Plio–Pleistocene vicariance across arid Australia in the ‘Spiny Knob-tailed Geckos’ (Nephrurus asper group), with the description of a new species from western Queensland
Paul M. Oliver A * , Stephen C. Donnellan B and Bee F. Gunn
A * , Stephen C. Donnellan B and Bee F. Gunn  C
C
A Centre for Planetary Health and Food Security, Griffith University, 170 Kessels Road, Brisbane, Qld 4121, and Biodiversity and Geosciences Program, Queensland Museum, South Brisbane, Qld 4101, Australia.
B South Australian Museum, North Terrace, Adelaide, SA 5000, Australia.
C Royal Botanic Gardens Victoria, Birdwood Avenue, Melbourne, Vic. 3004, Australia.
Australian Journal of Zoology 69(6) 216-228 https://doi.org/10.1071/ZO22008
Submitted: 28 February 2022 Accepted: 1 July 2022 Published: 25 October 2022
© 2021 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Across Australia’s monsoon tropics and vast arid zone isolated regions or ‘islands’ of upland or rocky habitat are home to disjunct populations of many taxa of plants and animals. Comparative analyses of lineages that occur across these habitat islands provide opportunities to understand when and how environmental change drove isolation and diversification across arid Australia. Here we present an analysis of mitochondrial genetic diversity across disjunct populations of geckos in the Nephrurus asper group. Dating analyses suggest that disjunct and genetically divergent populations spanning the northern half of Australia diverged through the Plio–Pleistocene. Based on the timing of divergence and current habitat associations we hypothesise that species in this lineage were isolated by the expansion of unsuitable arid-zone habitats from the late Pliocene onwards. Across most areas, these barriers appear to be sandy or stony deserts. However, in eastern Australia genetically divergent populations are separated by grassland on flat vertisol-dominated soils (‘blacksoils’), suggesting that these habitats also expanded during the late Pliocene aridification. Finally, we show that western Queensland populations formerly referred to N. asper are genetically divergent and diagnosable on the basis of colour pattern and, herein, recognise these populations as a distinct species. https://zoobank.org/urn:lsid:zoobank.org:pub:9508CAAA-D014-452D-A3DA-325851615FA7
Keywords: aridification, biogeography, blacksoil, Nephrurus eromanga sp. nov, refugia, sandy deserts, stony deserts, vicariance, gecko.
Introduction
Upland ranges with associated stable rocky substrates in many different biomes are often inhabited by animals that show high levels of genetic diversity and localised endemism (Andreone et al. 2003; Couper and Hoskin 2008; Oliver et al. 2017; Simó-Riudalbas et al. 2017). In Australia upland rocky habitat ‘islands’ across the Monsoon Tropics and Arid Zone are home to a plethora of endemic lineages (Rosauer et al. 2016; McDonald et al. 2021), especially amongst gekkonid lizards (Fujita et al. 2010; Pepper et al. 2011; Oliver and McDonald 2016; Ashman et al. 2018; Laver et al. 2018). In contrast, lineages in surrounding habitats such as woodlands and sandplains tend to show shallower structure over wider areas (Pepper et al. 2011). However, the depth and number of lineages in rocky ranges can vary across regions and taxa. The vast Kimberley and Pilbara regions of Western Australia show high levels of endemism, deeply divergent lineages and considerable evidence of within-region speciation and phenotypic diversification (Ashman et al. 2018; Moritz et al. 2018; Noble et al. 2018; Oliver et al. 2019). In contrast, diversity in the smaller and more arid Central Uplands regions is more attenuated, with less evidence of extensive intraregional diversification (Oliver and McDonald 2016; McDonald et al. 2021).
Timeframes of divergence and patterns of distribution across rocky habitat islands spanning Australia also show considerable variation. For instance, in both the Australian Central Uplands and southern Kimberley, it has been shown that co-occurring saxicoline endemics may have isolation histories linked to both older (Miocene) and more recent (Plio–Pleistocene) periods of aridification (Oliver and McDonald 2016; Oliver et al. 2017). These data indicate that overlapping patterns of endemism in rocky ranges may be underpinned by varying evolutionary histories, and highlight the need to generate comparative datasets from multiple taxa to understand drivers of endemism and the impacts of environmental change.
The geckos in the Nephrurus asper group are large to very large geckos that are often, but not always, associated with rocky areas. They can be readily distinguished from other Nephrurus by the combination of prominent tubercles comprising multiple enlarged conical scales on the flanks and across the body, large size (SVL typically over 100 mm) and a greatly reduced tail with no autotomy planes. The three recognised species in this group are distributed across four largely disjunct areas spanning the northern (tropical to subtropical) half of Australia. Nephrurus asper occurs in two areas in Queensland, N. amyae occurs in the ranges of Central Australia and Nephrurus sheai occurs in the Kimberley and ‘Top End’ regions (Fig. 1) (Couper and Gregson 1994). The latter two species are entirely associated with rocky habitats, but N. asper is more widespread: some populations in eastern Queensland occur in heathlands and woodlands and often take refuge in other hard substrates such as fallen logs. All species in this complex are padless and relatively poor climbers, and observations from captive animals suggest that, unlike some other lineages of Nephrurus, they are not dependent on mesic microrefugia (Porter 2008).
Oliver and Bauer (2011) presented a phylogenetic analysis of the entire genus Nephrurus and estimated likely Plio–Pleistocene divergence between the three recognised and widely disjunct species in the N. asper group. Subsequent phylogenomic analyses have also suggested late Miocene to early Pliocene divergence of taxa in the N. asper group (Skipwith et al. 2019). However, Oliver and Bauer (2011) only sparsely sampled each of the three recognised taxa, and particularly lacked samples from apparently disjunct populations of N. asper in western Queensland, and N. sheai from the ‘Top End’ region in the Northern Territory. In a subsequent work N. sheai showed little genetic diversity across the Kimberley region, one of only two rock-associated lizards in this study that was not deeply structured (Oliver et al. 2017). Patterns of genetic diversity across most of the range of N. asper remained unassessed.
Here we assemble and present a more comprehensive analysis of mitochondrial DNA diversity across the N. asper group, including more sampling, and exemplars from all disjunct populations. We focus on (a) comparing genetic diversity within and between geographically disjunct clusters and (b) estimating the timeframe over which the N. asper group became restricted to isolated patches of habitat. Based on these genetic data and targeted morphological comparisons we also recognise populations from arid western Queensland as a distinct species.
