Birds pre-adapted to a road in a heterogeneous and contiguous old-growth forest: a point transect study
Graham R. Fulton A B * , Jutta Beher C and Hugh P. Possingham A
A B * , Jutta Beher C and Hugh P. Possingham A
A
B
C
Abstract
Roads are present globally across all habitats and their negative impacts on the landscape are being increasingly reported. Yet often more is known about the identity of roadkill than how avian assemblages are impacted by roads. This study used 100 paired point counts by the road and 400 m into the forest interior to assess if the assemblages were different and determine what species may be impacted by the road. The study was undertaken along a highway cut through one of the world’s tallest forests – old-growth karri (Eucalyptus diversicolor) forest of south-western Australia. There was no overall significant difference in species richness and abundance between road and forest interior sites, although a small number of species (4.3%) did demonstrate preferences. Overall, we suggest that the limited significant differences resulted from: (1) the narrowness of the road with the forest canopy frequently extended fully across and (2) the natural variation found in eucalypt forests, which has aided the birds as a pre-adaptation to the presence of this road – because eucalypt forests are a heterogeneous array of streams and forest heterogeneity.
Keywords: extinction filter, forest birds, forest heterogeneity, karri forest, landscape ecology, population ecology, road ecology.
Introduction
Road ecology has become more important to nature conservation with an ever-increasing road network, which is evidenced by the consistent growth of the literature on roads (Linsdale 1929; Bennett 1991; Erritzoe et al. 2003; Fulton et al. 2008; Fahrig and Rytwinski 2009; Morelli et al. 2014; Alamgir et al. 2017; Cooke et al. 2020). The term road ecology was coined by Richard Forman who observed that roads are the only spatial element that all landscapes have in common (Forman 1998). Roads cover large areas, linearly and spatially, and they can have significant downstream effects within the landscape (Forman et al. 2003; Coffin 2007; Goosem 2007; Laurance et al. 2009). Road ecology can be considered at the landscape level (Raiter et al. 2018; Cooke et al. 2020), or at the road itself and within its immediate surroundings (Forman and Alexander 1998; Fulton et al. 2008). An empirical review (Fahrig and Rytwinski 2009) and a UK wide study (Cooke et al. 2020) both asserted that the effects of roads and traffic are strong enough to merit their mitigation.
Unlike the natural breaks inherent in the landscape such as streams and topographic contours, roads are subject to constant human activity and ongoing disturbances (Bennett 1991; Goosem 2007). Road traffic kills animals via direct impact (Fulton et al. 2008). Roads may act as a barrier to movement and isolate populations (Goosem 2001; Jones and Bond 2010). They may inhibit animals with specialised adaptations: such as those that are strictly arboreal or adapted to flying in dense vegetation (Laurance et al. 2009). Roads may repel some species or facilitate invasions by other species able to take advantage of the altered conditions (Brown et al. 2006; Laurance et al. 2009; Šálek et al. 2010; Cooke et al. 2020).
The zone directly adjacent to the road, the verges and forest edges, bear the immediate and ongoing brunt of this impact. The depth and degree of impact will depend on the type of impact and the sensitivity of the species present (Forman et al. 1997; Šálek et al. 2010). Road noise is one of many possible demographic or genetic barriers (Reijnen et al. 1995; Forman 1998; Ware et al. 2015). Such a barrier has been confirmed in some studies (Pocock and Lawrence 2005) and refuted in others (Summers et al. 2011). Some studies found roads increased abundances of birds (e.g. Morelli et al. 2014) by providing an additional resource. For example, Šálek et al. (2010) found roads created greater habitat heterogeneity in structurally poor forests and influenced greater abundance and diversity with some birds better able to exploit the change in the habitat. Yet other studies report negative effects at roads (e.g. Fahrig and Rytwinski 2009; Cooke et al. 2020). For example, more than just quantifying positive and negative effects, some studies have found birds adapting to the presence of roads (Husby 2017), evolving due to road mediated fatalities (Brown and Bomberger Brown 2013), or colonising roads and urban areas as invasive species (Møller et al. 2012). Invasive colonising assumes a pre-adaptation to the new environment, roads being novel, and being favoured over previous natural habitats (Møller et al. 2012). Overall, the varied results and the variety of studies indicate that complex relationships exist between different species and different habitats or landscapes. This variety of possibilities suggests that different species will use the road differently in different circumstances, thus they will be impacted by or use the road in varied ways.
In Australia, early studies of birds that frequent roads were historically and primarily undertaken in agricultural areas, which have narrow road-side strips of vegetation (Carrick 1963; Vestjens 1973; Arnold and Weeldenburg 1990; Lepschi 1992). However, more recently forest habitats have been considered: temperate (Fulton et al. 2008), tropical (Laurance et al. 2009) and subtropical urban forests (Pell and Jones 2015). Roads traversing large contiguous native forests and national parks are of interest because these habitats often harbour greater numbers of threatened species. Studies in such biodiverse locations can provide baseline data on how these roads are used by avian assemblages and have implications for their conservation and management (Ramp et al. 2006; Goosem 2007; Fulton et al. 2008; Laurance et al. 2009).
The current study focused on a relatively narrow two-lane highway (7 m plus 4 m verges) in south-western Australia, which traversed a landscape of contiguous and heterogeneous old-growth karri (Eucalyptus diversicolor) forest. It aimed to assess if avian species incidence and/or abundance on a simple highway in this old-growth forest was different from that of the forest interior, by using randomly selected and repeated point counts.
Materials and methods
Study site
This study was conducted using point counts (Verner 1985; Ralph et al. 1995) along a 50 km section of the South Western Highway, a primary highway, which traverses mature karri forest through the south-west of Western Australia. The study section of the highway commenced at the intersection of Wheatley Coast Road and South Western Highway (34°27′05.6″S, 116°14′13.7″E) and followed the South Western Highway in a south-easterly direction through Greater Dordagup, Shannon and Mount Franklin National Parks, for 50 km, ending at 34°46′03.7″S 116°30′05.6″E (Fig. 1). It was chosen because it is a region of old-growth forest with a full ecologically functional assemblage of fauna, including birds (Abbott 1999). Old growth or mature karri forest, which occurs only in the south-western corner of Australia, has two structurally distinct layers of vegetation: a dense understorey to 12 m and an open overstorey to 60 m with the tree crowns of the karri above. Karri grows over 90 m in height, making it one of the tallest trees in the world (Wardell-Johnson and Williams 2000). The karri forest within the survey area intergrades with patches of jarrah (Eucalyptus marginata) and wandoo (Eucalyptus wandoo) forest on areas of poorer soils. This type of heterogeneity is common in eucalypt landscapes (Moore et al. 1993; Wardell-Johnson and Horwitz 1996).
