Does the photoluminescence of rat fur influence interactions in the field?
Linda M. Reinhold A * , David T. Wilson
A * , David T. Wilson  B and Tasmin L. Rymer
B and Tasmin L. Rymer  A C *
A C *
A
B
C
Handling Editor: Laura Wilson
Abstract
While the photoluminescence of mammal fur is widespread, any potential function based on its optical properties remains speculative. Using paired photoluminescent and non-photoluminescent real-fur rat models in a field experiment, we aimed to test whether nocturnal vertebrates reacted differently to blueish-white photoluminescent fur than to non-photoluminescent fur. Remote cameras were set out in three different habitats (farmland, rainforest and woodland) in the Wet Tropics of Far North Queensland, Australia, over three full moon and three new moon phases. We recorded what species interacted with the models and counted the number of interactions with each model to calculate pair-wise differences of interactions with photoluminescent and non-photoluminescent models. No animal group (marsupial, placental mammal or avian) showed a preference for either model, on either new or full moon, suggesting that they either cannot detect a difference, or that preference is not based on photoluminescent properties. These findings do not support a hypothesis of selective pressure from nocturnal vertebrates acting on the trait of blueish-white photoluminescence in mammal fur.
Keywords: fluorescence, hair, mammal, moon, nocturnal, phosphorescence, terrestrial, visual function.
Introduction
The appearance of animals can be driven by evolutionary pressures on visual signals (Endler 1992). The colouration of mammal fur can be beneficial in crypsis, aposematism and social signalling, among others (Caro 2013). Mostly limited to the drabness of melanin, some mammals use a simple achromatic contrast of white (absence of melanin) alongside darker fur for signalling in dim light (crepuscular and/or nocturnal landscapes: Penteriani and Delgado 2017). However, recent observations of brightly photoluminescent (fluorescent and/or phosphorescent) mammals have attracted considerable media attention, and several authors have proposed that ventral or whole-body fur photoluminescence may have a visual function as a signal for nocturnal–crepuscular mammals (Kohler et al. 2019; Anich et al. 2021; Olson et al. 2021; Pynne et al. 2021).
Four hypotheses have been proposed for a visual signalling function of photoluminescence (Kohler et al. 2019). Kohler et al. (2019) hypothesised that photoluminescence is adaptive in nocturnal–crepuscular species. This was based on observations of North and Central American flying squirrels (Glaucomys spp.), which are nocturnal–crepuscular, active all year round in low light (Dolan and Carter 1977; Wells-Gosling and Heaney 1984), have clear ocular lenses (Yolton et al. 1974) and photoluminescent fur. In contrast, ground-dwelling squirrels are diurnal, hibernate in winter, have yellow ocular lenses, and are non-photoluminescent (Kohler et al. 2019). However, yellow lens colouration is not common across all diurnal mammals (Hammond 2012; Douglas and Jeffery 2014). Kohler et al. (2019) suggested that clear ocular lenses, which transmit ultraviolet light (Yolton et al. 1974) may give stronger low-light vision to nocturnal squirrels (i.e. increased photosensitivity), whereas Marshall and Johnsen (2017) explained that yellow long-pass ocular filters could facilitate the viewing of photoluminescence rather than hinder it, as they narrow the spectral range of absorbance, thereby enhancing contrast. At least for nocturnal flying squirrels, a clear ocular lens would only be beneficial for letting more light into the eye. This photosensitivity could counteract the likely inability of flying squirrels to see the pink colour of their photoluminescence (Carvalho et al. 2006). Sensitivity to particular wavelengths is also determined by the density and distribution of photoreceptor proteins in the retina (Hunt et al. 2007; McDonald et al. 2020). While the ability to see ultraviolet light is likely ancestral in vertebrates (Hunt et al. 2007), flying squirrels have lost the cone pigment that would have enabled them to see ultraviolet light (Carvalho et al. 2006), and have mostly rod-based vision (Jacobs 1993). However, fur photoluminescence transmutes ultraviolet–violet light into longer-wavelength colours, negating any involvement of ultraviolet vision. Animals do not need to see the ultraviolet excitation wavelengths, only the visible-spectrum emission wavelengths, or at least a contrast against the background, to discern photoluminescence.
Kohler et al. (2019) also hypothesised that photoluminescence could be a consequence of remaining active in snowy winter landscapes, where snow cover would reflect ultraviolet light, thereby boosting the photoluminescence of the ventral surface of the flying squirrels. However, Toussaint et al. (2023) recorded pink photoluminescence in flying squirrels from the warm climates of south-east Asia, and photoluminescent fur had also been recorded from other mammal species inhabiting snow-free landscapes (e.g. Udall et al. 1964; Nicholls and Rienits 1971).
Both Kohler et al. (2019) and Pynne et al. (2021) proposed a visual function for photoluminescence in intraspecific communication. To date, there has been no empirical evidence to describe a functional role of photoluminescence for communication in mammals. Although tested in other animals, this hypothesis has only been trialled using artificial ultraviolet lighting to boost photoluminescence, or in natural lighting but with artificial photoluminescent paint (Arnold et al. 2002; Lim et al. 2007; Gerlach et al. 2014; Douglas et al. 2021).
