Sexual dimorphism and reproductive biology of commercially harvested oriental rat snakes (Ptyas mucosa: Colubridae) from West Java
Amir Hamidy A , Evy Arida B , Noor Laina Maireda C , Alamsyah Elang Nusa Herlambang A , Awal Riyanto A , Mumpuni D *
D *
A
B
C
D
Abstract
Large (>3 m) slender-bodied rat snakes are abundant in agroecosystems of southern Asia and are heavily exploited for their skins and meat. We examined 216 specimens killed at commercial facilities in Cirebon, West Java, to quantify morphological and reproductive traits and evaluate harvest sustainability by comparing this sample to one taken 25 years previously. The snakes we examined were mostly adults, and mostly male. Females were less heavy-bodied than males of the same body length, matured at a larger size than males, but attained smaller maximum sizes. Reproduction and energy (fatbody) storage was seasonal in both sexes, with females containing oviductal eggs primarily during the annual wet season. Clutch size averaged 12.6 eggs and increased with maternal body size. Comparison of the two samples taken 25 years apart revealed strong similarity in sex ratio, the numerical preponderance of adults, body sizes at sexual maturation and mean adult body sizes, sexual dimorphism in body length and mass, reproductive seasonality in females, mean fecundity, and the relationship between fecundity and maternal body size. That consistency suggests that commercial harvesting over the intervening period has not affected the biological traits of rat snake populations, consistent with a sustainable level of offtake.
Keywords: Colubridae, Indonesia, reproductive cycles, snake ecology, sustainability, tropical, utilisation, wildlife trade.
Introduction
Disproportionate research effort focused on temperate-zone versus tropical organisms has resulted in a paucity of information about the biology of tropical species (Titley et al. 2017). For example, even abundant tropical snakes are poorly known compared to many temperate-zone species (Brown and Shine 2002) – and this is true even for large-bodied taxa that are harvested for commercial purposes (Murphy 2007). The species that we studied fits these criteria: it is widely distributed through southern Asia, is very large (to around 3 m total length: Kopstein 1938) and thousands of these animals are culled every year for commercial use, primarily for meat, skins, and Traditional Chinese Medicines (Sugardjito et al. 1998; Auliya 2010). Nonetheless, empirical data on the species’ biology are scarce.
Information on the ecological attributes of harvested tropical snakes is valuable not only to fill a gap in basic understanding. Such data also can clarify the degree to which that harvest is sustainable. Field surveys to quantify abundances and life-history parameters can enable population-viability analyses, but robust estimates of the critical parameters can be logistically difficult or impossible to attain in tropical reptiles (e.g. Shine and Harlow 1999; Natusch et al. 2019, 2020). Information on the attributes of harvested specimens offers a simpler and more achievable means of data collection and can illuminate sustainability of harvesting in two ways. First, a knowledge of life-history traits can suggest a species’ resilience to the annual offtake of animals for commercial purposes – for example, a population’s ability to replace culled animals will be reduced by late maturation and low reproductive output (Natusch et al. 2016). Likewise, a species that exploits anthropogenically disturbed habitats and has broad dietary habits likely will fare better than will an ecological specialist. Second, repeated surveys of culled animals through time can detect changes caused by unsustainable levels of harvest. For example, a population that is being driven to low abundances by overintense harvesting may show a shift in culled animals towards smaller (younger) specimens, and/or to a different sex ratio (reflecting increased effort to capturing less easily available or less commercially attractive sectors of the population), or to reduced fecundity or body condition (reflecting harvesting from marginal rather than prime habitats of the exploited species).
Despite the ease and low cost of quantitative surveys of culled animals at processing facilities, such studies have rarely been conducted for tropical reptiles. In South America, sustainability issues have been examined for taxa such as tegu lizards (Tupinambis spp.: Fitzgerald 1994; Mieres and Fitzgerald 2006) and yellow anaconda (Euectes notaeus) (Camera et al. 2020). In Indonesia, repeated surveys at processing facilities suggest that the harvest has not caused any shift in monitored parameters of reticulated pythons (Malayopython reticulatus) but has been associated with a reduction in body condition, size at maturation and reproductive frequency in female blood pythons (Python brongersmai) (Natusch et al. 2019, 2020). In the present study, we address this issue with data on a large species of colubrid snake (Ptyas mucosa) that is subject to intense commercial harvest in West Java. The magnitude of this harvest has raised concerns about sustainability, but original data are scarce (Auliya 2010). For Java (where harvesting is most intense), the only detailed study of harvested specimens was conducted in 1994–1996 (Boeadi et al. 1998), supplemented by subsequent small data sets plus anecdotal information from collectors and traders (Sidik 2006; Auliya 2010). In the present study we describe morphology and reproductive output of P. mucosa in West Java based on sampling from 2020 to 2021, and we compare those attributes to those recorded for the same species in Java 25 years earlier (Boeadi et al. 1998).
