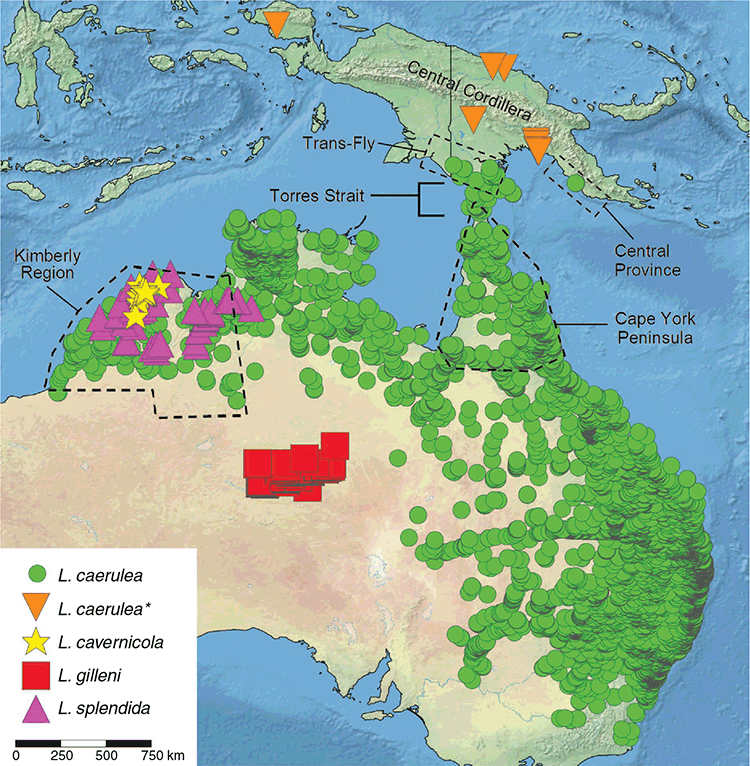
Many frogs (at least 14 nominal species) are shared between Australia and New Guinea (Menzies 2006); however, the phylogeographic history of none of these species has been assessed. Most of these frogs have distributions centred on savannah or monsoonal rainforest habitats and have relatively restricted ranges in southern New Guinea (Menzies 2006). A small number of species that are widespread in New Guinea are more closely associated with rainforest and have restricted ranges in northern Australia (e.g. Litoria eucnemis: Richards et al. 2010). One taxon that shows a distributional pattern that does not neatly fit this dichotomy is Litoria caerulea (popularly referred to as the green tree frog). This species is widespread across the northern and eastern parts of Australia in seasonally wet–dry habitats but tends to be absent from rainforests (Fig. 1). Within New Guinea L. caerulea is well known from the savannahs of the Trans-Fly and around Port Moresby (Tyler 1968; Menzies 2006). Less well known are a series of records from geographically distant areas on both the north and south sides of New Guinea’s Central Cordillera (Fig. 1). Generally, these are from ‘non-savannah’ habitats such as swampy lowland rainforest in high-rainfall areas (Fig. 2a, b) (Dahl et al. 2013; S. J. Richards, pers. obs.). These locations are much wetter, less strongly seasonal, and more densely vegetated than the savannah habitats typically occupied by the Litoria caerulea group in far southern New Guinea and in northern and eastern Australia (Fig. 2c, d).
The distribution of frogs currently referred to L. caerulea is thus relatively unusual, and no other predominantly savannah taxon in Australia has multiple isolated populations extending across New Guinea in rainforest (Williams et al. 2008; Joseph et al. 2019). One hypothesis to explain the scattered records across New Guinea is anthropogenic dispersal. Litoria caerulea often lives in close association with people in both northern Australia and New Guinea (P. M. Oliver, S. J. Richards, E. N. Rittmeyer, pers. obs) and is often accidentally transported in fruit shipments (O’Dwyer et al. 2000; Hartigan et al. 2012). Some New Guinean reptiles also show evidence of past anthropogenic dispersal events, e.g. Carlia (Austin et al. 2011). An alternative hypothesis is that these are natural genetically, ecologically and geographically distinct populations. Here we address these alternative hypotheses by investigating: (1) levels of genetic divergence between populations of L. caerulea across New Guinea and Australia; and (2) testing for morphological differentiation across savannah and non-savannah populations of L. caerulea.
Materials and methods
Sampling
New specimens were collected by hand at night by spotlighting or audiolocation of calling males, humanely field-killed using standard practices (immersion in aqueous MS-222) (Gamble 2014), fixed in formalin, and stored in ethanol. Genetic samples and comparative material used in this study (Table S1, Supplementary Material) are primarily stored at the following institutions: Australian National Wildlife Collection (ANWC), Canberra; Australian Museum (AMS), Sydney; and the South Australian Museum/Australian Biological Tissues Collections (SAMA/ABTC), Adelaide. Some unregistered material uses the acronym SJR, demarcating original field number of Stephen J. Richards. One paratype (SJR15355) will be repatriated to the Papua New Guinea National Museum (PNGNM).
Recordings of calls were made with a Sony TCM-5000 tape recorder and an Edirol R09 digital recorder with a Sennheiser K6 power module and ME66 microphone and were analysed using the sound-analysis package Avisoft SASLab Pro. Air temperature (to the nearest 0.1°C) was measured adjacent to the calling frog with a Digital Thermometer. Terminology and acoustic analysis procedures follow Köhler et al. (2017). Coordinates of localities were obtained using the GPS Datum WGS84.
Genetic data and phylogenetic analyses
Frozen or alcohol-preserved tissues were available from 63 nominal L. caerulea from 33 locations (Table S1, Fig. 1). Additional taxa in the L. caerulea group were also included: L. cavernicola (n = 3), L. gilleni (n = 3) and L. splendida (n = 24). DNA was extracted using a Puregene DNA isolation kit (Gentra Systems, Minneapolis, MN, USA) following manufacturer protocols for DNA purification from solid tissue. A fragment of the mitochondrial genome, including the 3′ end of the NADH dehydrogenase subunit 4 (ND4) gene and the tRNA genes tRNAHis, tRNASer and the 5′ end of tRNALeu (hereafter referred to as ND4), was amplified and sequenced using the forward primers 5- TGACTACCAAAAGCTCATGTAGAAGC-3 or 5-ACCTATGACTACCAAAAGCTCATGTAGAAGC-3 with the reverse primer 5-CATTACTTTTTACTTGGATTTGCACCA-3. Our final genetic dataset comprised 696 bp from the ND4 gene plus 86 bp of tRNA. These were aligned using the MUSCLE algorithm (Edgar 2004) with default settings as implemented in JalView 2.11.0 (Waterhouse et al. 2009), and subsequently checked by eye for missense mutations and correct reading frames.
Partitioning and model strategies were selected using PartitionFinder 2 (Lanfear et al. 2016) run on the CIPRES Web portal ver. 3.1. Model selection using AICc suggested a single data partition combining 1st, 2nd, and 3rd codon and tRNA regions and using the TRN+G model. Phylogenetic analyses for the full mitochondrial dataset were implemented on the CIPRES Web portal and estimated by standard maximum-likelihood (RAxML ver. 8) (Stamatakis 2014) using the GTR-CAT model (the TRN model is not implemented in RAxML). In addition, a neighbour-net phylogenetic network was constructed in SplitsTree ver. 4.15.1v.5 (Huson 1998) with 1000 bootstraps. Uncorrected p-distances were calculated in MEGA X with standard error estimates from 1000 bootstrap replicates and subsequently exported to calculate average divergences within and between clades and species (Kumar et al. 2018).
BEAST ver. 2.5 (Bouckaert et al. 2019) was used to obtain preliminary estimates for the timing of divergence events between populations of L. caerulea in Australia and New Guinea and speciation events within Australia. While presenting these analyses, we acknowledge that additional data from the nuclear genome are required to validate hypotheses about geneflow and divergence times. Because BEAST assumes that each included tip represents an independent population, sampling for L. caerulea was reduced to a single exemplar for each genetically distinctive or geographically disjunct population. These data were aligned with additional data from four species from the Litoria gracilenta group, which is the sister lineage to the L. caerulea group (Rosauer et al. 2009).
