The influence of meal size on the digestive energetics of Gould’s wattled bat, Chalinolobus gouldii
Melissa J. Walker A B E , Stephen R. Griffiths
A B E , Stephen R. Griffiths  A C , Christopher S. Jones
A C , Christopher S. Jones  D and Kylie A. Robert
D and Kylie A. Robert  A C
A C
A Department of Ecology, Environment and Evolution, La Trobe University, Bundoora, Vic. 3086, Australia.
B Hawkesbury Institute for the Environment, Western Sydney University, Richmond, NSW 2753, Australia.
C Research Centre for Future Landscapes, La Trobe University, Bundoora, Vic. 3086, Australia.
D Arthur Rylah Institute for Environmental Research, Department of Environment, Land, Water and Planning, Heidelberg, Vic. 3084, Australia.
E Corresponding author. Email: m.walker2@westernsydney.edu.au
Australian Journal of Zoology 67(6) 331-338 https://doi.org/10.1071/ZO20028
Submitted: 1 May 2020 Accepted: 14 September 2020 Published: 8 October 2020
Abstract
Although variation in meal size is known to have an impact on digestive energetics, there is limited information on how it influences metabolic rate and energy assimilation in insectivorous bats. We investigated the influence of meal size, representing 10% or 20% of an individual’s weight, on the digestive energetics of Gould’s wattled bat, Chalinolobus gouldii (n = 61 bats). Using open-flow respirometry, we recorded a median resting metabolic rate of 2.0 mL g–1 h–1 (n = 51, range = 0.4–4.8) at an air temperature of 32°C. Median postprandial metabolic rate peaked at 6.5 (range = 3.4–11.6, n = 4) and 8.2 (range = 3.8–10.6, n = 7), representing 3.3- and 4.1-fold increases from resting metabolic rate for the two meal sizes. Using bomb calorimetry, we calculated the calorific value of the two meal sizes, and the calories lost during digestion. Following gut passage times of 120 min (range = 103–172, n = 15) and 124 min (range = 106–147, n = 12), C. gouldii assimilated 88.0% (range = 84.6–93.8, n = 5) and 93.3% (range = 84.0–99.4, n = 10) of the kilojoules available from the 10% and 20% meal sizes, respectively. When fed ad libitum, C. gouldii consumed a mean of 23.2% of their body weight during a single sitting (n = 18, range = 6.3–34.1%). Overall, digestive energetics were not significantly different between 10% or 20% meal sizes. The ability to ingest small and large meals, without compromising the rate or efficiency of calorie intake, indicates that free-ranging C. gouldii are likely limited by food available in the environment, rather than the ability to assimilate energy.
Keywords: calorific value, Chalinolobus gouldii, digestive efficiency, Gould’s wattled bat, gut capacity, gut passage time, insectivorous bat, metabolic rate, oxygen consumption.
Introduction
Meal size is known to affect digestive energetics. Large meals require more energy to digest, reduce digestive efficiency and increase the time that the meal is retained in the body (McCue 2006; Secor 2009). Understanding the impact that meal size has on digestive energetics is particularly important for small insectivorous mammals, as variation in the abundance and diversity of their invertebrate prey resources show significant temporal variation (e.g. hourly, nightly, monthly and seasonally) (Milne et al. 2005), and terrestrial insect abundance is in decline (van Klink et al. 2020). Further, foraging behaviours could be better described if the impact of meal size on calorific uptake is better understood, particularly for species where energy demands are high, i.e. species with high surface area to volume ratios resulting in high rates of heat loss.
Bats are an interesting model taxon as they have evolved a range of morphological and physiological modifications in order to maintain a positive energy balance. Compared with their similarly sized non-volant terrestrial counterparts, bats have a reduced digestive system, an adaptation presumed advantageous as it reduces gut passage time and increases flight performance by reducing weight and/or the energy required to maintain tissue (Caviedes-Vidal et al. 2007; Roswag et al. 2012). A reduced gut passage time increases the amount of food that can be consumed, which is reportedly in the range 20–30% of body weight per night for some bat species (Kunz 1974). High rates of food consumption also help to cover costs associated with flight and high surface area to volume ratios, resulting in high rates of heat loss and concurrent high mass-specific metabolic rates (MR) (Thomas and Suthers 1972; Winter and Von Helversen 1998; Geiser 2006; Geiser and Stawski 2011). The ecophysiology of insectivorous bats has been well studied, particularly in the context of thermal energetics; mitigating heat-energy lost to the environment is an important survival strategy of small insectivorous bats (Geiser 2006; Turbill and Geiser 2006). Fewer studies have focussed on bat digestive energetics.
Studies on the energetics of digestion in bats generally have a narrow focus. The focus may include MR (Morris et al. 1994; Matheson et al. 2010), the amount of food that can be consumed in a single sitting (gut capacity) (Kunz 1974; Bell et al. 1986; Kurta et al. 1989), the amount of chemical energy assimilated during digestion compared with the amount of energy present in a meal (digestive efficiency) (Barclay et al. 1991), and time taken for food to pass through the gastrointestinal tract (gut passage time) (Buchler 1975; Sibly 1981; Rydell and Baagøe 1994; Roswag et al. 2012). Little attention has been given to the impact of meal size on digestive energetics, and few studies have investigated how doubling a meal size influences the various components of bat digestive energetics. For example, Matheson et al. (2010) found that feeding to 50% (9.8% of body weight) or 100% (17.3% of body weight) satiation increased the time that little brown bats, Myotis lucifugus, spent normothermic before entering torpor, and that this method of feeding did not affect the rate of body cooling or MR during torpor. On the other hand, Welch et al. (2015) found that feeding fish-eating myotis, Myotis vivesi, a 3-g meal compared with a 1.5-g meal (representing 10.4% and 5.3% of body weight, respectively) increases MR and the scope of the metabolic response.
