Evolutionary biogeography of Australian jumping spider genera (Araneae : Salticidae)
Barry J. Richardson
Australian National Insect Collection, National Research Collections Australia, CSIRO, Canberra, ACT 2600, Australia. Email: barry.richardson@csiro.au
Australian Journal of Zoology 67(3) 162-172 https://doi.org/10.1071/ZO20023
Submitted: 29 April 2020 Accepted: 13 July 2020 Published: 28 July 2020
Journal Compilation © CSIRO 2019 Open Access CC BY-NC
Abstract
Phylogenetic relationships and estimated dates of origin, plus distributional, ecological and morphological data for salticid genera were used to examine a series of hypotheses related to the evolution of the Australian salticid fauna. Though independent, the time patterns of evolution of genera in Australia and South America were similar, while that for Northern Hemisphere taxa differed. In each case the production of new genera occurred during the warmer parts of the mid Tertiary but not during cooler and drier times. Asian elements entered Australia as early as 31 million years ago, long before the collision of the Australasian and Asian continental plates. Endemic and derivatives of Asian genera were similarly distributed across Australian biomes. However, arriving taxa were more successful when conditions matched their mesic origins (tropical), but less so when different (temperate). While endemic genera often extended their ranges into drier environments by increasing the number of species, recent arrivals did so by extending the range of individual species. Maximum Parsimony analyses of a range of presumed adaptive, morphological and ecological characters showed these did not reflect genus-level processes; however, the analysis did show all endemic genera had mesic origins.
Additional keywords: Gondwana, macroevolution, Miocene fauna, Oligocene fauna, South America, spiders.
Introduction
The processes occurring during the evolutionary responses of continental faunas to changing environmental conditions are of general interest in a time of changing climate (e.g. Forrest et al. 2015; Clotten et al. 2019). The dynamic Tertiary climatic history of the Australian continent provides opportunities to explore evolutionary responses to such changes in the past. As a consequence of their studies of the processes occurring during the Australian Tertiary, Byrne et al. (2011) suggested that further insights into the evolutionary biogeography of Australia would be obtained if an interdisciplinary approach similar to theirs was used but based on other groups. In their view, such work should move beyond studies based on higher vertebrates and plants; for example, by exploring the evolutionary responses of the continental invertebrate fauna to changing conditions. A dated phylogenetic tree (Bodner and Maddison 2012; Zhang and Maddison 2013) of many of the world’s salticid (Araneae, Salticidae) genera, which includes many Australian genera, is now available. This information in concert with distributional, ecological and morphological data (Richardson et al. 2006; Richardson et al. 2019) for Australian jumping spider genera makes the family a good candidate for such work and this opportunity is taken up here.
The Tertiary history of the Australian continent has been studied by many workers and can be briefly summarised in the following way (Byrne et al. 2008, 2011; Bowman et al. 2010; Black et al. 2012; Crisp and Cook 2013; Crayn et al. 2015; Greenwood and Christophel 2015; Rix et al. 2015; Cassis et al. 2017). In the early Tertiary, the Australasian Plate moved northwards, though Australia was still connected to Antarctica and hence South America. The final break with Antarctica came in the Eocene, isolating Australia from the south. Thereafter the continent continued to move closer to Asia with the plates ultimately colliding. Throughout this process, climate changed, with relatively sudden cooling at the end of the Eocene and a similarly sudden warming in the late Oligocene. There was a further sudden cooling and aridification in the mid-Miocene, which continued to deepen until the present (Fig. 1). Together with the gradual northward movement of the continent, this has led to a generally drier and more seasonal landmass. The warm, mesic Gondwanan forests that had previously dominated the continent were gradually replaced over much of Australia, starting in the Oligocene, by sclerophyllous forests and woodlands. Eventually extensive grasslands and deserts developed by the Pliocene. While the warm, wet forest remains in parts of north-eastern Australia, derived, cold, wet forests developed in the south-east. Parallel to these changes, areas of the northern part of the continent were covered with tropical rainforest in the east and monsoonal vegetation further west. By 33 million years ago (mya) the Australian Plate had moved close enough to south-east Asia for the first biological exchanges to occur, though extensive exchanges did not occur until the collision of the Australian and Asian Plates ~20 mya. A series of summaries of the information available on the biogeography of Australian invertebrates in presented in Ebach (2017). The very limited information on Australian arachnid biogeography is summarised in Harvey et al. (2017), who advocate increased effort to study patterns and processes in the extremely rich Australian arachnid fauna. Most of these studies have addressed processes at the species rather than genus level.
|
Unlike most spider families, jumping spiders are of relatively recent origin (the crown age of the Salticidae is estimated at ~47 mya: Bodner and Maddison 2012) and, in Australia, potentially include elements of both Gondwanan and Asian origin. These include radiations of genera in Australia (called ‘Endemic’ genera hereafter, though several of these genera have since extended their range to much of the rest of the world, e.g. Neon Simon, 1876 and Myrmarachne MacLeay, 1839), radiations of Australian species though part of otherwise Asian genera plus single endemic Australian species belonging to Asian genera (called ‘Partial’ genera hereafter) and, finally, Asian species also present in Australia (called ‘Shared’ species hereafter). The question arises as to the similarities and differences in evolutionary dynamics between these different groups when confronted with the Australian environment.
Analyses of the evolution of Australia’s invertebrate fauna face a serious difficulty: an estimated 71% of the invertebrate species of Australia are yet to be collected and/or described (Chapman 2009; Cassis et al. 2017). Only 500 of the estimated 1500 species of Australian jumping spiders have been described (Richardson 2019). An alternative is to study genera instead; measurements at the generic level have been extensively used in the study of the evolution of taxa or faunas over geological time but the assumption that they are valid surrogates of species-level processes has been questioned (Hendricks et al. 2014). As is to be expected given the present state of knowledge of salticids, genera have been proposed on the basis of the common gestalt of the species included and the presence of significant gaps in morphology between related taxa. Usually the basis for the change in gestalt is unknown, though occasionally, for example in the case of the adaptations to life under bark in Holoplatys Simon, 1885, the reason is clear.
The interpretation of the observed patterns of changes at the generic level depends in part on the processes by which genera develop. Is the gestalt of a genus distinct simply as a result of the gradual accumulation of ‘random’ species-level divergences and extinctions extended over time within a lineage? In this case, generic patterns in related taxa on different continents would be expected to have evolved independently. Alternatively, do genera arise through macroevolutionary processes as part of an adaptive response to changes in conditions (e.g. Lemen and Freeman 1984; Jablonski 2005; Sepkoski 2012)? In this case one would predict similar evolutionary patterns in geographically isolated subsets of taxa that were subjected to similar changes in conditions (e.g. Segar et al. 2020). The independence of the endemic Australian salticid fauna from that on other continents since the Eocene, combined with worldwide changes in climatic conditions, allows the patterns of independent evolution of genera in each area to be compared, and hypotheses about the formation of genera to be tested. Furthermore, predictions about the expected evolutionary patterns in distribution across habitats and in adaptive morphological and ecological characters can also be tested based on these alternate hypotheses about the formation of genera: if genera arise as macroevolutionary responses to conditions, one would expect genus-level rather than species-level patterns in these characteristics. The availability of a worldwide, genera-level, dated phylogeny allows such questions to be explored using the Salticidae.