Kohler et al. (2019), Anich et al. (2021), Olson et al. (2021) and Pynne et al. (2021) all hypothesised that photoluminescence is involved in an antipredator context. In this context, Kohler et al. (2019) suggested that the photoluminescence of North and Central American flying squirrels could be Batesian mimicry to resemble photoluminescent pink owls. Whereas the flying squirrels themselves may be colour-blind (Carvalho et al. 2006), owls may be able to discern colour in low light (Martin 1974; Potier et al. 2020). However, reddish photoluminescence may only be emitted in response to a strong excitation light; if the excitation light is weak and therefore the emission dim, then the photoluminescence will only appear whitish, rendering the ability to see pink inconsequential (Harvey 1957). Fur photoluminescence has also been suggested to be a form of crypsis, where flying squirrels may appear camouflaged against photoluminescent lichen (Kohler et al. 2019). Pynne et al. (2021) also suggested camouflage against an unspecified background for pocket gophers (Geomyidae), citing soil photoluminescence as a possible source of luminophores. Photoluminescence may also act as aposematism, as suggested for caterpillars (Antheraea polyphemus) (Czarnecki et al. 2022).
To determine if photoluminescence is ecologically significant, Marshall and Johnsen (2017) proposed that five conditions should be met: (1) luminophores occur in a visible location; (2) the appropriate excitation wavelength is available, and the emission wavelength is visually relevant; (3) the emission wavelength is at maximal sensitivity to the viewer; (4) natural lighting conditions required for excitation are available; and (5) visually directed behaviours change when the photoluminescence is muted. Thus, all hypotheses for a visual function of photoluminescent fur in crepuscular–nocturnal environments principally rest on the premise that natural moonlight or twilight is strong enough to activate the luminophores in fur, and that the mammals themselves, or their predators, must be able to detect the photoluminescence excited by natural light.
The strength of short-wavelength emissions from bright sunlight is enough to excite most natural photoluminescence (Marshall and Johnsen 2017). However, subtle photoluminescence may be masked by the reflectance of bright sunlight (Viitala et al. 1995). At twilight, the overpowering middle wavelengths of the sun taper off, allowing lesser-intensity wavelengths to become more dominant without so much interference from reflection (McFarland and Munz 1975; Endler 1993). It is the shorter wavelengths that have the potential to excite photoluminescence that would stand out against an otherwise dark and monochromatic background (Pohland 2007).
The irradiance of the full moon is approximately 1000 times less than that of twilight, and it lacks the defined peaks of blue and red light, instead mimicking the more gradual spectrum of daylight (McFarland and Munz 1975; Johnsen et al. 2006). Excitation by full moonlight has been tested experimentally and shown to trigger the natural photoluminescence of scorpions (Vaejovis sp.), with nocturnal flying insects reacting to photoluminescent scorpions on a full moon but not on a new moon (Kloock 2005). However, subsequent experiments on other photoluminescent scorpions (Centruroides granosus) found that their house cricket (Acheta domesticus) prey did not react to photoluminescence or lack thereof in either laboratory trials with moonlight simulation or in natural outdoor lighting under a half moon (Gálvez et al. 2020).
The plausibility of the excitation of photoluminescence by relatively low-intensity ambient light in a visual function role also relies on the adequate visual sensitivity of the observer to detect the emitted photons. The notion that nocturnal animals may have highly sensitive vision had previously been overlooked, with studies predominantly focusing on the importance of olfaction for mammals and hearing for birds of prey (Penteriani and Delgado 2017). However, an emerging body of research is beginning to understand more about the evolution of nocturnal-specific visual systems and indicates that nocturnal landscapes are visually rich in detail to nocturnal animals (Warrant 2004; O’Carroll and Warrant 2017). In addition, marsupial and placental mammals differ in the evolutionary retention of visual pigments, with marsupials possessing a third type of cone photoreceptor (Arrese et al. 2002). While some predictions can be made from eye anatomy about the range of vision of an animal, behavioural trials are required to confirm the functional vision of the animal (Jacobs 1993; Arrese et al. 2006; O’Carroll and Warrant 2017).
Therefore, we investigated whether wild nocturnal animals preferentially choose to interact with a blueish-white photoluminescent model compared to a non-photoluminescent model. We deployed pairs of real-fur rat models, one photoluminescent and the other not, and recorded the initial interactions on full moon versus new moon nights. It is possible that the use of a single species (brown rat, Rattus norvegicus) to represent generic photoluminescent fur may affect species interactions with the models generally. Similar-sized species could avoid the models due to increased perception of competition (Brown et al. 2022), regardless of responses to photoluminescence. However, it is equally plausible that animals might approach the models due to local enhancement (Range and Huber 2007) or due to species differences in neophilia and neophobia (Bergman and Kitchen 2009).
If the ability to detect blueish-white photoluminescence is ubiquitous in mammals, we had several predictions: (1) We predicted that, if full moonlight was strong enough to excite the photoluminescence in mammal fur, as it was for Kloock’s (2005) scorpions, and if nocturnal vertebrates can see this photoluminescence, wild animals would demonstrate a preference for one model type under a full moon, but not under a new moon. (2) If mammals use photoluminescent fur as a means of intraspecific communication that is more visible to themselves than to their predators, as suggested by Kohler et al. (2019), we expected that similar-sized mammals would interact more with photoluminescent models, whereas interactions from predators such as dogs (Canis familiaris), cats (Felis catus) and owls (Strigiformes) would show no difference. (3) Alternatively, if photoluminescent fur acts as a camouflage mechanism, as suggested by Kohler et al. (2019), Anich et al. (2021), Olson et al. (2021) and Pynne et al. (2021), and if the substrate on which the models were placed was similarly photoluminescent, then we expected that the photoluminescent models would receive fewer interactions than the non-photoluminescent models on a full moon when their photoluminescence was activated. (4) Finally, if photoluminescent fur acts as aposematism, then we expected photoluminescent models would receive fewer interactions specifically from predators. If, however, the ability to detect photoluminescence is species-specific, as birds, marsupials and placental mammals have different visual systems, then we expected species-specific responses in the same directions as described above (e.g. for intraspecific communication, we expected heightened responses to photoluminescent models, but only for rodents specifically).
