Intra- and interspecific agonistic behaviour in hatchling Australian freshwater crocodiles (Crocodylus johnstoni) and saltwater crocodiles (Crocodylus porosus)
Matthew L. Brien A B D , Grahame J. Webb A B , Jeffrey W. Lang C and Keith A. Christian AA Research Institute for the Environment and Livelihoods, Charles Darwin University, Darwin, NT 0909, Australia.
B Wildlife Management International Pty Ltd, PO Box 530, Karama, NT 0813, Australia.
C Department of Fisheries, Wildlife and Conservation Biology, University of Minnesota, Saint Paul, MN 55108, USA.
D Corresponding author. Email: mbrien@wmi.com.au
Australian Journal of Zoology 61(3) 196-205 https://doi.org/10.1071/ZO13035
Submitted: 6 May 2013 Accepted: 1 July 2013 Published: 24 July 2013
Abstract
We examined agonistic behaviour in hatchling Australian freshwater crocodiles (Crocodylus johnstoni) at 2 weeks, 13 weeks, and 50 weeks after hatching, and between C. johnstoni and saltwater crocodiles (Crocodylus porosus) at 40–50 weeks of age. Among C. johnstoni, agonistic interactions (15–23 s duration) were well established by two weeks old and typically involved two and occasionally three individuals, mostly between 17 : 00 and 24 : 00 hours in open-water areas of enclosures. A range of discrete postures, non-contact and contact movements are described. The head is rarely targeted in contact movements with C. johnstoni because they exhibit a unique ‘head raised high’ posture, and engage in ‘push downs’. In contrast with C. porosus of a similar age, agonistic interactions between C. johnstoni were conducted with relatively low intensity and showed limited ontogenetic change; there was also no evidence of a dominance hierarchy among hatchlings by 50 weeks of age, when the frequency of agonistic interactions was lowest. Agonistic interactions between C. johnstoni and C. porosus at 40–50 weeks of age were mostly low level, with no real exclusion or dominance observed. However, smaller individuals of both species moved slowly out of the way when a larger individual of either species approached. When medium- or high-level interspecific interactions did occur, it was between similar-sized individuals, and each displayed species-specific behaviours that appeared difficult for contestants to interpret: there was no clear winner or loser. The nature of agonistic interactions between the two species suggests that dominance may be governed more strongly by size rather than by species-specific aggressiveness.
Introduction
Agonistic behaviour is any behaviour relating to aggression, including threat, display, attack, submission and flight (Tinbergen 1952, 1953; Wilson 1975). Agonistic behaviour plays a crucial role in determining access to resources such as food, shelter and mates in many species of bird (Drummond 2001), reptile (Phillips et al. 1993), crustacean (Huber and Kravitz 1995), fish (Genner et al. 1999), and amphibian (Staub 1993). For many of these species, agonistic behaviour starts shortly after birth and is important in the formation of dominance hierarchies and in determining future rates of growth and survival (Tinbergen 1953; de Waal and Luttrell 1989; Drummond 2006). Therefore, studies of agonistic behaviour during early development can greatly improve our understanding of the social structuring and population dynamics of a species.
The role of agonistic behaviour in interspecific competition is not as well understood. It can be expected to occur between species that exist in sympatry and utilise similar resources (Brown and Wilson 1956; Ricklefs 1990), and may well contribute to divergence in morphology, behaviour and ultimately niche partitioning (Brown and Wilson 1956; Adams 2004), as proposed for mammals (Stoecker 1972; Pereira and Kappeler 1997; Aguirre et al. 2002), birds (Schoener 1965; Murray 1971; Smith 1990), salamanders (Jaeger 1972; Adams 2004), fish (Newman 1956) and crustaceans (Bovbjerg 1970).
Knowledge of agonistic behaviour in crocodilians is mostly associated with adults and breeding biology, in wild and captive situations (Cott 1961; Pooley 1969; Garrick and Lang 1977; Garrick et al. 1978; Vliet 1989; Thorbjarnarson 1991). With saltwater crocodiles (Crocodylus porosus), which are considered the most aggressive of extant crocodilian species (Lang 1987), they begin life in hatchling crèches and dominance hierarchies become established within the first three weeks of life (Brien et al. 2013). Intolerance of conspecifics has long been implicated in their dispersal from crèches after a few months, and in the later spatial separation of juveniles and adults (Webb et al. 1977; Messel et al. 1981; Lang 1987). The degree to which agonistic behaviour occurs in less aggressive crocodilian species is unknown.
Australian freshwater crocodiles (Crocodylus johnstoni) are regarded as one of the least aggressive crocodilians (Lang 1987). Like C. porosus, they exist in crèches after hatching, but unlike C. porosus, juvenile and adult C. johnstoni often congregate at high densities during the annual dry season (Webb et al. 1983a; Kennett and Christian 1993). They thus appear much more tolerant of conspecifics, although some discrete behaviours such as tail raking, are implicated in dominance hierarchies (Webb et al. 1983c). Although C. johnstoni and C. porosus have largely allopatric distributions in northern Australia, they also coexist within zones of sympatry in some rivers and wetlands (Webb et al. 1983a). Despite the larger size of adult C. porosus, and the strong possibility that adult C. porosus may control C. johnstoni populations in such zones (Webb et al. 1983a), juveniles of each species live together with similar size-specific morphology, diet and spatial needs (Messel et al. 1981; Webb et al. 1983a), which is reasonably uncommon in situations where crocodilian distributions overlap (Ouboter 1996; Thorbjarnarson et al. 2006).
The present study was undertaken with two primary aims. First, to describe agonistic behaviour in hatchling C. johnstoni, and determine whether the nature and ontogeny is similar to that observed in hatchling C. porosus (Brien et al. 2013). Second, to examine how C. johnstoni and C. porosus respond during agonistic interactions involving both species.
