Fire regimes drive population trends of a threatened lizard in the central and western deserts of Australia
Darren M. Southwell A B * , Danae Moore C , Steve McAlpin D , Edward M. J. Blackwood
A B * , Danae Moore C , Steve McAlpin D , Edward M. J. Blackwood  E , Andrew Schubert F ,
E , Andrew Schubert F ,  H I and Rachel M. Paltridge B J
H I and Rachel M. Paltridge B J A
B
C
D
E
F
G
H
I
J
Abstract
Animal and plant populations in arid regions fluctuate in size and extent in response to rainfall, fire and predation. Understanding the influence of these drivers on the status and trends of populations is crucial to implementing effective conservation actions.
In this study, we quantified the long-term drivers and trends in populations of a threatened lizard, the great desert skink (Liopholis kintorei; Tjakura), in the central and western deserts of Australia.
We collated 23 years (2002–2023) of active Tjakuṟa burrow count data from 31 sites clustered in the following four regions: Yulara, Newhaven Wildlife Sanctuary, Uluṟu–Kata Tjuṯa National Park and Kiwirrkurra Indigenous Protected Area. We fitted a negative binomial regression model in a Bayesian framework to estimate trends in active burrow counts over time and quantified the effect of rainfall, mean annual normalised difference vegetation index (NDVI), time since fire and fire extent on active burrow counts.
Our results showed contrasting trends in Tjakuṟa active burrow counts across the four regions. At Kiwirrkurra, Newhaven Wildlife Sanctuary and Yulara, active burrow counts increased consistently at rates of 35% (0.298; 95% CI 0.099–0.471), 18% (0.168; 95% CI 0.029, 0.314) and 5% per year (0.045; 95% CI 0.017, 0.073) respectively. In contrast, active burrow counts in Uluṟu–Kata Tjuṯa National Park increased from 2002 to 2012 before steadily decreasing. Across all sites, fire was the most important predictor of active Tjakuṟa burrow counts, with a significant positive effect of time since fire (0.108; 95% CI 0.014–0.204) and a strong negative effect of fire extent in the previous year (−0.111; 95% CI −0.243 to −0.026).
Our results have highlighted the importance of delivering ongoing planned fire management programs that avoid burning vegetation directly at and around Tjakura burrow systems, while providing a patch mosaic across the surrounding landscape.
We recommend that monitoring of Tjakura burrows be standardised across regions and that site covariates, especially measures of predation pressure, be monitored to further understand drivers of population trends.
Keywords: arid zone, burrow counts, fire regime, great desert skink, long-term monitoring, population modelling, Tjakura, wildfire.
Introduction
Understanding the status and trends of plant and animal populations is crucial to implementing effective conservation actions (Yoccoz et al. 2001). General biodiversity inventories, targeted species surveys and ongoing monitoring of biodiversity are needed to improve knowledge about where species are located, how they are trending and whether management interventions are working (Possingham et al. 2012; Lindenmayer and Likens 2018). Identifying the ecological processes and mechanisms driving trends is also important for prescribing conservation actions and understanding how populations and communities might respond to environmental change (Didham et al. 2007; Brook et al. 2008). However, despite the need for long-term and robust biodiversity monitoring, there are few long-term datasets available for plants and animals at risk of extinction (Scheele et al. 2019; Lavery et al. 2021), particularly in remote and inaccessible regions such as deserts (Durant et al. 2014).
Quantifying trends and drivers of plant and animal populations in arid regions is especially challenging. Surveys must often be conducted in remote and inaccessible environments subject to extreme weather events (Southwell et al. 2023a), limiting the spatial and temporal coverage of sampling. Many arid-zone species are sparsely distributed across large areas, meaning that biodiversity monitoring programs must span multiple management jurisdictions and land tenures (Legge et al. 2024). This can lead to inconsistent survey effort and sampling protocols across sites, increasing rates of observer error and limiting what can be inferred about population trends. Furthermore, detecting trends in arid-zone species is complicated by high rates of natural variability in both the size and extent of species populations (Letnic and Dickman 2010; Yang et al. 2010; Dickman et al. 2018). Monitoring should be conducted regularly enough to understand the natural year-to-year variation in population extent and size, and over periods that are sufficiently long enough to be teased apart from long-term underlying trends.
High variability in the population size and extent of arid zone species is primarily driven by processes such as rainfall, fire and predation. Episodic rainfall events stimulate plant productivity and growth, which in turn drives increases in animal populations such as rodents (Bennison et al. 2018), other small mammals (Letnic and Dickman 2010) and lizards (Letnic et al. 2004). As these populations increase, predators such as foxes and cats also increase, shaping species communities by limiting the density and range of prey (Letnic et al. 2005). In addition, fire is an important process in arid regions that regulates populations of plants and animals (Bowman et al. 2009). In the deserts of Australia, the likelihood of fire is influenced by both cumulative rainfall since fire and antecedent rainfall accumulated over 24 months (Allan and Southgate 2002; Van Etten and Burrows 2018; Ruscalleda-Alvarez et al. 2023). Fires spread easily once spinifex cover exceeds 30% (Burrows et al. 2016) or when two consecutive summers of above-average rainfall result in a continuity of ephemeral fuels (Verhoeven et al. 2020). Since the cessation of Indigenous land management in many arid regions around the world, large-scale fire events have become more widespread, likely contributing to the decline of many threatened arid-zone species (Letnic et al. 2004; Bird et al. 2005).
In arid regions, rainfall, predation and fire are likely to interact with one another to influence the spatial extent and long-term trends of animal populations (Greenville et al. 2014). For example, periods of high rainfall will likely increase vegetation productivity, which in turn will increase food resources and shelter from predators for some species, while at the same time increase fire risk (Pastro et al. 2013). There has been considerable interest from conservation ecologists in untangling interactions between fire and predation risk, particularly in the fire-prone ecosystems of the arid and wet–dry tropical regions of central and northern Australia (Stobo-Wilson et al. 2020; Doherty et al. 2022; Doherty et al. 2023). Management of fire and predators might help uncouple these processes from other drivers such as rainfall. Understanding the relative contribution of such processes operating on threatened species at multiple scales is important for understanding the likely effectiveness of management for improving population trends.
