Green turtle (Chelonia mydas) hatching success at Raine and Heron Islands
David T. Booth A *
A *
A School of Biological Sciences, The University of Queensland, Brisbane, Qld 4072, Australia.
Australian Journal of Zoology 70(6) 211-215 https://doi.org/10.1071/ZO23013
Submitted: 27 March 2023 Accepted: 2 June 2023 Published: 28 June 2023
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Raine Island, the world’s largest green turtle nesting location, has low nest hatching success. The main causes of this low hatching success are thought to be nest destruction by subsequent nesting females, and inundation of nests during high tides and storm surges. But even nests that are protected from nest destruction and inundation appear to have relatively low hatching success, with most of the embryo mortality occurring early in incubation. Here, I compare hatching success and developmental phase of embryo death of protected ‘dry’ nests from Raine Island (RI) with similar nests from Heron Island (HI), a nesting location previously reported as having high hatching success. Nests at both sites were sampled close to the peak time of nesting (December). Twenty-eight nests were sampled at RI and 14 nests at HI. Nest temperatures were cooler during the first week of incubation at HI (median 26.9°C) than at RI (median 30.1°C), but three-days-in-a-row maximum nest temperatures were higher at HI (median 36.0°C) than at RI (median 33.5°C). I found the hatching success of sampled nests at both locations was similar, ~70%, but most embryo death occurred early in incubation at RI (median 16.5%) compared to HI (median 3.8%), but late in incubation at HI (median 4.9%) compared to RI (median 0.2%).
Keywords: Chelonia mydas, embryonic mortality, hatching success, Heron Island, nests, nest temperature, sea turtle, Raine Island.
Introduction
Raine Island in the northern Great Barrier Reef is the location of the world’s largest green turtle (Chelonia mydas) nesting aggregation and the nesting site for the vast majority of the northern Great Barrier Reef genetic stock of green turtles (Limpus et al. 2003; Limpus 2008). The large number of females nesting on this small island and the resultant high nest density means that many nests are destroyed by subsequently nesting females, particularly in high female visitation years (Limpus et al. 2003; Coffee and Robertson 2021). Despite this, in the three years that hatching success was assessed in nests that were located by holes created by escaping hatchlings (1979, 1983 and 1984), hatching success was high, with means of 76%, 85% and 85% respectively (Limpus et al. 2003). The proportion of eggs with no signs of visible embryo development (Phase 1 development) in these nests was low, varying between 6% and 18% (Limpus et al. 2003). It was not until the 1996–1997 nesting season that the inundation of low-lying nests was identified as a major cause of incubation failure (Limpus et al. 2003), and embryo mortality due to inundation due to high tides, storm surges and heavy rain has persisted (Coffee and Robertson 2021). The hatching success and therefore recruitment of hatchlings from Raine Island appears to have declined since the mid-1980s, varying between 30 and 60% depending on the female visitation rate (Coffee and Robertson 2021). A definitive reason for the decline in hatching success is unknown, but unusually high rates of embryo death very early during incubation may partly explain the overall low hatching success (Booth et al. 2020, 2021, 2022). Here, I compare the hatching success, and the early and late incubation embryo death rates of green turtle nests from Raine Island with those from Heron Island in the southern Great Barrier Reef where hatching success is generally greater (Limpus 2008) to determine if there are genuine differences in early- and late-stage embryo death rates between the two locations.
Materials and methods
This study uses data obtained from two sites while conducting other studies: (1) Raine Island, located in the northern Great Barrier Reef (11.5904°S, 144.0352°E), during the 2018–2019 nesting season (Booth et al. 2020, 2022), and (2) Heron Island in the southern Great Barrier Reef (23.4422°S, 151.9146°E) during the 2019–2020 and 2020–2021 nesting seasons (Smith et al. 2021; Young et al. unpubl. data). Only data from nests laid in December were compared to ensure comparisons between the two sites were commensurate because embryo mortality and hatching success vary with the month of nesting at Raine Island: hatching success decreases later in the nesting season (Booth et al. 2020, 2022). Methods were essentially the same at both sites. Females emerging to nest were followed and, once egg laying was complete, all eggs were collected from the nest by hand and place into a plastic bucket. The clutch of eggs was taken a distance of 50–200 m to a site on the beach where artificial nests were dug by hand so that the bottom of the nest was located 70 cm below the sand surface. Eggs were counted and placed into the nest, and once 50 eggs had been placed into the nest a temperature data logger (iButtonTM Maxim USA, Model DS1922L, resolution of 0.06°C, accuracy ± 0.5°C) programmed to log temperature once per hour was placed amongst the eggs and the remainder of the clutch placed into the nest. Sand was then back-filled on top of the eggs to the level of the beach surface. On Raine Island three 50 mm × 50 mm × 1800 mm wooden stakes were hammered in around the nest to a depth of 1 m to protect the nest from disturbance by subsequent nesting females; on Heron Island a single wooden stake 25 mm × 25 mm × 1000 mm was placed 50 cm from the nest to mark its position. On a subsequent field trip, typically one or two weeks after the average 55-day incubation period, the nests were excavated, the temperature-data loggers recovered, and the number of empty shells (=hatched eggs) and unhatched eggs were counted. I calculated hatchling success using the following equation (Miller 1999):
All unhatched eggs were opened and the development phase of the dead embryo determined visually using the six-phase guide described in Booth et al. (2020).