Materials and methods
Subjects and housing
In December 2011, 70 hatchling Australian freshwater crocodiles (Crocodylus johnstoni), in three age cohorts, were provided by Wildlife Management International (Darwin, Australia). All had been captured in the wild from crèches when <1 week after hatching (based on size and extent of yolk scar healing), and were almost certainly siblings. The first cohort (n = 25) contained individuals from five different clutches (20 males, 5 females), two weeks after hatching, while the second (13 weeks after hatching; n = 25; 19 males, 6 females) and third (50 weeks after hatching; n = 25; 20 males, 5 females) cohorts contained hatchlings from a further six and seven different clutches, respectively, that were of similar size and age. These age cohorts were based on the early life history of C. johnstoni, which is similar to that described for C. porosus (Brien et al. 2013). This also enabled direct comparisons. Using the same crocodiles throughout the study was initially considered but not feasible, due to the highly variable nature of growth within and between different clutches for this species and crocodilians in general, which can lead to significant differences in size and high rates of mortality during the first year of life under captive conditions (Webb et al. 1983b; Brien et al. 2013).
Hatchling C. johnstoni of different ages were transferred to experimental enclosures and housed in groups of five individuals for the duration of the study. For each animal, snout–vent length (SVL to the front of the cloaca, in millimetres), total length (TL, in millimetres), and body mass (BM, in grams) were measured and a uniquely numbered metal webbing tag (Small animal tag 1005-3: National Band and Tag Co.) was attached to the rear back right foot. Mean size of crocodiles at the initiation of observations were: 2-week-olds: 110.5 ± 0.5 mm SVL, 245.3 ± 1.1 mm TL, and 42.7 ± 0.8 g mass; 13-week-olds: 147.0 ± 1.7 mm SVL, 316.8 ± 2.8 mm TL and 76.3 ± 1.8 g mass; 50-week-olds: 305.5 ± 1.7 mm SVL, 618.8 ± 2.3 mm TL, and 628.2 ± 14.5 g mass.
In February 2012, 12 hatchling C. porosus at 40 weeks after hatching (10 male, 2 female) and 12 hatchling C. johnstoni of a similar size and age (50 week after hatching, 9 male, 3 female) were also provided by Wildlife Management International. However, while the three age cohorts of C. johnstoni were all captured from the wild, the 40-week-old C. porosus were from seven different clutches of eggs artificially incubated in captivity (see Brien et al. 2013). In the wild, this is the age and/or size at which C. johnstoni and C. porosus are naturally found occupying similar habitat (Webb et al. 1983a). Hatchlings of both species were transferred to experimental enclosures and housed in six groups of four individuals (two C. porosus and two C. johnstoni), each containing two similar-size ‘large’ crocodiles of each species (CJ: 345.2 ± 3.7 mm SVL, 700.5 ± 7.4 mm TL, 957.2 ± 34.7 g mass; CP: 348.8 ± 3.1 mm SVL, 709.7 ± 6.7 mm TL, 952.2 ± 24.8 g mass) and two similar size ‘small’ crocodiles of each species (CJ: 305.5 ± 1.7 mm SVL, 618.8 ± 2.3 mm TL, 628.2 ± 14.5 g mass; CP: 300.3 ± 2.8 mm SVL, 624.0 ± 3.9 mm TL, 632.2 ± 10.8 g mass).
The fibreglass enclosures in which the experimental groups (C. johnstoni only, C. johnstoni and C. porosus combined) were housed were box-shaped (170 × 100 × 50 cm), with a land area (70 × 100 cm) that gradually sloped down to a water area (100 × 100 cm; ≤8 cm deep). Therefore, all hatchlings were at a density of 2.9 animals m–2, which was considered very low on the basis of previous studies (Riese 1991; Mayer 1998). A ‘hide area’ (Riese 1991; Mayer 1998; Davis 2001) was provided in each enclosure, and was constructed with eight lengths of PVC pipe (80 cm long; 10 cm diameter) strapped together and on legs, centrally positioned in the water (partly immersed) and overhanging the land. Water temperatures were maintained at 30−32°C, and air temperatures were 26−32°C, with a natural light cycle. All animals were fed chopped red meat supplemented with dicalcium phosphate (4%) and a multivitamin supplement (1%), which is the standard diet fed to hatchling C. johnstoni and C. porosus in captivity. Enough food for each individual was made available throughout the night (16:00–17 : 00 hours), which is when hatchling C. johnstoni and C. porosus are known to feed in captivity and in the wild (Lang 1987; Brien et al. 2013). Waste removal and cleaning occurred the following morning (08:00–09 : 00 hours) when the water was changed. Hatchlings of both species were subject to the same raising conditions (enclosure, temperature, density, and husbandry) before and during the experiments.
Recording behaviour
Wide-angle infrared CCTV cameras (Signet, 92.6°) in each enclosure were used to record all behaviour on digital video recorders (Signet 4CH QV-8104). For the three cohorts of C. johnstoni, a recording period lasted 15 h (17:00–08 : 00 hours), and was conducted on three consecutive nights for each group (45 h per group). To allow for settling, the recordings were taken 4–5 days after the crocodiles were placed in the new experimental enclosures. This night-time sampling period was based on previous recordings (hundreds of hours) that revealed no agonistic behaviour in C. johnstoni hatchlings between 08 : 00 and 17 : 00 hours, when they were inactive and under cover. For the mixed-species groups, recordings were taken only during the first two nights (17:00–08 : 00 hours) when, from previous experience, the level of aggression and frequency of interactions is typically high. Despite the documented importance of vocalisations during crocodilian communication at all life stages (Lang 1987), especially among hatchlings and juveniles, no audio was recorded during this study due to limitations of the recording equipment.