In this paper, we assessed the long-term trends and drivers of a threatened lizard in arid Australia, the great desert skink (Liopholis kintorei). The species is a large orange (or sometimes grey) skink that lives in communal family burrow systems confined to fire-prone arid environments in the central and western deserts of Australia. Populations are threatened by feral cats (Felis catus) and the red fox (Vulpes vulpes), along with severe and extensive wildfire (Moore et al. 2018). The species is known by a number of names in Australian Aboriginal languages, namely, Tjakuṟa in the Pitjantjatjara, Yankunytjatjara and Ngaanyatjarra languages, Warrarna in Warlpiri language, Tjalapa by Pintupi speakers, Mulyamiji in the Manyjilijarra language spoken by Martu, Nampu in the language of the Mantjintjarra Ngalia people and Aran spoken by the Anmatjere (McAlpin 2001; Indigenous Desert Alliance 2022). Here, we use Tjakuṟa because this name covers the highest number of populations from the South Australian Aṉangu Pitjantjatjara Yankunytjatjara (APY) Lands to southern Northern Territory, and across to the Ngaanyatjarra Lands of Western Australia.
Populations of Tjakuṟa have been monitored by Indigenous desert ranger groups and conservation organisations for the past two decades because of the species’ cultural significance to Aboriginal peoples (Pearson et al. 2001; Indigenous Desert Alliance 2022) and in response to evidence of population declines (McAlpin 2001; Indigenous Desert Alliance 2022). Fire and predators have been managed in some regions to benefit Tjakuṟa and studies have shown negative effects of fire and predation on Tjakuṟa burrow activity (Moore et al. 2015; Cadenhead et al. 2016; Moore et al. 2018). However, these studies have generally focused on single populations over short time frames. Analysis of Tjakuṟa data across the full spatial and temporal extent of monitoring would provide further insight into the drivers of long-term population trends.
Here, we (1) collated Tjakuṟa monitoring data from the central and western deserts of arid Australia, (2) estimated trends in Tjakuṟa across geographically distinct populations, and (3) estimated the relative effect of processes driving population trends. In total, we collated Tjakuṟa burrow count data from 31 monitoring sites clustered in four regions in arid Australia, some of which have been monitored for over two decades. For each site, we extracted the dynamic variables, cumulative rainfall, mean annual normalised difference vegetation index (NDVI), time since fire and fire extent. Using a regression model, we quantified trends in active burrow counts across the four regions and the relative effect of the dynamic variables on active Tjakuṟa burrow counts. In light of our results, we provide advice on how a standardised Tjakuṟa monitoring program might be implemented across the species range. Understanding the drivers of Tjakuṟa population trends across space and time will inform conservation decision-making for the species, paving the way for species recovery and more cost-effective management.
Materials and methods
Study species
Tjakuṟa is a large, scincid lizard with an average snout–vent length of 200 mm and a body mass of up to 350 g (McAlpin 2001; Chapple 2003). The diet of Tjakura consists of a wide variety of invertebrates, small vertebrates, and the leaves, flowers and fruits of plants (McAlpin 2001; Thuo et al. 2024). They construct complex burrow systems with up to 20 entrances within a 10 m radius, and can be occupied by up to 10 individuals (McAlpin et al. 2011; Dennison 2015). Active burrows are conspicuous and easy to distinguish from diggings and burrows made by other species because of the distinctive nature of the communal latrine. Individuals forage only a few hundred metres from their burrow system but may disperse 0–4 km to establish elsewhere (McAlpin 2011). Predation by feral cats and the red fox are thought to threaten populations along with severe and extensive fire (Moore et al. 2018). The species is listed as Vulnerable under the Environment Protection and Biodiversity Conservation Act (EPBC) 1999 and by the International Union for Conservation of Nature (IUCN 2014).
Study area
Tjakuṟa are thought to occupy a vast area of approximately 770,000 km2 of the central and western deserts of Australia (Fig. 1), although this distribution is known to be patchy, with their presence being recorded at fewer than 100 localities (Indigenous Desert Alliance 2022). The occupied region of Australia is arid with irregular rainfall events ranging from 200 mm annually in the south of the species range to 400 mm in the north-west. Tjakuṟa most commonly occupy spinifex-dominated sand plains and dune swales, but are also found in spinifex-dominated paleo-drainage lines and undulating gravelly downs with spinifex.
Estimated geographic range of Tjakura in Australia (left panel, grey shading) and location of 31 monitoring sites in (a) Yulara (yellow dots) and Uluṟu–Kata Tjuṯa National Park (pink polygons), (b) Kiwirrkurra, and (c) Newhaven Wildlife Sanctuary. The grey shading in a–c shows the number of fires since 2000 in and around the monitoring sites.

Tjakuṟa monitoring
Tjakuṟa populations are monitored by counting the number of active burrows within designated search areas rather than surveying for the individuals themselves, because this provides an index of abundance that is cost-effective, non-invasive and builds on the tracking skills of Indigenous desert rangers. A burrow system is confirmed to be a Tjakuṟa burrow only if evidence of a latrine can be found and is only recorded as active if fresh Tjakuṟa tracks are observed or there are fresh (black not grey) scats in the latrine.
We collated active Tjakuṟa burrow counts from 31 sites in four regions of arid Australia: Yulara (11 sites), Australian Wildlife Conservancy’s Newhaven Wildlife Sanctuary (eight sites; hereafter Newhaven), Kiwirrkurra Indigenous Protected Area (three sites; hereafter Kiwirrkurra) and Uluṟu–Kata Tjuṯa National Park (nine sites; hereafter Uluṟu; Fig. 1). Newhaven is a privately owned wildlife sanctuary; Uluṟu is a jointly managed national park; Yulara is a privately owned tourism area; and Kiwirrkurra is an Indigenous Protected Area. Fire management is conducted in all four regions to protect infrastructure, flora and fauna, but there are different approaches to fire management in each and varying levels of capacity for wildfire suppression.
Most sites were surveyed annually in late summer (February/March) when Tjakuṟa are most active and burrows are most visible because of accumulated scats in the latrine and burrow maintenance activity over the active season. The exception is Yulara, which was monitored in November. The number of years that sites were monitored, area searched, and search methodology varied across the four regions. Uluṟu and Yulara have been monitored from 2001 to 2023, whereas monitoring at Newhaven and Kiwirrkurra commenced in 2014 and 2016 respectively. Sites of 4 ha in size were searched at Yulara (approximately 260 × 160 m); 30 ha sites were searched in Kiwirrkurra (approximately 300 × 1000 m), whereas sites in Uluṟu ranged from 13 to 289 ha. At Newhaven, a consistent 10 m belt transects were searched inside 50 ha sites approximately 500 m wide and 1000 m long, resulting in a search area of 7.5 ha. Although sites were searched systematically, the search pattern, number of observers, experience of observers and time spent searching varied across site-years and was not recorded routinely.