Methods
Ethics statement
This field experiment was conducted under Queensland Department of Environment and Science Research Permit no. WA0036056, under the Nature Conservation (Animals) Regulation 2020. All study sites were located on private property, with permission from the landowners. The study was approved by the James Cook University Animal Ethics Committee (approval no. A2768), and in compliance with the Australian Code for the Care and Use of Animals for Scientific Purposes. The hairspray used on the models was designed to be safe for use on human hair, so was not expected to have harmful effects on other species. Wild animals were free to interact with the models or not, and at no time experienced any unexpected adverse events.
Study sites
The study took place between September 2021 and March 2022. Three habitats (described below) on the Atherton Tablelands (Far North Queensland, Australia) were selected to encompass different conditions of sky light, with minimal interference from city skyglow. Faunal composition was factored into site choice to include both ground-dwelling mammals that were of similar size to the models, and nocturnal avian predators. Each site was sampled six times, during three new moon periods and three full moon periods. Due to logistical constraints, the farmland and rainforest sites were sampled concurrently for the first three months, and the woodland site was sampled separately for the second three months.
The open farmland site (17°14′46″S, 145°31′39″E) encompassed two properties separated by a dirt road, 9 km east of the small town of Atherton (bordering Kairi). The site has some skyglow visible from Atherton, but no local lighting, and provided for full moon illumination under an open sky. The farm on the northern side was a recently harvested sugar cane (Saccharum sp.) field bordering a Rhodes grass (Chloris gayana) field on the adjoining farm to the east, with fields of legumes at the northern corner. This area was relatively flat. The farm to the south was a young avocado (Persea americana) plantation bounded by a dirt road, an older avocado plantation, harvested sugar cane and a fenced, treed creek line and cattle (Bos taurus) paddock that the block sloped down towards.
The rainforest site (17°17′22″S, 145°38′16″E) encompassed two adjoining hilly private properties in secondary rainforest backing onto a creek, 8 km south-east of the small town of Yungaburra. The canopy was mostly closed but not dense, allowing dappled light through. Each property had household dwellings on rainforest acreage, but there was minimal interference from artificial lighting.
The woodland site (17°21′26″S, 145°19′48″E) comprised ironbark (Eucalyptus sp.), red bloodwood (Corymbia gummifera) and lemon-scented gum (C. citriodora) woodland, with Cypress pine (Callitris sp.) thickets, and an understorey of native grasses and forbs. The canopy was open, and the site sloped from a granite range down to an annual creek. The woodland habitat provided a mix of filtered light. Being 10 km from the small town of Herberton and in an off-grid part of Watsonville, it experienced no interference from artificial skyglow.
Rat model preparation
We selected brown rats primarily for their ease at obtaining sufficient pelts to run this experiment. Although the brown rat is a non-native species to Australia, it does occur in the wild in the Wet Tropics bioregion, and elsewhere is known to be preyed on by native predators, including northern quolls (Dasyurus hallucatus) (Pellis and Officer 1987) and eastern grass owls (Tyto longimembris) (Clulow et al. 2011). In addition, when we compared the fur photoluminescence of the rats to native rodents and antechinuses, it was similar in intensity and blueish-white in colour, indicating that brown rat fur would be an adequate model.
Thirty-six frozen brown rats of mixed sex and colour, bred locally on the Atherton Tablelands, were purchased from a commercial supplier (Bugs Alive, Cairns, Australia) because roadkill carcasses were rarely intact enough to secure the numbers of pelts required. Rats were used because their fur is highly photoluminescent (Rebell et al. 1956; Udall et al. 1964). All rats, regardless of visible pelt colour (white, brown or black), displayed bright blueish-white photoluminescence when exposed to 365–410 nm ultraviolet–violet light, with the photoluminescence of white fur most prominent. Rats were skinned, and the pelts salted before being fitted over a non-photoluminescent grey or black PVC model rat (20 cm straight head–body length). The PVC feet and tail remained exposed. Pelts were stitched into place and craft eyes fitted on the head. Model rats were allowed to air-dry in a dark, air-conditioned (~24°C; 50–65% relative humidity) room for 2–3 weeks. Finished models were paired by sex, colour and size so that both rats in a pair looked similar. Nine pairs were white, two pairs were white with light brown hoods, two pairs were brown, two pairs were black, and one pair was grey. The remaining four rats were used as spares.
