Tracking the spread of the eastern dwarf tree frog (Litoria fallax) in Australia using citizen science
Jodi J. L. Rowley A B * and Corey T. Callaghan
A B * and Corey T. Callaghan  C
C
A Australian Museum Research Institute, Australian Museum, 1 William Street, Sydney, NSW 2010, Australia.
B Centre for Ecosystem Science, School of Biological, Earth and Environmental Sciences (BEES), University of New South Wales, Sydney, NSW 2052, Australia.
C Department of Wildlife Ecology and Conservation, Fort Lauderdale Research and Education Center, University of Florida, Davie, FL 33314-7719, USA.
Australian Journal of Zoology 70(6) 204-210 https://doi.org/10.1071/ZO23012
Submitted: 21 March 2023 Accepted: 31 May 2023 Published: 28 June 2023
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
An increasing number of species are establishing populations outside of their native ranges, often with negative ecological and economic impacts. The detection and surveillance of invasive species presents a huge logistical challenge, given the large spatial regions in which new populations can appear. However, data collected through citizen science projects are increasingly recognised as a valuable source for detection and monitoring of invasive species. We use data from a national citizen science project, FrogID, to quantify the spread of the eastern dwarf tree frog (Litoria fallax) outside its historical native range in Australia. Of 48 012 records of L. fallax in the FrogID database, 485 were located far outside the historical native range of the species. L. fallax has established geographically large populations hundreds of kilometres away from its native range, and these appear to be spreading in extent over time. These populations have resulted in novel species co-occurrences, with L. fallax now co-occurring with at least two frog species not present in their native range. Although the impacts of the invasive populations of L. fallax remain unknown, our work highlights the value in leveraging citizen science projects to detect and monitor native species that can become invasive far outside their historical range.
Keywords: amphibian, biodiversity, community science, ecology, frogs, invasive species, monitoring, range expansion, species detection, species interactions.
Introduction
The global distribution of biodiversity is being dramatically altered because of human modification of the environment, and international trade. Plant and animal species are being transported outside their native ranges and an increasing number of species are establishing populations in these new localities. The establishment of such species can have environmental impacts ranging from single species to ecosystem-level effects (Grosholz 2002). And in some instances, they can have serious economic impacts (Bradshaw et al. 2016). Although many invasive species are introduced from countries outside their native range, some native species also establish invasive populations outside their native range (Simberloff 2010). These invasive native species have been the subject of far less attention, but their impacts may be similarly severe (Davis 2009).
Timely data on the spatial and temporal distribution of invasive species are vital to manage existing populations (Giovos et al. 2019) and detect potential establishment of populations in new regions (Reaser et al. 2020). However, the availability of such data is hindered by enormous logistical and resource limitations in structured scientific surveys, resulting in large gaps in knowledge of invasion patterns (Crall et al. 2010; Giovos et al. 2019). Citizen science projects focused on biodiversity can provide scientifically robust and reliable data at broad spatial and temporal scales, comparable to professionally collected or expert-derived data (Lewandowski and Specht 2015; Aceves-Bueno et al. 2017; Callaghan et al. 2020). Consequently, such citizen science data are increasingly being harnessed to monitor or guide surveillance of invasive species (Giovos et al. 2019; Koen and Newton 2021; Dart et al. 2022).
Here, we use data from a national citizen science project across Australia – FrogID (Rowley et al. 2019) – to detect the eastern dwarf tree frog (L. fallax) outside its historical native range in Australia. Using approximately 57 months of data from FrogID, we report detections of the species outside its native range, including the persistence and likely spread of established populations, and document new frog co-occurrences in its range.
Materials and methods
Study species
The eastern dwarf tree frog (Litoria fallax) is a small (~2.5 cm body length), commonly encountered frog native to eastern Australia, from central-eastern Queensland to the border of New South Wales (NSW). L. fallax was not detected outside its native range until the 1990s (Supplementary Fig. S1). The first record of the species outside its native range was in a wetland in the suburb of Moorabbin, in Melbourne, Victoria, in 1999 (Gillespie and Clemann 2000), and in 2010 it was recorded for the first time in north-eastern Victoria (Michael and Johnson 2016). The persistence and expansion of L. fallax throughout the suburbs of Melbourne was also documented by Bevelander (2014). The species has also established an invasive population in Guam (Christy et al. 2007).
