Camp site habitat preferences of the little red flying-fox (Pteropus scapulatus) in Queensland
Stewart L. Macdonald A B , Matthew Bradford C , Adam McKeown D , Eric Vanderduys E , Andrew Hoskins F G and David Westcott C
A B , Matthew Bradford C , Adam McKeown D , Eric Vanderduys E , Andrew Hoskins F G and David Westcott C
A CSIRO Land and Water, Townsville, Qld 4811, Australia.
B College of Science and Engineering, James Cook University, Townsville, Qld 4811, Australia.
C CSIRO Land and Water, Atherton, Qld 4883, Australia.
D CSIRO Land and Water, Waite Campus, SA 5064, Australia.
E CSIRO Land and Water, Brisbane, Qld 4000, Australia.
F CSIRO Health and Biosecurity, Townsville, Qld 4811, Australia.
G Corresponding author. Email: andrew.hoskins@csiro.au
Australian Journal of Zoology - https://doi.org/10.1071/ZO20079
Submitted: 9 September 2020 Accepted: 4 October 2021 Published online: 12 November 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
Urban flying-fox camps are a major source of human–wildlife conflict, producing noise, odour, vegetation damage, property damage, and concerns about disease. Although there is a significant demand in many communities for bat camps to be dispersed, there is limited information on how such dispersal can be conducted effectively. Determining the habitat characteristics flying-foxes use when selecting a camp site is key to understanding why they establish camps where they do and to where they might move if dispersed. We characterised little red flying-fox (LRFF) camp habitat at two spatial scales: floristics and vegetation structure at the local scale, and climatic and landscape characteristics at the broad scale. We found weak associations with local-scale tree and shrub height and cover, and stronger associations with increased Normalised Difference Vegetation Index (a measure of ‘greenness’) and decreased distance to nearest watercourse. These relationships were not strong enough to explain all variation in the model, suggesting that there are other factors, such as social cues, that could also influence camp site selection. Our results suggest that minor modifications to existing or proposed camp sites will be unlikely to repel or attract LRFFs, as other factors are likely to play key roles in the formation of camp sites for this species.
Keywords: species-distribution modelling, little red flying-fox, Pteropus scapulatus, roost, camp, habitat preferences.
Introduction
As humans continue to modify the world, they increasingly come into conflict with wildlife (Madden 2004; Nyhus 2016). Such conflict occurs when the coexistence of people and animals results in competition for space or other resources (e.g. damage to crops: Pérez and Pacheco 2006), health issues (e.g. disease transmission or depredation of people: Waltner-Toews 2017; Kushnir and Packer 2019), and/or impact on amenity/happiness (e.g. unwanted noise: Roberts et al. 2011). Proper management of human–wildlife conflict first requires an understanding of the animals’ resource requirements. In some situations, humans may unwittingly modify their urban environments in a way that increases their attractiveness to certain animal species (McDonald‐Madden et al. 2005; Parris and Hazell 2005). Regular watering regimes, a wide variety of ornamental or fruit trees that may flower/fruit year-round, and tall shade trees all provide utility or amenity for both humans and wildlife, setting the scene for human–wildlife conflict. This conflict is exemplified by flying-foxes in urban environments (Tait et al. 2014).
Human–flying-fox conflict
Flying-foxes (Pteropus spp.) and their relatives are common across the Old World tropics, and often form large camps (or roosts) in areas that are also inhabited by people, across the full range of urban settings from capital cities (e.g. Melbourne Botanic Gardens, Victoria, Australia) to smaller country towns (Markus and Hall 2004; Tait et al. 2014; Gulraiz et al. 2015; Westcott et al. 2015). Although in many places these camps are tolerated, in others, including in Australia, the presence of flying-foxes leads to conflict with nearby humans. Camps can regularly be home to thousands of flying-foxes and can produce considerable noise, odour, vegetation damage, and property damage. In recent years, the risk of disease transmission from flying-foxes to domestic animals and humans has also become highly publicised and has resulted in additional concerns (e.g. Halpin et al. 1999). Given these negative aspects of urban camps, it is no surprise that substantial amounts of time and effort have gone into the management of flying-fox camps.
Camp management
In Australia, urban flying-fox camps have been a cause of concern since the 1790s when heat stress mortality resulted in the deaths of thousands of flying-foxes (Tench 1793). In the intervening years, the management response to urban camps has been to disturb them in an attempt to force the animals to leave. Until about the 1950s or 1960s, this process almost invariably involved lethal methods, usually guns and explosives (Anon. 1890; Ratcliffe 1931; Lunney and Moon 1997). As the various flying-fox species received legislative protection and the ineffectiveness of lethal methods became increasingly obvious (e.g. Hall 2002; Westcott et al. 2015), other methods have been sought. These have included the use of vegetation removal, lights, noise, smoke, water sprinklers/jets, and helicopters (Roberts and Eby 2013).
Although disturbing camps may result in the animals leaving the camp, these dispersals have often been unsuccessful in the long term because the departure was only temporary or because the animals relocated to another nearby and still undesirable urban location (Roberts et al. 2011; Roberts and Eby 2013). This outcome has led to: (1) management of vegetation (e.g. removal) to reduce attractiveness of the original camp site; and (2) the establishment of new camp sites increasingly being employed in concert with dispersals to achieve suitable outcomes. Such approaches, however, are implicitly based on the assumption that flying-foxes have a preference for particular camp site characteristics and that the presence or absence of these characteristics will make a site more or less attractive. It is not clear that this assumption is well founded, given that camps occur in a broad range of environments. To better manage flying-fox camp sites, we first need to understand if and how vegetation and landscape characteristics influence the selection of camp sites.
Camp site selection
The major factor influencing flying-fox distribution across the landscape is thought to be the distribution of feeding resources (Westcott et al. 2015), which, for nectivorous species such as the little red flying-fox (LRFF; Pteropus scapulatus), are primarily flowering plants. The ephemeral nature of these resources across the landscape means that to exploit them, flying-foxes must be highly mobile. Telemetry studies of Australian flying-foxes reveal that they can move great distances (up to hundreds of kilometres per night: e.g. Welbergen et al. 2020; unpubl. data, CSIRO, this study), both as part of their daily foraging and also over longer periods (Roberts et al. 2012; Westcott et al. 2015). A consequence of the extreme mobility of individual flying-foxes is that individual flying-foxes change camp frequently, with the average individual staying at a camp for an average of just 10–14 days (Westcott et al. 2015; Westcott et al. unpubl. data; CSIRO, unpubl. data, this study). Not surprisingly, given this high individual mobility, flying-fox camps are highly dynamic in nature, with the number of flying-foxes present and the boundary of camps changing through time – sometimes dramatically over periods of days or weeks. Little red flying-foxes can move into camps in huge numbers at certain times of the year (e.g. >1 000 000 LRFFs were at the Irvinebank, Qld, camp for two months in early 2018 and >500 000 were in Tolga, Qld, also in 2018: E. Vanderduys, pers. obs.). The reasons for this are not always clear though this is likely related to resource availability (unpubl. data, CSIRO, this study) and aggregating for mating during the breeding season (A. McKeown, pers. obs.).
So, what makes a site attractive to flying-foxes? There is a clear social aspect to camp choice – the highly aggregated nature of flying-fox distribution points to the fact that sites with flying-foxes are highly attractive to them. But there may also be biotic and abiotic characteristics that are required for the establishment of a camp (trees being an obvious requirement). Previous studies of flying-fox habitat selection have focussed on foraging habitat (e.g. Giles et al. 2018), with few studies having examined roosting habitat preferences. Studies have generally found that camps are established in tall, dense vegetation, often near watercourses (Hall and Richards 2000). Radio-tracking of black flying-foxes (BFF; Pteropus alecto) in Australia’s Northern Territory found that animals preferentially roosted in bamboo, rainforest, and mangroves (Palmer and Woinarski 1999). The same study also found seasonal shifts in habitat use, with flying-foxes mainly using bamboo in the dry season and rainforest in the wet season. All known LRFF and BFF camps investigated by Tidemann et al. (1999) around Kakadu National Park in the Northern Territory were found in dense riparian vegetation, including mangroves and bamboo. A study of Pteropus lylei and P. vampyrus camps in Thailand found that camp sites were more likely to be found close to rivers and were commonly established in bamboo and mangroves (Thanapongtharm et al. 2015). A study in Bangladesh found a preference by Pteropus giganteus for roosting in bamboo (Hahn et al. 2014). Bamboo accounts for only a small proportion of habitat within the range of flying-foxes in Australia, but vegetation with similar characteristics (i.e. dense, tall, structurally complex) is available. Thus, multiple studies have suggested that many species of flying-fox have a preference for roosting in dense vegetation near watercourses. Outside of these natural habitats, flying-fox camps are often established in urban areas such as well watered parks and botanical gardens (e.g. Parris and Hazell 2005; Krystufek 2009; Tait et al. 2014). It is these urban camps that cause the most conflict with humans and that are the target of intensive management actions such as camp modifications or dispersals.
