Coronaviruses and Australian bats: a review in the midst of a pandemic
Alison J. Peel A G , Hume E. Field B C , Manuel Ruiz Aravena D , Daniel Edson E , Hamish McCallum A , Raina K. Plowright D and Diana Prada F
A G , Hume E. Field B C , Manuel Ruiz Aravena D , Daniel Edson E , Hamish McCallum A , Raina K. Plowright D and Diana Prada F
A Environmental Futures Research Institute, Griffith University, Nathan, Qld 4111, Australia.
B EcoHealth Alliance, New York, NY 10001, USA.
C School of Veterinary Science, The University of Queensland, Gatton, Qld 4343, Australia.
D Department of Microbiology and Immunology, Montana State University, Bozeman, MT 59717, USA.
E Department of Agriculture, Water and the Environment, Canberra, ACT 2601, Australia.
F School of Veterinary Medicine, Murdoch University, Perth, WA 6150, Australia.
G Corresponding author. Email: a.peel@griffith.edu.au
Australian Journal of Zoology 67(6) 346-360 https://doi.org/10.1071/ZO20046
Submitted: 8 June 2020 Accepted: 24 July 2020 Published: 2 September 2020
Journal Compilation © CSIRO 2019 Open Access CC BY
Abstract
Australia’s 81 bat species play vital ecological and economic roles via suppression of insect pests and maintenance of native forests through pollination and seed dispersal. Bats also host a wide diversity of coronaviruses globally, including several viral species that are closely related to SARS-CoV-2 and other emergent human respiratory coronaviruses. Although there are hundreds of studies of bat coronaviruses globally, there are only three studies of bat coronaviruses in Australian bat species, and no systematic studies of drivers of shedding. These limited studies have identified two betacoronaviruses and seven alphacoronaviruses, but less than half of Australian species are included in these studies and further research is therefore needed. There is no current evidence of spillover of coronaviruses from bats to humans in Australia, either directly or indirectly via intermediate hosts. The limited available data are inadequate to determine whether this lack of evidence indicates that spillover does not occur or occurs but is undetected. Conversely, multiple international agencies have flagged the potential transmission of human coronaviruses (including SARS CoV-2) from humans to bats, and the consequent threat to bat conservation and human health. Australia has a long history of bat research across a broad range of ecological and associated disciplines, as well as expertise in viral spillover from bats. This strong foundation is an ideal platform for developing integrative approaches to understanding bat health and sustainable protection of human health.
Introduction
The global COVID-19 pandemic resulting from the emergence of a novel coronavirus, SARS-CoV-2 in late 2019 (Zhu et al. 2020) has refocussed attention on coronaviruses of bat origin. Bats host a wide diversity of coronaviruses (Cui et al. 2007; Vijaykrishna et al. 2007; Drexler et al. 2014; Anthony et al. 2017a; Lacroix et al. 2017; Wong et al. 2019; Latinne et al. 2020), including viral species linked to emergent human respiratory syndrome coronaviruses (Cui et al. 2019). SARS-CoV-2 has close nucleotide sequence identity with coronaviruses isolated from bats in Yunnan Province in China (Hu et al. 2017; Zhou et al. 2020). The epidemiological link between early COVID-19 cases and a Wuhan wet market (Chen et al. 2020; Li et al. 2020) led to an initial hypothesis that transmission from bats and recombination in an unknown intermediate host sold in the market might be involved (Zhang et al. 2020); however, the links to intermediate hosts are unclear (Boni et al. 2020), and it is possible that the market was the site of a superspreading event after the emergence of SARS-CoV-2 elsewhere. This scenario parallels the emergence of severe acute respiratory syndrome (SARS) in China in 2002 (Ksiazek et al. 2003); the search for the origins of the aetiological coronavirus (SARS-CoV) of that pandemic (Guan et al. 2003; Tu et al. 2004; Kan et al. 2005; Poon et al. 2005; Tang et al. 2006) and the cluster of SARS-related betacoronaviruses subsequently described in Rhinolophus species in China (Lau et al. 2005; Li et al. 2005) provides the background for the current focus on bats in the search for the origins of SARS-CoV-2.
Australia has a diverse bat community, with 81 species representing both suborders and 9 families (Reardon et al. 2015). The majority of Australian species are smaller insectivorous bats with little public profile and generally limited public contact opportunity. In contrast, large, arboreal, colonially roosting pteropodid bats (commonly known as flying-foxes) are regularly present, and prominent, in eastern Australian cities and towns. This bat community has been the focus of a diverse range of research studies, including their genetic and evolutionary history (e.g. Godthelp et al. 1992; Webb and Tidemann 1996), ecology (e.g. McKenzie and Rolfe 1986; Lumsden and Bennett 2005; Eby and Law 2008), physiology (e.g. Hosken and Withers 1997; Welbergen et al. 2008), immunology (e.g. Zhang et al. 2013; Zhou et al. 2016; Schountz et al. 2017), and infectious disease emergence (Gould et al. 1998; Halpin et al. 2000; Barr et al. 2012; Kessler et al. 2018) and dynamics (e.g. Field et al. 2015; Plowright et al. 2015; Paez et al. 2017; Edson et al. 2019; Peel et al. 2019).
The vital ecosystem services provided by bats through insect pest control (e.g. Kolkert et al. 2020), pollination (e.g. Law and Lean 1999; Eby and Law 2008) and seed dispersal (e.g. Moran et al. 2009) are often overshadowed in public perception by their potential to act as reservoirs of zoonotic viruses, such as Hendra virus, Australian bat lyssavirus and Menangle virus. Most research on bat-borne viruses in Australia (~200 papers to date) has focussed on these pathogens, with only three coronavirus surveillance studies (Smith et al. 2016; Holz et al. 2018; Prada et al. 2019). Even though none of Australia’s bat species have distributions overlapping with species that are known hosts of SARS-related viruses, most of the chiropteran genera in which zoonotic-related coronaviruses have been detected in China and elsewhere are represented in Australia (e.g. Rhinolophus spp., Hipposideros spp., Chaerophon spp., Pipistrellus spp.) (Tang et al. 2006; Pfefferle et al. 2009; Tong et al. 2009).
Against this background, we first seek to review the current state of knowledge of bat coronaviruses globally and within Australia, identifying key areas in need of further research. Second, we canvass the potential for spillover of endemic Australian bat coronaviruses to humans and the potential for transmission of SARS-CoV-2 from infected humans into Australian bat populations.
Coronaviruses in bats worldwide
Bats naturally host viruses from two of the four genera of coronaviruses: alpha- and betacoronaviruses – the other two (delta- and gammacoronaviruses) are predominantly found in birds (Woo et al. 2009, 2010; Su et al. 2016) (Fig. 1). The diversity of coronaviruses circulating in bat populations is the highest detected to date in any mammalian host group (Drexler et al. 2014). Empirical detections and estimates suggest the existence of 2–5 unique coronaviruses per bat species, and potentially up to 7000 coronaviruses worldwide when ~1400 bats species are considered (Anthony et al. 2017a; Willoughby et al. 2017; Latinne et al. 2020; Simmons and Cirranello 2020).

|
All of the known zoonotic coronaviruses with origins in bats are betacoronaviruses, belonging either to the Sarbecovirus subgenus (i.e. the SARS-related coronaviruses [SARSr-CoV]: SARS-CoV-1, SARS-CoV-2), or the Merbecovirus subgenus (MERS-CoV) (Fig. 1). Although the specific conditions resulting in the spillover of SARS-CoV-1, SARS-CoV-2 and MERS-CoV remain unresolved, the closest evolutionary relatives to these viruses have been identified in bat hosts. Viruses with 88–91% nucleotide identity to SARS-CoV-1 have been amplified from horseshoe bat species (Rhinolophus sinicus, R. ferrumequinum, R. macrotis and R. pearsoni), supporting Rhinolophus bats as the ancestral reservoir origin of SARS (Lau et al. 2005; Li et al. 2005; He et al. 2014; Zhou et al. 2020; Zhou et al. 2020a). The viruses most closely related to SARS-CoV-2 identified so far include lineages of sarbecoviruses circulating in bat populations in China: RaTG13 CoV isolated from Rhinolophus affinis (Zhou et al. 2020b) and RmYN02 CoV detected in Rhinolophus malayanus (Zhou et al. 2020). Beyond Rhinolophidae, SARS-related coronaviruses have also been detected in bats from the Hipposideridae and Vespertilionidae in Europe and Asia, and the Molossidae in Asia (Drexler et al. 2014; Fan et al. 2019; Wong et al. 2019). At least three bat families have been identified as hosts of Merbecoviruses to date, though most detections have been in bats from the Vespertilionidae (reviewed in Wong et al. 2019).
Existing bat coronavirus studies demonstrate several factors with apparent influence on coronavirus detectability and diversity. Coronavirus detection rates are statistically significantly higher in specimens collected from bats in human-altered landscapes (e.g. particularly associated with animal usage in hunting, trade, etc), compared with those from undisturbed environments, suggesting the influence of environmental conditions on bat–coronavirus interactions (Anthony et al. 2017a). Coronavirus diversity is higher where bat diversity is also high, suggesting an intimate and long evolutionary history of interactions. This is evident at the continental scale (bat coronavirus diversity is highest in South-east Asia, northern Latin America, and south-west, equatorial and east Africa) and also within continents (Anthony et al. 2017a; Wong et al. 2019; Joffrin et al. 2020). Phylogenetic analyses of bat coronavirus genetic sequences show that closely related coronaviruses are commonly shared among closely related bat families (Drexler et al. 2014). For instance, in Mexico, coronaviruses in vespertilionid bats cluster together with coronaviruses in molossid bats (Anthony et al. 2013). This relationship supports an influence of cospeciation and host-switching in the ecology and evolution of bats and their coronaviruses (Cui et al. 2007; Joffrin et al. 2020; Latinne et al. 2020). Both alpha- and betacoronaviruses have been detected across all continents occupied by bats, yet in North America, betacoronaviruses have been detected in Mexico but are notably absent in bats in the USA and Canada (Anthony et al. 2013; Wong et al. 2019). It is important to note that sampling efforts are not globally uniform, and therefore such absences may not necessarily represent true diversity and abundance of circulating coronaviruses (Anthony et al. 2017a). This may also affect analyses that indicate that the number of coronavirus species detected per bat family varies by continent in Africa and Asia (with high rates of detection in Pteropodidae in Africa; high rates of detection in Miniopteridae, Vespertilionidae and Pteropodidae in Asia), but bat family did not influence rate of detection in Latin America (Anthony et al. 2017a).