Map showing the track of the South Western Highway through karri forest, in south-western Australia.
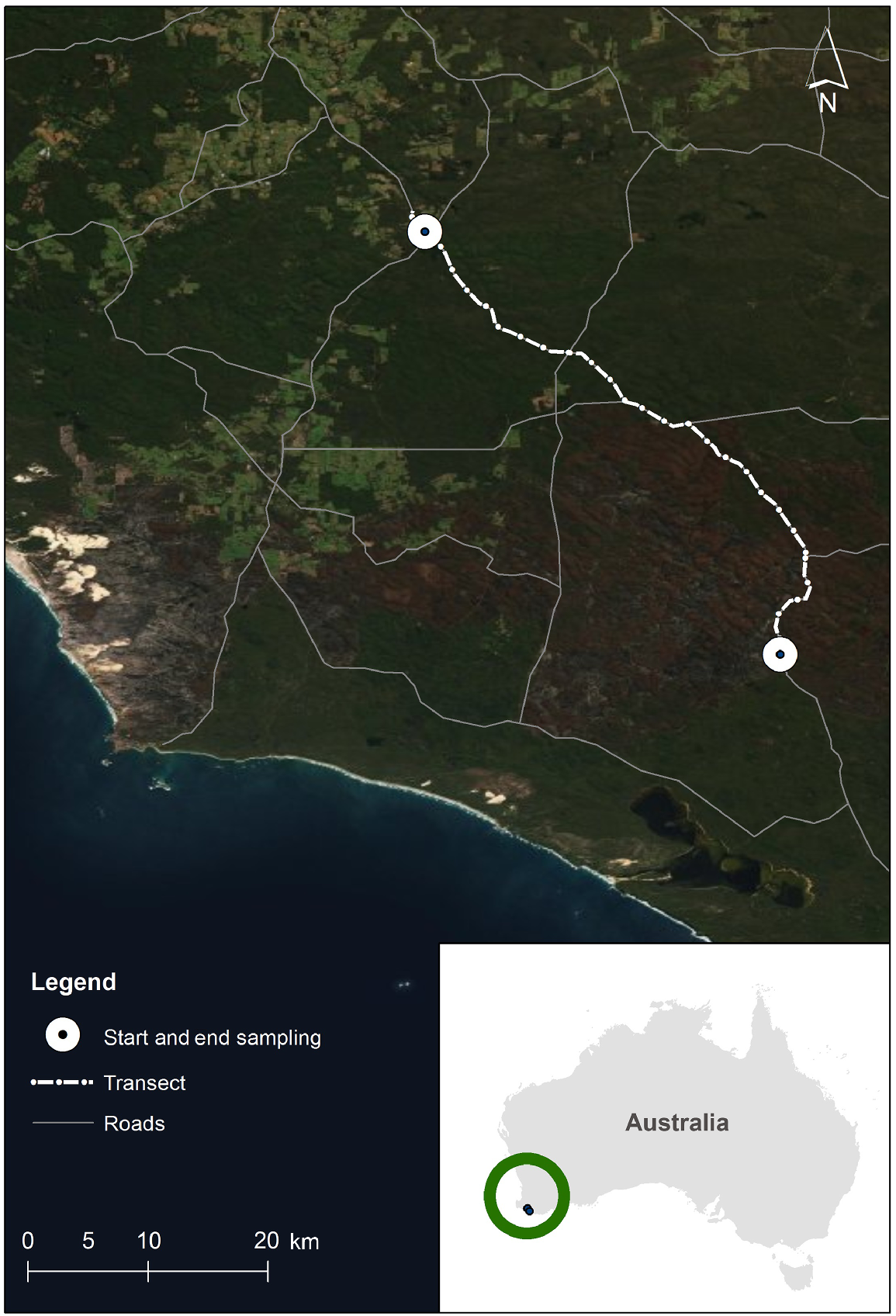
The study section of the South Western Highway, between Manjimup and Walpole, developed from an impassable track in about 1918 to a formed and gravelled road completed by about 1922 (Anon 1921). The road was sealed in 1968 (Department of Main Roads, Western Australia in litt.). During the study, the South Western Highway was a two lane bitumen road (one lane in either direction: width 7 m, plus 4 m verges on either side), without fences between the road and the forest. Its verges (cleared edges) were approximately 4 m wide and consisted of either gravel or short grass and gravel. The verges then gave way to the native forest. The forest canopy frequently overhung part of the road, sometimes completely across (unquantified) (Fig. 2). The mean number of cars and heavy vehicles that used this part of the highway were calculated from data supplied by Main Roads Western Australia. The mean number of vehicles that used this section of the highway per day, for the three years, July–June 2004/2005, 2005/2006 and 2006/2007, was 519 (s.e. 71), of which 6.8% (s.e. 1.5) were heavy vehicles (Main Roads Western Australia 2014). The speed limit on this section of the highway is mostly 100 km/h (there are some short slower sections).
Choice of study site
We chose to focus our study on this section of road, in this forest, because secondary roads had dissimilar characteristics (such as road and verge width, road surface (sealed or unsealed), vehicular type and speed), all of which would have made them poor replicates and would have introduced a range of external effects into the study. In addition, secondary roads typically led deeper into the forest, usually for short distances and were predominantly unsealed. The natural heterogeneity and extent of the old-growth forest available in the national parks and forest reserves of this study was also a factor in its selection, because heterogeneity of forest type and old-growth support the presence of a diverse assemblage of birds (e.g. Fulton 2013; Fulton and Lawson 2021), which in turn provides a more transferable result with regard to species and species-groups to analogous situations. Moreover, the natural heterogeneity, present as the intergradation of the various forest types matches well with the natural patchiness present in all Australian forests (Moore et al. 1993; Barrett et al. 1994; Wardell-Johnson and Horwitz 1996). Thus, only this single road was chosen because it is the only main road through this dominant forest type, in this region of Australia.
Point counts
In total, 36 paired point counts (road plus forest interior = 1 paired point count) were censused over 10 months from July 2005 to April 2006, on 21 survey days with a total of 100 paired point counts surveyed. Point counts consisted of monitoring 10 min each by the road and at a point 400 m into the forest interior on both sides of the road. The 400 m distance was chosen because car noise was inaudible to the surveyor’s ear this far into the forest, due to the dense lower layer of vegetation. It generally took about 10 min to travel from the road to the interior sampling point. Between two and six (mode: five) paired points were sampled each survey day. The sampling points were selected where access into the forest interior was facilitated by walking tracks, streams, unpaved forest roads or disused roads that were largely overgrown. In general, the forest was impenetrable due to deep and dangerous litter, which consisted of large fallen branches that were covered with other litter and could not be seen. If a gravel track was used for access then the positions of the point count would be moved as far as possible away from this track to minimise its influence, usually about 50 m. No cars were seen on these gravel tracks during the transects. In total, 36 points of entry into the forest interior were discovered and used. These were then selected randomly for surveying using a random number generator, which drew one point at a time from the 36 points. All points were surveyed over the course of the study. The same set of paired points was never surveyed twice on the same day. These paired points were approximately evenly spaced along a 50 km transect (mean 1.3 km, s.e. 0.2 km, range 0.1–4.8 km). The two sites separated by only 100 m were not both used on the same day. All points were situated in mature karri forest.