Topology and timeframes of divergence were estimated using the uncorrelated lognormal model, Yule speciation prior for 20 million generations, sampling every 20 000, with the first 20% of trees discarded as burn-in, giving a total of 800 trees from which to estimate topological support. There are no published fossil data that provide constraints for the nodes within this relatively limited subgroup within the Pelodryadidae. However, Duellman et al. (2016) estimated basal divergence splits within the L. caerulea group of approximately 7.5 mya, so we used this as a secondary calibration in our analyses, with a 95% posterior distribution of 5–10 mya.
Morphology
Measurements were taken from a total of 79 nominal L. caerulea, including 19 from savannah habitats in southern New Guinea, and 11 from non-savannah habitats in New Guinea. Material of L. cavernicola (n = 6), L. gilleni (n = 9) and L. splendida (n = 10) was also measured. Probable juvenile (smaller than the smallest male with vocal slits) or highly contorted specimens were not measured, even if genotyped. The following measurements (in millimetres) were taken using digital calipers: snout to urostyle length (SUL), from tip of snout to tip of urostyle; tibia length (TL), from outer edge of flexed knee to posterior edge of limb; head length (HL), from posterior edge of tympanic ring to tip of snout; head width (HW) at centres of tympana; eye–snout length (ESN), from anterior corner of eye to tip of snout; eye–naris length (EN) from anterior corner of eye to centre of nostril; internarial width (IN), distance between centres of nostrils; horizontal diameter of tympanic ring (TYM); horizontal diameter of eye (EYE); third finger width (3FW), width of toepad on third finger at widest point; and fourth toe width (4TW), width of toepad on fourth toe at widest point. Additional comparative data on body size, colouration and extent of webbing were taken from Tyler et al. (1977), Tyler and Davies (1979), Richards and Oliver (2006), Kraus (2018) and Oliver et al. (2019b). Due to slight differences from previous publications in the method for measuring IN and EN, we did not compare ratios involving these characters. To determine whether consistent differences in key ratios were apparent between lineages, all morphological variables were log-transformed. Body, head and limb measurements were then corrected for body size by regressing against principal component 1 from a principal component analysis (PCA). To visualise patterns of univariate variation across populations, we used boxplots of the size-corrected morphological data. All morphological analyses were conducted in R (R Core Team 2013).
Results
Mitochondrial phylogenetic relationships and divergence dates
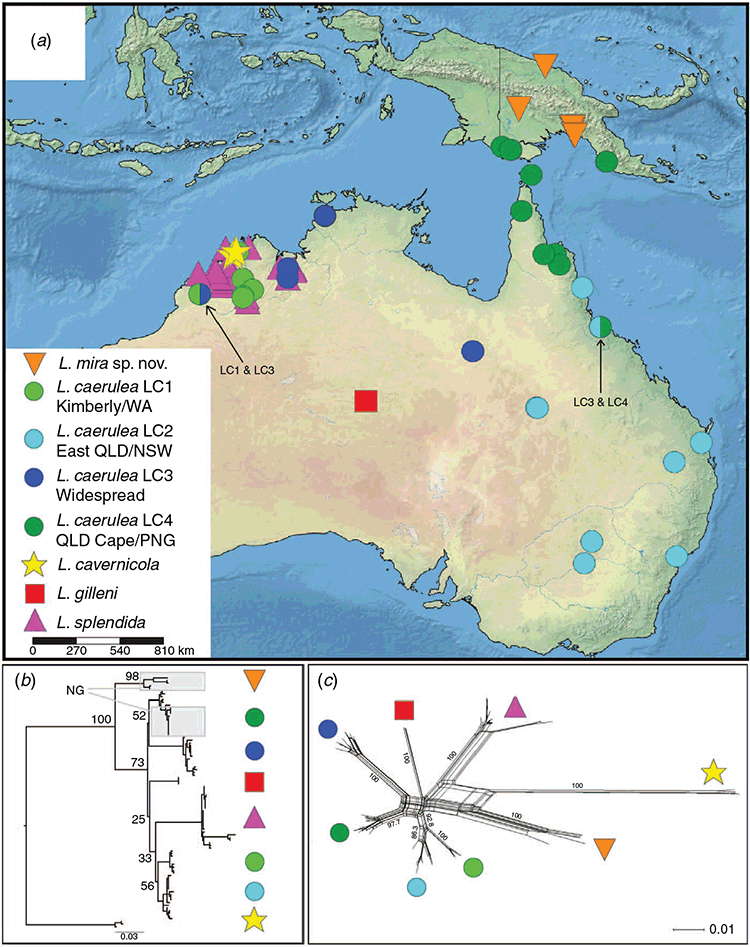
Within the L. caerulea group, L. cavernicola was the sister taxon to all other species in all phylogenetic analyses, and showed the highest genetic divergence from other members of the species group (mean p-distances 0.12–0.14) (Table 1). The remaining lineages (L. gilleni, L. splendida and all samples referred to L. caerulea) formed a strongly supported clade (Figs 3, 4). Within this clade, samples of L. caerulea from non-savannah localities in New Guinea were the sister lineage to remaining populations of L. caerulea + L. gilleni + L. splendida with moderate to strong support. Samples of non-savannah L. caerulea – derived from one locality north of the Central Cordillera and two clusters of localities south of the Cordillera – showed moderate genetic divergence between sites (between-site p-distances ranged across 0.026–0.030).

|
The remaining samples of L. caerulea formed four geographically cohesive and strongly supported clades, but with unresolved relationships between them (Fig. 3a–c): LC1 – the Kimberley of north-western Australia; LC2 – across New South Wales and much of Queensland; LC3 – across the Monsoonal Tropics from north-eastern Western Australia through the Northern Territory and into western Queensland; LC4 – north Queensland, Torres Strait and isolated populations in the Trans-Fly and Central Province savannah regions of southern New Guinea. Average uncorrected p-distances between these four lineages ranged from 0.044 to 0.059 (Table 1). These mitochondrial lineages show overlap in places, and were sampled in sympatry at Townsville, Queensland (LC2, LC4) and at Derby, Western Australia (LC1, LC3) (Fig. 4). Previous allozyme analysis (Donnellan et al. 2000) did not detect any diagnostic-level differentiation in pairwise comparisons within either of these pairs of clades. Phylogenetic relationships between these four lineages of savannah L. caerulea plus L. gilleni and L. splendida were unresolved (Figs 3, 4).
Within the savannah L. caerulea clade occurring across Cape York Peninsula and New Guinea (LC4), genetic divergence between Central Province and Trans-Fly populations was negligible (0–4 bp differing across the 782 bp of aligned data), and the most common haplotype (7 out of 11 samples from southern New Guinea) was shared across the Trans-Fly and Port Moresby regions. New Guinean samples were most genetically similar to a sample from Waraber Island in Torres Strait. The clade comprising samples from New Guinea and Torres Strait was in turn nested within a cluster of lineages from northern Queensland.
Dating analyses estimated that the non-savannah L. caerulea in New Guinea diverged from the ancestor of savannah L. caerulea, L. gilleni and L. splendida during the Pliocene (Fig. 4). New Guinean and northern Queensland samples of the savannah populations were not added into the dating analyses because genetic data indicate that until very recently they represented a single population, making phylogeny-based divergence dating inappropriate. Indeed, the low level of genetic divergence between samples from the Trans-Fly, Central Province, and northern Australia implies very recent connectivity across these areas. A late Pliocene to early Pleistocene timeframe for the radiation of L. caerulea, L. gilleni and L. splendida across savannahs of northern Australia is inferred by us. In contrast, L. cavernicola from the north-western Kimberley is inferred to have diverged from the remainder of the complex during the late Miocene.