Materials and methods
Genetic data generation
Genetic samples were accessed from the following institutions: Australian National Wildlife Collection (ANWC), Canberra; Queensland Museum (QM), Brisbane; and the South Australian Museum/Australian Biological Tissues Collections (SAMA/ABTC), Adelaide. Some additional unregistered material uses the acronyms CCM or PMO, demarcating field and collection numbers for tissue-only samples currently stored at the Australian National University. Sequence data (new and existing) were compiled from 17 nominal N. asper, 10 N. amyae and 23 N. sheai (Appendix S1). These data were aligned with data for other species in Nephrurus, Underwoodisaurus and Uvidicolus presented elsewhere (Oliver and Bauer 2011; Oliver et al. 2017) (Appendix S2).
For new samples, DNA was extracted using a Puregene DNA isolation kit (Gentra Systems, Minneapolis, MN, USA) following manufacturer protocols for DNA purification from solid tissue. A fragment of the mitochondrial genome, including the 3′ end of the NADH dehydrogenase subunit 2 (ND2) gene and the tRNA gene, was amplified and sequenced using the forward primers 5′-AAGCTTTCGGGGCCCATACC-3′ and with the reverse primer 5′-CTAAAATRTTRCGGGATCGAGGCC-3′. Our final genetic dataset comprised 939 bp from the ND2 gene. These were aligned using the MUSCLE algorithm (Edgar 2004) and subsequently checked by eye for missense mutations and correct reading frames.
Population genetics and phylogenetic analyses
Pairwise sequence divergences (uncorrected p-distances) were calculated in MEGA 6.06 (Kumar et al. 2018). Lineage relationships within the N. asper group were also visualised by generating phylogenetic networks of mtDNA using the Neighbor–Net algorithm as implemented in SplitsTree v4.10 (Huson and Bryant 2006). Further phylogenetic analyses to visualise the full dataset (all samples) were implemented using IQtree ver. 1.6.12 (Nguyen et al. 2015) using the automatic estimation of model parameters (−nt AUTO) and fast bootstrapping options (− bb 1000).
To estimate phylogenetic relationships and divergence history across major lineages we used BEAST v.2.6 (Bouckaert et al. 2014). Because BEAST assumes that each included tip represents an evolutionarily independent population, sampling was reduced to a single exemplar for each genetically distinctive or geographically disjunct population. While presenting these analyses, we acknowledge that additional data from the nuclear genome are required to validate hypotheses about geneflow and population divergence times. Third codon sites were removed from BEAST analyses to reduce the potential for saturation at these sites to confound branch length estimation. Topology and timeframes of divergence were estimated using the strict-clock model and Yule speciation prior for 50 million generations, sampling every 10 000 generations, with the first 20% of trees discarded as burn-in. For BEAST analyses data were partitioned by codon and the HKY+G model was applied to each partition.
There are no published fossil data that provide time constraints for the nodes within this relatively limited subgroup within the Carphodactylidae. However, Oliver and Bauer (2011) estimated basal divergence splits within Nephrurus. Based on dates from that study we applied a secondary prior with a normal distribution to the basal splits for the genus Nephrurus (mean 14.5, sigma 2.0) mya. To further scaffold the tree we also applied an additional normal prior to the age of the smooth knobtail gecko clade (node spanning N. levis and N. deleani, mean 10.0, sigma 1.4).
Morphology
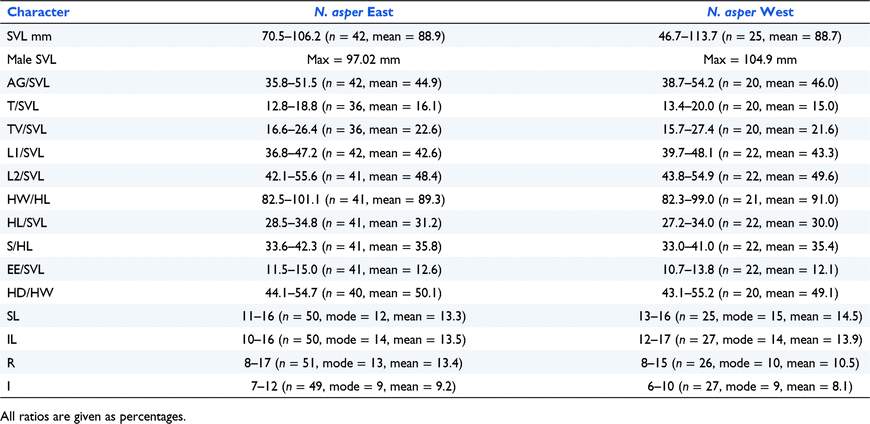
Genetic data (see below) indicated that there were particularly deep divergences within populations currently referred to N. asper. We collected morphological and meristic data in order to investigate whether there was corroborating evidence to support the divergence of these populations. Measurement protocols followed Couper and Gregson (1994): SVL – snout to vent length (mm); AG – axilla to groin (mm); L1 – length of forelimb, axilla to tip of longest digit (mm); L2 – length of hindlimb, groin to tip of longest digit (mm); T – tail length, from constriction at base to tip (mm); TV – tail length, from vent to tip (mm); KW – knob width (mm); TA – number of tail annuli; HL – head length, snout to dorsal edge of ear opening (mm); HW – head width, above ear opening (mm); HD – head depth, maximum depth (mm); S – snout length, tip of snout to anterior edge of orbit (mm); NL – neck length, axilla to posterior margin of ear opening (mm); SL – number of supralabial scales; IL – number of infralabial scales; R – number of granular scales in direct contact with the dorsal edge of the rostral scale; I – number of interorbital scales; and, EE – eye to ear, posterior margin of orbit to anterior margin of ear opening (mm). For comparisons of overall body size and ratios we focused on animals that were likely to be mature – using a SVL >70 mm as a cut-off. Morphological examinations were based on material in the Australian Museum (AMS), Queensland Museum (QM), Museums Victoria (NMV), South Australian Museum (SAMA) and Western Australian Museum (WAM) (Appendix S3).