Materials and methods
Study area and species
Cirebon (6°42′26″S, 108°33′27″E) is a port city in West Java, surrounded primarily by agricultural land such as rice paddies, irrigation canals and fish ponds. The air temperature is high year-round (all monthly mean maxima >25°C) but with rainfall concentrated in a monsoonal ‘wet season’ from November to March (annual rainfall 2120 mm: Climate-data-org). The city and nearby areas are home to several snake-processing facilities, which receive live wild-caught snakes from agricultural workers (with a few full-time collectors, at least seasonally) and kill and process those snakes on a daily basis at their facilities (see Kurniawan et al. 2018; Kusrini et al. 2022).
One of the largest colubrid snakes worldwide, the oriental rat snake (Ptyas mucosa) often attains >3 m in total length (Wall 1921; Pope 1935; Kopstein 1938; Bergman 1952; Auliya 2010). Java comprises the eastern edge of the species’ geographic distribution, that extends westwards through to Iran and includes large areas of other countries including India, Pakistan, Myanamar, Laos, Thailand and Vietnam (Auliya 2010). Slender in build, these snakes are brown dorsally with darker bands on the posterior body (see Fig. 1). Common in highly disturbed habitats, oriental rat snakes feed primarily on amphibians and rodents (Boeadi et al. 1998; Sidik 2006; Pal et al. 2012). Females lay eggs primarily during the wet season, as do most snake species in ricefield habitats (Kopstein 1938), and the young snakes grow rapidly to attain sexual maturity at about 9 months of age (Auliya 2010). Because of their large body sizes, these snakes are heavily exploited for their skins and meat, and for traditional medicine, across much of southern Asia (e.g. Auliya 2010; Kurniawan et al. 2018; Kusrini et al. 2022). The skins of the species are used to manufacture a range of leather products, such as belts and handbags, for both domestic and international sale. The meat is sold for human consumption. Historically, the Hong Kong Special Administrative Region of the People’s Republic of China and mainland China have been primary markets for meat of this species.
Data acquisition
In March, September, October and November 2020 and in February, March and May 2021, we visited five processing facilities around the city of Cirebon. After snakes were humanely euthanised we measured them (snout–vent length [SVL] and tail length), weighed them, and determined sex by everting hemipenes. After the snakes were skinned, we examined their internal organs to assess sexual maturity (based on thickened efferent ducts in males and thickened oviducts in females) and counted oviductal eggs and enlarged (>8 mm) ovarian follicles. We allocated a score of 0–3 for size of the abdominal fat bodies, and treated this as an ordinal variable in our analyses. We measured lengths and widths of enlarged ovarian follicles and testes, and we calculated volume of the right testis based on the formula for a prolate spheroid (4/3π (half testis length) × (half testis width)2) (Harlow and Taylor 2000).
Analysis
We used JMP Pro 16.1.0 to analyse our data. Logistic regression was used to analyse influences on nominal variables (sex, maturity, reproductive state), and Chi-square contingency tests were used to compare sex ratio to a null hypothesis of 50:50. We used ANOVA and ANCOVA to analyse continuous dependent variables, after ln-transformation in cases where distributions of raw data were non-normal. We included interaction terms in ANOVAs and ANCOVAs, but then deleted non-significant interaction terms and recalculated main effects. To compare our data to that gathered by Boeadi et al. (1998), we first deleted outliers for clutch size and body mass, then applied the same analytical techniques to the combined data set (using older versus recent study as a factor) as we had for the more recent study.
Results
Sample size
We obtained data on 216 oriental rat snakes, with the largest samples from Pak Mukti (N = 90) and Pak Daru (N = 83), and 9–24 specimens from the other three facilities.
Demography
Most of the snakes that we examined were sexually mature (60 of 84 females = 71%; 129 of 132 males = 98%). Thus, the proportion of adults was higher in males than in females (χ2 = 32.46, 1 d.f., P < 0.0001), and the overall sex ratio was male-biased (132 males, 84 females = 61% male; χ2 = 10.67, 1 d.f., P < 0.001).