Morphology
Univariate analyses of size-corrected morphological data indicated that the non-savannah L. caerulea differed from remaining populations assigned to L. caerulea in head and digit proportions, especially relative head width and, to a lesser extent, head length (Fig. 5). Non-savannah L. caerulea also had smaller maximum sizes than savannah L. caerulea (maximum male SUL length 70.8 mm versus 77.3 mm, maximum female SUL 79.6 mm versus 99.7 mm). All specimens of non-savannah L. caerulea also showed a consistently dark-brown dorsal colouration in both life and preservative and were never green, as observed in other populations of L. caerulea.
Due to small sample sizes and uncertain phylogenetic affinities of many animals examined for morphology, we did not undertake further analyses to look for evidence of differentiation between other, less genetically divergent, lineages of L. caerulea identified. Likewise, as the New Guinean savannah populations of L. caerulea were not genetically divergent from Australian populations, we did not undertake analyses to assess whether they were morphologically divergent.
Discussion
How the biotas of Australia and New Guinea have been linked in space and time is a focal question of Australasian biogeography (Schodde and Calaby 1972; Schweizer et al. 2015; Peñalba et al. 2019). Resolving how connectivity patterns vary across taxa with differing origins (Australian or Melanesian), ecologies and levels of vagility is particularly important for understanding the expansion and contraction of savannah and rainforest habitats across northern Sahul. Our study is the first to present phylogenetic and phylogeographic data for a nominal frog taxon occurring across these two regions, and it provides evidence for both established and novel biogeographic hypotheses and, as we will argue, recognition of a previously undescribed species.
Phylogeography and history of the savannahs of Sahul
Across the Trans-Fly and northern Australia, low genetic divergence has been detected in many taxa (Joseph et al. 2019; Peñalba et al. 2019), and is not surprising given that the two landmasses have been continuous until very recently. Low genetic divergence is especially pronounced in species associated with open savannah woodlands of northern Australia and the Trans-Fly region (Wüster et al. 2005; Doughty et al. 2007; Joseph et al. 2019). Our data indicate that in New Guinea savannahs L. caerulea is restricted to the Trans-Fly and Central Province and shows very low genetic divergence between both areas and with Cape York Peninsula. Low genetic divergence across multiple taxa with varying vagility (Wüster et al. 2005; Joseph et al. 2019; Peñalba et al. 2019) strongly indicates that terrestrial savannah corridor(s) of some description existed between Australia and New Guinea until very recently. In most cases (including L. caerulea) genetic data also indicate that savannah lineages have higher genetic diversity and probable centres of origin in Australia, with recent migration and/or isolation of northern outliers in New Guinea (Joseph et al. 2019).
The nature of historical connections among the savannahs within New Guinea is less well understood (Joseph et al. 2019). Several savannah taxa occur in both the Trans-Fly and Central Province (and a few extend to isolated savannah remnants in Oro and Milne Bay Provinces), even though the intervening area is currently dominated by the floodplains and lowland rainforest of the Fly and Purari River deltas (Joseph et al. 2019). This raises questions as to how and when savannah lineages moved or were isolated across this region. In L. caerulea one haplotype is shared between the Trans-Fly and Central Province, suggesting very recent geneflow. Recent anthropogenic movement between these regions could be invoked to explain low genetic diversity. However, both populations have been known for decades, suggesting that they have not been recently dispersed by human activities. Furthermore, at least two savannah-associated elapid snake taxa with similarly disjunct distributions in the Trans-Fly and Central Province also show negligible genetic diversity (Wüster et al. 2005; Maddock et al. 2017). Together, these data suggest that savannah connectivity existed through areas of Gulf and Western Provinces now covered in rainforest. The nature of this connection remains to be investigated but the overall patterns of disjunct distributions and low genetic diversity suggest that the nature and extent of the rainforest–savannah interface in southern New Guinea has undergone major shifts through recent climatic change.
While not the central focus of this study, our data indicate that the L. caerulea group in Australia also warrants further investigation from both taxonomic and phylogeographic perspectives. Both L. gilleni and L. splendida appear to be closely related to L. caerulea. Their inferred divergence levels suggest Pleistocene speciation, range shifts and extinction across the Australian savannah and arid biomes. Nuclear datasets are required to further resolve patterns of gene flow and relationships between these seemingly closely related lineages and to test whether additional undescribed taxa are involved. The overall implication of a dynamic and shallow history across the interface of the Australia Monsoon Tropics and arid zone mirrors other recent studies of the Australian herpetofauna (Afonso Silva et al. 2017; Oliver et al. 2019a). In contrast, L. cavernicola from the comparatively higher-rainfall zone of the north-western Kimberley appears to be a relatively early (late Miocene) offshoot in the L. caerulea group that is now ecologically specialised and relatively restricted. This taxon further emphasises that the high rainfall zone of the north-western Kimberley has mediated long-term persistence and endemism in many taxa, especially those closely associated with rocky microhabitats and refugia (Oliver et al. 2013; Laver et al. 2018; Rosauer et al. 2018).
Pliocene divergences between Australia and New Guinea
Typically, in Australasian biogeography more mesic habitats are considered sources for lineages that have colonised expanding dry habitats (summarised in Byrne et al. 2011). The savannahs of northern Sahul are much drier than the rainforests in New Guinea despite close geographic and biological links. In this context, the presence of the widespread non-savannah L. caerulea in the lowland swamp forests of New Guinea is somewhat surprising. All other lineages in the L. caerulea group inhabit savannah or at least seasonally arid habitats, most are restricted to Australia, and the lineage tends to be absent or rare in rainforests in eastern Australia (S. J. Richards, pers. obs.). There are two, potentially not mutually exclusive, hypotheses for this apparently anomalous pattern of distribution and relationships across biomes.
First, the L. caerulea group may have shifted into savannahs relatively recently. The two most-divergent lineages in this clade are non-savannah L. caerulea (lowland swamp and rainforests) and L. cavernicola, a relictual cave specialist species from north-western Australia. Both are associated with relatively wet and hot areas with annual rainfall over 1000 mm. This raises the possibility that the shallower clade comprising L. caerulea, L. gilleni and L. splendida may have only radiated into seasonally drier habitats relatively recently. Our preliminary chronogram (Fig. 4) for the L. caerulea group also suggests an early Pliocene divergence between New Guinea and northern Australia. While there has never been a formal analysis of patterns of temporal disjunctions between sister lineages across these two areas, some studies have identified the very late Miocene or early Pliocene as a time of exchange, especially for mesic taxa (Aplin et al. 1993; Norman et al. 2018).
Second, we can find at least one other example of a taxon with likely origins in savannah habitats in Australia occurring in higher-rainfall areas of New Guinea. This is in the plant genus Seringia (Cheek et al. 2018), wherein the northernmost taxon – and only member of this genus in New Guinea – is associated with ultramafic soils in western New Guinea. In both Litoria and Seringia the northernmost taxon occurs in a habitat that is ecologically distinctive and relatively species poor. Non-savannah L. caerulea appear to be closely associated with lowland swamp forest that has a relatively simple community of widespread frogs that often cope well with disturbed or open habitats. As has been noted elsewhere in Australasia, ecological opportunity in the form of distinctive and/or species-poor habitats may increase the probability of outwardly anomalous northward biogeographic shifts (Oliver et al. 2018).