Both Byrne et al. (2011) and Crisp et al. (2011), in their studies of Australian biogeography, urged the use of hypothesis testing rather than description. Accordingly, this study uses datasets based on an invertebrate group to test hypotheses relating to the evolutionary responses to continental climate changes and the resulting ecological and morphological responses in the production of new genera. It does so by addressing the following hypotheses partially derived from Byrne et al. (2011):
-
That the dates of origin of Australian genera are related to times of climate and vegetation change.
-
That the dates of origin of the Australian salticid genera match those of salticid genera in other parts of the world and reflect the pattern of changes to climate and vegetation.
-
That Australian genera with different histories (i.e. ‘endemic’, ‘partial’ or ‘shared’) adapt to the changing states of Australian environments in different fashions.
-
That radiations in drier environments derive from mesic-zone ancestors and no mesic-zone genera derive from drier environments.
-
That mesic biomes are more resistant to overseas colonisation than arid or monsoonal biomes.
-
That genera increase their range by increasing the number of included species.
-
That genus-level patterns should be apparent in adaptive morphological and ecological characteristics.
Materials and methods
Published molecular trees based on 28s, Actin 5C, 16sND1, and CO1 provide phylogenetic predictions at the generic level for the whole of the Salticidae.
Estimated phylogenetic relationships and dates relevant to the present study were extracted from Bodner and Maddison (2012, fig. 8) for the crown date of the Astioida and for genera in four of the included tribes (Myrmarachnini, Neonini, Astinini and Mopsini: 10 genera) and from Zhang and Maddison (2013, fig. 3) for the Euophryini (7 genera). Some genera, too poorly known or more recently described, have been omitted. Overall, for this part of the study, 17 of the 19 Australian genera in these groups were used, with a further three Pacific genera excluded. The estimated ages of a further 12 endemic Australian genera, not in these clades, led to a combined total of 29 dated Australian genera. The estimated ages of South American and Northern Hemisphere genera were extracted from the same sources.
Previously compiled information (Richardson et al. 2019; Genus Information Sheets) on the number of described species, macrohabitat and microhabitat inhabited by each genus (52 genera), plus a set of presumed adaptive morphological characteristics (maximum body length, longest leg, leg 1 tibial spine size and number, paturon size and direction), of the Australian salticid genera were extracted and used in this study (restricted to 17 genera when combined with the Australian phylogenies). These characters reflect the size and nature of prey and microhabitats, rather than the usual genital characters used in taxonomic studies. These datasets were combined into a characteristics matrix for analysis.
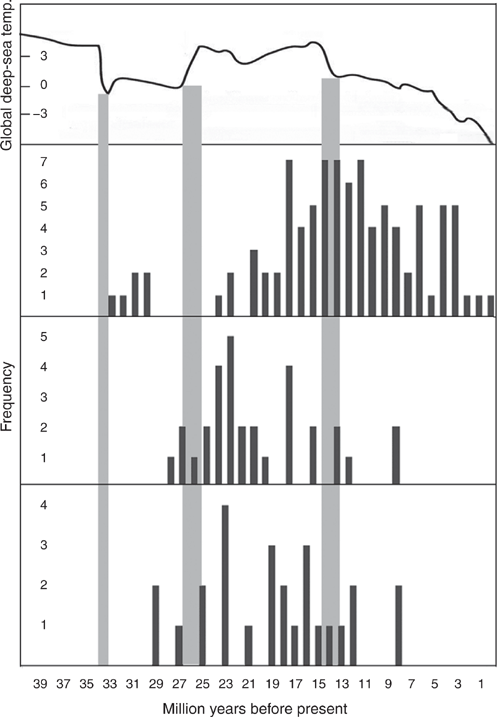
Relevant Tertiary climate information and vegetation changes were taken from Crisp et al. (2004), Byrne et al. (2011), and Crisp and Cook (2013). Barlow (1985) proposed a system of 33 Natural Regions (Fig. 2) which were later refined and described in detail as Regional Landscape Units (RLU) by Bridgewater (1987). The predicted geographical distribution, based on museum records and made using BIOCLIM, of each genus in terms of RLUs were taken from Richardson et al. (2006) except where the taxon has been subjected to more recent research. Revised values were then obtained using the same BIOCLIM modelling methodology used previously. The cluster analysis in Richardson et al. (2006) classifies these RLUs into three groups on the basis of shared salticid genera. These reflect the traditional, mesic (Bassian), sclerophyllous (Eremaean) and monsoonal (Torresian) zones of Australia (Fig. 2), except that, based on salticid genera, the Darling and Riverina Landscape units are included in the mesic rather than the sclerophyllous zone. This is due to salticid species from wetter areas using the riverine vegetation of the Murray–Darling system to extend their range into otherwise drier areas.
|
Character states were mapped onto the astioid and euophryine chronograms and analysed using Maximum Parsimony (MP) with all character states unordered using the Mesquite 3.6 package (Maddison and Maddison 2018). Character states of the ‘parent’ genus (predicted state(s) held by the immediate higher node) of each genus and that of the stem of each radiation were estimated. Frequency histograms of the dates of origin of Australian genera, South American genera and Northern Hemisphere genera were generated from the chronograms of Bodner and Maddison (2012: fig. 8) and Zhang and Maddison (2013: fig. 3) and compared using non-parametric Wilcoxon ranked sum tests. The relationships between the ages, distributions, and number of species were examined using correlations and rank sum tests.
Results
The characteristics matrix developed is given as Supplementary Material (Table S1).
Relationships between the dates of origin of genera and changes in climate and vegetation
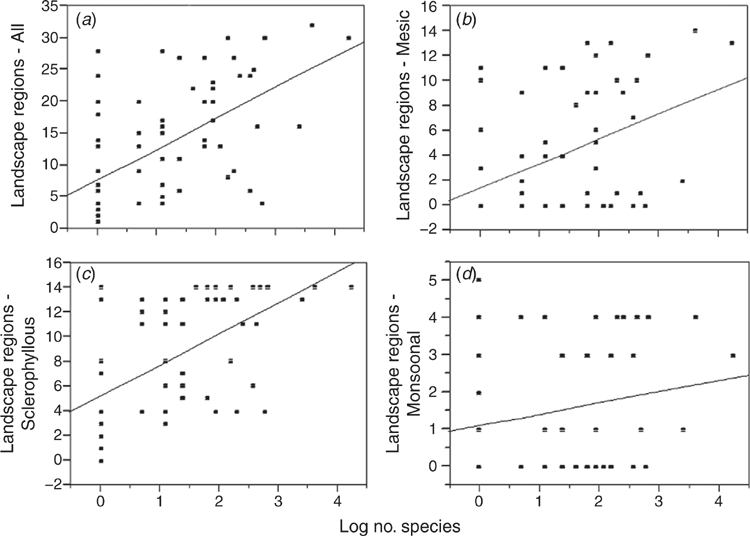
Fig. 3 shows the accumulation of present Australian genera through time, based on the estimated stem dates of origin of each genus. No present-day genera older than mid Oligocene are known and only two genera with estimated dates of origin before the late Oligocene warming are known. The estimated origins of modern genera range from the mid-Oligocene through the early Miocene until the drop in world temperatures beginning in the mid-Miocene. The accumulation curve shows a steady rate of accumulation of new genera throughout the early Miocene without evidence of any ‘burst’ of production following the change in climate.