FrogID dataset
We used data from the national citizen science project FrogID (Rowley et al. 2019; Rowley and Callaghan 2020), based on a smartphone app that allows users to submit audio recordings of calling frogs with associated metadata including location, time, and date. The species calling in each submission are then identified to species-level by experts in frog call identification. FrogID submissions typically contain the advertisement calls of more than one species of frog. The average number of frog species calling in a single recording is 1.6, and for recordings with at least one species of frog present is 2.6, with a maximum of 13 species per submission. We exported data from the FrogID database on 25 July 2022 and used data from 10 November 2017 until 30 June 2022.
The presumed native range of L. fallax was obtained from the Australian Frog Atlas (Cutajar et al. 2022) with invasive range removed based upon historical (<1980) records of the species in the Atlas of Living Australia (ALA; ala.org.au). The true historical range of L. fallax is unknown but is likely to be relatively reliable as it is well-sampled (the fifth most well-sampled frog species in Australia: ALA), and distinctive in both appearance and advertisement call. All records of L. fallax outside of this range were deemed out of range.
To examine the distribution of invasive L. fallax in relation to population density, we used population density (per square kilometre) from the Gridded Population of the World, Ver. 4 (GPWv4), Revision 11. We extracted human population density values for each FrogID record of L. fallax and conducted a Wilcoxon rank sum test in R to determine if there was any difference in human population density for records inside versus outside the presumed native range of L. fallax. We calculated Extent of Occurrence (EOO) for L. fallax using FrogID data and the package ConR in R (Dauby et al. 2017).
Results
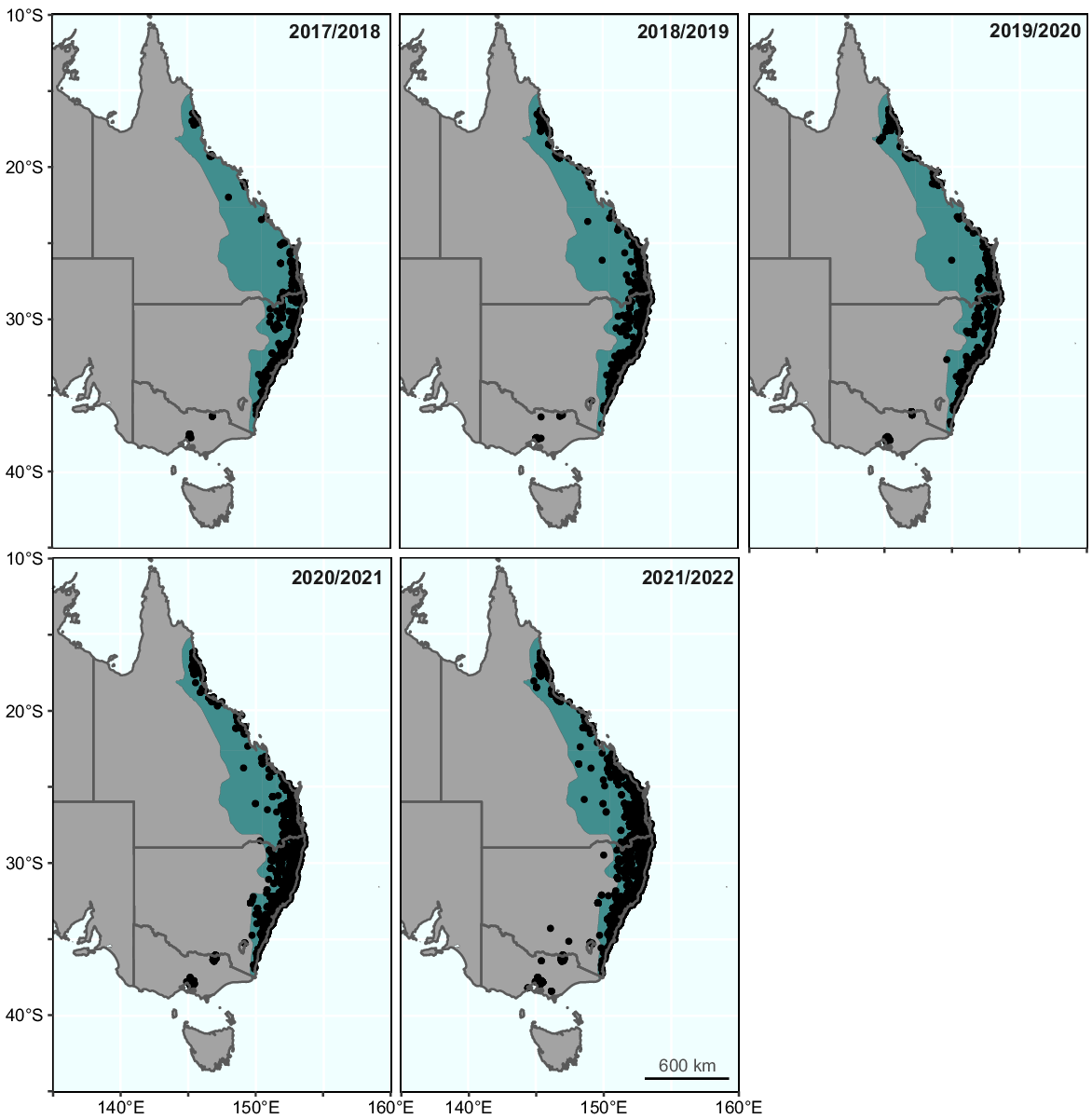
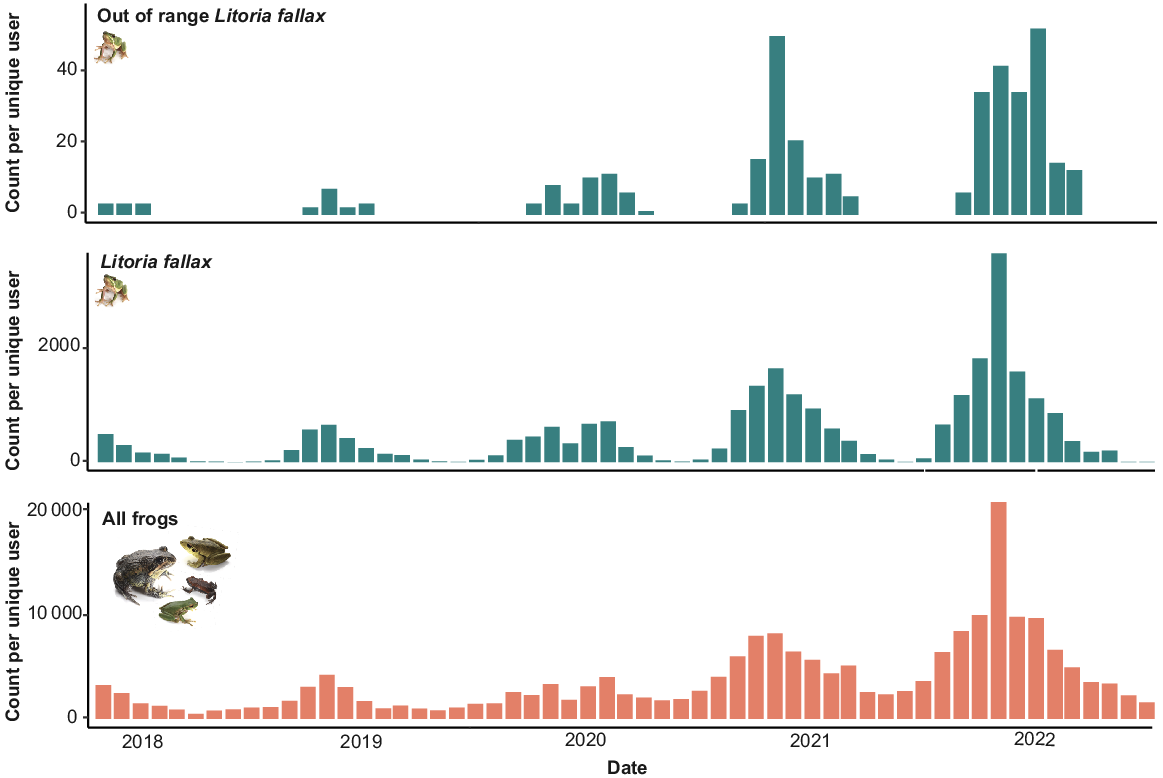
In total, there were 48 012 records of L. fallax in the FrogID database, representing 7% of all frog records in the dataset. Of these records, 495 were located outside the presumed native range of the species (Fig. 1). Most of these were in Victoria (422), with fewer records in NSW (62), and the ACT (11). Records of L. fallax outside of its native range have been documented every year since the project launched in 2017, and the number of records and unique users that have recorded out-of-range L. fallax has increased over time, as have records of L. fallax and all frog species (Fig. 2).
Records of Litoria fallax (black circles) in Australia from the FrogID project over time. Each panel spans 1 July to 30 June and represents an entire breeding season for the species (except for 2017/2018, which spanned 10 November 2017 to 30 June 2018). The approximate native range of Litoria fallax is indicated in green.