Gould’s wattled bat, Chalinolobus gouldii, is a common and widespread insectivorous bat in Australia (Churchill 2008). This 15-g vespertilionid is a generalist aerial insectivore (Hosken and Withers 1997) that hunts a broad range of nocturnal invertebrate prey (Churchill 2008; Straka 2015). We sought to characterise components of digestive energetics for C. gouldii, following the ingestion of a meal representing 10% or 20% of an individual’s body weight. We used open-flow respirometry to measure: (1) MR (resting metabolic rate, RMR; and postprandial metabolic rate, PMR), with a focus on the time taken to reach the peak of the PMR following ingestion, and the scope of the peak (the peak of the PMR expressed as a factor of the RMR). Using bomb calorimetry, we calculated the calorific value of the two meal sizes, and the energy remaining in scats following digestion, to determine (2) digestive efficiency. We also investigated (3) gut passage time, and (4) gut capacity. We hypothesised that the energy required to digest the 20% meal would be double that required for the 10% (McCue 2006; Secor 2009; Matheson et al. 2010; Welch et al. 2015) and that this would be reflected in an increase in PMR. Additionally, we predicted that digestive efficiency would be lower for the 20% meal than for the 10% meal, and that gut passage time would be greater for the 20% meal than for the 10% meal.
Materials and methods
Collection and housing
We collected 61 adult C. gouldii, 25 males and 36 non-gravid females (mean (±s.e.) body weight = 15.4 ± 0.2 g). The bats were retrieved from artificial roosts (bat boxes) at the La Trobe University Wildlife Sanctuary, Victoria, Australia (37.7160°S, 145.0498°E), between 4 May and 22 July 2016 (Austral autumn and winter). Captive bats were transported ~1 km to a laboratory at La Trobe University, where morphometric data were recorded, including age, weight at the time of capture (g), and forearm length (mm). The bats used in this study are part of a larger, ongoing study (Griffiths et al. 2017, 2020), and had previously been marked with bat-bands (Australasian Bird and Bat Banding Authority) or microchips (Trovan ID-100 passive integrated nanotransponder) (Godinho et al. 2015).
For experimentation, captive bats were weighed to the nearest 0.01 g; bats were deemed postabsorptive after fasting for >12 h (Kovtun and Zhukova 1994). Meal sizes of either 10% or 20% of individual body weight were determined from each bat’s fasted weight; some individuals were fed both meal sizes and contributed to multiple experiments in this study. The meals consisted of farmed mealworms, Tenebrio molitor, with a mean calorific content of 1.5 ± 0.4 kJ g–1 and weight of 0.2 ± 0.02 g (n = 5). Outside of experimental periods, bats roosted freely in soft-sided pet enclosures (46 × 38 × 41 cm) on a 12 h : 12 h L : D photoperiod, at ambient temperatures (22–24°C), and were fed until satiated. Bats were held for a maximum of four days, and were released at the La Trobe University Wildlife Sanctuary after sunset.
Metabolic rate
We used 58 bats in the metabolic experiments. Of these, 51 bats contributed to RMR (19 females, 32 males), 27 bats were fed the 10% meal size (16 females, 11 males), 22 bats were fed the 20% meal size (12 females, 10 males), and 9 bats were sham-fed (3 males, 6 females). We used four metabolic chambers to conduct open-flow respirometry experiments (mesh-lined Perspex tubes, 200 mm length and 45 mm diameter, 233 mL volume; Qubit systems, Ontario, Canada). Three of the four chambers each contained a single bat; the fourth chamber was left empty and used as a baseline reference chamber. All four chambers were held in an incubator (Galaxy 170 S, New Brunswick, Eppendorf AG, Germany) and oriented vertically to simulate a natural roosting position. The incubator was set to 32°C at all times to ensure bats remained euthermic (Hosken and Withers 1997). During each trial, the three bats were acclimated in their chamber for 1 h, during which time they were monitored by passive infrared cameras placed inside the incubator (1/3 CCD colour surveillance security camera with 6-IR LED night-vision; Jaycar, Rydalmere, NSW), which provided a live-video-feed to an external multichannel Digital Video Recorder (DVR; Pro Master AS-H268-16D1, Aussie Surveillance, Echuca, Victoria). Outside air was pumped in through a length of tubing and passed through drierite to decrease relative humidity (Qubit drying column, Qubit Systems, Ontario, Canada). The temperature of the dry air was increased as it passed through coiled tubing inside of the incubator before reaching the chambers (a preventative measure to stop the bats from entering torpor).
An open-flow respirometer (Qubit Systems Q-Box RP1LP High Range Respiration Package, Qubit Systems, Ontario, Canada) was used to maintain a constant flow rate of air (347 mL min–1), and analyse the concentrations of oxygen and carbon dioxide entering and exiting the chambers. RMR and PMR were determined using the rate of oxygen consumed ( ) to carbon dioxide produced (
) to carbon dioxide produced ( ) as calculated by Qubit output (C901 Logger Pro Software, Qubit Systems, Ontario, Canada) (in mL min–1 using Eqns 1 and 2, and converted to mL g–1 h–1):
) as calculated by Qubit output (C901 Logger Pro Software, Qubit Systems, Ontario, Canada) (in mL min–1 using Eqns 1 and 2, and converted to mL g–1 h–1):


where FR is the gas flow rate (in mL–1 min), FeO2 and FeCO2 are the fractional excurrent O2 and CO2 concentration, respectively, and FiO2 and FiCO2 are the fractional incurrent O2 and CO2 concentration, respectively.
