Male copulation frequency, sperm competition and genital damage in the golden orb-web spider (Nephila plumipes)
Jutta M. Schneider A G , Marie E. Herberstein B , Matthew J. Bruce B C , Michael M. Kasumovic D , Melissa L. Thomas E F and Mark A. Elgar EA Zoological Institute and Museum, University of Hamburg, Martin-Luther-King Platz 3, 20146 Hamburg, Germany.
B Department of Biological Sciences, Macquarie University, North Ryde, NSW 2109, Australia.
C Present address: Behavioural Biology and Helmholtz Institute, Utrecht University, PO Box 80086, 3508 TB Utrecht, The Netherlands.
D Department of Life Sciences, University of Toronto at Scarborough, Toronto, ON M1C 1A4, Canada.
E Department of Zoology, University of Melbourne, Vic. 3010, Australia.
F Present address: School of Animal Biology, University of Western Australia, Crawley, WA 6009, Australia.
G Corresponding author. Email: js@gilgamesh.de
Australian Journal of Zoology 56(4) 233-238 https://doi.org/10.1071/ZO08041
Submitted: 21 April 2008 Accepted: 18 November 2008 Published: 22 December 2008
Abstract
Copulation in many sexually cannibalistic spiders is associated with a loss of function of the male reproductive organs and, as a consequence, males that survive sexual cannibalism may nevertheless be unable to subsequently copulate successfully. Sexual cannibalism is common in the Australian golden orb-web spider (Nephila plumipes), in which the tip of the conductor typically breaks during copulation. Thus, male mating frequency may be physiologically limited to two females, irrespective of the male’s ability to avoid cannibalism or the opportunity to locate and court additional, receptive females. Laboratory experiments revealed that the likelihood of the conductor breaking depends upon the copulatory history of the female insemination duct: males were more likely to break their conductor if they inseminated a ‘virgin’ rather than ‘mated’ insemination duct. However, the choice of insemination duct did not influence the duration of copulation or quantity of sperm transferred. In field populations, the proportion of males with both conductors broken increased during the course of the mating season, but while males with broken conductors did not copulate successfully with virgin females, they were nevertheless observed on the webs of immature females. We suggest that male N. plumipes with broken conductors on the webs of females are most likely mate guarding, as this appears to be the most effective mechanism of securing paternity.
Introduction
Male reproductive success is determined, in part, by the number of females with whom the male mates and the mating frequency of his mating partner. It is widely held that selection favours males that can secure the most mating partners (e.g. Andersson 1994), although contemporary studies now focus on paternity, which must include the male’s ability to either prevent and/or engage in sperm competition that arises from polyandry (e.g. Simmons 2001; Arnqvist and Rowe 2005). Some of these male adaptations may reduce or even eliminate the opportunities for male polygyny (see Fromhage et al. 2005). Such mating systems are thought to be particularly likely in species without paternal care, and where the costs of mate search for males are high (e.g. Andrade 2003). However, if the probability that males can find mates is low, then it is unlikely that males will need to defend their paternity (Fromhage et al. 2005). Fromhage et al. (2005) resolved this paradox theoretically, showing that monogyny, as a means of protecting paternity, is favoured when the sex ratio is male-biased, but not necessarily as a result of high search costs.
Sexual cannibalism, in which the female kills and consumes her mating partner shortly before, during or after copulation, places unambiguous limits on male mating frequency and the behaviour is often discussed in the context of a particularly dramatic manifestation of sexual conflict over mating frequency (Elgar 1992, 1998; Elgar and Schneider 2004; Prenter et al. 2006). Monogyny may also be a typical consequence of sexual cannibalism (Fromhage et al. 2005). For example, male orb-web spiders of the genus Argiope mate with a single female by making a single insertion with each of their pedipalps (intromittant organs), and will always fall victim to the sexually cannibalistic female after the second insertion (Sasaki and Iwahashi 1995; Foellmer and Fairbairn 2003). Similarly, male red-back spiders (Latrodectus hasselti) typically fall victim to the sexually cannibalistic female after the second insertion (Forster 1992). While male red-backs apparently benefit from sexual cannibalism through protecting and/or increasing their paternity share (e.g. Andrade 1996), the benefits of sexual cannibalism to male Argiope are less clear (Fromhage and Schneider 2003; Schneider et al. 2006).