Results
Genetic structuring within recognised species
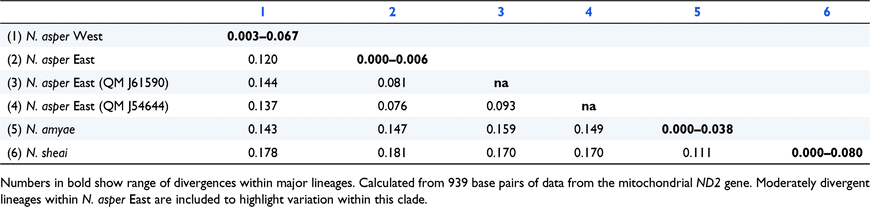
We were able to obtain genetic data from samples spanning the range of all three taxa in the N. asper group, although the density of sampling varied greatly, data for northern Queensland being particularly sparse. All three recognised taxa in the N. asper group showed evidence of geographically structured intraspecific genetic diversity (Table 1). Nephrurus sheai showed comparatively low genetic divergence across the densely sampled Kimberley region of Western Australia (p-distances 0.00–0.029). In contrast, two samples (Fig. 2) from the sparsely sampled Northern Territory were moderately divergent from populations in the Kimberley (mean p-distances 0.070–0.077), and also showed moderate intragroup divergence (p-distance 0.048). In N. amyae, genetic structuring was relatively low (0.00–0.036). Notably, a single animal from an apparently disjunct population in the Peterman Ranges of far eastern Western Australia showed divergences from other localities (0.00–0.038) comparable to those within the more densely sampled MacDonnell Ranges area (0.00–0.035).
|
|
|
Nephrurus asper showed the deepest, and the most complex patterns of genetic divergence (Fig. 2, Table 1). Samples from western Queensland (‘N. asper West’) showed high divergence (p-distances 0.11–0.15) from populations to the east (‘N. asper East’); these p-distance estimates are comparable to those between the recognised taxa N. amyae and N. sheai (mean p-distances 0.11). Within N. asper West, samples from the north and south provided further evidence of genetic structuring (p-distances 0.064–0.067). The remaining samples in N. asper East showed very low genetic divergence across the south (p-distances 0.00–0.006), although two singletons from the sparsely sampled northern regions were more divergent, both from each other, and from all other samples of N. asper East (p-distances 0.073–0.093).
Phylogenetic relationships and divergence dating
Maximum likelihood and Bayesian phylogenetic analyses all supported the monophyly of each of the recognised species (Fig. 3, Fig. SI1). Patterns of phylogenetic relationship between the three recognised species were not strongly supported. However, genetic distance values (Table 1) and Bayesian trees (Fig. 3) using a reduced set of lineages tended to suggest that N. sheai and N. amyae are most closely related and may be sister taxa, with N. asper being more genetically divergent.
Dating analyses suggested that the divergence and isolation of populations in the N. asper group largely occurred across the Plio–Pleistocene (Fig. 3). Mean divergence ages for three recognised taxa were concentrated in the Pliocene. Likewise, the divergence between N. asper West and N. asper East was also estimated to have occurred in the Pliocene. In contrast, divergence age estimates for remaining lineages within N. amyae, N. sheai and N. asper East were shallower and concentrated in the Pleistocene.
Morphology
Analyses of morphological variation focused on the genetically most divergent lineages in populations currently ascribed to N. asper. We found no consistent differences in scale counts or relative proportions between the two major lineages of N. asper (Table 2). In body size (SVL) there was some evidence that the maximum size of N. asper West was slightly larger than that of N. asper East for both males and females (Table 2).
|
With respect to colouration, all species and most lineages are quite variable. In preservative N. asper East shows considerable variation in base colouration of the head, dorsum and upper surfaces of limbs (usually medium to dark brown or grey) and overlapping pattern (spotted, banded, reticulated or combinations of the above) (Fig. 4a). As Couper and Gregson (1994) noted, N. asper East from Cape York Peninsula in north Queensland tend to be boldly marked with dark and light bands. Based on specimens, photographs and records on the Atlas of Living Australia (www.ala.org.au, accessed 10 January 2002), these boldly banded animals occur as least as far south as the Einasleigh Uplands.
Nephrurus asper West is less variable (Fig. 4b). This form consistently has a much lighter base colouration in preservative than other populations of N. asper (fawn to mid-brown versus darker brown to mid-dark grey) and a strongly contrasting dark-brown band or saddle on the nape (versus typically without a strongly contrasting brown saddle on nape).
In life the dorsal colouration of N. asper West is orange or reddish and the dark-brown saddle is always apparent (Fig. 5), whereas N. asper East is much darker, typically brown or dark grey, and often lacks a clearly defined saddle. Examination of photographs of live animals also suggested that the iris of N. asper East tends towards pale lilac, whereas that of N. asper West tends towards pale grey. However, examination of more specimens in life is required to better understand how consistently differentiated eye colouration is.
Discussion
Comparative analyses of lineages that occur across disjunct ‘islands’ or rocky or refugial habitat spanning the Australian Arid Zone provide opportunities to understand how and when environmental change isolated formerly continuous populations (e.g. Shoo et al. 2008). Oliver and McDonald (2016) suggested that relictual vertebrate taxa in the Central Upland regions of the Australian Arid Zone could be broadly divided into older lineages potentially isolated by earlier phases of aridification during the Miocene versus younger endemic species and populations isolated by more recent spread of grasslands and other arid-zone habitats since the onset of the Plio–Pleistocene (Byrne et al. 2008). We concede that divergence estimates based on mitochondrial DNA and secondary calibrations have numerous potential sources of uncertainty; however, our analyses suggest that taxa in the N. asper group fall in the latter group, and have been isolated by Plio–Pleistocene environmental change. This pattern of Plio–Pleistocene vicariance across the inland ranges mirrors at least some other relict taxa in the Australian Arid Zone, and especially the Central Uplands (Pepper et al. 2013; Oliver et al. 2014). Dated phylogenies for these allopatric gecko populations suggest more connectivity between populations at or around the early Pliocene mesic pulse (Sniderman et al. 2016).
Plio–Pleistocene divergences between relictual Arid Zone populations (such as we observe here in the N. asper group) raise questions as to how connectivity between these now isolated regions was severed. Throughout most of their range species in the N. asper group are associated with hard rocky substrates, and, typically, areas of outcropping rock. The most obvious contemporary potential habitat barrier between the distributions of N. amyae, N. asper West and N. sheai is large areas of sandy deserts, especially the Tanami and Simpson Deserts. A suite of other bird and reptile taxa that seem similarly dependent on woodlands or rocky ranges for suitable habitat show comparable divergence dates or distributions (Christidis et al. 2010; Pepper et al. 2013; Oliver et al. 2014). These distributions suggest that the expansion of sandy desert systems may explain disjunct populations in the N. asper group. A process of sandy desert expansion has similarly been invoked to explain disjunct populations of karst-associated gecko species along the edge of the Kimberley in Western Australia (Oliver et al. 2019). However, dating analyses have not pushed age estimates for dunefields in the Australian Arid Zone older than approximately one million years (reviewed in Pepper and Keogh 2021), younger than our estimates for divergence ages for species in the N. asper group. Whether relatively young age estimates for dune systems are an artefact of the unstable nature of these systems and geographic biases in where data have been collected, or a genuine indicator that they are a young component of the Australian Arid Zone is unclear (Pepper and Keogh 2021).