Following acclimation, and before sunset and the start of the active-phase, we recorded RMR. We sampled every minute; however, metabolic responses were later refined to 10-min periods, using a 2-min window, so that all MR are presented as the median and range at every 10-min interval (e.g. 9 to 11 min covered the 10-min interval and 19 to 21 min covered the 20-min interval). RMR was recorded by sampling one bat for ~30 min, so as to best capture bats at rest, followed by a measurement of the reference chamber until the sample was equal to previous baseline values. We then continuously sampled the second bat for 30 min, and so on until all bats had been sampled. We measured RMR from 60 min to 150 min after bats entered the chambers; however, bats became restless after 120 min, likely as the active phase had started, and much of these data were excluded. After collecting RMR, the three bats were removed from the chambers, and two bats were simultaneously offered their premeasured meals using forceps. The third bat was offered the ends of forceps for the same amount of time as the two fed bats, but was not provided with food, thereby providing a ‘sham-fed’ control (Welch et al. 2015). Feeding occurred at sunset to simulate natural feeding times. PMR was sampled once the three bats were returned to their chambers; samples were recorded from each chamber every minute for 30 min, with a brief (<1 min) measurement of the reference chamber in between each switch. The first chamber was sampled twice to give a total of 120 min.
Any data from moving or agitated bats, noted by monitoring and recording bats on the external DVR, were removed from the dataset (primarily after 120 min of collecting RMR or 80 min of collecting PMR). Linear mixed-effects models were used to evaluate the effect of treatments on the change in metabolic rate from RMR to PMR. Linear mixed models were used in preference to non-linear alternatives as they provided the best model fit for the data. All data compilation and analyses were conducted using R 3.6.0 (R Core Team 2019) through R-Studio (RStudio Team 2020). Metabolic rates were square-root transformed before modelling to ensure a normal data distribution. Effects are shown on the transformed data scale. The number of RMR and PMR measurements per animal, per treatment, varied, but this variation is captured in the level of uncertainty in the effects. Meal treatments (sham 10%, sham 20%, 10% and 20%) and treatment period (before and after feeding), and their interaction, as well as sample time (min) and its interaction with period, and animal weight and sex, were included as fixed effects in the model. Animal ID was included as a random effect. We ran the model using the lmer function in the lme4 package (Bates et al. 2015), using the lmerTest package (Kuznetsova et al. 2017) for significance reporting for weight and sex. The fixed effects of sample time and weight were scaled to allow comparisons.
Post hoc contrasts between treatment factors were conducted on the model using the emmeans function in the emmeans package (Lenth 2020), with significance level of 0.05. Two separate tests were conducted on the same model: (1) to determine the change in the response variable from before (RMR) to after (PMR) treatment; and (2) to determine the comparative effects of treatments on PMR values. The coefficient estimates indicate that the effect size of the treatment and confidence intervals (95%) were approximated as the coefficient ± two times the standard error.
Gut passage time, digestive efficiency and gut capacity
Experiments to determine gut passage time and digestive efficiency ran consecutively with metabolic experiments. Bats placed inside metabolic chambers were passively monitored using cameras mounted within the incubator (video system described above). The time (min) taken from being fed to first evidence of a scat being passed was recorded as gut passage time. Bats were removed from the chambers at the conclusion of the metabolic experiment, and housed individually in calico bags, within the soft-sided pet enclosure. Any scat voided in the chambers, or in the calico bags over the next 12 h, were collected to calculate digestive efficiency. The calorific value of mealworms and scat were obtained using a bomb calorimeter (6400 oxygen bomb calorimeter, Parr Instrument Co., Moline, Illinois, USA). Scat samples were dried over 48 h at ambient temperatures and a sample of mealworms (n = 5) were euthanised at –20°C for 20 min and dried at 50°C for 4 h before analysis. Calorific values were calculated using the gross heat of combustion (Eqn 3):

where W = energy equivalent of the calorimeter being used; T = observed temperature rise; e1 = heat produced by burning the nitrogen portion of the air trapped in the bomb to form nitric acid; e2 = heat produced by the formation of sulfuric acid from the reaction of sulfur dioxide, water and oxygen; e3 = heat produced by the heating wire and cotton thread; m = mass of the sample.
Control combustions were conducted using five cigarette rolling papers per combustion (Tally-Ho, 0.2 ± 0.0 g and 26.2 ± 0.0 kJ g–1). In addition to acting as a control, the papers helped to form a pellet, along with cotton thread, for each scat and mealworm sample; the calorific value and mass of the papers was later subtracted, with the thread accounted for by the calorimeter. We calculated a mean calorific value for a mealworm (1.5 ± 0.4 kJ g–1, n = 5), multiplied by the number of mealworms consumed by each bat per meal (an average of 6 worms and 8.9 ± 2.4 kJ g–1 for the 10% meal size, and an average 12 worms and 17.9 ± 4.8 kJ g–1 for the 20% meal size). We subtracted the calorific value of the lost energy (in the scat from each bat) from the calorific value of each bat’s meal to determine calorie assimilation. A metabolisable energy coefficient (MEC) (Sibly 1981) was used to account for uncorrected endogenous, gaseous or urinary losses (Eqn 4):
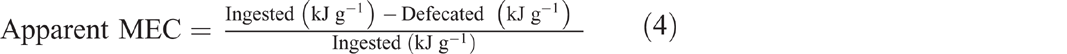
Due to modest sample sizes and single samples per bat, responses to the meal sizes for gut passage time (min) and digestive efficiency (%) were analysed using t-tests across grouped data and did not account for animal weight or sex. Raw data are presented for clarity and results for digestive efficiency and gut passage time are presented as median and range.