Because incubation temperature is well known to affect embryonic death rate and early phase embryos are more sensitive to high temperatures than late phase embryos (Booth 2017), possible relationships between nest temperature and embryo death were explored. In particular, the relationships between average nest temperature during the first week of incubation (which corresponds to Phase 1 embryos), and the temperature averaged over three days in a row during the warmest temperatures experienced during incubation (corresponding to Phase 6 embryos as metabolic heat generation, which elevates nest temperature, is greatest during this development phase) were explored.
Statistical analysis
Hatching success, mortality rate at different developmental phases, and nest temperatures were compared between Raine Island and Heron Island using non-parametric Kolmogorov–Smirnov tests because hatching success and mortality rates were measured as fractions, and nest temperature variances were significantly different at Raine and Heron Islands. Spearman rank order correlation analysis was used to test for possible relationships between nest temperature and embryo death during different developmental phases. All statistical tests were performed using Statistica© ver. 13.2 Software (TIBCO, Palo Alto, CA, USA).
Results
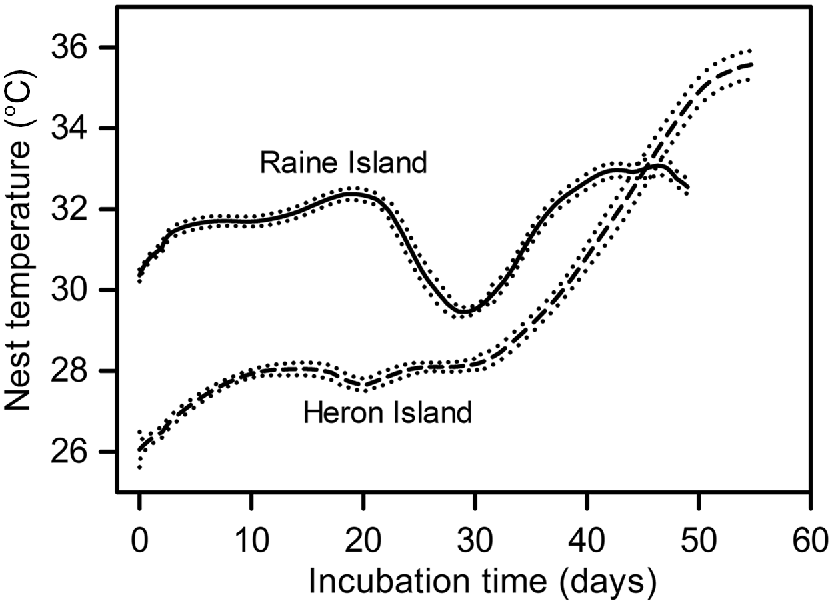
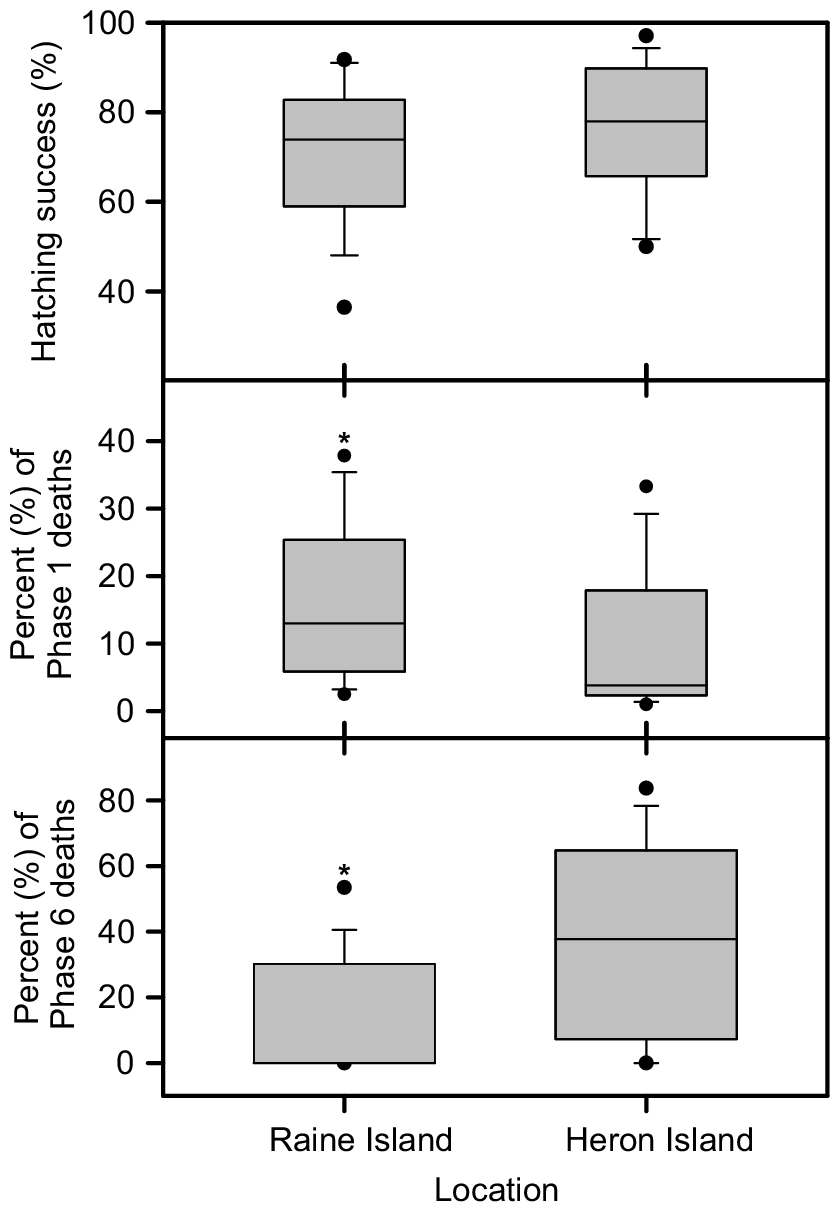
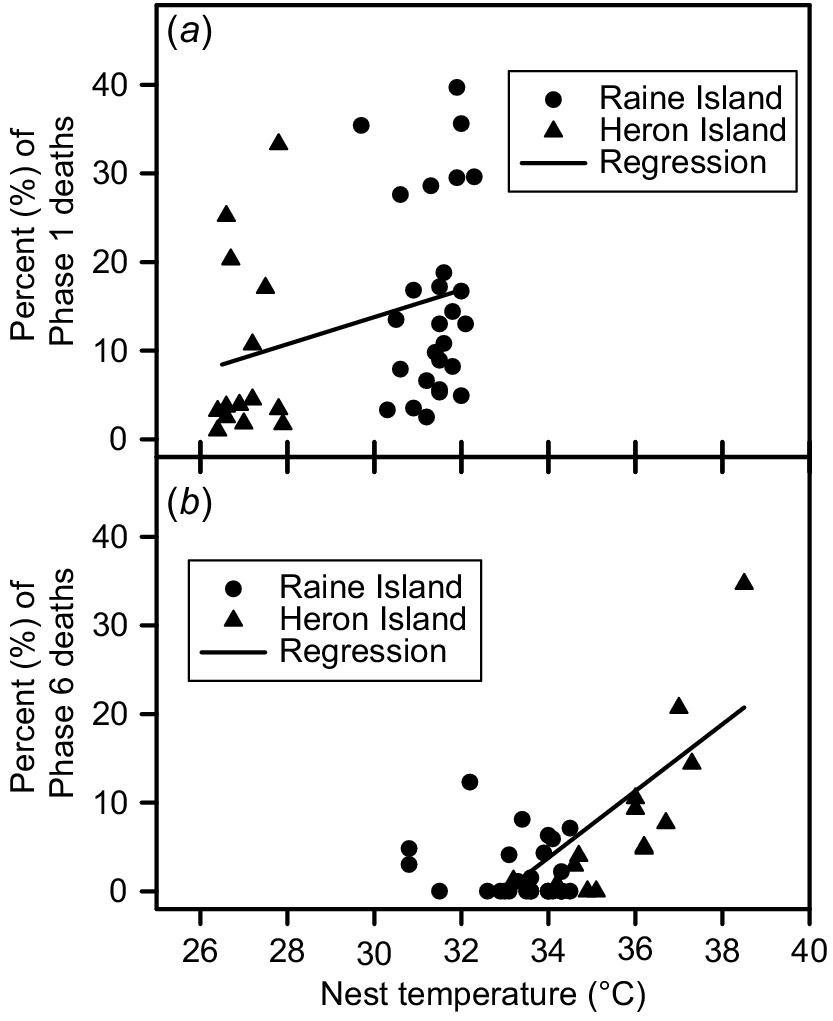
