Classifying relationships that define interactions between native and invasive species in Australian ecosystems
Joshua L. Gaschk A * and Christofer J. Clemente
A * and Christofer J. Clemente  A B
A B
A School of Science and Engineering, University of the Sunshine Coast, 90 Sippy Downs Drive, Sippy Downs, Qld 4556, Australia.
B Global-Change Ecology Research Group, University of the Sunshine Coast, 90 Sippy Downs Drive, Sippy Downs, Qld 4556, Australia.
Abstract
Australia was isolated for approximately 40 million years from the presence of eutherian predation until the introduction of the dingo (Canis familiaris; 4000 years ago), foxes (Vulpes vulpes; 1871) and feral cats (Felis catus; post-1788). The arrival of these invasive species coincides with the decline and extinction of many native mammals, specifically within the critical weight range (35–5500 g). These extinctions are likely a result of competition and predation, where locomotor performance and the associated behaviours contribute largely to overall fitness. We used the population responses of native fauna in the presence of introduced predators to establish a research framework. Introduction/extinction timelines, predator diets, and prey occurrence were used to identify invasive/native relationships where predation may define the population outcome. We then examined the locomotor performance of these species using current data (maximum speeds). Consumption of prey items does not seem to be associated with the probability of the predator encountering the prey. Dingoes had the most variable mammalian prey of all invasive predators, likely due to higher maximal speeds. Feral cats favour Dasyuridae and smaller species, preying upon these prey groups more than dingoes and foxes. The role of locomotor performance in invasive ecology is not well understood; we identified relationships for further exploration.
Keywords: Australian natives, diet, eutherian predation, invasive ecology, mammals, marsupials, maximum speeds, paired comparisons.
Introduction
Every continent is currently experiencing the threat of invasive species through anthropogenic vectors (Elton 2000). These invaders cannot wholly create a novel niche within this new ecosystem and must establish themselves within the food web (Elton 2000; Pimentel et al. 2001). The invaders’ presence increases the pressure within established trophic levels; horizontally, as they compete with species for resources, and vertically, by either increasing predation pressure on lower trophic levels or increasing mortality rates of species that prey upon them (e.g. poisoning via consumption of cane toads, Rhinella marina) (Phillips and Shine 2006; Clout and Russell 2008). The novelty of the invader in their new environment can aid in their success, especially in island populations (Duffy and Capece 2012). Successful invaders can benefit from being absent throughout the evolutionary processes that have formed their new ecosystem; for example, evolving in ecosystems with stronger evolutionary drivers can afford an advantage over native species (Elton 2000; Flannery 2002; Wilson et al. 2018). The advantage invasive species have is evident in Australian ecosystems, particularly when exploring the impact these have on native species that lie within the critical weight range (35–5500 g) (Burbidge and McKenzie 1989). Feral cats (Felis catus) alone are implicated in the extinction and decline of 97 species of mammals but are also predicted to kill 182.9 million reptiles and almost 400 million birds annually (Woinarski et al. 2015, 2017, 2018). The predation pressure in Australia has not been limited to cats, with foxes (Vulpes vulpes) and dingoes (Canis familiaris) playing a role in the mass extinction and decline of Australian native fauna (Allen and Fleming 2012; Allen and Leung 2012; Doherty et al. 2019; Fairfax 2019). The success of these three introduced predators over native species reveals some level of advantage that has permitted their establishment; however, the nature of their advantage is unclear.
The advantage these invasive predators possess could result from a performance difference in locomotory ability between predator and the native prey (Arnold 1983; Garland and Losos 1994; Irschick and Garland 2001). Locomotion is a function of morphology and physiology, which influences many species’ traits directly: the inability to move effectively in a specific habitat will result in a decrease in fitness (Clemente et al. 2019). Behavioural choices behind locomotion can also affect performance and overall fitness in an environment, for example, choosing to run faster at the cost of increasing the chance of slipping on substrate (Wilson et al. 2014; Wynn et al. 2015; Wheatley et al. 2018). Thus, the role of locomotor performance in predation is likely to be central, as prey and predator engage in pursuits that usually require close to maximal performance for survival. Within pursuit dynamics, speed and acceleration benefit the predator (closing the gap), whereas the ability to manoeuvre unpredictably without sacrificing speed is important for the prey (making the predator miss) (Wilson et al. 2017, 2018). This creates an evolutionary arms race that results in prey and predator increasing in overall athleticism (Wilson et al. 2018). Without tandem evolution, an introduced predator could either be outmatched (lesser athletic ability) or overpowering (greater athletic ability) in comparison to native fauna (Zenni and Nuñez 2013). A native species being overpowered (or outgunned) has previously been described as a level of prey naivety, the other two being inability to perceive the predation risk and inappropriate antipredator responses (both behavioural) (Banks and Dickman 2007). The physical aspect of prey naivety has not been explored within Australian invasive/native relationships and it is unknown whether a performance gap exists.
Australian ecosystems have undergone significant changes through the arrival of both Indigenous Australian and European settlements, including the introduction of associated species (Flannery 2002; Prowse et al. 2014). The purpose of this paper is to explore these relationships, identifying the presence of predator/prey interactions, the outcome of these interactions at a population level, and the locomotor abilities of the involved species. These findings will allow us to determine whether athleticism plays a role in species decline in Australia. Firstly, we explore the historical dynamics of invasive/native interactions. We examine the timing of introductions and extinctions and whether these introductions are associated with the direct decline or extinction of individual species. Next, we explore the present-day dynamics of predator/prey relationships using the dietary data of three invasive predators (cats, foxes, dingoes) and compare these to the occurrence data of prey species to understand predation relative to prey availability. This will reveal whether consumption of prey is based on the probability of encounter. Thirdly, we will characterise and identify predator/prey pairs based on dietary data and the persistence of the prey. Lastly, we explore the potential of locomotor capacity to explain the persistence of prey items in these predator/prey pairs.
Invasion timeline
Within the Pleistocene, humans were the first alien mammals whose arrival in Australia resulted in an observable effect on the ecosystem (Fig. 1) (Saltré et al. 2016). The next significant introduction was the dingo, arriving as camp dogs with Asiatic seafarers approximately 4000 years ago (Prowse et al. 2014). It is suggested that the dingo was never truly domesticated by the Indigenous Australians but formed loose relationships, and this permitted the dingo to disperse over the entire Australian mainland in 100–500 years (Tindale 1974; Gollan 1984). Dingoes were the first introduced placental carnivores, and their arrival has been linked to direct competition and extinction of the similarly sized thylacine (Thylacinus cynocephalus) and the smaller Tasmanian devil (Sarcophilus harrisii) from mainland Australia (Letnic et al. 2012; Prowse et al. 2014). The disappearance of both Dasyuromorphia from mainland Australia occurred at least 25 000 years after the Pleistocene megafaunal extinction and approximately two millennia before European settlement (Fig. 1a). Despite the climate and human intensification foreshadowing the dingo’s arrival, the competition presented by them would have likely impacted the population levels for both these carnivorous native species (Brown 2006; Allen and Fleming 2012; Allen and Leung 2012; Prowse et al. 2014). While still a contentious topic, the survival of these two native species in Tasmania (thylacine until human culling), where dingoes were never introduced, support theories of the dingo’s involvement (Letnic et al. 2012).
The introduction and extinction of mammalian species and megafauna in Australia. (a) The mass extinction of Australian megafauna and (b) the mass extinction surrounding European settlement. Circles represent the most probable time of introduction of the species or actual timing in cases that are known. The bars represent the most probable window of time of introduction. Blue diamonds and bars represent native Australian fauna that have gone extinct (some bars in Years BP represent a genus, not individual species). Orange triangles and bars represent the introduction of invasive mammals, brown triangles and bars represent the arrival of humans. An asterisk indicates extinctions from mainland Australia, still present in Tasmania. More detail on the methods can be found in the Supplementary material Appendix 1.