We fed 18 captive bats, separate from the metabolic and gut passage time experiments, to determine gut capacity. Bats were fed individually; mealworms were offered to the hand-held bat on the end of forceps, and the bat was permitted to eat to satiation. The cumulative weight of mealworms consumed (i.e. gut capacity) was recorded. Gut capacity is presented as a percentage of individual body weight.
Results
We characterised components of the digestive energetics of C. gouldii in response to meal sizes representing 10% or 20% of individual body weight. We found a significant increase in PMR from RMR for both meal sizes, and a significant 1.3-fold difference in metabolic scope between meal sizes. Unexpectedly, all other components of C. gouldii’s digestion were mostly unaffected by meal size.
Metabolic rate
RMR values were similar across bats and across periods before treatment (median = 2.0, range = 0.4–4.8) (Fig. 1). PMR values peaked after 30 min following both the 10% and 20% meal treatments but these peaks were inconsistent among individuals. This meant that the full dataset suggested a relatively flat response over time after treatment and both the meal treatments had not returned to RMR levels by the end of the experiment (120 min) (Fig. 1). For bats fed the 10% meal size, the highest median  values occurred 100 min (n = 4) after treatment (median = 6.5, range = 3.4–11.6), whereas the highest median
values occurred 100 min (n = 4) after treatment (median = 6.5, range = 3.4–11.6), whereas the highest median  value for the 20% meal size occurred 70 min (n = 7) after treatment (median = 8.2, range = 3.8–10.6) (Fig. 1). However, near-peak
value for the 20% meal size occurred 70 min (n = 7) after treatment (median = 8.2, range = 3.8–10.6) (Fig. 1). However, near-peak  values were recorded for both the 10% and 20% meal sizes from 40 min, suggesting that peak levels were reached at 40–70 min after treatment (Fig. 1). Initial comparisons showed weak relationships between MR (
values were recorded for both the 10% and 20% meal sizes from 40 min, suggesting that peak levels were reached at 40–70 min after treatment (Fig. 1). Initial comparisons showed weak relationships between MR ( mL h–1) and weight (R2BEFORE = 0.03, R2AFTER = 0.003, d.f. = 168, P > 0.05) or MR (
mL h–1) and weight (R2BEFORE = 0.03, R2AFTER = 0.003, d.f. = 168, P > 0.05) or MR ( mL g–1 h–1) by sex (Fig. 2). Linear mixed models indicated that there was a significant positive effect of the two meal treatments (10%: d.f. = 270, P < 0.0001; 20%: d.f. = 269, P < 0.0001) on MR from RMR to PMR (Fig. 3a). There was no significant effect of the sham meals on MR (sham 10%: d.f. = 254, P = 0.18; sham 20%: d.f. = 256, P = 0.94) (Fig. 3a). There was no effect of weight (effect = 0.04, d.f. = 84, P = 0.52) or sex (effect = 0.09, d.f. = 52, P = 0.45) on MR. When comparing PMR values only, the 20% meal size had a significantly greater effect on MR than the 10% meal size (effect = –0.28, d.f. = 268, P = 0.004) (Fig. 3b). As expected, the meal sizes had a significantly larger effect on PMR than their sham counterparts (10%: effect = 0.60, d.f. = 219, P = 0.003; 20%: effect = 0.87, d.f. = 174, P < 0.0001) but there was no significant difference between effects of the two sham meals (effect = –0.007, d.f. = 161, P = 1.0) (Fig. 3b). The scope of the peak (the peak of the PMR expressed as a factor of the RMR) increased to 3.3-fold and 4.1-fold above the RMR following 10 and 20% meal sizes, respectively.
mL g–1 h–1) by sex (Fig. 2). Linear mixed models indicated that there was a significant positive effect of the two meal treatments (10%: d.f. = 270, P < 0.0001; 20%: d.f. = 269, P < 0.0001) on MR from RMR to PMR (Fig. 3a). There was no significant effect of the sham meals on MR (sham 10%: d.f. = 254, P = 0.18; sham 20%: d.f. = 256, P = 0.94) (Fig. 3a). There was no effect of weight (effect = 0.04, d.f. = 84, P = 0.52) or sex (effect = 0.09, d.f. = 52, P = 0.45) on MR. When comparing PMR values only, the 20% meal size had a significantly greater effect on MR than the 10% meal size (effect = –0.28, d.f. = 268, P = 0.004) (Fig. 3b). As expected, the meal sizes had a significantly larger effect on PMR than their sham counterparts (10%: effect = 0.60, d.f. = 219, P = 0.003; 20%: effect = 0.87, d.f. = 174, P < 0.0001) but there was no significant difference between effects of the two sham meals (effect = –0.007, d.f. = 161, P = 1.0) (Fig. 3b). The scope of the peak (the peak of the PMR expressed as a factor of the RMR) increased to 3.3-fold and 4.1-fold above the RMR following 10 and 20% meal sizes, respectively.