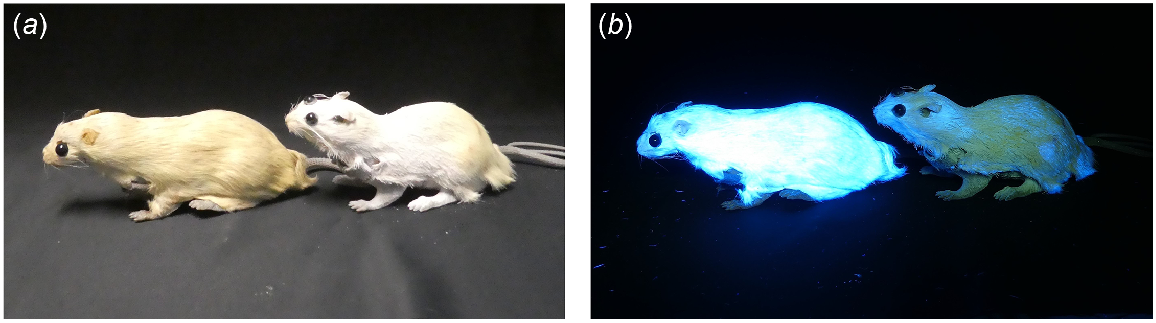
To remove luminophores from one rat of each prepared rat model pair (n = 16), pelts were washed in 50°C tap water in a laundry tub for 1.5 h, with several kettles of boiling water poured over them. This method was a practical way of replicating studies that reduced tryptophan metabolite photoluminescence in fur to approximately one-third of its previous observable intensity (Rebell 1966; Nicholls and Rienits 1971). Photoluminescence was further extinguished using ultraviolet-protectant hairsprays (‘Clarins UVB UVA high protection 30 Sun Care Oil Mist’ hair oil; ‘Batiste dry shampoo and colour protect, with UV filter to protect fade’ for white rat pelts; ‘Batiste dry shampoo beautiful brunette’ and ‘divine dark’, and ‘Tony and Guy brunette’ hairspray for brown pelts). When illuminated with 365–410 nm torches, the suppressant methods used were effective in removing the glow, with the photoluminescent rat appearing much brighter than the non-photoluminescent rat (Fig. 1). Regular ‘Schwarzkopf’ or ‘Woolworths homebrand’ hairspray was then sprayed over all models to mask differing odours. When rain showers were forecast, model rats were also sprayed with ‘Maseur Weather Guard boot and garment spray’. The suppression of photoluminescence in the fur of the non-photoluminescent models was checked before each field session to ascertain that there was a marked difference in photoluminescence within each pair of rats. Ultraviolet-protectant hairsprays were reapplied as needed. Damaged rats were repaired or replaced with spare matching rats as necessary.
Remote camera set-up
Sixteen remote cameras were used (n = 13 PR700 20MP 1080P 120° Detecting Range Hunting Trail Camera Waterproof Hunting Scouting Camera with Auto IR Filter for Wildlife Monitoring; n = 2 Anaconda 16MP Trail Camera Camo; n = 1 16MP 1080P Hunting Trail Camera Infrared Security Night Vision Waterproof Cam). Different camera models were used because some cameras failed prior to commencement. Videos were chosen over photographs or marks left on models to better capture animal behaviours (Akcali et al. 2019). All cameras had a trigger speed of 0.2–0.6 s, with a 20 m passive infrared (PIR) sensing distance. Cameras were set with high sensitivity and to record in 1080P resolution infrared video with a 2 s delay between videos. Videos were set to record for 20 s.
Setting of remote cameras was adapted from Gillespie et al. (2015). Cameras were placed 70 cm above the ground facing a small clearing and tilted downwards to frame the model pairs. Any long grass obscuring either the cameras or the models was trimmed, but not removed completely, to reduce the chance of false triggers. For each camera station, the model pair (one photoluminescent and one non-photoluminescent model) was set on natural ground (mostly dirt, grass or leaf litter) 1.5 m directly in front of the camera post/tree. Non-photoluminescent synthetic black cord was used to tether the models to the camera post/tree to prevent them from being carried off by predators. Models were placed two body lengths apart (=40 cm), facing each other and the camera at a 45° angle. We acknowledge that this proximity could mean that animals may have avoided both models if photoluminescence serves as a warning (but see Results). Within each habitat, half of the camera stations had the photoluminescent model on the left, and half had the photoluminescent model on the right. The side on which the photoluminescent model was placed was alternated once within each habitat to reduce bias. Pair sides were kept consistent within each full/new moon pair.
Experimental design
The study mostly followed the experimental design of Kloock (2005) but was adapted for interactions with vertebrates rather than flying insects. Within each habitat, camera stations were set out for three nights at a time at each full moon and new moon phase, apart from one full and new moon set at the woodland site, which were each left out for four nights because the full moon fell more evenly over four nights than three. Weather was mostly clear or partly cloudy; however, it cannot be known whether there was a cloud over the moon at the time of each interaction.
Camera stations were set as far apart as possible within the confines of each property. For the open farmland site, four cameras were set at 200 m intervals on a line of old fence posts dividing the upper adjoining fields, and four cameras were set at 150 m intervals on the fence posts bounding the lower creek line or dirt road. In the rainforest site, four cameras were set 100 m apart along narrow tracks on each of the two properties. In the woodland site, initially 13 camera stations were spaced 100–200 m apart along tracks. This number was reduced to nine stations towards the end of the experiment as some rat models were damaged irreparably. Only these nine stations were used in the statistical analyses for the woodland site.
Behavioural observations
Interactions between wild animals and the model pairs were scored only on first approach; i.e. the rat model that was interacted with first, regardless of subsequent interactions with the other model. This ensured the greatest chance of the interactions being based on sight, and before the infrared light from the camera interfered with natural illumination. Interactions where animals were foraging in the leaf litter and accidentally touched a model in the process of sniffing food from the ground were not counted. Video sequences more than 10 min apart, or where there was a group size of two or more animals in the same frame interacting with models, were counted as separate events. If an animal was observed coming back to the models in numerous consecutive videos, only the first model interaction was scored. Wild animals were identified to species where possible. We also categorised animals into broad taxon groups (bird, marsupial or placental mammal). Only interactions between sunset and sunrise were used in the analyses. While lighting phase (golden hour, civil twilight, nautical twilight, astronomical twilight or dark) was recorded for each interaction, sample sizes were not sufficient to allow for robust statistical analyses.
Pair differences
All statistical analyses were conducted using RStudio (RStudio Team 2020, ver. 1.0.153; R Core Team 2020, ver. 4.1.2). Following Kloock (2005), we calculated the pair difference for each camera station at each habitat as the number of first interactions with the photoluminescent model minus the number of first interactions with the non-photoluminescent model. More first interactions with the photoluminescent model indicated a positive pair difference, while more first interactions with the non-photoluminescent model indicated a negative pair difference. Data were tested for normality (Shapiro-Wilk test) and transformed using the orderNorm function (bestNormalize package, Peterson 2022). We first ran a linear mixed effects model (LMER; lmerTest package, Kuznetsova et al. 2022) with Gaussian distribution to assess whether pair differences were affected by moon phase. Habitat, replicate and moon phase were included as fixed effects, and camera number was included as a random effect. We then ran a second model (as moon phase and replicate had no significant effect, see Results) with habitat and animal type as fixed effects only. Significant differences in the main effects were identified using Tukey’s post hoc tests (emmeans package, Lenth et al. 2022).