The phylogeographic significance of New Guinea’s Central Cordillera
The Central Cordillera of New Guinea has been hypothesised to be an important barrier to dispersal of lowland taxa; however, results to date suggest timings of genetic divergence ranging from Pleistocene to Miocene (e.g. Unmack et al. 2013; Georges et al. 2014; Oliver et al. 2017; Tallowin et al. 2018; Natusch et al. 2020). The genetic divergences between northern and southern populations of non-savannah L. caerulea are too deep to be explained as an artefact of human dispersal, and furthermore all populations occur in relatively remote areas. Conversely, genetic divergences are shallow enough that they likely postdate even the younger estimate for timing of the uplift of the Central Cordillera approximately 3 million years ago (Quarles van Ufford and Cloos 2005). The Litoria caerulea group as a whole also appears to be a strictly lowland taxon, so recent dispersal across the high, wet and cool mountain ranges of central New Guinea is unlikely. We suggest this genetic pattern again indicates that there is (or was) connectivity across lowland regions either to the western or eastern end of the Central Cordillera. We hypothesise that the west seems more likely, as the vast areas of lowland swamp forests across western New Guinea are inaccessible and little surveyed, and continue to reveal novel and poorly known frog taxa (Oliver et al. 2021). We hypothesise that non-savannah L. caerulea may occur widely through this area. This said, we concede that further sampling is required to better explain the low divergence between northern and southern populations of non-savannah L. caerulea. However, the emergent pattern certainly emphasises that the role of the Central Cordillera in shaping (or reinforcing) divergence between lowland populations in New Guinea varies greatly across taxa.
Systematics
Pending a compelling resolution of generic boundaries within Pelodryadidae the species described herein is assigned to Litoria based on its having a horizontal pupil. The combination of genetic and morphological data presented here indicate that non-savannah L. caerulea from across New Guinea is a morphologically and genetically distinct taxon. This taxon also shows phylogeographic structuring between scattered sites both south and north of the Central Cordillera, suggesting that these populations diverged before widespread human settlement and movement across New Guinea. These datasets therefore refute the hypothesis that the distribution of non-savannah L. caerulea can be explained by human-mediated range shifts (at least for the sample sites for which we have genetic information). The type locality for L. caerulea is ‘New South Wales’, in eastern Australia (Tyler 1968), and no names are available for the undescribed populations of the L. caerulea group from New Guinea (Tyler 1968); hence, we here present a formal description of the non-savannah form as a new species.
Litoria mira Oliver, Rittmeyer, Torkkola,Dahl, Donnellan & Richards, sp. nov.
(Chocolate tree frog)
(Figs 6–8)

|
Holotype
SAMA R71114 (field no. SJR15133), adult male, Purari River Basin (7.3518°S, 145.1904°E), Gulf Province, Papua New Guinea, 30 m a.s.l., collected by S. Richards and E. Nagombi on 13 February 2016.
Paratypes
All from Papua New Guinea (n = 10). SAMA R70446 (field no. SJR 14869), subadult female; SAMA R71655 (SJR15015), adult female, upper Strickland River basin (6.2763°S, 142.1022°E), 110 m. a.s.l., Western Province, collected by K. Aplin and S. Richards between 4 and 10 August 2013; SAMA R71657– R71658, QM J97086 (SJR8879–SJR8880, 8913) adult males, Wamangu Village (3.7870°S, 143.6520°E), East Sepik Province, collected by C. Dahl between 26 and 30 October 2004; SAMA R71117 (SJR15085), female, Purari River Basin (7.4910°S, 145.2189°E), 130 m a.s.l., Gulf Province, collected by S. Richards and E. Nagombi 4 February 2016; SAMA R71116 (SJR15320), female, Purari River Basin (7.7892°S, 145.2664°E), 5 m a.s.l., collected by S. Richards and C. Dahl 9 July 2016; SAMA R71656 (SJR15336), male, SAMA R71115 (SJR15353), male, PNGNM (SJR15355), female, Purari River Basin (7.8566°S, 145.3308°E), 100 m a.s.l., Gulf Province, collected by S. Richards and C. Dahl between 15 and 18 July 2016.
Diagnosis
Litoria mira can be distinguished from all other Litoria by the unique combination of moderately large size (male SUL up to 70.8, female SUL up to 79.6 mm); vomerine teeth present; webbing on hand extending no further than base of penultimate phalanx on fourth finger; limbs without prominent white or yellow lateral folds or ornamentation; lip lacking a white stripe; limbs relatively short and robust (TL/SUL 0.41–0.48); parotoid gland present, but not prominent and not fused with prominent gland on top of head; head relatively narrow (HW/HL 0.91–1.0), distinctly tapering from body; dorsal colouration uniformly brown, without white or yellow spots; small violet patch of skin at postero-ventral edge of eye; and ventral surfaces of limbs, torso and throat with moderate to extensive regions densely stippled with dark to medium brown.
Description of holotype
Adult male with the following measurements (mm): SUL 70.8; TL 29.4; HL 19.9; HW 20.7; ES 8.9; EN 6.5; IN 5.2; EYE 7.3; TYM 4.1; 3FW 3.9; 4TW 3.5. Body robust (Fig. 6), head approximately as wide as long (HL/HW 0.96), narrower than body in dorsal view; snout truncate in dorsal view, rounded in lateral view; labial region very slightly flared; loreal region steeply sloping and slightly concave; canthus rostralis indistinct, weakly curved; nares rounded, much closer to tip of snout than to eyes, oriented laterally, barely visible in dorsal view. Choanae moderate sized, ovoid, separated by a distance ~1.5 times their width; vomerine teeth in rows of 5 or 6 clustered along posterior edge of bifid elevated ridge medial to choanae. Eyes rather small (EYE/SUL 0.10), moderately prominent in dorsal and lateral views, pupil horizontal. Tympanum moderately sized (TYM/SUL 0.58), annulus moderately distinct and raised, dorsal edge obscured by prominent thick downwards-curving supratympanic fold that extends from anterodorsal edge of eye to supra-axillary junction. Skin on dorsal and lateral surfaces of body, head, throat, anterior abdomen, arms and extremities of legs smooth; on posterior abdomen and upper leg coarsely rugose.
Arms robust, without skin folds or ornamentation; fingers with relative lengths III > IV > II > I (Fig. 7a); discs prominent, with circum-marginal grooves, wider than penultimate phalanx on all digits, subarticular tubercles rounded, indistinct, one on digits I and two on II–IV, supernumerary tubercles absent, prominent palmar tubercle at base of digit I. Nuptial excrescence in single small, very indistinct, squarish pad at base of digit I (Fig. 7b). All digits with prominent lateral flanges, webbing basal between I and II, reaching first subarticular tubercles between digits II and III, and reaching second subarticular tubercles between III and IV.
Legs robust, short (TL/SUL 0.42), without skin folds or ornamentation, relative lengths of toes IV > V > III > II > I; terminal discs prominent and expanded, with circum-marginal grooves, wider than penultimate phalanges on all digits; subarticular tubercles rounded, moderately prominent, one on I–II and V, two on III–IV, prominent ovoid inner metatarsal tubercle at base of digit I. All digits webbed, webbing extends just beyond first subarticular tubercle between I–II, to base of disc on II and to first subarticular tubercle on III, to base of disc on outside of III, and to base of penultimate phalanx on both sides of IV and to base of disc on inside of V. Dermal fringes present on all digits.
Dorsal colouration in preservative dark brown over all surfaces with no obvious pattern, upper hindlimbs and digits slightly paler brown than torso. Ventral surfaces pale buff, with large area of dark brown on throat and smaller patches of lighter brown on upper torso, lower forelimbs and across hindlimbs and toes.
Variation
Mensural variation for the six males, four females and one subadult in the type series is presented in Table 2. Females are larger than males. The smallest female (SAMA R70446, SUL 66.7 mm) does not have fully developed eggs. In colouration, all preserved specimens show the same uniformly dark-brown colouration, with tone varying slightly; larger specimens tend to be slightly paler. Venter always pale buff with brown mottling of varying extent and intensity on the throat, anterior portion of torso, fore and hindlimbs, plantar surfaces of webbing and digits.

|
Appearance in life
Generally similar in life as in preservative. Photographs of the holotype (SAMA R70446) show the same uniformly brown dorsal colouration in life (Fig. 6) as in preservative, with the outer edge of the tympanum more sparsely pigmented, and lower lateral regions and extremities of limbs slightly more sparsely pigmented and overall lighter brown. Hidden and exposed surfaces of hindlimbs are uniform brown (Fig. 7c). SAMA R71655 has a greyish dorsal ground colour in life with fine light-brown mottling and maculations. This specimen is dark brown in preservative. All specimens have a small light-violet patch of skin at the posteroventral edge of the eye in life, which appears to be diagnostic for the species. Iris silvery grey, with dense black vermiculations and flecks.