Records per unique user over time from the FrogID project from 10 November 2017 to 30 June 2022 for out-of-range Litoria fallax, all Litoria fallax, and all frog species.
Our analysis documents the continued persistence and likely spread of populations around Melbourne (‘Melbourne population’; >400 km outside native range) and northern Victoria, documenting the population in northern Victoria extending into NSW for the first time in November 2019 (‘Northern Victoria/Albury population’; >220 km outside its native range: Figs 1, 3 and S2). L. fallax was also documented in Canberra, in the ACT, for the first time (>50 km outside its native range) – a single record in 2018 and then five additional records each in the 2020/2021 spring/summer and 2021/2022 spring/summer. In 2021, L. fallax records were obtained from Griffith (>300 km outside its native range) and Wagga Wagga in NSW (>200 km outside its native range), and in 2022, the first records of L. fallax from Mirboo North in South Gippsland, Victoria, were received (>300 km outside its native range). Out-of- range records of L. fallax have a higher human population density (median of 139 people per square kilometre) compared to records within the presumed native range of L. fallax (median of 53 people per square kilometre: W = 11 023 390, P < 0.001) (Figs 3 and S3).
Records of Litoria fallax (black circles) from the FrogID project in south-eastern Australia, from 10 November 2017 to 30 June 2022, and population density (number of persons per square kilometre; Gridded Population of the World v4).