Results
General observations
Eleven species of marsupial, at least nine species of placental mammal and four species of bird interacted a combined total of 142 times with the models between sunset and sunrise (Supplementary Table S1). Only dogs, cats and northern long-nosed bandicoots (Perameles pallescens) were recorded interacting in all habitats. Rodents interacted with the models in the farmland and rainforest sites, but not in the woodland site. Northern quolls (Fig. 2) were observed only in the woodland site, where they interacted enthusiastically with the models. Eastern grass owls were observed interacting only during one moon session, in the open farmland site.
Pair differences
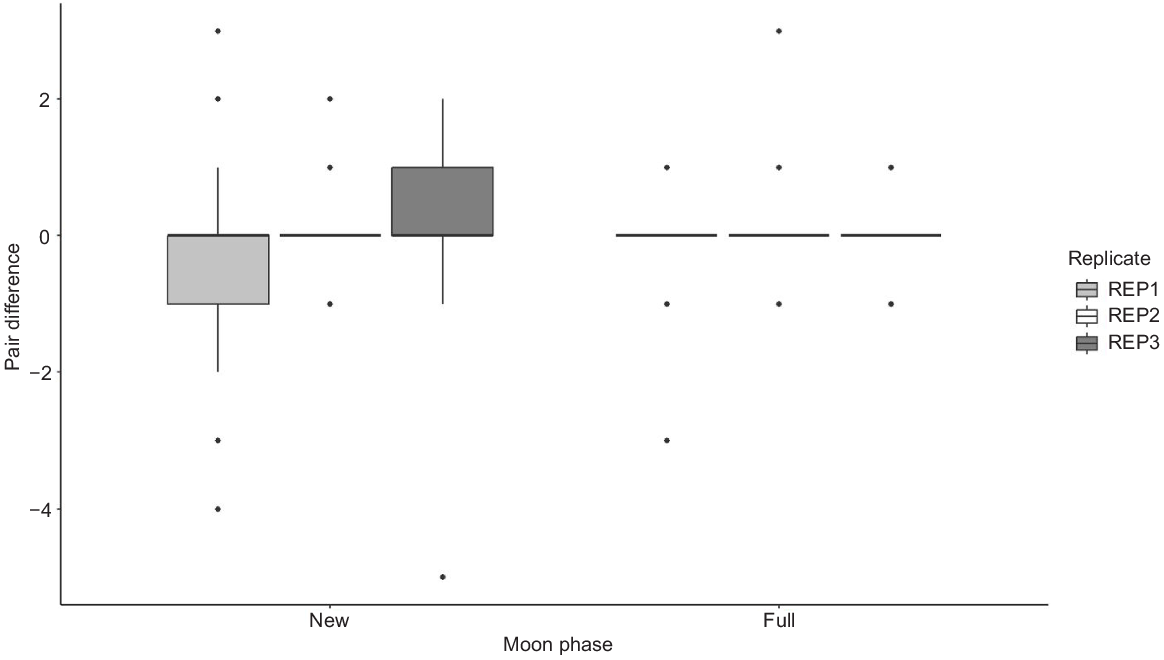
There was an overall pair difference of zero (i.e. equal numbers of interactions with each model type) when pooled for both moon phases. There were no significant effects of habitat (LMER: χ22 = 0.60, P = 0.739), replicate (χ22 = 1.35, P = 0.508), moon phase (χ21 = 0.48, P = 0.488) (Fig. 3) or camera number (χ21 = 0.03, P = 0.872) on the pair differences. In addition, there was no significant effect of habitat (F2,4 = 0.10, P = 0.907) or animal type (F2,4 = 0.93, P = 0.466) on the pair differences.
Discussion
With speculation increasing about a visual function for fur photoluminescence, this study aimed to test whether wild nocturnal vertebrates would respond to the natural photoluminescence of real fur in natural lighting. The experimental design loosely followed that of Kloock (2005), who concluded that scorpion photoluminescence could be detected by flying insects during a full moon. In this study, we only tested the response of nocturnal vertebrates to blueish-white rat fur photoluminescence, which is likely caused by tryptophan metabolites (Rebell et al. 1957). Consequently, the responses observed here may be specific for these types of luminophores. However, the longer excitation wavelengths of porphyrin luminophores, leading to pink-orange-red photoluminescence in species such as flying squirrels (Toussaint et al. 2023), could elicit a different response, such as Batesian mimicry or crypsis (Kohler et al. 2019), and warrant further investigation.
In contrast to Kloock’s (2005) study on the response of flying insects to scorpion photoluminescence, we found no significant difference in preference for non-photoluminescent over photoluminescent rat models for any habitat, moon phase or animal type (birds, marsupials or placentals). There are two possible explanations for these findings: (1) the light of the full moon was not strong enough to excite the photoluminescence in the model fur to a level where it was visible to nocturnal mammals and birds, contrary to Kloock’s (2005) observations for nocturnal flying insects; or (2) the lack of distinction could mean that, even if nocturnal vertebrates can detect the photoluminescence, it does not affect their behaviour and they have no preference for or against it. Although intraspecific communication with live rats of the same species was not tested, our findings indicate that blueish-white photoluminescence in the fur of nocturnal mammals does not provide a visual function for either communication between similar-sized mammals, or predator avoidance, as suggested by several recent studies (Kohler et al. 2019; Anich et al. 2021; Olson et al. 2021; Pynne et al. 2021). The lack of behavioural change in response to the trialled moonlight activation of photoluminescence does not meet Marshall and Johnsen’s (2017) criteria for ecological significance.