Agonistic interactions
An agonistic interaction was defined as any interaction between individuals in which aggression and intolerance appeared to be signalled by postures or actions by one or more individuals. An aggressive individual was one that made deliberate advances towards another, or that made physical contact with another. Each agonistic interaction was described in terms of whether one or both contestants engaged in aggression, and the intensity (low, medium, high) demonstrated. Low-intensity interactions appeared accidental, occurring when individuals lying together disturbed each other when moving, or if one swam into another under water. Medium- and high-intensity interactions appeared intentional, with one individual approaching another with the apparent goal of initiating an agonistic interaction. High-intensity interactions were distinguished from medium-intensity interactions by the display of more intense contact behaviours. The behaviour exhibited, the intensity of interaction (low, medium, high), the location (water, hide, land), the time, duration of interaction and outcome were all obtained from the videos, as previously described for C. porosus (Brien et al. 2013).
Classification of behaviour
Behavioural observations recorded during these experiments were used to create an inventory of agonistic behaviours for C. johnstoni hatchlings, similar to that described for hatchling C. porosus (Brien et al. 2013). The descriptions are based on a series of basic postures, modified by movement of body parts, or of the whole animal, and whether visual signals or actual contact was involved. Some of these behaviours have been described in studies with adult crocodilians (Garrick and Lang 1977; Lang 1987).
Statistical analyses
All statistical analyses were performed using JMP 8.0 statistical software (SAS Institute 2010). Where appropriate, data were checked for normality (Shapiro–Wilk’s test) and homoscedasticity (Cochran’s test) before statistical analysis. A repeated-measures ANOVA was used for comparison of means between 2-, 13-, and 50-week-old hatchlings, with consecutive night of recording (n = 3) as the repeated measure. A Pearson’s Chi-square test was used to compare intensity, outcome, contact made, number of individuals being aggressive, and how often the instigator won, where sample sizes were adequate. To test whether variation in the frequency of interactions could be explained by time of day, the results were grouped into one of three periods: dusk (17:00–20 : 00 hours), night (20:00–06 : 00 hours), and dawn (06:00–08 : 00 hours). A significance level of P < 0.05 was used for all statistical tests. All means are reported ± one standard error with sample sizes.
Results
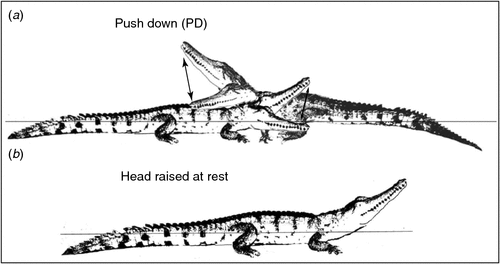
Most agonistic interactions between hatchling C. johnstoni involved two individuals in open water, with none observed on the land or near food (on land). However, there were two interactions at 13 weeks and three at 50 weeks in which 3–4 individuals were involved in an agonistic interaction. Some interactions appeared to occur accidentally when individuals lying together disturbed each other when moving off, or if one swam into another underwater. However, interactions were also initiated either by one or both individuals moving towards each other in a series of short, rapid, deliberate advance movements (RA). In response to RA, one or both individuals would adopt a series of other agonistic behaviours that involved the adoption of some discrete postures that varied in the intensity of expression (Table 1; Fig. 1).

|
|
The adoption of such postures could be abandoned at any time by either slow (SF) or rapid flight (RF), ending the agonistic interaction. Alternatively, the signals emanating from the postures could be intensified with body movements, such as light jaw claps (LJC) or tail wagging (TW), which were displays not involving physical contact with combatants. If the interaction was still not terminated by flight of one or both animals, the behaviour intensified further, with contact movements such as head pushing (HP), or biting (B), occasionally combined in different ways with intense tail wagging (TWB), or side head striking (SHS), until one or both individuals took flight. Another contact movement unique to C. johnstoni was a push down (PD) (Fig. 1). When crocodiles were not engaged in aggression and were at rest, they typically lay with their head raised up on an angle, appearing as if on look out (Fig. 1).
Aggression
An aggressive individual was defined as one that made deliberate advances towards another and/or made physical contact with another animal. Each agonistic interaction was examined to quantify whether one or both contestants engaged in aggression, and whether this differed across age classes. The number of interactions in which both individuals appeared aggressive was similar for all age classes (χ2 = 2.48, d.f. = 2, P > 0.05), with 31.7% at 2 weeks, 41.2% at 13 weeks and 40% at 50 weeks. The number of separate movements or steps in the RA did not change significantly with age (F2,12 = 1.04, P > 0.05), with a mean of 1.9 ± 0.10 steps (mean of means; n = 15, range = 0–4) per interaction.
Frequency
The mean number of agonistic interactions per group per night at two weeks of age was 6.9 ± 0.42 (mean of means; n = 5, range = 5–10). The frequency of agonistic interactions was significantly lower among the older age cohorts (F2,10 = 20.55, P < 0.05), with 5.7 ± 0.35 (n = 5, range = 4–9) at 13 weeks, and 2.8 ± 0.86 (n = 5, range = 2–5) at 50 weeks.
Timing
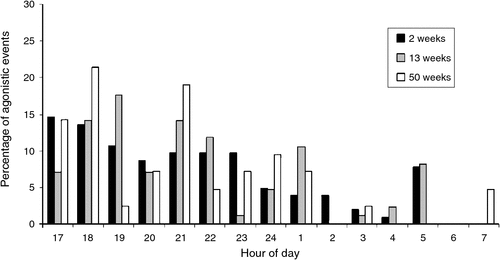
For all three age cohorts combined, the frequency of agonistic interactions at dusk (17:00–20 : 00 hours), night (20:00–06 : 00 hours) and dawn (06:00–08 : 00 hours) varied significantly (F2,11 = 6.99, P < 0.05). Most agonistic interactions occurred throughout the night (17:00–02 : 00 hours), and were lower during the early morning (Fig. 2). Outside of agonistic interactions, hatchlings of all ages would often lie together in contact, in the water, in groups of 2–5 individuals. The 2- and 13-week-old hatchlings would retreat under the hide and were rarely visible after 06 : 00 hours.
|
Postures
The postures displayed in agonistic interactions, as deemed to be aggressive and non-aggressive, varied among the three age cohorts (Table 2). At two weeks old, all hatchlings typically remained LIW (~80%) during an interaction (Table 2). The number of aggressive hatchlings observed in an HRH posture during an agonistic interaction was significantly higher among the older age cohorts (χ2 = 29.06, d.f. = 2, P < 0.05), while the number of non-aggressive individuals observed in an HRH posture during an interaction was highest among 50-week-olds (χ2 = 10.39, d.f. = 2, P < 0.05) (Table 2). The number of hatchlings with MA was higher among the older age cohorts for both aggressive and non-aggressive individuals (Table 2).