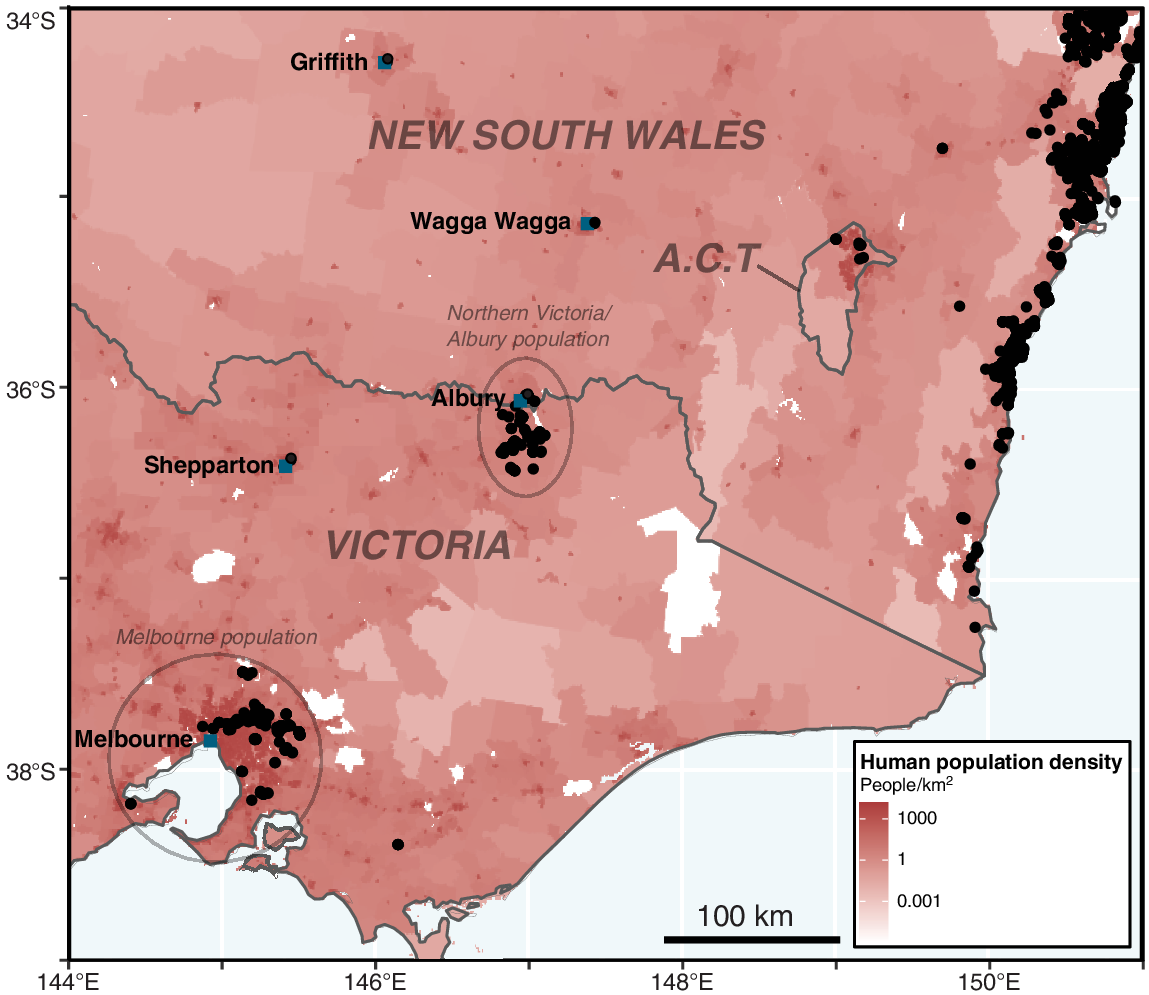
The known geographic extent of the Melbourne and northern Victoria/Albury L. fallax populations have increased over the duration of the FrogID project and are now expansive: with an extent of occurrence of approximately 1751 km2 and 789 km2 respectively (Fig. S4). As a result of these invasive populations, L. fallax now co-occurs (calling in close proximity) with species not found within its native range. L. fallax was detected co-occurring at the same site as 84 other frog species within its native range (Table S1), and 15 species outside of its native range, two of which were novel co-occurrences: Crinia sloanei (four records from four unique users in the Albury area of NSW) and Geocrinia victoriana (one record from the Melbourne area of Victoria).
Discussion
L. fallax is increasingly being detected outside its presumed native range, and establishing populations that are not only self-sustaining, but appear to be spreading in geographic extent. The spread of L. fallax well outside its presumed native range has previously been documented (Gillespie and Clemann 2000; Bevelander 2014; Michael and Johnson 2016), but our data provide evidence of the further spread of known invasive populations and the potential establishment of previously undocumented populations as well as the potential of citizen science to track this spread. However, increased sampling effort (i.e. the increased rate of FrogID submissions) and/or detectability (i.e. increased calling in wetter weather) in recent years may have contributed to the detection of these populations. It is possible that they were previously present but undetected.