The hypothesis that photoluminescence may enhance camouflage against a substrate (Kohler et al. 2019) does not appear relevant for ground-dwelling mammals in the Wet Tropics showing blueish-white photoluminescence. While many organic and inorganic substances have a mild background glow (Tomalia et al. 2019), the varying dirt, leaf litter and grass substrates on which the model pairs were placed had insignificant photoluminescence compared to that of rat fur when observed with the same torches (see Reinhold (2021) for photographs of live mammals against natural backgrounds, and Reinhold (2023) for photographs of two species of roadkill mammals against dirt/gravel and grass backgrounds, both on the Atherton Tablelands). This observation questions the likelihood of ground-dwelling mammal blueish-white photoluminescence functioning as camouflage. The lack of avoidance of our photoluminescent models suggests that aposematism is also not a likely function for mammalian fur photoluminescence.
Nocturnal mammal activity has been shown to decrease with increasing moon illumination, suggesting that prey animals may trade-off foraging and predation risk during full, but not new, moon periods (Clarke 1983; Linley et al. 2021). We found a similar response for the number of interactions (Fig. S1). However, we found no difference in the pair choice with moon phase, indicating that animals, when they did interact, did not favour one model over the other, providing further support for a lack of blueish-white photoluminescence being used as a warning or an attractant. The tendency for prey-sized mammals to avoid open spaces on full moon nights would also reduce the opportunity to display their photoluminescence, further suggesting a lack of visual function for photoluminescent fur.
Our predictions were based on the ability to observe blueish-white photoluminescence either being ubiquitous to mammals or being species-specific. The expression of photoluminescence in mammal fur is common (Reinhold et al. 2023). Of the 16 wild mammal species identified in this study, the 10 that have also been examined with ultraviolet–violet light all display fur photoluminescence (either pink and/or blueish-white) to some degree (Reinhold 2021, 2023). In addition, diverse mammal taxa have photoluminescence effected by similar luminophores in their fur (e.g. tryptophan metabolites in brown rats and common brushtail possums (Trichosurus vulpecula): Rebell 1966; Nicholls and Rienits 1971; porphyrins in bandicoots and northern quolls: Reinhold 2023). Therefore, detection and use of photoluminescence in visual communication is unlikely to be species-specific. However, sample sizes and interactions were quite low, and species differences may have been masked by pooling sample sizes of different species. Targeted studies focusing specifically on Rattus species or northern quolls could be insightful. However, targeting these species specifically would require extensive amounts of time in the field. While laboratory studies could be informative, they are limited by the need for unfiltered natural lighting. Additional studies focused on pink-orange-red porphyrin photoluminescence would add insights to understanding whether none, all, or just some, fur photoluminescence may serve a visual function. The abundance of varying excitation wavelengths in twilight or daylight may also provide conditions conducive to photoluminescence display that were not met by moonlight.
This is the first study on vertebrates testing photoluminescence of real mammal fur in natural lighting conditions in the field. This is also the first study to test whether the photoluminescence of fur is preferentially selected by nocturnal mammals or birds, that is, whether it has the potential for a visual function. We found no evidence for a visual function for blueish-white photoluminescence in the fur of nocturnal mammals, highlighting that without behavioural tests, a trait function should not automatically be assumed.
Data availability
The data that support this study are available in Research Data JCU at https://doi.org/10.25903/3sdj-5076.
Declaration of funding
This project was funded by the North Queensland Wildlife Trust, James Cook University College of Science and Engineering, and an Australian Government Research Training Program Scholarship granted to LMR. The funding sources had no involvement in the preparation of the data or manuscript or the decision to submit for publication.
Acknowledgements
For access to study sites, thanks to Alan Gillanders, Alison Faigniez, Laurie and Lauren Ross, Lyn Kattenberg, Ellen, Aldo and Roger Pezzelato, Dr Scott Burnett and Jacqueline Nolen. Prof Kristofer Helgen gave helpful advice in the planning stages of this experiment. Two anonymous reviewers provided comments that improved the manuscript.
References
Akcali CK, Adan Pérez-Mendoza H, Salazar-Valenzuela D, Kikuchi DW, Guayasamin JM, Pfennig DW (2019) Evaluating the utility of camera traps in field studies of predation. PeerJ 7, e6487.
| Crossref | Google Scholar | PubMed |
Anich PS, Anthony S, Carlson M, Gunnelson A, Kohler AM, Martin JG, Olson ER (2021) Biofluorescence in the platypus (Ornithorhynchus anatinus). Mammalia 85, 179-181.
| Crossref | Google Scholar |
Arnold KE, Owens IPF, Marshall NJ (2002) Fluorescent signaling in parrots. Science 295, 92.
| Crossref | Google Scholar | PubMed |
Arrese CA, Hart NS, Thomas N, Beazley LD, Shand J (2002) Trichromacy in Australian marsupials. Current Biology 12, 657-660.
| Crossref | Google Scholar | PubMed |
Arrese CA, Beazley LD, Neumeyer C (2006) Behavioural evidence for marsupial trichromacy. Current Biology 16, R193-R194.
| Crossref | Google Scholar | PubMed |
Bergman TJ, Kitchen DM (2009) Comparing responses to novel objects in wild baboons (Papio ursinus) and geladas (Theropithecus gelada). Animal Cognition 12, 63-73.