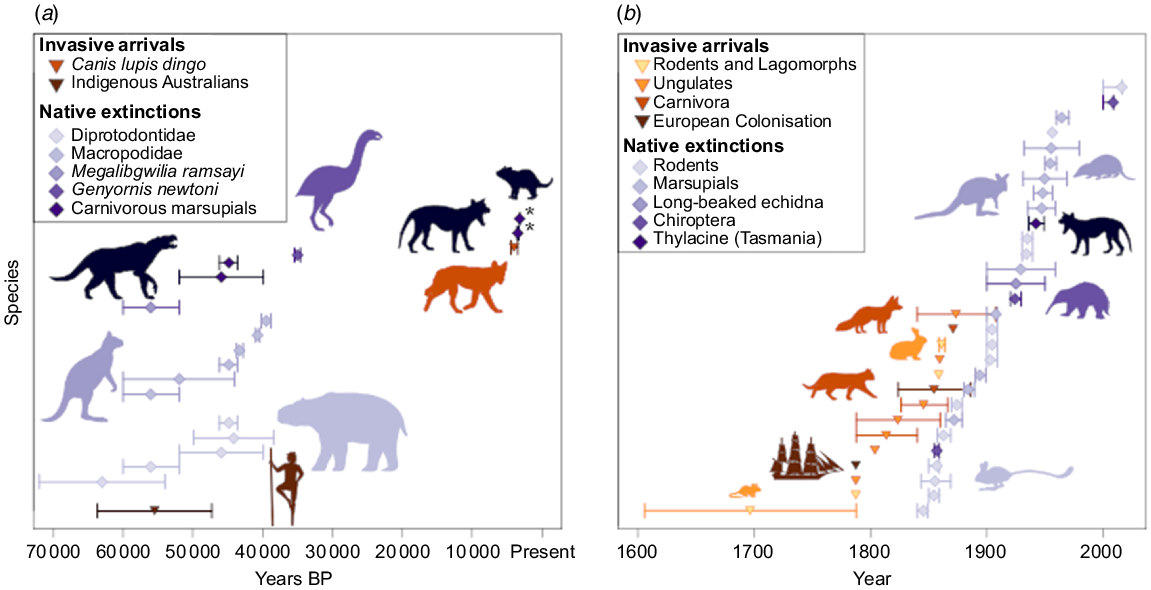
Another major invasion event followed the European settlement of Australia in 1788, along with which came a myriad of invaders (Woinarski et al. 2015). Some were domestic animals (ungulates), others occupied niches alongside human development (rodents and felids), and others were introduced for sport (canids and lagomorphs) (Fig. 1b) (Dickman 1996). The red fox and domestic cat were some of the more impactful introductions on the Australian ecosystems (Chisholm and Taylor 2010). These two species have been implicated in multiple extinctions where they have been introduced (Woinarski et al. 2019). Cats arrived with European settlement in 1788; however, they were not initially released, making the timing of their introduction and dispersal into Australian ecosystems unclear (Dickman 1996). Some have argued that cats were introduced when Dutch sailors became shipwrecked on the shores of Australia; however, there is evidence to suggest that feral populations of cats did not exist in Australia for many years after European settlement (Abbott 2002). While there is no reliable estimate for the dispersal of cats across Australia, records propose there were feral populations in Western Australia by 1907 (Shortridge 1936). Their current distribution, success, and ability to revert to a feral nature could mean a similar dispersal timeline to foxes (Dickman 1996). The red fox was introduced in 1871, at roughly the same time as the rabbits and hares, taking only 100 years to appear on the western side of Arnhem Land (Dickman 1996; Fairfax 2019).
Diets of invasive predators
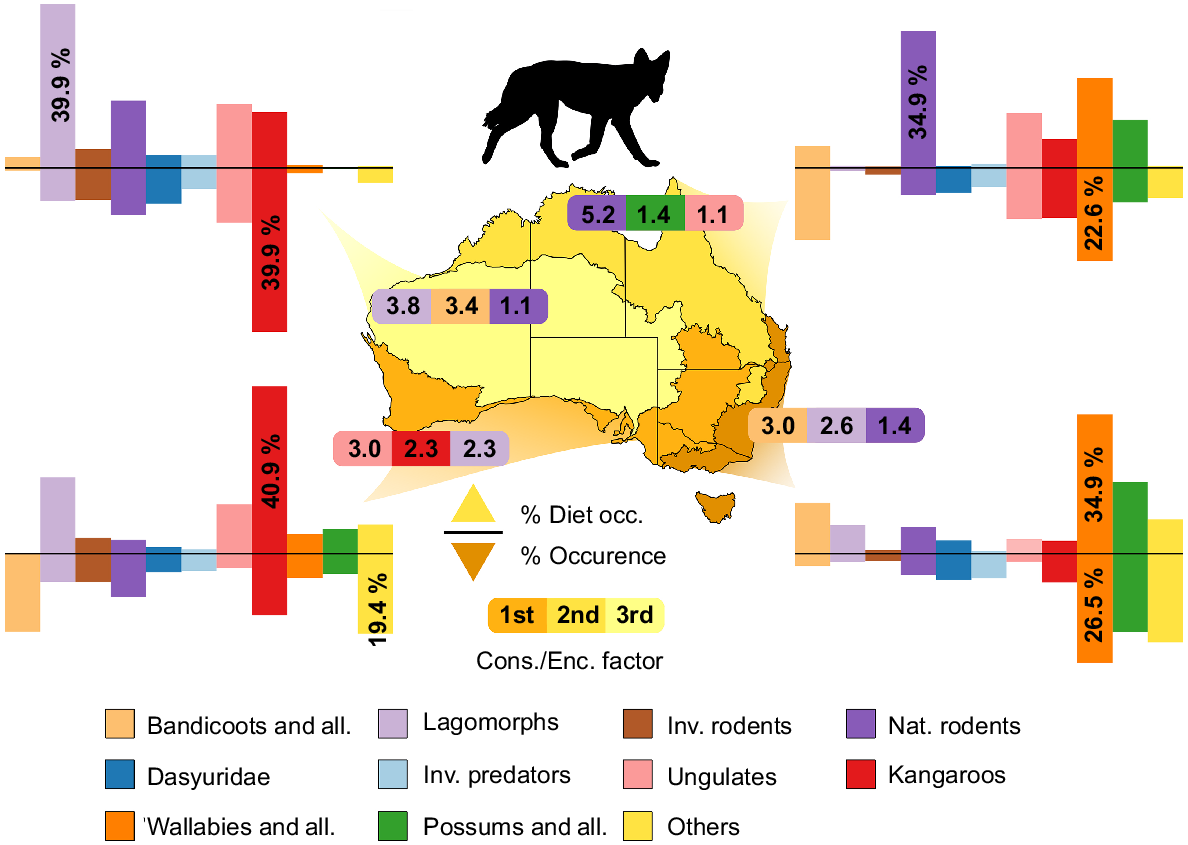
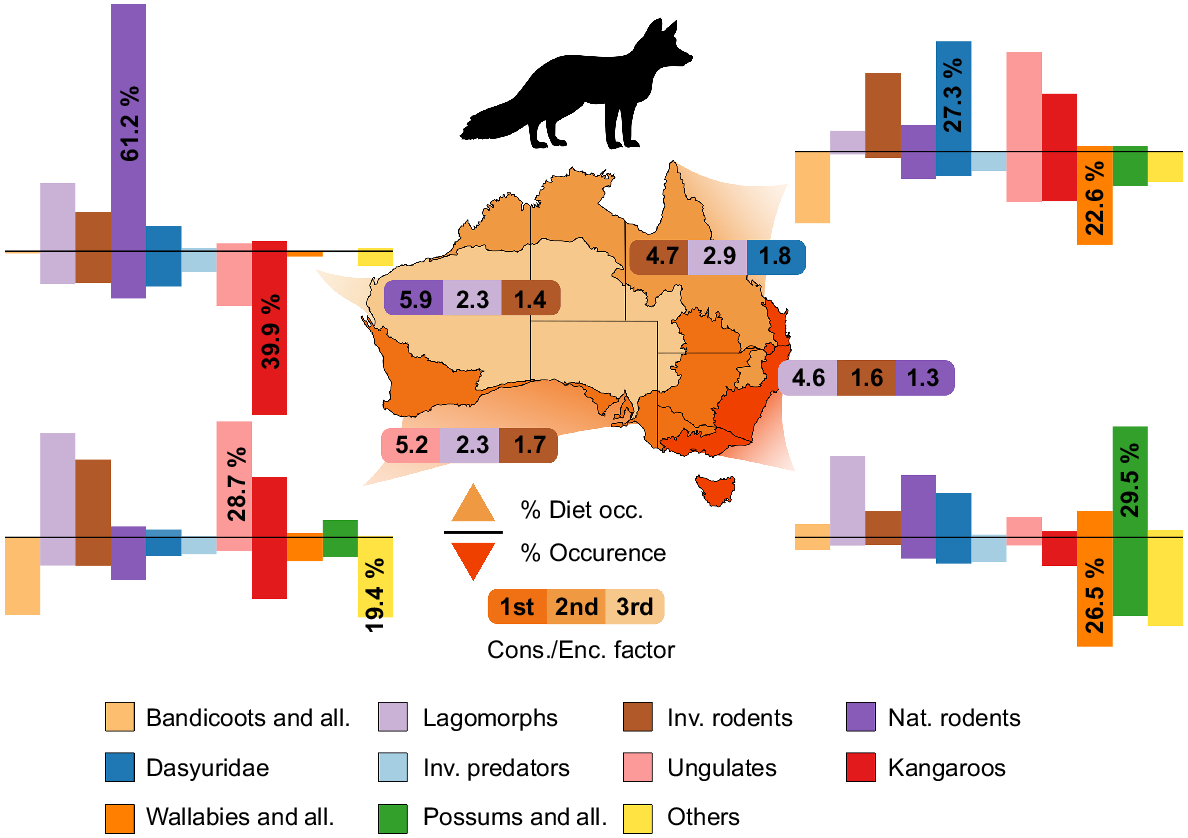
Diet compositions for three invasive predators (dingoes, foxes, and feral cats) were collated from research examining the stomach or scat contents of these species from around Australia (see Supplementary material Appendix 3). A search was performed on both Web of Science and Scopus for each species, and it resulted in 117, 140 and 104 unique studies for dingoes, foxes, and cats, respectively. Resulting searches were refined based on the availability of data, collection methods, and location of study. Overall, the frequency of prey-occurrence data from 105 studies resulted in 90 735 prey-occurrences from 177 different mammalian species in the stomachs or scats of all three invasive predators. Diet data were divided into four bioregions (Arid, Tropical, Temperate/Mediterranean, and Temperate – lighter to darker shades respectively in Figs 2, 3, 4) and were based on the Interim Biogeographical Regionalisation for Australia, ver. 7 (IBRA 2020), configured akin to Kittel and Austin (2016). The data from Mediterranean Forests, Woodland and Scrub were collated with Temperate Grasslands, Savannas and Shrublands because of the minimal dietary studies undertaken in these areas across all species. Tropical/Subtropical areas were also combined for this reason. Prey species were divided into groups Bandicoots and Allies, Dasyuridae, Invasive predators, Lagomorphs, Native Rodents, Invasive Rodents, Kangaroos, Wallabies and Allies, Possums and Allies, Ungulates, and Others (more detail on species inclusion can be found in Table S1). Groups were assigned based on the phylogeny, size, and ecology of the species. Observational occurrence data of all species were obtained from the Atlas of Living Australia in January 2022. Only the observational occurrence data of species that were identified in the diet of cats, foxes, and dingoes were obtained. Data were organised into the same bioregions as the dietary-occurrence data using the decimal longitude and latitude of the sighting. The percentage observational occurrence in each region was calculated for each group (Figs 2, 3, 4). The consumption to encounter factor was calculated by dividing the dietary occurrence by the bioregion occurrence of an individual group, then dividing by the average of all groups in each bioregion. This provides a factor of how much more likely the predator is to consume the prey group per chance of encounter (the Cons./Enc. factor for all groups, invasive predators, and bioregions can be found in Table S2). The numbers represented as ungulates in occurrence data do not include animals from farms.