Sexual dimorphism
Mean snout–vent lengths were greater in males than in females both in the total sample (mean female SVL = 1296.7 mm, s.e. = 19.6, N = 84; mean male SVL = 1466 mm, s.e. = 18.1, N = 132) and if analysis is restricted to adult snakes only (female mean = 1380.2 mm, s.e. = 14.4, N = 60; male mean = 1479.4 mm, s.e. = 17.0, N = 129; ANCOVA on ln-transformed values F1,214 = 33.91, P = 0.0001) (see Fig. 2a). Similarly, mean body mass was higher in males both in the total sample (females = 688.9 g, s.e. = 29.8, N = 83; males = 1076.2 g, s.e. = 37.3, N = 130; ANOVA on ln-transformed values F1,211 = 44.87, P < 0.0001) and also among adults only (females = 805.3 g, s.e. = 28.8, N = 59; males = 893.8 g, s.e. = 34.8, N = 99; F1,184 = 18.10, P < 0.0001) (see Fig. 2b).
Sexual size dimorphism in the oriental rat snake (Ptyas mucosa) from western Java. Panel (a) shows distributions of snout–vent lengths for male and female snakes. Panel (b) shows equivalent data for body mass. Panel (c) shows body mass relative to body length for the two sexes.
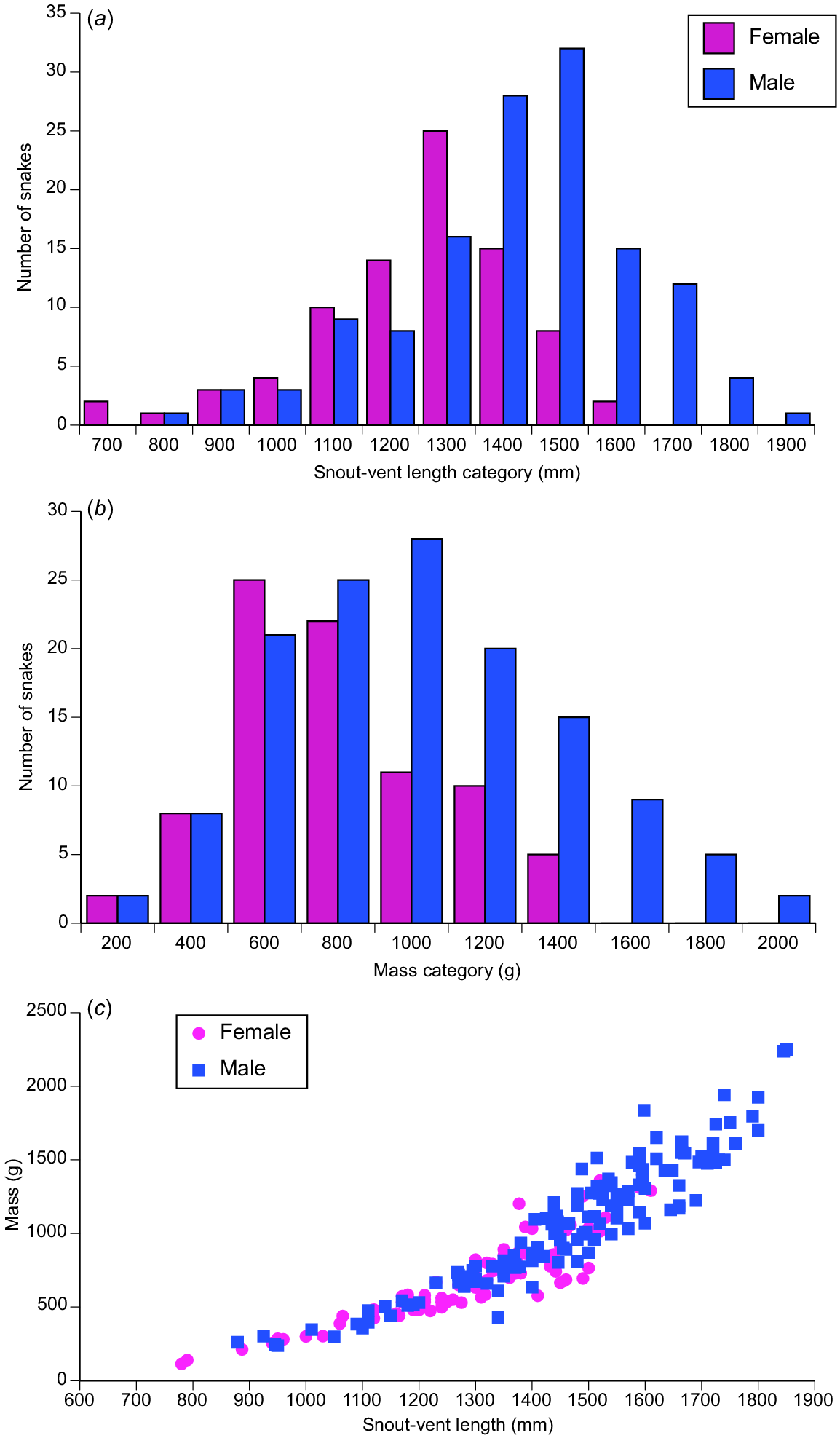
ANCOVA of ln(body mass) relative to snout–vent length showed that males were more heavy-bodied than females (based on total sample: interaction sex × ln(SVL), F1,209 = 0.11, P = 0.74; main effect of sex F1,210 = 9.23, P < 0.0001). Tail length relative to SVL did not differ significantly between the sexes (interaction sex × ln(SVL), F1,198 = 0.87, P = 0.35; main effect sex, F1,199 = 2.23, P = 0.14).