Methods of observation
We designed the study to use aural identifications (bird calls) because birds were often hidden by dense vegetation. All observations were made by one person (GF). These identifications were double-checked by visual observations when possible, which in all cases confirmed that bird calls were not misinterpreted. In most cases birds were seen though this was not quantified. The point counts were conducted from first light to approximately 0900 hours, after which time bird calls decreased markedly and thus surveying was ceased. All point counts were area-limited to a 25 m circle laterally with no other distance to the observer recorded. This distance was chosen because it was too hard to see any further through the forest at ground level. Yet birds within this distance were clearly audible. Birds much higher in the karri tree crowns were included though they might have been 75 m away vertically. The tree crowns are more open than the undergrowth, making it possible to see more than 75 m above and identify birds with field binoculars. Points at the roadside clearly had less vegetation, with the road providing greater visual access; since cars were few in the early mornings they did not impede aural identifications. We acknowledge that visual observations are likely to be more reliable at the road points than the forest interior points, but this is why we employed the use of aural cues to identify and count birds.
Statistical analyses
The observed effect size was used to show the general magnitude of the difference between road and forest interior points as a complementary measure to the statistical significance of the P-values. Post hoc power was also computed from the observed effects to complement interpreting the results of the statistical significance.
Definitions: Incidence is the frequency of occurrence of a species. Abundance is the total number of individuals of a species. Paired-samples t-tests were used to compare both the overall incidence and abundance of the assemblage at the road and the forest interior using SPSS ver. 25. Chi-square Goodness of Fit tests were used to compare incidence and abundance by species. Chi-square was corrected for continuity following Yates (1934), Lowry (1998–2019) and Zar (1999); P was non-directional and d.f. = 1 in all cases. Chi-square tests were performed with the Vassar Website for Statistical Computation (Lowry 1998–2019). Power (1−β) and effect size (w) were quantified using G*Power 3.1.9.4. Cohen (1988) defines the Chi-square effect size (w) as the square root of the standardised Chi-square statistic, stating that the effect is small at 0.10, medium at 0.30 and large at 0.50. A Bonferroni correction was excluded from the multiple Chi-square tests following Moran (2003), who argued against this correction on mathematical, logical and practical grounds, finding that it can lead to falsely accepting null hypotheses in multiple tests by inflating Type II errors. He proposed reporting all P-values and making reasonable and logical interpretations of the data.
Results
Community
No significant community-wide effects were found between the bird assemblages at the road with the forest interior. A total abundance of 2137 individual birds was recorded over the 100 paired point counts, with 1065 birds recorded by the road and 1072 recorded at the forest interior, which was not significantly different using a paired-samples t-test for the road (M = 17.46, s.d. = 25.52) and the forest interior (M = 17.57, s.d. = 23.71) (t (60) = −0.09, 2-tailed P = 0.93). There was a total of 1186 recorded incidences of species – 614 by the road and 572 at the forest interior, which was not significantly different using a paired-samples t-test for the road (M = 10.06, s.d. = 13.92) and the forest interior (M = 9.38, s.d. = 12.45) (t (60) = 1.04, 2-tailed P = 0.30) (Table 1). In total, 61 species were recorded, 56 by the road and 53 at the forest interior (Table 1).
| Species | Incidence | Abundance | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rd | 400 m | Total n | χ2 | P | Effect size (w) | Power (1−β) | Rd | 400 m | Total n | χ2 | P | Effect size (w) | Power (1−β) | ||
| Australian shelduck Tadorna tadornoides | 1 | 0 | 1 | 0.00 | 1.00 | 1.00 | 0.17 | 2 | 0 | 2 | 0.50 | 0.48 | 1.00 | 0.29 | |
| Australian wood duck Chenonetta jubata | 2 | 0 | 2 | 0.50 | 0.48 | 1.00 | 0.29 | 2 | 0 | 2 | 0.50 | 0.48 | 1.00 | 0.29 | |