Male golden orb-web spiders (Nephila plumipes) are frequent victims to sexual cannibalism (Schneider and Elgar 2002), and there are no obvious paternity benefits of sexual cannibalism to the male victim (Schneider and Elgar 2001, 2002; Schneider et al. 2001). Sexual cannibalism in N. plumipes does not terminate copulation and several lines of evidence suggest that a morphological feature of the male’s genitalia is an adaptation associated with sexual conflict over the duration of copulation. The pedipalps (or palps) of males have a conductor with a peculiarly curved ending and a triangular process near the terminal end. Typically, the tip of the conductor, including the process, breaks during mating and remains inside the female genital tract. However, these broken conductors do not act as mating plugs (Schneider et al. 2001): males are able to insert their palp into an insemination duct that contains a broken conductor and can fertilise her eggs, although the conductor is more likely to break if it is inserted in an unused insemination duct. This contrasts with the situation in the congeneric N. fenestrata, in which males that leave remains of their conductor within the female insemination duct enjoy a paternity advantage (Fromhage and Schneider 2006). Nevertheless, the process on the conductor of N. plumipes may allow males to copulate for longer than is in the interest of the female, because males remain in copula even as the female attempts to drag him off, wrapping him with silk and biting him (Schneider and Elgar 2001; Schneider et al. 2001). Paternity share in this species is associated with the duration of copulation (Schneider and Elgar 2001; Schneider et al. 2001).
The webs of females of N. plumipes are aggregated and webs may share both vegetation and, in some instances, support lines (Elgar 1989). The mortality associated with the movement between webs has been estimated from disappearance data (see Kasumovic et al. 2007), but this is likely to be trivial within aggregations that may contain more than a dozen female webs (Elgar 1989), and may not be high between adjacent aggregations. The sex ratio on individual webs can be very heavily skewed, with individual females being host to numerous males who may engage in competitive contests (Elgar and Fahey 1996). There is an opportunity for sperm competition because females can mate with several males (Elgar et al. 2003b).
Critical to understanding male and female mating strategies of this species is the potential mating frequency of males and, in particular, whether they can mate more than once. For example, a male’s strategy in response to the risk of sperm competition will depend upon his capacity to copulate more than twice – preventing sperm competition may be more effective than attempting to engage in sperm competition (see Simmons 2001). The aim of this study was to establish the potential mating frequency of males, and to investigate the association between sperm transfer and paternity success. We also document the mating histories of males located on the webs of sexually mature and immature females in a natural population over the duration of the mating season.
Methods
Collection, culturing and measuring captive individuals
Subadult female and both subadult and adult male N. plumipes were collected in January and March, 2000 from a large, single population near Gosford, Australia. Males were collected as adults from the webs of females or as subadults from their own webs. Most of the females were housed in separate Perspex frames (100 cm by 75 cm by 20 cm), where they built typical orb-webs; the remaining females were kept individually in 750-mL plastic cups. The females were watered and fed ~10 bushflies (Lucilia cuprina) three days per week. Females were measured and weighed shortly after they matured. We used callipers to measure the total body length (total cephalothorax to abdomen) and the width of the cephalothorax across the dorsal eyes. The female was immobilised by covering her with plastic film (Glad-wrap™). In the laboratory, males were maintained individually in 250-mL cups on a diet of Drosophila. They were weighed and their body length was measured to the nearest 0.5 mm. Each male was inspected for the condition of his conductors.