Coronaviruses in Australian bats
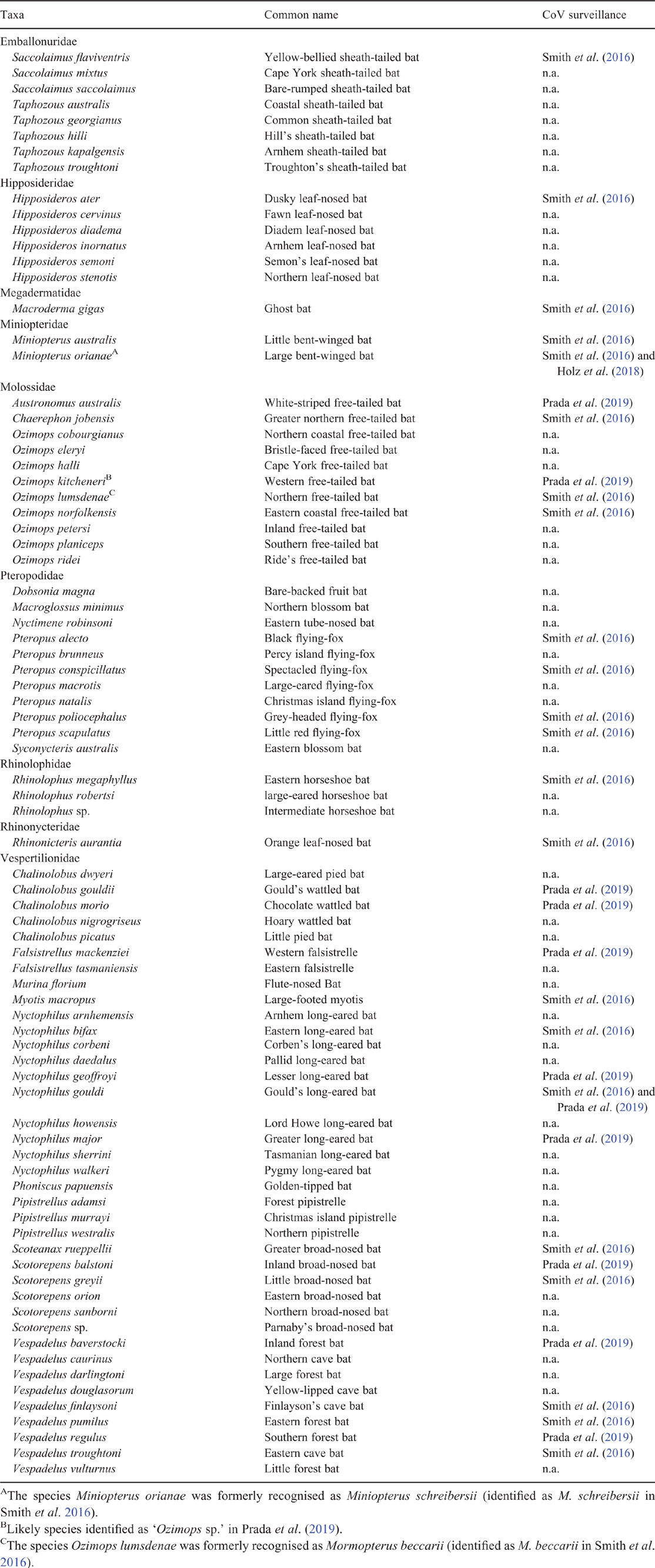
Coronavirus diversity and antibody prevalence in Australian bats is known from two cross-sectional studies on bat faeces or rectal swabs, encompassing 35 Australian bat species across 17 genera and 8 families (Smith et al. 2016; Prada et al. 2019) (Table 1, Fig. 2). Based on PCR amplification and sequencing of a 396–440 bp fragment of the RNA-dependent RNA polymerase (RdRp) gene, Smith et al. (2016) and Prada et al. (2019) detected two betacoronaviruses (exclusively from pteropodiformes) and seven alphacoronaviruses (mainly from vespertilionid bats) across 15 Australian bat species. Full genome sequences from four of the seven alphaviruses amplified from host species have been subsequently obtained and made publicly available (Prada et al. 2019). To date, there is no evidence of any SARS-like viruses (Sarbecoviruses) or MERS-like viruses (Merbecoviruses) in any Australian bat species, though cross-reactive antibodies have been detected (Smith et al. 2016; W. Boardman, pers. comm.) and further surveillance is currently underway.
In agreement with other studies globally (Drexler et al. 2014; Lacroix et al. 2017), strains amplified from Australian bats have strong host associations, with sequences clustering by host taxonomic affinity instead of sampling site (Fig. 1). For example, betacoronaviruses amplified from Pteropus alecto and Rhinonicteris aurantia were more similar to other sequences amplified from Asian bats of the same taxonomic order than to each other.
Notable differences are also evident in Australian bat coronavirus studies; for example, despite high viral prevalences of betacoronaviruses in rhinolophid bats in China (e.g. 39%: Lau et al. 2005) and elsewhere (e.g. 32%: Drexler et al. 2010), no betacoronaviruses have been detected in Australian rhinolophids. Despite an extensive sampling effort of Rhinolophus megaphyllus (n = 506), only one bat tested positive by PCR and cross-regional seroprevalence was low (6.7%; 95% CI = 4–9%) (Smith et al. 2016). The single positive result was an alphacoronavirus with 99% nucleotide sequence identity with Miniopterus bat coronavirus HKU8 and which was widespread in concurrently sampled Miniopterus australis and M. schreibersii (now renamed M. orianae), suggesting that R. megaphyllus may not be the primary reservoir host for this viral species. Similarly, there has been no detection of betacoronaviruses in Australian Hipposideros species, even though SARSr-CoV have been amplified from this genera in Africa (Pfefferle et al. 2009; Quan et al. 2010). In Australia, no active viral shedding was detected in Hipposideros ater (n = 56), and seroprevalence was very low (1.5%; 95% CI = 0.2–8.3%). Equally surprising as the lack of representative Australian betacoronavirus strains within these genera is the small number of strains and lack of host-specific alphacoronaviruses detected within the broader Pteropodiformes group, since alphacoronaviruses generally have greater detection rates and a widespread global distribution (Wong et al. 2019).
Sample sizes and the employed primer set are congruent with global studies (Tsuda et al. 2012; Moreira-Soto et al. 2015; Lacroix et al. 2017; Joffrin et al. 2020), but broader sampling would help determine how representative these existing studies are, and how sample sizes, sample type, number of sampling sites, sampling season, number of bat species surveyed, or the implemented PCR assay have affected detection rates. For example, an additional study focussed in Australian populations of Miniopterus orianae yielded no coronavirus detections from oropharyngeal swabs (Holz et al. 2018). This lack of detection is likely due to decreased sensitivity of coronavirus detection in oropharyngeal swabs, because the route of coronavirus shedding in bats is predominantly through faeces (Wacharapluesadee et al. 2013; Wong et al. 2019). By comparison, rectal swabs collected from the same species in south-east Queensland and the Northern Territory were positive for coronavirus RNA in 26.5% and 10.2% of individuals, respectively (Smith et al. 2016) (Table 1). Only small numbers of individuals of several Australian bat species have been screened for coronaviruses, and an additional 46 species have not yet been screened at all (Table 2). A meta-analysis of bat coronavirus studies globally identified that a sampling effort of ~400 individuals per species is required to capture the full diversity of coronaviruses present in each species (Anthony et al. 2017a). Only one Australian species has been sampled to this intensity (R. megaphyllus, n = 506), and the single detection in this species suggests that this target sample number must be replicated across sites and seasons before firm conclusions are drawn on a species’ viral diversity.
|
Spillover potential of Australian bat-borne coronaviruses
Various processes must align in space and time for viruses to successfully spill over from a natural reservoir host to alternative hosts (Plowright et al. 2017). The viruses must be circulating within reservoir host populations, be shed and survive within the environment at a time and place where novel hosts may be exposed, and, finally, overcome any structural or immune barriers to infection and replication within that host. These cross-species transmission events can involve one-directional transmission from one species to another (e.g. bat to civet to human, as is suspected for SARS-CoV-1), or circular transmission between two or more host species (e.g. a shared virus between two bat host species; conceptually illustrated in Fig. 3). While coronaviruses are known to be circulating within Australian bat populations, there have been no reports of their spillover to humans, either directly or via an intermediate host. Some evidence exists for bat–bat cross-species transmission events between sympatric species within the studied communities (in fact, they appear to be somewhat common) (Fig. 3), yet sustained infection within the new host and further transmission within populations may be limited. For instance, high nucleotide similarity (100%) was detected between sequences amplified from M. australis and R. megaphyllus, yet marked differences existed between the species in the proportion of active infections (RNA detection; 46.7% versus 1.7%, respectively) and historical infections (coronavirus antibodies) (53% versus 8.2%, respectively) (Smith et al. 2016). These data are indicative of a cross-infection event from the vespertilionid bat M. australis into the pteropodiforme R. megaphyllus and warrant further investigation. Similarly, cross-infections were also detected in south-west Western Australian bat communities, with tree hollows proposed as a source of transmission between sympatric bat species (Prada et al. 2019). One alphacoronavirus strain detected in Chalinolobus gouldii had a spike protein with a higher sequence similarity to the coronavirus that causes Porcine Endemic Diarrhoea in pigs (Jung and Saif 2015) than to any other virus of bat origin (Prada et al. 2019). Since the spike protein determines host tropism, the ability of the spike protein to enter mammalian cells other than bat cells is currently under investigation (Haynes et al., unpubl. data). In general, however, the spatiotemporal shedding dynamics of Australian bat-borne coronaviruses and their capacity to infect cells and overcome immune systems of human or other animal species are unknown, making it challenging to assess whether spillover does not occur, or does not cause disease, or occurs but is simply not detected.