| Pacific black duck Anas superciliosa | 1 | 0 | 1 | 0.00 | 1.00 | 1.00 | 0.17 | 2 | 0 | 2 | 0.50 | 0.48 | 1.00 | 0.29 | |
| Common bronzewing Phaps chalcoptera A | 1 | 4 | 5 | 0.80 | 0.37 | 0.60 | 0.27 | 1 | 4 | 5 | 0.80 | 0.37 | 0.60 | 0.27 | |
| Brush bronzewing P. elegans A | 0 | 1 | 1 | 0.00 | 1.00 | 1.00 | 0.17 | 0 | 2 | 2 | 0.50 | 0.48 | 1.00 | 0.29 | |
| Tawny frogmouth Podargus strigoides | 2 | 0 | 2 | 0.50 | 0.48 | 1.00 | 0.29 | 2 | 0 | 2 | 0.50 | 0.48 | 1.00 | 0.29 | |
| Red-tailed black-cockatoo Calyptorhynchus banksii A | 5 | 4 | 9 | 0.00 | 1.00 | 0.11 | 0.06 | 16 | 11 | 27 | 0.60 | 0.44 | 0.19 | 0.16 | |
| Carnaby’s black-cockatoo Zanda latirostris A | 1 | 0 | 1 | 0.00 | 1.00 | 1.00 | 0.17 | 1 | 0 | 1 | 0.00 | 1.00 | 1.00 | 0.17 | |
| Baudin’s black-cockatoo Z. baudinii A | 19 | 15 | 34 | 0.26 | 0.61 | 0.12 | 0.07 | 59 | 35 | 94 | 5.62 | 0.02* | 0.26 | 0.70 | |
| Purple-crowned lorikeet Glossopsitta porphyrocephala | 4 | 1 | 5 | 0.80 | 0.37 | 0.60 | 0.27 | 6 | 13 | 19 | 1.90 | 0.17 | 0.37 | 0.36 | |
| Western rosella Platycercus icterotis A | 14 | 19 | 33 | 0.76 | 0.49 | 0.15 | 0.14 | 30 | 30 | 60 | 0.00 | 1.00 | 0.00 | 0.05 | |
| Australian ringneck Barnardius zonarius A | 42 | 31 | 73 | 1.36 | 0.24 | 0.15 | 0.25 | 83 | 53 | 136 | 6.18 | 0.01* | 0.22 | 0.73 | |
| Red-capped parrot Purpureicephalus spurius A | 3 | 3 | 6 | 0.00 | 1.00 | 0.00 | 0.05 | 4 | 5 | 9 | 0.00 | 1.00 | 0.11 | 0.06 | |
| Horsfield’s bronze-cuckoo Chalcites basalis | 1 | 1 | 2 | 0.00 | 1.00 | 0.00 | 0.05 | 1 | 2 | 3 | 0.00 | 1.00 | 0.00 | 1.00 | |
| Pallid cuckoo Heteroscenes pallidus | 3 | 1 | 4 | 0.26 | 0.61 | 0.50 | 0.17 | 3 | 2 | 5 | 0.00 | 1.00 | 0.00 | 1.00 | |
| Fan-tailed cuckoo Cacomantis flabelliformis | 14 | 15 | 29 | 0.00 | 1.00 | 0.03 | 0.05 | 18 | 16 | 34 | 0.02 | 0.89 | 0.06 | 0.06 | |
| Southern boobook Ninox boobook | 2 | 0 | 2 | 0.50 | 0.48 | 1.00 | 0.29 | 2 | 0 | 2 | 0.50 | 0.48 | 1.00 | 0.29 | |
| Barn owl Tyto alba | 1 | 0 | 1 | 0.00 | 1.00 | 1.00 | 0.17 | 1 | 0 | 1 | 0.00 | 1.00 | 1.00 | 0.17 | |
| Laughing kookaburra Dacelo novaeguineae | 39 | 24 | 63 | 3.12 | 0.08 | 0.24 | 0.47 | 58 | 42 | 100 | 2.26 | 0.13 | 0.16 | 0.36 | |
| Sacred kingfisher Todiramphus sanctus | 0 | 1 | 1 | 0.00 | 1.00 | 1.00 | 0.17 | 0 | 1 | 1 | 0.00 | 1.00 | 1.00 | 0.17 | |
| Rufous treecreeper Climacteris rufus | 7 | 15 | 22 | 2.22 | 0.14 | 0.52 | 0.68 | 8 | 16 | 24 | 2.26 | 0.15 | 0.33 | 0.37 | |
| Red-winged fairy-wren Malurus elegans | 36 | 27 | 63 | 1.02 | 0.31 | 0.14 | 0.21 | 62 | 56 | 118 | 0.22 | 0.64 | 0.05 | 0.09 | |
| White-browed scrubwren Sericornis frontalis | 27 | 31 | 58 | 0.16 | 0.69 | 0.07 | 0.08 | 43 | 43 | 86 | 0.00 | 1.00 | 0.00 | 0.05 | |
| Weebill Smicrornis brevirostris | 2 | 2 | 4 | 0.00 | 1.00 | 0.00 | 0.05 | 3 | 4 | 7 | 0.00 | 1.00 | 0.14 | 0.07 | |
| Western gerygone Gerygone fusca | 1 | 1 | 2 | 0.00 | 1.00 | 0.00 | 0.05 | 1 | 1 | 2 | 0.00 | 1.00 | 0.00 | 0.05 | |
| Yellow-rumped thornbill Acanthiza chrysorrhoa | 1 | 0 | 1 | 0.00 | 1.00 | 1.00 | 0.17 | 1 | 0 | 1 | 0.00 | 1.00 | 1.00 | 0.17 | |
| Inland thornbill A. apicalis | 33 | 38 | 71 | 0.35 | 0.64 | 0.07 | 0.09 | 55 | 57 | 112 | 0.00 | 1.00 | 0.02 | 0.05 | |
| Western thornbill A. inornata | 2 | 3 | 5 | 0.00 | 1.00 | 0.02 | 0.05 | 2 | 5 | 7 | 0.58 | 0.45 | 0.43 | 0.21 | |
| Spotted pardalote Pardalotus punctatus | 1 | 2 | 3 | 0.00 | 1.00 | 0.33 | 0.09 | 3 | 3 | 6 | 0.00 | 1.00 | 0.00 | 0.05 | |
| Striated pardalote P. striatus | 20 | 14 | 34 | 1.06 | 0.39 | 0.18 | 0.18 | 22 | 24 | 46 | 0.00 | 1.00 | 0.04 | 0.06 | |
| Western spinebill Acanthorhynchus superciliosus | 1 | 2 | 3 | 0.00 | 1.00 | 0.33 | 0.09 | 1 | 2 | 3 | 0.00 | 1.00 | 0.00 | 1.00 | |
| Singing honeyeater Gavicalis virescens | 2 | 1 | 3 | 0.00 | 1.00 | 0.33 | 0.09 | 3 | 1 | 4 | 0.26 | 0.61 | 0.50 | 0.17 | |
| Western wattlebird Anthochaera lunulata | 2 | 3 | 5 | 0.00 | 1.00 | 0.02 | 0.05 | 2 | 26 | 28 | 18.90 | 0.00* | 0.86 | 0.99 | |
| Red wattlebird A. carunculata | 51 | 46 | 97 | 0.16 | 0.69 | 0.05 | 0.41 | 100 | 83 | 183 | 1.40 | 0.24 | 0.09 | 0.24 | |
| Brown honeyeater Lichmera indistincta | 0 | 1 | 1 | 0.00 | 1.00 | 1.00 | 0.17 | 0 | 1 | 1 | 0.00 | 1.00 | 1.00 | 0.17 | |
| New Holland honeyeater Phylidonyris novaehollandiae | 49 | 34 | 83 | 2.36 | 0.12 | 0.18 | 0.36 | 98 | 104 | 202 | 0.12 | 0.73 | 0.03 | 0.07 | |
| White-cheeked honeyeater P. niger | 10 | 12 | 22 | 0.04 | 0.84 | 0.09 | 0.07 | 19 | 23 | 42 | 0.22 | 0.64 | 0.10 | 0.09 | |
| Brown-headed honeyeater Melithreptus brevirostris | 1 | 5 | 6 | 1.50 | 0.22 | 0.67 | 0.37 | 1 | 7 | 8 | 3.12 | 0.08 | 0.75 | 0.56 | |