The composition of mammalian prey in dingo and wild dog (Canis familiaris) diets across four bioregions in Australia: Arid, Tropical, Temperate/Mediterranean, and Temperate (lightest to darkest, respectively). The positive y-axis represents the number of occurrences each group has been recorded in either scats or stomachs of dingoes, and the negative y-axis represents the number of occurrences of each group based on data from Atlas of Living Australia (ALA) and represents an abundance score. Dietary data were compiled from papers listed in Table S1 and Occurrence data were collated from ALA (January 2022). The Cons./Enc. Factor represents how much more likely the predator is to consume the prey group per chance of encounter compared to other groups; the top three in each bioregion are represented. More detailed information regarding how the species were categorised and what species are represented in each group can be found in Table S1.
The composition of mammalian prey in fox (Vulpes vulpes) diets across four bioregions in Australia: Arid, Tropical, Temperate/Mediterranean, and Temperate (lightest to darkest, respectively). The positive y-axis represents the number of occurrences in the diet of foxes, and the negative y-axis represents the number of occurrences in the bioregion. The Cons./Enc. Factor represents how much more likely the predator is to consume the prey group per chance of encounter; the top three in each bioregion are represented. More detail can be found in Table S1.
The composition of mammalian prey in feral cat (Felis catus) diets across four bioregions in Australia: Arid, Tropical, Temperate/Mediterranean, and Temperate (lightest to darkest, respectively). The positive y-axis represents the number of occurrences in the diet of cats, and the negative y-axis represents the number of occurrences in the bioregion. The Cons./Enc. Factor represents how much more likely the predator is to consume the prey group per chance of encounter; the top three in each bioregion are represented. More detail can be found in Table S1.

Bioregion occurrence
The Kangaroo group was the most prominent in Arid bioregions and accounted for 39.9% of all occurrence data (Figs 2, 3, 4). Ungulates and Native Rodent groups showed occurrence percentages of 13.3% and 11.5% respectively, whereas Dasyurids, Lagomorphs, and Invasive Rodents represented between 7 and 9% of occurrences. All other groups were under-represented in Arid zones (<5%). In Tropical bioregions, the Wallaby group represented the most, with 22.6% of all occurrences. Bandicoot, Ungulate and Kangaroo groups were well represented (17.5%, 12.4%, and 12.1%, respectively). Invasive rodents and Lagomorphs seemed to be severely under-represented (1.6%, 0.7%), and all other groups ranged between 4.7 and 8.4%. In Mediterranean bioregions, the Others group was the highest represented (19.4%), with the Bandicoot group a close second (18.9%). Kangaroo and Native Rodent groups were the only other groups significantly represented (>10%); all other groups ranged between 3 and 7% occurrence. Lastly, in Temperate bioregions, Wallaby, Possum and Others groups made up for over 65% of occurrence data (26.5%, 21.5%, and 19%, respectively). All other groups in Temperate bioregions were represented with between 2 and 7% of occurrences. Lagomorphs were not well represented in the occurrence data; whether this was an accurate representation of their occurrence in these ecosystems, or whether there is a bias against reporting their presence is unknown.
Dingo diets
Dingo dietary studies were distributed across all four bioregions, with a large focus on Arid and Temperate zones. Lagomorphs were the most represented in Arid zone dingo diets (39.9%; Fig. 2), with Native Rodents, Ungulates, and Kangaroos contributing significant proportions (16.4%, 15.5%, 13.6%, respectively). The Kangaroo group seemed to be under-represented in dingo diets compared with the occurrence in Arid bioregions, and Lagomorphs were 3.8 times more likely to be consumed relative to their observational occurrence. In Tropical bioregions, the Native rodents group (34.9%) was the most common in dingo diets, but this was closely followed by the Wallaby group (22.9%). Wallabies were greatly represented in the occurrence data, thus their representation in the dietary data is possibly due to a greater availability. The consumption/encounter factor for Native rodents was 5.2 in Tropical bioregions, which suggests that dingoes more often successfully consume rodents after encountering them. Dingoes consumed more wallabies (34.9%) in Temperate bioregions; however, the Bandicoot group (12.8%) had the greatest consumption to encounter factor (3.0). In the Mediterranean bioregion, the Kangaroo group contributed 40.9% to dingo diets whereas Lagomorphs (18.6%) and Ungulates (12.1%) represented the next two most consumed groups. These three groups were also the three most likely to be consumed once encountered in this bioregion. Although solitary dingoes can survive by feeding on rabbits, rodents, carrion, and small marsupials, they are also capable of hunting in small packs to bring down larger prey items like kangaroos and ungulates. Dingo diet and occurrence data reflect the variability in ecology and social/hunting behaviour (Thomson 1992).
Fox diets
The diets of foxes have rarely been studied in Tropical bioregions (n = 77 diet occurrences), which is likely due to their scarcity in these regions; however, they have been heavily studied in Temperate climates (n = 29 546) and so the data in tropical regions might not be an accurate representation (Fig. 3). Native Rodents occurred 61.2% of the time in fox diet studies in Arid bioregions despite them contributing only 11.5% of observational occurrence data in this region. Thus, foxes were 5.9 times more likely to consume Native Rodents when encountering them than the average of that region. In Mediterranean bioregions, 28.7% of all fox stomachs and scats contained animals from the Ungulates group. Lagomorphs, Invasive Rodent, and Kangaroo groups also contributed large proportions to their diet (25.8%, 19.5%, 15.0%, respectively). Consumption of Ungulate and Kangaroo by foxes in this area is probably linked to predation of young, and scavenging (Moore et al. 1966; Lugton 1993; Greentree et al. 2000). As for dingo diets, the diets of foxes were much more variable in Temperate bioregions; the Possum group (29.5%; arboreal) represented the largest proportion of diets in that region; however, their occurrence was also quite high. This group represents arboreal specialists, where morphology that increases stability in arboreal environments likely results in their reduced locomotor ability along the ground (Gaschk et al. 2019). The predator/prey relationship that foxes share with these arboreal species likely represents a common target that is relatively easy to catch than terrestrial specialists like wallabies and bandicoots.
Cat diets
Native rodents were prominent in cat diets in Arid, Tropical and Mediterranean bioregions (40.5%, 39.5%, and 27.1%; Fig. 4), but accounted for only 4.0% of their diet in Temperate areas. In Arid and Tropical bioregions, they also had increased consumption per encounter (3.3 and 3.0, respectively). Dasyurids also featured in the diets of cats in Arid, Tropical and Mediterranean bioregions, showing an increased consumption/encounter in Tropical and Mediterranean bioregions (2.7 and 2.2). Cat dietary data supported the theory that they prefer prey less than 200 g, focusing mainly on the groups comprising smaller-sized species (rodents and dasyurids) (Dickman 1996). However, cats also seemed to be good at consuming Lagomorphs and Invasive rodents. Lagomorphs (49.4%) were the most prominent dietary item in Temperate regions, followed by Invasive Rodents (30.6%), whereas both Dasyurids and Native Rodents were under-represented (2.36%, 1.93%). Lagomorphs were also more prominent in cat diets from Arid and Mediterranean bioregions (24.8%, 29.5%). Interestingly, the highest rate of consumption of Lagomorphs occurred in Temperate forests, where, presumably, the groups with smaller body sizes (native rodents and dasyurids) would benefit from well-developed shrub coverage for protection from predators (McElhinny et al. 2006). The density of shrubs that would increase the protection of Melomys and Antechinus would be less of an advantage to the much larger lagomorph. However, in arid zones where native rodents were much more favoured to lagomorphs, the open spaces between the sparse shrubs may benefit the speeds of lagomorphs and disadvantage native rodents and small dasyurids.