Insights from other bat–viral systems and geographic regions will be key in targeting further research in Australia. The origin of SARS-CoV-2, the chain of transmission to humans, and direct evidence for the involvement of an intermediate host are yet to be established. Pathogen transmission between species cannot occur without every condition, in a hierarchical series of events, lining up (Plowright et al. 2017). Hence, if intermediate hosts are required, then even if diverse viruses are present in bats and peaks of shedding generate pathogen pressure for bat-borne coronaviruses, spillover may not be able to occur without the presence of these competent intermediate hosts from which human exposure occurs. There are multiple reports of transmission of bat-borne coronaviruses to various laboratory animal species in experimental settings (Gretebeck and Subbarao 2015), domestic and wild felids across a range of settings (Shi et al. 2020; US Department of Agriculture Animal and Plant Health Inspection Service 2020), and to non-bat wildlife species utilised in wildlife farming and trade (Shi and Hu 2008; Gretebeck and Subbarao 2015). There are also examples of spillover of coronaviruses from bats to livestock or farmed domestic species: in camels, associated with the emergence of MERS in 2012 in Saudi Arabia (Anthony et al. 2017b), and in pigs, associated with the emergence of Swine Acute Diarrhoea Syndrome (SADS) in 2016 in southern China that resulted in the deaths of ~25 000 pigs across four farms (Zhou et al. 2018). The novel SADS coronavirus detected in pigs that caused this outbreak shared 96–98% sequence identity with SADS-related bat coronaviruses present in ~10% of Rhinolophus spp. bats in the same region. This event was preceded by at least three other examples of bat–pig viral spillover: transmission of Nipah virus from Pteropus spp. flying foxes to pigs in Malaysia (Chua et al. 2000), Reston ebolavirus in pigs in the Philippines and China (Jayme et al. 2015), and Menangle virus from Pteropus poliocephalus to pigs in Australia (Philbey et al. 1998). The latter example highlights that bat–pig viral transmission events are feasible within an Australian context. Spillover events are more likely to succeed where susceptible intermediate hosts are stressed and immunocompromised and where ongoing transmission is facilitated by high-density cohousing and poor on-farm biosecurity (Lindahl and Grace 2015).
The potential for direct bat–human transmission of coronaviruses remains unclear (Fig. 3). There is no molecular evidence for direct spillover; however, Wang et al. (2018) found serological evidence of human exposure to SARS-related coronaviruses in 6 of 218 (2.7%) rural people living close to caves in which SARS-related coronaviruses had been identified in bats. None of the positive individuals had a history of exposure to SARS-CoV-1 virus during the 2002–03 outbreak and none could recall any clinical symptoms consistent with SARS-CoV-1 infection in the past 12 months, suggesting the possibility of subclinical or mild clinical infection. Uncertainty remains as to whether these findings could represent transmission via an intermediate host, but they are sufficient to warrant inclusion of direct bat–human transmission routes in coronavirus spillover risk assessments.
Several human–bat interaction scenarios could theoretically present the opportunity for spillover of bat coronaviruses to humans in Australia, including where people are occupationally exposed to bats and bat body fluids. Bat rescuers and rehabilitators would likely be at high risk of exposure because of the number of animals they handle, the length of time that animals are frequently in care, and the often physically close nature of contact between carer and bat (particularly orphan flying-foxes). Indeed, following the emergence of Hendra virus in 1994 (which spilled from flying-foxes to horses to humans), health authorities recognised that bat carers and others working with bats were an ideal sentinel group to screen for serological evidence of exposure to Hendra virus; however, no evidence of exposure was found (Selvey et al. 1996).
Given the tangible threats to human health and the unknown risks that emergent coronaviruses pose to wild bat populations, future surveillance efforts on bat taxa traditionally associated with zoonotic strains should prioritise species common to the human/urban interface. Additionally, the commonality and marked variability of alphacoronavirus infections among insectivorous bats, provide an opportunity to further investigate drivers of cross-species spillover, as well as pathogen persistence, viral shedding, transmission routes and their associations to the host ecology and health (Jeong et al. 2017; Smith 2015).
Potential establishment of novel coronaviruses in Australian bat populations following human-to-bat transmission
SARS-CoV-2 is expected to persist as the world’s fifth endemically circulating human coronavirus (Kissler et al. 2020), and it is unlikely to be the last novel coronavirus linked to bat origins. Widespread transmission within human populations of viruses that are either bat-borne, or whose progenitors originate in bats, raises the possibility of reverse zoonotic spillover (Fig. 3) of these viruses from humans into novel bat or other animal hosts that have no evolutionary association with the circulating strains. Bat organisations internationally have responded to this threat by advocating, at a minimum, for increased biosecurity protocols when handling bats for research or rehabilitation (IUCN Bat Specialist Group 2020; Olival et al. 2020; Runge et al. 2020). In some cases, this has extended to blanket bans on handling wild bats. Establishment of SARS-CoV-2, or other endemic human coronaviruses from people into Australian bat populations, could have serious public health implications, via establishment of an adjunct source of human infection (via ‘spillback’: Fig. 3) or via recombination events with endemic bat coronavirus strains. Bat welfare, conservation, and management, already contentious issues in Australia (Degeling and Kerridge 2012; Kung et al. 2015), would likely be further compromised. The likelihood of human-to-bat transmission of SARS-CoV-2 in Australia is likely to reflect levels of community transmission (at the time of writing (4 June 2020), there had been a total of ~7240 cases of COVID-19 in Australia (~28 per 100 000 individuals), and detection rates for new cases had dropped to an average of 13 per day: Australian Government Department of Health 2020). However, the likelihood of transmission would be expected to increase proportionately with increases in human cases, which has subsequently been observed in a ‘second wave’ in the State of Victoria (Australian Government Department of Health 2020). Given the dynamic nature of the COVID-19 pandemic, and the potentially serious consequences of a human–bat transmission event, now is an ideal time to be discussing practices to mitigate the risk of human-to-bat transmission of endemic and exotic coronaviruses more broadly.
The risk of human-to-bat transmission will vary with the emergence of each new human-transmitted coronavirus, and also over space and time. Important human factors to consider in assessing this risk include prevalence in the human population and the proportion of asymptomatic carriers, as well as the likely transmission route, infectious period, and virus survival characteristics (Olival et al. 2020). Conditional on exposure of a bat to an infected human, successful human-to-bat transmission will depend on individual bat and species susceptibility, which may be based on cross-reactive immunity to circulating bat coronaviruses, receptor sequence similarity across species, or alternative transmission mechanisms yet to be elucidated (Olival et al. 2020; Wildlife Health Australia, unpubl. data). Finally, the frequency and patterns of contact among people with different occupational or recreational exposure to bats (e.g. researchers, ecological consultants, bat rehabilitators, veterinary staff, and recreational cavers) and the different bat species they interact with, will determine groups at greatest risk of initiating or receiving spillover. Regardless, direct human-to-bat contact might not be necessary for spillover: since SARS-CoV-2 can be shed in faeces and may not be wholly inactivated by wastewater treatment processes, Franklin and Bevins (2020) have hypothesised that wastewater might be a potential route of transmission when human case numbers are high.
Discussion
Australian bats are not known to host Merbecoviruses or Sarbecoviruses with demonstrated zoonotic and pandemic potential. Nevertheless, the patchy coverage of coronavirus studies to date should alert us to the possibility that related viruses might be detected with increased sampling intensity. The wide variation in both viral RNA (0–46%) and antibody (0–100%) prevalence detected across species, space and time from existing cross-sectional studies also suggests that point-estimates of prevalence should be interpreted with care (Becker et al. 2019). Such dynamic systems can be understood only through detailed spatiotemporal surveillance of coronaviruses in Australasian bat populations (Plowright et al. 2019).
Globally, coronaviruses are widely distributed across bat species and populations. Few studies, however, include sufficient spatial or temporal sampling design or host ecological factors to infer the drivers of transmission. Point prevalence of coronaviruses in wild bat populations varies considerably among sites, seasons, age categories and physiological stages of bats, with values of up to 60% of infected individuals (Montecino-Latorre et al. 2020). For instance, in a study in Pteropus lylei in Thailand, Wacharapluesadee et al. (2018) report higher prevalence of coronaviruses in juveniles than adults, with detections only in adults in January, only in juveniles in April and no differences among both age categories between May and October. Smith (2015) identified significantly higher anti-coronavirus antibody prevalence in M. schreibersii (now M. orianae) and R. megaphyllus in summer and in female adult bats. Comparable, but non-significant, trends were observed for PCR detection of coronaviruses. Similar age and seasonal effects have been reported for coronavirus shedding in other bat species: in some studies, juveniles present up to 16 times higher probability of shedding virus than adults, with a peak during the weaning season (Montecino-Latorre et al. 2020). These age, seasonal and physiologically related infection status and shedding patterns of coronaviruses show similarities to the dynamic of infections by Henipaviruses in Pteropus bats with seasons and life stages of high physiological stress playing a role in infection and shedding risk (Plowright et al. 2008; Field et al. 2015; Paez et al. 2017; Brook et al. 2019; Edson et al. 2019). These extensive data on the ecological drivers of Hendra virus shedding in Australia could provide a foundation upon which to base deeper investigations into the drivers of coronavirus shedding. An increase in coronavirus studies in Australian bats would provide an indication of the broad viral diversity, the potential for novel spillover risks, as well as a baseline comparison for efforts to detect human–bat transmission of CoV.