Copulatory behaviour, ejaculate size and paternity
We staged copulations by gently placing a male in the lower corner of the frame, using a small paint-brush. Typically, the male walked up the side of the frame, eventually encountering one of the support threads of the orb-web. He then traversed the web to the hub, where he would wait on the opposite side of the female. We noted when the male reached the edge of the web and the hub. Males rarely move from this location unless the female captures a prey item (Elgar and Fahey 1996; see also Fromhage and Schneider 2005b) and males in this study mated only after the female had captured a prey item larger than a bushfly. We used Sarcophagus flies and in most cases, the male followed the female when she approached the prey and the male mated while the female inserted her fangs into the prey. Since the flies were relatively large, it took the females over 30 s until the prey was immobilised and could be wrapped. This was enough time for most males to copulate and safely jump off the female.
We measured the duration of copulation from the time the male inserted his pedipalp until he or the female dislocated it from the genital pore. Males of N. plumipes insert their pedipalp ipsilaterally – the right-hand palp is inserted into the right-hand insemination duct (note that Schneider and Elgar (2001) incorrectly assumed a contralateral insertion pattern, although this did not affect the general conclusions of their study). We carefully observed which pedipalp the male used and into which genital pore he inserted his palp. After a preset schedule, singly mated females were assigned to have their used or their unused genital pore blocked with BluTack® (Bostik, Australia), a non-toxic reusable putty. The females were anaesthetised and a tiny ball of BluTack® was placed inside one opening of the epigynum using a pair of fine needles. The female was gently returned onto her web and, once she had recovered, a virgin male was introduced and the same procedure as described above commenced with the second male.
After mating, the males were removed from the web and preserved in alcohol. Used and unused pedipalps were removed and carefully torn with forceps under the dissecting microscope and the sperm-carrying duct was ruptured. The pedipalps were then individually placed in a centrifuge tube with 120 µL of saline solution (Casyt®tone, Schärfe System). The tubes were centrifuged for 60 s at 5000g. To achieve a homogeneous distribution each sample was treated in an ultrasonicator. To avoid cross-contamination and sample loss we used indirect processing in a cup booster especially designed for small volumes (Bandelin UW 2070). Ultrasonication was conducted four times, each for 30 s at 40% power with a break of 30 s between ultrasonication to avoid overheating of samples. After centrifuging for another 60 s at 5000 g we gently vortexed for 60 s. A 12-µL sample was removed from the centre of the sample and placed on a Neubauer improved double-chamber haematocytometer (=0.3 mm3). Spermatozoa were counted under a light microscope at a magnification of ×400. The values reported are estimates of the total number of sperm in the palp.
Pedipalp integrity and copulation success
The females collected from Sydney were cultured as above. We randomly allocated each female to one of two treatments, in which a male, with either both of his pedipalps intact or both of his pedipalps broken, was introduced onto her web. Adult males were collected from the field, their pedipalps assessed and randomly allocated to females. The males were released onto the web and allowed to cohabit with the female for 2 h. We then applied an electric toothbrush to the web to simulate prey vibrations. Females reacted by attacking the toothbrush, which provided males with the opportunity to attempt to copulate with her.
Field observations
We collected male N. plumipes from the webs of females from a population in the suburban parks around Macquarie University, Sydney, New South Wales, during the summer of 2005. Three samples of males were obtained with 12 days interim between samples. These sample times reflect different times of the breeding season (8 and 10 February, 22 and 24 February, and 7 March 2005). We collected males from the webs of juvenile, penultimate and adult females, first recording the distance of the male to the central hub, and then measuring the size of the male (average patella–tibia length of the front legs) and the status (broken or intact) of each pedipalp.
Results
Copulation frequency and pedipalp damage
A male’s mating behaviour depended upon the state of his pedipalps. Six (of 10) males with intact pedipalps mated with the virgin female, but only one of 11 males with broken pedipalps mated (Fisher’s exact P = 0.024). The single mating among males with broken pedipalps lasted for less than 5 s, which is considerably less than the average duration of copulation in this species (see below) and thus may not have been an effective copulation. The Fisher’s exact probability is reduced to 0.004 when this brief copulation is redesignated as no copulation.