In this study we examine the factors influencing little red flying-fox camp site selection at two spatial scales: broad scale, using large spatial datasets (e.g. satellite imagery), and local scale, using vegetation structure within a camp as determined by onsite assessment. By correlating the location of known LRFF camps with environmental values (e.g. climate, vegetation) and comparing this with non-camp locations in the surrounding area, we can build a model of the habitat preferences for LRFFs and predict the location of other suitable habitat across the state. Importantly, the comparison with non-camp locations enables not only the identification of the habitat and environmental features associated with flying-fox camps but also determination of the strength of the preference for these characteristics.
Understanding the habitat preferences for LRFF camp sites should enable us to: (1) alter existing urban areas to make them less attractive to flying-foxes; (2) predict where new flying-fox camps might be established; (3) alter existing habitat to make it more attractive to flying-foxes to encourage the establishment of camps in areas that will reduce human–flying-fox conflict; and (4) determine whether flying-foxes are limited by suitable habitat to judge whether dispersal is appropriate generally or only in specific cases.
Methods
Study species
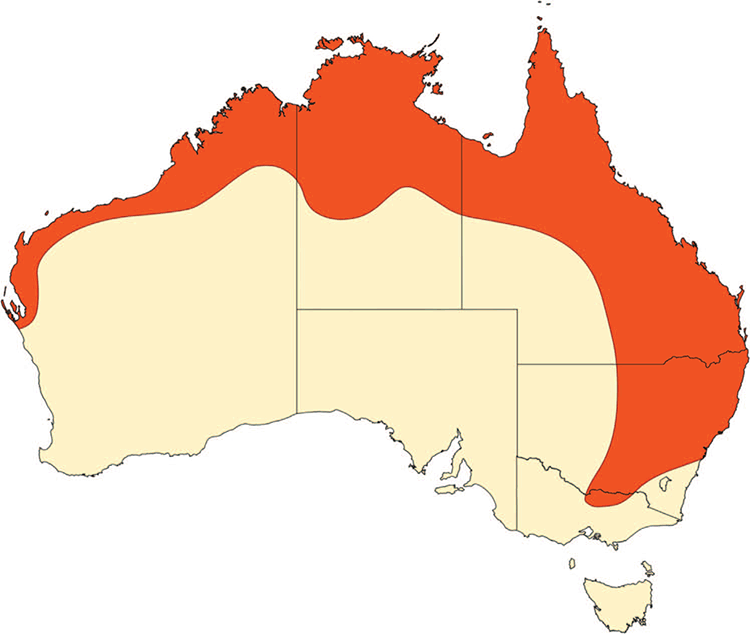
We focussed on the habitat preferences of the little red flying-fox (LRFF), as part of a larger project looking at the ecology and management of this species, particularly in urban areas. This species has the widest distribution of flying-foxes in Australia (Fig. 1), occurring from Victoria to northern Queensland and west to Shark Bay in Western Australia. This species also occurs much further inland than other species. Current work has found that, while LRFFs can move up to 80 km a night from their camp site to their feeding grounds (plus the return trip back to camp), the average maximum one-way distance to feeding grounds is ~20 km (CSIRO, unpubl. data, this study).
Identifying camps
We collated a list of camp locations for LRFFs from the following major sources:
National Flying-Fox Monitoring Program
Camps in the National Flying-fox Monitoring Program (NFFMP) (Westcott et al. 2015) database are listed along with some information on the size and use of the camp. In each case they represent camps used for long periods by multiple animals. A subset of these camps is monitored quarterly to track changes in population numbers over time. While grey-headed and spectacled flying-foxes are the main priorities for monitoring, LRFF camps within the range of those two species are also recorded and monitored as time/logistics permit.
Satellite telemetry
New camps were identified from satellite tracking as part of this current LRFF project. Between December 2016 and March 2020, 52 adult LRFFs (36 male, 16 female) were fitted with tracking collars with Microwave Telemetry Argos transmitters (9.5 g) attached. Diurnal locations of tagged animals were identified as potential camps, and classified as: (1) Class 1 – low confidence of being a camp: multiple fixes from only one tagged animal at the same location on only one day; (2) Class 2 – moderate confidence of being a camp: multiple fixes from one tagged animal at the same location over multiple days; and (3) Class 3 – high confidence of being a camp: multiple fixes from multiple tagged animals at the same location over multiple days, or at least one fix per day from at least one tagged animal for 10 consecutive days. Camps identified through telemetry were visited and assessed for size and usage wherever possible. Accessibility to some sites, particularly those in northern Queensland, was limited, meaning that some could be assessed only by helicopter and some could not be assessed directly at all.
Queensland Herbarium
The Queensland Herbarium camp location database has been collated from records from the Queensland Parks and Wildlife Service’s WildNet database, the Queensland Herbarium’s QBERD database, and from experts (e.g. zoologists, botanists, wildlife carers, apiarists) who responded to an email request for information on camps that was distributed broadly through research, NGOs, and flying-fox contacts and groups.
The final dataset, with all camps from the above three sources, contained 338 possible LRFF camps in Queensland. These camps were split into three usage categories based on historical survey data (e.g. NFFMP camps), satellite telemetry data, and/or expert opinion. Category 3 camps (n = 67) were those deemed to be the most important camps, in terms of number of animals using the site and/or the amount of time the camp is occupied. Category 2 (n = 122) and category 1 (n = 149) camps are deemed to be of moderate or lower importance, with category 1 potentially representing solitary roost sites associated with foraging locations rather than camps. The 271 category 2 and 3 camp site locations were then used for habitat assessment at two scales: local scale and broad scale.
Local-scale habitat assessment – vegetation assessment
Vegetation assessments were conducted at 143 Category 2 and 3 camps, sampling as many different geographic regions and broad habitat types across Queensland as possible. Most sites were assessed in person by one of our team members at the location (n = 127), but a small number of camps were assessed by our team from notes provided by someone else (n = 10), from a distance (e.g. through binoculars) if access was impossible (n = 6), from a helicopter (n = 5), or from a photograph (n = 1). Camp sites were assessed for the presence of the following vegetation layers: emergent trees (present or absent), tree layer 1 (T1) (all camp sites had at least a T1 layer), tree layer 2 (T2), shrub layer, and ground layer (grass and other low vegetation).
For each layer, the following habitat characteristics were assessed: maximum height above ground level in metres, percentage foliage projective cover, and names of dominant (one species) and subdominant (up to two) species. This methodology follows the standard Queensland Herbarium CORVEG vegetation assessment protocol (Sattler and Williams 1999).
Because the presence of LRFFs at each of these sites was confirmed before or during the assessment, these camp-present sites represent utilised habitat. The characteristics of these presence sites are then compared with absence or background sites.
Local-scale habitat assessment — background site assessment
All Queensland Herbarium CORVEG sites (i.e. sites at which the Queensland Herbarium has conducted standardised vegetation assessments) within 20 km of any LRFF camp were used as background sites (i.e. camp-absent sites to represent available habitat within an ecologically relevant distance of a known camp).
CORVEG site data are more detailed than the camp site data collected as part of this project. To make the two datasets comparable, the following changes were made to CORVEG site data: if both tree layers 2 and 3 were present, their cover values were combined into a new T2 cover layer by taking the larger value and adding half of the smaller value; and if both shrub layers 1 and 2 were present, their cover values were combined into a single shrub cover layer by taking the larger value and adding half of the smaller value.