Reproductive seasonality
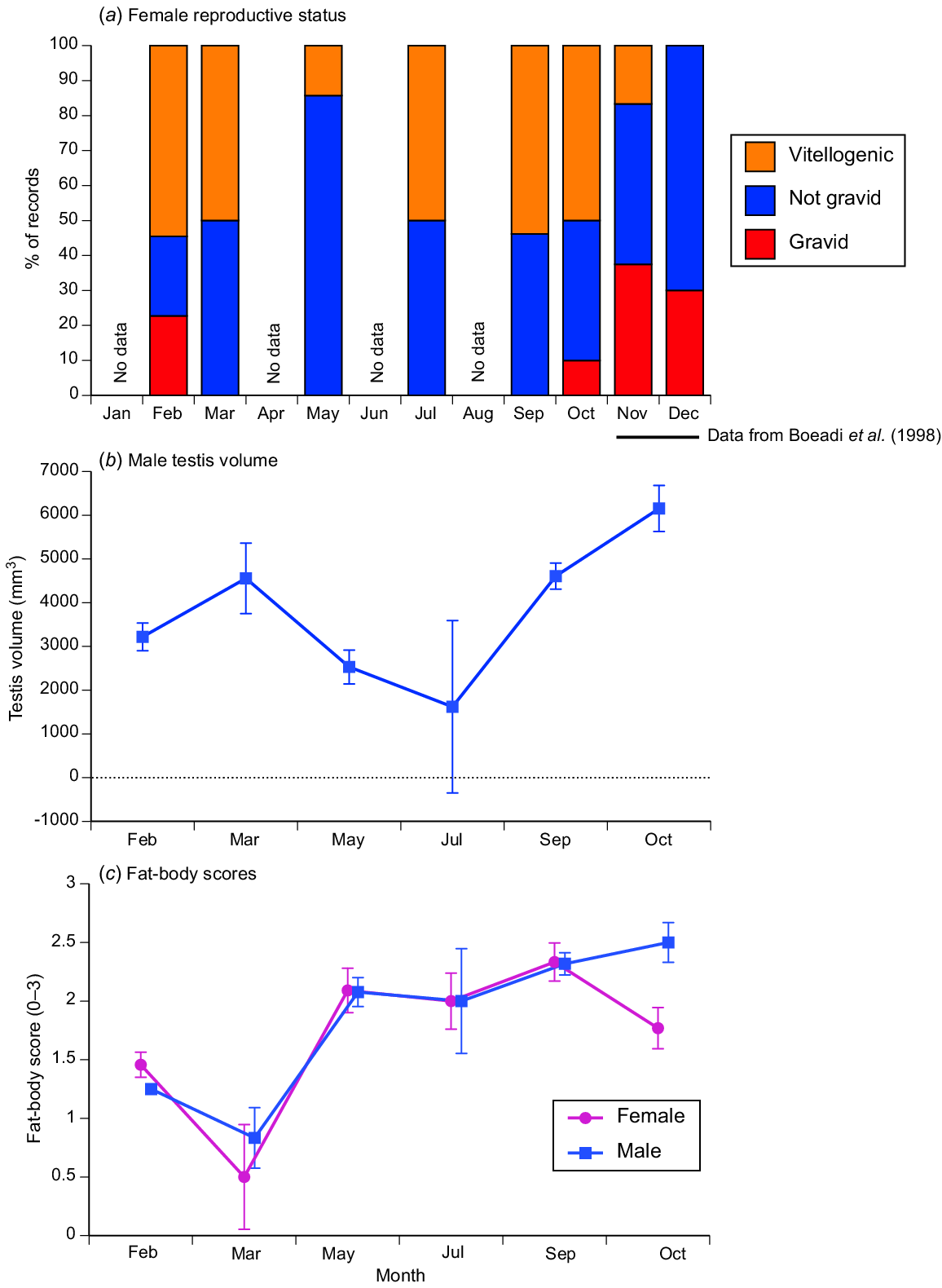
Females with oviductal eggs were recorded in October and February (before and during the wet season), with most females non-reproductive during the mid-dry season (March to September: see Fig. 3a). Testis volume varied among months (ANOVA on ln-transformed values: F5,124 = 6.39, P < 0.0001), being lower in the dry season (May–July) than at other times of the year (Fig. 3b). Monthly variation in length of the largest ovarian follicle was marginally non-significant (F4,33 = 2.48, P = 0.06). Our scores for fatbody size varied among months, and did so differently in males and females (treating this score as an ordinal variable: interaction sex × month, F5,203 = 2.28, P < 0.05) (see Fig. 3c). Fat bodies increased throughout the dry season in both sexes, but fell in the early wet season (October) in females but not in males (Fig. 3c).