The current limited information and uncertainty about potentially zoonotic viruses, including coronaviruses, in Australian bats leaves openings for misinformation and fear. For example, in April 2020, a Victorian state member of Parliament called for the grey-headed flying-fox (P. poliocephalus) colony in Yarra Bend National Park to be removed because of a perceived disease threat, including COVID-19 (Rimmer 2020). Further investigations of the diversity, distribution, and zoonotic potential of coronaviruses in Australian bats are needed to allow a rational assessment of the risk, if any, of potential future spillover to humans or livestock, or spillback of SARS-CoV-2 to endemic bats.
Investigations of spillover potential of coronaviruses between Australian bats and humans should be framed in a broader ecological context. Describing the viruses that infect Australian bats is a first step, but without the ecological conditions that promote pathogen shedding, contact with susceptible hosts (intermediate or otherwise), and human behaviours that allow cross-species transmission, spillover may be unlikely. Landscape change is a key factor modulating all of these risk factors for disease emergence (Kessler et al. 2018; Johnson et al. 2020). The ecological integrity of landscapes affects bat distribution and health, and hence contact with other species and pathogen shedding dynamics. With increasing rates of emergence of pathogens from bats associated with rapid environmental change (Johnson et al. 2020) and amplified stressors as a result of climate change, the links between environmental stress and spillover need more attention. Environmental changes not only drive bat virus emergence, they can decrease the ecosystem services that bats provide that directly benefit humans. Investigations of disease risk need to consider the holistic interaction of bats, their infections, and their environment.
Conclusion
Australia has a unique global advantage with its long history of bat research across a broad range of ecological and associated disciplines, as well as its expertise in viral spillover from bats. We must utilise this foundation to develop integrative approaches to understanding bat health and sustainable protection of human health.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors thank Craig Smith (Biosecurity Queensland) for providing the unpublished Pteropus betacoronavirus sequence in Fig. 1. AJP was supported by an ARC DECRA fellowship (DE190100710). This manuscript was not supported by any specific funding. The findings and conclusions in this publication are those of the authors and should not be construed to represent any official Department of Agriculture, Water and the Environment or Australian Government determination or policy.
References
Anthony, S. J., Ojeda-Flores, R., Rico-Chávez, O., Navarrete-Macias, I., Zambrana-Torrelio, C. M., Rostal, M. K., Epstein, J. H., Tipps, T., Liang, E., Sanchez-Leon, M., Sotomayor-Bonilla, J., Aguirre, A. A., Ávila-Flores, R., Medellín, R. A., Goldstein, T., Suzán, G., Daszak, P., and Lipkin, W. I. (2013). Coronaviruses in bats from Mexico. The Journal of General Virology 94, 1028–1038.| Coronaviruses in bats from Mexico.Crossref | GoogleScholarGoogle Scholar | 23364191PubMed |
Anthony, S. J., Johnson, C. K., Greig, D. J., Kramer, S., Che, X., Wells, H., Hicks, A. L., Joly, D. O., Wolfe, N. D., Daszak, P., Karesh, W., Lipkin, W. I., Morse, S. S., Consortium, P., Mazet, J. A. K., and Goldstein, T. (2017a). Global patterns in coronavirus diversity. Virus Evolution 3, vex012.
| Global patterns in coronavirus diversity.Crossref | GoogleScholarGoogle Scholar | 28630747PubMed |
Anthony, S. J., Gilardi, K., Menachery, V. D., Goldstein, T., Ssebide, B., Mbabazi, R., Navarrete-Macias, I., Liang, E., Wells, H., Hicks, A., Petrosov, A., Byarugaba, D. K., Debbink, K., Dinnon, K. H., Scobey, T., Randell, S. H., Yount, B. L., Cranfield, M., Johnson, C. K., Baric, R. S., Lipkin, W. I., and Mazet, J. A. K. (2017b). Further evidence for bats as the evolutionary source of Middle East Respiratory Syndrome coronavirus. mBio 8, e00373-–17.
| Further evidence for bats as the evolutionary source of Middle East Respiratory Syndrome coronavirus.Crossref | GoogleScholarGoogle Scholar | 28377531PubMed |
Australian Government Department of Health (2020). Coronavirus (COVID-19) at a glance. Available at: https://www.health.gov.au/resources/publications/coronavirus-covid-19-at-a-glance [accessed 4 June 2020].
Barr, J. A., Smith, C., Marsh, G. A., Field, H. E., and Wang, L. F. (2012). Evidence of bat origin for Menangle virus, a zoonotic paramyxovirus first isolated from diseased pigs. The Journal of General Virology 93, 2590–2594.
| Evidence of bat origin for Menangle virus, a zoonotic paramyxovirus first isolated from diseased pigs.Crossref | GoogleScholarGoogle Scholar | 22915696PubMed |
Becker, D. J., Crowley, D. E., Washburne, A. D., and Plowright, R. K. (2019). Temporal and spatial limitations in global surveillance for bat filoviruses and henipaviruses. Biology Letters 15, 20190423.
| Temporal and spatial limitations in global surveillance for bat filoviruses and henipaviruses.Crossref | GoogleScholarGoogle Scholar | 31822244PubMed |
Boni, M. F., Lemey, P., Jiang, X., Lam, T. T.-Y., Perry, B., Castoe, T., Rambaut, A., and Robertson, D. L. (2020). Evolutionary origins of the SARS-CoV-2 sarbecovirus lineage responsible for the COVID-19 pandemic. bioRxiv
Brook, C. E., Ranaivoson, H. C., Broder, C. C., Cunningham, A. A., Héraud, J.-M., Peel, A. J., Gibson, L., Wood, J. L. N., Metcalf, C. J., and Dobson, A. P. (2019). Disentangling serology to elucidate henipa- and filovirus transmission in Madagascar fruit bats. Journal of Animal Ecology 88, 1001–1016.
| Disentangling serology to elucidate henipa- and filovirus transmission in Madagascar fruit bats.Crossref | GoogleScholarGoogle Scholar | 30908623PubMed |
Chen, N., Zhou, M., Dong, X., Qu, J., Gong, F., Han, Y., Qiu, Y., Wang, J., Liu, Y., Wei, Y., Xia, J. a., Yu, T., Zhang, X., and Zhang, L. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395, 507–513.
| Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study.Crossref | GoogleScholarGoogle Scholar | 32007143PubMed |
Chua, K. B., Bellini, W. J., Rota, P. A., Harcourt, B. H., Tamin, A., Lam, S. K., Ksiazek, T. G., Rollin, P. E., Zaki, S. R., Shieh, W. J., Goldsmith, C. S., Gubler, D. J., Roehrig, J. T., Eaton, B., Gould, A. R., Olson, J., Field, H., Daniels, P., Ling, A. E., Peters, C. J., Anderson, L. J., and Mahy, B. W. J. (2000). Nipah virus: a recently emergent deadly paramyxovirus. Science 288, 1432–1435.
| Nipah virus: a recently emergent deadly paramyxovirus.Crossref | GoogleScholarGoogle Scholar | 10827955PubMed |
Cui, J., Han, N., Streicker, D., Li, G., Tang, X., Shi, Z., Hu, Z., Zhao, G., Fontanet, A., Guan, Y., Wang, L., Jones, G., Field, H. E., Daszak, P., and Zhang, S. (2007). Evolutionary relationships between bat coronaviruses and their hosts. Emerging Infectious Diseases 13, 1526–1532.
| Evolutionary relationships between bat coronaviruses and their hosts.Crossref | GoogleScholarGoogle Scholar | 18258002PubMed |
Cui, J., Li, F., and Shi, Z.-L. (2019). Origin and evolution of pathogenic coronaviruses. Nature Reviews. Microbiology 17, 181–192.
| Origin and evolution of pathogenic coronaviruses.Crossref | GoogleScholarGoogle Scholar | 30531947PubMed |
Degeling, C., and Kerridge, I. (2012). Hendra in the news: public policy meets public morality in times of zoonotic uncertainty. Social Science & Medicine 82, 156–163.
Drexler, J. F., Gloza-Rausch, F., Glende, J., Corman, V. M., Muth, D., Goettsche, M., Seebens, A., Niedrig, M., Pfefferle, S., Yordanov, S., Zhelyazkov, L., Hermanns, U., Vallo, P., Lukashev, A., Muller, M. A., Deng, H., Herrler, G., and Drosten, C. (2010). Genomic characterization of severe acute respiratory syndrome-related coronavirus in European bats and classification of coronaviruses based on partial RNA-dependent RNA polymerase gene sequences. Journal of Virology 84, 11336–11349.
| Genomic characterization of severe acute respiratory syndrome-related coronavirus in European bats and classification of coronaviruses based on partial RNA-dependent RNA polymerase gene sequences.Crossref | GoogleScholarGoogle Scholar | 20686038PubMed |
Drexler, J. F., Corman, V. M., and Drosten, C. (2014). Ecology, evolution and classification of bat coronaviruses in the aftermath of SARS. Antiviral Research 101, 45–56.
| Ecology, evolution and classification of bat coronaviruses in the aftermath of SARS.Crossref | GoogleScholarGoogle Scholar | 24184128PubMed |
Eby, P., and Law, B. (2008). Ranking the feeding habitat of grey-headed flying foxes for conservation management. Department of Environment and Climate Change (NSW) and The Department of Environment, Water, Heritage and the Arts, Sydney.