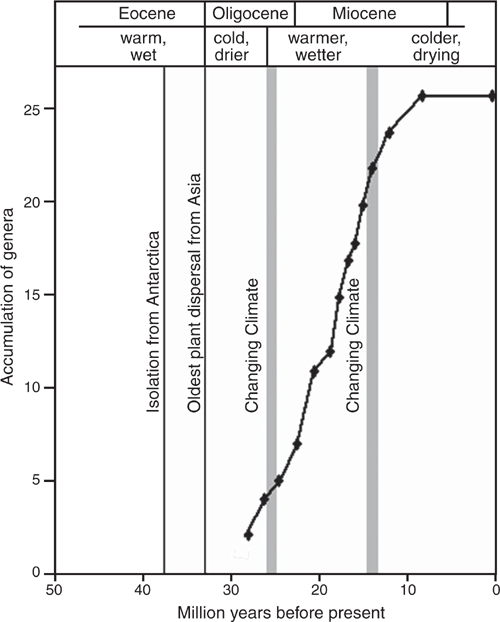
|
Comparison of the dates of origin of present-day genera in South America, the Northern Hemisphere and Australia (Fig. 1) shows that the patterns for Australia and South America are similar but that the pattern for the Northern Hemisphere differs. The dates of origin [x ± s.e. (n) (95% C.I.)] of South American euophryine genera [20.9 ± 0.9 (31) (19.0–22.7) mya] are of a similar age and distribution as those of the Australian fauna [19.1 ± 1.2 (29) (16.8–21.5), Wilcoxon rank sum test P = 0.19 n.s.]. The dates of origin of the present-day genera of these southern continents differed significantly from those from the Northern Hemisphere [14.2 ± 0.7 (93) (12.8–15.5)] (Wilcoxon rank sum test, P = 0.0001), where the evolution of genera started earlier, peaked later and continued well into the Pliocene.
How have Australian genera with different histories adapted to different Australian environments?
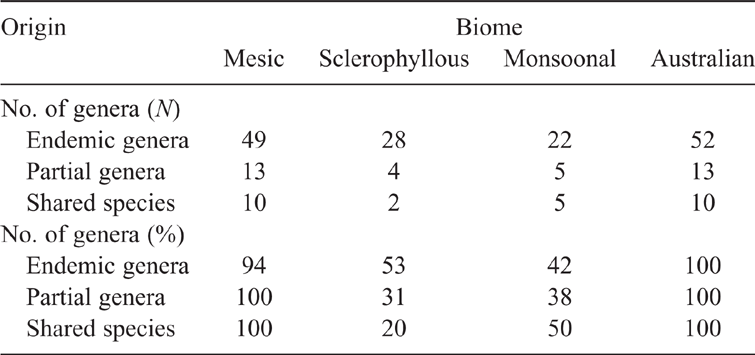
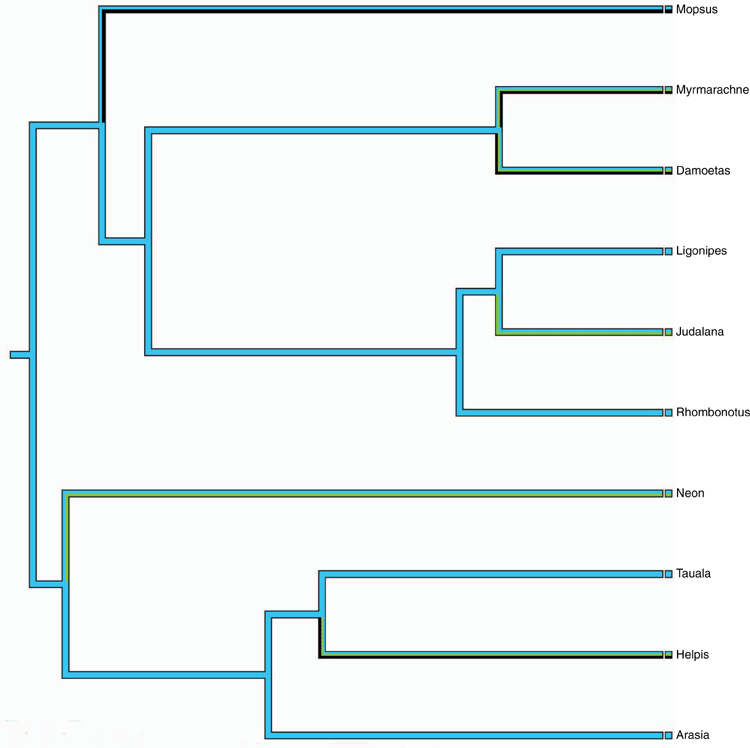
The number of genera using Mesic, Sclerophyllous or Monsoonal biomes by each of the Endemic, Partial and Shared ancestral groups are summarised in Table 1. With three exceptions, all genera are found in the Mesic biome and half or fewer in the other biomes. Shared species seem to have had only limited success invading the Sclerophyllous biome. All Endemic genera or their immediate ancestors (as predicted by the MP analysis) are found in Mesic environments (Fig. 4; Supplementary Material, Fig S1). The MP analyses of the other presumed adaptive characters were uninformative as most genera carried multiple character states, there was little variation across each radiation, or a distinct character state was held by a small clade within a wider monomorphic radiation (Supplementary Material, Table S1). The first of these options apparently reflects species-level rather than genus-level adaptive changes.
|
|
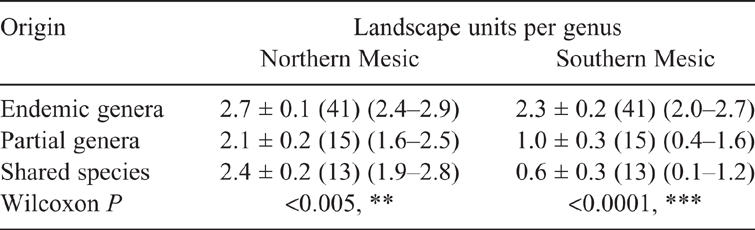
The average numbers of RLUs predicted to be occupied by a genus by Origin (Endemic, Partial or Shared) are shown in Table 2. Endemic genera occupy significantly more Mesic RLUs than the other two groups while there is no difference in the other environments. This raises the question as to whether the non-Endemic genera of tropical origin are less able to adapt to more temperate regions. Table 3 shows the patterns of use of north-eastern and south-eastern Mesic areas by Origin. While all three groups, though differing significantly, are able to effectively use the tropical areas, Partial and Shared taxa are much less successful at adapting to temperate regions.