Native responses
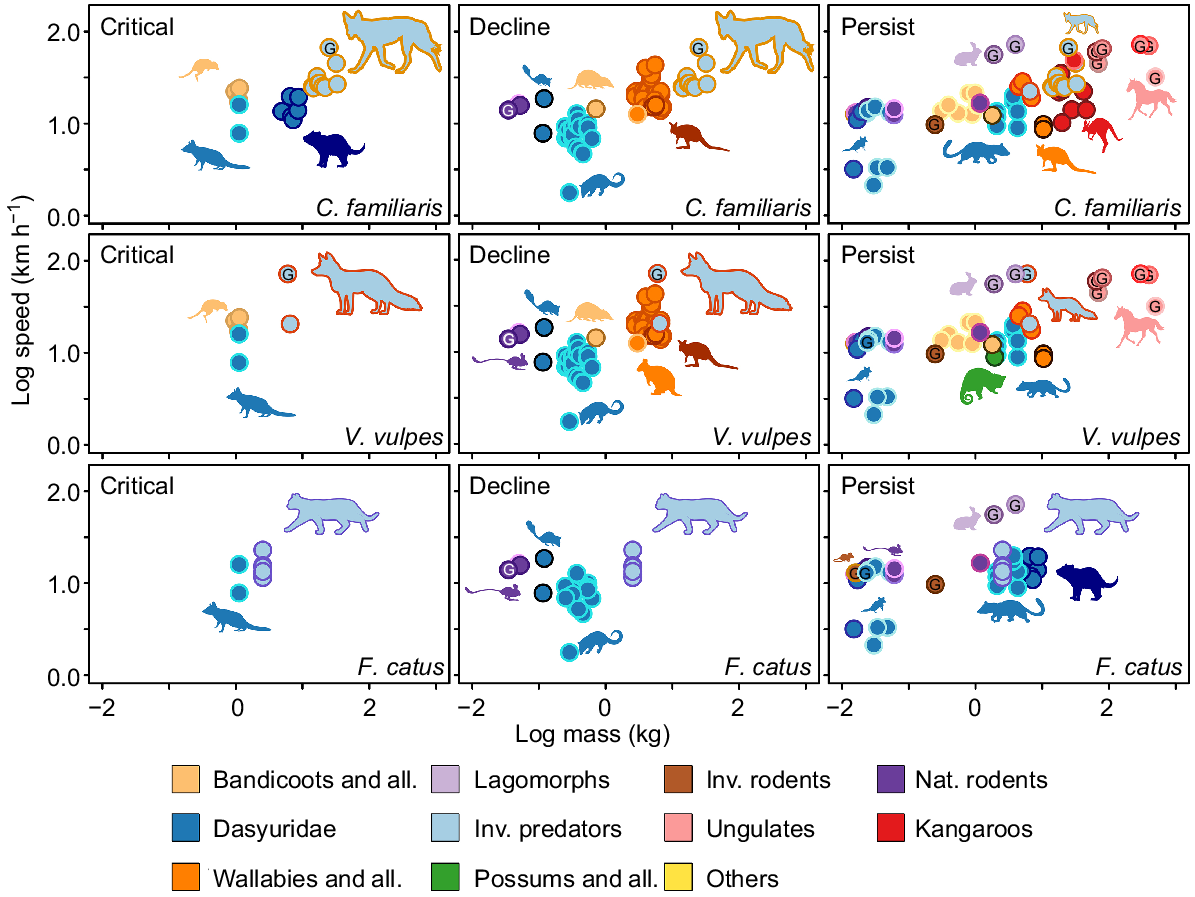
Predation and competition are important in food webs; the response of native species to invaders can reflect this (David et al. 2017). We surveyed the literature and identified Australian native faunal responses in three categories. Range extirpation, literary evidence, IUCN status and predator susceptibility from Radford et al. (2018) were used to determine population responses to invaders. Native populations have been categorised into critical, decline or persist (Fig. 5). ‘Critical’ species held an Extinct or Critical IUCN classification, were severely extirpated, or had been recorded with high susceptibility to a predator. ‘Decline’ species held an Endangered or Vulnerable IUCN status, showed moderate range extirpation, or had a moderate susceptibility to a predator. ‘Persist’ species held a Near Threatened or Least Concern IUCN status, showed minimal range extirpation, and minimal susceptibility despite being a common prey item. Native species were then divided between the three introduced predators (cats, foxes, and dingoes), based on range overlap, diet, invasion/extinction timeline, and records in the literature (i.e. hunting behaviour, predator/prey ecology).
The invasive eutherian predators and their relationship with native fauna that are defining Australian ecosystems. Native fauna were divided into Critical, Decline, or Persist based on their IUCN status, timing and magnitude of extirpation, and predation susceptibility (Radford et al. 2018). The relationships between native and invasive fauna were defined by predator diet within native distribution, distribution overlaps, timing of introduction (invasive) and extinction (native), and reports in the literature of interactions.

Response to dingoes
Because of the protracted presence of dingoes in Australian ecosystems, it can seem difficult to implicate them in the decline of current species (since 1788); however, the impact of dingoes on native fauna has been explored (Allen and Fleming 2012; Allen and Leung 2012). Species such as the pig-footed bandicoot (Chaeropus ecaudatus) were recorded as rare and in decline by Indigenous Australians before European settlement (Seebeck 1990). As discussed previously, the timing of the arrival of C. familiaris implicates it in the extinction of the Tasmanian devil and the thylacine from mainland Australia, its presence increasing competitive pressures on the two large carnivorous marsupials of the time (Guiler 1970; Johnson and Wroe 2003; Fillios et al. 2012; Letnic et al. 2012; White et al. 2018). And the prevalence of wallaby-like prey items in temperate and tropical region dingo diets suggests that the bridled nailtail wallaby (Onychogalea fraenata) could have been a frequent prey item for dingoes. Despite the contention, their effect on native Australian mammals is supported.
Petrogale species are more common in dingo diets than in those of other invasive predators, with most of these species currently restricted to rocky outcrops due to predation (Lavery et al. 2021). The historical ranges of species such as the golden bandicoot (Isoodon auratus) and the greater bilby (Macrotis lagotis) have also reduced significantly (Zenger et al. 2005; Southgate 2007). Both species inhabited ranges that extended into the Arid bioregions of Australia, where dingoes have some success consuming this prey group (Fig. 2).
Other species appear to have retained much of their historical range, despite their presence in the diet of dingoes. These include red-necked wallabies (Macropus rufogriseus), swamp wallabies (Wallabia bicolor), rufous bettongs (Aepyprymnus rufescens) and northern brown bandicoots (Isoodon macrourus) (Fig. 5). These species appear to be similar in form and function to species that have declined or are functionally/regionally extinct.
Locomotor forms, hopping (macropods) and bounding (Perameles and dasyurids), are present in all three levels of population outcome. Therefore, it is likely to be intraspecific variation that would cause some species to decline while others remain largely untouched. Thus, the comparison of locomotor forms would be a great example of the limitations of pursuit and escape in different morphologies, body size, and locomotor forms.
Response to foxes
Foxes are mesopredators with a notoriety that rivals feral cats, particularly in urban, periurban and island habitats (White et al. 2006). They are opportunistic feeders, often responsible for hunting juvenile ungulates and raiding aviaries (Baker et al. 2006; Coman 1973). In addition, both introduced and native mammals still make up large proportions of their diets (Fig. 3). Since their introduction, the radiation of foxes in Australia has been well documented (Dickman 1996). Their dispersal westward was synchronised with extinctions and declines in species such as the toolache wallaby (Macropus greyi), the crescent nail-tail wallaby (Onychogalea lunata), and the numbat (Myrmecobius fasciatus) (Friend 1990; Flannery 2002; Scholtz and DeSantis 2020). The timing of fox dispersal also implicates them in extinctions of various other small–medium sized marsupials including the broad-faced potoroo (Potorous platyops), the eastern hare-wallaby (Lagorchestes leporides), and the desert bandicoot (Perameles eremiana) (Fig. 1) (Burbidge and Woinarski 2016).
Previously reported relationships of foxes with species that have kept some of their former range, including quokkas (Setonix brachyurus), western quolls (Dasyurus geoffroii), or tammar wallabies (Notomacropus eugenii), help understand why foxes are so dominant in the Australian landscapes and whether habitat complexity could be altered to favour native species (Catling and Burt 1995). The relationship between fox, quokka and habitat has already been explored, finding that foxes are less effective at capturing quokkas in densely vegetated swamps (i.e. increased habitat complexity) (Hayward et al. 2005a, 2005b). Whether this is related to locomotor performance is yet to be determined.