Edson, D., Peel, A. J., Huth, L., Mayer, D. G., Vidgen, M. E., McMichael, L., Broos, A., Melville, D., Kristoffersen, J., de Jong, C., McLaughlin, A., and Field, H. E. (2019). Time of year, age class and body condition predict Hendra virus infection in Australian black flying foxes (Pteropus alecto). Epidemiology and Infection 147, e240.
| Time of year, age class and body condition predict Hendra virus infection in Australian black flying foxes (Pteropus alecto).Crossref | GoogleScholarGoogle Scholar | 31364577PubMed |
Fan, Y., Zhao, K., Shi, Z.-L., and Zhou, P. (2019). Bat coronaviruses in China. Viruses 11, 210.
| Bat coronaviruses in China.Crossref | GoogleScholarGoogle Scholar |
Field, H. E., Jordan, D., Edson, D., Morris, S., Melville, D., Parry-Jones, K., Broos, A., Divljan, A., McMichael, L. A., Davis, R., Kung, N., Kirkland, P., and Smith, C. (2015). Spatiotemporal aspects of Hendra virus infection in pteropid bats (flying-foxes) in eastern Australia. PLoS One 10, e0144055.
| Spatiotemporal aspects of Hendra virus infection in pteropid bats (flying-foxes) in eastern Australia.Crossref | GoogleScholarGoogle Scholar | 26060997PubMed |
Franklin, A. B., and Bevins, S. N. (2020). Spillover of SARS-CoV-2 into novel wild hosts in North America: a conceptual model for perpetuation of the pathogen. The Science of the Total Environment 733, 139358.
| Spillover of SARS-CoV-2 into novel wild hosts in North America: a conceptual model for perpetuation of the pathogen.Crossref | GoogleScholarGoogle Scholar | 32416535PubMed |
Godthelp, H., Archer, M., Cifelli, R., Hand, S. J., and Gilkeson, C. F. (1992). Earliest known Australian Tertiary mammal fauna. Nature 356, 514–516.
| Earliest known Australian Tertiary mammal fauna.Crossref | GoogleScholarGoogle Scholar |
Gould, A. R., Hyatt, A. D., Lunt, R., Kattenbelt, J. A., Hengstberger, S., and Blacksell, S. D. (1998). Characterisation of a novel lyssavirus isolated from pteropid bats in Australia. Virus Research 54, 165–187.
| Characterisation of a novel lyssavirus isolated from pteropid bats in Australia.Crossref | GoogleScholarGoogle Scholar | 9696125PubMed |
Gretebeck, L. M., and Subbarao, K. (2015). Animal models for SARS and MERS coronaviruses. Current Opinion in Virology 13, 123–129.
| Animal models for SARS and MERS coronaviruses.Crossref | GoogleScholarGoogle Scholar | 26184451PubMed |
Guan, Y., Zheng, B. J., He, Y. Q., Liu, X. L., Zhuang, Z. X., Cheung, C. L., Luo, S. W., Li, P. H., Zhang, L. J., Guan, Y. J., Butt, K. M., Wong, K. L., Chan, K. W., Lim, W., Shortridge, K. F., Yuen, K. Y., Peiris, J. S. M., and Poon, L. L. M. (2003). Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science 302, 276–278.
| Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China.Crossref | GoogleScholarGoogle Scholar | 12958366PubMed |
Halpin, K., Young, P. L., and Field, H. E. (2000). Isolation of Hendra virus from pteropid bats: a natural reservoir of Hendra virus. Journal of General Virology 81, 1927–1932.
| Isolation of Hendra virus from pteropid bats: a natural reservoir of Hendra virus.Crossref | GoogleScholarGoogle Scholar | 10900029PubMed |
He, B., Zhang, Y., Xu, L., Yang, W., Yang, F., Feng, Y., Xia, L., Zhou, J., Zhen, W., Feng, Y., Guo, H., Zhang, H., and Tu, C. (2014). Identification of diverse alphacoronaviruses and genomic characterization of a novel severe acute respiratory syndrome-like coronavirus from bats in China. Journal of Virology 88, 7070–7082.
| Identification of diverse alphacoronaviruses and genomic characterization of a novel severe acute respiratory syndrome-like coronavirus from bats in China.Crossref | GoogleScholarGoogle Scholar | 24719429PubMed |
Holz, P. H., Lumsden, L. F., Druce, J., Legione, A. R., Vaz, P., Devlin, J. M., and Hufschmid, J. (2018). Virus survey in populations of two subspecies of bent-winged bats (Miniopterus orianae bassanii and oceanensis) in south-eastern Australia reveals a high prevalence of diverse herpesviruses. PLoS One 13, e0197625.
| Virus survey in populations of two subspecies of bent-winged bats (Miniopterus orianae bassanii and oceanensis) in south-eastern Australia reveals a high prevalence of diverse herpesviruses.Crossref | GoogleScholarGoogle Scholar | 30303979PubMed |
Hosken, D. J., and Withers, P. C. (1997). Temperature regulation and metabolism of an Australian bat, Chalinolobus gouldii (Chiroptera: Vespertilionidae) when euthermic and torpid. Journal of Comparative Physiology. B, Biochemical, Systemic, and Environmental Physiology 167, 71–80.
| Temperature regulation and metabolism of an Australian bat, Chalinolobus gouldii (Chiroptera: Vespertilionidae) when euthermic and torpid.Crossref | GoogleScholarGoogle Scholar | 9051907PubMed |
Hu, B., Zeng, L.-P., Yang, X.-L., Ge, X.-Y., Zhang, W., Li, B., Xie, J.-Z., Shen, X.-R., Zhang, Y.-Z., Wang, N., Luo, D.-S., Zheng, X.-S., Wang, M.-N., Daszak, P., Wang, L.-F., Cui, J., and Shi, Z.-L. (2017). Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathogens 13, e1006698.
| Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus.Crossref | GoogleScholarGoogle Scholar | 29190287PubMed |
IUCN Bat Specialist Group (2020). IUCN SSC Bat Specialist Group (BSG) recommendations to reduce the risk of transmission of SARS-CoV-2 from humans to bats in bat rescue and rehabilitation centers. MAP: Minimize, Assess, Protect. Living Document Version 1.1, released 15 July 2020. Available at: https://www.iucnbsg.org/bsg-publications.html [accessed 15 July 2020].
Jayme, S. I., Field, H. E., de Jong, C., Olival, K. J., Marsh, G., Tagtag, A. M., Hughes, T., Bucad, A. C., Barr, J. A., Azul, R. R., Retes, L. M., Foord, A., Yu, M., Cruz, M. S., Santos, I. J., Lim, T. M. S., Benigno, C. C., Epstein, J. H., Wang, L.-F., Daszak, P., and Newman, S. H. (2015). Molecular evidence of Ebola Reston virus infection in Philippine bats. Virology Journal 12, 107.
| Molecular evidence of Ebola Reston virus infection in Philippine bats.Crossref | GoogleScholarGoogle Scholar | 26184657PubMed |
Jeong, J, Smith, C. S., Peel, A. J., Plowright, R. K., Kerlin, D. H., McBroom, J, and McCallum, H (2017). Persistent infections support maintenance of a coronavirus in a population of Australian bats (Myotis macropus). Epidemiology and Infection 145, 2053–2061.
| 28528587PubMed |
Joffrin, L., Goodman, S. M., Wilkinson, D. A., Ramasindrazana, B., Lagadec, E., Gomard, Y., Minter, G. L., Santos, A. D., Schoeman, M. C., Sookhareea, R., Tortosa, P., Julienne, S., Gudo, E. S., Mavingui, P., and Lebarbenchon, C. (2020). Bat coronavirus phylogeography in the western Indian Ocean. Scientific Reports 10, 6873.
| Bat coronavirus phylogeography in the western Indian Ocean.Crossref | GoogleScholarGoogle Scholar | 32327721PubMed |
Johnson, C. K., Hitchens, P. L., Pandit, P. S., Rushmore, J., Evans, T. S., Young, C. C. W., and Doyle, M. M. (2020). Global shifts in mammalian population trends reveal key predictors of virus spillover risk. Proceedings of the Royal Society B: Biological Sciences 287, 20192736.
| 32259475PubMed |
Jung, K., and Saif, L. J. (2015). Porcine epidemic diarrhea virus infection: etiology, epidemiology, pathogenesis and immunoprophylaxis. Veterinary Journal 204, 134–143.
| Porcine epidemic diarrhea virus infection: etiology, epidemiology, pathogenesis and immunoprophylaxis.Crossref | GoogleScholarGoogle Scholar |
Kan, B., Wang, M., Jing, H., Xu, H., Jiang, X., Yan, M., Liang, W., Zheng, H., Wan, K., Liu, Q., Cui, B., Xu, Y., Zhang, E., Wang, H., Ye, J., Li, G., Li, M., Cui, Z., Qi, X., Chen, K., Du, L., Gao, K., Zhao, Y.-t., Zou, X.-z., Feng, Y.-J., Gao, Y.-F., Hai, R., Yu, D., Guan, Y., and Xu, J. (2005). Molecular evolution analysis and geographic investigation of severe acute respiratory syndrome coronavirus-like virus in palm civets at an animal market and on farms. Journal of Virology 79, 11892–11900.
| Molecular evolution analysis and geographic investigation of severe acute respiratory syndrome coronavirus-like virus in palm civets at an animal market and on farms.Crossref | GoogleScholarGoogle Scholar | 16140765PubMed |
Kessler, M. K., Becker, D. J., Peel, A. J., Justice, N. V., Lunn, T., Crowley, D. E., Jones, D. N., Eby, P., Sánchez, C. A., and Plowright, R. K. (2018). Changing resource landscapes and spillover of henipaviruses. Annals of the New York Academy of Sciences 1429, 78–99.