Twenty-eight nests were sampled at Raine Island (RI) during December 2018 and 14 nests were sampled at Heron Island (HI) (eight during December 2019, six during December 2020). Nest temperatures were cooler during the first week of incubation (P < 0.001) at HI (mean 27.0°C, median 26.9°C) compared to RI (mean 30.3°C, median 30.1°C,), but three-days-in-a-row maximum nest temperatures were higher (P < 0.001) at HI (mean 35.9°C, median 36.0°C) compared to RI (mean 33.2°C, median 33.5°C) (Fig. 1). Nest hatching success was similar (P > 0.10) at HI (mean 76.4%, median 78.0%) and at RI (mean 64.8%, median 67.8%) (Fig. 2). However, the proportion of deaths that occurred during Phase 1 of embryonic development (corresponding with the first week of incubation) was lower (P < 0.005) at HI (mean 9.4%, median 3.8%) than at RI (mean 24.6%, median 16.5%) (Fig. 2). The maximum three-days-in-a-row temperature in all monitored nests occurred during the last week of incubation, by which time surviving embryos had reached Phase 6 of development. The proportion of embryos that died during Phase 6 of development was higher (P < 0.01) at HI (mean 8.3%, median 4.9%) than at RI (mean 2.4%, median 0.2%) (Fig. 2). There was a weak correlation between average incubation temperature during the first week of incubation and Phase 1 embryonic death (Fig. 3a). There appears to be a bimodal pattern in Phase 1 deaths. At Heron Island, the data are clustered above and below 10%, whereas at Raine Island data are clustered above and below 20%. The proportion of Phase 6 embryo death averaged 3% for three-days-in-a-row maximum temperatures between 28°C and 33°C, and then increased remarkably (Fig. 3b).
Mean temperature traces for green turtle nests sampled in December from 28 nests on Raine Island and 14 nests on Heron Island. Dotted lines indicate ± standard errors of the mean.
Hatching success, percentage of developmental Phase 1 and developmental Phase 6 embryo deaths from 28 Raine Island and 14 Heron Island green turtle nests sampled in December. Solid lines inside the box medians, boundaries of box 25% and 75% percentiles, cross bars 10% and 90% percentiles, and dots 5% and 95% percentiles. Asterisks indicate a significant difference between Raine Island and Heron Island nests.
(a) Relationship between precentage of developmental Phase 1 embryo death and average nest temperature during the first week of incubation for 28 Raine Island and 14 Heron Island green turtle nests sampled in December. The Spearman rank-order correlation was significant (Rs = 0.44, t(N − 2) = 3.042, P = 0.004, N = 42). (b) Relationship between percentage of developmental Phase 6 embryo death and average nest temperature during the three-days-in-a-row maximum nest temperature for 28 Raine Island and 14 Heron Island green turtle nests sampled in December. The Spearman rank-order correlation for temperatures above 33°C was significant (Rs = 0.47, t(N − 2) = 2.999, P = 0.005, N = 34).