Site covariates
We extracted the following four time-varying covariates at monitoring sites that are thought to drive Tjakuṟa population trends: rainfall, NDVI, time since fire and fire extent. Rainfall is thought to be an important driver of reptile population structure in central Australia because it can promote the availability of food resources, increase vegetation cover and influence the incidence of fire (Letnic et al. 2004; Pastro et al. 2013). Given that our raw data show that Tjakuṟa were able to persist during years of drought, we hypothesised that rainfall accumulated over a 12–24 month period is most relevant to Tjakuṟa burrow activity through changes in spinifex cover and fire risk; however, the optimal length of this temporal window is uncertain. We therefore downloaded raster tiles of daily rainfall interpolated at 5 km resolution developed by the Climate Hazards Center available from the chirps package (De Sousa et al. 2020) in R ver. 4.3.1 (R Core Team 2023) and calculated the cumulative rainfall over two periods preceding surveys, namely, 12 and 24 months (November–October for Yulara; March–February for Newhaven, Uluṟu and Kiwirrkurra).
There is a growing body of literature demonstrating that vegetation productivity is a good predictor of stability and persistence of animal populations in arid and semi-arid environments because it can provide a more direct measure of vegetation condition, resource availability and shelter than does rainfall (Selwood et al. 2018; Young et al. 2022). NDVI is a common remotely sensed index available at high spatial and temporal resolution. We extracted raster layers of NDVI collected by the Moderate Resolution Imaging Spectroradiometer (MODIS) satellite for each region at 250 m resolution by using the MODISTools package in R (Hufkens 2023). We calculated the mean NDVI in the 12 and 24 months preceding surveys. Raw rainfall and NDVI data are plotted against burrow counts in Supplementary Figs S1–S3.
Fire is thought to be a key driver of Tjakuṟa population dynamics, with previous studies suggesting that long-unburnt habitat supports larger populations (Moore et al. 2015, 2018). Fire mapping is conducted annually at each region by using Landsat satellite imagery. We rasterised each map at 30 m resolution (Yulara/Uluṟu 1987–2023; Newhaven 2000–2020 and Kiwirrkurra 2000–2023) and calculated two fire metrics at sites, namely, fire extent and time since fire. To calculate fire extent, we calculated the proportion of cells that burnt within the boundary of each site each year. To calculate time since fire, we calculated the number of years since at least 5% of cells burnt within a site. Although Tjakuṟa have a home range of a few hundred metres (McAlpin 2011), we did not buffer sites when calculating the fire metrics because we were primarily interested in the incidence of fire at the monitored burrows.
Trends and drivers of active burrow counts
We fitted generalised additive models (GAMs) to active burrow count density data by using the mgcv package in R (Wood 2011), with ‘years’ as a predictor variable, to explore non-linear trends in the number of active burrows across the four regions over time. Exploring the response shape of ecological data with GAMs has been recommended as an important first step in the ecological modelling literature (Hastie and Tibshirani 1990; Wintle et al. 2005a).
We then quantified linear trends and drivers in Tjakuṟa active burrow counts by fitting a regression model in a Bayesian framework. Prior to model fitting, we calculated the pairwise correlation among variables and found that none were highly correlated (|r| < 0.7; Dormann et al. (2013) (Supplementary Tables S1, S2). A chi-squared test suggested that burrow counts did not fit a Poisson distribution (P < 0.05). We therefore modelled active burrow counts by using a negative binomial regression model, which assumed that for each site i and year j, the number of active burrows is described as
where is the mean number of active burrows and p is the overdispersion parameter. The mean number of active burrows is modelled as a log regression, as follows:
where is a random intercept for sites by region, is a linear trend in burrow counts by region, is the effect of cumulative rainfall in a temporal window preceding surveys, is the effect of mean NDVI in a temporal window preceding surveys, is the effect of time since fire in the previous year, is an indicator variable specifying whether a fire occurred (1) or not (0) in the previous year, is the effect of fire extent in the previous year, is an offset term to account for unequal site areas, accounts for first-order temporal correlation among burrow counts in successive years, and and are site and year random effects respectively.
All covariates were scaled by their mean and standard deviation except for fire extent, which was log transformed. We fitted the model in R (ver. 4.3.1; R Core Team 2023) by using Markov-chain Monte Carlo sampling (MCMC) in the rjags package for Bayesian inference (Sturtz et al. 2010). We ran two parallel chains for 100,000 iterations with the burn-in set to 50,000 and thinning set to two. We assessed model convergence using the coda package (Plummer et al. 2006) and ensured that R-hat values were <1.1 (Brooks and Gelman 1998). We assigned vague normal priors N (0,10−6) (mean and precision parameterisation) for parameters α and β. The means of the priors for the random effects were set at zero, with a standard deviation assigned a uniform distribution U (0,100).
Given there was uncertainty in the most ecologically relevant temporal window from which to extract rainfall and NDVI, we compared two competing models: one where rainfall and NDVI were calculated in the 24 months preceding surveys, and a second, where rainfall and NDVI were calculated in the 12 months beforehand. For each model, we calculated the deviance information criterion (DIC) and the Watanabe Akaike information criterion (WAIC) and assumed that the model with the lowest BIC and WAIC was ‘best’ (Pastro et al. 2013; Gelman et al. 2014).
Results
Active burrow counts
The density of active burrows varied across the four regions. The lowest active burrow density was observed at Uluṟu (0.13/ha), where very large areas were searched (up to 289 ha), followed by Yulara (0.37/ha in 4 ha sites) and Kiwirrkurra (0.40/ha in 30 ha sites). In contrast, the highest density was recorded at Newhaven, with an estimated 1.76 active burrows per hectare recorded over a 7.5 ha search area. Overall, the average density of active burrows across all sites and years was 0.51/ha.
Competing models
The model with rainfall and NDVI calculated over a 24 month window preceding surveys received DIC and WAIC values of 2095.9 and 1886.2 respectively, which were both less than the model with these variables calculated over a 12 month window (DIC = 2101.3; WAIC = 1887.4). We therefore present results from the 24 month model below and provide the results for the 12 month model in Fig. S4.