|
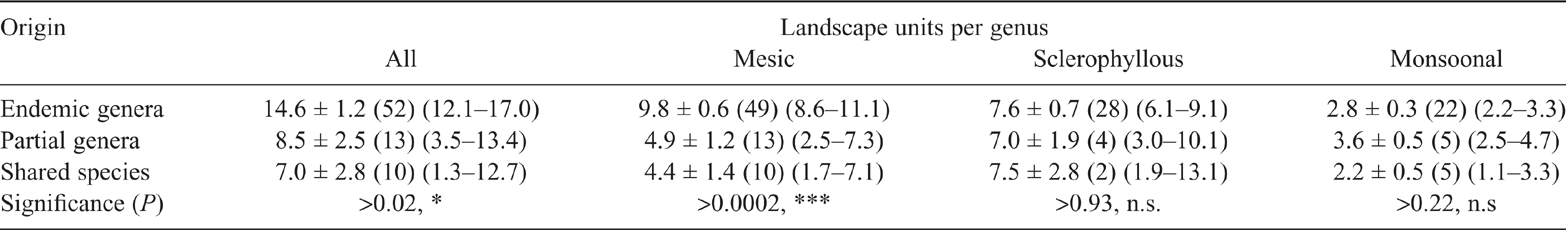
The bivariate fit of the logarithm of the number of species in each genus against the predicted number of RLUs inhabited is shown in Fig. 5. The slope of the regression line is significant for the combined biomes and for the Mesic and Sclerophyllous biomes, but not for the Monsoonal biome. In each case, genera with a large number of species are always widely distributed while genera with low numbers of species may be narrowly or widely distributed.
There is no significant difference between the log number of species in each genus and the origin (i.e. Endemic etc.) of the genus (P > 0.29, n.s.). There is no correlation between the estimated ages of endemic genera and either the logarithm of the number of species they contain or the predicted number of RLUs they occupy (r2 = 0.005, slope of the regression line P < 0.71, n.s., and r2 = –0.0004, slope P < 0.91, n.s.). There is no relationship between maximum size and number of landscape units occupied (r2 = 0.025, slope P > 0.18, n.s.). There is no significant difference between size and origin (Endemic 7.31 ± 0.45 (52) (6.41–8.21); Partial 7.46 ± 0.90 (13) (5.66–9.36); Shared 7.30 ± 1.03 (10) (5.25–9.35); Wilcoxon P > 0.92).
Discussion
The estimated dates of origin of each of the genera have certain limitations. First, sequences and hence dates are not available for many genera and these could not be included in some of the analyses. Second, the estimated dates are of the stem (origin) of a genus, not the crown radiation (Crisp and Cook 2013). Genera such as Servaea Simon, 1887 and Mopsus Karsch, 1878 with very long stems may well have been part of now-lost radiations of genera. Dated species-level molecular-based phylogenies are not available for Australian genera and consequently no within-genus analyses were possible. Finally, single, molecular-based estimates of age have quite wide confidence limits. However, this study is based on molecular trees that include several estimates (9 and 18, respectively: Bodner and Maddison 2012; Zhang and Maddison 2013). As these estimates are internally consistent, they are considered useful when used in combined analyses as they have provided a coherent picture of overall processes.
Review of hypotheses
1. That the dates of origin of Australian genera are related to times of climate and vegetation change
This hypothesis is supported. Two genera arose in the mid Oligocene and new genera steadily accumulate from the late Oligocene through to the mid-Miocene (Fig. 1). The evolution of further genera then slowed and stopped with no new genera evolving after ~8 mya. These estimated dates parallel marked changes in climate, with new genera steadily appearing between the worldwide late Oligocene warming (25–26 mya) and the mid-Miocene cooling (13–15 mya).
The opportunity for the direct arrival of Euophryini salticids from Antarctica/South America (Fig. 1) ceased with the final separation of Antarctica from Australia, perhaps as recently as 34 mya. The earliest estimated date of origin of an extant Australian genus likely derived from Gondwanan origins is 29 mya, and of the stem date of the Australasian Euophryini radiation is 31 mya (Zhang and Maddison 2013), three million years after the link to Antarctica was broken. Opportunities for the origin of endemic Australasian plant genera derived from Northern Hemisphere precursors started ~33 mya (Crayn et al. 2015) and increased over time with the closer approach of the Australasian plate to Asia. The estimated date of origin of the oldest extant Australian endemic salticid genus derived from Northern Hemisphere sources is 29 mya and that of the Australasian Astioida stem is 31 mya (Bodner and Maddison 2012), making them amongst the earliest arrivals of any faunal group from Asia, if the estimated dates are accepted.
No Eocene Gondwanan genus survives to the present, and presumably these warm, wet-adapted forms were severely challenged by the rapid drop in temperature and drier conditions at the beginning of the Oligocene that led to the development and spread of open, sclerophyllous and xeromorphic vegetation (Crisp et al. 2004; Crisp and Cook 2013). The first modern salticid genera appear after a two- or three-million-year lag, with independent radiations of euophryine and astioid salticids both beginning at that time. Time lags have been observed for other groups following sudden changes in conditions (e.g. Brennan and Oliver 2017; Folk et al. 2019). Like events at the end of the Eocene, the severe, cooling and drying, environmental changes from the mid-Miocene onwards did not lead to the production of new genera adapted to these harsher conditions, even with a time lag.
The few molecular-based studies of the evolution of other, non-salticid, Australian arachnid taxa have been directed towards the times of diversification of species and this has largely occurred from the late Miocene onwards (e.g. Boyer et al. 2016; Harms et al. 2018). A study of Australian huntsman spiders (Deleninae) reports the origin of three of the four genera examined occurred in the early Miocene (Agnarsson and Rayor 2013). Differences in taxonomic rankings can further confuse interpretation (e.g. Oberski et al. 2018). The study of Australian assassin spiders (Archaeidae) by Rix and Harvey (2012a) was based on a single Mesozoic genus; however, their phylogenetic analysis showed that the Australian lineage separated into a series of clades in the very late Oligocene or early Miocene, at a time similar to that of the origin of the Australian salticid genera although, as in the other studies, modern species evolved more recently. One of these clades has since been described as a new genus (Rix and Harvey 2012b). Significant early Miocene radiations have also been reported in other, non-arachnid groups (e.g. Crisp et al. 2004; Marin et al. 2013; Skinner et al. 2013; Brennan and Oliver 2017; Oberski et al. 2018).
The steady, early Miocene accumulation of genera (Fig. 3) provides no evidence in the present study of a ‘burst’ in the production of new novel forms following significant changes in conditions (Slater and Friscia 2019) in the late Oligocene. However, genera may be the wrong taxonomic level for such analyses in salticids (Harmon et al. 2010) and species-level analyses may show such a response.
2. That the dates of origin of the Australian salticid genera match the dates of origin of salticid genera in other parts of the world and reflect the pattern of changes to climate and vegetation
This hypothesis was supported. The dates of origin of South American genera (Fig. 1) show a pattern that does not differ significantly from that found in Australia. Even though evolution on these two continents was independent by the early Oligocene, evolution of new extant genera on both continents began in the mid Oligocene and tapered off in the mid-Miocene. The Northern Hemisphere pattern of dates of origin of salticid genera differs significantly from the pattern in the Southern Hemisphere. While the earliest present-day genera also arise in the Oligocene, the production of new genera peaks in the late Miocene and continues into the Pliocene.