Gut passage time, digestive efficiency and gut capacity
We obtained samples from 27 bats to determine gut passage time; 15 bats were fed a 10% meal size (6 females, 9 males), and 12 bats were fed a 20% meal size (6 females, 6 males). There was no significant difference in gut passage time between the meal sizes (t = 0.6, d.f. = 22.6, P = 0.5); bats fed 10% had a median gut passage time of 120 min (range = 103–172), while those fed 20% retained food for 124 min (range = 106–147) (Fig. 4a). To determine digestive efficiency, and using apparent MEC, we sampled 15 bats, 5 fed at 10% (2 females, 3 males) and 10 fed at 20% (4 females, 6 males). Digestive efficiency between meal sizes was not significant (t = –1.5, d.f. = 10.3, P = 0.2); median digestive efficiencies were 88.0% (range = 84.6–93.8) and 93.3% (range = 84.0–99.4) for the 10% and 20% meal sizes, respectively (Fig. 4b). After feeding 18 bats to satiation, we found that gut capacity ranged from 6.3 to 34.1% of an individual’s body weight. The average gut capacity was a 3.5-g meal, or 23% of the bat’s body weight.
Discussion
Limited empirical information exists on the impact of meal size on the digestive energetics of insectivorous bats. We found that the scope for C. gouldii fed 10% of their body weight (median = 6.5 mL g–1 h–1) was more than three times the RMR (median = 2.0 mL g–1 h–1) and more than quadruple the RMR for individuals fed the 20% meal size (median = 8.2 mL g–1 h–1). The peak of the PMR following the consumption of the 20% meal size was 1.3-fold the peak of the PMR following the consumption of the 10% meal. The time taken for either meal size to pass through the digestive tract, and the calories absorbed from those meals, were not significantly different. The ability to ingest small and large meals, without compromising the rate or efficiency of calorie intake, indicates that free-ranging C. gouldii are likely limited by food available in the environment, rather than the ability to assimilate energy.
Metabolic rate
The mean RMR of postabsorptive C. gouldii in our study falls within the range reported previously for this species. Hosken and Withers (1997) found the postabsorptive BMR of a euthermic C. gouldii was 1.44 ± 0.08 mL g–1 h–1. More recently, Codd et al. (2000) reported a mean MR of 6.95 ± 0.58 mL g–1 h–1 for inactive male C. gouldii, and 9.59 ± 0.56 mL g–1 h–1 for warm, active bats; however, their higher MRs reflect that the bats in that study were not postabsorptive. In this study, the effects of the different meal sizes on MR were significantly different when comparing PMR values, and were higher than the peaks seen in the metabolic response of other species. Gould’s long-eared bat, Nyctophilus gouldi, peaked at 5.6 mL g–1 h–1 when fed 15% of its body weight (Morris et al. 1994), and M. vivesi peaked at 83.2 ± 7.0 and 103.0 ± 14.8 mL h–1 after being fed 5.3% and 10.4% of its body weight, respectively (Welch et al. 2015). The time taken for C. gouldii to reach peak PMR (40–70 min) was faster than observed for the similarly sized N. gouldi (8.0–10.5 g) at 90–120 min (Morris et al. 1994), and slower than the much larger M. vivesi (28.8 ± 0.7 g) at 25–37 min (Welch et al. 2015).
We expected a significant increase from RMR to the peak PMR for each meal size, and that the scope of the metabolic response would double between meal sizes (McCue 2006; Secor 2009). Although the peak PMR was significantly higher than RMR for both meal sizes, the 20% meal (4.1 times the RMR) was only 1.3 times greater than the scope of the 10% meal (3.3 times the RMR). In this study, the meal sizes provided to C. gouldii elicited almost identical scopes to M. vivesi, provided with shrimp (Welch et al. 2015). Despite feeding a proportionately less amount of shrimp to M. vivesi than we fed to C. gouldii, accounting for 5.3% and 10.4% of the body weight (28.8 ± 0.7 g) of M. vivesi compared with our 10% and 20% of body weight, M. vivesi had a scope of 3.0 and 4.3 for each meal size, representing a 1.3 difference between meals. It is possible that we underestimate the peak and scope, given our sampling regime.
Components of digestion
Mealworm larvae are not a natural prey item for any bat species and are therefore an equally novel food source for all bats used in captive studies examining digestion. Gut passage time and digestive efficiency of C. gouldii in our study are comparable to those of other captive bat species fed mealworms, which range from 46 min for the little brown bat, M. lucifugus (Buchler 1975), to 204 min for the lesser noctule, Nyctalus leisleri (Roswag et al. 2012). The gut passage time that we observed for C. gouldii (120 min and 124 min for the 10% and 20% meal sizes, respectively) were most comparable to the similarly sized (14 g) particoloured bat, Vespertilio murinus (102 min: Rydell and Baagøe 1994). Given the generalist nature of their diet, it seems fitting that gut passage time in C. gouldii is longer than that of other bat species with more specialised diets (sensu Roswag et al. 2012). The digestive efficiency of the C. gouldii in our study is also comparable to those of other captive bat species fed mealworms, reportedly in the range 88–92% (Brisbin 1966; O’Farrell et al. 1971; Barclay et al. 1991; Webb et al. 1993). We considered that the lack of significance observed in gut passage time and digestive efficiency between meal sizes may have been due to meal sizes being too small. We therefore examined gut capacity, as there were no previous reports of this for C. gouldii. We found mean gut capacity to be 23%; however, it is unlikely we underfed our bats, given that the mean gut capacity was only 3% more than our large meal size. Further, gut capacity for C. gouldii is comparable to other small bat species; for example, adult cave myotis, Myotis velifer, consume 20–30% of their body weight, although this species was consuming natural prey items (Kunz 1974).