All CORVEG sites that had zero tree and shrub layers (e.g. grasslands) were removed. Many sites lacked one or more of the vegetation layers (e.g. many mangrove sites had only a T1 layer). For such sites, the height and cover values for the missing layers were recorded as zeroes (i.e. height = 0 m; percentage cover = 0%). The final background dataset contained a total of 4011 camp-absent sites.
Local-scale habitat assessment — analysis
All analysis was conducted in ver. 3.5.2 of the R statistical programming environment. Logistic regression was performed using a generalised linear model with a binomial error distribution. Because of the many zeroes in our presence/absence data, we used a cloglog (complementary log–log) link function (note: we also used a logit link function, but this produced qualitatively similar results and so is not presented). This modelled camp presence/absence as a function of the nine habitat characteristics (i.e. presence/absence of emergent trees, plus height and proportion of cover for layers T1, T2, S, and G).
The initial, full logistic regression model included all nine structural characteristics. Additional models were then run with subsets of variables to determine how removing variables affected the model’s performance, as measured by Akaike’s Information Criterion (AIC), adjusted for finite sample size (AICc; see Brewer et al. 2016). AICc is a measure of how well a model performs given the number of variables it includes. Including a variable that does not contribute meaningfully (or contributes only slightly) to the performance of the model will result in an increased AICc value, indicating poorer model performance (i.e. the added complexity of the model with the additional variable is not justified, given the small increase in model performance it provides). The model with the lowest AICc value is deemed to have the best performance. A total of 512 models were evaluated using the dredge function in the MuMIn package in R.
The best-performing model was then run 1000 times, each time using a randomly selected subset of training data (75% of the camp-present data and nine random camp-absent sites for each camp-present site), to test how robust the resultant model was.
Broad-scale habitat assessment – modelling
Four modelling techniques – (1) generalised linear model (GLM), (2) random forest (RF), (3) maximum entropy (MAXENT), and (4) support vector machine (SVM) – that are commonly employed in species distribution modelling (e.g. Elith and Leathwick 2009) were used to develop a set of broad-scale distribution models using camp site locations and standard environmental layers of near-surface air temperature, precipitation, vegetation greenness, and proximity to watercourses. A total of 10 000 random background points were selected from within the LRFF geographic range (Fig. 1) and used as pseudoabsence locations (i.e. it was assumed that no LRFF camps were present at these locations). Note that these pseudoabsence points are different from the background points used in the local-scale analysis above but serve the same purpose. At each of these points, values were extracted from the relevant environmental layers. Pseudoabsence points were at least 150 m from the nearest presence point. In brief, each technique models the likelihood of the presence/absence of a flying-fox camp as a function of the environmental variables. Modelling was conducted with only category 2 and 3 LRFF camp sites (i.e. those sites that experts deemed to have a higher probability of representing actual camps as opposed to ephemeral roost sites; n = 271).
Broad-scale habitat assessment – environmental layer selection
A range of environmental data layers was considered based on our understanding of the conditions likely to influence camp site selection. All layers were available at, or resampled (using a nearest-neighbour algorithm) to, a resolution of 0.001° (giving a cell size of ~100 m). These layers are summarised below.
Temperature and humidity have a large influence on flying-foxes, with mass mortality events taking place during periods of temperature extremes (Welbergen et al. 2008). BioClim layers take standard measures of temperature and precipitation (averaged over the 30-year period from 1961 to 1990) and perform simple transformations to make them more biologically relevant (Kriticos et al. 2012). For example, daily maximum temperatures are used to assess seasonality of temperature by calculating the average temperature reached during the three-month period of highest temperature (i.e. the average summer temperature: BioClim10). An Australia-wide relative humidity layer (Williams et al. 2010) was also included.
A distance-to-watercourse layer was created in ArcGIS by running the Euclidean distance function on the national Geofabric V2 surface hydrology vector layer (BOM 2016). This resulted in a raster file with 25-m cells, with the value of each cell being the distance in metres from that cell to the nearest watercourse. The original hydrolines layer contains both permanent and ephemeral waterways, and both were used to generate the distance-to-watercourse layer used in the final model. As such, this layer represents the distance to the nearest watercourse, not necessarily the distance to the nearest water.
The Normalised Difference Vegetation Index (NDVI) represents the ‘greenness’ of a pixel from remotely sensed imagery having red and near-infrared spectral data available. Greenness is of interest because how much green light is reflected correlates with the amount (a greater amount of vegetation will reflect more green light) and type of vegetation at a site. Google Earth Engine was used to create an NDVI layer from 30 m Landsat 8 satellite imagery. Median values were extracted from the daily satellite imagery for 1–31 July 2019 (median values were used to remove outliers such as cloud cover).
In our study region, many of these environmental layers were highly correlated with each other, as is often the case in niche modelling (De Marco and Nóbrega 2018). After selecting those variables with the clearest biological relevance and then discarding as many as needed to ensure that no pairs of variables used in the model had correlation coefficients >0.65 (Fig. 2), we were left with four environmental layers: maximum near-surface temperature of the warmest quarter (BioClim05); annual mean precipitation (BioClim12); Normalised Difference Vegetation Index (NDVI); and distance to nearest watercourse.
Broad-scale habitat assessment – model performance
To test how repeatable the modelling was, each model was run 100 times with a randomised subset of the presence and absence points. In each iteration, 75% of the 189 presence points (category 2 and 3 camps) were randomly selected for training, with the remaining 25% used to validate the model. For each presence point, nine background points were randomly selected from the pool of 10 000 available background points (i.e. presence points represented 10% of the total number of points used to train the model). Each model was tested using the validation data, with the area under the curve calculated to test goodness-of-fit.
Once statistics about goodness-of-fit and the contribution of each environmental variable to the model were calculated via bootstrapping, the final model was generated using the technique that had the best performance, with all presence data (i.e. training data plus testing data) and again using nine random background points for each presence point. This model was used to predict the spatial distribution of habitat suitable for little red flying-fox camps across Queensland.
Results
Camp site locations and summary statistics
Our final camp list contained 338 sites (Fig. 3). Of these, 149 were visited and subjected to a vegetation assessment for use in the local-scale analysis. All category 2 (n = 122) and 3 (n = 67) camps were used in the broad-scale habitat suitability modelling.
Table 1 summarises key landscape metrics for LRFF camps. Camps were, on average, 21.6 km away from the nearest neighbouring camp, 11.8 km away from the nearest protected area (e.g. National Park), and 261.3 m away from the nearest watercourse (permanent or ephemeral). Camps had a mean Normalised Difference Vegetation Index of 0.45. This absolute value is less important than the relative NDVI values of the camp and its surrounding habitat (i.e. camps are located in those parts of the landscape that are greener than their surrounds).

|
Local-scale habitat assessment – camp vegetation
Little red flying-foxes were found to camp in a wide variety of vegetation types, ranging from short mangrove stands next to salt water, to tall riparian eucalypts along freshwater courses, through to trees in urban parks. A total of 56 tree species was recorded as the dominant species in the 143 category 2 and 3 camps for which we had vegetation data. The top 10 species, accounting for 68% of camps, were: Eucalyptus tereticornis (forest red gum, 28 camps), E. camaldulensis (river red gum; 12 camps), Melaleuca quinquenervia (broad-leaved paperbark; 12 camps), M. leucadendra (weeping paperbark; 12 camps), Rhizophora stylosa (spotted mangrove; 10 camps), Casuarina cunninghamiana (river oak; 9 camps), Avicennia marina (grey mangrove; 6 camps), Melaleuca bracteata (black tea-tree, 5 camps), Corymbia tessellaris (Moreton Bay ash, 4 camps), and Tamarindus indica (tamarind, an exotic species, 4 camps). Nine of the top 10 tree species were Australian native species, with the one exotic species being T. indica. The most common genera were: Eucalyptus (45 camps, 7 spp.), Melaleuca (35 camps, 8 spp.), Casuarina (11 camps, 2 spp.), Rhizophora (11 camps, 2 spp.), Avicennia (8 camps, 1 sp.), and Corymbia (5 camps, 2 spp.).