Trends in active burrow counts
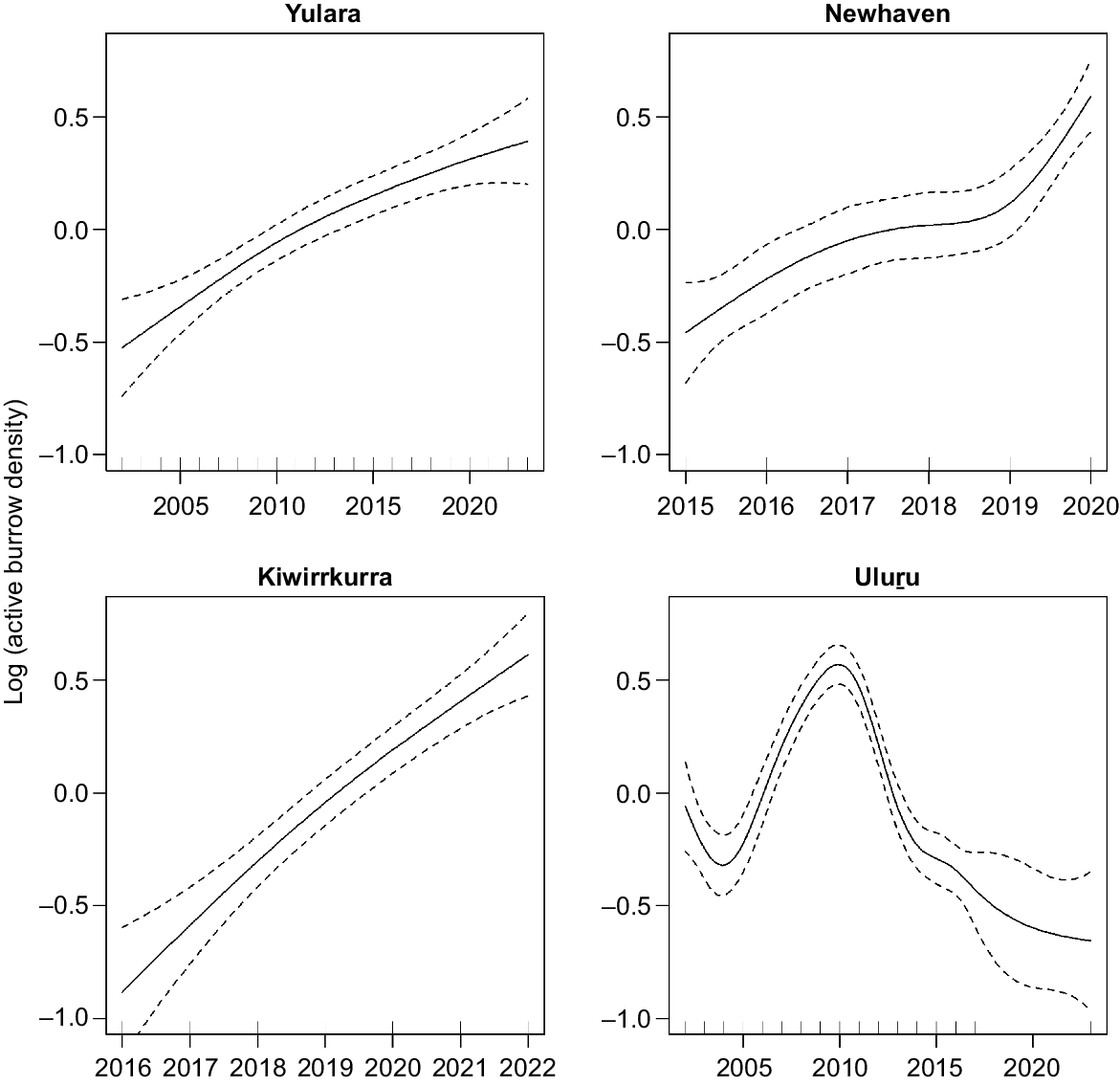
Trends in Tjakura active burrow counts were inconsistent across the four regions (Figs 2, 3). Our negative binomial regression model and GAMs suggested that Kiwirrkurra had a consistent increase in active burrow counts at a rate of 35% (0.298; 95% CI 0.099–0.471), although this estimate had a higher degree of uncertainty because only three sites have been monitored for 6 years. We found similar evidence for an increase in active burrow activity at Newhaven and Yulara at rates of 18% (0.168; 95% CI 0.029, 0.314) and 5% per year (0.045; 95% CI 0.017, 0.073) respectively. Our GAMs showed that these increases were linear in Kiwirrkurra, Yulara and Newhaven over the monitoring periods; however, the increase at Newhaven was influenced by a sharp increase in burrows in 2020. In contrast, the GAMs showed a highly non-linear trend in burrow counts at Uluṟu; active burrow counts increased steadily from 2002 to 2012, before decreasing consistently until 2023. When modelled in our regression model, active burrow counts at Uluṟu have declined at a rate of −2.18% per year (−0.022; 95% CI −0.055, 0.011) over the monitoring period from 2002 to 2023.
Response of GAMs fitted to active burrow count data separately for the four regions with years as a predictor variable.
The trend (i.e. the effect of year) in active burrow counts at Yulara, Newhaven, Kiwirrkurra and Uluṟu and the effect of four site covariates (total rainfall in the 24 months preceding surveys, mean NDVI in 24 months preceding surveys, time since fire and fire extent) as estimated by the negative binomial regression model. The error bars represent 95% credible intervals.

Drivers of trends
The two fire variables, namely, time since fire and fire extent, were strongly associated with trends in Tjakuṟa active burrow counts (Fig. 3). Time since fire had a significant positive association with active burrow counts (0.108; 95% CI 0.014, 0.204) (i.e. the 95% credible intervals did not overlap with zero), whereas the log of fire extent in the previous year had a strong negative impact on burrow counts (−0.111; 95% CI −0.243, 0.026). We found weak support for a positive effect of 24-month total rainfall on active burrow counts (0.032; 95% CI −0.061, 0.125), although this effect was not statistically significant because a large portion of the posterior distribution overlapped zero. Similarly, the effect of mean NDVI over the 24-month window prior to surveys was uncertain (−0.038; 95% CI −0.159, 0.084).
Discussion
Quantifying drivers and trends in species populations is crucial for informing conservation land management actions. In this study, we (1) collated Tjakuṟa active burrow count data from 31 sites across four regions of arid Australia, (2) estimated trends in active burrow counts across each region, and (3) estimated the effect of time-varying site covariates on active burrow count trends. Pooling data across the full spatial and temporal extent of monitoring maximised what could be inferred about the processes driving Tjakuṟa population trends. Although the known distributional range of Tjakuṟa has contracted and localised population declines and extinctions have been documented (McAlpin 2001), we found contrasting trends in active burrow counts across the four regions. Active burrow counts at Yulara, Newhaven and Kiwirrkurra followed the same general pattern, increasing steadily over time despite differences in site areas and search methods. Whereas the trend at Uluṟu, which is only 5–10 km away from Yulara, differed considerably, with a steady increase in active burrow counts from 2002 to 2012, before consistent declines across sites to lower levels of activity in 2023.