Stony deserts in the eastern Australian Arid Zone are estimated to be older than dunefields, with evidence dating their origin back to at least two to three million years ago (Fujioka et al. 2005) – a timeframe more consistent with inferred Pliocene aridification and vicariance in the N. asper group. This suggests that these expanding habitat types may have also formed a barrier for formerly connected populations in taxa such as the N. asper group. However, better understanding the environmental drivers of vicariance across the Australian Arid Zone will likely require additional estimates of age of key desert landforms in Australia, and more detailed multigene dating analyses to refine estimates of divergence timing across isolated populations.
In the eastern portion of Australia the separation between the two main lineages in N. asper seems to be correlated with extensive areas of relatively flat and featureless tussock grasslands on vertisol dominated soils. These landforms, often referred to as ‘blacksoils’ or ‘Mitchell Grass Downs’ dominate large areas of eastern and northern Australia and have a distinctive biota (Ford 2022). However, our knowledge of the history of this habitat type and its associated biota is very scant. The timing of divergence of lineages within N. asper suggests that as for stony deserts, the late Pliocene and early Pleistocene may have also been a period of marked expansion of blacksoil grasslands. Unfortunately, although many mesic-zone taxa show evidence of turnover at the ‘Carpentarian Gap’ or ‘Carpentarian Barrier’ to the north of the blacksoils (Cracraft 1991), we are unaware of any other taxa that show divergence partitioned by blacksoil habitats in eastern Australia. This limits our ability to test the hypothesis of Plio–Pleistocene formation and expansion of blacksoil grasslands in a comparative framework. An alternative approach may involve comparative analyses of speciation ages between the many endemic blacksoil taxa and their sister lineages in surrounding habitats.
Systematics
The genetic data presented here indicate that N. asper West and N. asper East are as genetically divergent as other lineages recognised as species in the N. asper group, specifically N. amyae and N. sheai. The observed genetic distances between lineages in N. asper are also comparable to those of two other sister species pairs of Nephrurus: N. cinctus and N. wheeleri (p-distances ∼10%), and N. deleani and N. laevissimus (p-distances ∼8%) (Kealley et al. 2020). These more shallowly divergent sister species pairs also show consistent differences in colour pattern and scalation. The two main clades of N. asper are similarly diagnosable by aspects of colour pattern, but not by scalation. On the basis of genetic and morphological divergence and differentiation, we recognise the two main lineages with N. asper as distinct species and provide diagnoses and descriptions below.
The type locality of N. asper is Peak Downs in eastern Queensland, hence this name is referrable to N. asper East. The holotype is an adult male with SVL of 91.2, a weak shoulder saddle and indistinct banding on the body (Fig. SI2). In these aspects of colour pattern it is consistent with variation shown by N. asper East, not N. asper West. There is considerable colour pattern variation in N. asper East. Boldly banded N. asper East from Cape York Peninsula may also show a combination of genetic divergence and a distinctive colour pattern; however, we refrain from recognising this population as a distinct taxon for two reasons. First, the genetic divergence of banded animals from the remaining populations of N. asper in eastern Australia is relatively shallow, and, second, we have relatively little genetic information from areas such as the Einsleigh Uplands where strongly banded and weakly banded animals occur in close proximity. Hence we do not know how patterns of genetic diversity correlate with colour pattern variation in key areas of contact and whether genetic isolation is maintained. A number of recent studies of the taxonomy of the Australian herpetofauna have emphasised the importance of carefully characterising colour pattern variation against genetic diversity patterns before naming taxa (Rabosky et al. 2014; Kealley et al. 2018; Esquerré et. al. 2021). For the N. asper group in north Queensland we currently lack sufficient sampling for an adequate analysis of this nature.
Following Kaiser et al. (2013), position statements from the Australian Society of Herpetologists (ASH 2016), and in accordance with a large number of active herpetofaunal taxonomists (Wüster et al. 2021), we do not consider selected nomenclatural acts published after 1 January 2000, even if these may have priority under the rules of the International Code of Zoological Nomenclature.
All taxa considered below are readily identified as members of the genus Nephrurus by the presence of a prominent swollen knob at the tip of the tail. They can be identified as members of the N. asper group by the combination of prominent tubercles consisting of multiple enlarged conical scales on the flanks and across the body, large size (SVL typically over 100 mm) and a greatly reduced tail with no autotomy planes.
Nephrurus asper Günther, 1876
Holotype
NHMUK (formerly BMNH) 1946.8.23.34. from Peak Downs, Queensland.
Diagnosis
Nephrurus asper can be distinguished from other taxa in the N. asper group by the following combination of characters: moderate size (maximum SVL males ∼ 97 mm, females ∼ 106 mm); grey or mid- to dark-brown base colouration on dorsal and lateral surfaces of head, torso and limbs; narrow to wide pale dorsal crossbands or broken series of blotches along dorsum; dark-brown saddle on the nape absent or if present not contrasting strongly with surrounding dorsal colouration; digits light grey or light brown, unbanded, but typically with some grey or dark brown flecks; and basal scales surrounding each tubercle uniform in height and less than half the height of the scale they enclose.
Nephrurus eromanga Oliver, Donnellan & Gunn, sp. nov.
(Eromanga Basin Knob-Tail Gecko)
https://zoobank.org/urn:lsid:zoobank.org:act:7FA107EE-175C-44E6-B61E-3C59A60E4100
Holotype
QM J97592 (formerly SAMA R42602), 4 km N. of Diamantina Station (23°44′S, 141°08′E), collected by B. Miller, G. Armstrong and J. Birrell on 12 October 1993.