| White-naped honeyeater M. lunatus | 22 | 20 | 42 | 0.02 | 0.89 | 0.05 | 0.06 | 52 | 47 | 99 | 0.16 | 0.69 | 0.05 | 0.08 | |
| White-browed babbler Pomatostomus superciliosus | 2 | 7 | 9 | 1.78 | 0.18 | 0.56 | 0.38 | 3 | 26 | 29 | 16.68 | 0.00* | 0.79 | 0.99 | |
| Varied sittella Daphoenositta chrysoptera | 1 | 2 | 3 | 0.00 | 1.00 | 0.33 | 0.09 | 7 | 6 | 13 | 0.00 | 1.00 | 0.08 | 0.06 | |
| Black-faced cuckoo-shrike Coracina novaehollandiae | 2 | 1 | 3 | 0.00 | 1.00 | 0.33 | 0.09 | 4 | 3 | 7 | 0.00 | 1.00 | 0.14 | 0.07 | |
| Crested shrike-tit Falcunculus leucogaster | 4 | 10 | 14 | 1.78 | 0.18 | 0.43 | 0.36 | 6 | 15 | 21 | 3.04 | 0.08 | 0.43 | 0.50 | |
| Golden whistler Pachycephala pectoralis | 42 | 35 | 77 | 0.64 | 0.50 | 0.09 | 0.13 | 65 | 63 | 128 | 0.00 | 1.00 | 0.02 | 0.05 | |
| Grey shrike-thrush Colluricincla harmonica | 23 | 36 | 59 | 2.44 | 0.19 | 0.22 | 0.39 | 36 | 50 | 86 | 1.96 | 0.16 | 0.16 | 0.33 | |
| Dusky woodswallow Artamus cyanopterus | 1 | 1 | 2 | 0.00 | 1.00 | 0.00 | 0.05 | 1 | 2 | 3 | 0.00 | 1.00 | 0.00 | 1.00 | |
| Grey butcherbird Cracticus torquatus | 1 | 1 | 2 | 0.00 | 1.00 | 0.00 | 0.05 | 1 | 2 | 3 | 0.00 | 1.00 | 0.00 | 1.00 | |
| Australian magpie Gymnorhina tibicen | 8 | 4 | 12 | 0.76 | 0.38 | 0.33 | 0.21 | 15 | 6 | 21 | 3.04 | 0.08 | 0.43 | 0.50 | |
| Grey currawong Strepera versicolor | 6 | 2 | 8 | 1.12 | 0.29 | 0.50 | 0.29 | 9 | 4 | 13 | 1.24 | 0.27 | 0.38 | 0.28 | |
| Grey fantail Rhipidura albiscapa | 28 | 38 | 66 | 1.52 | 0.27 | 0.15 | 0.23 | 43 | 72 | 115 | 6.82 | 0.01* | 0.25 | 0.77 | |
| Willie wagtail R. leucophrys | 3 | 1 | 4 | 0.26 | 0.61 | 0.50 | 0.17 | 3 | 1 | 4 | 0.26 | 0.61 | 0.50 | 0.17 | |
| Australian raven Corvus coronoides | 30 | 14 | 44 | 5.12 | 0.02* | 0.36 | 0.67 | 41 | 16 | 57 | 10.10 | 0.00* | 0.44 | 0.91 | |
| Restless flycatcher Myiagra inquieta | 1 | 1 | 2 | 0.00 | 1.00 | 0.00 | 0.05 | 1 | 2 | 3 | 0.00 | 1.00 | 0.00 | 1.00 | |
| Scarlet robin Petroica boodang | 8 | 6 | 14 | 0.08 | 0.78 | 0.14 | 0.08 | 12 | 9 | 21 | 4.04 | 0.44 | 0.14 | 0.10 | |
| Red-capped robin P. goodenovii | 0 | 1 | 1 | 0.00 | 1.00 | 1.00 | 0.17 | 0 | 2 | 2 | 0.50 | 0.48 | 1.00 | 0.29 | |
| Western yellow robin Eopsaltria griseogularis | 0 | 3 | 3 | 0.00 | 1.00 | 1.00 | 0.41 | 0 | 3 | 3 | 0.00 | 1.00 | 1.00 | 0.41 | |
| White-breasted robin Quoyornis georgianus | 17 | 20 | 37 | 0.01 | 0.75 | 0.08 | 0.08 | 19 | 26 | 45 | 0.80 | 0.37 | 0.16 | 0.18 | |
| Silvereye Zosterops lateralis | 7 | 6 | 13 | 0.00 | 1.00 | 0.08 | 0.06 | 13 | 35 | 48 | 9.18 | 0.00* | 0.46 | 0.89 | |
| Tree martin Petrochelidon nigricans | 4 | 3 | 7 | 0.00 | 1.00 | 0.14 | 0.07 | 15 | 7 | 22 | 2.22 | 0.14 | 0.36 | 0.40 | |
| Mistletoebird Dicaeum hirundinaceum | 2 | 1 | 3 | 0.00 | 1.00 | 0.33 | 0.09 | 2 | 1 | 3 | 0.26 | 0.61 | 0.00 | 1.00 | |
| Red-eared firetail Stagonopleura oculata A | 1 | 2 | 3 | 0.00 | 1.00 | 0.33 | 0.09 | 2 | 2 | 4 | 0.00 | 1.00 | 0.00 | 0.05 | |
Column headings and abbreviations: Rd, point counts at the road; 400 m, point counts 400 m into forest; Total n, the total incidences and abundances recorded at the road and interior; χ2, Chi-square; P, * denotes a significant effect; Effect size (w), (small ≈ 0.10, medium ≈ 0.30 and large ≈ 0.50); Power (1−β), the probability that the test correctly rejects the null hypothesis.
Species
The null hypothesis of equal incidence was rejected for only one of the 61 species (1.6%), the Australian raven (Corvus coronoides), which occurred more frequently at the road. Equal abundance was rejected for seven of the 61 species (11.5%) (Table 1). Of these, three (4.9%) had greater abundances by the road and four (6.6%) had greater abundances in the forest interior. However, contradictory results were noted for western wattlebird (Anthochaera lunulata) and silvereye (Zosterops lateralis) (both with greater abundance in the forest interior). Both species had highly significant P-values for their measures of abundance at P < 0.01; yet for incidence both species were non-significant at P = 1.00, with very low observed effect values of 0.02 and 0.08 respectively (Table 1).
Species absent from either road or forest interior
In total, 13 species of 61 observed species (21.3%) were either absent from the road or the forest interior: five (8.2%) were absent from the road and eight (13.1%) were absent from the forest interior. None had total incidence or abundance counts > 3 and none had their null hypotheses rejected (Table 1).