Non-contact and contact movements
After assuming a posture, hatchlings often graduated to non-contact movements by displaying a series of LJCs with or without TW. LJCs appeared to indicate aggressive intent, while TW was also displayed by aggressive individuals and appeared to forewarn (threaten) of an impending contact movement. Non-aggressive individuals also engaged in TW, which appeared to signal anticipation of an attack by an approaching individual. TW often occurred after LJC, and increased in intensity as an interaction escalated. LJCs were displayed only by aggressive individuals at two weeks of age, while the display of TW by aggressive and non-aggressive individuals, despite being infrequent, was displayed at both two weeks and 50 weeks of age (Table 3).
Contact movements were often adopted after non-contact movements. The frequency of HPs was significantly higher at two weeks of age (F2,12 = 3.60, P < 0.05), while the frequency of PDs was slightly higher at 13 and 50 weeks of age (F2,12 = 0.76, P < 0.05) (Table 3). The frequency of Bs was similar for all age cohorts (F2,12 = 0.34, P > 0.05), while SHSs were infrequent (Table 3). The number of agonistic interactions in which contact was made did not change significantly with age (χ2 = 0.70, d.f. = 2, P > 0.05), with an average of 90% for all three age groups (2 weeks: 92.7%; 13 weeks: 92.5%; 50 weeks: 90.3%).
Intensity
While low-intensity interactions appeared accidental by definition, both medium- and high-intensity interactions appeared deliberate. High-intensity interactions were distinguished from medium-intensity interactions by the display of more intense contact movements, in the form of TWB. Most interactions between hatchlings of all ages were mostly of low or medium intensity, with only two high-level interactions observed between hatchlings at 50-weeks-old. The proportion of low- and medium-level interactions did not differ with age (χ2 = 2.98, d.f. = 2, P > 0.05).
Duration and outcome
The mean duration of an agonistic interaction was similar between 2-week- (16.1 ± 1.32 s, 5–120 s), 13-week- (15.6 ± 0.97 s, 5–50 s) and 50-week-old hatchlings (23.0 ± 3.3 s, 6–88 s) (F2,12 = 0.31, P > 0.05). The instigator in most agonistic interactions was usually the winner, and this did not change with age (χ2 = 2.14, d.f. = 2, P > 0.05).
The outcome of an agonistic interaction did not differ between the three age groups (χ2 = 19.85, d.f. = 8, P > 0.05). The most common outcome was that both individuals stood their ground (37.7%), or the loser, defined as the less aggressive individual and the first to back down, took flight slowly (SF: 27.7%). Very few interactions (4.5%) resulted in the loser taking flight rapidly (RF).
Interspecific agonistic interactions
Among groups containing hatchling C. johnstoni (1 small, 1 large) and C. porosus (1 small, 1 large) of a similar size and age, a mean of 11.2 ± 1.2 (mean of means, range = 8–16) agonistic interactions was observed mostly (46.3%) around dusk (17:00–20 : 00 hours). Most of these interactions (74%) were low intensity and involved both species and sizes, with biting the most common behaviour (92.4%). Individuals of both species were commonly observed lying together or near each other, with no obvious partitioning, exclusion or dominance observed. However, size did vary within groups and if a larger animal approached a smaller one, regardless of species, the smaller individual would move slowly out of the way in response. There was also a clear distinction between the two species while at rest, with C. porosus remaining LIW and C. johnstoni lying with the head raised (Fig. 1).
The few medium- and high-intensity agonistic interactions observed occurred between individuals of the same size (different species). During these interactions, individuals displayed a species-specific pattern of behaviour, described for C. johnstoni at 50-weeks-old in this study, and for C. porosus at 40-weeks-old in Brien et al. (2013). The typical pattern involved C. porosus rapidly advancing (RA) towards C. johnstoni. However, unlike agonistic interactions between two C. porosus, where this behaviour clearly signals dominance and elicits rapid flight (RF) in the other animal, C. johnstoni would stand its ground and appeared unclear about the intended message. In some cases, due to the lack of response in C. johnstoni, the interaction would end. However, if C. porosus continued to RA, C. johnstoni would respond by lifting its head high in the air (HRH). The interaction would then escalate with C. porosus remaining low in the water (LIW), biting (B) and side head striking (SHS), while C. johnstoni would push down (PD) on the top of C. porosus and occasionally bite (B). This resulted in the two individuals moving together in a circular motion and becoming entangled. Both individuals appeared confused by this species–specific behaviour, and the interaction would end with both lying together, often entangled, or swimming away slowly with no clear winner.
Discussion
Within two weeks after hatching, captive C. johnstoni hatchlings tolerated high levels of close contact with each other with little evidence of aggression. C. johnstoni at all ages (2-, 13-, and 50-weeks-old) regularly grouped together throughout the evening. Agonistic interactions typically involved contact between two or more individuals, but were of low intensity, and halved in frequency by the end of the first year. Clear dominance or submissive outcomes from an interaction were infrequent, and there was no evidence of a dominance hierarchy within groups by 50 weeks of age.
Agonistic behaviour
Hatchling C. johnstoni exhibited a diverse repertoire of postures (n = 3), non-contact movements (n = 5) and contact movements (n = 6) that were utilised in agonistic interactions. All of these behaviours were displayed by individuals at two weeks of age, and most were utilised in similar contexts, with few ontogenetic changes during the first year of life. None of the behaviours appeared to be sex specific, and while vocalisations may play an important role in agonistic interactions, audio was not recorded in this study. One behaviour, a push-down contact movement (PD) in C. johnstoni has never been observed in C. porosus hatchlings under the same conditions. The other 13 behaviour components listed in Table 4 were shared between the two species.