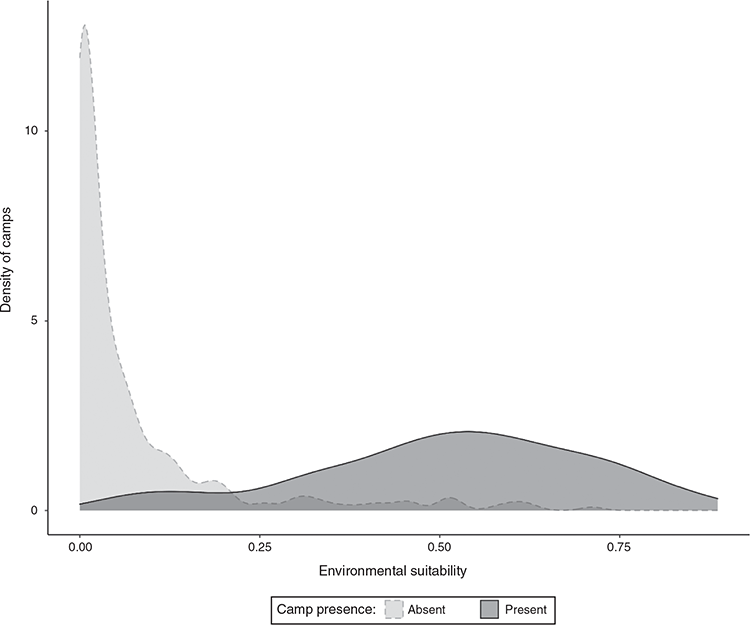
The height of the T1 tree layer ranged from 4 to 40 m (mean ± s.d. = 19.9 ± 8.9 m), and T2 ranged from 3 to 25 m (mean ± s.d. = 9.9 ± 4.8 m) (Fig. 4). While half of the camps were estimated to be within 200 m of water, some camps were as far away as 15 km (mean ± s.d. = 1.3 ± 2.7 km) (Fig. 5).
|
Local-scale habitat assessment – vegetation structure preferences
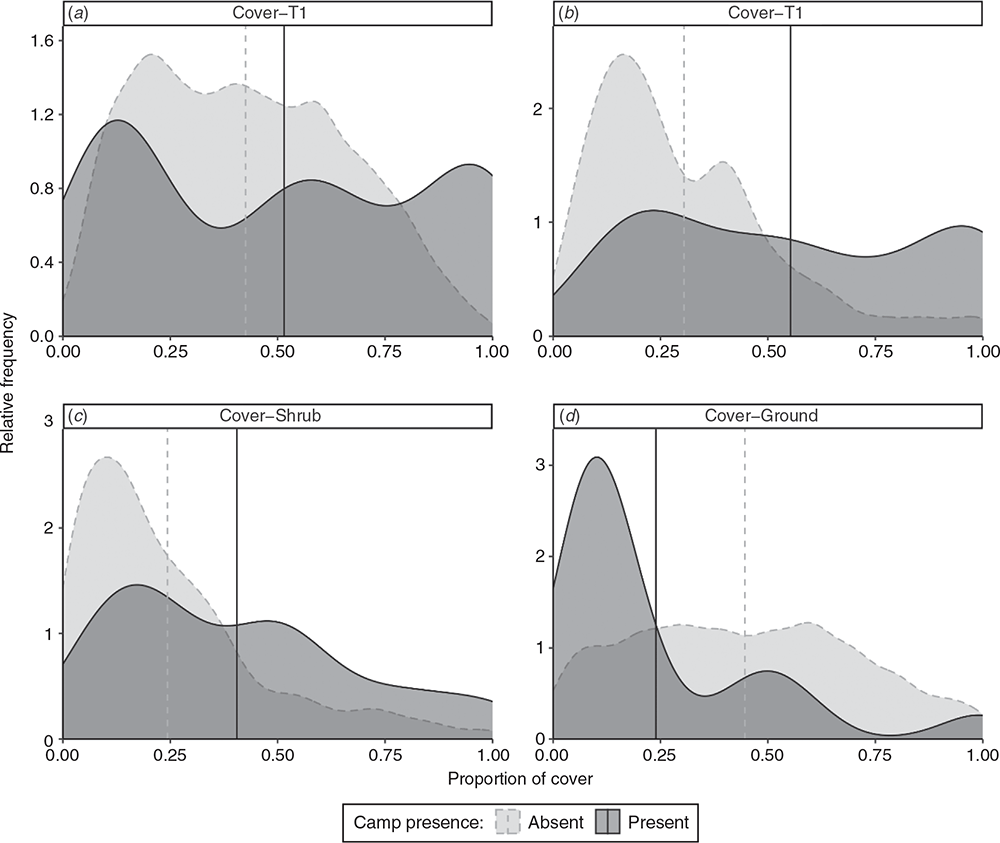
Comparison of the structural values taken from random background points (camp-absent) to those same values taken at camp sites (camp-present) provides an indication of the strength of LRFF selection for a particular habitat characteristic relative to its availability in the landscape. If camp sites are distributed randomly through the landscape, the used values will follow a similar pattern to the available values. If, however, camps are not distributed randomly, used values will skew differently. For example, Fig. 6d shows the proportion of cover of the ground layer at camp-present and camp-absent sites. That the camp-present peak between 0 and 0.25 is much higher than the camp-absent curve shows that more camp sites have much less groundcover than camp-absent sites in the surrounding area. However, camp sites typically have higher levels of cover for shrub and both tree layers (Fig. 6a–c). Likewise, there is a small trend towards camps having taller shrub and tree layers (Fig. 7).
Local-scale habitat assessment – regression model
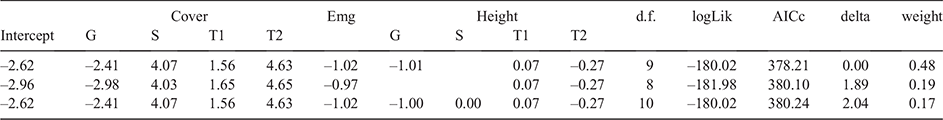
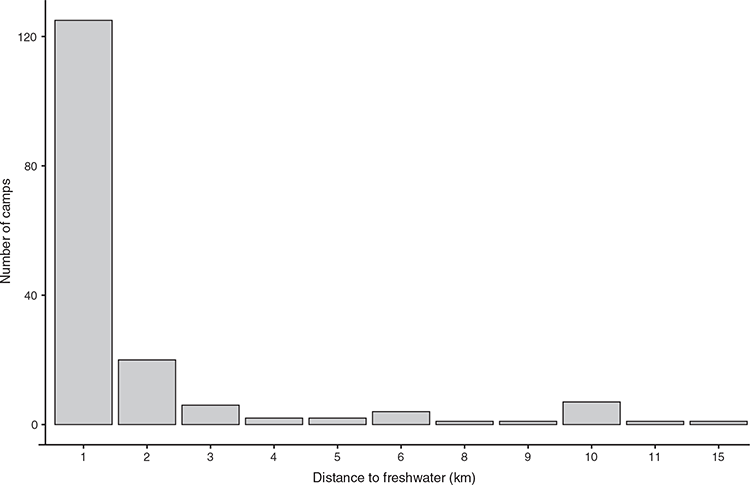
Initially, a full model containing all nine structural characteristics (i.e. emergent presence/absence, plus height and cover for T1, T2, S, and G layers) was developed. Model selection was then run on the full model to assess how removing variables affected model performance (Table 2). The top model contained all structural characteristics except shrub layer height. The second-best model excluded shrub layer height and ground layer height. The third-best model contained all nine variables.