Comparisons
Readily distinguished from all Litoria species outside the L. caerulea group by its combination of large size (adult SUL >60 mm), limbs without prominent skinfolds or lateral ornamentation, absence of a prominent white lip stripe, nictitating membrane without flecks or reticulations, enlarged paratoid gland above tympanum obvious in life, webbing on fingers never extending beyond penultimate subarticular tubercle, snout rounded in lateral view and plain-brown dorsal colouration without any pattern.
Litoria mira differs from L. caerulea in its smaller maximum size (maximum male SUL length 70.8 versus 77.3 mm, maximum female SUL 79.6 versus at least 99.7 mm), generally narrower head (HL/HW mean 0.97, range 0.91–1.0 versus mean 0.9, range 0.78–0.98); presence of a small violet patch of skin at posterio-ventral edge of the eye in life (versus absent), more extensive brown ventral pigmentation on throat, belly and limbs (versus scattered and generally found on throat region only); and consistently dark-brown dorsal colouration in life (Fig. 6, 8a, b) and preservative (versus typically green (Fig. 8c), brownish green or sometimes brown in life, and often blue in preservative). In life, photographs of two individuals also suggest that L. mira has dark-brown hidden surfaces on the thighs (Fig. 7c) (versus typically pink or yellow in L. caerulea); however, data from more specimens are required to further test the strength of this character.
The three other species in the Litoria caerulea group do not occur in New Guinea, and all lack a pale-violet patch of skin at the posterior-ventral edge of the eye. L. mira further differs from L. cavernicola in having a plain dark-brown dorsum (versus usually olive green: Fig. 8d), smooth to moderately granular dorsal skin (versus strongly granular) and larger size (maximum male SUL length 70.8 versus 51.0 mm, maximum female SUL 79.6 versus 57.0 mm); from L. gilleni by its plain dark-brown dorsum (versus olive green or brown with yellow or white spots: Fig. 8e); and from L. splendida by its smaller maximum size (maximum male SUL length 70.8 versus 106.0 mm, maximum female SUL 79.6 versus 118.0 mm), much-less-prominent parotoid gland above eye and snout (versus always apparent in both areas on adults), and dark-brown dorsal colouration in life (versus green with scattered white or yellow flecks: Fig. 8f).
Distribution
The type series originates from three localities spanning provinces north and south of New Guinea’s Central Cordillera (Fig. 1). These scattered records, and low genetic divergence between samples, suggest that the species may occur more widely in the difficult-to-access lowland swamp forests across the island of New Guinea.
Tyler (1968) lists uncatalogued specimens of L. caerulea in the Australian Museum and Australian National University from near Maprik Village in northern Papua New Guinea that we have not been able to locate. These specimens are also most likely to be L. mira. We have been unable to examine one further specimen of ‘L. caerulea’ (RMNH 12370) from Erokwero on the Bird’s Head Peninsula (Vogelkop) in West Papua Province listed by Tyler (1968). It requires further investigation to confirm which member of the L. caerulea group occurs in far western New Guinea.
Habitat and ecology
Collecting localities are in both disturbed and undisturbed lowland swamp forest or swampy rainforest (Fig. 2a, b). The species was typically observed perched on branches within 3 m of the ground.
The advertisement call is a deep, rasping bark repeated in long series, ‘crawk, crawk, crawk …’ (Fig. 9), that is indistinguishable to the ear from that of L. caerulea. Five call series from SAMA R71658 recorded at an air temperature of 25°C contain 33–48 calls (=notes) (mean = 40.4, s.d. = 6.58) produced at a rate of 2.6–2.8 calls s–1 (mean = 2.66, s.d. = 0.08) lasting a total of 13–17.8 s (mean = 14.96, s.d. = 2.51). The first call of each series is the softest and shortest, and amplitude of calls subsequently increases during each series (Fig. 9a) except for the last call, which may have a distinctly lower amplitude than the calls preceding it. Calls last 0.10–0.17 s, and contain 18–23 pulses produced at a rate of ~150 pulses s–1.

|
Other Litoria collected in sympatry with L. mira at sites in southern New Guinea included the widespread lowland species L. auae, L. chloristona, L. congenita, L. infrafrenata, L. pygmaea and L. thesaurensis and in northern New Guinea included L. chrisdahli, L. infrafrenata, L. mucro, L. pygmaea and L. thesaurensis.
Suggested IUCN red list status
Litoria mira is known from widely separated sites in difficult-to-access and relatively undisturbed areas of lowland New Guinea. There are large areas of suitable lowland habitat in the intervening areas, and human population density in most of this region remains low. We suggest that the species be listed as Least Concern.
Etymology
The name mira is the feminine form of the Latin adjective mirum, for surprised or strange, stemming from our surprise in discovering an undescribed member of the predominately Australian L. caerulea group occurring widely across lowland swampy rainforest in New Guinea.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
Fieldwork in Papua New Guinea was supported by Total E&P PNG Limited, ExxonMobil PNG Limited (EMPNG), and Conservation International. We also thank the Papua New Guinea National Research Institute (NRI) and the Papua New Guinea Conservation and Environment Protection Authority (CEPA) for providing relevant visas, research approvals and export permissions. SJR is particularly grateful to Barnabas Wilmott (CEPA) and Georgia Kaipu (NRI) for their assistance. We thank Mark Hutchinson, Carolyn Kovach, Jodi Rowley, Patrick Couper and Andrew Amey for providing access to specimens in their care. CD was partially supported by grants to Vojtech Novotny from the Czech Ministry of Education (CZ.1.07/2.3.00/20.0064, LH11008), the UK Darwin Initiative, and the US National Science Foundation (DEB0211591), and is also grateful to the Wildlife Conservation Society (PNG) and the New Guinea Binatang Research Center for their support. ER was supported by grants from the National Science Foundation (DEB 1402285). PMO was supported by grants from the Australian Research Council, and genetic data collection was supported by a grant from the Australian Biological Resources Survey to SD. Finally, we acknowledge the numerous field assistants for their help and the forest owners for permission to work on their lands.
References
Afonso Silva, A. C., Bragg, J. G., Potter, S., Fernandes, C., Coelho, M. M., and Moritz, C. (2017). Tropical specialist vs. climate generalist: diversification and demographic history of sister species of Carlia skinks from northwestern Australia. Molecular Ecology 26, 4045–4058.| Tropical specialist vs. climate generalist: diversification and demographic history of sister species of Carlia skinks from northwestern Australia.Crossref | GoogleScholarGoogle Scholar | 28543871PubMed |
Aplin, K. P., Baverstock, P. R., and Donnellan, S. C. (1993). Albumin immunological evidence for the time and mode of origin for the New Guinea terrestrial mammal fauna. Science in New Guinea 19, 131–145.
| Albumin immunological evidence for the time and mode of origin for the New Guinea terrestrial mammal fauna.Crossref | GoogleScholarGoogle Scholar |
Austin, C. C., Rittmeyer, E. N., Oliver, L. A., Andermann, J. O., Zug, G. R., Rodda, G. H., and Jackson, N. D. (2011). The bioinvasion of Guam: inferring geographic origin, pace, pattern and process of an invasive lizard (Carlia) in the Pacific using multi-locus genomic data. Biological Invasions 13, 1951–1967.