Drivers of active burrow count trends
Our analysis showed no clear associations between accumulated rainfall and mean NDVI over 24 months as drivers of Tjakuṟa active burrow counts. This is not surprising, given that rainfall over a 24 month period could have both negative and positive impacts on Tjakuṟa populations. On the one hand, high rainfall over a 24 month period may result in an increase in the amount of ground vegetation and therefore the incidence of fire, which in turn affects predation pressure, having a negative impact on Tjakuṟa populations. On the other hand, an increase in the amount of vegetation cover, if remaining unburnt, may reduce effectiveness of predation at the burrow systems. In addition, it is possible that high rainfall increases the amount of food resources available to Tjakuṟa, increasing reproductive success and survivorship. However, the latter scenario is unlikely to be a major driver of active burrow counts, given our raw data showed that Tjakuṟa populations were able to persist at Newhaven, Yulara and Kiwirrkurra during periods of drought with record-low rainfall (e.g. 42.2 mm at Newhaven in 2019). This might have been because termites (one of their primary food source) feed on dead spinifex material during dry periods, maintaining a reliable food source, and/or because reptiles have low metabolic rates and ability to become inactive during periods of stress. Other skink species have been found to maintain high survivorship but low recruitment in dry years, before achieving increased reproductive success post-drought, followed by high adult mortality after successive above-average rainfall years, likely owing to increased predation pressure (James 1991). The asynchronous recovery of predator and prey is thought to be a critical driver of post-drought recovery trends (Ruiz et al. 2022).
In contrast, we found stronger support for both fire metrics as drivers of active burrow count trends. Our result that time since fire is positively associated with active burrow counts and fire extent is negatively associated is consistent with previous studies (McAlpin 2001; Moore et al. 2015; Indigenous Desert Alliance 2022). For example, broadscale surveys at Newhaven showed that 84% of 78 burrows located in unburnt spinifex habitat were active, whereas just 8% of burrows found in habitat that had been burnt within 2 years were in use (Moore et al. 2015). Similarly, 82% of 78 burrows at Uluṟu became inactive within 4 months of being burnt in 2002 (Director of National Parks 2013). In 2012, when most sites were burnt by large fires, 98% of 37 burrows surveyed also became inactive within 4 months (Director of National Parks 2013). At the Yulara sites, Paltridge and Eldridge (2021) found that 83% of burrows became inactive within 12 months of a fire in 2019, compared with an average of 30% of unburnt burrows becoming inactive each year (range = 12–55%). Taken together, these studies have provided strong support for a negative effect of fire on the number of active Tjakura burrows.
Fire is likely to be an important moderator of Tjakura populations because fire induced changes to vegetation, such as reduced ground cover, influence predator–prey encounters (Janssen et al. 2007). With their conspicuous burrows and predictable behaviour of emerging around sunrise and sunset, Tjakuṟa are potentially at high risk of predation from predators that learn burrow locations and become specialised in hunting this species (Moore et al. 2018). This risk is likely to increase in a freshly burnt landscape given the hunting efficiency of some predators, such as feral cats, increases under these conditions (Dickman 1996; McGregor et al. 2015). For example, Tjakuṟa were found to survive the immediate impacts of fire and burrows remained active for at least 1 month after their burrows were burnt in experimental burns at Newhaven (Moore et al. 2015). However, more than half the burrows became inactive in the following 3 months (Moore et al. 2015), coinciding with high rates of predation visits to Tjakuṟa burrows by feral cats (Moore et al. 2018). Prey species that rely on habitat structure for avoiding predation often experience increased predation rates and lower survival rates in recently burnt areas (Doherty et al. 2022). Although solid evidence remains elusive, we believe that if fire has reduced the vegetation cover around Tjakuṟa burrows, predator hunting success is very likely to be enhanced.
The contrasting trends in active Tjakuṟa burrow counts may reflect the implementation of different fire regimes and suppression capabilities across the four regions. We found that all regions with increasing active burrow count trends (Yulara, Newhaven, Kiwirrkurra) have had active fire management programs and/or capacity for quick wildfire suppression that have mostly succeeded in excluding fire from key Tjakuṟa monitoring sites. For example, very few of the Kiwirrkurra and Newhaven sites have been burnt since 2001, and at Yulara, 7 of the 11 monitoring plots remained unburnt since 2005. In contrast, large fires burnt Tjakuṟa habitat at Uluṟu in 2012, 2019 and 2023, affecting 3, 6 and 1 of the 9 sites respectively. Although only 25% of the mapped active burrows across the monitoring area were burnt in the 2012 fire, the population has been declining ever since. The divergent trends in active burrow counts and fire histories at Uluṟu compared with the other regions support the hypothesis that Tjakuṟa populations are driven by fire, and that reducing the frequency and extent of large unplanned fires in and around burrows can prevent population declines.
Introduced predator control, specifically feral cat control, has been conducted alongside fire management at Newhaven, Yulara and Kiwirrkurra, over at least the past 5 years to reduce predation pressure on Tjakuṟa populations. For example, at Yulara, 153 cats have been culled from Tjakuṟa habitat since 2018 and, at Newhaven, 180 cats were culled from Tjakuṟa habitat between 2014 and 2022, with 69% (n = 124) being culled during the 3 years of drought (2018–2020). Over the same period, more than 200 cats were removed from an area that includes the Tjakuṟa sites but also extends out to a 20 km buffer around the population. The much stronger focus on cat control in Tjakuṟa habitat in the three regions, alongside active fire management and suppression, is likely to have contributed to the observed increases in Tjakuṟa burrow trends, especially during drought years. This suggests that cat control both after fires and during drought periods may have supported their persistence by reducing predation pressure at a time when post-fire vegetation cover was limited, or during drought periods when the absence of small mammals made Tjakuṟa a target for predators. We initially included a binary term for predator control in our model (1 = control, 0 = no control); however, records were limited and even when information was available, there was uncertainty about the intensity of control that had been implemented. This made it very difficult to assign a meaningful value to every site-year. We were therefore reluctant to include this term in our model and strongly recommend that future monitoring record both the presence and/or activity of predators and management effort.
Fire has the potential to affect Tjakuṟa populations by reducing food availability. Tjakuṟa are known to feed on invertebrates, ground beetles, mosquitoes, termites and plant material (McAlpin 2001; Thuo et al. 2024). A recent eDNA study by Thuo et al. (2024) at Uluṟu found that food items did not differ significantly among age groups, seasons or time since fire. However, the availability of these resources may be influenced by longer time since fire periods or under different environmental conditions and warrants further research. For example, harvester termites (Drepanotermes perniger) can survive on underground stores of spinifex in habitats where spinifex can rapidly resprout after fire, but may go locally extinct in habitats dominated by spinifex species that can regenerate only through the germination of seed (obligate seeder; (Perry 1972; Abensperg-Traun et al. 1996). Although little can be done to improve food availability after large unplanned fires, further research could explore whether the diet of Tjakuṟa shifts immediately following large, intense fires.