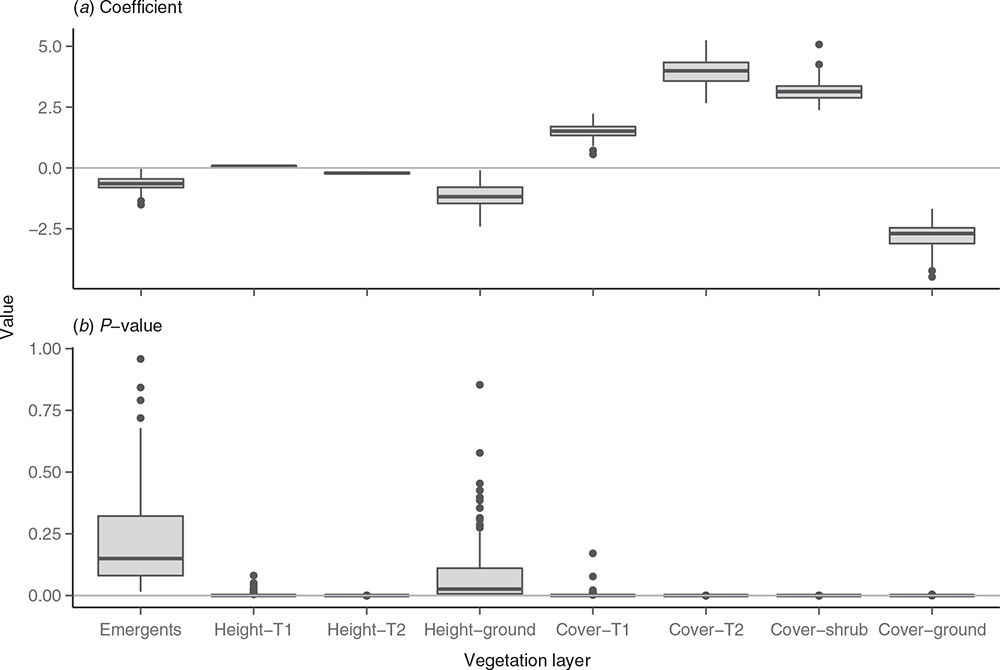
To test the repeatability of the model, the top model was run 1000 times with a random subset of the data (107 camp-present sites and 963 camp-absent sites on each iteration). Lower variance in the model coefficients (Fig. 8a) and P-values (Fig. 8b) for a vegetation layer indicates more confidence that that layer does indeed have a meaningful relationship with little red flying-fox camp presence or absence.
Table 3 shows mean coefficients and P-values for each vegetation characteristic. The four vegetation characteristics that consistently exhibited statistical significance (P < 0.05) were T2 height, T2 cover, shrub cover, and ground cover. Two additional characteristics (T1 height and T1 cover) had mean P-values <0.001. This shows that flying-fox camps are associated with increased T1 height and cover, increased shrub canopy cover, decreased levels of ground layer cover, and a decreased T2 layer height.
Broad-scale habitat assessment – model selection and variable ranking
The modelling technique that consistently performed best was the Random Forest algorithm, followed by MAXENT, GLM, and SVM (Fig. 9).

|
In a Random Forest model, the importance of each predictor variable is expressed as a percentage increase in the Mean Squared Error when the values of that variable are randomly permutated (note: these percentages do not add up to 100%). Importance rankings for each of the included variables are shown in Table 4, where larger numbers indicate more importance. Normalised Difference Vegetation Index was the most important variable, following by (in order) maximum temperature of the warmest month, annual precipitation, and distance to nearest watercourse.

|
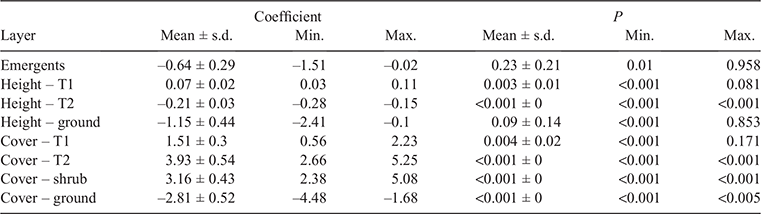
For each environmental variable included in the model, density plots were generated (Fig. 10) to compare the range of available values (camp-absent) to the range of utilised values (camp-present). In the plots below, if camp sites were located randomly across the landscape (i.e. if LRFFs had no strong preferences for where they camp), the two curves would be expected to have a similar shape. When the curves are not similar, it suggests that habitat selection preferences are operating. In this case, LRFF camps are located in greener, cooler areas with higher annual precipitation and that are closer to watercourses than would be expected by chance.
The effect of each individual environmental variable on the probability of occurrence across the range of that variable’s values is shown in Fig. 11. Probability of occurrence decreases with increasing maximum temperature of the warmest month (Fig. 11a), increases with increasing annual precipitation (Fig. 11b), increases with increasing NDVI (Fig. 11c), and decreases sharply with increasing distance from the nearest watercourse (Fig. 11d).
Relative humidity was not included in the model because it was highly correlated with other variables. Humidity is, however, thought to influence heat stress in flying-foxes (Welbergen 2017; Briscoe et al. 2020), with higher humidity meaning flying-foxes are less able to thermoregulate effectively, leading to higher mortality. Little red flying-fox camps are typically located in areas with higher-than-average humidity (Fig. 12). This is likely to be related to the thicker, greener vegetation in which they roost, and the close proximity of camps to watercourses.
Broad-scale habitat assessment – predicting habitat suitability
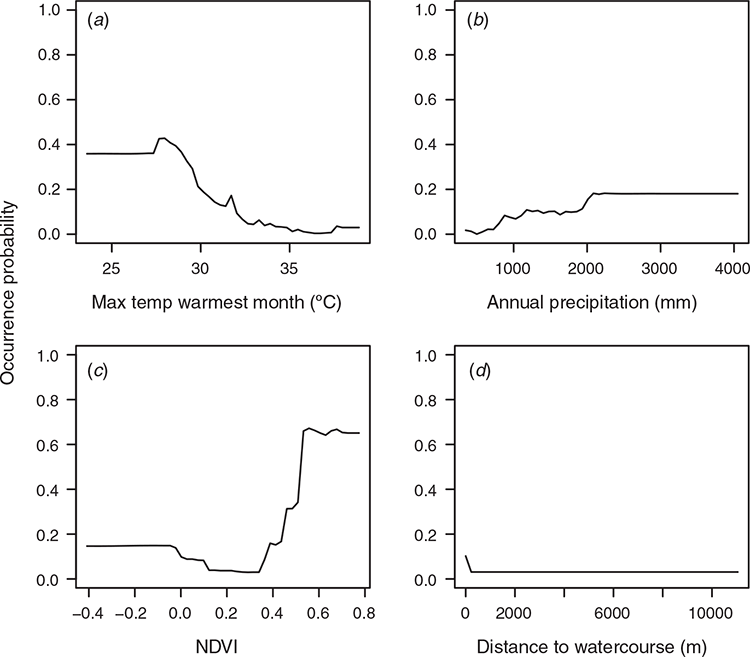
The best-performing model (Random Forest) was used to predict suitability for LRFF camp sites across Queensland (Fig. 13). Most camps (66%) are located in areas with suitability values 0.5 or higher (Fig. 14), the total state-wide area of which is 28 489 km2 (Table 5).

|
Discussion
Environmental features of camps
This modelling and analysis sought to identify the environmental characteristics associated with little red flying-fox camp sites at both the local (on the scale of metres) and landscape (kilometres) scale.
At the local scale, little red flying-fox camps were associated with: (1) a marginally taller canopy; (2) greater canopy and subcanopy cover, which are probably the most important characteristics as they provide structure to roost on and protection from sun, wind, and aerial predators such as birds of prey; (3) a marginally taller shrub layer with greater cover; and (4) a shorter, less dense groundcover layer, possibly due to increased canopy and shrub cover, and/or damage from falling branches and flying-fox faecal drop. Combined, these four characteristics correspond to forest locations with higher-than-average cover and suggest that sites that offer the animals comparatively high protection from the environment (e.g. protection from high temperatures, wind, predators) are preferred.
At the landscape scale, camps were found in locations that were strongly associated with four environmental traits: a vegetation feature, two climatic features, and a landscape context feature. These were: (1) lower maximum temperature of the warmest month, suggesting that LRFFs select camp sites to avoid temperature extremes; (2) higher annual precipitation; (3) higher NDVI, a measure of the amount of living, green vegetation at the site; and (4) shorter distance to nearest watercourse, including ephemeral watercourses. The importance of this variable was relatively small, possibly because watercourses are very common within the range of LRFFs (i.e. the availability of watercourses is not a limiting factor).