Lying either on land or in water with the head raised up at an angle (~30°) was commonly observed among C. johnstoni. This posture, not associated with agonistic behaviour, makes them appear as if they are on ‘look out’. This behaviour has been observed for C. johnstoni hatchlings in the wild (R. Somaweera, pers. comm.), and may indicate a heightened vigilance in a species that remains small and more vulnerable to predation and interspecific competition for an extended period. It was not observed in C. porosus, which tend to remain low in the water (LIW) while at rest (Brien et al. 2013).
Push downs (PD) were displayed by either one or both C. johnstoni during an agonistic interaction with a head raised high (HRH) posture, with hatchlings often moving together in a circling motion as they attempted to push each other down. This behaviour increased in frequency by 13 and 50 weeks of age and is very similar to what has been reported for several species of salamander (Staub 1993; Davis 2002) and snakes (Carpenter 1977), with the apparent aim of pinning the other individual down in a ritualised display of dominance (Staub 1993; Davis 2002).
Raising the head, or ‘snout lifting’, has been reported among larger subadults and adults of several crocodilian species, including C. johnstoni and C. porosus (Webb and Manolis 1989), as a typical submissive response common to most species studied (Lang 1987). It appears that the raising of the head and/or trunk may also initially be an attempt at bluffing an opponent, but eventually, with age, signals submission. The transition appears to occur more rapidly in hatchling C. porosus than in C. johnstoni (Brien et al. 2013).
C. johnstoni rarely targeted the head of another individual during an interaction, and often raised the head out of the way (HRH posture), especially when both individuals were aggressive. This tendency to avoid contact with the head and the prevalence of less injurious forms of contact (HP and PD) among C. johnstoni, especially as they get older, may be linked to skull morphology. During the initial post-hatching period, the shape of the snout in C. johnstoni is not noticeably different from that of C. porosus, and the frequency of agonistic interactions and behaviours used is similar (Brien et al. 2013). However, as C. johnstoni grow, the snout becomes considerably narrower, which coincides with a decrease in the frequency of agonistic interactions and an increase in the frequency of push downs (PD) and head pushes (HP).
As the long, narrow snouts of crocodilian species such as C. johnstoni are structurally weaker than those of species with broader snouts (e.g. C. porosus) (McHenry et al. 2006; Pierce et al. 2008), this makes them more vulnerable to broken or damaged jaws during conflict (Huchzermeyer 2003), which can impair or prevent their ability to feed, and can even be fatal (Webb et al. 1983c). The use of less invasive contact behaviours and avoidance of the head may both have an adaptive evolutionary significance in C. johnstoni, and perhaps with other slender-snouted species of crocodilian, which are often specialist fish eaters (Lang 1987).
Intraspecific agonistic interactions
The frequency and nature of agonistic interactions between C. johnstoni at two weeks of age were similar to those seen between C. porosus hatchlings at one week of age, when both species were maintained in captivity under comparable conditions (Brien et al. 2013). In C. johnstoni, agonistic interactions decreased in frequency slightly by 13 weeks, and were reduced to less than one-half of the initial frequency by 50 weeks. Agonistic interactions involving contact (>90%) occurred at all ages, but were typically two-sided and symmetrical, with each individual engaging in the same behaviour, e.g. push-downs (PD).
Interactions between hatchling C. johnstoni occurred largely between 17 : 00 and 24 : 00 hours, with individuals at two and 13 weeks of age retreating under the hide boards by 06 : 00 hours and not being seen again. In comparison, hatchling C. porosus of all ages (1, 13, 40 weeks), had a characteristic dusk (17:00–20 : 00 hours) and dawn (06:00–08 : 00 hours) pattern of agonistic interactions and were regularly observed out in open areas up until 08 : 00 hours. That hatchling C. johnstoni would retreat under the hide almost an hour before sunrise may again be linked to the greater vulnerability of hatchling C. johnstoni to predation due to their smaller size.
Agonistic interactions between hatchling C. johnstoni typically involved two individuals, and on occasion even up to three or four at 13 and 50 weeks of age. The number of interactions in which both individuals were aggressive increased slightly with age (30–40% of interactions), as did the duration of an interaction, while the number of times contact was actually made did not change (90% of interactions). Almost all interactions between hatchling C. johnstoni were of low to medium intensity, while the outcome varied, often resulting in either both individuals standing their ground or the loser taking flight slowly. While most interactions resulted in the instigator winning (76.4%), this was far lower than for hatchling C. porosus older than one week old (Brien et al. 2013).
The formation of dominance hierarchies is often important in limiting the cost of interactions in species that exist together in social groups, while in more solitary species, dominance is usually absent and individuals will engage in higher levels of aggression (Bekoff 1974, 1977; Huntington and Turner 1987; Krause and Ruxton 2002). Although C. johnstoni in the wild are generally solitary, in some habitats they are often forced to live together at higher densities during certain times of the year due to lower water levels (Webb et al. 1983a), while they also show a high degree of site fidelity and communal nesting (Webb et al. 1983a; Tucker et al. 1997).
While there was no evidence of a dominance hierarchy among C. johnstoni by 50 weeks of age in this study, agonistic interactions were relatively infrequent and of a lower intensity, which may reflect a different social strategy enabling them to coexist at certain times of the year. In comparison, C. porosus under captive conditions had well defined dominance hierarchies despite being largely solitary in the wild. While it is possible that these results are a consequence of captivity, and that dominance may develop at a later stage in C. johnstoni, it does indicate that juveniles of these two species employ very different social strategies.