Paratypes
QM J4525–6, Kuridala, S of Cloncurry (21°17′S, 140°30′E); QM J70473 Tick Hill, 25 km SSE of Duchess (21°38′47″S, 139°55′47″E); QM J31976, QMJ35040, Winton (22°23′S, 143°02′E); QM J5727, Lucknow Stn, W of Winton (22°43′S, 140°55′E); QM J28699, Cork Stn via Winton (22°56′S, 142°18′E); QM J9912, Longreach (23°26′S, 144°15′E); AMS R120094, Mayne Junction Hotel, approx. 40 km N of Diamantina Lakes Stn (23°33′S, 141°22′E); QM J31545, Winton fossil site (23°50′S, 142°15′E); QM J7878, Jundah (24°50′S, 143°04′E); SAMA R42600, 6 km N of Diamantina Stn (23°43′S, 141°08′E); SAMA R42601, 4 km N of Diamantina Stn (23°44′S, 141°08′E); QM J741, Diamantina Lakes (23°46′S, 141°08′E); QM J83532–3, Homestead on Westerton Stn (24°03′S, 142°49′30″E); QM J10526, Jundah (24°50′S, 143°04′E); SAMA R42603, 85 km W of Windorah (25°21′S, 141°50′E); SAMA R55288–9 The Monument, west side of Mt Bruce (23°45′S, 139°55′E).
Referred material
WAM R55552, Fermoy Homestead, 88 km S of Winton (23°10′S, 143°02′E); WAM R55601–2, Fermoy Homestead, 88 km S of Winton (23°10′S, 143°02′E); AMS R110544, AMS R110562, camp, 14 km NE of Scott’s Tank, Diamantina Lakes, NW of Windorah (23°45′S, 141°40′E); AMS R113116, Diamantina Lakes Stn, SW of Winton (23°41′S, 141°11′E).
Diagnosis
Nephrurus eromanga, sp. nov. can be distinguished from other taxa in the N. asper group by the following combination of characters: moderately large size (maximum SVL males ∼105 mm, females ∼114 mm); fawn to mid-brown (reddish in life) dorsal colouration on dorsal and lateral surfaces of head, torso and limbs; narrow to wide pale dorsal crossbands or broken series of blotches; dark-brown saddle on the nape, which extends posterior to the forelimbs and contrasts strongly with the base colouration on the back the head and remainder of torso; digits lacking dark-brown or grey bands or flecks; and basal scales surrounding each tubercle uniform in height and less than half the height of the scale they enclose.
Description
SVL (mm): 46.7–113.7 (n = 25, mean = 88.7). Proportions (as % SVL for specimens >70 mm SVL): L1 39.7–48.1 (n = 22, mean = 43.4); L2 43.8–54.9 (n = 22, mean = 49.6); AG 38.7–54.2 (n = 20, mean = 46.0); T 13.4–20.0 (n = 20, mean = 15.0); TV 15.7–27.4 (n = 20, mean = 21.6); HL 27.2–34.0 (n = 22, mean = 30.0); HW 23.9–30.1 (n = 21, mean = 27.2); HD 10.7–15.2 (n = 21, mean = 13.4); S 9.2–11.8 (n = 22, mean = 10.6); EE 10.7–13.8 (n = 22, mean = 12.1); NL 6.7–21.0 (n = 21, mean = 14.9).
Head. Large and deep (HW 82.3–99% HL, n = 21, mean = 91.0; HD 43.1–55.2% HW, n = 20, mean = 49.1), covered with small, round to hexagonal, juxtaposed scales. Posteriorly bearing scattered tubercles intermixed with the smaller scales. Each tubercle consists of a high central scale, circled by a ring of smaller basal scales. Tubercles most prominent on the nape. Dorsal skin co-ossified with skull. Nostril small, opening upwards and backwards. Eye very large and protruding with overhanging supraciliary ridge, pupil vertical. Ear vertically elongate, tympanum deeply recessed. Rostral scale small, with 8–15 (n = 26, mode = 10, mean = 10.5) scales in direct contact with dorsal edge. Interorbital scales 6–10 (n = 27, mode 9, mean 8.1). Supralabial scales 13–16 (n = 25, mode 15, mean 14.5). Infralabial scales 12–17 (n = 27, mode = 14, mean = 13.9).
Neck. Broad.
Body. Stout, dorsal and ventral surfaces with small granular scalation. Granular scales intermixed with larger conical tubercles on dorsal and lateral surfaces. Lower flanks and ventral surface with scattered, slightly raised rosettes which vary from being pronounced to barely discernible
Limbs. Long and slender, bearing enlarged tubercles on dorsal surfaces. Tubercles on thighs largest, with uniform basal scales less than half as high as the central scale. Digits short, cylindrical, undilated distally and terminating in a non-retractile claw. Third toe on hindlimb longest.
Tail. Short, moderately depressed, constricted at base and terminating in a globular, kidney-shaped knob (KW 17.9–39.4%T, n = 20, mean = 30.9). Usually four rows of tubercles present on dorsal and lateral surfaces. Uniform basal scales surrounding caudal tubercles less than half as high as the central scale. Caudal annuli 9–12 (n = 20, mode = 10, mean 10.3).
Colouration
Body (in spirit), relatively pale, fawn to mid brown on the dorsal surface, with a broad dark-brown saddle on the nape that extends a short distance posterior to the forelimbs. Narrow, pale-creamish cross-bands are usually present between head and hindlimbs (absent in some individuals). They may be continuous or broken into a series of spots with each spot centred on a dorsal tubercle. A series of fine black lines form a netted pattern over the dorsal surface; these are strongest in small individuals but are often obscure, or absent, in larger individuals. Ventral surface off white.
Limbs. Relatively plain fawn or light brown or with obscure pale lines/blotches, enlarged tubercles often paler than granular interspaces.
Digits. Pale fawn without distinctive banding or flecking.
Tail. As for body, with an obscure, broad, pale cross band on proximal half.
Head. Covered dorsally and laterally with fine black reticulations which form an intricate pattern; these are most prominent in smaller individuals and fade to varying degrees in larger specimens.
In life colouration and pattern very similar to that of preserved specimens. Base colouration of dorsum fawn to reddish-brown, with pale creamish cross-bands or series of blotches, and further extensive dark greyish-brown frosting and vermiculations, dark-grey network pattern particularly prominent and continuous on head. The nuchal saddle is dark brown, and contrasts strongly against surrounding areas. Limbs same base colour as dorsum, but with much less pattern. Iris pale grey.
Particulars of holotype
QM J97592 (all measurements in millimetres): SVL 96.0, L1 42.4, L2 48.0, AG 40.7, T 14.6, TV 24.6, HL 29.6, HW 26.0, HD 12.9, EE 11.6, S 10.5, NL 15.4, tail annuli 10, supralabials 15, infralabials 16. The colouration this specimen is typical of the species in preservative (SI3).