There was no significant difference in either the size or weight of the virgin females allocated to males with broken (11.9 ± 0.3 mm; 0.389 ± 0.040 g) or intact (11.8 ± 0.3 mm, t17 = 0.20, P > 0.8; 0.370 ± 0.033 g, t17 = 0.34, P > 0.7) pedipalps. However, males with broken pedipalps were significantly larger (4.9 ± 0.1 mm) than those with intact pedipalps (4.2 ± 0.2 mm, t19 = 3.33, P = 0.003).
Copulatory behaviour and sperm use
A general linear model with treatment (unused versus used spermatheca) as the factor and number of sperm in the unused pedipalp, male body mass and copulation duration as covariates explained 80% of the variation in the number of sperm that remained in the used pedipalp. The variation in the number of remaining sperm was explained by the duration of copulation (F1,17 = 6.17, P = 0.024) and the number of sperm in the unused palp (F1,17 = 51.62, P < 0.0001). The influence of body mass was not significant (F1,17 = 2.14, P = 0.162). The number of sperm that a male transferred was not significantly influenced by the treatment (F1,17 = 3.38, P = 0.083) despite a relatively large size effect. In other words, males forced to copulate into a used spermatheca (n = 13) retained a mean of 93 333 sperm in their pedipalp while males that mated into an unused spermatheca (n = 18) retained, on average, two-thirds as many (62 904) sperm. Males that mated into a used side may have copulated for a shorter period so that the significant covariate (copulation duration) in the model would reduce the strength of the factor. We tested this possibility by removing copulation duration from the model but treatment remained non-significant.
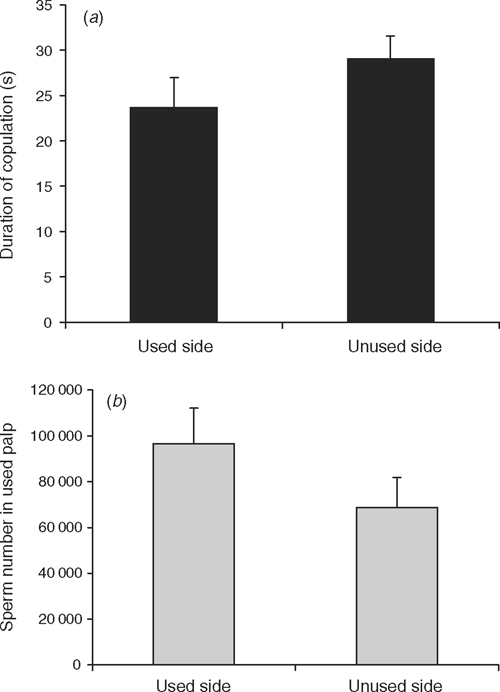
Computing individual comparisons, we found that the duration of copulation was not influenced by whether a male copulated into a previously used or unused side (t27 = –1.21, P = 0.24) (Fig. 1a). Qualitatively more sperm remained in the palps of males that mated into used sides but the difference was again not statistically significant (t22 = –1.36, P = 0.19) (Fig. 1b).
|
Most males that mated into an unused reproductive tract broke off the tip of the conductor. The lack of variance precluded further analysis of the effects of a broken-off part on copulation duration and sperm numbers. As shown previously, males that mated into a used reproductive tract were less likely to break off the tip of the conductor (Table 1).

|
Field observations
The number of males with neither, one or both pedipalps intact varied across both the mating status of the female and the sampling time (Table 2). In general, a greater proportion of males had both pedipalps intact earlier than later in the summer (χ2 = 14.7, P < 0.005). Males with both pedipalps intact were typically found more frequently on the webs of immature than mature females, but this association was more marked in the second sampling period (Fisher’s exact P = 0.001) than in the first (Fisher’s exact P = 0.07) or third (Fisher’s exact P = 0.08) sampling period. It is important to note that the proportion of males with one or both pedipalps intact is likely to be underestimated, since many males that mated would have subsequently fallen victim to their sexually cannibalistic mate. Nevertheless, it is notable that 5% of males found on the webs of immature females had both palps broken.