Limitations
Our study contained some limitations that warrant further research. First, uneven site areas ranging from 4 to 289 ha may have resulted in uneven search effort per unit area, with smaller sites likely being searched more thoroughly than larger ones. It is therefore unclear whether our reported difference in burrow densities across regions reflects true differences or is an artefact of site area or search protocol. We accounted for differences in site areas in our model with an offset term but had incomplete information on other aspects of survey effort, such as the number of observers, time of survey, or duration of survey. Future monitoring should record these details for inclusion in the modelling. Second, although surveys were mostly completed in late summer (February/March), when Tjakuṟa are most active and burrows most visible, the level of observer skill may have varied, resulting in burrows being missed (false-absence), misidentified as active (false-positives) or misidentified as belonging to different species (false-positives). Further, the rate of false-absences might have varied over time (e.g. as ground cover increases or search effort changes), giving false impressions of a trend. Both false-positives and false-negatives can be reduced by ensuring observers are appropriately trained prior to surveys and by standardising search methodologies across sites (e.g. searching equal areas for a fixed time). We recommend that a subset of sites are repeatedly surveyed within a year to quantify rates of false-negatives (i.e. detectability) (Wintle et al. 2005b).
Time since fire and fire extent are only two ways to characterise fire regimes. Our analysis could be extended to include other metrics of fire patchiness or interactions between fire and rainfall, such as the amount of rainfall since the last fire (Ruscalleda-Alvarez et al. 2023). We calculated time since fire and fire extent within each site boundary. This approach was chosen because we believed that Tjakuṟa burrow activity within sites is predominantly driven by the impact that fire has on vegetation cover in and around burrows, and the subsequent effect this has on predation risk. It is possible that larger scale effects of fire exist; for example, the occurrence of fire at a landscape-scale might influence predator densities, and in turn, predation risk at burrows. This could be explored by calculating fire metrics using very large buffers around sites (>1 km); however, this approach was complicated by the proximity of sites and very large differences in site size. These considerations highlight the complex interactions between time since fire, fire frequency and fire size, and the challenge extracting comparable site information from unequal site areas. Standardising the area of sites and survey methodology would remove some of these challenges (Southwell et al. 2023b).
Conclusions
Our study highlighted the important role that long-term monitoring can play in the conservation of species and ecological systems. Long-term studies that monitor populations, population drivers or environmental variables at multiple sites across decades by using modern statistical approaches allow us to gain a deep understanding of how ecosystems operate and respond to driving processes (Lindenmayer et al. 2014). However, our study focussed on Tjakuṟa populations in only four regions, three of which have had intense fire management and predator control over the past decade. Thus, we stress that our estimates of Tjakuṟa active burrow trends are not likely to be representative of the species across its broader distribution. We expect that most populations of Tjakuṟa that have not been actively managed for fire and predators are in decline. We recommend that standardised Tjakuṟa monitoring be expanded across the species’ known range, and assessment of covariates such as predation pressure and ground cover are built into the monitoring program to provide a more representative view of trends and drivers of the broader population (Southwell et al. 2023b).
Since the cessation of Indigenous peoples land management in many arid regions around the world, large-scale fire events have become more widespread, resulting in the decline in many threatened arid-zone species (Letnic et al. 2004; Bird et al. 2005; Santos et al. 2022). Our results demonstrated that when fire extent and frequency are reduced through fire management programs and the majority of vegetation, specifically ground cover, is maintained in a mature vegetative state in close proximity to burrows, Tjakuṟa active burrow counts increase or remain stable. Our results support the importance and need for land managers responsible for the conservation of Tjakuṟa to actively conduct planned burning programs, including fine-scale burns, and suppression of fire when required, to reduce fire extent and frequency in and around burrows. We recommend avoiding burning vegetation directly at and around Tjakuṟa burrow systems, but conducting ongoing burning in adjacent areas to maintain a variety of spinifex ages within dispersal distance of extant burrows. Additional broadscale burning in the surrounding landscape will reduce the risk of wildfires encroaching into Tjakuṟa habitat. Fire management in conjunction with predator control will not only benefit Tjakuṟa but will also improve the persistence of many other species in arid Australia.
Data availability
The data that support this study were obtained from the Australian Wildlife Conservancy, Parks Australia and the Kiwirrkurra Ranger Program by permission/licence. Data will be shared upon reasonable request to the corresponding author with permission from these organisations.
Declaration of funding
This project is supported with funding from the Australian Government under the National Environmental Science Program’s Resilient Landscapes Hub.
Acknowledgements
We thank Parks Australia, the Kiwirrkurra Community, Australian Wildlife Conservancy and Yulara for providing Tjakuṟa burrow data. The survey method for monitoring Tjakuṟa described in this study is based on identification and interpretation of tracks, scats and burrows. We are grateful to the many expert trackers who have shared their tracking skills, knowledge and stories with us and specifically acknowledge the generous teachings of Alice Nampitjinpa, Christine Michaels, Mitjili Napangangka, Yalti Napangati, Yukultji Napangati, Payu West and Mantua James.
References
Abensperg-Traun M, Smith GT, Arnold GW, Steven DE (1996) The effects of habitat fragmentation and livestock-grazing on animal communities in remnants of gimlet Eucalyptus salubris woodland in the Western Australian wheatbelt. I. Arthropods. Journal of Applied Ecology 33(6), 1281-1301.
| Crossref | Google Scholar |
Bennison K, Godfree R, Dickman CR (2018) Synchronous boom–bust cycles in central Australian rodents and marsupials in response to rainfall and fire. Journal of Mammalogy 99(5), 1137-1148.
| Crossref | Google Scholar |
Bird DW, Bird RB, Parker CH (2005) Aboriginal burning regimes and hunting strategies in Australia’s Western Desert. Human Ecology 33(4), 443-464.
| Crossref | Google Scholar |
Bowman DMJS, Balch JK, Artaxo P, Bond WJ, Carlson JM, Cochrane MA, D’Antonio CM, DeFries RS, Doyle JC, Harrison SP, Johnston FH, Keeley JE, Krawchuk MA, Kull CA, Marston JB, Moritz MA, Prentice IC, Roos CI, Scott AC, Swetnam TW, van der Werf GR, Pyne SJ (2009) Fire in the Earth system. Science 324(5926), 481-484.
| Crossref | Google Scholar | PubMed |
Brook BW, Sodhi NS, Bradshaw CJA (2008) Synergies among extinction drivers under global change. Trends in Ecology & Evolution 23(8), 453-460.
| Crossref | Google Scholar | PubMed |
Brooks SP, Gelman A (1998) General methods for monitoring convergence of iterative simulations. Journal of Computational and Graphical Statistics 7(4), 434-455.
| Crossref | Google Scholar |
Cadenhead NCR, Kearney MR, Moore D, McAlpin S, Wintle BA (2016) Climate and fire scenario uncertainty dominate the evaluation of options for conserving the great desert skink. Conservation Letters 9(3), 181-190.