Comparisons with other species in the N. asper group
Nephrurus eromanga, sp. nov. can be distinguished from N. sheai by the colour pattern of its digits (digits unbanded versus digits strongly marked with alternating bands of brown and white) and from N. sheai and N. amyae by its smaller size (maximum SVL 114 mm versus 121 mm N. sheai, 135 mm N. amyae). It is further distinguished from N. amyae by the spinosity of the tubercules on its rump and thighs (moderately spinose versus extremely spinose) and the arrangement of the basal scales surrounding the tubercles on the rump and thighs (scales are uniform in size and less than half the height of the central scale versus basal scales are irregular in size and some are more than half the height of the central scale).
Nephrurus eromanga, sp. nov. is similar to N. asper; however, it is separated from this species by its slightly larger size (maximum SVL 114 mm versus 106 mm for N. asper), and by aspects of dorsal colour pattern (fawn or reddish brown on dorsal and lateral surfaces of head, torso and limbs and with a strongly contrasting and wide, dark saddle on nape versus darker brown to mid-dark grey without a strongly contrasting saddle on nape). It is also genetically distinct from N. asper (mean p-distance of 12% in the ND2 gene).
Distribution and ecology
Nephrurus eromanga occurs in a broad area of central western Queensland (corresponding to the distribution of N. asper West in Fig. 1), extending from Windorah in the south, to Winton in the east and Dajarra in the north-west. It is typically associated with rocky breakaway and mesa country where it lives on the ground in areas with rocky scree or boulders (Fig. 6).
It has a relatively large range and occurs in at least one protected area (Diamantina National Park). There is no reason to assume that grazing or other land uses in its range will lead to significant declines of this species in the near future. The possibility that species may be vulnerable to climatic change and more frequent extremes of high temperatures cannot be discounted on available data, but there is also no evidence to suggest that this is the case. On this basis we suggest an IUCN status of Least Concern.
Etymology
Named after the Eromanga Basin, a Mesozoic sedimentary basin in inland eastern Australia that entirely circumscribes the distribution of the species. The name Eromanga is in turn derived from the small town of Eromanga (at which this species does not occur). Eromanga is used as a noun in apposition.
Supplementary material
Supplementary material is available online.
Data availability
The data that support this study are available in GenBank (registrations OP184875–OP184896).
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
Funding for genetic work and analyses was provided by the grants from the Australian Research Council (DE140100220 and LP120100081) and by Broken Hill Petroleum (BHP) as part of Project Digital Infrastructure Growth (DIG).
Acknowledgements
We thank Angus Emmott, Chris Jolly and Steve Wilson for providing images and information on these geckos, and Peter Waddington and the Queensland Museum for providing images. We especially thank Patrick Couper for providing, expanding and summarising his extensive morphological dataset for the N. asper group. Graham Armstrong, Peter McDonald and Phillip Skipwith all provided assistance with fieldwork. Paul Doughty provided images and data pertaining to the holotype of N. asper. Fieldwork and collecting in the Northern Territory and Western Australia was undertaken under ANU ethics approvals issued to Professor Craig Moritz (A2012/14), and with relevant permits from the Northern Territory Department of Natural Resources (58454) and Western Australian Department of Parks and Wildlife (SF009862, SF009270). We thank Leo Joseph and Paul Doughty for their constructive reviews.
References
Andreone, F, Glaw, F, Nussbaum, RA, Raxworthy, CJ, Vences, M, and Randrianirina, JE (2003). The amphibians and reptiles of Nosy Be (NW Madagascar) and nearby islands: a case study of diversity and conservation of an insular fauna. Journal of Natural History 37, 2119–2149.| The amphibians and reptiles of Nosy Be (NW Madagascar) and nearby islands: a case study of diversity and conservation of an insular fauna.Crossref | GoogleScholarGoogle Scholar |
ASH (2016) Position of the Australian Society of Herpetologists on the increasing proliferation of names for taxa without adequate diagnosis or description and published without the benefits of peer review. (Australian Society of Herpetologists Inc., Position Statement, No. 2) Available at https://static1.squarespace.com/static/5448a9abe4b0ad6dc5e6fe6d/t/577e45a029687fd477a8375a/1467893157321/ASH_taxonomic_position_statement.pdf. [Accessed 5 February 2022]
Ashman, LG, Bragg, JG, Doughty, P, Hutchinson, MN, Bank, S, Matzke, NJ, Oliver, P, and Moritz, C (2018). Diversification across biomes in a continental lizard radiation. Evolution 72, 1553–1569.
| Diversification across biomes in a continental lizard radiation.Crossref | GoogleScholarGoogle Scholar |
Bouckaert, R, Heled, J, Kühnert, D, Vaughan, T, Wu, CH, Xie, D, Suchard, MA, Rambaut, A, and Drummond, AJ (2014). BEAST 2: a software platform for Bayesian evolutionary analysis. PLoS Computational Biology 10, e1003537.
| BEAST 2: a software platform for Bayesian evolutionary analysis.Crossref | GoogleScholarGoogle Scholar |
Byrne, M, Yeates, DK, Joseph, L, Kearney, M, Bowler, J, Williams, MAJ, Cooper, S, Donnellan, SC, Keogh, JS, Leys, R, Melville, J, Murphy, DJ, Porch, N, and Wyrwoll, K-H (2008). Birth of a biome: insights into the assembly and maintenance of the Australian arid zone biota. Molecular Ecology 17, 4398–4417.
| Birth of a biome: insights into the assembly and maintenance of the Australian arid zone biota.Crossref | GoogleScholarGoogle Scholar |
Christidis, L, Rheindt, FE, Boles, WE, and Norman, JA (2010). Plumage patterns are good indicators of taxonomic diversity, but not of phylogenetic affinities, in Australian grasswrens Amytornis (Aves: Maluridae). Molecular Phylogenetics and Evolution 57, 868–877.
| Plumage patterns are good indicators of taxonomic diversity, but not of phylogenetic affinities, in Australian grasswrens Amytornis (Aves: Maluridae).Crossref | GoogleScholarGoogle Scholar |
Couper, PJ, and Gregson, RAM (1994). Redescription of Nephrurus asper Gunther, and description of N. amyae sp. nov. and N. sheai sp. nov. Memoirs of the Queensland Museum 37, 67–81.