Taken at face value, these features suggest that LRFFs are seeking sites that offer protection from the environment (vegetation cover, access to water, protection from thermal extremes). Given the physiological implications of the very large surface-area-to-volume ratio of flying-foxes (Briscoe et al. 2020), this makes sense; well known mass mortality events occur in flying-foxes when temperatures reach extreme highs (Welbergen et al. 2008).
While these four environmental characteristics are only somewhat correlated with each other (Fig. 2), they are highly correlated with other environmental characteristics (e.g. NDVI is highly correlated with relative humidity). As such, it is important to remember that the relationships between LRFF camps and these environmental characteristics are correlative, not causative. It is highly likely that other environmental characteristics influence LRFF camp site selection, but our analysis has tried to distill these down to the most important environmental correlates: those that are ecologically relevant and that can provide some predictive power for modelling with data that are readily available for our study region (i.e. Queensland), and that can help inform camp management actions.
The wetter, moister habitat in which most LRFF camps are found is less likely to burn, as seen with grey-headed flying-fox camps (some of which are shared by LRFFs) during the 2019–2020 summer bushfires (unpubl. data, CSIRO, this study). While a large amount of habitat was burnt, only four of 67 surveyed GHFF camp sites were affected by fire (A. McKeown, pers. obs.).
The preference for wetter/greener areas may be apparent to anyone who has visited at least a few flying-fox camps. Fig. 15a–c shows the Mary Valley, Queensland, camp at various spatial scales. Satellite imagery shows the camp located in one section of the greener vine thicket that is surrounded by much drier/browner open woodland habitat. Fig. 15d is a photograph taken from ground level at a different camp (Copmanhurst, New South Wales), but that shows similarly structured vegetation to that found at the Mary Valley camp (dense vegetation with a thick canopy cover). The photographs of both camps show vegetation damage (broken and dead branches) caused by the large numbers of bats roosting at these sites.
Camp site selection
Our modelling shows that while many areas in the landscape are suitable, they do not contain camps. This suggests two things. First, that the availability of camp habitat is not limiting the selection of camps for LRFF at the state-scale, though in some areas (particularly in the west) this may not be the case. Second, that while suitable habitat may act to constrain the choice of camps, the choice of the specific site is likely to be a function of factors other than the environmental features considered in this analysis. Key candidates for these factors include: (1) the distribution and abundance of resources relative to the camp site; and/or (2) social influences such as knowledge of the use of the site in the past or the presence of other animals at the site serving to precipitate camp formation.
If suitable sites differ in their proximity to resources, we might expect that LRFFs would choose sites where the trade-off between resource availability and resource accessibility is optimised. In other words, locations that minimised the cost of accessing resources (i.e. the distance that little red flying-foxes need to fly each night to their foraging grounds) relative to the benefit gained (i.e. the amount of food available at a foraging location). This would suggest that camp sites are likely to be located centrally relative to the distribution of resources in a region. This aspect is currently under investigation.
However, the availability of large areas of suitable habitat (Fig. 13) suggests that while focusing on locations that optimise returns on foraging may further constrain the choice, even then, in most parts of the LRFF range, this would not necessarily highlight one particular camp location out of the possible suite of available sites. At this point, a significant influence is likely to be either the memory of the past use of a site by little red flying-foxes (although we have no data on LRFF longevity, other flying-fox species can live for over 30 years: Wilkinson and South 2002) and/or the current presence of other flying-foxes (LRFF or otherwise) serving to attract others and resulting in camp formation. Coloniality in flying-foxes could confer several advantages. Information about feeding resources could be exchanged, either through communication at the camp or simply by following another flying-fox as it heads out to forage. While information transfer was not demonstrated in a study of insectivorous bats (Kerth et al. 2001), this has not been studied in flying-foxes. Many flying-fox species are known to forage together (including LRFFs), so information transfer could conceivably be a benefit of roosting communally. Other benefits could include protection from predators (i.e. safety in numbers) or cooperative breeding.
All of these influences may occur simultaneously, with the camp site selection process probably involving multiple individuals and operating at a variety of temporal and spatial scales with at least the following steps: (1) find a region with adequate foraging resources; (2) within this region, find areas that provide efficient (or adequate) access to resources; (3) within these areas, find potential camp sites with appropriate habitat features; (4) of these sites, prefer those that have a known occupation history; and (5) of these, prefer those that are currently occupied by other flying-foxes.
The above steps are also likely to operate over multiple temporal scales. Camps are often ‘abandoned’ for part of the year and then reused, or abandoned for multiple years before being occupied again. This may be due to changes in the seasonal or long-term availability of feeding resources in the area, or due to changes in camp suitability over the shorter term. Over time, camp sites that were once highly suitable might decrease in suitability (due to, for example, habitat modification or climate change). Despite this decrease in suitability, flying-foxes may continue to roost there due to their familiarity with the site – they’ve roosted there for many years, and may continue to do so even though there might now be better sites available within the local region. This could be due to flying-foxes just looking for sites that are ‘good enough’, rather than trying to select sites that optimise all criteria. If flying-foxes (1) have a wide tolerance for camp site habitat, (2) are mainly driven by food availability in the surrounding region, and (3) have strong long-term memory for camp locations, then camp site management will always be difficult. However, there are actions that can be taken to better manage problematic flying-fox camps.
Implications for camp management
While larger-scaled movements are likely to be influenced by the availability of foraging habitat, altering roosting habitat is increasingly seen as an integral part of the management of human–flying-fox conflict. Management actions might aim to create new roost sites or to make part or all of existing sites more or less attractive to the animals. Given that there is no indication that suitable camp sites are a limiting factor, it is unlikely that modifying a potential roost site to enhance its suitability alone will result in a flying-fox colony immediately taking up residence. However, recent management experience makes it clear that flying-foxes can be encouraged to move within a camp and, in some circumstances, to new sites at specific locations. For example, the dispersal of Melbourne’s Royal Botanic Gardens camp evolved from a dispersal that scattered the animals across the city and beyond into an exercise of ‘herding’ the coalescing remnants along the Yarra River to their current location at Yarra Bend, several kilometres away from their original Botanic Gardens camp (Roberts et al. 2011). These experiences suggest that patient, directed dispersals are likely to be most successful, particularly when trying to relocate animals to distant sites.
Management of vegetation in these locations (both within an existing camp or at a new location) to make it more attractive should increase both the probability of successfully relocating the animals and of keeping them in the new location. Our results suggest that management actions or choice of sites should focus on the following: (1) greenness, including the amount of canopy cover and structure; (2) open water for drinking and cooling; and (3) avoidance of climatic extremes.
Camp habitat augmentation
Despite the uncertainty of its effectiveness, it may be worthwhile modifying habitat to increase suitability/attractiveness to LRFFs (and, likely, other flying-fox species) to encourage them to roost in a location more acceptable to humans. Ideally, sites along watercourses would be identified early on in the town planning process and buffers set aside to ensure that no infrastructure encroaches on these areas likely to be used by flying-foxes. This potential roosting habitat could then be modified using the following guidelines (note: not all suggestions will apply to all sites).
Increase vegetation greenness. While the NDVI values of camp sites are significantly higher than those of non-camp sites, NDVI is a proxy. Increasing a site’s NDVI values by establishing a lush grass layer is unlikely to be of use to roosting bats. Instead, efforts should be directed towards increasing the volume of vegetation at a site, to provide shade and roosting structure. Ensure that all trees are well watered, especially during times of drought and especially young plants (see below). Greenness (as determined by NDVI) can be measured over time to assess changes using time-series remote sensing data such as satellite imagery (Donohue et al. 2009).
Prepare for future vegetation turnover by planting additional trees of appropriate species. For camps, it is more important to plant/manage trees that provide structure rather than food. If planting in an existing camp site, using the same tree species that flying-foxes are currently roosting in would be most appropriate (assuming that the existing tree species are well suited to that local environment). Although planting native trees should be encouraged, many flying-fox camps are dominated by species that are not native to the local region (e.g. large figs in parks and botanical gardens).