Interspecific agonistic interactions
Agonistic interactions between C. johnstoni and C. porosus in this study involved only two individuals and were mostly low level, with no real exclusion or dominance observed. However, smaller individuals of both species moved slowly out of the way when a larger individual approached. Occasional medium- or high-level interactions occurred between the same-sized (different species) individuals in a group, and involved both individuals displaying a species–specific pattern of behaviour with no clear winner of the contest.
These results were unexpected, as observations of intraspecific aggression suggested that C. porosus would dominate C. johnstoni due to a higher level of aggression, and the use of more injurious forms of contact. Instead, it suggests that interspecific interactions between these two species may be governed largely by size, which has also been found among different species of crayfish (Vorburger and Ribi 1999) and trout (Newman 1956) during staged interactions. Given that C. porosus reaches a much larger adult size, it is likely that C. johnstoni may avoid C. porosus in the wild.
Since the recovery of the saltwater crocodile population in the Northern Territory from hunting (Fukuda et al. 2011), there appears to have been displacement of C. johnstoni by C. porosus from areas they previously occupied (Messel et al. 1981; Webb et al. 1983a). Increased numbers of C. porosus were found to be negatively correlated with the abundance of C. johnstoni, suggesting competitive exclusion (Messel et al. 1981; Webb et al. 1983a). This situation has also been described in several sympatric species of lizards (Jenssen 1973), salamanders (Jaeger 1972; Keen and Sharp 1984), birds (Williams and Batzli 1979) and mammals (Terman 1974), with removal of the competitively superior species resulting in increased habitat use by the inferior one.
This study describes agonistic behaviour in C. johnstoni for the first time and provides new insights into the nature of agonistic behaviour between C. porosus and C. johnstoni at one year of age, a time at which they would naturally start occurring together in the wild. Several clear differences exist in morphology, resource use, and behaviour, between C. porosus and C. johnstoni, which may have been shaped by interspecific agonistic interactions. As the larger, more dominant species, C. porosus may have played a significant role in this divergent evolution.
Acknowledgements
We thank Charlie Manolis, Ruchira Somaweera, and Jemeema Brien for their help in different phases of the project. We also thank Wildlife Management International for the supply of animals, use of facilities and logistical support. Wildlife Management International funding through the Rural Industries Research and Development Corporation is gratefully acknowledged. Funding for equipment (cameras, digital video recorders) was provided through a Holsworth Wildlife Research Endowment (ANZ Trustees Foundation), a Northern Territory Research and Innovation Board student grant, an IUCN Crocodile Specialist Group student grant, and Charles Darwin University. Experimental protocols were approved by the Charles Darwin University Animal Ethics Committee (Animal ethics permit no. A11003).
References
Adams, D. C. (2004). Character displacement via aggressive interference in Appalachian salamanders. Ecology 85, 2664–2670.| Character displacement via aggressive interference in Appalachian salamanders.Crossref | GoogleScholarGoogle Scholar |
Aguirre, L. F., Herrel, A., van Damme, R., and Matthysen, E. (2002). Ecomorphological analysis of trophic niche partitioning in a tropical savannah bat community. Proceedings of the Royal Society of London. Series B, Biological Sciences 269, 1271–1278.
| Ecomorphological analysis of trophic niche partitioning in a tropical savannah bat community.Crossref | GoogleScholarGoogle Scholar |
Bekoff, M. (1974). Social play in coyotes, wolves, and dogs. Bioscience 24, 225–230.
| Social play in coyotes, wolves, and dogs.Crossref | GoogleScholarGoogle Scholar |
Bekoff, M. (1977). Mammalian dispersal and the ontogeny of individual behavioural phenotypes. American Naturalist 111, 715–732.
| Mammalian dispersal and the ontogeny of individual behavioural phenotypes.Crossref | GoogleScholarGoogle Scholar |
Bovbjerg, R. V. (1970). Ecological isolation and competitive exclusion in two crayfish (Orconectes virilis and Orconectes immunis). Ecology 51, 51225–51236.
Brien, M. L., Webb, G. J., Lang, J. W., McGuinness, K. A., and Christian, K. A. (2013). Born to be bad: agonistic behaviour in hatchling saltwater crocodiles (Crocodylus porosus). Behaviour 150, 737–762.
Brown, W. L., and Wilson, E. O. (1956). Character displacement. Systematic Biology 5, 49–64.
Carpenter, C. C. (1977). Communication and displays of snakes. American Zoologist 17, 217–223.
Cott, H. B. (1961). Scientific results of an inquiry into the ecology and economic status of the Nile crocodile (Crocodylus niloticus) in Uganda and northern Rhodesia. The Transactions of the Zoological Society of London 29, 211–356.
| Scientific results of an inquiry into the ecology and economic status of the Nile crocodile (Crocodylus niloticus) in Uganda and northern Rhodesia.Crossref | GoogleScholarGoogle Scholar |
Davis, B. M. (2001). Improved nutrition and management of farmed crocodiles – hatchling to harvest. Australian Government Rural Industries Research and Development Corporation. RIRDC Project No. 01/123.
Davis, T. M. (2002). An ethogram of intraspecific agonistic and display behaviour for the wandering salamander, Aneides vagrans. Herpetologica 58, 371–382.
| An ethogram of intraspecific agonistic and display behaviour for the wandering salamander, Aneides vagrans.Crossref | GoogleScholarGoogle Scholar |
de Waal, F. B. M., and Luttrell, L. M. (1989). Toward a comparative socioecology of the genus Macca: different dominant styles in rhesus and stumptail macaques. American Journal of Primatology 19, 83–109.
| Toward a comparative socioecology of the genus Macca: different dominant styles in rhesus and stumptail macaques.Crossref | GoogleScholarGoogle Scholar |
Drummond, H. (2001). The control and function of agonism in avian broodmates. Advances in the Study of Behavior 30, 261–301.
| The control and function of agonism in avian broodmates.Crossref | GoogleScholarGoogle Scholar |
Drummond, H. (2006). Dominance in vertebrate broods and litters. The Quarterly Review of Biology 81, 3–32.
| Dominance in vertebrate broods and litters.Crossref | GoogleScholarGoogle Scholar | 16602272PubMed |
Fukuda, Y., Webb, G., Manolis, C., Delaney, R., Letnic, M., Lindner, G., and Whitehead, P. (2011). Recovery of saltwater crocodiles following unregulated hunting in tidal rivers of the Northern Territory, Australia. Journal of Wildlife Management 75, 1253–1266.
| Recovery of saltwater crocodiles following unregulated hunting in tidal rivers of the Northern Territory, Australia.Crossref | GoogleScholarGoogle Scholar |
Garrick, L. D., and Lang, J. W. (1977). Social signals and behaviours of adult alligators and crocodiles. American Zoologist 17, 225–239.