The dietary composition of free-ranging versus captive bats is likely to influence gut passage time and digestive efficiency. For example, Barclay et al. (1991) found that little brown bat, M. lucifugus, long-eared myotis, Myotis evotis, and long-legged myotis, Myotis volans, each had significantly lower digestive efficiencies when fed moths compared with their counterparts fed mealworms. Reduced digestive efficiency for bats fed novel prey species are likely the result of variation in (1) prey body parts, such as legs, wings and the head, being more difficult to digest given the presence of chitin (Webb et al. 1993; Straka 2015), and (2) the composition of the prey (fats, protein and carbohydrates) (Finke 2002). As a natural diet for C. gouldii consists of up to 90% lepidopteran species (Straka 2015), natural prey is likely more difficult to digest than mealworms. Conversely, undescribed morphological adaptations may mean that C. gouldii can digest natural prey with equal efficiency as the mealworm larvae. For example, the big brown bat, Eptesicus fuscus, has an oesophagus lined with a keratinised, callus-like epithelium, plus an absence of a sphincter into the stomach, which mitigates the need to break down chitin (Neuweiler 2000). Free-ranging C. gouldii consuming a natural diet comprising a range of invertebrate taxa would likely have a different digestive efficiency than the captive bats fed mealworms in this study. Future research could examine digestive efficiency of C. gouldii when consuming different prey types.
An ability to digest and assimilate energy from a variety of meal sizes, with equal efficiency, may be of benefit to small bats in the context of gut passage time and digestive efficiency. However, high metabolic rate following the ingestion of a large meal may affect how energy saving mechanisms, such as torpor, are employed. In this study, the 20% meal was higher for all aspects of digestive energetics, but only PMR differences were significant. Larger meals result in a higher metabolic rate and can affect the time that is spent normothermic before entering into torpor (Matheson et al. 2010). Given that C. gouldii employ torpor as an energy saving mechanism (Hosken and Withers 1997), and energy assimilation is impaired by torpor (Speakman and Rowland 1999), it is expected that torpor use would be postponed until digestion is complete. The time taken to enter into torpor may be increased for C. gouldii following the consumption of a larger meal size, given that the PMR was significantly high. Consequently, there could be a fine balance between consuming large meals and obtaining more energy, and offsetting torpor use as an energy saving mechanism.
Conclusions
Here we provide insight on the impact that meal size has on the digestive energetics of a common, moderately sized, insectivorous bat. The peak of the PMR following the ingestion of food more than doubled from RMR; however, the difference between the scopes of the meals was less than double. Unexpectedly, doubling meal size did not significantly decrease digestive efficiency or increase gut passage time. Results of this study indicate that C. gouldii does not pay a high cost to consume larger meals, and the relatively low cost to digest large meals, coupled with high energy assimilation, does not limit prey intake. Further research could investigate whether meal type affects digestion and, if there is an effect, how variation in prey availability may vary the digestive response seasonally. A broader range of insectivorous bat species should be studied, and comparisons between species with more or less specialised diets could be beneficial. Furthermore, future research would do well to investigate the relationship between consumption of different meal sizes and time spent normothermic before torpor, as this energy-saving strategy is important in understanding the energy budget of bats. Our results provide insight into the relationship that exists between digestive energetics, physiology and ecology of insectivorous bats. We highlight the importance of investigating a variety of factors contributing to digestive energetics of insectivorous bats.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We thank R. Bender, D. Eastick, A. Dimovski, S. Omond, A. Monagle, and E. Jones for their assistance during fieldwork, and the staff at the La Trobe Wildlife Sanctuary and the La Trobe Department of Ecology, Environment and Evolution for their research support. We thank the two anonymous reviewers whose comments and suggestions helped to improve and clarify this manuscript. All animal capture, handling and experimental procedures were carried out under ethics approval from the La Trobe University Animal Ethics Committee (project AEC13-30), and under a scientific research permit from the Department of Environment, Land, Water and Planning (permit no. 10006790). This study was funded by the Holsworth Wildlife Research Endowment (grant to SRG).
References
Barclay, R. M. R., Dolan, M. A., and Dyck, A. (1991). The digestive efficiency of insectivorous bats. Canadian Journal of Zoology 69, 1853–1856.| The digestive efficiency of insectivorous bats.Crossref | GoogleScholarGoogle Scholar |
Bates, D., Maechler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67, 1–48.
| Fitting linear mixed-effects models using lme4.Crossref | GoogleScholarGoogle Scholar |
Bell, G. P., Bartholomew, G. A., and Nagy, K. A. (1986). The roles of energetics, water economy, foraging behavior, and geothermal refugia in the distribution of the bat, Macrotus californicus. Journal of Comparative Physiology. B, Biochemical, Systemic, and Environmental Physiology 156, 441–450.
| The roles of energetics, water economy, foraging behavior, and geothermal refugia in the distribution of the bat, Macrotus californicus.Crossref | GoogleScholarGoogle Scholar |
Brisbin, I. L. (1966). Energy-utilization in a captive hoary bat. Journal of Mammalogy 47, 719–720.
| Energy-utilization in a captive hoary bat.Crossref | GoogleScholarGoogle Scholar |
Buchler, E. R. (1975). Food transit time in Myotis lucifugus Chiroptera: Vespertilionidae. Journal of Mammalogy 56, 252–255.
| Food transit time in Myotis lucifugus Chiroptera: Vespertilionidae.Crossref | GoogleScholarGoogle Scholar | 1113046PubMed |
Caviedes-Vidal, E., McWhorter, T. J., Lavin, S. R., Chediack, J. G., Tracy, C. R., and Karasov, W. H. (2007). The digestive adaptation of flying vertebrates: high intestinal paracellular absorption compensates for smaller guts. Proceedings of the National Academy of Sciences of the United States of America 104, 19132–19137.
| The digestive adaptation of flying vertebrates: high intestinal paracellular absorption compensates for smaller guts.Crossref | GoogleScholarGoogle Scholar | 18025481PubMed |
Churchill, S. (2008). ‘Australian Bats.’ 2nd edn. (Allen and Unwin: Sydney.)