Several species are able to persist, despite the presence of foxes, including arboreal species. Foxes seem to be able to exploit arboreal species in temperate biomes more than any other group of prey, and more than cats and dingoes (Fig. 3). The arboreal species that are present in fox diets include species that utilise terrestrial environments (Phalangeridae), but also numerous gliders (Petauridae). This predation has been investigated further, finding a significant increase in possum abundance during targeted fox baiting in Victoria (Dexter and Murray 2009).
The predation of arboreal species by foxes could represent an easier prey choice, as arboreal species can often be vulnerable on the ground (Gaschk et al. 2019). Thus, the distinction in locomotion performance between arboreal specialists (possums, more frequently caught by foxes) in comparison to terrestrial specialists (bandicoots, less frequently caught by foxes), could determine if arboreal species are vulnerable along the ground. Further, the addition of lagomorphs in this comparison could explain why they seem to be consumed more regularly despite being under-represented in the bioregion occurrence data.
Response to cats
Although they are the smallest of the three invasive predators, feral cats have been implicated in the extinction of many species, to which they are a novel threat (Woinarski et al. 2019). Much of their diet is suggested to be due to chance encounters and prey abundance; however, the bioregion occurrence data do not support this theory (Fig. 4). While they are capable of hunting possums and bandicoots, they seem to be much more efficient at consuming smaller animals like dasyurids, lagomorphs, and rodents, as has already been reported (Fig. 4) (Dickman 1996). Thus, Notomys species, both the Darling Downs and broad-cheeked hopping mice, would have been likely prey targets for feral cats when they were extant (Fig. 5).
Species that have undergone significant range extirpation, like the crested mulgara (Dasycercus cristicauda) and the kowari (Dasyuroides byrnei), are present in feral cat diets (Woolley et al. 2013; Zichy-Woinarski et al. 2014). Dasyurids were a significant part of cat diets in Arid and Mediterranean areas and had consumption to encounter factors greater than 1.0 within these species’ distributions (Fig. 5). The importance of habitat complexity for mulgaras has been previously explored, finding that they are more abundant in higher complexity (Masters 1993). The higher complexity would aid in escape from feral cats, thus the reduction in complexity could explain their reduced distribution.
While feral cats have been implicated in many species declines, there are quite a number that, despite sharing similarities with the declined or extinct species, have persisted in their distributions. For example, the fat tailed dunnart (Sminthopsis crassicaudata) and the desert mouse (Pseudomys desertor) share similar distributions to species mentioned above but have not experienced a similar reduction in distribution.
The locomotor relationship for cats, is not about how devastating they can be to smaller species (<200 g). Feral cats would likely have an athletic advantage over such species – a notion that is already significantly supported (Legge et al. 2017; Woinarski et al. 2017, 2018, 2019). However, the preference for smaller prey species means feral cats would compete with the native carnivorous marsupials for resources (likely interference competition) (Glen and Dickman 2008; Pascoe et al. 2012). Competition is shown to contribute to declines in species where novel animals are involved (Ruscoe et al. 2011). The spotted-tail quoll (D. maculatus) is still present in much of its historical distribution; however, all other quolls – northern quoll (Dasyurus hallucatus), western quoll (D. geoffroii), eastern quoll (D. viverrinus) – have experienced significant decline (Jones et al. 2001). The relationship between feral cats and quolls is a great example of how the presence of eutherian predators, with different performance capabilities and locomotor behaviours, can affect native predators. For example, are feral cats athletically better, or do they just make different behavioural choices in terms of foraging and pursuits?
Predator/prey speeds
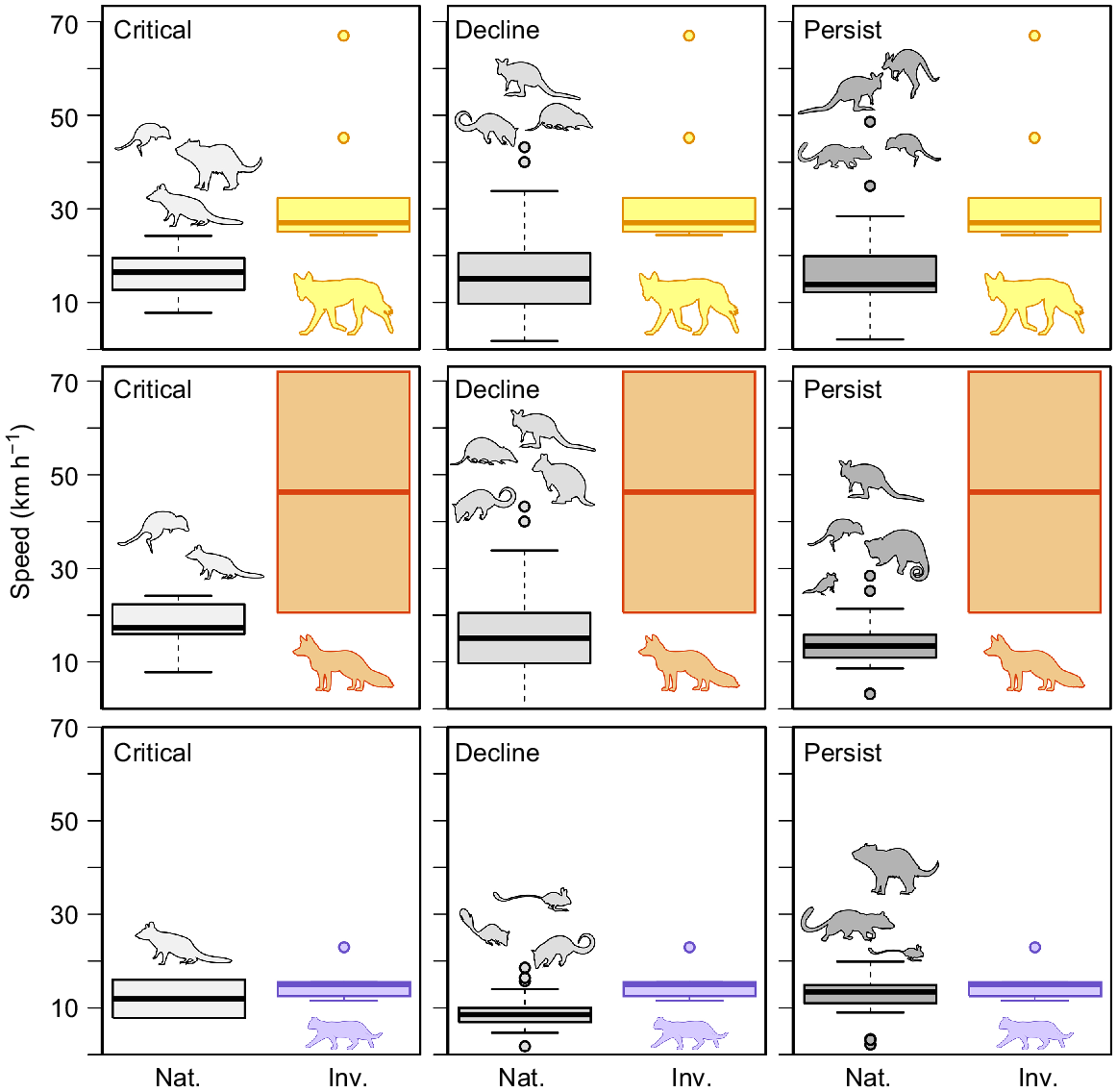
The extent to which locomotor performance can contribute to the outcome of predator/prey outcomes is of significance. Cats, foxes, and dogs (cf. dingoes) are all capable of speeds over 20 km h−1 (Fig. 6). Cats were, on average, much slower than foxes and dogs; this is likely because of the difficulty in making cats run at top speeds. The top speed recorded here was 22.9 km h−1 and was produced by an individual running between two known distances on a sporting field (see Supplementary material Appendix 1 for additional methods). The fox speeds of 72 km h−1 were obtained from Garland (1983), which seems to be an overestimate. We obtained a speed of 20.5 km h−1 for a fox running on a sporting field (see Supplementary material Appendix 1). This overestimation likely extended to lagomorphs also (estimated 40–72 km h−1: Garland 1983), with later studies suggesting that domestic rabbits were only able to reach speeds of 18 km h−1 running on a treadmill (Simons 1997), and European hares reached 36 km h−1 while being chased by sighthounds (Kuznetsov et al. 2017). The Garland (1983) speed for Canis familiaris was likely from greyhounds, which are reported to reach speeds around 68.4 km h−1 (Hudson et al. 2012); however, greyhounds are much larger than dingoes. The dog speeds collated here report dogs between 14 and 22 kg running at top speeds of 24–32 km h−1 and were acquired from trained agility dogs and dogs pursuing balls (Haagensen et al., in press). The speeds for the spotted tail quoll and Tasmanian devil were recorded from released animals using video analysis to determine speed, and while this is likely close to maximal speeds for the spotted tail quoll, the Tasmanian devil was likely not stimulated to maximum performance during escapes, so its escape speeds could be slight underestimates (see Supplementary material Appendix 1). Cat speeds were significantly faster than those of northern quolls (t11 = 5.55, P < 0.001), but were not significantly different from those of spotted-tail quolls and Tasmanian devils (Fig. 6). Dog speeds were significantly faster than those of Dasyurids (t9 = 5.08, P < 0.001), Bandicoot and Allies (t10 = 3.48, P < 0.01), Native Rodents (t9 = 4.17, P < 0.01), and Wallabies and Allies (t9 = 2.88, P = 0.016) (Fig. 6).