|
Pooling the data revealed that males that had both pedipalps intact were generally further from the hub (127.8 ± 9.5 mm, n = 246) than males that had at least one broken pedipalp (84.2 ± 19.1 mm, n = 62, t306 = 2.04, P = 0.04). There was no difference in the size of males with both pedipalps intact (0.510 ± 0.004 g) compared with those that had at least one broken pedipalp (0.517 ± 0.009 g, t326 = 0.71, P > 0.48).
Discussion
The tip of the conductor of male golden orb-web spiders typically breaks during copulation and consequently male mating frequency may be physiologically limited to one or two females, irrespective of the males’ opportunities for locating and courting additional females. The proportion of males with both conductors broken increased during the course of the mating season, presumably reflecting larger numbers of males securing two copulations. Males with two broken conductors did not copulate successfully with virgin females but, curiously, these males were nevertheless observed on the webs of immature females. The likelihood of the conductor breaking depends upon the copulation history of the female insemination duct: males were more likely to break their conductor if they inseminated a ‘virgin’ rather than ‘mated’ insemination duct. However, the choice of insemination duct did not significantly influence the quantity of sperm transferred.
There are remarkable similarities between the mating system of N. plumipes and that of other sexually cannibalistic spiders, including the redback spider (L. hasselti) and species of the orb-web genus Argiope. Males of these species are typically cannibalised by the female during their second copulation, and male Argiope and L. hasselti are apparently physiologically incapable of using a palp more than once (Forster 1992; Sasaki and Iwahashi 1995; Andrade 1996; Foellmer and Fairbairn 2003; Herberstein et al. 2005; Snow and Andrade 2005). Sexual cannibalism is also common in N. plumipes (Schneider and Elgar 2001, 2002; Schneider et al. 2001; Elgar et al. 2003b) but, unlike red-backs and Argiope, it is apparently not inevitable after the male’s second copulation. This is also the case for N. fenestrata, in which males defend their females from rivals after their second and final copulation (Fromhage and Schneider 2005a).
Sexual cannibalism in L. hasselti may benefit the male through protecting and/or enhancing his paternity share, and thus allows males to maximise the benefits of monogyny (Fromhage et al. 2005). Such benefits of sexual cannibalism are not so obvious in Argiope (Fromhage and Schneider 2003; Schneider et al. 2006), despite the apparent complicity of the male during their second copulation. Nevertheless, male A. bruennichi damage their paired pedipalps during mating, which clearly limits them to two copulations but also increases and/or protects their paternity share (Nessler et al. 2007a, 2007b) and provides benefits of monogyny. In contrast, sexual cannibalism in N. plumipes more likely reflects a sexual conflict of interest over the duration of copulation (Schneider et al. 2001). However, prolonging the duration of copulation and leaving the remains of the genitalia in the insemination duct does not provide much paternity protection (cf. Fromhage and Schneider 2006), and thus other mechanisms, such as mate guarding, are required. Perhaps the functionally sterile males found on the webs of mature females are attempting to protect their paternity by repelling rival males from the female.
Our collections of adult males revealed that males with broken pedipalps were generally larger than males with intact pedipalps. There are several explanations. First, larger males may be more likely to survive copulations that involve pedipalp breakage than smaller males. However, earlier experiments reveal no size-related patterns of male survival of sexual cannibalism in N. plumipes, either before or during copulation (Schneider and Elgar 2001, 2002; Schneider et al. 2001; Elgar et al. 2003b). Alternatively, larger males may be more likely to mate with the female than smaller males, either because females fail to copulate with smaller males, or because larger males exclude their smaller rivals. There is extensive evidence that large body size conveys an advantage in direct competitive interactions between male spiders (see Elgar 1998), including Nephila (Elgar and Fahey 1996; Elgar et al. 2003a).