Seasonality of reproductive and fatbody traits in the oriental rat snake (Ptyas mucosa) from Java. Upper panel (a) shows relative numbers of adult females with enlarged ovarian follicles (vitellogenic), oviductal eggs (gravid) or neither of these conditions (not gravid). Panel (b) shows mean volume of the right testis in adult males (calculated from the formula for a prolate spheroid (4/3π (half testis length) × (half testis width)2) (Harlow and Taylor 2000), and Panel (c) shows data on our ordinal scores for fatbody size (subjectively rated from 0 to 3, representing no visible fat through to extensive fat stores) in both sexes. In panels (b) and (c), vertical bars show one standard error.
Fecundity and size at maturity
Counts of enlarged vitellogenic follicles and oviductal eggs yielded 32 records of clutch size, with a range of 6–19 (mean = 12.56, s.e. = 0.59, median = 12, N = 32). Clutch sizes increased with maternal SVL (r2 = 0.15, P < 0.037, N = 32).
For female snakes, the largest juvenile was 1275 mm SVL whereas the smallest adult was 1150 mm SVL. Equivalent figures for males were 945 mm and 950 mm. Thus, females matured at larger body sizes than males, despite attaining smaller maximum SVLs (1590 versus 1660 mm).
Comparisons through time
Using raw data from the study of Boeadi et al. (1998), we compared the sample from 1994–1995 (176 snakes) to that from 2019–2021 (216 snakes). Broadly, those samples were similar, as detailed below.
Of 74 females in the earlier study, 64 (86%) were adult. Of 102 males, all were adult (100%). As in the recent sample, then, the collection was male-biased (58% male, versus 50:50 – χ2 = 14.61, 1 d.f., P < 0.0001), and sex ratios of the two studies did not differ significantly from each other (χ2 = 0.40, 1 d.f., P = 0.53).
Mean SVLs of the total sample from the earlier study were 1315 mm for females (s.e. = 14.9, N = 74) and 1415 mm (s.e. = 17.4, N = 102) for males. For adult animals only from the earlier study, equivalent figures were females 1348.2 (s.e. = 10.7, N = 64) and males 1415.4 (s.e. = 17.4, N = 102). These numbers are very similar to those obtained in the more recent sample (ANOVA on ln(SVL), with sex and study as factors: interaction sex × study, F1,388 = 3.31, P = 0.07; main effect of study, F1,389 = 0.67, P = 0.42; main effect of sex, F1,389 = 48.12, P < 0.0001). That is, males averaged larger than females in both studies (see Fig. 4a).
A comparison of body sizes of adult oriental rat snakes (Ptyas mucosa) examined at processing facilities in Java in 1994–1996 (Boeadi et al. 1998) and in 2020–2021 (current study). Panel (a) shows mean and associated standard error for snout–vent lengths, and panel (b) shows equivalent data for body mass.