The available top speed (km h−1) and mass data (kg) for marsupials and eutherians that were present in the diets of Felis catus, Vulpes vulpes and Canis familiaris, compared with speeds of the invasive predators. Data were log-transformed. Centre colours of the dots represent the groups that were defined in Figs 2, 3. The outside colour of the dots signifies different species or genera. Species were categorised into critical, decline and persist based on their IUCN conservation status and local extinctions discussed in the invasion timeline (Fig. 1). Species present in each predator pane were relevant to the dietary data and likelihood of predation (e.g. cats aren’t likely to hunt the livestock found in their diets and are likely consumed via scavenging). A G within dots denotes that the data are from Garland (1983). Other data were collated from Dawson and Taylor (1973); Alexander and Vernon (1975); Baudinette et al. (1976, 1978, 1992, 1993); Baudinette (1977); Cavagna et al. (1977); Thompson et al. (1980); Bennett (1987); Garland et al. (1988); Griffiths (1989); Biewener and Baudinette (1995); Biewener (1998); Kram and Dawson (1998); Webster and Dawson (2003); Biewener et al. (2004); McGowan et al. (2005, 2007, 2008); Kim et al. (2014); Clemente et al. (2019).
Speed affords the predator an advantage during interactions of pursuit and, as such, many predators have evolved to be more powerful and explosive in terms of acceleration and top speeds (Wilson et al. 2018). Dogs have a speed advantage over most Australian fauna, with only some of the larger wallabies and kangaroos showing comparable speeds (Fig. 7). For this reason, the speed advantage in dogs could explain why their mammalian diet is the most variable of the three introduced predators in Australia(Fig. 2). However, speed of dingoes may only partially contribute to prey variability as size and sociality of the species may also be involved. Both eastern quolls and spotted-tail quolls persist only in Tasmania (absent from foxes and dingoes); however, the smaller and slower northern quoll, still present on mainland Australia, has declined across much of its former distribution. Although other effects are contributing to this decline, it has been reported that the population density of northern quolls is reduced in habitats with much less complexity (Oakwood 2000; Clemente et al. 2019). These less complex environments afford a greater advantage to a much faster predator because it is the agility, or turning ability, of the prey that increases the chance of a successful evasion (Wilson et al. 2018). If the predator does not have to weave through a complex environment, or the distance to a refuge is too great, the advantage afforded by speed is increased (Clarke et al. 1993; Wilson et al. 2018). This is evident with cats, and the smaller dasyurids (dunnarts, Antechinus) and native mice (Notomys, Pseudomys), which feature less often in cat diets in temperate biomes where the foliage and shrubbery are consistently more complex (McElhinny et al. 2006). Thus, while speed is important, it does not reveal the whole story of pursuit locomotion; other locomotor aspects of pursuit (e.g. cornering ability, acceleration) are also important; however, these are less commonly studied.
The available top speeds (km h−1) for native Australian fauna that were present in the diets of Felis catus (purple), Vulpes vulpes (orange), and Canis familiaris (yellow), compared with speeds of the invasive predators. The boxplot is the amalgamated speeds from the data presented in Fig. 5. Invasive prey items were excluded.
Conclusion
By reviewing the temporal, diet, occurrence, and locomotor literature of invasive species C. familiaris, V. vulpes, and F. catus and their native prey items, we have highlighted relationships of significant interest to conservation with potential to further our understanding of Australian ecosystems. We have compiled data to detail relationships between species where an introduced predator has resulted in extinction, greatly reduced distributions, or moderately to no effect on native species. By combining dietary data of our invasive predators with the occurrence data of prey items across Australia, we have shown that diet is not always determined by the apparent occurrence of a prey item. Further, our research highlights that larger predators consume prey with more variable locomotor capacities, with the prey of dingoes being more variable than both foxes and cats. However, the current knowledge of animal speeds in the literature is precarious, highlighting the need for additional studies to determine the extent to which locomotor capacity can influence invasive species success. A better understanding of the biomechanical relationships identified in this research (Fig. 5) might better inform conservation strategies for the protection of Australia’s native fauna.
Acknowledgements
The authors acknowledge the Australian Living Atlas for providing the data for occurrences of native fauna. We also thank Dr Robert Cieri, Professor Philip Withers, and Lauren Thornton for their help throughout the editing process.
References
Abbott I (2002) Origin and spread of the cat, Felis catus, on mainland Australia, with a discussion of the magnitude of its early impact on native fauna. Wildlife Research 29(1), 51-74.
| Crossref | Google Scholar |
Alexander RM, Vernon A (1975) The mechanics of hopping by kangaroos (Macropodidae). Journal of Zoology 177(2), 265-303.
| Crossref | Google Scholar |
Allen BL, Fleming PJS (2012) Reintroducing the dingo: the risk of dingo predation to threatened vertebrates of western New South Wales. Wildlife Research 39(1), 35-50.
| Crossref | Google Scholar |
Allen BL, Leung LK-P (2012) Assessing predation risk to threatened fauna from their prevalence in predator scats: dingoes and rodents in arid Australia. PLoS ONE 7(5), e36426.
| Crossref | Google Scholar |
Arnold SJ (1983) Morphology, performance and fitness. American Zoologist 23(2), 347-361.
| Crossref | Google Scholar |
Baker P, Furlong M, Southern S, Harris S (2006) The potential impact of red fox Vulpes vulpes predation in agricultural landscapes in lowland Britain. Wildlife Biology 12(1), 39-50.
| Crossref | Google Scholar |
Banks PB, Dickman CR (2007) Alien predation and the effects of multiple levels of prey naiveté. Trends in Ecology & Evolution 22(5), 229-230 [author reply 230-1].
| Crossref | Google Scholar |
Baudinette RV (1977) Locomotory energetics in a marsupial, Setonix brachyurus. Australian Journal of Zoology 25(3), 423-428.
| Crossref | Google Scholar |
Baudinette RV, Nagle KA, Scott RAD (1976) Locomotory energetics in dasyurid marsupials. Journal of Comparative Physiology 109(2), 159-168.
| Crossref | Google Scholar |
Baudinette RV, Seymour RS, Orbach J (1978) Cardiovascular responses to exercise in the brush-tailed possum. Journal of Comparative Physiology 124(2), 143-147.
| Crossref | Google Scholar |
Baudinette RV, Snyder GK, Frappell PB (1992) Energetic cost of locomotion in the tammar wallaby. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology 262(5), R771-R778.
| Crossref | Google Scholar |
Baudinette RV, Halpern EA, Hinds DS (1993) Energetic cost of locomotion as a function of ambient temperature and during growth in the marsupial Potorous tridactylus. The Journal of Experimental Biology 174(1), 81-95.
| Crossref | Google Scholar |
Bennett MB (1987) Fast locomotion of some kangaroos. Journal of Zoology 212(3), 457-464.
| Crossref | Google Scholar |
Biewener AA (1998) Muscle-tendon stresses and elastic energy storage during locomotion in the horse. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 120(1), 73-87.
| Crossref | Google Scholar |
Biewener A, Baudinette R (1995) In vivo muscle force and elastic energy storage during steady-speed hopping of tammar wallabies (Macropus eugenii). The Journal of Experimental Biology 198(9), 1829-1841.
| Crossref | Google Scholar |
Biewener AA, McGowan C, Card GM, Baudinette RV (2004) Dynamics of leg muscle function in tammar wallabies (M. eugenii) during level versus incline hopping. Journal of Experimental Biology 207(2), 211-223.
| Crossref | Google Scholar |
Brown OJF (2006) Tasmanian devil (Sarcophilus harrisii) extinction on the Australian mainland in the mid-Holocene: multicausality and ENSO intensification. Alcheringa: An Australasian Journal of Palaeontology 30(sup1), 49-57.
| Crossref | Google Scholar |
Burbidge AA, McKenzie NL (1989) Patterns in the modern decline of western Australia’s vertebrate fauna: causes and conservation implications. Biological Conservation 50(1–4), 143-198.
| Crossref | Google Scholar |
Burbidge AA, Woinarski J (2016) Perameles eramiana. In ‘The IUCN Red List of Threatened Species 2016. (IUCN): e.T16570A21965953’. Available at https://dx.doi.org/10.2305/IUCN.UK.2016-3.RLTS.T16570A21965953.en [Accessed 18 October 2022]
Catling PC, Burt RJ (1995) Studies of the ground-dwelling mammals of eucalypt forests in south-eastern New South Wales: the effect of habitat variables on distribution and abundance. Wildlife Research 22(3), 271-288.