Localities of L. fallax outside their native range were associated with areas of high human population density, which is likely the result of human-mediated transport, possibly via horticultural products and fresh fruit. Large numbers of native frogs are accidentally translocated around Australia, with over 7000 frogs per annum translocated into New South Wales alone in shipments of bananas, most of which were subsequently released into the local environment (O’Dwyer et al. 2000). Their small body size and habit of sheltering in vegetation, often well away from water, along with their relatively high tolerance of urbanisation (Liu et al. 2021) makes this species particularly susceptible to translocation outside of its native range.
The impacts of the invasive populations of L. fallax remain unknown. The introduction of amphibian species to novel environments can cause declines and genetic changes in native taxa (Riley et al. 2003), transmit disease or change disease dynamics in frog communities (Strauss et al. 2012), and have economic costs (impacts reviewed by Kraus 2015). Given that populations of L. fallax may have a high prevalence of the amphibian chytrid fungus (Batrachochytrium dendrobatidis) and not suffer population declines as a result (Kriger and Hero 2007), it is possible that introduced populations of L. fallax are acting as disease amplifiers or reservoir hosts (e.g. Rivera et al. 2019).
We report on potential novel species co-occurrences as a result of the invasive populations of L. fallax, one of which is the threatened Sloane’s froglet (Crinia sloanei). Continued use of FrogID may allow a greater understanding of any impacts, particularly on this and other threatened native species in the new range of L. fallax, such as the southern bell frog (Litoria raniformis). Although fieldwork will be necessary to elucidate the impact of these novel co-occurrences, continued collection of FrogID data will assist in better understanding the impact of invasive frog species on local frog communities.
We demonstrate the utility of citizen science in detecting and monitoring an invasive frog species in Australia. Although we focus on a single species, the FrogID project is also detecting many other native species outside their historical ranges (Rowley et al. 2019). The project allows local communities to advance our understanding of invasive frog species, including the detection of calling individuals and their subsequent establishment and spread, for example for other invasive species in Australia such as the introduced cane toad (Rhinella marina) (Rowley et al. 2019). Community groups are already actively using FrogID to monitor sites and species over time (i.e. Crinia sloanei in the Albury area of NSW by the Sloane’s Champions: Rowley et al. 2019) and communicating spatial and temporal priorities to participants could be used to further optimise data collection (Callaghan et al. 2023; Thompson et al. 2023), including for invasive species monitoring.
This study adds to the growing body of research confirming the ability of citizen science projects in collecting biodiversity records, including invasive species records, across large spatial scales (Giovos et al. 2019; Koen and Newton 2021). In addition, using citizen science to detect invasive species has benefits outside of the data themselves – citizen science has been demonstrated to engage people with science, increasing their awareness of environmental issues (Weber 2000; Jordan et al. 2011; Bonney et al. 2016).
Data availability
The complete raw dataset is not fully available due to sensitivities in relation to locations of rare or threatened species and citizen scientist information (Rowley and Callaghan 2020). However, the data, with sensitive species’ localities removed or buffered, are made available annually (Rowley and Callaghan 2020; data available through GBIF: https://doi.org/10.15468/wazqft and FrogID: https://www.frogid.net.au/explore). Maps of the current range of L. fallax in Australia are available as part of the Australian Frog Atlas (https://zenodo.org/record/6544829). A shapefile of the presumed historical (<1980) range of L. fallax is also available (https://zenodo.org/record/7933987).
Declaration of funding
We thank the Citizen Science Grants of the Australian Government and the Impact Grants program of IBM Australia for providing funding and resources to help build the initial FrogID App; the generous donors who have provided funding for the project, including the James Kirby Foundation, the Vonwiller Foundation, the NSW Biodiversity Conservation Trust, the Department of Planning and Environment – Water and the Saving our Species program as Supporting Partners.
Acknowledgements
We thank the Museum and Art Gallery of the Northern Territory, Museums Victoria, Queensland Museum, South Australian Museum, Tasmanian Museum and Art Gallery, and Western Australian Museum as FrogID partner museums; the many Australian Museum staff and volunteers who make up the FrogID team; and, most importantly, the thousands of citizen scientists across Australia who have volunteered their time to record frogs.