| The bioinvasion of Guam: inferring geographic origin, pace, pattern and process of an invasive lizard (Carlia) in the Pacific using multi-locus genomic data.Crossref | GoogleScholarGoogle Scholar |
Bouckaert, R., Vaughan, T. G., Barido-Sottani, J., Duchêne, S., Fourment, M., Gavryushkina, A., Heled, J., Jones, G., Kühnert, D., De Maio, N., Matschiner, M., Mendes, F. K., Müller, N. F., Ogilvie, H. A., Du Plessis, L., Popinga, A., Rambaut, A., Rasmussen, D., Siveroni, I., Suchard, M. A., Wu, C. H., Xie, D., Zhang, C., Stadler, T., and Drummond, A. J. (2019). BEAST 2.5: an advanced software platform for Bayesian evolutionary analysis. PLoS Computational Biology 15, e1006650.
| BEAST 2.5: an advanced software platform for Bayesian evolutionary analysis.Crossref | GoogleScholarGoogle Scholar | 30958812PubMed |
Byrne, M., Steane, D. A., Joseph, L., Yeates, D. K., Jordan, G. J., Crayn, D., Aplin, K., Cantrill, D. J., Cook, L. G., Crisp, M. D., Keogh, J. S., Melville, J., Moritz, C., Porch, N., Sniderman, J. M. K., Sunnucks, P., and Weston, P. H. (2011). Decline of a biome: evolution, contraction, fragmentation, extinction and invasion of the Australian mesic zone biota. Journal of Biogeography 38, 1635–1656.
| Decline of a biome: evolution, contraction, fragmentation, extinction and invasion of the Australian mesic zone biota.Crossref | GoogleScholarGoogle Scholar |
Cheek, M., Wanma, J., Jitmau, M., and Jebb, M. (2018). Seringia (Byttneriaceae/Malvaceae-Byttnerioideae) new to Southeast Asia and S. botak endangered in Indonesian New Guinea grassland and savannah. Blumea – Biodiversity, Evolution and Biogeography of Plants 63, 150–156.
| Seringia (Byttneriaceae/Malvaceae-Byttnerioideae) new to Southeast Asia and S. botak endangered in Indonesian New Guinea grassland and savannah.Crossref | GoogleScholarGoogle Scholar |
Dahl, C., Richards, S. J., and Novotny, V. (2013). The Sepik River (Papua New Guinea) is not a dispersal barrier for lowland rain-forest frogs. Journal of Tropical Ecology 29, 477–483.
| The Sepik River (Papua New Guinea) is not a dispersal barrier for lowland rain-forest frogs.Crossref | GoogleScholarGoogle Scholar |
Donnellan, S. C., Tyler, M. J., Monis, P., Barclay, A., and Medlin, A. (2000). Do skin peptide profiles reflect speciation in the Australian treefrog Litoria caerulea (Anura: Hylidae)? Australian Journal of Zoology 48, 33–46.
| Do skin peptide profiles reflect speciation in the Australian treefrog Litoria caerulea (Anura: Hylidae)?Crossref | GoogleScholarGoogle Scholar |
Doughty, P., Maryan, B., Donnellan, S. C., and Hutchinson, M. N. (2007). A new species of taipan (Elapidae: Oxyuranus) from central Australia. Zootaxa 1422, 45–58.
| A new species of taipan (Elapidae: Oxyuranus) from central Australia.Crossref | GoogleScholarGoogle Scholar |
Duellman, W. E., Marion, A. B., and Hedges, S. B. (2016). Phylogenetics, classification, and biogeography of the treefrogs (Amphibia: Anura: Arboranae). Zootaxa 4104, 1–109.
| Phylogenetics, classification, and biogeography of the treefrogs (Amphibia: Anura: Arboranae).Crossref | GoogleScholarGoogle Scholar | 27394762PubMed |
Edgar, R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32, 1792–1797.
| MUSCLE: multiple sequence alignment with high accuracy and high throughput.Crossref | GoogleScholarGoogle Scholar | 15034147PubMed |
Fisher, D. O., Dickman, C. R., Jones, M. E., and Blomberg, S. P. (2013). Sperm competition drives the evolution of suicidal reproduction in mammals. Proceedings of the National Academy of Sciences of the United States of America 110, 17910–17914.
| Sperm competition drives the evolution of suicidal reproduction in mammals.Crossref | GoogleScholarGoogle Scholar | 24101455PubMed |
Gamble, T. (2014). Collecting and preserving genetic material for herpetological research. Herpetolgical Circular No. 41. Society for the Study of Amphibian and Reptiles, Salt Lake City, UT.
Georges, A., Zhang, X., Unmack, P., Reid, B. N., Le, M., and Mccord, W. P. (2014). Contemporary genetic structure of an endemic freshwater turtle reflects Miocene orogenesis of New Guinea. Biological Journal of the Linnean Society 111, 192–208.
| Contemporary genetic structure of an endemic freshwater turtle reflects Miocene orogenesis of New Guinea.Crossref | GoogleScholarGoogle Scholar |
Hartigan, A., Peacock, L., Rosenwax, A., Phalen, D. N., and Šlapeta, J. (2012). Emerging myxosporean parasites of Australian frogs take a ride with fresh fruit transport. Parasites & Vectors 5, 208.
| Emerging myxosporean parasites of Australian frogs take a ride with fresh fruit transport.Crossref | GoogleScholarGoogle Scholar |
Huson, D. H. (1998). SplitsTree: analyzing and visualizing evolutionary data. Bioinformatics 14, 68–73.
| SplitsTree: analyzing and visualizing evolutionary data.Crossref | GoogleScholarGoogle Scholar | 9520503PubMed |
Irestedt, M., Batalha-Filho, H., Roselaar, C. S., Christidis, L., and Ericson, P. G. P. (2016). Contrasting phylogeographic signatures in two Australo-Papuan bowerbird species complexes (Aves: Ailuroedus). Zoologica Scripta 45, 365–379.
| Contrasting phylogeographic signatures in two Australo-Papuan bowerbird species complexes (Aves: Ailuroedus).Crossref | GoogleScholarGoogle Scholar |
Jones, M. R., and Torgersen, T. (1988). Late quaternary evolution of Lake Carpentaria on the Australia–New Guinea continental shelf. Australian Journal of Earth Sciences 35, 313–324.
| Late quaternary evolution of Lake Carpentaria on the Australia–New Guinea continental shelf.Crossref | GoogleScholarGoogle Scholar |
Joseph, L., Bishop, K. D., Wilson, C. A., Edwards, S. V., Iova, B., Campbell, C. D., Mason, I., and Drew, A. (2019). A review of evolutionary research on birds of the New Guinean savannas and closely associated habitats of riparian rainforests, mangroves and grasslands. Emu 119, 317–330.
| A review of evolutionary research on birds of the New Guinean savannas and closely associated habitats of riparian rainforests, mangroves and grasslands.Crossref | GoogleScholarGoogle Scholar |
Köhler, J., Jansen, M., Rodríguez, A., Kok, P. J. R., Toledo, L. F., Emmrich, M., Glaw, F., Haddad, C. F. B., Rödel, M.-O., and Vences, M. (2017). The use of bioacoustics in anuran taxonomy: theory, terminology, methods and recommendations for best practice. Zootaxa 4251, 1–124.
| The use of bioacoustics in anuran taxonomy: theory, terminology, methods and recommendations for best practice.Crossref | GoogleScholarGoogle Scholar | 28609991PubMed |
Kraus, F. (2018). Taxonomy of Litoria graminea (Anura: Hylidae), with descriptions of two closely related new species. Zootaxa 4457, 264–284.
| Taxonomy of Litoria graminea (Anura: Hylidae), with descriptions of two closely related new species.Crossref | GoogleScholarGoogle Scholar | 30314169PubMed |
Kumar, S., Stecher, G., Li, M., Knyaz, C., and Tamura, K. (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution 35, 1547–1549.
| MEGA X: molecular evolutionary genetics analysis across computing platforms.Crossref | GoogleScholarGoogle Scholar | 29722887PubMed |
Lanfear, R., Frandsen, P. B., Wright, A. M., Senfeld, T., and Calcott, B. (2016). Partitionfinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Molecular Biology and Evolution 34, 772–773.
| Partitionfinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses.Crossref | GoogleScholarGoogle Scholar |
Laver, R., Doughty, P., and Oliver, P. (2018). Origins and patterns of endemism in two specialised lizard lineages from the Australian Monsoonal Tropics (Oedura spp.). Journal of Biogeography 45, 142–153.
| Origins and patterns of endemism in two specialised lizard lineages from the Australian Monsoonal Tropics (Oedura spp.).Crossref | GoogleScholarGoogle Scholar |
Macqueen, P., Seddon, J. M., Austin, J. J., Hamilton, S., and Goldizen, A. W. (2010). Phylogenetics of the pademelons (Macropodidae: Thylogale) and historical biogeography of the Australo-Papuan region. Molecular Phylogenetics and Evolution 57, 1134–1148.
| Phylogenetics of the pademelons (Macropodidae: Thylogale) and historical biogeography of the Australo-Papuan region.Crossref | GoogleScholarGoogle Scholar | 20727976PubMed |
Maddock, S. T., Childerstone, A., Fry, B. G., Williams, D. J., Barlow, A., and Wüster, W. (2017). Multi-locus phylogeny and species delimitation of Australo-Papuan blacksnakes (Pseudechis Wagler, 1830: Elapidae: Serpentes). Molecular Phylogenetics and Evolution 107, 48–55.
| Multi-locus phylogeny and species delimitation of Australo-Papuan blacksnakes (Pseudechis Wagler, 1830: Elapidae: Serpentes).Crossref | GoogleScholarGoogle Scholar | 27637992PubMed |
Menzies, J. (2006). ‘The Frogs of New Guinea and the Solomon Islands.’ (Pensoft: Sofia, Moscow.)
Mitchell, K. J., Pratt, R. C., Watson, L. N., Gibb, G. C., Llamas, B., Kasper, M., Edson, J., Hopwood, B., Male, D., Armstrong, K. N., Meyer, M., Hofreiter, M., Austin, J., Donnellan, S. C., Lee, M. S. Y., Phillips, M. J., and Cooper, A. (2014). Molecular phylogeny, biogeography, and habitat preference evolution of marsupials. Molecular Biology and Evolution 31, 2322–2330.
| Molecular phylogeny, biogeography, and habitat preference evolution of marsupials.Crossref | GoogleScholarGoogle Scholar | 24881050PubMed |
Natusch, D. J. D., Esquerré, D., Lyons, J. A., Hamidy, A., Lemmon, A. R., Moriarty Lemmon, E., Riyanto, A., Keogh, J. S., and Donnellan, S. (2020). Species delimitation and systematics of the green pythons (Morelia viridis complex) of Melanesia and Australia. Molecular Phylogenetics and Evolution 142, 106640.
| Species delimitation and systematics of the green pythons (Morelia viridis complex) of Melanesia and Australia.Crossref | GoogleScholarGoogle Scholar |
Nix, H. A. (1982). Environmental determinants of biogeography and evolution in Terra Australis. In ‘Evolution of the Flora and Fauna of Arid Australia’. (Eds W. R. Barker, and P. J. M. Greenslade.) pp. 47–66. (Peacock Publications: Frewville, SA.)
Norman, J. A., Christidis, L., and Schodde, R. (2018). Ecological and evolutionary diversification in the Australo-Papuan scrubwrens (Sericornis) and mouse-warblers (Crateroscelis), with a revision of the subfamily Sericornithinae (Aves: Passeriformes: Acanthizidae). Organisms, Diversity & Evolution 18, 241–259.
| Ecological and evolutionary diversification in the Australo-Papuan scrubwrens (Sericornis) and mouse-warblers (Crateroscelis), with a revision of the subfamily Sericornithinae (Aves: Passeriformes: Acanthizidae).Crossref | GoogleScholarGoogle Scholar |
O’Dwyer, T. W., Buttemer, W. A., and Priddel, D. M. (2000). Inadvertent translocation of amphibians in the shipment of agricultural produce into New South Wales: its extent and conservation implications. Pacific Conservation Biology 6, 40–45.
| Inadvertent translocation of amphibians in the shipment of agricultural produce into New South Wales: its extent and conservation implications.Crossref | GoogleScholarGoogle Scholar |
Oliver, P. M., Laver, R. J., Smith, K. L., and Bauer, A. M. (2013). Long-term persistence and vicariance within the Australian Monsoonal Tropics: the case of the giant cave and tree geckos (Pseudothecadactylus). Australian Journal of Zoology 61, 462–468.
| Long-term persistence and vicariance within the Australian Monsoonal Tropics: the case of the giant cave and tree geckos (Pseudothecadactylus).Crossref | GoogleScholarGoogle Scholar |
Oliver, P. M., Iannella, A., Richards, S. J., and Lee, M. S. Y. (2017). Mountain colonisation, miniaturisation and ecological evolution in a radiation of direct-developing New Guinea frogs (Choerophryne, Microhylidae). PeerJ 5, e3077.
| Mountain colonisation, miniaturisation and ecological evolution in a radiation of direct-developing New Guinea frogs (Choerophryne, Microhylidae).Crossref | GoogleScholarGoogle Scholar | 28382230PubMed |
Oliver, P. M., Blom, M. P. K., Cogger, H. G., Fisher, R. N., Richmond, J. Q., and Woinarski, J. C. Z. (2018). Insular biogeographic origins and high phylogenetic distinctiveness for a recently depleted lizard fauna from Christmas Island, Australia. Biology Letters 14, 20170696.
| Insular biogeographic origins and high phylogenetic distinctiveness for a recently depleted lizard fauna from Christmas Island, Australia.Crossref | GoogleScholarGoogle Scholar | 29899126PubMed |
Oliver, P. M., Ashman, L. G., Bank, S., Pratt, R. C., Tedeschi, L. G., Laver, R. J., Pratt, R. C., Tedeschi, L. G., and Moritz, C. C. (2019a). On and off the rocks: persistence and ecological diversification in a tropical Australian lizard radiation. BMC Evolutionary Biology 19, 81.
| On and off the rocks: persistence and ecological diversification in a tropical Australian lizard radiation.Crossref | GoogleScholarGoogle Scholar | 30894117PubMed |
Oliver, P. M., Richards, S. J., and Donnellan, S. C. (2019b). Two new species of treefrog (Pelodrydidae: Litoria) from southern New Guinea elucidated by DNA barcoding. Zootaxa 4609, 469–484.
| Two new species of treefrog (Pelodrydidae: Litoria) from southern New Guinea elucidated by DNA barcoding.Crossref | GoogleScholarGoogle Scholar |
Oliver, P. M., Günther, R., Tjaturadi, B., and Richards, S. J. (2021). A new species of large green treefrog (Litoria, Pelodryadidae) from Papua, Indonesia. Zootaxa 4903, 117–126.
| A new species of large green treefrog (Litoria, Pelodryadidae) from Papua, Indonesia.Crossref | GoogleScholarGoogle Scholar |
Peñalba, J. V., Joseph, L., and Moritz, C. (2019). Current geography masks dynamic history of gene flow during speciation in northern Australian birds. Molecular Ecology 28, 630–643.
| Current geography masks dynamic history of gene flow during speciation in northern Australian birds.Crossref | GoogleScholarGoogle Scholar | 30561150PubMed |
Quarles van Ufford, A. Q., and Cloos, M. (2005). Cenozoic tectonics of New Guinea. AAPG Bulletin 89, 119–140.
| Cenozoic tectonics of New Guinea.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2013). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: http://www.R-project.org/
Reeves, J. M., Chivas, A. R., García, A., Holt, S., Couapel, M. J. J., Jones, B. G., Cendón, D. I., and Fink, D. (2008). The sedimentary record of palaeoenvironments and sea-level change in the Gulf of Carpentaria, Australia, through the last glacial cycle. Quaternary International 183, 3–22.