Discussion
Lack of an assemblage-level effect – a pre-adaptation from natural heterogeneity
Overall, the impact of the road on the avifaunal composition of old-growth karri forest was relatively small compared to the findings of other studies, with only seven of the 61 species (11.5%) affected by the road. In comparison, Reijnen et al. (1995) found 60% of bird species to have reduced density at roads, Pocock and Lawrence (2005) found 67% of bird species demonstrated either edge or interior preferences in relation to roads, Morelli et al. (2015) determined that the percentage of birds demonstrating positive and negative responses were similar, and Peris and Pescador (2004) determined that 45% of bird species had statistically different breeding densities among different types of roads. In this study the overall abundance and diversity of birds was not significantly altered by the road, although a small number of species were affected. Notably a study of clear-felled edges related to logging in the same karri forests (Atkinson 2003) also failed to find a distinct avifaunal assemblage in logged gaps of a similar size to the gap caused by the road in this study; instead, finding that the gap assemblages matched the mature karri forest assemblage. Yet, in the current study, an ad hoc explanation for the lack of an assemblage-level effect would be that the road was narrow (7 m plus 4 m verges) and had been in place for 87 years (sealed for 37 years) and the canopy frequently extended fully across the road, thus the forest was contiguous and the road width did not pose a significant barrier or disturbance to the assemblage (Fig. 2). Additionally, the age of the road may be important, with some birds developing behavioural adaptations (Husby 2017), whereas some species have evolved physical characteristics that help them avoid collisions with vehicles (Brown and Bomberger Brown 2013). However, in this study, the evolution of physical traits across the assemblage seems unlikely. Beyond these hypotheses we suggest that the natural heterogeneity found in eucalypt forests has aided the birds as a pre-adaptation to the presence of this road. We argue that the birds were adapted to a heterogeneous habitat of streams and natural forest heterogeneity. Heterogeneity is typical of eucalypt forests in general (Moore et al. 1993; Barrett et al. 1994; Wardell-Johnson and Horwitz 1996). Pre-adaptation is not a novel proposition. A parallel yet broader global finding was provided by Betts et al. (2019) when they proposed their extinction filter, which mediates the effects of fragmentation. They found that fragmentation-sensitive species were three times more common in regions with low rates of historical disturbance. They predicted that species that have evolved and survived in disturbed environments will persist in the face of new disturbances such as habitat loss and fragmentation, including adapting to edge habitats. Natural disturbances such as patchy wildfire may exert such evolutionary pressure and with the natural heterogeneity present in eucalypt forests this may provide the pre-adaptation to the road seen in this study.
Fahrig and Rytwinski (2009) reviewed the effect of roads on animal abundance and found many studies without a statistically significant effect. Yet, in their study ‘negative effects’ still outweighed ‘no effect’ by about 2:1 (114:56), which places the current study in the minority. Some Australian studies have found disparate effects. Maron and Kennedy (2007) did not find a difference in avian assemblages between secondary roads and forest interior sites, in either cypress pine (Callitris glaucophylla) forest or spotted gum (Corymbia citriodora) forest. However, they did find differences in avian assemblages between their forest types, highlighting that forest heterogeneity had a greater impact than roads. Pell and Jones (2015) found roads may act as barriers to small insectivorous birds less suited to direct flight, in subtropical eucalypt forest. Such birds preferentially used a forested overpass, avoiding direct crossings of a major arterial road. In support, they found stronger fliers such as silvereye (Z. lateralis) and striated pardalote (Pardalotus striatus) made more direct flights across the road, although they also made use of the forested overpass. In stark contrast to other studies, Pocock and Lawrence (2005) found 9% of bird species occurred only at the road and 21% occurred only in the forest interior. Yet their study was limited, spatially and temporally, to very open and fragmented box–ironbark eucalypt woodland where visual and aural impacts from the road could penetrate quite easily into the interior. Overall, habitat quality and heterogeneity appear to be more important in controlling avian assemblages than roads, which is in general agreement with this study’s findings for the South Western Highway and karri forest.
Species exhibiting positional preferences
The Australian raven was the only species to show a significant habitat preference for the road by both species incidence and abundance counts. It benefits from carrion, including invertebrates, along the road and it is known to have benefitted from roads cut into forests (Rowley and Vestjens 1973; Abbott 1999; Fulton et al. 2008) and in built-up areas (Sazima 2020; Fulton 2021). It was the most commonly detected bird on roadsides in northern jarrah (E. marginata) forest, in south-western Australia, again associated with carrion (Fulton et al. 2008).
Two other introduced and invasive species were present in this study, Australian magpie (Gymnorhina tibicen) and laughing kookaburra (Dacelo novaeguineae), although neither were statistically more frequent in either habitat. Yet, Australian magpie had medium to high observed effect sizes favouring the road. Historically, it was not recorded in the primeval karri forest and has introduced itself into the forest via roads and over time (Abbott 1999); it prefers more open habitats (Higgins et al. 2006). The laughing kookaburra is an introduced and invasive species in south-western Australia. It was introduced in 1897 (Long 1972) and probably reached the study area by 1927 (Johnstone and Storr 1998; Abbott 1999). It is a perch-pounce predator that benefits from the cleared and open road verges (Recher et al. 1985). Yet in contrast to expectations it was not more common at the road, its low observed effect sizes agreeing with a lack of statistical significance.
Only two of the nine granivorous species identified in this study, Baudin’s black-cockatoo (Zanda baudinii) and Australian ringneck (Barnardius zonarius), were significantly more abundant at the road. Dehiscent grain from endemic trees is more easily detected on roads and their verges, through a lack of dense vegetation (see argument in Fulton et al. (2008), based on Stoneman (1992)). The expectation that granivorous species would be more common and exhibit a clear preference for the road did not eventuate for the other seven granivorous species present. The two species that were significantly more abundant at the road were also the most numerously detected granivorous species. Baudin’s black-cockatoo was recorded more at the road because it cries loudly as it follows roads through the forest. It was observed on the ground twice, both times on road verges: once to drink from pooled water and once to swallow small gravel to assist with digestion (unpubl. data), which indicates that it does use the road as a resource, making it vulnerable to roadkill. The Australian ringneck was the third most abundantly detected species in the study and the most abundantly detected granivorous species.
Of the four species that showed significant preferences for the forest interior, two species were based on the very low observed effect sizes for their incidence counts and two species were unquestioned and discussed, as follows: the white-browed babbler (Pomatostomus superciliosus) exhibited the greatest proportional preference of any species, to either habitat, being 8.7 times more abundant at the forest interior points. Cale (2003), found the white-browed babbler’s reproductive success was dependent on remnant size and shape, with road verges providing the lowest quality habitat, which suggests that roads are unsuitable for this species and are thus avoided. The other species clearly favouring the forest interior, the grey fantail (Rhipidura albiscapa), has had the understorey of eucalypt forest reported as its primary habitat (Johnstone and Storr 2005). It has also been reported responding negatively to traffic, with lower counts near busier roads and higher counts at less busy roads (Parris and Schneider 2008).
The two species that had questionable significant preferences for the forest interior were the western wattlebird (A. lunulata) and the silvereye (Z. lateralis). The western wattlebird’s significant abundance contrasted with its incidence P-value of 1.00. Likewise, its very low observed effect size for incidence of 0.02 contrasted with its high observed effect size for abundance of 0.86. Similarly, the silvereye’s low observed effect size for incidence of 0.08 contrasted with its high observed effect size for abundance of 0.46. Moreover, the silvereye’s incidence was greater by only one at the road than the forest interior. In both cases, flocks of birds increased their abundance counts while the flock itself stands as a single incidence. Thus, the abundance counts were skewed well above the low incidence counts.