Couper, P, and Hoskin, C (2008). Litho-refugia: the importance of rock landscapes for the long-term persistence of Australian rainforest fauna. Australian Zoologist 34, 554–560.
| Litho-refugia: the importance of rock landscapes for the long-term persistence of Australian rainforest fauna.Crossref | GoogleScholarGoogle Scholar |
Cracraft, J (1991). Patterns of diversification within continental biotas: hierarchical congruence among the areas of endemism of Australian vertebrates. Australian Systematic Botany 4, 211–227.
| Patterns of diversification within continental biotas: hierarchical congruence among the areas of endemism of Australian vertebrates.Crossref | GoogleScholarGoogle Scholar |
Edgar, RC (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32, 1–15.
| MUSCLE: multiple sequence alignment with high accuracy and high throughput.Crossref | GoogleScholarGoogle Scholar |
Esquerré, D, Donnellan, SC, Pavón-Vázquez, CJ, Fenker, J, and Keogh, JS (2021). Phylogeography, historical demography and systematics of the world’s smallest pythons (Pythonidae, Antaresia). Molecular Phylogenetics and Evolution 161, 107181.
| Phylogeography, historical demography and systematics of the world’s smallest pythons (Pythonidae, Antaresia).Crossref | GoogleScholarGoogle Scholar |
Ford F (2022) Tropical and subtropical grasslands, savannas and shrublands: northeastern Australia. Available at https://www.worldwildlife.org/ecoregions/aa0707
Fujioka, T, Chappell, J, Honda, M, Yatsevich, I, Fifield, K, and Fabel, D (2005). Global cooling initiated stony deserts in central Australia 2–4 Ma, dated by cosmogenic 21Ne-10Be. Geology 33, 993–996.
| Global cooling initiated stony deserts in central Australia 2–4 Ma, dated by cosmogenic 21Ne-10Be.Crossref | GoogleScholarGoogle Scholar |
Fujita, MK, McGuire, JA, Donnellan, SC, and Moritz, C (2010). Diversification and persistence at the arid–monsoonal interface: Australia-wide biogeography of the Bynoe’s gecko (Heteronotia binoei; Gekkonidae). Evolution 64, 2293–2314.
| Diversification and persistence at the arid–monsoonal interface: Australia-wide biogeography of the Bynoe’s gecko (Heteronotia binoei; Gekkonidae).Crossref | GoogleScholarGoogle Scholar |
Huson, DH, and Bryant, D (2006). Application of phylogenetic networks in evolutionary studies. Molecular Biology and Evolution 23, 254–267.
| Application of phylogenetic networks in evolutionary studies.Crossref | GoogleScholarGoogle Scholar |
Kaiser, H, Crother, BI, Kelly, CMR, Luiselli, L, O’Shea, M, Ota, H, Passos, P, Schleip, WD, and Wüster, W (2013). Best practices: In the 21st Century, taxonomic decisions in herpetology are acceptable only when supported by a body of evidence and published via peer-review. Herptological Review 44, 8–23.
Kealley, L, Doughty, P, Pepper, M, Keogh, JS, Hillyer, M, and Huey, J (2018). Conspicuously concealed: revision of the arid clade of the Gehyra variegata (Gekkonidae) group in Western Australia using an integrative molecular and morphological approach, with the description of five cryptic species. PeerJ 6, e5334.
| Conspicuously concealed: revision of the arid clade of the Gehyra variegata (Gekkonidae) group in Western Australia using an integrative molecular and morphological approach, with the description of five cryptic species.Crossref | GoogleScholarGoogle Scholar |
Kealley, L, Doughty, P, Edwards, D, and Brennan, IG (2020). Taxonomic assessment of two pygopodoid gecko subspecies from Western Australia. Israel Journal of Ecology and Evolution 66, 126–141.
| Taxonomic assessment of two pygopodoid gecko subspecies from Western Australia.Crossref | GoogleScholarGoogle Scholar |
Kumar, S, Stecher, G, Li, M, Knyaz, C, and Tamura, K (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution 35, 1547–1549.
| MEGA X: molecular evolutionary genetics analysis across computing platforms.Crossref | GoogleScholarGoogle Scholar |
Laver, RJ, Doughty, P, and Oliver, PM (2018). Origins and patterns of endemic diversity in two specialized lizard lineages from the Australian Monsoonal Tropics (Oedura spp.). Journal of Biogeography 45, 142–153.
| Origins and patterns of endemic diversity in two specialized lizard lineages from the Australian Monsoonal Tropics (Oedura spp.).Crossref | GoogleScholarGoogle Scholar |
McDonald, PJ, Jobson, P, Köhler, F, Nano, CEM, and Oliver, PM (2021). The living heart: climate gradients predict desert mountain endemism. Ecology and Evolution 11, 4366–4378.
| The living heart: climate gradients predict desert mountain endemism.Crossref | GoogleScholarGoogle Scholar |
Moritz, CC, Pratt, RC, Bank, S, Bourke, G, Bragg, JG, Doughty, P, Keogh, JS, Laver, RJ, Potter, S, Teasdale, LC, Tedeschi, LG, and Oliver, PM (2018). Cryptic lineage diversity, body size divergence, and sympatry in a species complex of Australian lizards (Gehyra). Evolution 72, 54–66.
| Cryptic lineage diversity, body size divergence, and sympatry in a species complex of Australian lizards (Gehyra).Crossref | GoogleScholarGoogle Scholar |
Nguyen, L-T, Schmidt, HA, von Haeseler, A, and Minh, BQ (2015). IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution 32, 268–274.
| IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies.Crossref | GoogleScholarGoogle Scholar |
Noble, C, Laver, RJ, Rosauer, DF, Ferrier, S, and Moritz, C (2018). Phylogeographic evidence for evolutionary refugia in the Gulf sandstone ranges of northern Australia. Australian Journal of Zoology 65, 408–416.
| Phylogeographic evidence for evolutionary refugia in the Gulf sandstone ranges of northern Australia.Crossref | GoogleScholarGoogle Scholar |
Oliver, PM, and Bauer, AM (2011). Systematics and evolution of the Australian knob-tail geckos (Nephrurus, Carphodactylidae, Gekkota): plesiomorphic grades and biome shifts through the Miocene. Molecular Phylogenetics and Evolution 59, 664–674.