| Crossref | Google Scholar | PubMed |
Brown GE, Crane AL, Demers EE, Chivers DP, Ferrari MCO (2022) Uncertain foraging opportunities and predation risk exert additive effects on induced neophobia in cichlids. Animal Behaviour 186, 21-28.
| Crossref | Google Scholar |
Caro T (2013) The colours of extant mammals. Seminars in Cell & Developmental Biology 24, 542-552.
| Crossref | Google Scholar | PubMed |
Carvalho LdS, Cowing JA, Wilkie SE, Bowmaker JK, Hunt DM (2006) Shortwave visual sensitivity in tree and flying squirrels reflects changes in lifestyle. Current Biology 16, R81-R83.
| Crossref | Google Scholar | PubMed |
Clarke JA (1983) Moonlight’s influence on predator/prey interactions between short-eared owls (Asio flammeus) and deermice (Peromyscus maniculatus). Behavioral Ecology and Sociobiology 13, 205-209.
| Crossref | Google Scholar |
Clulow S, Peters KL, Blundell AT, Kavanagh RP (2011) Resource predictability and foraging behaviour facilitate shifts between nomadism and residency in the eastern grass owl. Journal of Zoology 284, 294-299.
| Crossref | Google Scholar |
Czarnecki C, Manderino R, Parry D (2022) Reduced avian predation on an ultraviolet-fluorescing caterpillar model. The Canadian Entomologist 154, e10.
| Crossref | Google Scholar |
Dolan PG, Carter DC (1977) Glaucomys volans. Mammalian Species 78, 1-6.
| Crossref | Google Scholar |
Douglas RH, Jeffery G (2014) The spectral transmission of ocular media suggests ultraviolet sensitivity is widespread among mammals. Proceedings of the Royal Society B: Biological Sciences 281, 20132995.
| Crossref | Google Scholar |
Douglas HD, III, Ermakov IV, Gellermann W (2021) Brighter is better: bill fluorescence increases social attraction in a colonial seabird and reveals a potential link with foraging. Behavioral Ecology and Sociobiology 75, 144.
| Crossref | Google Scholar |
Endler JA (1992) Signals, signal conditions, and the direction of evolution. The American Naturalist 139, S125-S153.
| Crossref | Google Scholar |
Endler JA (1993) The color of light in forests and its implications. Ecological Monographs 63, 1-27.
| Crossref | Google Scholar |
Gálvez D, Nieto C, Samaniego P (2020) Test of the prey-attraction hypothesis for the scorpion fluorescence. Neotropical Biodiversity 6, 172-177.
| Crossref | Google Scholar |
Gerlach T, Sprenger D, Michiels NK (2014) Fairy wrasses perceive and respond to their deep red fluorescent coloration. Proceedings of the Royal Society B: Biological Sciences 281, 20140787.
| Crossref | Google Scholar |
Hammond BR (2012) The visual effects of intraocular colored filters. Scientifica 2012, 424965.
| Crossref | Google Scholar |
Hunt DM, Carvalho LS, Cowing JA, Parry JWL, Wilkie SE, Davies WL, Bowmaker JK (2007) Spectral tuning of shortwave-sensitive visual pigments in vertebrates. Photochemistry and Photobiology 83, 303-310.
| Crossref | Google Scholar | PubMed |
Jacobs GH (1993) The distribution and nature of colour vision among the mammals. Biological Reviews of the Cambridge Philosophical Society 68, 413-471.
| Crossref | Google Scholar | PubMed |
Johnsen S, Kelber A, Warrant E, Sweeney AM, Widder EA, Lee RL, Jr, Hernández-Andrés J (2006) Crepuscular and nocturnal illumination and its effects on color perception by the nocturnal hawkmoth Deilephila elpenor. Journal of Experimental Biology 209, 789-800.
| Crossref | Google Scholar | PubMed |
Kloock CT (2005) Aerial insects avoid fluorescing scorpions. Euscorpius – Occasional Publications in Scorpiology 2005 1-7.
| Crossref | Google Scholar |
Kohler AM, Olson ER, Martin JG, Anich PS (2019) Ultraviolet fluorescence discovered in New World flying squirrels (Glaucomys). Journal of Mammalogy 100, 21-30.
| Crossref | Google Scholar |
Kuznetsova A, Brockhoff PB, Christensen RHB, Jensen SP (2022) Package ‘lmerTest’. Available at https://cran.r-project.org/web/packages/lmerTest/lmerTest.pdf
Lenth R, Buerkner P, Giné-Vázquez I, Herve M, Jung M, Love J, Miguez F, Riebl H, Singmann H (2022) Package ‘emmeans’. Available at https://cran.r-project.org/web/packages/emmeans/emmeans.pdf
Lim MLM, Land MF, Li D (2007) Sex-specific UV and fluorescence signals in jumping spiders. Science 315, 481.
| Crossref | Google Scholar | PubMed |
Linley GD, Pauligk Y, Marneweck C, Ritchie EG (2021) Moon phase and nocturnal activity of native Australian mammals. Australian Mammalogy 43, 190-195.
| Crossref | Google Scholar |
Marshall J, Johnsen S (2017) Fluorescence as a means of colour signal enhancement. Philosophical Transactions of the Royal Society B: Biological Sciences 372, 20160335.
| Crossref | Google Scholar | PubMed |
Martin GR (1974) Color vision in the tawny owl (Strix aluco). Journal of Comparative and Physiological Psychology 86, 133-141.
| Crossref | Google Scholar | PubMed |
McDonald B, Geiger B, Vrla S (2020) Ultraviolet vision in Ord’s kangaroo rat (Dipodomys ordii). Journal of Mammalogy 101, 1257-1266.