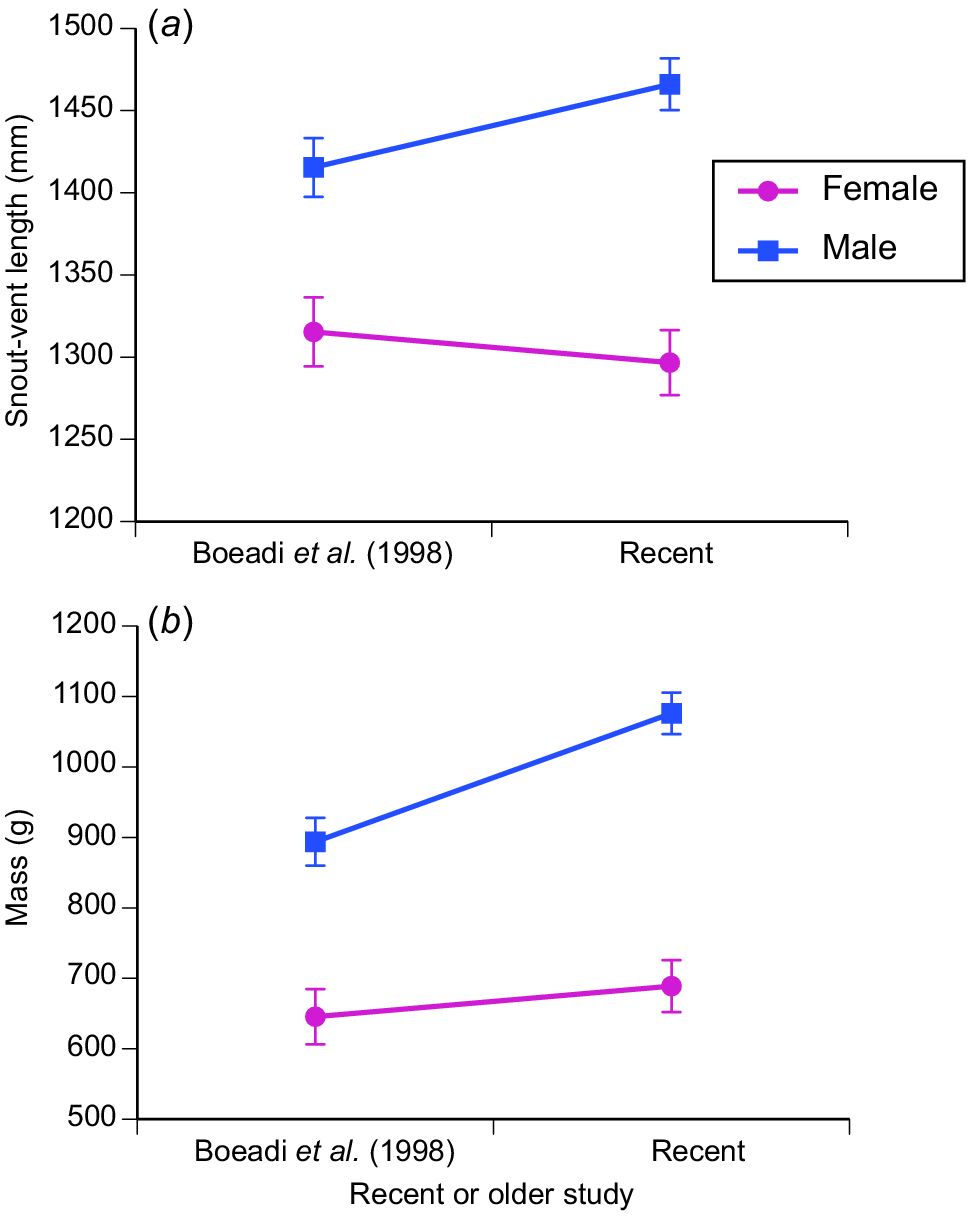
The same ANOVA design applied to ln(body mass) showed strong sexual dimorphism (males heavier: F1,383 = 71.23, P < 0.0001) but also a greater mean mass in the more recent study than the earlier one (F1,383 = 5.70, P < 0.02; interaction F1,383 = 2.84, P = 0.09). That is, snakes of both sexes were heavier in the recent study (Fig. 4b). Unsurprisingly given the above results, ANCOVA with ln(SVL) as covariate, ln(mass) as dependent variable and study as the factor, showed that snakes were more heavy-bodied at the same SVL in the more recent study than in the earlier one (females: interaction study × ln(SVL), F1,153 = 7.93, P < 0.006; for males: interaction study × ln(SVL), F1,226 = 19.95, P < 0.0001).
The two studies were conducted at different times of year, so combining the data provides a clearer picture of seasonality. The proportion of females carrying oviductal eggs peaked during the wet season (October–February) (see Fig. 3a). We have no data on follicle or testis volumes, or fatbody scores, for the earlier sample.
The mean clutch size from the study by Boeadi et al. (12.65, s.e. 0.54, range 7–17, N = 23) was very similar to that found in the current study (F1,53 = 0.01, P = 0.92). In an ANCOVA with SVL included as a covariate, fecundity increased with maternal SVL among adult females (F1,51 = 7.53, P < 0.009) in a similar way in both samples (interaction study × SVL, F1,51 = 1.53, P = 0.22; main effect of study, F1,51 = 0.49, P = 0.49). Likewise, sizes at sexual maturation were similar in the two samples (from the study by Boeadi et al., largest immature female 1210 mm SVL; no immature males; smallest adult female 1185 mm, male 980 mm).