As the dates for the origin of genera on all three regions come from the work of Maddison and his coworkers and the description of salticid genera in both hemispheres were developed by a common suite of taxonomists, it seems clear that the similarities and differences in patterns seen are real, not due to systematic differences in generic concepts or to the methodology used for date estimation.
The termination of production of new genera in Australia and South America ~12–15 mya coincides with a relatively sudden, cooling change in world climates. This was compounded with the increasing height of the Andes in South America (Le Roux 2012; Jordan et al. 2014; Groeneveld et al. 2017; Carrapa et al. 2019) and the continuing northward movement of Australia. These changes led to increased aridification on both continents.
While the same general pattern of cooling in the late Miocene is found in both the Northern and Southern Hemispheres there are differences in timing (Herbert et al. 2016) with the changes in the Northern Hemisphere occurring later than those in the south. The changes in sea surface temperatures in the southern temperate (30–50°S) ocean matched those of the far northern seas (>50°N) while those of the northern temperate seas (30–50°N) were similar to those in the tropics. In the north, forests occurred as far north as Greenland until sea-ice developed in the Pliocene (4 mya: Clotten et al. 2019) and glaciation even later. The development of new genera in the north then ceased. Changes in the rate of production of new genera in both the north and the south are correlated with the different timing of changing climates and the associated biological consequences in each hemisphere.
The disappearance of older genera at some point following the cooling at the end of the Eocene and the failure of the production of new genera following the mid-Miocene or later cooling suggests that harsher conditions and the associated vegetation changes did not suit salticids. The production of new genera occurred under warmer and wetter conditions and ceased in different areas as conditions cooled and dried at different times. While late Miocene climate changes leading to large-scale shifts in vegetation and landscape coincided with large changes in other groups in the terrestrial fauna, this was not the case at the generic level for the salticid faunas of the southern continents. In Australia this process may have been replaced by either increased adaptive speciation within genera (e.g. Brennan and Keogh 2018) or by the arrival of Asian genera preadapted to conditions in northern Australia, pre-empting the evolution of new endemic genera.
It is clear that all three regional faunas responded by independently evolving new genera in similar fashions to similar changes in conditions and, apparently, macroevolutionary processes were occurring. If true, the evolution of genera may not be a surrogate for species evolution but at least in part reflects generic-level (i.e. macroevolutionary) processes. It is possible, however, that species-level processes are being detected. The parallel changes in circumstances may lead to the accumulation of new species in each region, ultimately resulting in new genera developing through drift. It would be of interest to know the dates of origin of the crown radiations of salticid species in Australia relative to the origins of the stems.
3. That Australian genera with different histories (i.e. Endemic, Partial and Shared) adapt to the changing states of Australian environments in different fashions
This hypothesis was supported. The northward movement of Australia to the point where exchanges were possible also led to the slow development of monsoonal and mesic woodlands and forests climatically similar to those to the north of Australia. It might be predicted that Partial and Shared genera would be better suited to these northernmost habitats and less so to those elsewhere in Australia.
The patterns of distribution by origin of genera between biomes are generally similar (Table 1), with almost all genera being found in the Mesic biome and about half in the other biomes. The only possible differences were with Shared species. These recent arrivals of Asian origin may be less common in the Sclerophyllous biome and more common in the Monsoonal biome. However, the number of genera is small and also the salticid fauna of the Monsoonal Region is very poorly known (e.g. Richardson 2017).
Success within Australia can be measured by the number of RLUs that a genus inhabits (Faurby and Antonelli 2018). If RLUs rather than Biomes are analysed (Table 2), Endemic genera occupy, on average, twice the number of Mesic Landscape Units occupied by Partial or Shared taxa. All groups are equally successful in the Sclerophyllous and Monsoonal biomes, presumably because these more recently derived biomes were equally available to genera from all three groups.
Examination of the distribution of Mesic RLUs used by genera within the north-eastern (tropical) and south-eastern (temperate) areas (Table 3) shows that the Partial and Shared groups are less successful in temperate areas than they are in the tropics. They have not, however, been as competitive against the temperate endemic fauna, or perhaps have not had sufficient time to adapt to cooler conditions. As in the intercontinental comparisons, salticid genera seem to be sensitive to climatic conditions. It would be informative to compare the dates of origin of species in each group.
The number of Australian species in each genus varies widely and no significant relationship between origin (Partial or Endemic) and numbers of species was detected.
4. That radiations in drier environments derive from mesic-zone ancestors and no mesic-zone genera derive from drier environments
This hypothesis, after Byrne et al. (2011), was supported. Examination of the MP analyses for each of the two endemic radiations (Fig. 4 and Supplementary Material, Fig. S1) shows that each genus, or its immediate ancestor, is predicted to have occurred in mesic forest. There is no case of the immediate ancestor of a modern genus being from sclerophyllous habitats.
5. That mesic biomes are more resistant to overseas colonisation than arid or monsoonal biomes
This hypothesis was supported. The Endemic genera make more effective use of the range of mesic environments than the more recent arrivals (Table 3). Invaders already adapted to tropic mesic environments have been able to successfully gain a foothold there but they have been far less successful in extending into temperate environments. Endemic faunas in Mesic biomes do seem to be resistant to invasion, as predicted by Byrne et al. (2011), but only if the invaders are not preadapted to such areas. Their inability to invade these areas means that they do not have the opportunity to evolve so as to effectively compete there.
6. That genera increase their range by increasing the number of included species
This hypothesis was only partly supported. There is a highly significant relationship between the number of species in each genus and the number of RLUs used (Fig. 5a). Genera with large numbers of species are widely distributed whereas genera with low numbers of species may be narrowly or widely distributed. This pattern is most strongly seen in the Mesic biome (Fig. 5b) and less so the Sclerophyllous biome (Fig. 5c). There is no significant pattern in the poorly-known Monsoonal biome (Fig. 5d). Genera with low numbers of species and small distributions are rare or little known genera, or relatively recent colonists from overseas. The genera with few species and large distributions are a mixture of Shared species (Menemerus Simon, 1868, Pellenes Simon, 1876 and Bianor Peckham & Peckham, 1886) and Endemic genera (Apricia Richardson, 2016; Clynotis Simon, 1901 and Zebraplatys Zabka, 1992).
There is no significant relationship between the estimated age of a genus and either the number of species in it or the predicted number of RLUs it inhabits. Even though time is related to increasing aridity, it has not been a general driver for the evolution of new genera or of the range of climate niches inhabited.
7. That genus-level patterns should be apparent in adaptive morphological and ecological characteristics
This hypothesis was not supported. The MP analyses of microhabitat usage and of presumed adaptive morphological characteristics showed that these did not reflect generic-level processes (i.e. the macroevolutionary development of distinct adaptive clades) but lower, presumably species-level, adaptive evolutionary responses (Harmon et al. 2019).
Final observations
The lack of within-genus molecular studies meant that this work was limited to the origins of stem relationships between genera and that the timing and patterns of crown radiations within genera are unknown. As a consequence, the question of whether significant still-extant species radiations occurred in the early Miocene at the same time as the development of the genera or only later under increasingly drier conditions could not be addressed.