The only clear pattern in absentees was that they all had very low incidences and abundances ≤3. The absences thus appear to be related to the rarity of the birds that may stem from their paucity in karri forest or difficulty in detecting them. For example, the three nocturnal birds detected at the road were not detected at the forest interior. Yet, this was a diurnal study inappropriate for detecting nocturnal birds. Perhaps greater visibility at the road facilitated their detection there. Notably, the only ducks found in this study were detected taking advantage of water pooled on the road. Pooled water is thought to attract different species to bathe and drink (Linsdale 1929). While such pooled water may not be available as frequently at the forest interior, ducks do use tree hollows in the forests for nesting – despite this, detecting them at the forest interior in breeding hollows, over 10 min point counts, seems much less likely than at the road.
While this study appears extensive with 100 paired point counts, the counts for rare and uncommon birds remained low. Further to this, some contradictory within-species results were seen for statistically significant P-values and observed effect sizes, between incidences and abundances. In general, statistical power was considered low for many species. Notably, unrelated avifaunal studies involving differences between clear-felled gaps and old-growth in karri forests were affected similarly with low statistical power (Wardell-Johnson and Williams 2000; Williams et al. 2001). Future avifaunal studies in karri forest might take this limitation into consideration and increase their sampling efforts to increase statistical power.
Conclusion
The karri forest’s avian assemblage was largely unchanged by the presence of a highway. Some species were affected and these may become the focus of further studies. For example, Baudin’s black-cockatoo (Z. baudinii), is threatened and its use of the road may increase its rate of decline. In general, the road’s narrowness and the presence of the overarching canopy that often fully extended across the road likely facilitated avian movement and abundances. However, the natural heterogeneity of karri and other eucalypt forests may have pre-adapted the avifauna to the presence of a road.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Acknowledgements
We thank Keith Morris, Graeme Liddelow and the Department of Parks and Wildlife who provided subsidised accommodation near the forest. Thank you to Beatrice Tyers of Main Roads Western Australia for advice on the numbers of cars using the road. We thank Denis Saunders for commenting on a draft manuscript. We acknowledge the Nyoongar people (Bibbulman and Minang language groups), the traditional owners of the land where these observations were made.
References
Abbott I (1999) The avifauna of the forests of south-west Western Australia: changes in species composition, distribution and abundance following anthropogenic disturbance. CALM Science Supplement 5, 1-175.
| Google Scholar |
Alamgir M, Campbell MJ, Sloan S, Goosem M, Clements GR, Mahmoud MI, et al. (2017) Economic, socio-political and environmental risks of road development in the tropics. Current Biology 27(20), R1130-R1140.
| Crossref | Google Scholar | PubMed |
Arnold GW, Weeldenburg JR (1990) Factors determining the number and species of birds in road verges in the wheatbelt of Western Australia. Biological Conservation 53, 295-315.
| Crossref | Google Scholar |
Barrett GW, Ford HA, Recher HF (1994) Conservation of woodland birds in a fragmented rural landscape. Pacific Conservation Biology 1, 245-256.
| Crossref | Google Scholar |
Betts MG, Wolf C, Pfeifer M, Banks-Leite C, Arroyo-Rodríguez V, Ribeiro DB, et al. (2019) Extinction filters mediate the global effects of habitat fragmentation on animals. Science 366(6470), 1236-1239.
| Crossref | Google Scholar | PubMed |
Brown CR, Bomberger Brown M (2013) Where has all the road kill gone? Current Biology 23, R233-R234.
| Crossref | Google Scholar | PubMed |
Brown GP, Phillips BL, Webb JK, Shine R (2006) Toad on the road: use of roads as dispersal corridors by cane toads (Bufo marinus) at an invasion front in tropical Australia. Biological Conservation 133, 88-94.
| Crossref | Google Scholar |
Cale PG (2003) The influence of social behaviour, dispersal and landscape fragmentation on population structure in a sedentary bird. Biological Conservation 109, 237-248.
| Crossref | Google Scholar |
Coffin AW (2007) From roadkill to road ecology: a review of the ecological effects of roads. Journal of Transport Geography 15, 396-406.
| Crossref | Google Scholar |
Cooke SC, Balmford A, Johnston A, Newson SE, Donald PF (2020) Variation in abundances of common bird species associated with roads. Journal of Applied Ecology 57, 1271-1282.
| Crossref | Google Scholar |
Erritzoe J, Mazgajski TD, Rejt Ł (2003) Bird casualties on European roads – a review. Acta Ornithologica 38, 77-93.
| Crossref | Google Scholar |
Fahrig L, Rytwinski T (2009) Effects of roads on animal abundance: an empirical review and synthesis. Ecology and Society 14, 21.
| Crossref | Google Scholar |
Forman RTT (1998) Road ecology: a solution for the giant embracing us. Landscape Ecology 13, III-V.
| Crossref | Google Scholar |
Forman RTT, Alexander LE (1998) Roads and their major ecological effects. Annual Review of Ecology and Systematics 29, 207-231.
| Crossref | Google Scholar |
Forman RTT, Friedman DS, Fitzhenry D, Martin JD, Chen AS, Alexander LE (1997) Ecological effects of roads: toward three summary indices and an overview for North America. In ‘Proceedings habitat fragmentation and infrastructure’. (Eds K Canters, A Piepers, D Hendriks-Heersma) pp. 40–54. (Ministry of Transport, Public Works and Water Management: Delft, The Netherlands)
Fulton GR (2013) Woodland birds persisting in least disturbed environment: birds of Dryandra Woodland 1953–2008. Pacific Conservation Biology 19, 58-75.
| Crossref | Google Scholar |
Fulton GR (2021) Matching theories on kleptoparasitism to a complex avian event in Perth, Western Australia. Journal of the Royal Society of Western Australia 104, 41-44.
| Google Scholar |
Fulton GR, Lawson J (2021) Birds respond to woodland type, soil and mesic gradients in heterogeneous woodlands at Dryandra. Australian Journal of Zoology 68, 55-61.
| Crossref | Google Scholar |
Fulton GR, Smith M, Na CM, Takahashi S (2008) Road ecology from a road-side assemblage of forest birds in south-western Australia. Ornithological Science 7, 47-57.
| Crossref | Google Scholar |
Goosem M (2001) Effects of tropical rainforest roads on small mammals: inhibition of crossing movements. Wildlife Research 28, 351-364.
| Crossref | Google Scholar |
Goosem M (2007) Fragmentation impacts caused by roads through rainforests. Current Science 93, 1587-1595.