| Changing resource landscapes and spillover of henipaviruses.Crossref | GoogleScholarGoogle Scholar | 30138535PubMed |
Kissler, S. M., Tedijanto, C., Goldstein, E., Grad, Y. H., and Lipsitch, M. (2020). Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science 368, 860–868.
| Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period.Crossref | GoogleScholarGoogle Scholar | 32291278PubMed |
Kolkert, H., Andrew, R., Smith, R., Rader, R., and Reid, N. (2020). Insectivorous bats selectively source moths and eat mostly pest insects on dryland and irrigated cotton farms. Ecology and Evolution 10, 371–388.
| Insectivorous bats selectively source moths and eat mostly pest insects on dryland and irrigated cotton farms.Crossref | GoogleScholarGoogle Scholar | 31988733PubMed |
Ksiazek, T. G., Erdman, D., Goldsmith, C. S., Zaki, S. R., Peret, T., Emery, S., Tong, S., Urbani, C., Comer, J. A., Lim, W., Rollin, P. E., Dowell, S. F., Ling, A.-E., Humphrey, C. D., Shieh, W.-J., Guarner, J., Paddock, C. D., Rota, P., Fields, B., DeRisi, J., Yang, J.-Y., Cox, N., Hughes, J. M., LeDuc, J. W., Bellini, W. J., Anderson, L. J., and Group, S. W. (2003). A novel coronavirus associated with severe acute respiratory syndrome. The New England Journal of Medicine 348, 1953–1966.
| A novel coronavirus associated with severe acute respiratory syndrome.Crossref | GoogleScholarGoogle Scholar | 12690092PubMed |
Kumar, S., Stecher, G., Li, M., Knyaz, C., and Tamura, K. (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution 35, 1547–1549.
| MEGA X: molecular evolutionary genetics analysis across computing platforms.Crossref | GoogleScholarGoogle Scholar | 29722887PubMed |
Kung, N. Y., Field, H. E., McLaughlin, A., Edson, D., and Taylor, M. (2015). Flying-foxes in the Australian urban environment – community attitudes and opinions. One Health 1, 24–30.
| Flying-foxes in the Australian urban environment – community attitudes and opinions.Crossref | GoogleScholarGoogle Scholar | 28616461PubMed |
Lacroix, A., Duong, V., Hul, V., San, S., Davun, H., Omaliss, K., Chea, S., Hassanin, A., Theppangna, W., Silithammavong, S., Khammavong, K., Singhalath, S., Greatorex, Z., Fine, A. E., Goldstein, T., Olson, S., Joly, D. O., Keatts, L., Dussart, P., Afelt, A., Frutos, R., and Buchy, P. (2017). Genetic diversity of coronaviruses in bats in Lao PDR and Cambodia. Infection, Genetics and Evolution 48, 10–18.
| Genetic diversity of coronaviruses in bats in Lao PDR and Cambodia.Crossref | GoogleScholarGoogle Scholar | 27932284PubMed |
Latinne, A., Hu, B., Olival, K. J., Zhu, G., Zhang, L., Li, H., Chmura, A. A., Field, H. E., Zambrana-Torrelio, C., Epstein, J. H., Li, B., Zhang, W., Wang, L.-F., Shi, Z., and Daszak, P. (2020). Origin and cross-species transmission of bat coronaviruses in China. bioRxiv
Lau, S. K. P., Woo, P. C. Y., Li, K. S. M., Huang, Y., Tsoi, H. W., Wong, B. H. L., Wong, S. S. Y., Leung, S. Y., Chan, K. H., and Yuen, K. Y. (2005). Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proceedings of the National Academy of Sciences of the United States of America 102, 14040–14045.
| Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats.Crossref | GoogleScholarGoogle Scholar |
Law, B. S., and Lean, M. (1999). Common blossom bats (Syconycteris australis) as pollinators in fragmented Australian tropical rainforest. Biological Conservation 91, 201–212.
| Common blossom bats (Syconycteris australis) as pollinators in fragmented Australian tropical rainforest.Crossref | GoogleScholarGoogle Scholar |
Li, W., Shi, Z., Yu, M., Ren, W., Smith, C., Epstein, J. H., Wang, H., Crameri, G., Hu, Z., Zhang, H., Zhang, J., McEachern, J., Field, H. E., Daszak, P., Eaton, B. T., Zhang, S., and Wang, L.-F. (2005). Bats are natural reservoirs of SARS-like coronaviruses. Science 310, 676–679.
| Bats are natural reservoirs of SARS-like coronaviruses.Crossref | GoogleScholarGoogle Scholar | 16195424PubMed |
Li, Q., Guan, X., Wu, P., Wang, X., Zhou, L., Tong, Y., Ren, R., Leung, K. S. M., Lau, E. H. Y., Wong, J. Y., Xing, X., Xiang, N., Wu, Y., Li, C., Chen, Q., Li, D., Liu, T., Zhao, J., Liu, M., Tu, W., Chen, C., Jin, L., Yang, R., Wang, Q., Zhou, S., Wang, R., Liu, H., Luo, Y., Liu, Y., Shao, G., Li, H., Tao, Z., Yang, Y., Deng, Z., Liu, B., Ma, Z., Zhang, Y., Shi, G., Lam, T. T. Y., Wu, J. T., Gao, G. F., Cowling, B. J., Yang, B., Leung, G. M., and Feng, Z. (2020). Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. The New England Journal of Medicine 382, 1199–1207.
| Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia.Crossref | GoogleScholarGoogle Scholar | 31995857PubMed |
Lindahl, J. F., and Grace, D. (2015). The consequences of human actions on risks for infectious diseases: a review. Infection Ecology & Epidemiology 5, 30048.
| The consequences of human actions on risks for infectious diseases: a review.Crossref | GoogleScholarGoogle Scholar |
Lumsden, L. F., and Bennett, A. F. (2005). Scattered trees in rural landscapes: foraging habitat for insectivorous bats in south-eastern Australia. Biological Conservation 122, 205–222.
| Scattered trees in rural landscapes: foraging habitat for insectivorous bats in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
McKenzie, N. L., and Rolfe, J. K. (1986). Structure of bat guilds in the Kimberley mangroves, Australia. Journal of Animal Ecology 55, 401.
| Structure of bat guilds in the Kimberley mangroves, Australia.Crossref | GoogleScholarGoogle Scholar |
Montecino-Latorre, D., Goldstein, T., Gilardi, K., Wolking, D., Wormer, E. V., Kazwala, R., Ssebide, B., Nziza, J., Sijali, Z., Cranfield, M., Consortium, P., and Mazet, J. A. K. (2020). Reproduction of East-African bats may guide risk mitigation for coronavirus spillover. One Health Outlook 2, 2.
| Reproduction of East-African bats may guide risk mitigation for coronavirus spillover.Crossref | GoogleScholarGoogle Scholar |
Moran, C., Catterall, C. P., and Kanowski, J. (2009). Reduced dispersal of native plant species as a consequence of the reduced abundance of frugivore species in fragmented rainforest. Biological Conservation 142, 541–552.
| Reduced dispersal of native plant species as a consequence of the reduced abundance of frugivore species in fragmented rainforest.Crossref | GoogleScholarGoogle Scholar |
Moreira-Soto, A., Taylor-Castillo, L., Vargas-Vargas, N., Rodríguez-Herrera, B., Jiménez, C., and Corrales-Aguilar, E. (2015). Neotropical bats from Costa Rica harbour diverse coronaviruses. Zoonoses and Public Health 62, 501–505.
| Neotropical bats from Costa Rica harbour diverse coronaviruses.Crossref | GoogleScholarGoogle Scholar | 25653111PubMed |
Olival, K. J., Cryan, P. M., Amman, B. R., Baric, R. S., Blehert, D. S., Brook, C. E., Calisher, C. H., Castle, K. T., Coleman, J. T. H., Daszak, P., Epstein, J. H., Field, H., Frick, W. F., Gilbert, A. T., Hayman, D. T. S., Ip, H. S., Karesh, W. B., Johnson, C. K., Kading, R. C., Kingston, T., Lorch, J. M., Mendenhall, I. H., Peel, A. J., Phelps, K. L., Plowright, R. K., Reeder, D. M., Reichard, J. D., Sleeman, J. M., Streicker, D. G., Towner, J. S., and Wang, L.-F. (2020). Possible risks of SARS-CoV-2 spillover from humans to free-ranging wildlife: a case study of bats. PLoS Pathogens 16, e1008758.
| Possible risks of SARS-CoV-2 spillover from humans to free-ranging wildlife: a case study of bats.Crossref | GoogleScholarGoogle Scholar |
Paez, D. J., Giles, J., McCallum, H., Field, H. E., Jordan, D., Peel, A. J., and Plowright, R. K. (2017). Conditions affecting the timing and magnitude of Hendra virus shedding across pteropodid bat populations in Australia. Epidemiology and Infection 145, 3143–3153.
| Conditions affecting the timing and magnitude of Hendra virus shedding across pteropodid bat populations in Australia.Crossref | GoogleScholarGoogle Scholar | 28942750PubMed |
Peel, A. J., Wells, K., Giles, J., Boyd, V., Burroughs, A., Edson, D., Crameri, G., Baker, M. L., Field, H., Wang, L.-F., McCallum, H., Plowright, R. K., and Clark, N. (2019). Synchronous shedding of multiple bat paramyxoviruses coincides with peak periods of Hendra virus spillover. Emerging Microbes & Infections 8, 1314–1323.
| Synchronous shedding of multiple bat paramyxoviruses coincides with peak periods of Hendra virus spillover.Crossref | GoogleScholarGoogle Scholar |
Pfefferle, S., Oppong, S., Drexler, J. F., Gloza-Rausch, F., Ipsen, A., Seebens, A., Müller, M. A., Annan, A., Vallo, P., Adu-Sarkodie, Y., Kruppa, T. F., and Drosten, C. (2009). Distant relatives of severe acute respiratory syndrome coronavirus and close relatives of human coronavirus 229E in bats, Ghana. Emerging Infectious Diseases 15, 1377–1384.