| Systematics and evolution of the Australian knob-tail geckos (Nephrurus, Carphodactylidae, Gekkota): plesiomorphic grades and biome shifts through the Miocene.Crossref | GoogleScholarGoogle Scholar |
Oliver, PM, and McDonald, PJ (2016). Young relicts and old relicts: a novel palaeoendemic vertebrate from the Australian Central Uplands. Royal Society Open Science 3, 160018.
| Young relicts and old relicts: a novel palaeoendemic vertebrate from the Australian Central Uplands.Crossref | GoogleScholarGoogle Scholar |
Oliver, PM, Laver, RJ, Smith, KL, and Bauer, AM (2014). Long-term persistence and vicariance within the Australian Monsoonal Tropics: the case of the giant cave and tree geckos (Pseudothecadactylus). Australian Journal of Zoology 61, 462–468.
| Long-term persistence and vicariance within the Australian Monsoonal Tropics: the case of the giant cave and tree geckos (Pseudothecadactylus).Crossref | GoogleScholarGoogle Scholar |
Oliver, PM, Laver, RJ, De Mello Martins, F, Pratt, RC, Hunjan, S, and Moritz, CC (2017). A novel hotspot of vertebrate endemism and an evolutionary refugium in tropical Australia. Diversity and Distributions 23, 53–66.
| A novel hotspot of vertebrate endemism and an evolutionary refugium in tropical Australia.Crossref | GoogleScholarGoogle Scholar |
Oliver, PM, Ashman, LG, Bank, S, Pratt, RC, Tedeschi, LG, Laver, RJ, Pratt, RC, Tedeschi, LG, and Moritz, CC (2019). On and off the rocks: persistence and ecological diversification in a tropical Australian lizard radiation. BMC Evolutionary Biology 19, 81.
| On and off the rocks: persistence and ecological diversification in a tropical Australian lizard radiation.Crossref | GoogleScholarGoogle Scholar |
Pepper, M, and Keogh, JS (2021). Life in the “dead heart” of Australia: the geohistory of the Australian deserts and its impact on genetic diversity of arid zone lizards. Journal of Biogeography 48, 716–746.
| Life in the “dead heart” of Australia: the geohistory of the Australian deserts and its impact on genetic diversity of arid zone lizards.Crossref | GoogleScholarGoogle Scholar |
Pepper, M, Ho, SYW, Fujita, MK, and Scott Keogh, J (2011). The genetic legacy of aridification: climate cycling fostered lizard diversification in Australian montane refugia and left low-lying deserts genetically depauperate. Molecular Phylogenetics and Evolution 61, 750–759.
| The genetic legacy of aridification: climate cycling fostered lizard diversification in Australian montane refugia and left low-lying deserts genetically depauperate.Crossref | GoogleScholarGoogle Scholar |
Pepper, M, Doughty, P, Fujita, MK, Moritz, C, and Keogh, JS (2013). Speciation on the rocks: integrated systematics of the Heteronotia spelea species complex (Gekkota; Reptilia) from western and central Australia. PLoS One 8, e78110.
| Speciation on the rocks: integrated systematics of the Heteronotia spelea species complex (Gekkota; Reptilia) from western and central Australia.Crossref | GoogleScholarGoogle Scholar |
Porter R (2008) ‘Keeping Australian Geckos.’ (ABK Publications: Burleigh BC, Qld, Australia)
Rabosky, DL, Hutchinson, MN, Donnellan, SC, Talaba, AL, and Lovette, IJ (2014). Phylogenetic disassembly of species boundaries in a widespread group of Australian skinks (Scincidae: Ctenotus). Molecular Phylogenetics and Evolution 77, 71–82.
| Phylogenetic disassembly of species boundaries in a widespread group of Australian skinks (Scincidae: Ctenotus).Crossref | GoogleScholarGoogle Scholar |
Rosauer, DF, Blom, MPK, Bourke, G, Catalano, S, Donnellan, S, Gillespie, G, Mulder, E, Oliver, PM, Potter, S, Pratt, RC, Rabosky, DL, Skipwith, PL, and Moritz, C (2016). Phylogeography, hotspots and conservation priorities: an example from the Top End of Australia. Biological Conservation 204, 83–93.
| Phylogeography, hotspots and conservation priorities: an example from the Top End of Australia.Crossref | GoogleScholarGoogle Scholar |
Shoo, LP, Rose, R, Doughty, P, Austin, JJ, and Melville, J (2008). Diversification patterns of pebble-mimic dragons are consistent with historical disruption of important habitat corridors in arid Australia. Molecular Phylogenetics and Evolution 48, 528–542.
| Diversification patterns of pebble-mimic dragons are consistent with historical disruption of important habitat corridors in arid Australia.Crossref | GoogleScholarGoogle Scholar |
Simó-Riudalbas, M, de Pous, P, Els, J, Jayasinghe, S, Péntek-Zakar, E, Wilms, T, Al-Saadi, S, and Carranza, S (2017). Cryptic diversity in Ptyodactylus (Reptilia: Gekkonidae) from the northern Hajar Mountains of Oman and the United Arab Emirates uncovered by an integrative taxonomic approach. PLoS One 12, e0180397.
| Cryptic diversity in Ptyodactylus (Reptilia: Gekkonidae) from the northern Hajar Mountains of Oman and the United Arab Emirates uncovered by an integrative taxonomic approach.Crossref | GoogleScholarGoogle Scholar |
Skipwith, PL, Bi, K, and Oliver, PM (2019). Relicts and radiations: phylogenomics of an Australasian lizard clade with east Gondwanan origins (Gekkota: Diplodactyloidea). Molecular Phylogenetics and Evolution 140, 106589.
| Relicts and radiations: phylogenomics of an Australasian lizard clade with east Gondwanan origins (Gekkota: Diplodactyloidea).Crossref | GoogleScholarGoogle Scholar |
Sniderman, JMK, Woodhead, JD, Hellstrom, J, Jordan, GJ, Drysdale, RN, Tyler, JJ, and Porch, N (2016). Pliocene reversal of late Neogene aridification. Proceedings of the National Academy of Sciences of the United States of America 113, 1999–2004.
| Pliocene reversal of late Neogene aridification.Crossref | GoogleScholarGoogle Scholar |
Wüster, W, Thomson, SA, O’Shea, M, and Kaiser, H (2021). Confronting taxonomic vandalism in biology: conscientious community self-organization can preserve nomenclatural stability. Biological Journal of the Linnean Society 133, 645–670.
| Confronting taxonomic vandalism in biology: conscientious community self-organization can preserve nomenclatural stability.Crossref | GoogleScholarGoogle Scholar |