Garrick, L. D., Lang, J. W., and Herzog, H. A. (1978). Social signals of adult American alligators. Bulletin of the American Museum of Natural History 160, 153–192.
Genner, M. J., Turner, G. F., Barker, S., and Hawkins, S. J. (1999). Niche segregation among Lake Malawi cichlid fishes? Evidence from stable istope signatures. Ecology Letters 2, 185–190.
| Niche segregation among Lake Malawi cichlid fishes? Evidence from stable istope signatures.Crossref | GoogleScholarGoogle Scholar |
Huber, R., and Kravitz, E. A. (1995). A quantitative analysis of agonistic behaviour in juvenile American lobsters (Homerus americanus). Brain, Behavior and Evolution 46, 72–83.
| A quantitative analysis of agonistic behaviour in juvenile American lobsters (Homerus americanus).Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK28%2FktVCrsw%3D%3D&md5=e3c1a927c61472b84cbd65b1b9eacb6dCAS | 7552224PubMed |
Huchzermeyer, F. W. (2003). ‘Crocodiles: Biology, Husbandry and Disease.’ (CABI Publishing: South Africa.)
Huntington, F., and Turner, A. (1987). ‘Animal Conflict.’ (Chapman and Hall: New York.)
Jaeger, R. G. (1972). Food as a limited resource in competition between two species of terrestrial salamanders. Ecology 53, 535–546.
| Food as a limited resource in competition between two species of terrestrial salamanders.Crossref | GoogleScholarGoogle Scholar |
Jenssen, T. A. (1973). Shift in the structural habitat of Anolis opalinus due to congeneric competition. Ecology 54, 863–869.
| Shift in the structural habitat of Anolis opalinus due to congeneric competition.Crossref | GoogleScholarGoogle Scholar |
Keen, W. H., and Sharp, S. (1984). Responses of a plethodontid salamander to conspecific and congeneric intruders. Animal Behaviour 32, 58–65.
| Responses of a plethodontid salamander to conspecific and congeneric intruders.Crossref | GoogleScholarGoogle Scholar |
Kennett, R., and Christian, K. (1993). Aestivation by freshwater crocodiles (Crocodylus johnstoni) occupying a seasonally ephemeral creek in tropical Australia. In ‘Herpetology in Australia’. (Eds D. Lunney and D. Ayers.) pp. 315–319. (Surrey Beatty: Sydney.)
Krause, J., and Ruxton, G. D. (2002). ‘Living in Groups.’ (Oxford University Press: Oxford.)
Lang, J. W. (1987). Crocodilian behaviour: implications for management. In ‘Wildlife Management: Crocodiles and Alligators’. (Eds G. J. W. Webb, S. C. Manolis and P. J. Whitehead.) pp. 273–294. (Surrey Beatty: Sydney.)
Mayer, R. (1998). Crocodile farming: research, development and on-farm monitoring. Australian Government Rural Industries Research and Development Corporation. RIRDC Publication No. DAQ-188A.
McHenry, C. R., Clausen, P. D., Daniel, W. J., Meers, M. B., and Pendharkar, A. (2006). Biomechanics of the rostrum in crocodilians: a comparative analysis using finite-element modelling. The Anatomical Record. Part A, Discoveries in Molecular, Cellular, and Evolutionary Biology 288A, 827–849.
| Biomechanics of the rostrum in crocodilians: a comparative analysis using finite-element modelling.Crossref | GoogleScholarGoogle Scholar |
Messel, H., Vorlicek, G. C., Wells, A. G., and Green, W. J. (1981). ‘Surveys of Tidal River Systems in the Northern Territory of Australia and their Crocodile Populations. Monograph 1. The Blyth–Cadell Rivers system study and the status of Crocodylus porosus in tidal waterways of northern Australia.’ pp. 454–459. (Pergamon Press: Australia.)
Murray, B. G. (1971). The ecological consequences of interspecific territorial behaviour in birds. Ecology 52, 414–423.
| The ecological consequences of interspecific territorial behaviour in birds.Crossref | GoogleScholarGoogle Scholar |
Newman, M. A. (1956). Social behaviour and interspecific competition in two trout species. Physiological Zoology 29, 64–81.
Ouboter, P. E. (1996). ‘Ecological Studies on Crocodilians in Suriname: Niche Segregation and Competition in Three Predators.’ (SPB Academic Publishing: Amsterdam and New York.)
Pereira, M. E., and Kappeler, P. M. (1997). Divergent systems of agonistic behaviour in lemurid primates. Behaviour 134, 225–274.
| Divergent systems of agonistic behaviour in lemurid primates.Crossref | GoogleScholarGoogle Scholar |
Phillips, J. A., Alberts, A. C., and Pratt, N. C. (1993). Differential resource use, growth, and the ontogeny of social relationships in the green iguana. Physiology & Behavior 53, 81–88.
| Differential resource use, growth, and the ontogeny of social relationships in the green iguana.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3s7msFyiuw%3D%3D&md5=8b9b7e18210557edfc6f50c59ae2ba9aCAS |
Pierce, S. E., Angielczyk, K. D., and Rayfield, E. J. (2008). Patterns of morphospace occupation and mechanical performance in extant crocodilian skulls: a combined geometric morphometric and finite element modelling approach. Journal of Morphology 269, 840–864.
| Patterns of morphospace occupation and mechanical performance in extant crocodilian skulls: a combined geometric morphometric and finite element modelling approach.Crossref | GoogleScholarGoogle Scholar | 18496856PubMed |
Pooley, A. C. (1969). Preliminary studies on the breeding of the Nile crocodile (Crocodylus niloticus) in Zululand. Lammergeyer 10, 22–44.