Discussion
The results of our study are consistent with earlier research but we extend the range of years and locations for which data are available, and the seasonal timing of sampling. As reported by earlier studies, P. mucosa in our sample from West Java attained large body sizes. Males matured at smaller sizes than females but attained larger maximum sizes, a common pattern in snake species in which males engage in bouts of physical combat for access to reproductive females (Shine 1994). In keeping with that pattern, male P. mucosa exhibit combat bouts (Carpenter 1986). Males also are more heavy-bodied than conspecific females, plausibly reflecting selection for muscular strength during combat (Bonnet et al. 1998). Despite high ambient temperatures year-round, reproductive cycles are strongly seasonal for P. mucosa, as for many other tropical snakes, reflecting monsoonal rainfall patterns that affect important parameters such as prey availability (Kopstein 1938) and soil moisture levels for egg incubation (Brown and Shine 2006). As in many other tropical snakes, maturation is attained rapidly and fecundity is high and increases with maternal body size (e.g. Brown and Shine 2002).
Perhaps the most interesting result of our analysis is the overall similarity – in every trait that we measured – between our data from 2020–2021 and those of a previous study from the same and surrounding areas gathered 25 years previously (Boeadi et al. 1998). Both studies used the same methodology, examining carcasses at commercial processing facilities. The two samples are not directly comparable because of differences in seasonal timing (Boeadi et al. sampled during the wet season only, whereas we obtained samples over the rest of the year) and location of sampling (Boeadi et al. obtained data from across several parts of Java, whereas our own sampling was restricted to western areas). Those temporal and spatial differences in sampling regimes make the similarity in results of the two studies even more impressive. The only significant divergence was that snakes were more heavy-bodied (i.e. in better body condition) in the recent sample than in the earlier one. In all other respects (body sizes, sexual dimorphism, fecundity, fecundity versus maternal body size), the two samples were nearly identical.
That apparent similarity in morphology and reproductive output over a 25-year period suggests that the intense commercial harvest of rat snakes over the intervening period (>2 million specimens culled; based on Indonesian annual quotas) has not altered population parameters to a measurable degree. That conclusion is consistent with Boeadi et al.’s (1998) prediction of harvest sustainability, based on biological attributes (e.g. dietary breadth, use of disturbed habitats). Given uncertainties about issues such as changing habitat types and catch-per-unit-effort, it is important to test such predictions by repeated sampling over long periods (Natusch et al. 2020). Encouragingly, our analysis suggests that this large rat snake remains abundant in agriculturally disturbed habitats of West Java, and that its fundamental life-history traits have not been substantially modified by the long history of intense commercial offtake.
Conflicts of interest
This research was partially funded by an initiative focused on the conservation of reptile species used for skins, which receives some of its funding from companies that use reptile skins (although those companies do not use skins of this species). Donors had no influence at any stage of this research.
Declaration of funding
This work was undertaken with support from the Southeast Asian Reptile Conservation Alliance (SARCA) and an Indonesian Education Scholarship (LPDP) awarded to Alamsyah Elang Nusa Herlambang.
Acknowledgements
We thank the Indonesian CITES Management Authority (Ministry of Environment and Forestry; KKH), the Indonesian CITES Scientific Authority (National Research and Innovation Agency of Indonesia; BRIN), the Natural Resources Conservation Agency of West Java (BBKSDA), the Indonesian Reptile Skin Exporters Association (AIRAI), the Indonesian Reptile Meat Exporters Association (APEKLI), and the Indonesian Herpetological Association (PHI) for facilitating this research. The research was carried out under permits B2252/IPH.1/KP.06.01/VI/2019, B4033/IPH.1/KP.06.01/X/2019, B2342/IPH.1/KP.06.01/VI/2019, B502/IPH.1/KP.06.01/II/2020, and B-51/IPH.1/KP.06.01/I/2020. Thanks to Slamet Priambada, Supomo, Ade Kurniadi Karim, Rini Aini, Ramdani Manurung, Farits Alhadi, Fata Habiburrahman Faz, Taufan Sulaiman, Alfonsus Toribio, Eko Saputro, Elika Boscha, Frendi Irawan, Quraisy Zakky, and Rohmat Subandriyo for field assistance. Thanks to Asep, Mukti, Lilis, Tosin, and Tasrip for allowing us to collect data from their facilities. This work was undertaken with support from the Southeast Asian Reptile Conservation Alliance (SARCA) and an Indonesian Education Scholarship (LPDP) awarded to Alamsyah Elang Nusa Herlambang.