Discussion
Although the number of nests sampled at both locations was relatively small, probably less than 5% of all nests laid in the month of December, there is no evidence to suggest the nests sampled were not typical of nests laid during December. Contrary to expectation, when nests were exposed to similar circumstances (i.e. laid in December, were not subjected to inundation, and remained undisturbed from subsequent nesting females), overall hatching success was similar at Raine Island and Heron Island, but the timing of the majority of embryo death varied between the two locations. The greatest mortality occurred very early during embryonic development in nests from Raine Island, but during late development in nests from Heron Island. High early embryonic mortality has been noted at Raine island previously (Booth and Dunstan 2018; Booth et al. 2021, 2022), but the cause of this mortality remains elusive, with egg infertility, high nest temperatures and extreme respiratory gas levels being investigated and dismissed as possible causes (Booth and Dunstan 2018; Booth et al. 2021, 2022). Although mean nest temperature was higher during the first week of incubation on Raine Island compared to Heron Island, it is unlikely to be the cause of the higher embryo death rate at this time because temperatures were still well within the optimal thermal conditions for incubation (27–32°C: Ackerman 1997; Howard et al. 2014) and the relationship between embryo mortality and temperature was weak during this period (Fig. 3a). However, there appears to be a dichotomy in these data, with a break in distribution at both Heron Island and Raine Island. Relatively high abundance of soil microorganisms, some of which may be parthenogenic, is a working hypothesis for the cause of high early embryo mortality at Raine Island (Booth et al. 2022). If this is the case, the data dichotomy may be caused by a threshold phenomenon where once the microbes reach a particular concentration, there is an increased likelihood of high mortality. This threshold hypothesis could be tested by comparing the abundance of sand microbes from nests with high and low early-stage mortality. Evidence supporting the microbial death hypothesis comes from observations that higher abundance of microbes and higher death rates have been found in sand at high density sea turtle nesting beaches (Honarvar et al. 2011; Bézy et al. 2014, 2015), and that hatching success of nests decreases at Raine Island towards the end of the nesting season when the abundance of sand microbes is expected to increase due to the accumulation of organic material in the sand from dead eggs and residual egg material left behind in the nest after hatchlings escape the nest (Booth et al. 2022). Because the number of green turtles nesting at major nesting sites can vary markedly from year to year, comparing hatching success from individual nests between high and low nest density seasons also informs this hypothesis. Early embryo survival and hatching success would be expected to be low during high nest density seasons and high during low nest density seasons. This trend is seen in data from Raine Island collected between 2011 and 2022 (Coffee and Robertson 2021), adding further support to this hypothesis. Further evidence for the sand microbe hypothesis causing lower hatching success at high nest densities might be found by comparing hatching success at sea turtle nesting beaches that have experienced large increases in nest density before and after the nest density increase. For example, the number of loggerhead turtle (Caretta caretta) nests on the island of Sal, Cape Verde, North Atlantic, has increased from 506 to 35 507 between 2008 and 2020 (Hays et al. 2022). The sand microbe hypothesis predicts that hatching success at this site would have decreased during this period. The greater mortality of mature embryos found in Heron Island nests is most likely caused by the high nest temperatures that were experienced at the end of incubation during the two years examined. There is clear evidence that once nest temperatures exceed 34°C for extended periods, embryo mortality increases (Fig. 3b) (Matsuzawa et al. 2002; Maulany et al. 2012).
The nests monitored on Raine Island for this report have a higher than average hatching success than for the entire nest population at this location because they were laid early in the nesting season when hatching success is greatest, were not disturbed by subsequently nesting females (as at other high nest density beaches, many nests are destroyed by subsequent nesting females), and were not inundated (considerable regions of the beach have nests that are exposed to seawater inundation (Coffee and Robertson 2021)). Hence, the overall nest hatching success at Raine Island will be considerably lower than reported for nests monitored in this study, particularly in high female visitation years, in which the proportion of nests destroyed by subsequent nesting females is high. In contrast, because of the much lower nest density on Heron Island, this source of embryo mortality is minimal, and hatching success in previous years when temperatures were cooler than those reported here, is greater, averaging ~80% (Limpus 2008). In conclusion, overall hatching success of the monitored green turtle nests at Raine Island and Heron Island were similar, ~70%, but the majority of embryo death occurred early in incubation at Raine Island and late in incubation at Heron Island.
Declaration of funding
Raine Island field work was funded by the Raine Island Recovery Project, which is a five-year, AU$7.95 million collaboration between BHP, the Queensland Government, the Great Barrier Reef Marine Park Authority, Wuthathi and Meriam Nation (Ugar, Mer, Erub) Traditional Owners and the Great Barrier Reef Foundation to protect and restore the island’s critical habitat to ensure the future of key marine species. Heron Island field work was funded by WWF Australia through sponsorship by Koala.com.