Ricklefs, R. E. (1990). ‘Ecology.’ 3rd edn. (Freeman: New York.)
Riese, G. (1991). Factors influencing the survival and growth of hatchling Crocodylus porosus in commercial crocodile farming. M.Sc. Thesis, University of Queensland, Brisbane.
SAS Institute (2010). ‘JMP Statistics and Graphics Guide.’ (SAS Institute Inc.: Cary, NC.)
Schoener, T. W. (1965). The evolution of bill size differences among sympatric congeneric species of birds. Evolution 19, 189–213.
| The evolution of bill size differences among sympatric congeneric species of birds.Crossref | GoogleScholarGoogle Scholar |
Smith, T. B. (1990). Resource use by bill morphs of an African finch: evidence for intraspecific competition. Ecology 71, 1246–1257.
| Resource use by bill morphs of an African finch: evidence for intraspecific competition.Crossref | GoogleScholarGoogle Scholar |
Staub, N. L. (1993). Intraspecific agonistic behaviour of the salamander Aneides flavipunctatus (Amphibia: Plethodontidae) with comparisons to other plethodontid species. Herpetologica 49, 271–282.
Stoecker, R. E. (1972). Competitive relations between sympatric populations of voles (Microtus montanus and M. pennsylvanicus). Journal of Animal Ecology 41, 311–329.
| Competitive relations between sympatric populations of voles (Microtus montanus and M. pennsylvanicus).Crossref | GoogleScholarGoogle Scholar |
Terman, M. R. (1974). Behavioural interactions between Microtus and Sigmodon: a model for competitive exclusion. Journal of Mammalogy 55, 705–719.
| Behavioural interactions between Microtus and Sigmodon: a model for competitive exclusion.Crossref | GoogleScholarGoogle Scholar |
Thorbjarnarson, J. B. (1991). Crocodylus acutus (American crocodile). Social behaviour. Herpetological Review 22, 130.
Thorbjarnarson, J., Mazzotti, F., Sanderson, E., Buitrago, F., Lazcano, M., Minkowski, K., Muñiz, M., Ponce, P., Sigler, L., Soberon, R., Trelancia, A. M., and Velasco, A. (2006). Regional habitat conservation priorities for the American crocodile. Biological Conservation 128, 25–36.
| Regional habitat conservation priorities for the American crocodile.Crossref | GoogleScholarGoogle Scholar |
Tinbergen, N. (1952). Derived activities: their causation, biological significance, origin, and emancipation during evolution. The Quarterly Review of Biology 27, 1–32.
| Derived activities: their causation, biological significance, origin, and emancipation during evolution.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaG38%2FmtVegsA%3D%3D&md5=227d34f94e54b76e5a5f0bab6e56ca62CAS | 14930222PubMed |
Tinbergen, N. (1953). ‘Social Behaviour in Animals: With Special Reference to Vertebrates.’ (Wiley: Oxford.)
Tucker, A. D., Limpus, C. J., McCallum, H. I., and McDonald, K. R. (1997). Movements and home ranges of Crocodylus johnstoni in the Lynd River, Queensland. Wildlife Research 24, 379–396.
| Movements and home ranges of Crocodylus johnstoni in the Lynd River, Queensland.Crossref | GoogleScholarGoogle Scholar |
Vliet, K. A. (1989). Social displays of the American alligator (Alligator mississippiensis). American Zoologist 29, 1019–1031.
Vorburger, C., and Ribi, G. (1999). Aggression and competition for shelter between a native and an introduced crayfish in Europe. Freshwater Biology 42, 111–119.
| Aggression and competition for shelter between a native and an introduced crayfish in Europe.Crossref | GoogleScholarGoogle Scholar |
Webb, G. J. W., and Manolis, S. C. (1989). ‘Crocodiles of Australia.’ (Reed Books: Sydney.)
Webb, G. J. W., Messel, H., and Magnusson, W. E. (1977). The nesting biology of Crocodylus porosus in Arnhem Land, northern Australia. Copeia 1977, 238–249.
| The nesting biology of Crocodylus porosus in Arnhem Land, northern Australia.Crossref | GoogleScholarGoogle Scholar |
Webb, G. J. W., Buckworth, R., and Manolis, S. C. (1983a). Crocodylus johnstoni and C. porosus coexisiting in a tidal river. Australian Wildlife Research 10, 639–650.
| Crocodylus johnstoni and C. porosus coexisiting in a tidal river.Crossref | GoogleScholarGoogle Scholar |
Webb, G. J. W., Buckworth, R., and Manolis, S. C. (1983b). Crocodylus johnstoni in a controlled-environment chamber: a raising trial. Australian Wildlife Research 10, 421–432.
| Crocodylus johnstoni in a controlled-environment chamber: a raising trial.Crossref | GoogleScholarGoogle Scholar |
Webb, G. J. W., Manolis, S. C., and Buckworth, R. (1983c). Crocodylus johnstoni in the McKinlay River area, NT. V. Abnormalities and injuries. Australian Wildlife Research 10, 407–420.
| Crocodylus johnstoni in the McKinlay River area, NT. V. Abnormalities and injuries.Crossref | GoogleScholarGoogle Scholar |
Williams, J. B., and Batzli, G. O. (1979). Competition among bark-foraging birds in central Illinois: experimental evidence. The Condor 81, 122–132.
| Competition among bark-foraging birds in central Illinois: experimental evidence.Crossref | GoogleScholarGoogle Scholar |
Wilson, E. O. (1975). ‘Sociobiology.’ (Belknap Press: Cambridge.)