| Distant relatives of severe acute respiratory syndrome coronavirus and close relatives of human coronavirus 229E in bats, Ghana.Crossref | GoogleScholarGoogle Scholar | 19788804PubMed |
Philbey, A. W., Kirkland, P. D., Ross, A. D., Davis, R. J., Gleeson, A. B., Love, R. J., Daniels, P. W., Gould, A. R., and Hyatt, A. D. (1998). An apparently new virus (family Paramyxoviridae) infectious for pigs, humans, and fruit bats. Emerging Infectious Diseases 4, 269–271.
| An apparently new virus (family Paramyxoviridae) infectious for pigs, humans, and fruit bats.Crossref | GoogleScholarGoogle Scholar | 9621197PubMed |
Plowright, R. K., Field, H. E., Smith, C., Divljan, A., Palmer, C., Tabor, G., Daszak, P., and Foley, J. E. (2008). Reproduction and nutritional stress are risk factors for Hendra virus infection in little red flying foxes (Pteropus scapulatus). Proceedings of the Royal Society B: Biological Sciences 275, 861–869.
| Reproduction and nutritional stress are risk factors for Hendra virus infection in little red flying foxes (Pteropus scapulatus).Crossref | GoogleScholarGoogle Scholar | 18198149PubMed |
Plowright, R. K., Eby, P., Hudson, P. J., Smith, I. L., Westcott, D. A., Bryden, W. L., Middleton, D., Reid, P. A., McFarlane, R. A., Martin, G., Tabor, G. M., Skerratt, L. F., Anderson, D. L., Crameri, G., Quammen, D., Jordan, D., Freeman, P., Wang, L.-F., Epstein, J. H., Marsh, G. A., Kung, N. Y., and McCallum, H. (2015). Ecological dynamics of emerging bat virus spillover. Proceedings of the Royal Society B: Biological Sciences 282, 20142124.
| Ecological dynamics of emerging bat virus spillover.Crossref | GoogleScholarGoogle Scholar | 25392474PubMed |
Plowright, R. K., Parrish, C. R., McCallum, H., Hudson, P. J., Ko, A. I., Graham, A. L., and Lloyd-Smith, J. O. (2017). Pathways to zoonotic spillover. Nature Reviews. Microbiology 15, 502–510.
| Pathways to zoonotic spillover.Crossref | GoogleScholarGoogle Scholar | 28555073PubMed |
Plowright, R. K., Becker, D. J., McCallum, H., and Manlove, K. (2019). Sampling to elucidate the dynamics of infections in reservoir hosts. Philosophical Transactions of the Royal Society B: Biological Sciences 374, 20180336.
| Sampling to elucidate the dynamics of infections in reservoir hosts.Crossref | GoogleScholarGoogle Scholar |
Poon, L. L. M., Chu, D. K. W., Chan, K. H., Wong, O. K., Ellis, T. M., Leung, Y. H. C., Lau, S. K. P., Woo, P. C. Y., Suen, K. Y., Yuen, K. Y., Guan, Y., and Peiris, J. S. M. (2005). Identification of a novel coronavirus in bats. Journal of Virology 79, 2001–2009.
| Identification of a novel coronavirus in bats.Crossref | GoogleScholarGoogle Scholar |
Prada, D., Boyd, V., Baker, M. L., O’Dea, M., and Jackson, B. (2019). Viral diversity of microbats within the South West Botanical Province of Western Australia. Viruses 11, 1157.
| Viral diversity of microbats within the South West Botanical Province of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Quan, P.-L., Firth, C., Street, C., Henriquez, J. A., Petrosov, A., Tashmukhamedova, A., Hutchison, S. K., Egholm, M., Osinubi, M. O. V., Niezgoda, M., Ogunkoya, A. B., Briese, T., Rupprecht, C. E., and Lipkin, W. I. (2010). Identification of a severe acute respiratory syndrome coronavirus-like virus in a leaf-nosed bat in Nigeria. mBio 1, e00208-10.
| Identification of a severe acute respiratory syndrome coronavirus-like virus in a leaf-nosed bat in Nigeria.Crossref | GoogleScholarGoogle Scholar | 21063474PubMed |
Reardon, T. B., Armstrong, K. N., and Jackson, S. M. (2015). A current taxonomic list of Australian Chiroptera. Australasian Bat Society. Available at: http://ausbats.org.au/taxonomic-list/4589345107 [accessed 4 June 2020].
Rimmer, M. (2020). ‘Bat people’ say the unique mammals need to be respected, not feared. SBS The Feed. Available at: https://www.sbs.com.au/news/the-feed/bat-people-say-the-unique-mammals-need-to-be-respected-not-feared [accessed 4 June 2020].
Runge, M. C., Grant, E. H. C., Coleman, J. T. H., Reichard, J. D., Gibbs, S. E. J., Cryan, P. M., Olival, K. J., Walsh, D. P., Blehert, D. S., Hopkins, M. C., and Sleeman, J. M. (2020). Assessing the risks posed by SARS-CoV-2 in and via North American bats – decision framing and rapid risk assessment. U.S. Geological Survey Open-File Report 2020-1060. Available at: http://pubs.er.usgs.gov/publication/ofr20201060 [accessed 4 June 2020].
Schountz, T., Baker, M. L., Butler, J., and Munster, V. (2017). Immunological control of viral infections in bats and the emergence of viruses highly pathogenic to humans. Frontiers in Immunology 8, 1098.
| Immunological control of viral infections in bats and the emergence of viruses highly pathogenic to humans.Crossref | GoogleScholarGoogle Scholar | 28959255PubMed |
Selvey, L., Taylor, R., Arklay, A., and Gerrard, J. (1996). Screening of bat carers for antibodies to equine morbillivirus. Communicable Diseases Intelligence 20, 477–478.
Shi, Z., and Hu, Z. (2008). A review of studies on animal reservoirs of the SARS coronavirus. Virus Research 133, 74–87.
| A review of studies on animal reservoirs of the SARS coronavirus.Crossref | GoogleScholarGoogle Scholar | 17451830PubMed |
Shi, J., Wen, Z., Zhong, G., Yang, H., Wang, C., Huang, B., Liu, R., He, X., Shuai, L., Sun, Z., Zhao, Y., Liu, P., Liang, L., Cui, P., Wang, J., Zhang, X., Guan, Y., Tan, W., Wu, G., Chen, H., and Bu, Z. (2020). Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science 368, 1016–1020.
| Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2.Crossref | GoogleScholarGoogle Scholar | 32269068PubMed |
Simmons, N. B., and Cirranello, A. L. (2020). Bat species of the world: a taxonomic and geographic database. Available at: www.batnames.org [accessed 4 June 2020].
Smith, C. (2015). Australian bat coronaviruses. Ph.D. Thesis, University of Queensland, Brisbane, Australia.
Smith, C. S., de Jong, C. E., Meers, J., Henning, J., Wang, L. F., and Field, H. E. (2016). Coronavirus infection and diversity in bats in the Australasian region. EcoHealth 13, 72–82.
| Coronavirus infection and diversity in bats in the Australasian region.Crossref | GoogleScholarGoogle Scholar | 27048154PubMed |
Su, S., Wong, G., Shi, W., Liu, J., Lai, A. C. K., Zhou, J., Liu, W., Bi, Y., and Gao, G. F. (2016). Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends in Microbiology 24, 490–502.
| Epidemiology, genetic recombination, and pathogenesis of coronaviruses.Crossref | GoogleScholarGoogle Scholar | 27012512PubMed |
Tang, X. C., Zhang, J. X., Zhang, S. Y., Wang, P., Fan, X. H., Li, L. F., Li, G., Dong, B. Q., Liu, W., Cheung, C. L., Xu, K. M., Song, W. J., Vijaykrishna, D., Poon, L. L. M., Peiris, J. S. M., Smith, G. J. D., Chen, H., and Guan, Y. (2006). Prevalence and genetic diversity of coronaviruses in bats from China. Journal of Virology 80, 7481–7490.
| Prevalence and genetic diversity of coronaviruses in bats from China.Crossref | GoogleScholarGoogle Scholar | 16840328PubMed |
Tong, S., Conrardy, C., Ruone, S., Kuzmin, I. V., Guo, X., Tao, Y., Niezgoda, M., Haynes, L., Agwanda, B., Breiman, R. F., Anderson, L. J., and Rupprecht, C. E. (2009). Detection of novel SARS-like and other coronaviruses in bats from Kenya. Emerging Infectious Diseases 15, 482–485.
| Detection of novel SARS-like and other coronaviruses in bats from Kenya.Crossref | GoogleScholarGoogle Scholar | 19239771PubMed |
Tsuda, S., Watanabe, S., Masangkay, J. S., Mizutani, T., Alviola, P., Ueda, N., Iha, K., Taniguchi, S., Fujii, H., Kato, K., Horimoto, T., Kyuwa, S., Yoshikawa, Y., and Akashi, H. (2012). Genomic and serological detection of bat coronavirus from bats in the Philippines. Archives of Virology 157, 2349–2355.
| Genomic and serological detection of bat coronavirus from bats in the Philippines.Crossref | GoogleScholarGoogle Scholar | 22833101PubMed |
Tu, C., Crameri, G., Kong, X., Chen, J., Sun, Y., Yu, M., Xiang, H., Xia, X., Liu, S., Ren, T., Yu, Y., Eaton, B. T., Xuan, H., and Wang, L.-F. (2004). Antibodies to SARS coronavirus in civets. Emerging Infectious Diseases 10, 2244–2248.
| Antibodies to SARS coronavirus in civets.Crossref | GoogleScholarGoogle Scholar | 15663874PubMed |
US Department of Agriculture Animal and Plant Health Inspection Service (2020). USDA statement on the confirmation of COVID-19 in a tiger in New York. United States Department of Agriculture, Animal and Plant Health Inspection Service. Available at: https://www.aphis.usda.gov/aphis/newsroom/news/sa_by_date/sa-2020/ny-zoo-covid-19 [accessed 4 June 2020].