Codd, J. R., Slocombe, N. C., Daniels, C. B., Wood, P. G., and Orgeig, S. (2000). Periodic fluctuations in the pulmonary surfactant system in Gould’s wattled bat (Chalinolobus gouldii). Physiological and Biochemical Zoology 73, 605–612.
| Periodic fluctuations in the pulmonary surfactant system in Gould’s wattled bat (Chalinolobus gouldii).Crossref | GoogleScholarGoogle Scholar | 11073796PubMed |
Finke, M. D. (2002). Complete nutrient composition of commercially raised invertebrates used as food for insectivores. Zoo Biology 21, 269–285.
| Complete nutrient composition of commercially raised invertebrates used as food for insectivores.Crossref | GoogleScholarGoogle Scholar |
Geiser, F. (2006). Energetics, thermal biology, and torpor in Australian bats. In ‘Functional and Evolutionary Ecology of Bats’. (Eds A. Zubaid, G. F. McCracken, and T. H. Kunz.) pp. 5–22. (Oxford University Press.)
Geiser, F., and Stawski, C. (2011). Hibernation and torpor in tropical and subtropical bats in relation to energetics, extinctions, and the evolution of endothermy. Integrative and Comparative Biology 51, 337–348.
| Hibernation and torpor in tropical and subtropical bats in relation to energetics, extinctions, and the evolution of endothermy.Crossref | GoogleScholarGoogle Scholar | 21700575PubMed |
Godinho, L. N., Lumsden, L. F., Coulson, G., and Griffiths, S. R. (2015). Network analysis reveals cryptic seasonal patterns of association in Gould’s wattled bats (Chalinolobus gouldii) roosting in bat-boxes. Behaviour 152, 2079–2105.
| Network analysis reveals cryptic seasonal patterns of association in Gould’s wattled bats (Chalinolobus gouldii) roosting in bat-boxes.Crossref | GoogleScholarGoogle Scholar |
Griffiths, S. R., Bender, R., Godinho, L. N., Lentini, P. E., Lumsden, L. F., and Robert, K. A. (2017). Bat boxes are not a silver bullet conservation tool. Mammal Review 47, 261–265.
| Bat boxes are not a silver bullet conservation tool.Crossref | GoogleScholarGoogle Scholar |
Griffiths, S. R., Lumsden, L. F., Robert, K. A., and Lentini, P. E. (2020). Nest boxes do not cause a shift in bat community composition in an urbanised landscape. Scientific Reports 10, 6210.
| Nest boxes do not cause a shift in bat community composition in an urbanised landscape.Crossref | GoogleScholarGoogle Scholar | 32277114PubMed |
Hosken, J. D., and Withers, P. C. (1997). Temperature regulation and metabolism of an Australian bat, Chalinolobus gouldii (Chiroptera: Vespertilionidae) when euthermic and torpid. Journal of Comparative Physiology. B, Biochemical, Systemic, and Environmental Physiology 167, 71–80.
| Temperature regulation and metabolism of an Australian bat, Chalinolobus gouldii (Chiroptera: Vespertilionidae) when euthermic and torpid.Crossref | GoogleScholarGoogle Scholar |
Kovtun, M., and Zhukova, N. (1994). Feeding and digestion intensity in chiropterans of different trophic groups. Folia Zoologica 43, 377–386.
Kunz, T. H. (1974). Feeding ecology of a temperate insectivorous bat (Myotis velifer). Ecology 55, 693–711.
| Feeding ecology of a temperate insectivorous bat (Myotis velifer).Crossref | GoogleScholarGoogle Scholar |
Kurta, A., Bell, G. P., Nagy, K. A., and Kunz, T. H. (1989). Energetics of pregnancy and lactation in free-ranging little brown bats (Myotis lucifugus). Physiological Zoology 62, 804–818.
| Energetics of pregnancy and lactation in free-ranging little brown bats (Myotis lucifugus).Crossref | GoogleScholarGoogle Scholar |
Kuznetsova, A., Brockhoff, P., and Christensen, R. (2017). lmerTest package: tests in linear mixed effects models. Journal of Statistical Software 82, 1–26.
| lmerTest package: tests in linear mixed effects models.Crossref | GoogleScholarGoogle Scholar |
Lenth, R. (2020) emmeans: estimated marginal means, aka least-squares means. R Package version 1.4.5. Available at: https://CRAN.R-project.org/package=emmeans [accessed 24 September 2020]
Matheson, A. L., Campbell, K. L., and Willis, C. K. (2010). Feasting, fasting and freezing: energetic effects of meal size and temperature on torpor expression by little brown bats Myotis lucifugus. The Journal of Experimental Biology 213, 2165–2173.