| Crossref | Google Scholar |
Chapple DG (2003) Ecology, life-history, and behavior in the Australian Scincid genus Egernia, with comments on the evolution of complex sociality in lizards. Herpetological Monographs 17, 145-180.
| Crossref | Google Scholar |
De Sousa K, Sparks AH, Ashmall W, van Etten J, Solberg SØ (2020) Chirps: API client for the CHIRPS precipitation data in R. The Journal of Open Source Software 5(51), 2419.
| Crossref | Google Scholar |
Dickman CR (1996) Impact of exotic generalist predators on the native fauna of Australia. Wildlife Biology 2(3), 185-195.
| Crossref | Google Scholar |
Didham RK, Tylianakis JM, Gemmell NJ, Rand TA, Ewers RM (2007) Interactive effects of habitat modification and species invasion on native species decline. Trends in Ecology & Evolution 22(9), 489-496.
| Crossref | Google Scholar | PubMed |
Doherty TS, Geary WL, Jolly CJ, Macdonald KJ, Miritis V, Watchorn DJ, Cherry MJ, Conner LM, González TM, Legge SM, Ritchie EG, Stawski C, Dickman CR (2022) Fire as a driver and mediator of predator–prey interactions. Biological Reviews 97(4), 1539-1558.
| Crossref | Google Scholar | PubMed |
Doherty TS, Watchorn DJ, Miritis V, Pestell AJL, Geary WL (2023) Cats, foxes and fire: quantitative review reveals that invasive predator activity is most likely to increase shortly after fire. Fire Ecology 19(1), 22.
| Crossref | Google Scholar |
Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré G, Marquéz JRG, Gruber B, Lafourcade B, Leitão PJ, Münkemüller T, McClean C, Osborne PE, Reineking B, Schroder B, Skidmore AK, Zurell D, Lautenbach S (2013) Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36(1), 27-46.
| Crossref | Google Scholar |
Durant SM, Wacher T, Bashir S, Woodroffe R, De Ornellas P, Ransom C, Newby J, Abáigar T, Abdelgadir M, El Alqamy H, Baillie J, Beddiaf M, Belbachir F, Belbachir-Bazi A, Berbash AA, Bemadjim NE, Beudels-Jamar R, Boitani L, Breitenmoser C, Cano M, Chardonnet P, Collen B, Cornforth WA, Cuzin F, Gerngross P, Haddane B, Hadjeloum M, Jacobson A, Jebali A, Lamarque F, Mallon D, Minkowski K, Monfort S, Ndoassal B, Niagate B, Purchase G, Samaïla S, Samna AK, Sillero-Zubiri C, Soultan AE, Price MRS, Pettorelli N (2014) Fiddling in biodiversity hotspots while deserts burn? Collapse of the Sahara’s megafauna. Diversity and Distributions 20(1), 114-122.
| Crossref | Google Scholar |
Gelman A, Hwang J, Vehtari A (2014) Understanding predictive information criteria for Bayesian models. Statistics and Computing 24(6), 997-1016.
| Crossref | Google Scholar |
Greenville AC, Wardle GM, Tamayo B, Dickman CR (2014) Bottom-up and top-down processes interact to modify intraguild interactions in resource-pulse environments. Oecologia 175(4), 1349-1358.
| Crossref | Google Scholar | PubMed |
Hufkens K (2023) The MODISTools package: an interface to the MODIS land products subsets web services. Available at https://doi.org/10.5281/zenodo.7551165
IUCN (2014) IUCN Red List of Threatened Species. Available at http://www.iucnredlist.org
James CD (1991) Population dynamics, demography and life history of sympatric scincid lizards (Ctenotus) in central Australia. Herpetologica 47(2), 194-210.
| Google Scholar |
Janssen A, Sabelis MW, Magalhães S, Montserrat M, Van der Hammen T (2007) Habitat structure affects intraguild predation. Ecology 88(11), 2713-2719.
| Crossref | Google Scholar | PubMed |
Lavery T, Lindenmayer D, Blanchard W, Carey A, Cook E, Copley P, MacGregor NA, Melzer R, Nano C, Prentice L, Scheele BC, Sinclair S, Southwell D, Stuart S, Wilson M, Woinarski J (2021) Counting plants: the extent and adequacy of monitoring for a continental-scale list of threatened plant species. Biological Conservation 260, 109193.
| Crossref | Google Scholar |
Legge S, Indigo N, Southwell DM, Skroblin A, Nou T, Young AR, Dielenberg J, Wilkinson DP, Brizuela-Torres D, Aṉangu Pitjantjatjara Yankunytjatjara, Birriliburu Rangers, Backhouse B, Silva CG, Arkinstall C, Lynch C, Central Land Council Rangers, Curnow CL, Rogers DJ, Moore D, Ryan-Colton E, Benshemesh J, Schofield J, Kanyirninpa Jukurrpa, Karajarri Rangers, Moseby K, Tuft K, Bellchambers K, Bradley K, Webeck K, Kimberley Land Council Land and Sea Management Unit, Kiwirrkurra Rangers, Tait L, Lindsay M, Dziminski M, Newhaven Warlpiri Rangers, Ngaanyatjarra Council Rangers, Ngurrara Rangers, Jackett N, Nyangumarta Rangers, Nyikina Mangala Rangers, Parna Ngururrpa Aboriginal Corporation, Copley P, Paltridge R, Pedler RD, Southgate R, Brandle R, van Leeuwen S, Partridge T, Newsome TM, Wiluna Martu Rangers, Yawuru Country Managers (2024) The arid zone monitoring project: combining Indigenous ecological expertise with scientific data analysis to assess the potential of using sign-based surveys to monitor vertebrates in the Australian deserts. Wildlife Research 51(9), WR24070.
| Crossref | Google Scholar |
Letnic M, Dickman CR (2010) Resource pulses and mammalian dynamics: conceptual models for hummock grasslands and other Australian desert habitats. Biological Reviews 85(3), 501-521.
| Crossref | Google Scholar | PubMed |
Letnic M, Dickman CR, Tischler MK, Tamayo B, Beh C-L (2004) The responses of small mammals and lizards to post-fire succession and rainfall in arid Australia. Journal of Arid Environments 59(1), 85-114.
| Crossref | Google Scholar |
Letnic M, Tamayo B, Dickman CR (2005) The responses of mammals to La Niña (El Niño Southern Oscillation)-associated rainfall, predation, and wildfire in central Australia. Journal of Mammalogy 86(4), 689-703.
| Crossref | Google Scholar |
Lindenmayer DB, Blanchard W, McBurney L, Blair D, Banks SC, Driscoll DA, Smith AL, Gill AM (2014) Complex responses of birds to landscape-level fire extent, fire severity and environmental drivers. Diversity and Distributions 20(4), 467-477.