Vijaykrishna, D., Smith, G. J. D., Zhang, J. X., Peiris, J. S. M., Chen, H., and Guan, Y. (2007). Evolutionary insights into the ecology of coronaviruses. Journal of Virology 81, 4012–4020.
| Evolutionary insights into the ecology of coronaviruses.Crossref | GoogleScholarGoogle Scholar | 17267506PubMed |
Wacharapluesadee, S., Sintunawa, C., Kaewpom, T., Khongnomnan, K., Olival, K. J., Epstein, J. H., Rodpan, A., Sangsri, P., Intarut, N., Chindamporn, A., Suksawa, K., and Hemachudha, T. (2013). Group C betacoronavirus in bat guano fertilizer, Thailand. Emerging Infectious Diseases 19, 1349–1351.
| Group C betacoronavirus in bat guano fertilizer, Thailand.Crossref | GoogleScholarGoogle Scholar | 23880503PubMed |
Wacharapluesadee, S., Duengkae, P., Chaiyes, A., Kaewpom, T., Rodpan, A., Yingsakmongkon, S., Petcharat, S., Phengsakul, P., Maneeorn, P., and Hemachudha, T. (2018). Longitudinal study of age-specific pattern of coronavirus infection in Lyle’s flying fox (Pteropus lylei) in Thailand. Virology Journal 15, 38.
| Longitudinal study of age-specific pattern of coronavirus infection in Lyle’s flying fox (Pteropus lylei) in Thailand.Crossref | GoogleScholarGoogle Scholar | 29463282PubMed |
Wang, N., Li, S.-Y., Yang, X.-L., Huang, H.-M., Zhang, Y.-J., Guo, H., Luo, C.-M., Miller, M., Zhu, G., Chmura, A. A., Hagan, E., Zhou, J.-H., Zhang, Y.-Z., Wang, L.-F., Daszak, P., and Shi, Z.-L. (2018). Serological evidence of bat SARS-related coronavirus infection in humans, China. Virologica Sinica 33, 104–107.
| Serological evidence of bat SARS-related coronavirus infection in humans, China.Crossref | GoogleScholarGoogle Scholar | 29500691PubMed |
Webb, N., and Tidemann, C. R. (1996). Mobility of Australian flying-foxes, Pteropus spp. (Megachiroptera): evidence from genetic variation. Proceedings of the Royal Society B: Biological Sciences 263, 497–502.
| Mobility of Australian flying-foxes, Pteropus spp. (Megachiroptera): evidence from genetic variation.Crossref | GoogleScholarGoogle Scholar | 8637931PubMed |
Welbergen, J. A., Klose, S. M., Markus, N., and Eby, P. (2008). Climate change and the effects of temperature extremes on Australian flying-foxes. Proceedings of the Royal Society B: Biological Sciences 275, 419–425.
| Climate change and the effects of temperature extremes on Australian flying-foxes.Crossref | GoogleScholarGoogle Scholar | 18048286PubMed |
Willoughby, A., Phelps, K., Consortium, P., and Olival, K. (2017). A comparative analysis of viral richness and viral sharing in cave-roosting bats. Diversity 9, 35.
| A comparative analysis of viral richness and viral sharing in cave-roosting bats.Crossref | GoogleScholarGoogle Scholar |
Wong, A., Li, X., Lau, S., and Woo, P. (2019). Global epidemiology of bat coronaviruses. Viruses 11, 174.
| Global epidemiology of bat coronaviruses.Crossref | GoogleScholarGoogle Scholar |
Woo, P. C. Y., Lau, S. K. P., Huang, Y., and Yuen, K.-Y. (2009). Coronavirus diversity, phylogeny and interspecies jumping. Experimental Biology and Medicine 234, 1117–1127.
| Coronavirus diversity, phylogeny and interspecies jumping.Crossref | GoogleScholarGoogle Scholar |
Woo, P. C. Y., Huang, Y., Lau, S. K. P., and Yuen, K.-Y. (2010). Coronavirus genomics and bioinformatics analysis. Viruses 2, 1804–1820.
| Coronavirus genomics and bioinformatics analysis.Crossref | GoogleScholarGoogle Scholar |
Zhang, G., Cowled, C., Shi, Z., Huang, Z., Bishop-Lilly, K. A., Fang, X., Wynne, J. W., Xiong, Z., Baker, M. L., Zhao, W., Tachedjian, M., Zhu, Y., Zhou, P., Jiang, X., Ng, J., Yang, L., Wu, L., Xiao, J., Feng, Y., Chen, Y., Sun, X., Zhang, Y., Marsh, G. A., Crameri, G., Broder, C. C., Frey, K. G., Wang, L.-F., and Wang, J. (2013). Comparative analysis of bat genomes provides insight into the evolution of flight and immunity. Science 339, 456–460.
| Comparative analysis of bat genomes provides insight into the evolution of flight and immunity.Crossref | GoogleScholarGoogle Scholar | 23258410PubMed |
Zhang, T., Wu, Q., and Zhang, Z. (2020). Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Current Biology 30, 1346–1351.e2.
| Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak.Crossref | GoogleScholarGoogle Scholar | 32197085PubMed |
Zhou, P., Chionh, Y. T., Irac, S. E., Ahn, M., Ng, J. H. J., Fossum, E., Bogen, B., Ginhoux, F., Irving, A. T., Dutertre, C.-A., and Wang, L.-F. (2016). Unlocking bat immunology: establishment of Pteropus alecto bone marrow-derived dendritic cells and macrophages. Scientific Reports 6, 38597.
| Unlocking bat immunology: establishment of Pteropus alecto bone marrow-derived dendritic cells and macrophages.Crossref | GoogleScholarGoogle Scholar | 27934903PubMed |
Zhou, P., Fan, H., Lan, T., Yang, X.-L., Shi, W.-F., Zhang, W., Zhu, Y., Zhang, Y.-W., Xie, Q.-M., Mani, S., Zheng, X.-S., Li, B., Li, J.-M., Guo, H., Pei, G.-Q., An, X.-P., Chen, J.-W., Zhou, L., Mai, K.-J., Wu, Z.-X., Li, D., Anderson, D. E., Zhang, L.-B., Li, S.-Y., Mi, Z.-Q., He, T.-T., Cong, F., Guo, P.-J., Huang, R., Luo, Y., Liu, X.-L., Chen, J., Huang, Y., Sun, Q., Zhang, X.-L.-L., Wang, Y.-Y., Xing, S.-Z., Chen, Y.-S., Sun, Y., Li, J., Daszak, P., Wang, L.-F., Shi, Z.-L., Tong, Y.-G., and Ma, J.-Y. (2018). Fatal swine acute diarrhoea syndrome caused by an HKU2-related coronavirus of bat origin. Nature 556, 255–258.
| Fatal swine acute diarrhoea syndrome caused by an HKU2-related coronavirus of bat origin.Crossref | GoogleScholarGoogle Scholar | 29618817PubMed |
Zhou, H., Chen, X., Hu, T., Li, J., Song, H., Liu, Y., Wang, P., Liu, D., Yang, J., Holmes, E. C., Hughes, A. C., Bi, Y., and Shi, W. (2020). A novel bat coronavirus closely related to SARS-CoV-2 contains natural insertions at the S1/S2 cleavage site of the spike protein. Current Biology 30, 2196–2203.e3.
| A novel bat coronavirus closely related to SARS-CoV-2 contains natural insertions at the S1/S2 cleavage site of the spike protein.Crossref | GoogleScholarGoogle Scholar | 32416074PubMed |
Zhou, P., Yang, X.-L., Wang, X.-G., Hu, B., Zhang, L., Zhang, W., Si, H.-R., Zhu, Y., Li, B., Huang, C.-L., Chen, H.-D., Chen, J., Luo, Y., Guo, H., Jiang, R.-D., Liu, M.-Q., Chen, Y., Shen, X.-R., Wang, X., Zheng, X.-S., Zhao, K., Chen, Q.-J., Deng, F., Liu, L.-L., Yan, B., Zhan, F.-X., Wang, Y.-Y., Xiao, G.-F., and Shi, Z.-L. (2020a). Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. bioRxiv
Zhou, P., Yang, X.-L., Wang, X.-G., Hu, B., Zhang, L., Zhang, W., Si, H.-R., Zhu, Y., Li, B., Huang, C.-L., Chen, H.-D., Chen, J., Luo, Y., Guo, H., Jiang, R.-D., Liu, M.-Q., Chen, Y., Shen, X.-R., Wang, X., Zheng, X.-S., Zhao, K., Chen, Q.-J., Deng, F., Liu, L.-L., Yan, B., Zhan, F.-X., Wang, Y.-Y., Xiao, G.-F., and Shi, Z.-L. (2020b). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273.
| A pneumonia outbreak associated with a new coronavirus of probable bat origin.Crossref | GoogleScholarGoogle Scholar | 32015507PubMed |
Zhu, N., Zhang, D., Wang, W., Li, X., Yang, B., Song, J., Zhao, X., Huang, B., Shi, W., Lu, R., Niu, P., Zhan, F., Ma, X., Wang, D., Xu, W., Wu, G., Gao, G. F., and Tan, W. (2020). A novel coronavirus from patients with pneumonia in China, 2019. The New England Journal of Medicine 382, 727–733.
| A novel coronavirus from patients with pneumonia in China, 2019.Crossref | GoogleScholarGoogle Scholar | 31978945PubMed |