It is not clear whether males attempt to fertilise more than one female during their adult life, but several lines of evidence suggest that this is unlikely. First, our field data reveal that only a very small fraction of the males observed on the webs of sexually immature females had previously mated (evidenced by the presence of at least one broken palp), suggesting that very few mated males seek further mating opportunities with females that are about to become sexually receptive. Second, the webs of adult females had relatively few males with only one palp broken compared with those with both palps broken. While these data may be open to several interpretations, a simple explanation is that males that survive their first copulation quickly initiate a second copulation. Males of N. plumipes almost invariably copulate with a female only if she is consuming a prey item (Elgar and Fahey 1996), a behaviour that reduces the risk of sexual cannibalism (Fromhage and Schneider 2005b). Copulation is relatively brief in this species (Schneider and Elgar 2001; Schneider et al. 2001; Elgar et al. 2003b), so females are likely to be consuming the prey item after the first copulation has ceased, thereby providing an incentive for males to quickly initiate a second copulation. Finally, several studies of this and other species of Nephila reveal high levels of disappearance among males moving between webs (Vollrath 1980; Kasumovic et al. 2007; but see Fromhage et al. 2007). While interpreting these kinds of data is notoriously difficult, it is possible that mate-searching males suffer higher levels of mortality. The uncertainty of locating a second female, the competitive disadvantage of copulating once rather than twice with the same female (Elgar et al. 2003b), and the possibility of being cannibalised before inseminating the second female (Elgar and Fahey 1996; Schneider and Elgar 2001, 2002; Schneider et al. 2001), provides a compelling argument that selection will favour males that inseminate both reproductive tracts of a single female, rather than attempt to inseminate a single tract each of two females.
These data raise interesting questions about the mechanisms available to males of N. plumipes to influence patterns of paternity. Clearly, males of N. plumipes cannot improve their paternity success by repeatedly mating with the same female, unlike males of the congenor N. edulis (Schneider et al. 2000; Schneider and Elgar 2005; Elgar and Jones 2008; Jones and Elgar 2008), despite the possible increase in paternity share (see Elgar et al. 2003b). Sexual cannibalism per se does not provide a greater paternity share (Schneider and Elgar 2001; Schneider et al. 2001) or strongly reduce female receptivity (Elgar et al. 2003b). While the conductor breaks during copulation in N. plumipes (Schneider and Elgar 2001; Schneider et al. 2001; Elgar et al. 2003b), thereby reducing the male’s capacity to remate, it is not clear that this provides paternity benefits in the context of avoiding or engaging in sperm competition (Schneider et al. 2001), as is the case with other spiders (e.g. Nessler et al. 2007a, 2007b). Finally, there is little compelling evidence that males of N. plumipes strategically adjust their sperm investment according to the risk of sperm competition, which is perhaps not surprising given their limited opportunities for polygyny. Thus, it would appear that physical mate guarding is the primary mechanism of paternity protection in this species, which may explain the presence of functionally sterile males on the webs of mature females.
Acknowledgements
We are grateful for the support of the Australian Research Council (A19930103 and A19802502 to MAE; DP0662873 to MEH), the Deutsche Forschungsgemeinschaft (to JMS) and the Natural Sciences and Engineering Research Council of Canada (PGSB to MMK).
Andrade, M. C. B. (1996). Sexual selection for male sacrifice in the Australian redback spider. Science 271, 70–72.
| Crossref | GoogleScholarGoogle Scholar | CAS |
Elgar, M. A. (1989). Kleptoparasitism: a cost of aggregating for the orb-weaving spider Nephila edulis. Animal Behaviour 37, 1052–1055.
| Crossref | GoogleScholarGoogle Scholar |
Elgar, M. A. , and Fahey, B. (1996). Sexual cannibalism, competition, and size dimorphism in the orb-weaving spider Nephila plumipes Latreille (Araneae, Araneoidea). Behavioral Ecology 7, 195–198.
| Crossref | GoogleScholarGoogle Scholar |
Snow, L. S. E. , and Andrade, M. C. B. (2005). Multiple sperm storage organs facilitate female control of paternity. Proceedings of the Royal Society of London. Series B. Biological Sciences 272, 1139–1144.
| Crossref | GoogleScholarGoogle Scholar |

Vollrath, F. (1980). Male body size and fitness in the web-building spider Nephila clavipes. Zeitschrift für Tierpsychologie 53, 61–78.