| Crossref | Google Scholar |
Cavagna GA, Heglund NC, Taylor CR (1977) Mechanical work in terrestrial locomotion: two basic mechanisms for minimizing energy expenditure. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology 233(5), R243-R261.
| Crossref | Google Scholar |
Chisholm RA, Taylor R (2010) Body size and extinction risk in Australian mammals: an information-theoretic approach. Austral Ecology 35(6), 616-623.
| Crossref | Google Scholar |
Clarke MF, da Silva KB, Lair H, Pocklington R, Kramer DL, McLaughlin RL (1993) Site familiarity affects escape behaviour of the eastern chipmunk, Tamias striatus. Oikos 66(3), 533-537.
| Crossref | Google Scholar |
Clemente CJ, Dick TJM, Wheatley R, Gaschk J, Nasir AFAA, Cameron SF, Wilson RS (2019) Moving in complex environments: a biomechanical analysis of locomotion on inclined and narrow substrates. Journal of Experimental Biology 222(6), jeb189654.
| Crossref | Google Scholar |
Clout MN, Russell JC (2008) The invasion ecology of mammals: a global perspective. Wildlife Research 35(3), 180-184.
| Crossref | Google Scholar |
Coman BJ (1973) The diet of red foxes, Vulpes vulpes L., in Victoria. Australian Journal of Zoology 21(3), 391-401.
| Crossref | Google Scholar |
Dawson TJ, Taylor CR (1973) Energetic cost of locomotion in kangaroos. Nature 246(5431), 313-314.
| Crossref | Google Scholar |
Dexter N, Murray A (2009) The impact of fox control on the relative abundance of forest mammals in East Gippsland, Victoria. Wildlife Research 36(3), 252-261.
| Crossref | Google Scholar |
Dickman CR (1996) Impact of exotic generalist predators on the native fauna of Australia. Wildlife Biology 2(3), 185-195.
| Crossref | Google Scholar |
Doherty TS, Davis NE, Dickman CR, Forsyth DM, Letnic M, Nimmo DG, Palmer R, Ritchie EG, Benshemesh J, Edwards G, Lawrence J, Lumsden L, Pascoe C, Sharp A, Stokeld D, Myers C, Story G, Story P, Triggs B, Venosta M, Wysong M, Newsome TM (2019) Continental patterns in the diet of a top predator: Australia’s dingo. Mammal Review 49(1), 31-44.
| Crossref | Google Scholar |
Duffy DC, Capece P (2012) Biology and impacts of Pacific Island invasive species. 7. The domestic cat (Felis catus). Pacific Science 66(2), 173-212.
| Crossref | Google Scholar |
Fairfax RJ (2019) Dispersal of the introduced red fox (Vulpes vulpes) across Australia. Biological Invasions 21(4), 1259-1268.
| Crossref | Google Scholar |
Fillios M, Crowther MS, Letnic M (2012) The impact of the dingo on the thylacine in Holocene Australia. World Archaeology 44(1), 118-134.
| Crossref | Google Scholar |
Garland T (1983) The relation between maximal running speed and body mass in terrestrial mammals. Journal of Zoology 199(2), 157-170.
| Crossref | Google Scholar |
Garland T, Jr, Geiser F, Baudinette RV (1988) Comparative locomotor performance of marsupial and placental mammals. Journal of Zoology 215(3), 505-522.
| Crossref | Google Scholar |
Gaschk JL, Frère CH, Clemente CJ (2019) Quantifying koala locomotion strategies: implications for the evolution of arborealism in marsupials. Journal of Experimental Biology 222(24), jeb207506.
| Crossref | Google Scholar |
Glen AS, Dickman CR (2008) Niche overlap between marsupial and eutherian carnivores: does competition threaten the endangered spotted-tailed quoll? Journal of Applied Ecology 45(2), 700-707.
| Crossref | Google Scholar |
Greentree C, Saunders G, Mcleod L, Hone J (2000) Lamb predation and fox control in south-eastern Australia. Journal of Applied Ecology 37(6), 935-943.
| Crossref | Google Scholar |
Griffiths RI (1989) The mechanics of the medial gastrocnemius muscle in the freely hopping wallaby (Thylogale billardierii). Journal of Experimental Biology 147(1), 439-456.
| Crossref | Google Scholar |
Guiler ER (1970) Observations on the Tasmanian devil, Sarcophilus harrisii (Marsupialia: Dasyuridae) I. Numbers, home range, movements and food in two populations. Australian Journal of Zoology 18(1), 49-62.
| Crossref | Google Scholar |
Haagensen T, Gaschk JL, Schultz JT, Clemente CJ (in press) Exploring the limits to turning performance with size and shape variation in dogs. The Journal of Experimental Biology
| Crossref | Google Scholar |
Hayward MW, de Tores PJ, Augee ML, Banks PB (2005a) Mortality and survivorship of the quokka (Setonix brachyurus) (Macropodidae: Marsupialia) in the northern jarrah forest of Western Australia. Wildlife Research 32(8), 715-722 [In English].
| Crossref | Google Scholar |
Hayward MW, de Tores PJ, Banks PB (2005b) Habitat use of the quokka, Setonix brachyurus (Macropodidae: Marsupialia), in the northern jarrah forest of Australia. Journal of Mammalogy 86(4), 683-688 [In English].
| Crossref | Google Scholar |
Hudson PE, Corr SA, Wilson AM (2012) High speed galloping in the cheetah (Acinonyx jubatus) and the racing greyhound (Canis familiaris): spatio-temporal and kinetic characteristics. Journal of Experimental Biology 215(14), 2425-2434.
| Crossref | Google Scholar |
Irschick DJ, Garland T, Jr. (2001) Integrating function and ecology in studies of adaptation: investigations of locomotor capacity as a model system. Annual Review of Ecology and Systematics 32(1), 367-396.
| Crossref | Google Scholar |
Johnson CN, Wroe S (2003) Causes of extinction of vertebrates during the Holocene of mainland Australia: arrival of the dingo, or human impact? The Holocene 13(6), 941-948.
| Crossref | Google Scholar |
Jones ME, Rose RK, Burnett S (2001) Dasyurus maculatus. Mammalian Species 2001(676), 1-9.
| Crossref | Google Scholar |
Kim YK, Park J, Yoon B, Kim K-S, Kim S (2014) The role of relative spinal motion during feline galloping for speed performance. Journal of Bionic Engineering 11(4), 517-528.
| Crossref | Google Scholar |
Kittel RN, Austin AD (2016) New species of Australian arid zone chelonine wasps from the genera Phanerotoma and Ascogaster (Hymenoptera: Braconidae) informed by the ‘Bush Blitz’ surveys of national reserves. Journal of Natural History 50(3–4), 211-262.
| Crossref | Google Scholar |
Kram R, Dawson TJ (1998) Energetics and biomechanics of locomotion by red kangaroos (Macropus rufus). Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 120(1), 41-49.
| Crossref | Google Scholar |
Kuznetsov AN, Luchkina OS, Panyutina AA, Kryukova NV (2017) Observations on escape runs in wild European hare as a basis for the mechanical concept of extreme cornering with special inference of a role of the peculiar subclavian muscle. Mammalian Biology 84(1), 61-72.
| Crossref | Google Scholar |
Lavery TH, Eldridge M, Legge S, Pearson D, Southwell D, Woinarski JCZ, Woolley L-A, Lindenmayer D (2021) Threats to Australia’s rock-wallabies (Petrogale spp.) with key directions for effective monitoring. Biodiversity and Conservation 30(14), 4137-4161.
| Crossref | Google Scholar |
Legge S, Murphy BP, McGregor H, Woinarski JCZ, Augusteyn J, Ballard G, Baseler M, Buckmaster T, Dickman CR, Doherty T, Edwards G, Eyre T, Fancourt BA, Ferguson D, Forsyth DM, Geary WL, Gentle M, Gillespie G, Greenwood L, Hohnen R, Hume S, Johnson CN, Maxwell M, McDonald PJ, Morris K, Moseby K, Newsome T, Nimmo D, Paltridge R, Ramsey D, Read J, Rendall A, Rich M, Ritchie E, Rowland J, Short J, Stokeld D, Sutherland DR, Wayne AF, Woodford L, Zewe F (2017) Enumerating a continental-scale threat: how many feral cats are in Australia? Biological Conservation 206, 293-303.
| Crossref | Google Scholar |
Letnic M, Fillios M, Crowther MS (2012) Could direct killing by larger dingoes have caused the extinction of the thylacine from mainland Australia? PLoS ONE 7(5), e34877.
| Crossref | Google Scholar |
Lugton IW (1993) Diet of red foxes (Vulpes vulpes) in south-west New South Wales, with relevance to lamb predation. The Rangeland Journal 15(1), 39-47.
| Crossref | Google Scholar |
Masters P (1993) The effects of fire-driven succession and rainfall on small mammals in spinifex grassland at Uluru National Park, Northern Territory. Wildlife Research 20(6), 803-813.