| Crossref | Google Scholar |
McAlpin S, Duckett P, Stow A (2011) Lizards cooperatively tunnel to construct a long-term home for family members. PLoS ONE 6(5), e19041.
| Crossref | Google Scholar |
McGregor H, Legge S, Jones ME, Johnson CN (2015) Feral cats are better killers in open habitats, revealed by animal-borne video. PLoS ONE 10(8), e0133915.
| Crossref | Google Scholar |
Moore D, Kearney MR, Paltridge R, McAlpin S, Stow A (2015) Is fire a threatening process for Liopholis kintorei, a nationally listed threatened skink? Wildlife Research 42(3), 207-216.
| Crossref | Google Scholar |
Moore D, Kearney MR, Paltridge R, McAlpin S, Stow A (2018) Feeling the pressure at home: predator activity at the burrow entrance of an endangered arid-zone skink. Austral Ecology 43(1), 102-109.
| Crossref | Google Scholar |
Pastro LA, Dickman CR, Letnic M (2013) Effects of wildfire, rainfall and region on desert lizard assemblages: the importance of multi-scale processes. Oecologia 173(2), 603-614.
| Crossref | Google Scholar | PubMed |
Pearson D, Davies P, Carnegie N, Ward J (2001) The great desert skink (Egernia kintorei) in Western Australia: distribution, reproduction and ethno-zoological observations. Herpetofauna 31(1), 64-68.
| Google Scholar |
Perry DH (1972) Some notes on the termites (Isoptera) of Barrow Island and a check list of species. Western Australian Naturalist 12(3), 42-55.
| Google Scholar |
Plummer M, Best N, Cowles K, Vines K (2006) CODA: convergence diagnosis and output analysis for MCMC. R News 6, 7-11.
| Google Scholar |
R Core Team (2023) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at https://www.R-project.org/
Ruiz T, Carrias J-F, Bonhomme C, Farjalla VF, Jassey VEJ, Leflaive J, Compin A, Leroy C, Corbara B, Srivastava DS, Céréghino R (2022) Asynchronous recovery of predators and prey conditions resilience to drought in a neotropical ecosystem. Scientific Reports 12(1), 8392.
| Crossref | Google Scholar |
Ruscalleda-Alvarez J, Cliff H, Catt G, Holmes J, Burrows N, Paltridge R, Russell-Smith J, Schubert A, See P, Legge S (2023) Right-way fire in Australia’s spinifex deserts: an approach for measuring management success when fire activity varies substantially through space and time. Journal of Environmental Management 331, 117234.
| Crossref | Google Scholar |
Santos JL, Hradsky BA, Keith DA, Rowe KC, Senior KL, Sitters H, Kelly LT (2022) Beyond inappropriate fire regimes: a synthesis of fire-driven declines of threatened mammals in Australia. Conservation Letters 15(5), e12905.
| Crossref | Google Scholar |
Scheele BC, Legge S, Blanchard W, Garnett S, Geyle H, Gillespie G, Harrison P, Lindenmayer D, Lintermans M, Robinson N, Woinarski J (2019) Continental-scale assessment reveals inadequate monitoring for threatened vertebrates in a megadiverse country. Biological Conservation 235, 273-278.
| Crossref | Google Scholar |
Selwood KE, McGeoch MA, Clarke RH, Mac Nally R (2018) High-productivity vegetation is important for lessening bird declines during prolonged drought. Journal of Applied Ecology 55(2), 641-650.
| Crossref | Google Scholar |
Southwell D, Skroblin A, Moseby K, Southgate R, Indigo N, Backhouse B, Bellchambers K, Brandle R, Brenton P, Copley P, Dziminski MA, Galindez-Silva C, Lynch L, Newman P, Pedler R, Rogers D, Roshier D, Ryan-Colton E, Tuft K, Ward M, Zurell D, Legge S (2023a) Designing a large-scale track-based monitoring program to detect changes in species distributions in arid Australia. Ecological Applications 33(2), e2762.
| Crossref | Google Scholar |
Stobo-Wilson AM, Stokeld D, Einoder LD, Davies HF, Fisher A, Hill BM, Mahney T, Murphy BP, Scroggie MP, Stevens A, Woinarski JCZ, Bawinanga Rangers, Warddeken Rangers, Gillespie GR (2020) Bottom-up and top-down processes influence contemporary patterns of mammal species richness in Australia’s monsoonal tropics. Biological Conservation 247, 108638.
| Crossref | Google Scholar |
Sturtz S, Ligges U, Gelman A (2010) R2OpenBUGS: a package for running OpenBUGS from R. Available at http://openbugs.info/w/UserContributedCode
Thuo D, Macgregor NA, Merson SD, Scopel D, Keogh JS, Kenny J, Williams JL, Guest T, Swan S, McAlpin S, Joseph L (2024) Metabarcoding clarifies the diet of the elusive and vulnerable Australian tjakura (great desert skink, Liopholis kintorei). Frontiers in Ecology and Evolution 12, 1354138.
| Crossref | Google Scholar |
Verhoeven EM, Murray BR, Dickman CR, Wardle GM, Greenville AC (2020) Fire and rain are one: extreme rainfall events predict wildfire extent in an arid grassland. International Journal of Wildland Fire 29(8), 702-711.
| Crossref | Google Scholar |
Wintle BA, Elith J, Potts JM (2005a) Fauna habitat modelling and mapping: a review and case study in the Lower Hunter Central Coast region of NSW. Austral Ecology 30(7), 719-738.
| Crossref | Google Scholar |
Wintle BA, Kavanagh RP, McCarthy MA, Burgman MA (2005b) Estimating and dealing with detectability in occupancy surveys for forest owls and arboreal marsupials. Journal of Wildlife Management 69(3), 905-917.
| Crossref | Google Scholar |
Wood SN (2011) Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. Journal of the Royal Statistical Society Series B: Statistical Methodology 73(1), 3-36.
| Crossref | Google Scholar |
Yang LH, Edwards KF, Byrnes JE, Bastow JL, Wright AN, Spence KO (2010) A meta-analysis of resource pulse–consumer interactions. Ecological Monographs 80(1), 125-151.
| Crossref | Google Scholar |
Yoccoz NG, Nichols JD, Boulinier T (2001) Monitoring of biological diversity in space and time. Trends in Ecology & Evolution 16(8), 446-453.
| Crossref | Google Scholar |
Young AR, Selwood KE, Benshemesh J, Wright J, Southwell D (2022) Remotely sensed vegetation productivity predicts breeding activity and drought refuges for a threatened bird in semi-arid Australia. Animal Conservation 25(4), 566-581.
| Crossref | Google Scholar |