The origin and radiation of the Astioida (estimated to have occurred 31 mya and 25 mya respectively) occurred well before the Australasian and Asian plates collided (20 mya) and support the observation of Crayn et al. (2015) of the arrival of Asian plant taxa on the Australian plate as early as 33 mya. Apparently salticids were amongst the earliest Tertiary animals to colonise the Australian plate from the north.
The study of this invertebrate group shows a distinctive history of evolution during the Tertiary. It supports the view that studies beyond those based on higher vertebrates and plants are needed if the evolutionary biogeography of Australia is to be understood (Byrne et al. 2011). A series of hypotheses partially derived from Byrne et al. (2011) have been explored as part of this work. As a consequence, new or refined hypotheses can be proposed:
-
That invertebrate groups may have reached Australia from Asia and radiated here after 33 mya but before the collision of the Australasian and Asian plates ~20 mya.
-
That the evolutionary pattern of Tertiary radiations of invertebrate genera or equivalent may take place at the same time in Australasia and South America but follow a different pattern in the Northern Hemisphere.
-
That species-level evolution will show a gradual accumulation of species, the pattern varying between related genera; however, the evolution of new genera (or equivalent) will occur in a coordinated fashion.
-
That recent arrivals from the north are able to effectively enter and evolve in Australian tropical mesic areas, but are much less successful at adapting to temperate regions.
-
That present-day members of crown radiations in Australia appeared during cooler and drier environments of the late Miocene/Pliocene rather than at the time of the earlier (stem) radiations in the wetter and warmer early Miocene.
-
Australian invertebrate genera have mesic origins.
-
Older radiations use drier environments by the evolution of new, arid-adapted species, while more recent arrivals invade drier environments by extending the ranges of single species.
Though jumping spiders are a highly successful group of relatively recent origin, their evolution has been strongly influenced by changing climatic conditions. The development of cooler and drier conditions was quite challenging for them, leading to the loss of genera and/or the failure to evolve new ones. Studies at the within-genus level are needed, however, to understand their more recent evolutionary responses to climate change. The present Australian salticid fauna is one showing a successful diversity of many genera, often with radiations of species covering the entire range of Australian habitats, and is probably able, given time and habitat connectivity, to respond to modern continental climate change. However, if past history is any model, the development of new ways of living (i.e. genera) could take several million years.
Conflicts of interest
The author declares no conflicts of interest.
Acknowledgements
Thanks to Dr Bruce Halliday for many helpful discussions of the issues explored in this work. Dr Juanita Rodriguez is gratefully acknowledged for her help in carrying out the MP analyses. My thanks to Christine Richardson for preparing figures and for detailed editing of the manuscript. This research did not receive any specific funding.
References
Agnarsson, I., and Rayor, L. S. (2013). A molecular phylogeny of Australian huntsman spiders (Sparassidae, Deleninae): implications for taxonomy and social behaviour. Molecular Phylogenetics and Evolution 69, 895–905.| A molecular phylogeny of Australian huntsman spiders (Sparassidae, Deleninae): implications for taxonomy and social behaviour.Crossref | GoogleScholarGoogle Scholar | 23831456PubMed |
Barlow, B. A. (1985). A revised natural regions map for Australia. Brunonia 8, 387–392.
| A revised natural regions map for Australia.Crossref | GoogleScholarGoogle Scholar |
Black, K. H., Archer, M., Hand, S. J., and Godhelp, H. (2012). The rise of Australian marsupials: a synopsis of biostratigraphic, phylogenetic, palaeoecologic and palaeobiogeographic understanding. In ‘Earth and Life’ (Ed. J. A. Talent.) pp. 983–1078. (Springer Science + Business Media.)
Bodner, M. R., and Maddison, W. P. (2012). The biogeography and age of salticid spider radiations (Araneae: Salticidae). Molecular Phylogenetics and Evolution 65, 213–240.
| The biogeography and age of salticid spider radiations (Araneae: Salticidae).Crossref | GoogleScholarGoogle Scholar | 22735169PubMed |
Bowman, D. M. J. S., Brown, G. K., Braby, M. F., Brown, J. R., Cook, L. G., Crisp, M. D., Ford, F, Haberle, S, Hughes, J, Isagi, Y, Joseph, L, McBride, J, Nelson, G, and Ladiges, P. Y. (2010). Biogeography of the Australian monsoon tropics. Journal of Biogeography 37, 201–216.
| Biogeography of the Australian monsoon tropics.Crossref | GoogleScholarGoogle Scholar |
Boyer, S. L., Markle, T. M., Luxbacher, A. M., and Kozak, K. H. (2016). Historical refugia have shaped biogeographical patterns of species richness and phylogenetic diversity in mite harvestmen (Arachnida, Opiliones, Cyphophthalmi) endemic to the Australian wet tropics. Journal of Biogeography 43, 1400–1411.
| Historical refugia have shaped biogeographical patterns of species richness and phylogenetic diversity in mite harvestmen (Arachnida, Opiliones, Cyphophthalmi) endemic to the Australian wet tropics.Crossref | GoogleScholarGoogle Scholar |
Brennan, I. G., and Keogh, J. S. (2018). Miocene biome turnover drove conservative body size evolution across Australian vertebrates. Proceedings. Biological Sciences 285, 20181474.
| Miocene biome turnover drove conservative body size evolution across Australian vertebrates.Crossref | GoogleScholarGoogle Scholar | 30333208PubMed |
Brennan, I. G., and Oliver, P. M. (2017). Mass turnover and recovery dynamics of a diverse Australian continental radiation. Evolution 71, 1352–1365.
| Mass turnover and recovery dynamics of a diverse Australian continental radiation.Crossref | GoogleScholarGoogle Scholar | 28213971PubMed |
Bridgewater, P. B. (1987). The present Australian environment – terrestrial and freshwater. In ‘Fauna of Australia. Vol. 1A. General Articles’. (Eds G. R. Dyne, and D. W. Walton.) pp. 69–100. (AGPS: Canberra.)
Byrne, M., Yeates, D. K., Joseph, L., Kearney, M., Bowler, J., Williams, M. A. J., Cooper, S, Donnellan, S. C., Keogh, J. S., Leys, R, Melville, J, Murphy, D. J., Porch, N, and Wyrwoll, K.‐H. (2008). Birth of a biome: insights into the assembly and maintenance of the Australian arid zone biota. Molecular Ecology 17, 4398–4417.
| Birth of a biome: insights into the assembly and maintenance of the Australian arid zone biota.Crossref | GoogleScholarGoogle Scholar | 18761619PubMed |
Byrne, M., Steane, D. A., Joseph, L., Yeates, D. K., Jordan, G. J., Crayn, D, Aplin, K, Cantrill, D. J., Cook, L. G., Crisp, M. D., Keogh, J. S., Melville, J, Moritz, C, Porch, N, Sniderman, J. M. K., Sunnucks, P, and Weston, P. H. (2011). Decline of a biome: evolution, contraction, fragmentation, extinction and invasion of the Australian mesic zone biota. Journal of Biogeography 38, 1635–1656.
| Decline of a biome: evolution, contraction, fragmentation, extinction and invasion of the Australian mesic zone biota.Crossref | GoogleScholarGoogle Scholar |
Carrapa, B., Clementz, M., and Ran, F. (2019). Ecological and hydroclimate responses to strengthening of the Hadley circulation in South America during the late Miocene cooling. Proceedings of the National Academy of Sciences of the United States of America 116, 9747–9752.
| Ecological and hydroclimate responses to strengthening of the Hadley circulation in South America during the late Miocene cooling.Crossref | GoogleScholarGoogle Scholar | 31036635PubMed |
Cassis, G., Laffan, S. W., and Ebach, M. C. (2017). Biodiversity and bioregionalisation perspectives on the historical biogeography of Australia. In ‘Handbook of Australian Biogeography’. (Ed. M. C. Ebach.) pp. 1–16. (CRC Press: Boca Raton, USA.)