Acknowledgements
All Raine Island field work was approved by the Raine Island Scientific Advisory Group. I thank the many people from the Raine Island recovery program that assisted us in the Raine Island field work. We thank the traditional owner of Raine Island, Wuthathi and Meriam Nation (Ugar, Mer, Erub), for allowing access to Raine Island. I thank WWF Australia and Heron Island Research station staff and Larrisa Young for participating in field work at Heron Island. Animal ethics approval was granted for these studies by the University of Queensland NEWMA animal ethics committee, approval numbers SBS/267/17 and SBS/402/19/HERON ISLAND. Field work on Heron Island was performed under Scientific Purposes Permit PTU10-002377 issued by the Queensland Parks and Wildlife Service.
References
Bézy VS, Valverde RA, Plante CJ (2014) Olive Ridley sea turtle hatching success as a function of the microbial abundance and the microenvironment of in situ nest sand at Ostional, Costa Rica. Journal of Marine Biology 2014, 351921.
| Crossref | Google Scholar |
Bézy VS, Valverde RA, Plante CJ (2015) Olive Ridley sea turtle hatching success as a function of the microbial abundance in nest sand at Ostional, Costa Rica. PLoS ONE 10, e0118579.
| Crossref | Google Scholar |
Booth DT (2017) Influence of incubation temperature on sea turtle hatchling quality. Integrative Zoology 12, 352-360.
| Crossref | Google Scholar |
Booth DT, Dunstan A (2018) A preliminary investigation into the early embryo death syndrome (EEDS) at the world’s largest green turtle rookery. PLoS ONE 13(4), e0195462.
| Crossref | Google Scholar |
Booth DT, Dunstan A, Bell I, Reina R, Tedeschi J (2020) Low male production at the world’s largest green turtle rookery. Marine Ecology Progress Series 653, 181-190.
| Crossref | Google Scholar |
Booth DT, Dunstan A, Robertson K, Tedeschi J (2021) Egg viability of green turtles nesting on Raine Island, the world’s largest nesting aggregation of green turtles. Australian Journal of Zoology 69, 12-17.
| Crossref | Google Scholar |
Booth DT, Staines MN, Reina RD (2022) Sand characteristics do not influence hatching success of nests at the world’s largest green turtle rookery. Australian Journal of Zoology 69, 113-124.
| Crossref | Google Scholar |
Coffee OI, Robertson K (2021) Raine Island Recovery Project: 2020–2021 Season. Technical report to the Raine Island Scientific Advisory Committee and Raine Island Reference Group. Department of Environment and Science, Queensland Government, Brisbane, Qld, Australia. Available at https://nla.gov.au/nla.obj-3130289661/view [Accessed 15 May 2023]
Hays GC, Taxonera A, Renom B, Fairweather K, Lopes A, Cozens J, Laloë J-O (2022) Changes in mean body size in an expanding population of a threatened species. Proceedings of the Royal Society B: Biological Sciences 289, 20220696.
| Crossref | Google Scholar |
Honarvar S, Spotila JR, O’Connor MP (2011) Microbial community structure in sand on two Olive Ridley arribada nesting beaches, Playa La Flor, Nicaragua and Playa Nancite, Costa Rica. Journal of Experimental Marine Biology and Ecology 409, 339-344.
| Crossref | Google Scholar |
Howard R, Bell I, Pike DA (2014) Thermal tolerances of sea turtle embryos: current understanding and future directions. Endangered Species Research 26, 75-86.
| Crossref | Google Scholar |
Limpus CJ, Miller JD, Parmenter CJ, Limpus DJ (2003) The green turtle, Chelonia mydas, population of Raine Island and the northern Great Barrier Reef: 1843–2001. Memoirs of the Queensland Museum 49, 349-440.
| Google Scholar |
Matsuzawa Y, Sato K, Sakamoto W, Bjorndal K (2002) Seasonal fluctuations in sand temperature: effects on the incubation period and mortality of loggerhead sea turtle (Caretta caretta) pre-emergent hatchlings in Minabe, Japan. Marine Biology 140, 639-646.
| Crossref | Google Scholar |
Maulany RI, Booth DT, Baxter GS (2012) Emergence success and sex ratio of natural and relocated nests of Olive Ridley turtles from Alas Purwo National Park, East Java, Indonesia. Copeia 2012, 738-747.
| Crossref | Google Scholar |
Smith CE, Booth DT, Crosby A, Miller JD, Staines MN, Versace H, Madden-Hof CA (2021) Trialling seawater irrigation to combat the high nest temperature feminisation of green turtle Chelonia mydas hatchlings. Marine Ecology Progress Series 667, 177-190.
| Crossref | Google Scholar |