References
Aceves-Bueno E, Adeleye AS, Feraud M, Huang Y, Tao M, Yang Y, Anderson SE (2017) The accuracy of citizen science data: a quantitative review. The Bulletin of the Ecological Society of America 98, 278-290.
| Crossref | Google Scholar |
Bonney R, Phillips TB, Ballard HL, Enck JW (2016) Can citizen science enhance public understanding of science? Public Understanding of Science 25, 2-16.
| Crossref | Google Scholar |
Bradshaw CJA, Leroy B, Bellard C, Roiz D, Albert C, Fournier A, Barbet-Massin M, Salles J-M, Simard F, Courchamp F (2016) Massive yet grossly underestimated global costs of invasive insects. Nature Communications 7, 12986.
| Crossref | Google Scholar |
Callaghan CT, Roberts JD, Poore AGB, Alford RA, Cogger H, Rowley JJL (2020) Citizen science data accurately predicts expert-derived species richness at a continental scale when sampling thresholds are met. Biodiversity and Conservation 29, 1323-1337.
| Crossref | Google Scholar |
Callaghan CT, Thompson M, Woods A, Poore AGB, Bowler DE, Samonte F, Rowley JJL, Roslan N, Kingsford RT, Cornwell WK, Major RE (2023) Experimental evidence that behavioral nudges in citizen science projects can improve biodiversity data. BioScience 73, 302-313.
| Crossref | Google Scholar |
Christy MT, Clark CS, Gee DE, Vice D, Vice DS, Warner MP, Tyrrell CL, Rodda GH, Savidge JA (2007) Recent records of alien anurans on the Pacific island of Guam. Pacific Science 61, 469-483.
| Crossref | Google Scholar |
Crall AW, Newman GJ, Jarnevich CS, Stohlgren TJ, Waller DM, Graham J (2010) Improving and integrating data on invasive species collected by citizen scientists. Biological Invasions 12, 3419-3428.
| Crossref | Google Scholar |
Cutajar TP, Portway CD, Gillard GL, Rowley JJL (2022) Australian Frog Atlas: species’ distribution maps informed by the FrogID dataset. Technical Reports of the Australian Museum 36, 1-48.
| Crossref | Google Scholar |
Dart K, Latty T, Greenville A (2022) Citizen science reveals current distribution, predicted habitat suitability and resource requirements of the introduced African carder bee Pseudoanthidium (Immanthidium) repetitum in Australia. Biological Invasions 24, 1827-1838.
| Crossref | Google Scholar |
Dauby G, Stévart T, Droissart V, Cosiaux A, Deblauwe V, Simo-Droissart M, Sosef MSM, Lowry PP, II, Schatz GE, Gereau RE, Couvreur TLP (2017) ConR: an R package to assist large-scale multispecies preliminary conservation assessments using distribution data. Ecology and Evolution 7, 11292-11303.
| Crossref | Google Scholar |
Gillespie GR, Clemann N (2000) The eastern dwarf tree frog Litoria fallax (Peters) (Anura: Hylidae): a recent introduction to Victoria? The Victorian Naturalist 117, 60-62.
| Google Scholar |
Giovos I, Kleitou P, Poursanidis D, Batjakas I, Bernardi G, Crocetta F, Doumpas N, Kalogirou S, Kampouris TE, Keramidas I, Langeneck J, Maximiadi M, Mitsou E, Stoilas V-O, Tiralongo F, Romanidis-Kyriakidis G, Xentidis N-J, Zenetos A, Katsanevakis S (2019) Citizen-science for monitoring marine invasions and stimulating public engagement: a case project from the eastern Mediterranean. Biological Invasions 21, 3707-3721.
| Crossref | Google Scholar |
Grosholz E (2002) Ecological and evolutionary consequences of coastal invasions. Trends in Ecology & Evolution 17, 22-27.