Chapman, A. D. (2009). Numbers of Living Species in Australia and the World. 2nd Edn. A report for the Australian Biological Resources Study 2009. Australian Biodiversity Information Services, Toowoomba, Australia.
Clotten, C., Stein, R., Fahl, K., Schreck, M., Risebrobakken, B., and de Schepper, S. (2019). On the causes of Arctic sea ice in the warm early Pliocene. Scientific Reports 9, 989.
| On the causes of Arctic sea ice in the warm early Pliocene.Crossref | GoogleScholarGoogle Scholar | 30700730PubMed |
Crayn, D. M., Costion, C., and Harrington, M. G. (2015). The Sahul–Sunda floristic exchange: dated molecular phylogenies document Cenozoic intercontinental dispersal dynamics. Journal of Biogeography 42, 11–24.
| The Sahul–Sunda floristic exchange: dated molecular phylogenies document Cenozoic intercontinental dispersal dynamics.Crossref | GoogleScholarGoogle Scholar |
Crisp, M. D., and Cook, L. G. (2013). How was the Australian flora assembled over the last 65 million years? A molecular phylogenetic perspective. Annual Review of Ecology, Evolution, and Systematics 44, 303–324.
| How was the Australian flora assembled over the last 65 million years? A molecular phylogenetic perspective.Crossref | GoogleScholarGoogle Scholar |
Crisp, M., Cook, L., and Steane, D. (2004). Radiation of the Australian flora: what can comparisons of molecular phylogenies across multiple taxa tell us about the evolution of diversity in present-day communities? Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 359, 1551–1571.
| Radiation of the Australian flora: what can comparisons of molecular phylogenies across multiple taxa tell us about the evolution of diversity in present-day communities?Crossref | GoogleScholarGoogle Scholar | 15519972PubMed |
Crisp, M. D., Trewick, S. A., and Cook, L. G. (2011). Hypothesis testing in biogeography. Trends in Ecology & Evolution 26, 66–72.
| Hypothesis testing in biogeography.Crossref | GoogleScholarGoogle Scholar |
Ebach, M. C. (2017). ‘Handbook of Australian Biogeography.’ pp. 11–375. (CRC Press: Boca Raton, USA.)
Faurby, S., and Antonelli, A. (2018). Evolutionary and ecological success is decoupled in mammals. Journal of Biogeography 45, 2227–2237.
| Evolutionary and ecological success is decoupled in mammals.Crossref | GoogleScholarGoogle Scholar | 31217658PubMed |
Folk, R. A., Stubbs, R. L., Mort, M. E., Cellinese, N., Allen, J. M., Soltis, P. S., Soltis, D. E., and Guralnick, R. P. (2019). Rates of niche and phenotype evolution lag behind diversification in a temperate radiation. Proceedings of the National Academy of Sciences of the United States of America 116, 10874–10882.
| Rates of niche and phenotype evolution lag behind diversification in a temperate radiation.Crossref | GoogleScholarGoogle Scholar | 31085636PubMed |
Forrest, M., Eronen, J. T., Utescher, T., Knorr, G., Stepanek, C., Lohmann, G., and Hickler, T. (2015). Climate–vegetation modelling and fossil plant data suggest low atmospheric CO2 in the late Miocene. Climate of the Past 11, 1701–1732.
| Climate–vegetation modelling and fossil plant data suggest low atmospheric CO2 in the late Miocene.Crossref | GoogleScholarGoogle Scholar |
Greenwood, D. R., and Christophel, D. C. (2015). The origins and Tertiary history of Australian “tropical” rainforests. In ‘Tropical Rainforests: Past, Present and Future’. (Eds E. Bermingham, C. W. Dick, and C. Moritz.) pp. 322–335. (University of Chicago: Chicago.)
Groeneveld, J., Hendriks, J., Renema, W., McHugh, C. M., De Vleeschouwer, D., Christensen, B. A., Fulthorpe, C. S., Reuning, L, Gallagher, S. J., Bogus, K, Auer, G, Ishiwa, T, Expedition 356 Scientists (2017). Australian shelf sediments reveal shifts in Miocene Southern Hemisphere westerlies. Science Advances 3, e1602567.
| Australian shelf sediments reveal shifts in Miocene Southern Hemisphere westerlies.Crossref | GoogleScholarGoogle Scholar | 28508066PubMed |
Harmon, L. J., Losos, J. B., Davies, T. J., Gillespie, R. G., Gittleman, J. L., Jennings, W. B., Kozak, K. H., McPeek, M. A., Moreno‐Roark, F, Near, T. J., Purvis, A, Ricklefs, R. E., Schluter, D, Schulte, J. A., Seehausen, O, Sidlauskas, B. L., Torres‐Carvajal, O, Weir, J. T., and Mooers, A. Ø (2010). Early bursts of body size and shape evolution are rare in comparative data. Evolution 64, 2385–2396.
| Early bursts of body size and shape evolution are rare in comparative data.Crossref | GoogleScholarGoogle Scholar | 20455932PubMed |
Harmon, L. J., Andreazzi, C. S., Debarre, F., Drury, J., Goldberg, E. E., Martins, A. B., Melián, C. J., Narwani, A, Nuismer, S. L., Pennell, M. W., Rudman, S. M., Seehausen, O, Silvestro, D, Weber, M, and Matthews, B (2019). Detecting the macroevolutionary signal of species interactions. Journal of Evolutionary Biology 32, 769–782.
| Detecting the macroevolutionary signal of species interactions.Crossref | GoogleScholarGoogle Scholar | 30968509PubMed |
Harms, D., Laffan, S. W., and Ebach, S. A. (2018). Speciation patterns in complex subterranean environments: a case study using short-tailed whipscorpions (Schisomida: Hubbardiida). Biological Journal of the Linnean Society 125, 355–367.
| Speciation patterns in complex subterranean environments: a case study using short-tailed whipscorpions (Schisomida: Hubbardiida).Crossref | GoogleScholarGoogle Scholar |
Harvey, M. S., Rix, M. G., Harms, D., Giribet, G., Vink, C. J., and Walter, D. E. (2017). The biogeography of Australasian arachnids. In ‘Handbook of Australian Biogeography’ (Ed. M. C. Ebach.) pp. 241–267. (CRC Press: Boca Raton, USA.)