| The sedimentary record of palaeoenvironments and sea-level change in the Gulf of Carpentaria, Australia, through the last glacial cycle.Crossref | GoogleScholarGoogle Scholar |
Richards, S. J., and Oliver, P. M. (2006). Two new species of large green canopy-dwelling frogs (Anura: Hylidae: Litoria) from Papua New Guinea. Zootaxa 1295, 41–60.
| Two new species of large green canopy-dwelling frogs (Anura: Hylidae: Litoria) from Papua New Guinea.Crossref | GoogleScholarGoogle Scholar |
Richards, S. J., Hoskin, C., Cunningham, M., Mcdonald, K. R., and Donnellan, S. C. (2010). Taxonomic re-assessment of the Australian and New Guinean green-eyed treefrogs Litoria eucnemis, L. genimaculata and L. serrata (Anura: Hylidae). Zootaxa 2391, 33–46.
| Taxonomic re-assessment of the Australian and New Guinean green-eyed treefrogs Litoria eucnemis, L. genimaculata and L. serrata (Anura: Hylidae).Crossref | GoogleScholarGoogle Scholar |
Rosauer, D., Laffan, S. W., Crisp, M. D., Donnellan, S. C., and Cook, L. G. (2009). Phylogenetic endemism: a new approach for identifying geographical concentrations of evolutionary history. Molecular Ecology 18, 4061–4072.
| Phylogenetic endemism: a new approach for identifying geographical concentrations of evolutionary history.Crossref | GoogleScholarGoogle Scholar | 19754516PubMed |
Rosauer, D. F., Byrne, M., Blom, M. P. K., Coates, D. J., Donnellan, S., Doughty, P., Keogh, J. S., Kinloch, J., Laver, R. J., Myers, C., Oliver, P. M., Potter, S., Rabosky, D. L., Afonso Silva, A. C., Smith, J., and Moritz, C. (2018). Real-world conservation planning for evolutionary diversity in the Kimberley, Australia, sidesteps uncertain taxonomy. Conservation Letters 11, e12438.
| Real-world conservation planning for evolutionary diversity in the Kimberley, Australia, sidesteps uncertain taxonomy.Crossref | GoogleScholarGoogle Scholar |
Rowe, K. C., Reno, M. L., Richmond, D. M., Adkins, R. M., and Steppan, S. J. (2008). Pliocene colonization and adaptive radiations in Australia and New Guinea (Sahul): multilocus systematics of the old endemic rodents (Muroidea: Murinae). Molecular Phylogenetics and Evolution 47, 84–101.
| Pliocene colonization and adaptive radiations in Australia and New Guinea (Sahul): multilocus systematics of the old endemic rodents (Muroidea: Murinae).Crossref | GoogleScholarGoogle Scholar | 18313945PubMed |
Schodde, R., and Calaby, J. H. (1972). The biogeography of the Australopapuan bird and mammal faunas in relations to Torres Strait. In ‘Bridge and Barrier: the Natural and Cultural History of Torres Strait’. (Ed. D. Walker.) pp. 257–300. (Australian National University: Canberra.)
Schweizer, M., Wright, T. F., Peñalba, J. V., Schirtzinger, E. E., and Joseph, L. (2015). Molecular phylogenetics suggests a New Guinean origin and frequent episodes of founder-event speciation in the nectarivorous lories and lorikeets (Aves: Psittaciformes). Molecular Phylogenetics and Evolution 90, 34–48.
| Molecular phylogenetics suggests a New Guinean origin and frequent episodes of founder-event speciation in the nectarivorous lories and lorikeets (Aves: Psittaciformes).Crossref | GoogleScholarGoogle Scholar | 25929786PubMed |
Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313.
| RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies.Crossref | GoogleScholarGoogle Scholar | 24451623PubMed |
Tallowin, O. J. S., Tamar, K., Meiri, S., Allison, A., Kraus, F., Richards, S. J., and Oliver, P. M. (2018). Early insularity and subsequent mountain uplift were complementary drivers of diversification in a Melanesian lizard radiation (Gekkonidae: Cyrtodactylus). Molecular Phylogenetics and Evolution 125, 29–39.
| Early insularity and subsequent mountain uplift were complementary drivers of diversification in a Melanesian lizard radiation (Gekkonidae: Cyrtodactylus).Crossref | GoogleScholarGoogle Scholar |
Tallowin, O. J. S., Meiri, S., Donnellan, S. C., Richards, S. J., Austin, C. C., and Oliver, P. M. (2020). The other side of the Sahulian coin: biogeography and evolution of Melanesian forest dragons (Agamidae). Biological Journal of the Linnean Society 129, 99–113.
| The other side of the Sahulian coin: biogeography and evolution of Melanesian forest dragons (Agamidae).Crossref | GoogleScholarGoogle Scholar |
Todd, E. V., Blair, D., Georges, A., Lukoschek, V., and Jerry, D. R. (2014). A biogeographical history and timeline for the evolution of Australian snapping turtles (Elseya: Chelidae) in Australia and New Guinea. Journal of Biogeography 41, 905–918.
| A biogeographical history and timeline for the evolution of Australian snapping turtles (Elseya: Chelidae) in Australia and New Guinea.Crossref | GoogleScholarGoogle Scholar |
Tyler, M. J. (1968). Papuan hylid frogs of the genus Hyla. Zoölogische Verhandelingen 96, 1–203.
Tyler, M. J., and Davies, M. (1979). A new species of cave dwelling hylid frog from the Mitchell Plateau, Western Australia. Transactions of the Royal Society of South Australia 103, 149–154.
Tyler, M., Davies, M. M., and Martin, A. A. (1977). A new species of large, green tree frog from northern Western Australia. Transactions of the Royal Society of South Australia 101, 133–138.
Unmack, P. J., Allen, G. R., and Johnson, J. B. (2013). Phylogeny and biogeography of rainbowfishes (Melanotaeniidae) from Australia and New Guinea. Molecular Phylogenetics and Evolution 67, 15–27.
| Phylogeny and biogeography of rainbowfishes (Melanotaeniidae) from Australia and New Guinea.Crossref | GoogleScholarGoogle Scholar | 23313459PubMed |
Waterhouse, A. M., Procter, J. B., Martin, D. M., Clamp, M., and Barton, G. J. (2009). Jalview Version 2 – a multiple sequence alignment editor and analysis workbench. Bioinformatics 25, 1189–1191.
| Jalview Version 2 – a multiple sequence alignment editor and analysis workbench.Crossref | GoogleScholarGoogle Scholar | 19151095PubMed |
Williams, D. J., Shea, M. O., Daguerre, R. L., Pook, C. E., Wüster, W., Hayden, C. J., Mcvay, J. D., Paiva, O., Matainaho, L., Winkel, K. D., and Austin, C. C. (2008). Origin of the eastern brownsnake, Pseudonaja textilis (Duméril, Bibron and Duméril) (Serpentes: Elapidae: Hydrophiinae) in New Guinea: evidence of multiple dispersals from Australia, and comments on the status of Pseudonaja textilis pughi Hoser 2003. Zootaxa 1703, 47–61.
| Origin of the eastern brownsnake, Pseudonaja textilis (Duméril, Bibron and Duméril) (Serpentes: Elapidae: Hydrophiinae) in New Guinea: evidence of multiple dispersals from Australia, and comments on the status of Pseudonaja textilis pughi Hoser 2003.Crossref | GoogleScholarGoogle Scholar |
Wüster, W., Dumbrell, A. J., Hay, C., Pook, C. E., Williams, D. J., and Fry, B. G. (2005). Snakes across the Strait: Trans-Torresian phylogeographic relationships in three genera of Australasian snakes (Serpentes: Elapidae: Acanthophis, Oxyuranus, and Pseudechis). Molecular Phylogenetics and Evolution 34, 1–14.
| Snakes across the Strait: Trans-Torresian phylogeographic relationships in three genera of Australasian snakes (Serpentes: Elapidae: Acanthophis, Oxyuranus, and Pseudechis).Crossref | GoogleScholarGoogle Scholar | 15579378PubMed |