Provide a permanent water source that is large enough for flying-foxes to skim across the surface and free from obstructions to enable clear entry and exit flight paths. Unfortunately, we do not have any data that can be used to suggest what size would be ‘large enough’. If the area is next to a seasonal watercourse, placing a bund across the downstream side of the watercourse will allow water to pool there after it ceases flowing. This could be augmented with water from a bore or other supply.
Heatwaves are a well known cause of mass mortality events in flying-foxes. This is particularly true for black and spectacled flying-foxes, but LRFFs can also be affected (Briscoe et al. 2020). Passive ways to control temperatures at a site include ensuring that ample tree cover and structure is available so that animals can remain in the shade and select their preferred height to minimise their exposure. Active techniques can include spraying water to allow for evaporative cooling, but care must be taken to ensure that humidity is not increased to the point where evaporative cooling is no longer effective. This is an area of active research, but more data are needed before an optimal strategy can be developed. Such strategies will need to account for local site and weather conditions (e.g. spraying may be more effective on days with moderate wind to help with evaporative cooling).
Making existing habitat less attractive
Conversely, the management decision might be to make existing habitat less attractive to either encourage the animals to shift location within a camp or abandon the camp entirely, the extreme case of which would be the total removal of vegetation. A less extreme approach would be to: (1) cease watering a site if this occurs; (2) remove access to open water sources, e.g. empty park ponds or fountains, put nets over swimming pools; and (3) modify vegetation to thin the canopy, especially via the removal of horizontal branches that could be used for roosting.
Other methods that have been tried include using model predators (e.g. birds of prey) and positioning predator scents (e.g. python faecal matter) at the site camp site (Lunney and Moon 1997). However, flying-foxes may rapidly become accustomed to these fake predator cues, making these methods ineffective in the long term.
If attempts to encourage or discourage flying-foxes to/from a camp site are made, all relevant details and results should be recorded and published (or otherwise made available) so that future attempts can be informed by past successes/failures.
Conclusion
Although this study has been unable to comprehensively determine clear drivers for where little red flying-foxes choose to roost, we have identified site- and landscape-scale structural and environmental characteristics that are strongly correlated with known LRFF camp site locations. Identifying specific camp site characteristics that appear to be important to LRFFs will enable land managers to assess the suitability of their local region for LRFF roosting sites. This can be used to predict where LRFFs might establish a new camp (after an influx or dispersal event), and it can also be used to modify existing sites to make them more or less attractive to LRFFs. On-going work by CSIRO involving satellite tracking of LRFFs will continue to further our understanding of their movement patterns, enabling land and wildlife managers to better deal with human–flying-fox conflicts as they arise.
Data availability statement
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This project was in part funded by the Queensland Department of Science.
Acknowledgements
We thank staff members from the Queensland Herbarium for allowing access to their CORVEG data, with particular thanks to Theresa Eyre and Melanie Venz. Thanks also to Tim McVicar from CSIRO for helpful feedback on an earlier version of this manuscript.
References
Anon, (1890). Orchard Pests: Experiments on flying-foxes with explosives. Agricultural Gazette of New South Wales 1, 105–107.BOM (2016). Australian hydrological geospatial fabric (Geofabric) product. Available at: http://www.bom.gov.au/water/geofabric/download.shtml
Brewer, M. J., Butler, A., and Cooksley, S. L. (2016). The relative performance of AIC, AICC and BIC in the presence of unobserved heterogeneity. Methods in Ecology and Evolution 7, 679–692.
| The relative performance of AIC, AICC and BIC in the presence of unobserved heterogeneity.Crossref | GoogleScholarGoogle Scholar |
Briscoe, N., Ratnayake, H., and Kearney, M. (2020). Predicting heat stress of flying-foxes using a biophysical model. Technical report, University of Melbourne. 68 pp.
De Marco, P. Jr, and Nóbrega, C. C. (2018). Evaluating collinearity effects on species distribution models: an approach based on virtual species simulation. PLoS One 13, e0202403.
| Evaluating collinearity effects on species distribution models: an approach based on virtual species simulation.Crossref | GoogleScholarGoogle Scholar |
Donohue, R. J., McVicar, T. R., and Roderick, M. L. (2009). Climate-related changes in Australian vegetation cover as inferred from satellite observations for 1981–2006. Global Change Biology 15, 1025–1039.
| Climate-related changes in Australian vegetation cover as inferred from satellite observations for 1981–2006.Crossref | GoogleScholarGoogle Scholar |
Elith, J., and Leathwick, J. R. (2009). Species distribution models: ecological explanation and prediction across space and time. Annual Review of Ecology, Evolution, and Systematics 40, 677–697.
| Species distribution models: ecological explanation and prediction across space and time.Crossref | GoogleScholarGoogle Scholar |
Giles, J. R., Eby, P., Parry, H., Peel, A. J., Plowright, R. K., Westcott, D. A., and McCallum, H. (2018). Environmental drivers of spatiotemporal foraging intensity in fruit bats and implications for Hendra virus ecology. Scientific Reports 8, 9555.
| Environmental drivers of spatiotemporal foraging intensity in fruit bats and implications for Hendra virus ecology.Crossref | GoogleScholarGoogle Scholar | 29934514PubMed |
Gulraiz, T. L., Javid, A., Mahmood-Ul-Hassan, M., Maqbool, A., Ashraf, S., Hussain, M., and Daud, S. (2015). Roost characteristics and habitat preferences of Indian flying fox (Pteropus giganteus) in urban areas of Lahore, Pakistan. Turkish Journal of Zoology 39, 388–394.
| Roost characteristics and habitat preferences of Indian flying fox (Pteropus giganteus) in urban areas of Lahore, Pakistan.Crossref | GoogleScholarGoogle Scholar |
Hahn, M. B., Epstein, J. H., Gurley, E. S., Islam, M. S., Luby, S. P., Daszak, P., and Patz, J. A. (2014). Roosting behaviour and habitat selection of Pteropus giganteus reveal potential links to Nipah virus epidemiology. Journal of Applied Ecology 51, 376–387.
| Roosting behaviour and habitat selection of Pteropus giganteus reveal potential links to Nipah virus epidemiology.Crossref | GoogleScholarGoogle Scholar |
Hall, L. (2002). Management of Flying-fox camps: What have we learnt in the last twenty five years? In ‘Managing the Grey-headed Flying-fox: as a Threatened Species in NSW’. (Eds P. Eby, and D. Lunney.) pp. 215–224. (Royal Zoological Society of New South Wales: New South Wales.)
Hall, L. S., and Richards, G. (2000). ‘Flying Foxes: Fruit and Blossom Bats of Australia.’ (UNSW Press.)
Halpin, K., Young, P. L., Field, H., and Mackenzie, J. S. (1999). Newly discovered viruses of flying foxes. Veterinary Microbiology 68, 83–87.
| Newly discovered viruses of flying foxes.Crossref | GoogleScholarGoogle Scholar | 10501164PubMed |
Kerth, G., Wagner, M., and König, B. (2001). Roosting together, foraging apart: information transfer about food is unlikely to explain sociality in female Bechstein’s bats (Myotis bechsteinii). Behavioral Ecology and Sociobiology 50, 283–291.
| Roosting together, foraging apart: information transfer about food is unlikely to explain sociality in female Bechstein’s bats (Myotis bechsteinii).Crossref | GoogleScholarGoogle Scholar |
Kriticos, D. J., Webber, B. L., Leriche, A., Ota, N., Macadam, I., Bathols, J., and Scott, J. K. (2012). CliMond: global high resolution historical and future scenario climate surfaces for bioclimatic modelling. Methods in Ecology and Evolution 3, 53–64.
| CliMond: global high resolution historical and future scenario climate surfaces for bioclimatic modelling.Crossref | GoogleScholarGoogle Scholar |
Krystufek, B. (2009). Indian flying fox Pteropus giganteus colony in Peradeniya Botanical Gardens, Sri Lanka. Hystrix 20, 29–35.