| Google Scholar |
Husby M (2017) Traffic influence on roadside bird abundance and behaviour. Acta Ornithologica 52, 93-103.
| Crossref | Google Scholar |
Jones DN, Bond ARF (2010) Road barrier effect on small birds removed by vegetated overpass in South East Queensland. Ecological Management & Restoration 11, 65-67.
| Crossref | Google Scholar |
Laurance WF, Goosem M, Laurance SGW (2009) Impacts of roads and linear clearings on tropical forests. Trends in Ecology & Evolution 24, 659-669.
| Crossref | Google Scholar | PubMed |
Lepschi BJ (1992) Birds killed on a primary road in southern New South Wales. Corella 16, 75-77.
| Google Scholar |
Linsdale JM (1929) Roadways as they affect bird life. The Condor 31, 143-145.
| Crossref | Google Scholar |
Lowry R (1998–2019) Concepts and applications of inferential statistics. Available at http://faculty.vassar.edu/lowry/webtext.html [Viewed 5 August 2017]
Main Roads Western Australia (2014) South west traffic digest. Available at https://www.mainroads.wa.gov.au/Documents/Traffic%20Digest.u_3169031r_1n_D11%5E2398971.PDF [Viewed 25 October 2014]
Maron M, Kennedy S (2007) Roads, fire and aggressive competitors: determinants of bird distribution in subtropical production forests. Forest Ecology and Management 240, 24-31.
| Crossref | Google Scholar |
Møller AP, Diaz M, Flensted-Jensen E, Grim T, Ibáñez-Álamo JD, Jokimäki J, et al. (2012) High urban population density of birds reflects their timing of urbanization. Oecologia 170, 867-875.
| Crossref | Google Scholar | PubMed |
Moore ID, Norton TW, Williams JE (1993) Modelling environmental heterogeneity in forested landscapes. Journal of Hydrology 150, 717-747.
| Crossref | Google Scholar |
Moran MD (2003) Arguments for rejecting the sequential Bonferroni in ecological studies. Oikos 100, 403-405.
| Crossref | Google Scholar |
Morelli F, Beim M, Jerzak L, Jones D, Tryjanowski P (2014) Can roads, railways and related structures have positive effects on birds? – A review. Transportation Research Part D: Transport and Environment 30, 21-31.
| Crossref | Google Scholar |
Morelli F, Jerzak L, Pruscini F, Santolini R, Benedetti Y, Tryjanowski P (2015) Testing bird response to roads on a rural environment: a case study from Central Italy. Acta Oecologica 69, 146-152.
| Crossref | Google Scholar |
Parris KM, Schneider A (2008) Impacts of traffic noise and traffic volume on birds of roadside habitats. Ecology and Society 14, 29.
| Crossref | Google Scholar |
Pell S, Jones D (2015) Are wildlife overpasses of conservation value for birds? A study in Australian sub-tropical forest, with wider implications. Biological Conservation 184, 300-309.
| Crossref | Google Scholar |
Peris SJ, Pescador M (2004) Effects of traffic noise on paserine populations in Mediterranean wooded pastures. Applied Acoustics 65, 357-366.
| Crossref | Google Scholar |
Pocock Z, Lawrence RE (2005) How far into a forest does the effect of a road extend? Defining road edge effect in eucalypt forests of South-Eastern Australia. In ‘Proceedings of the 2005 International Conference on Ecology and transportation center for transportation and the environment’. (Eds CL Irwin, P Garrett, KP McDermott) pp. 397–405. (North Carolina State University: Raleigh)
Raiter KG, Prober SM, Possingham HP, Westcott F, Hobbs RJ (2018) Linear infrastructure impacts on landscape hydrology. Journal of Environmental Management 206, 446-457.
| Crossref | Google Scholar | PubMed |
Ramp D, Wilson VK, Croft DB (2006) Assessing the impacts of roads in peri-urban reserves: road-based fatalities and road usage by wildlife in the Royal National Park, New South Wales, Australia. Biological Conservation 129, 348-359.
| Crossref | Google Scholar |
Recher HF, Holmes RT, Schulz M, Shields J, Kavanagh R (1985) Foraging patterns of breeding birds in eucalypt forest and woodland of southeastern Australia. Australian Journal of Ecology 10, 399-419.
| Crossref | Google Scholar |
Reijnen R, Foppen R, Braak CT, Thissen J (1995) The effects of car traffic on breeding bird populations in woodland. III. Reduction of density in relation to the proximity of main roads. Journal of Applied Ecology 32, 187-202.
| Crossref | Google Scholar |
Rowley I, Vestjens WJM (1973) The comparative ecology of Australian corvids. V. Food. CSIRO Wildlife Research 18, 131-155.
| Crossref | Google Scholar |
Šálek M, Svobodová J, Zasadil P (2010) Edge effect of low-traffic forest roads on bird communities in secondary production forests in central Europe. Landscape Ecology 25, 1113-1124.
| Crossref | Google Scholar |
Sazima I (2020) Australian raven (Corvus coronoides) Scavenges on all five major vertebrate groups at Urban Sydney, Southeast Australia. Tropical Natural History 20, 89-94.
| Google Scholar |
Summers PD, Cunnington GM, Fahrig L (2011) Are the negative effects of roads on breeding birds caused by traffic noise? Journal of Applied Ecology 48, 1527-1534.
| Crossref | Google Scholar |
Vestjens WJM (1973) Wildlife mortality on a road in New South Wales. Emu - Austral Ornithology 73, 107-112.
| Crossref | Google Scholar |
Wardell-Johnson G, Horwitz P (1996) Conserving biodiversity and the recognition of heterogeneity in ancient landscapes: a case study from south-western Australia. Forest Ecology and Management 85, 219-238.
| Crossref | Google Scholar |
Wardell-Johnson G, Williams M (2000) Edges and gaps in mature karri forest, south-western Australia: logging effects on bird species abundance and diversity. Forest Ecology and Management 131, 1-21.
| Crossref | Google Scholar |
Ware HE, McClure CJW, Carlisle JD, Barber JR (2015) A phantom road experiment reveals traffic noise is an invisible source of habitat degradation. Proceedings of the National Academy of Sciences 112, 12105-12109.
| Crossref | Google Scholar |
Williams MR, Abbott I, Liddelow GL, Vellios C, Wheeler IB, Mellican AE (2001) Recovery of bird populations after clearfelling of tall open eucalypt forest in Western Australia. Journal of Applied Ecology 38, 910-920.
| Crossref | Google Scholar |
Yates F (1934) Contingency tables involving small numbers and the X2 test. Supplement to the Journal of the Royal Statistical Society 1, 217-235.
| Crossref | Google Scholar |