Hendricks, J. R., Saupe, E. E., Myers, C. E., Hermsen, E. J., and Allmon, W. D. (2014). The generification of the fossil record. Paleobiology 40, 511–528.
| The generification of the fossil record.Crossref | GoogleScholarGoogle Scholar |
Herbert, T. D., Lawrence, K. T., Tzanova, A., Peterson, L. C., Caballero-Gill, R., and Kelly, C. S. (2016). Late Miocene global cooling and the rise of modern ecosystems. Nature Geoscience 9, 843–847.
| Late Miocene global cooling and the rise of modern ecosystems.Crossref | GoogleScholarGoogle Scholar |
Jablonski, D (2005). Mass extinctions and macroevolution. Paleobiology 31, 192–210.
Jordan, T. E., Kirk-Lawler, N. E., Blanco, P. N., Rech, J. A., and Cosentino, N. J. (2014). Landscape modification in response to repeated onset of hyperarid paleoclimate states since 14 Ma, Atacama Desert, Chile. Geological Society of America Bulletin 126, 1016–1046.
| Landscape modification in response to repeated onset of hyperarid paleoclimate states since 14 Ma, Atacama Desert, Chile.Crossref | GoogleScholarGoogle Scholar |
Le Roux, J. P. (2012). A review of Tertiary climate changes in southern South America and the Antarctic Peninsula. Part 2: continental conditions. Sedimentary Geology 247–248, 21–38.
| A review of Tertiary climate changes in southern South America and the Antarctic Peninsula. Part 2: continental conditions.Crossref | GoogleScholarGoogle Scholar |
Lemen, C. A., and Freeman, P. W. (1984). The genus: a macroevolutionary problem. Evolution 38, 1219–1237.
| The genus: a macroevolutionary problem.Crossref | GoogleScholarGoogle Scholar | 28563772PubMed |
Maddison, W. P., and Maddison, D. R. (2018). Mesquite: a modular system for evolutionary analysis. Version 3.6. Available at: http://mesquiteproject.org [accessed September 2019].
Marin, J., Donnellan, S. C., Hedges, S. B., Doughty, P., Hutchinson, M. N., Cruaud, C., and Vidal, N. (2013). Tracing the history and biogeography of the Australian blindsnake radiation. Journal of Biogeography 40, 928–937.
| Tracing the history and biogeography of the Australian blindsnake radiation.Crossref | GoogleScholarGoogle Scholar |
Oberski, J. T., Sharma, P. P., Jay, K. R., Coblens, M. J., Lemon, K. A., Johnson, J. E., and Boyer, S. L. (2018). A dated molecular phylogeny of mite harvestmen (Arachnida: Opiliones: Cyphothalmi) elucidates ancient diversification dynamics in the Australian wet tropics. Molecular Phylogenetics and Evolution 127, 813–822.
| A dated molecular phylogeny of mite harvestmen (Arachnida: Opiliones: Cyphothalmi) elucidates ancient diversification dynamics in the Australian wet tropics.Crossref | GoogleScholarGoogle Scholar | 29935300PubMed |
Richardson, B. J. (2017). The nature and distribution of jumping spider (Aranaea: Salticidae) diversity on Pungalina and Seven Emu Stations. In ‘Pungalina Wetlands Scientific Study Report. Geography Monograph Series No. 14.’ pp. 47–56. (The Royal Geographical Society of Queensland: Brisbane.)
Richardson, B. J. (2019). Salticidae. Arachnida: Araneomorphae. Australian Faunal Directory. Australian Biological Resources Study, Canberra. Available at: https://biodiversity.org.au/afd/taxa/SALTICIDAE [accessed September 2019].
Richardson, B. J., Zabka, M., Gray, M. R., and Milledge, G. (2006). Distribution patterns of jumping spiders (Araneae: Salticidae) in Australia. Journal of Biogeography 33, 707–719.
| Distribution patterns of jumping spiders (Araneae: Salticidae) in Australia.Crossref | GoogleScholarGoogle Scholar |
Richardson, B. J., Whyte, R., and Zabka, M. (2019). A key to the genera of Australian jumping spiders (Araneae: Salticidae). Available at https://apps.lucidcentral.org/salticidae/ [accessed September 2019].
Rix, M. G., and Harvey, M. S. (2012a). Phylogeny and historical biogeography of ancient assassin spiders (Araneae: Archaeidae) in the Australian mesic zone: evidence for Miocene speciation within Tertiary refugia. Molecular Phylogenetics and Evolution 62, 375–396.
| Phylogeny and historical biogeography of ancient assassin spiders (Araneae: Archaeidae) in the Australian mesic zone: evidence for Miocene speciation within Tertiary refugia.Crossref | GoogleScholarGoogle Scholar | 22040763PubMed |
Rix, M. G., and Harvey, M. S. (2012b). Australian assassins, Part II: a review of the new assassin spider genus Zephyrarchaea (Araneae, Archaeidae) from southern Australia. ZooKeys 191, 1–62.
| Australian assassins, Part II: a review of the new assassin spider genus Zephyrarchaea (Araneae, Archaeidae) from southern Australia.Crossref | GoogleScholarGoogle Scholar |
Rix, M. G., Costion, C., and Harrington, J. D. (2015). Biogeography and speciation of terrestrial fauna in the south-western Australian biodiversity hotspot. Biological Reviews of the Cambridge Philosophical Society 90, 762–793.
| Biogeography and speciation of terrestrial fauna in the south-western Australian biodiversity hotspot.Crossref | GoogleScholarGoogle Scholar | 25125282PubMed |
Segar, S. T., Fayle, T. M., Srivastava, D. S., Lewinsohn, T. M., Lewis, O. T., Novotny, V., Kitching, R. L., and Maunsell, S. C. (2020). The role of evolution in shaping ecological networks. Trends in Ecology & Evolution 35, 454–466.
| The role of evolution in shaping ecological networks.Crossref | GoogleScholarGoogle Scholar |
Sepkoski, D. (2012). ‘Rereading the Fossil Record: The Growth of Paleobiology as an Evolutionary Discipline.’ (University of Chicago: Chicago.)
Skinner, A., Hutchinson, M. N., and Lee, M. S. Y. (2013). Phylogeny and divergence times of Australian Sphenomorphus group skinks (Scincidae, Squamata). Molecular Phylogenetics and Evolution 69, 906–918.
| Phylogeny and divergence times of Australian Sphenomorphus group skinks (Scincidae, Squamata).Crossref | GoogleScholarGoogle Scholar | 23810993PubMed |
Slater, G. J., and Friscia, A. R. (2019). Hierarchy in adaptive radiation: a case study using the Carnivora (Mammalia). Evolution 73, 524–539.
| Hierarchy in adaptive radiation: a case study using the Carnivora (Mammalia).Crossref | GoogleScholarGoogle Scholar | 30690704PubMed |
Zhang, J., and Maddison, W. P. (2013). Molecular phylogeny, divergence times and biogeography of spiders of the subfamily Euophryinae (Araneae: Salticidae). Molecular Phylogenetics and Evolution 68, 81–92.
| Molecular phylogeny, divergence times and biogeography of spiders of the subfamily Euophryinae (Araneae: Salticidae).Crossref | GoogleScholarGoogle Scholar | 23542001PubMed |