References
Bergman RAM (1952) L’anatomie du genre Ptyas a Java. Rivista di Biologia Coloniale Roma 12, 1-42.
| Google Scholar |
Boeadi , Shine R, Sugardjito , Amir M, Sinaga MH (1998) Biology of the commercially-harvested ratsnake (Ptyas mucosus) and cobra (Naja sputatrix) in central Java. Mertensiella 9, 99-104.
| Google Scholar |
Bonnet X, Shine R, Naulleau G, Vacher-Vallas M (1998) Sexual dimorphism in snakes: different reproductive roles favour different body plans. Proceedings of the Royal Society. Series B: Biological Sciences 265, 179-183.
| Crossref | Google Scholar |
Brown GP, Shine R (2002) Reproductive ecology of a tropical natricine snake, Tropidonophis mairii (Colubridae). Journal of Zoology 258(1), 63-72.
| Crossref | Google Scholar |
Brown GP, Shine R (2006) Why do most tropical animals reproduce seasonally? Testing hypotheses on an Australian snake. Ecology 87, 133-143.
| Crossref | Google Scholar | PubMed |
Camera BF, Quintana I, Strüssmann C, Waller T, Barros M, Draque J, Micucci PA, Miranda EBP (2020) Assessing the sustainability of yellow anaconda (Eunectes notaeus) harvest in northeastern Argentina. PLoS ONE 18(1), e0277629.
| Crossref | Google Scholar |
Carpenter CC (1986) An inventory of combat rituals in snakes. Smithsonian Herpetological Information Service 69, 1-18.
| Crossref | Google Scholar |
Fitzgerald LA (1994) The interplay between life history and environmental stochasticity: implications for the management of exploited lizard populations. American Zoologist 34(3), 371-381.
| Crossref | Google Scholar |
Harlow PS, Taylor JE (2000) Reproductive ecology of the jacky dragon (Amphibolurus muricatus): an agamid lizard with temperature-dependent sex determination. Austral Ecology 25(6), 640-652.
| Crossref | Google Scholar |
Kopstein F (1938) Ein Beitrag zur Eierkunde und zur Fortpflanzung der Malaiischen Reptilien. The Raffles Bulletin of Zoology 14, 81-167.
| Google Scholar |
Kurniawan N, Nugraha FA, Maulidi A, Firdaus AS, Kadafi AM, Kurnianto AS (2018) Snapshot of an on-going trade in reptile wholesaler, Kebumen, Central Java: preparation, trading, and conservation implications. Indonesian Journal of Environment and Sustainable Development 9, 9-14.
| Google Scholar |
Kusrini MD, Manurung R, Habiburrahman Faz A, Dwiputro A, Tajalli A, Nur Prasetyo H, Bayu Saputra P, Kennedi UF, Parikesit DW, Shine R, Natusch D (2022) Abundance, demography, and harvesting of water snakes from agricultural landscapes in West Java, Indonesia. Wildlife Research 50(4), 272-282.
| Crossref | Google Scholar |
Mieres MM, Fitzgerald LA (2006) Monitoring and managing the harvest of tegu lizards in Paraguay. Journal of Wildlife Management 70(6), 1723-1734.
| Crossref | Google Scholar |
Natusch DJD, Lyons JA, Mumpuni , Riyanto A, Shine R (2016) Jungle giants: assessing sustainable harvesting in a difficult-to-survey species (Python reticulatus). PLoS ONE 11(7), e0158397.
| Crossref | Google Scholar | PubMed |
Natusch DJD, Lyons JA, Riyanto A, Mumpuni , Khadiejah S, Shine R (2019) Detailed biological data are informative, but robust trends are needed for informing sustainability of wildlife harvesting: a case study of reptile offtake in Southeast Asia. Biological Conservation 233, 83-92.
| Crossref | Google Scholar |
Natusch DJD, Lyons JA, Mumpuni , Riyanto A, Shine R (2020) Harvest effects on blood pythons in North Sumatra. The Journal of Wildlife Management 84, 249-255.
| Crossref | Google Scholar |
Pal A, Dey S, Roy US (2012) Seasonal diversity and abundance of herpetofauna in and around an industrial city of West Bengal, India. Journal of Applied Sciences in Environmental Sanitation 7, 281-286.
| Google Scholar |
Shine R (1994) Sexual size dimorphism in snakes revisited. Copeia 1994, 326-346.
| Crossref | Google Scholar |
Shine R, Ambariyanto , Harlow PS, Mumpuni (1999) Reticulated pythons in Sumatra: biology, harvesting and sustainability. Biological Conservation 87(3), 349-357.
| Crossref | Google Scholar |
Sidik I (2006) Analisis Isi Perut Dan Ukuran Tubuh Ular Jali (Ptyas mucosus). Zoo Indonesia. Jurnal Fauna Tropika 15(2), 121-127.
| Google Scholar |
Sugardjito J, Boeadi , Amir M, Sinaga MH (1998) Assessment of harvest levels and status of the spitting cobra (Naja sputatrix) and the rat snake (Ptyas mucosus) in Central Java. Mertensiella 9, 105-110.
| Google Scholar |
Titley MA, Snaddon JL, Turner EC (2017) Scientific research on animal biodiversity is systematically biased towards vertebrates and temperate regions. PLoS ONE 12(12), e0189577.
| Crossref | Google Scholar | PubMed |