| Crossref | Google Scholar |
Nicholls EM, Rienits KG (1971) Tryptophan derivatives and pigment in the hair of some Australian marsupials. International Journal of Biochemistry 2, 593-603.
| Crossref | Google Scholar |
O’Carroll DC, Warrant EJ (2017) Vision in dim light: highlights and challenges. Philosophical Transactions of the Royal Society B: Biological Sciences 372, 20160062.
| Crossref | Google Scholar | PubMed |
Olson ER, Carlson MR, Ramanujam VMS, Sears L, Anthony SE, Anich PS, Ramon L, Hulstrand A, Jurewicz M, Gunnelson AS, Kohler AM, Martin JG (2021) Vivid biofluorescence discovered in the nocturnal springhare (Pedetidae). Scientific Reports 11, 4125.
| Crossref | Google Scholar | PubMed |
Pellis SM, Officer RCE (1987) An analysis of some predatory behaviour patterns in four species of carnivorous marsupials (Dasyuridae), with comparative notes on the eutherian cat Felis catus. Ethology 75, 177-196.
| Crossref | Google Scholar |
Penteriani V, Delgado MdM (2017) Living in the dark does not mean a blind life: bird and mammal visual communication in dim light. Philosophical Transactions of the Royal Society B: Biological Sciences 372, 20160064.
| Crossref | Google Scholar | PubMed |
Peterson RA (2022) Package ‘bestNormalize’. Available at https://cran.r-project.org/web/packages/bestNormalize/bestNormalize.pdf
Potier S, Mitkus M, Kelber A (2020) Visual adaptations of diurnal and nocturnal raptors. Seminars in Cell & Developmental Biology 106, 116-126.
| Crossref | Google Scholar | PubMed |
Pynne JT, Castleberry SB, Conner LM, Piper CW, Parsons EI, Gitzen RA, Duncan SI, Austin JD, McCleery RA (2021) Ultraviolet biofluorescence in pocket gophers. The American Midland Naturalist 186, 150-155.
| Crossref | Google Scholar |
Range F, Huber L (2007) Attention in common marmosets: implications for social-learning experiments. Animal Behaviour 73, 1033-1041.
| Crossref | Google Scholar |
Rebell G (1966) Kynurenine in rat hair. Nature 209, 913-914.
| Crossref | Google Scholar | PubMed |
Rebell G, Mahvi A, Lamb JH (1956) Paper chromatographic separation of fluorescent materials in normal rat hair. Journal of Investigative Dermatology 27, 259-262.
| Crossref | Google Scholar |
Rebell G, Lamb JH, Mahvi A, Lee HR (1957) The identification of L-kynurenine as the cause of fluorescence of the hair of the laboratory rat. Journal of Investigative Dermatology 29, 471-477.
| Crossref | Google Scholar | PubMed |
Reinhold L (2021) Mammals with fluorescent fur: observations from the Wet Tropics. North Queensland Naturalist 51, 1-8.
| Google Scholar |
Reinhold L (2023) Photoluminescence in fur. MPhil thesis, James Cook University, Cairns, Australia. Available at https://doi.org/10.13140/RG.2.2.12146.48325
Reinhold LM, Rymer TL, Helgen KM, Wilson DT (2023) Photoluminescence in mammal fur: 111 years of research. Journal of Mammalogy 104, 892-906.
| Crossref | Google Scholar | PubMed |
R Core Team (2020) R: a language and environment for statistical computing (Version 4.1.2). R Foundation for Statistical Computing, Vienna, Austria. Available at https://www.R-project.org
RStudio Team (2020) RStudio: integrated development for RStudio (Version 1.0.153). PBC, Boston, Massachusetts, United States of America. Available at https://www.rstudio.com
Tomalia DA, Klajnert-Maculewicz B, Johnson KA-M, Brinkman HF, Janaszewska A, Hedstrand DM (2019) Non-traditional intrinsic luminescence: inexplicable blue fluorescence observed for dendrimers, macromolecules and small molecular structures lacking traditional/conventional luminophores. Progress in Polymer Science 90, 35-117.
| Crossref | Google Scholar |
Toussaint SLD, Ponstein J, Thoury M, Métivier R, Kalthoff DC, Habermeyer B, Guilard R, Bock S, Mortensen P, Sandberg S, Gueriau P, Amson E (2023) Fur glowing under ultraviolet: in situ analysis of porphyrin accumulation in the skin appendages of mammals. Integrative Zoology 18, 15-26.
| Crossref | Google Scholar | PubMed |
Udall SL, Briggs FP, Pautzke CF, Janzen DH (1964) Fluorescence studies. In ‘Wildlife research: problems programs progress 1963. Activities of the branch of wildlife research in the Bureau of Sport Fisheries and Wildlife for the calendar year 1963. Circular 188’. p. 64. (United States Department of the Interior, Fish and Wildlife Service and Bureau of Sport Fisheries and Wildlife: Washington DC). Available at https://spo.nmfs.noaa.gov/sites/default/files/legacy-pdfs/CIRC188.pdf
Viitala J, Korplmäki E, Palokangas P, Koivula M (1995) Attraction of kestrels to vole scent marks visible in ultraviolet light. Nature 373, 425-427.
| Crossref | Google Scholar |
Warrant E (2004) Vision in the dimmest habitats on earth. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural and Behavioral Physiology 190, 765-789.
| Crossref | Google Scholar | PubMed |
Wells-Gosling N, Heaney LR (1984) Glaucomys sabrinus. Mammalian Species 229, 1-8.
| Crossref | Google Scholar |
Yolton RL, Yolton DP, Renz J, Jacobs GH (1974) Preretinal absorbance in sciurid eyes. Journal of Mammalogy 55, 14-20.
| Crossref | Google Scholar | PubMed |