| Crossref | Google Scholar |
Jordan RC, Gray SA, Howe DV, Brooks WR, Ehrenfeld JG (2011) Knowledge gain and behavioral change in citizen-science programs. Conservation Biology 25, 1148-1154.
| Crossref | Google Scholar |
Koen EL, Newton EJ (2021) Outreach increases detections of an invasive species in a crowdsourced monitoring program. Biological Invasions 23, 2611-2620.
| Crossref | Google Scholar |
Kraus F (2015) Impacts from invasive reptiles and amphibians. Annual Review of Ecology, Evolution, and Systematics 46, 75-97.
| Crossref | Google Scholar |
Kriger KM, Hero J-M (2007) The chytrid fungus Batrachochytrium dendrobatidis is non-randomly distributed across amphibian breeding habitats. Diversity and Distributions 13, 781-788.
| Crossref | Google Scholar |
Lewandowski E, Specht H (2015) Influence of volunteer and project characteristics on data quality of biological surveys. Conservation Biology 29, 713-723.
| Crossref | Google Scholar |
Liu G, Rowley JJL, Kingsford RT, Callaghan CT (2021) Species’ traits drive amphibian tolerance to anthropogenic habitat modification. Global Change Biology 27, 3120-3132.
| Crossref | Google Scholar |
Michael DR, Johnson G (2016) Notes on a naturalised population of the eastern dwarf tree frog Litoria fallax (Peters) (Anura: Hylidae) in north-east Victoria. Victorian Naturalist 133, 202-205.
| Google Scholar |
O’Dwyer TW, Buttemer WA, Priddel DM (2000) Inadvertent translocation of amphibians in the shipment of agricultural produce into New South Wales: its extent and conservation implications. Pacific Conservation Biology 6, 40-45.
| Crossref | Google Scholar |
Reaser JK, Burgiel SW, Kirkey J, Brantley KA, Veatch SD, Burgos-Rodríguez J (2020) The early detection of and rapid response (EDRR) to invasive species: a conceptual framework and federal capacities assessment. Biological Invasions 22, 1-19.
| Crossref | Google Scholar |
Riley SPD, Bradley Shaffer H, Randal Voss S, Fitzpatrick BM (2003) Hybridization between a rare, native tiger salamander (Ambystoma californiense) and its introduced congener. Ecological Applications 13, 1263-1275.
| Crossref | Google Scholar |
Rivera B, Cook K, Andrews K, Atkinson MS, Savage AE (2019) Pathogen dynamics in an invasive frog compared to native species. EcoHealth 16, 222-234.
| Crossref | Google Scholar |
Rowley JJL, Callaghan CT (2020) The FrogID dataset: expert-validated occurrence records of Australia’s frogs collected by citizen scientists. ZooKeys 912, 139-151.
| Crossref | Google Scholar |
Rowley JJL, Callaghan CT, Cutajar T, Portway C, Potter K, Mahony S, Trembath DF, Flemons P, Woods A (2019) FrogID: citizen scientists provide validated biodiversity data on frogs of Australia. Herpetological Conservation Biology 14, 155-170.
| Google Scholar |
Simberloff D (2010) Invasive species. In ‘Conservation biology for all’. (Eds NS Sodhi, PR Ehrlich) pp. 131–152. (Oxford Academic: Oxford) doi:10.1093/acprof:oso/9780199554232.001.0001
Strauss A, White A, Boots M (2012) Invading with biological weapons: the importance of disease-mediated invasions. Functional Ecology 26, 1249-1261.
| Crossref | Google Scholar |
Thompson MM, Moon K, Woods A, Rowley JJL, Poore AGB, Kingsford RT, Callaghan CT (2023) Citizen science participant motivations and behaviour: implications for biodiversity data coverage. Biological Conservation 282, 110079.
| Crossref | Google Scholar |
Weber EP (2000) A new vanguard for the environment: grass-roots ecosystem management as a new environmental movement. Society & Natural Resources 13, 237-259.
| Crossref | Google Scholar |