Kushnir, H., and Packer, C. (2019). Perceptions of risk from man-eating lions in southeastern Tanzania. Frontiers in Ecology and Evolution 7, 47.
| Perceptions of risk from man-eating lions in southeastern Tanzania.Crossref | GoogleScholarGoogle Scholar |
Lunney, D., and Moon, C. (1997). Flying-foxes and their camps in the remnant rainforests of north-eastern New South Wales. In ‘Australia’s Ever-Changing Forests III: Proceedings of the Third National Conference on Australian Forest History’. (Eds K. Frawley and J. Dargavel.) pp. 247–277. (CRES, ANU: Canberra.)
Madden, F. (2004). Creating coexistence between humans and wildlife: global perspectives on local efforts to address human–wildlife conflict. Human Dimensions of Wildlife 9, 247–257.
| Creating coexistence between humans and wildlife: global perspectives on local efforts to address human–wildlife conflict.Crossref | GoogleScholarGoogle Scholar |
Markus, N., and Hall, L. (2004). Foraging behaviour of the black flying-fox (Pteropus alecto) in the urban landscape of Brisbane, Queensland. Wildlife Research 31, 345–355.
| Foraging behaviour of the black flying-fox (Pteropus alecto) in the urban landscape of Brisbane, Queensland.Crossref | GoogleScholarGoogle Scholar |
McDonald‐Madden, E., Schreiber, E. S. G., Forsyth, D. M., Choquenot, D., and Clancy, T. F. (2005). Factors affecting grey‐headed flying‐fox (Pteropus poliocephalus: Pteropodidae) foraging in the Melbourne metropolitan area, Australia. Austral Ecology 30, 600–608.
| Factors affecting grey‐headed flying‐fox (Pteropus poliocephalus: Pteropodidae) foraging in the Melbourne metropolitan area, Australia.Crossref | GoogleScholarGoogle Scholar |
Nyhus, P. J. (2016). Human–wildlife conflict and coexistence. Annual Review of Environment and Resources 41, 143–171.
| Human–wildlife conflict and coexistence.Crossref | GoogleScholarGoogle Scholar |
Palmer, C., and Woinarski, J. C. Z. (1999). Seasonal roosts and foraging movements of the black flying-fox (Pteropus alecto) in the Northern Territory: resource tracking in a landscape mosaic. Wildlife Research 26, 823–838.
| Seasonal roosts and foraging movements of the black flying-fox (Pteropus alecto) in the Northern Territory: resource tracking in a landscape mosaic.Crossref | GoogleScholarGoogle Scholar |
Parris, K. M., and Hazell, D. L. (2005). Biotic effects of climate change in urban environments: the case of the grey-headed flying-fox (Pteropus poliocephalus) in Melbourne, Australia. Biological Conservation 124, 267–276.
| Biotic effects of climate change in urban environments: the case of the grey-headed flying-fox (Pteropus poliocephalus) in Melbourne, Australia.Crossref | GoogleScholarGoogle Scholar |
Pérez, E., and Pacheco, L. F. (2006). Damage by large mammals to subsistence crops within a protected area in a montane forest of Bolivia. Crop Protection 25, 933–939.
| Damage by large mammals to subsistence crops within a protected area in a montane forest of Bolivia.Crossref | GoogleScholarGoogle Scholar |
Ratcliffe, F. N. (1931). ‘The Flying-fox (Pteropus) in Australia.’ (Australian Government Printer.)
Roberts, B., and Eby, P. (2013). Review of past flying-fox dispersal actions between 1990–2013. Unpublished report. Available at https://www.environment.nsw.gov.au/resources/animals/flying-fox-2014-subs/flyingfoxsub-jenny-beatson-part2.pdf
Roberts, B. J., Eby, P., Catterall, C. P., Kanowski, J., and Bennett, G. (2011). The outcomes and costs of relocating flying-fox camps: insights from the case of Maclean, Australia. In ‘The Biology and Conservation of Australasian Bats’. (Eds B. Law, P. Eby, D. Lunney, and L. Lumsden.) pp. 277–287. (Royal Zoological Society of NSW: Mosman, NSW.)
Roberts, B. J., Catterall, C. P., Eby, P., and Kanowski, J. (2012). Long-distance and frequent movements of the flying-fox Pteropus poliocephalus: implications for management. PLoS One 7, e42532.
| Long-distance and frequent movements of the flying-fox Pteropus poliocephalus: implications for management.Crossref | GoogleScholarGoogle Scholar | 22880021PubMed |
Sattler, P., and Williams, R. (1999). The conservation status of Queensland’s bioregional ecosystems. Environmental Protection Agency, Brisbane.
Tait, J., Perotto-Baldivieso, H. L., McKeown, A., and Westcott, D. A. (2014). Are flying-foxes coming to town? Urbanisation of the spectacled flying-fox (Pteropus conspicillatus) in Australia. PLoS One 9, e109810.
| Are flying-foxes coming to town? Urbanisation of the spectacled flying-fox (Pteropus conspicillatus) in Australia.Crossref | GoogleScholarGoogle Scholar | 25295724PubMed |
Tench, W. (1793). A complete account of the settlement at Port Jackson. Available at: http://setis.library.usyd.edu.au/ozlit/pdf/p00044.pdf
Thanapongtharm, W., Linard, C., Wiriyarat, W., Chinsorn, P., Kanchanasaka, B., Xiao, X., Biradar, C., Wallace, R. G., and Gilbert, M. (2015). Spatial characterization of colonies of the flying fox bat, a carrier of Nipah Virus in Thailand. BMC Veterinary Research 11, 81.
| Spatial characterization of colonies of the flying fox bat, a carrier of Nipah Virus in Thailand.Crossref | GoogleScholarGoogle Scholar | 25880385PubMed |
Tidemann, C. R., Vardon, M. J., Loughland, R. A., and Brocklehurst, P. J. (1999). Dry season camps of flying-foxes (Pteropus spp.) in Kakadu World Heritage Area, north Australia. Journal of Zoology 247, 155–163.
| Dry season camps of flying-foxes (Pteropus spp.) in Kakadu World Heritage Area, north Australia.Crossref | GoogleScholarGoogle Scholar |
Waltner-Toews, D. (2017). Zoonoses, One Health and complexity: wicked problems and constructive conflict. Philosophical Transactions of the Royal Society B: Biological Sciences 372, 20160171.
| Zoonoses, One Health and complexity: wicked problems and constructive conflict.Crossref | GoogleScholarGoogle Scholar |
Welbergen, J. A. (2017). Responding to heat stress in flying-fox camps. Available at https://www.animalecologylab.org/uploads/4/4/9/0/44908845/ff_heat_stress_management_responses.pdf
Welbergen, J. A., Klose, S. M., Markus, N., and Eby, P. (2008). Climate change and the effects of temperature extremes on Australian flying-foxes. Proceedings of the Royal Society B: Biological Sciences 275, 419–425.
| Climate change and the effects of temperature extremes on Australian flying-foxes.Crossref | GoogleScholarGoogle Scholar | 18048286PubMed |
Welbergen, J. A., Meade, J., Field, H. E., Edson, D., McMichael, L., Shoo, L. P., Praszczalek, J., Smith, C., and Martin, J. M. (2020). Extreme mobility of the world’s largest flying mammals creates key challenges for management and conservation. BMC Biology 18, 101.
| Extreme mobility of the world’s largest flying mammals creates key challenges for management and conservation.Crossref | GoogleScholarGoogle Scholar | 32819385PubMed |
Westcott, D., McKeown, A., Parry, H., Parsons, J., Jurdak, R., Kusy, B., and Caley, P. (2015). Implementation of the national flying-fox monitoring program. Rural Industries Research and Development Corporation, Canberra. Available at http://www.environment.gov.au/node/16393
Wilkinson, G. S., and South, J. M. (2002). Life history, ecology and longevity in bats. Aging Cell 1, 124–131.
| Life history, ecology and longevity in bats.Crossref | GoogleScholarGoogle Scholar | 12882342PubMed |
Williams, K., Stein, J., Storey, R., Ferrier, S., Austin, M., Smyth, A., & Harwood, T. (2010). 0.01 degree stack of climate layers for continental analysis of biodiversity pattern, version 1.0. v2. CSIRO Data Collection.