| Crossref | Google Scholar |
McElhinny C, Gibbons P, Brack C, Bauhus J (2006) Fauna–habitat relationships: a basis for identifying key stand structural attributes in temperate Australian eucalypt forests and woodlands. Pacific Conservation Biology 12(2), 89-110.
| Crossref | Google Scholar |
McGowan CP, Baudinette RV, Biewener AA (2005) Joint work and power associated with acceleration and deceleration in tammar wallabies (Macropus eugenii). Journal of Experimental Biology 208(1), 41-53.
| Crossref | Google Scholar |
McGowan CP, Baudinette RV, Biewener AA (2007) Modulation of proximal muscle function during level versus incline hopping in tammar wallabies (Macropus eugenii). Journal of Experimental Biology 210(7), 1255-1265.
| Crossref | Google Scholar |
McGowan CP, Baudinette RV, Biewener AA (2008) Differential design for hopping in two species of wallabies. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 150(2), 151-158.
| Crossref | Google Scholar |
Moore RW, Donald IM, Messenger JJ (1966) Fox predation as a cause of lamb mortality. In ‘Proceedings of the Australian Society of Animal Production’. pp. 157–160. Available at https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.845.5092&rep=rep1&type=pdf
Oakwood M (2000) Reproduction and demography of the northern quoll, Dasyurus hallucatus, in the lowland savanna of northern Australia. Australian Journal of Zoology 48(5), 519-539.
| Crossref | Google Scholar |
Pascoe JH, Mulley RC, Spencer R, Chapple R (2012) Diet analysis of mammals, raptors and reptiles in a complex predator assemblage in the Blue Mountains, eastern Australia. Australian Journal of Zoology 59(5), 295-301.
| Crossref | Google Scholar |
Phillips BL, Shine R (2006) An invasive species induces rapid adaptive change in a native predator: cane toads and black snakes in Australia. Proceedings of the Royal Society B: Biological Sciences 273(1593), 1545-1550.
| Crossref | Google Scholar |
Pimentel D, McNair S, Janecka J, Wightman J, Simmonds C, O’Connell C, Wong E, Russel L, Zern J, Aquino T, Tsomondo T (2001) Economic and environmental threats of alien plant, animal, and microbe invasions. Agriculture, Ecosystems & Environment 84(1), 1-20.
| Crossref | Google Scholar |
Prowse TAA, Johnson CN, Bradshaw CJA, Brook BW (2014) An ecological regime shift resulting from disrupted predator–prey interactions in Holocene Australia. Ecology 95(3), 693-702.
| Crossref | Google Scholar |
Radford JQ, Woinarski JCZ, Legge S, Baseler M, Bentley J, Burbidge AA, Bode M, Copley P, Dexter N, Dickman CR, Gillespie G, Hill B, Johnson CN, Kanowski J, Latch P, Letnic M, Manning A, Menkhorst P, Mitchell N, Morris K, Moseby K, Page M, Ringma J (2018) Degrees of population-level susceptibility of Australian terrestrial non-volant mammal species to predation by the introduced red fox (Vulpes vulpes) and feral cat (Felis catus). Wildlife Research 45(7), 645-657.
| Crossref | Google Scholar |
Ruscoe WA, Ramsey DSL, Pech RP, Sweetapple PJ, Yockney I, Barron MC, Perry M, Nugent G, Carran R, Warne R, Brausch C, Duncan RP (2011) Unexpected consequences of control: competitive vs. predator release in a four-species assemblage of invasive mammals. Ecology Letters 14(10), 1035-1042.
| Crossref | Google Scholar |
Saltré F, Rodríguez-Rey M, Brook BW, Johnson CN, Turney CS, Alroy J, Cooper A, Beeton N, Bird MI, Fordham DA, Gillespie R, Herrando-Pérez S, Jacobs Z, Miller GH, Nogués-Bravo D, Prideaux GJ, Roberts RG, Bradshaw CJA (2016) Climate change not to blame for late Quaternary megafauna extinctions in Australia. Nature Communications 7(1), 10511.
| Crossref | Google Scholar |
Scholtz EJ, DeSantis LRG (2020) Invasive species, not environmental changes, restrict the population and geographical range of the quokka (Setonix brachyurus). Journal of Zoology 311(2), 106-115.
| Crossref | Google Scholar |
Shortridge GC (1936) Field notes (hitherto unpublished) on Western Australian mammals south of the Tropic of Capricorn (exclusive of Marsupialia and Monotremata), and records of specimens collected during the Balston expeditions (November 1904 to June 1907). Proceedings of the Zoological Society of London 106, 743-749.
| Crossref | Google Scholar |
Thomson PC (1992) The behavioural ecology of dingoes in north-western Australia. III. Hunting and feeding behaviour, and diet. Wildlife Research 19(5), 531-541.
| Crossref | Google Scholar |
Thompson SD, MacMillen RE, Burke EM, Taylor CR (1980) The energetic cost of bipedal hopping in small mammals. Nature 287(5779), 223-224.
| Crossref | Google Scholar |
Webster KN, Dawson TJ (2003) Locomotion energetics and gait characteristics of a rat-kangaroo, Bettongia penicillata, have some kangaroo-like features. Journal of Comparative Physiology B 173(7), 549-557.
| Crossref | Google Scholar |
Wheatley R, Clemente CJ, Niehaus AC, Fisher DO, Wilson RS (2018) Surface friction alters the agility of a small Australian marsupial. Journal of Experimental Biology 221(8), jeb172544.
| Crossref | Google Scholar |
White JG, Gubiani R, Smallman N, Snell K, Morton A (2006) Home range, habitat selection and diet of foxes (Vulpes vulpes) in a semi-urban riparian environment. Wildlife Research 33(3), 175-180.
| Crossref | Google Scholar |
White LC, Saltré F, Bradshaw CJA, Austin JJ (2018) High-quality fossil dates support a synchronous, Late Holocene extinction of devils and thylacines in mainland Australia. Biology Letters 14(1), 20170642.
| Crossref | Google Scholar |
Wilson RS, Niehaus AC, David G, Hunter A, Smith M (2014) Does individual quality mask the detection of performance trade-offs? A test using analyses of human physical performance. Journal of Experimental Biology 217(4), 545-551.
| Crossref | Google Scholar |
Wilson RS, Clemente CJ, Kasumovic M (2017) Teaching evolutionary principles using games: escape speeds, performance and life history trade-offs. Integrative and Comparative Biology 57(Supplement 21), e180.
| Google Scholar |
Wilson AM, Hubel TY, Wilshin SD, Lowe JC, Lorenc M, Dewhirst OP, Bartlam-Brooks HLA, Diack R, Bennitt E, Golabek KA, Woledge RC, McNutt JW, Curtin NA, West TG (2018) Biomechanics of predator–prey arms race in lion, zebra, cheetah and impala. Nature 554(7691), 183-188.
| Crossref | Google Scholar |
Woinarski JCZ, Burbidge AA, Harrison PL (2015) Ongoing unraveling of a continental fauna: decline and extinction of Australian mammals since European settlement. Proceedings of the National Academy of Sciences of the United States of America 112(15), 4531-4540.
| Crossref | Google Scholar |
Woinarski JCZ, Murphy BP, Legge SM, Garnett ST, Lawes MJ, Comer S, Dickman CR, Doherty TS, Edwards G, Nankivell A, Paton D, Palmer R, Woolley LA (2017) How many birds are killed by cats in Australia? Biological Conservation 214, 76-87.
| Crossref | Google Scholar |
Woinarski JCZ, Murphy BP, Palmer R, Legge SM, Dickman CR, Doherty TS, Edwards G, Nankivell A, Read JL, Stokeld D (2018) How many reptiles are killed by cats in Australia? Wildlife Research 45(3), 247-266.
| Crossref | Google Scholar |
Woinarski JCZ, Braby MF, Burbidge AA, Coates D, Garnett ST, Fensham RJ, Legge SM, McKenzie NL, Silcock JL, Murphy BP (2019) Reading the black book: the number, timing, distribution and causes of listed extinctions in Australia. Biological Conservation 239, 108261.
| Crossref | Google Scholar |
Woolley PA, Haslem A, Westerman M (2013) Past and present distribution of Dasycercus: toward a better understanding of the identity of specimens in cave deposits and the conservation status of the currently recognised species D. blythi and D. cristicauda (Marsupialia: Dasyuridae). Australian Journal of Zoology 61(4), 281-290.
| Crossref | Google Scholar |
Wynn ML, Clemente C, Nasir AFAA, Wilson RS (2015) Running faster causes disaster: trade-offs between speed, manoeuvrability and motor control when running around corners in northern quolls (Dasyurus hallucatus). Journal of Experimental Biology 218(3), 433-439.
| Crossref | Google Scholar |
Zenger KR, Eldridge MDB, Johnston PG (2005) Phylogenetics, population structure and genetic diversity of the endangered southern brown bandicoot (Isoodon obesulus) in south-eastern Australia. Conservation Genetics 6, 193-204.
| Crossref | Google Scholar |
Zenni RD, Nuñez MA (2013) The elephant in the room: the role of failed invasions in understanding invasion biology. Oikos 122(6), 801-815.
| Crossref | Google Scholar |