| Feasting, fasting and freezing: energetic effects of meal size and temperature on torpor expression by little brown bats Myotis lucifugus.Crossref | GoogleScholarGoogle Scholar | 20511531PubMed |
McCue, M. D. (2006). Specific dynamic action: a century of investigation. Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology 144, 381–394.
| Specific dynamic action: a century of investigation.Crossref | GoogleScholarGoogle Scholar |
Milne, D. J., Fisher, A., Rainey, I., and Pavey, C. R. (2005). Temporal patterns of bats in the top end of the Northern Territory, Australia. Journal of Mammalogy 86, 909–920.
| Temporal patterns of bats in the top end of the Northern Territory, Australia.Crossref | GoogleScholarGoogle Scholar |
Morris, S., Curtin, A. L., and Thompson, M. B. (1994). Heterothermy, torpor, respiratory gas exchange, water balance and the effect of feeding in Gould’s long-eared bat Nyctophilus gouldi. The Journal of Experimental Biology 197, 309–335.
| 7852907PubMed |
Neuweiler, G. (2000). ‘The Biology of Bats.’ (Oxford University Press: New York.)
O’Farrell, M. J., Studier, E. H., and Ewing, W. G. (1971). Energy utilization and water requirements of captive Myotis thysanodes and Myotis lucifugus (Chiroptera). Comparative Biochemistry and Physiology. Part A, Physiology 39, 549–552.
| Energy utilization and water requirements of captive Myotis thysanodes and Myotis lucifugus (Chiroptera).Crossref | GoogleScholarGoogle Scholar |
R Core Team (2019). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: https://www.R-project.org [accessed 18 September 2020].
Roswag, A., Becker, N. I., and Encarnação, J. A. (2012). Inter‐ and intraspecific comparisons of retention time in insectivorous bat species (Vespertilionidae). Journal of Zoology 288, 85–92.
| Inter‐ and intraspecific comparisons of retention time in insectivorous bat species (Vespertilionidae).Crossref | GoogleScholarGoogle Scholar |
RStudio Team (2020). RStudio: integrated development for R (RStudio, PBC: Boston, MA). Available at: http://www.rstudio.com [accessed 18 September 2020].
Rydell, J., and Baagøe, H. J. (1994). Vespertilio murinus. Mammalian Species Archive 467, 1–6.
Secor, S. M. (2009). Specific dynamic action: a review of the postprandial metabolic response. Journal of Comparative Physiology. B, Biochemical, Systemic, and Environmental Physiology 179, 1–56.
| Specific dynamic action: a review of the postprandial metabolic response.Crossref | GoogleScholarGoogle Scholar | 18597096PubMed |
Sibly, R. (1981). Strategies of digestion and defecation. In ‘Physiological Ecology: An Evolutionary Approach to Resource Use’. (Eds C. R. Townsend, and P. Calow.) pp. 109–139. (Sinauer Associates: Sunderland, MA.)
Speakman, J. R., and Rowland, A. (1999). Preparing for inactivity: how insectivorous bats deposit a fat store for hibernation. The Proceedings of the Nutrition Society 58, 123–131.
| Preparing for inactivity: how insectivorous bats deposit a fat store for hibernation.Crossref | GoogleScholarGoogle Scholar | 10343349PubMed |
Straka, T. M. (2015). The shared habitat: understanding and linking the needs of insectivorous bats and people at urban wetlands. Ph.D. Thesis, University of Melbourne.
Thomas, S. P., and Suthers, R. A. (1972). The physiology and energetics of bat flight. The Journal of Experimental Biology 57, 317–335.
Turbill, C., and Geiser, F. (2006). Thermal physiology of pregnant and lactating female and male long-eared bats, Nyctophilus geoffroyi and N. gouldi. Journal of Comparative Physiology. B, Biochemical, Systemic, and Environmental Physiology 176, 165–172.
| Thermal physiology of pregnant and lactating female and male long-eared bats, Nyctophilus geoffroyi and N. gouldi.Crossref | GoogleScholarGoogle Scholar | 16331479PubMed |
van Klink, R., Bowler, D. E., Gongalsky, K. B., Swengel, A. B., Gentile, A., and Chase, J. M. (2020). Meta-analysis reveals declines in terrestrial but increases in freshwater insect abundances. Science 368, 417–420.
| 32327596PubMed |
Webb, P., Speakman, J., and Racey, P. (1993). Defecation, apparent absorption efficiency, and the importance of water obtained in the food for water balance in captive brown long‐eared (Plecotus auritus) and Daubenton’s (Myotis daubentoni) bats. Journal of Zoology 230, 619–628.
| Defecation, apparent absorption efficiency, and the importance of water obtained in the food for water balance in captive brown long‐eared (Plecotus auritus) and Daubenton’s (Myotis daubentoni) bats.Crossref | GoogleScholarGoogle Scholar |
Welch, K. C., Otálora-Ardila, A., and Flores-Martínez, J. J. (2015). The cost of digestion in the fish-eating myotis (Myotis vivesi). The Journal of Experimental Biology 218, 1180–1187.
| The cost of digestion in the fish-eating myotis (Myotis vivesi).Crossref | GoogleScholarGoogle Scholar | 25911733PubMed |
Winter, Y., and Von Helversen, O. (1998). The energy cost of flight: do small bats fly more cheaply than birds? Journal of Comparative Physiology. B, Biochemical, Systemic, and Environmental Physiology 168, 105–111.
| The energy cost of flight: do small bats fly more cheaply than birds?Crossref | GoogleScholarGoogle Scholar | 9542147PubMed |






