A new device to reduce mammal predation on reptiles in pitfall traps
Andrea D. Stiglingh A * , Katherine E. Moseby B C , Georgina Neave
A * , Katherine E. Moseby B C , Georgina Neave  B D , Nathan Beerkens B E and Katherine Tuft
B D , Nathan Beerkens B E and Katherine Tuft  B
B
A
B
C
D
E
Abstract
Many vertebrate studies report predation from pit co-occupants as a source of mortality during pitfall surveys.
This study aims to assess the use of false-floors in pitfall traps to reduce the opportunistic predation of small reptiles by small mammals caught within the same pit.
Small-vertebrate surveys were conducted using pitfall traps in an arid landscape from 1998 to 2021. Between 2018 and 2021, wooden false-floors with 2 cm notches in their sides were placed inside pitfall traps to reduce the amount of reptile predation caused by small mammals co-occupying the same pit. The position of captured individuals, relative to the false-floor, were used to assess the capacity of false-floors to create an effective barrier between captured reptiles and mammals.
During the false-floor trial period (2018–2021), Pseudomys australis and Notomys alexis were identified as the key mammal species opportunistically predating on captured reptiles, collectively accounting for 54% of reptile predation incidents. Most of the N. alexis and P. australis captures were found above false-floors (92 and 70% of captures respectively), indicating that they were generally not able to access the prey refuge beneath. Reptile mortality from small mammal predation was significantly lower in pitfalls with false-floors (15% of reptile-mammal co-occupancy incidents) than in those without (60% of co-occupancy incidents). However, false-floors did not prevent all predation events because some mammals were able to access the compartment underneath the false-floors.
The false-floors provided an effective barrier between small reptiles and key mammal species caught in the same pit and reduced occurrences of small reptile predation.
False-floors can effectively be used as a tool to reduce reptile mortality during pitfall surveys. However, they also increased the time taken to set and check traps and we therefore suggest their use only during times of high mammal abundances, when the abundance of large rodents is high. The efficacy of false-floors at any particular site may be improved by trialling different-sized notches and construction materials.
Keywords: animal welfare, false-floors, herpetology survey, mammal survey, mortality, pitfall trapping, predation, reptile survey, safehaven.
Introduction
Animal mortality as a direct result of live-trapping methods used in fauna surveys, such as pitfall traps, cage traps and aluminium box-style traps (Elliott/Sherman), is an animal-welfare concern for research, particularly when monitoring animal populations of conservation concern (Stephens and Anderson 2014). In this context, trap mortalities can have larger implications for population stability and long-term monitoring trends (Stephens and Anderson 2014). Sources of animal mortality during live-trapping surveys include heat stress and desiccation (Jenkins et al. 2003; Read and Kearney 2016; Read et al. 2018), drowning (Aubry and Stringer 2000; Clemann 2006), starvation (Yunger et al. 1992), handling accidents (Read and Kearney 2016) and predation (Read and Kearney 2016; Waudby et al. 2019). Ongoing assessment and refinement of survey methods is necessary for reducing mortality risks and improving the health and wellbeing of captured individuals (Karraker 2001; Powell and Proulx 2003; Petit and Waudby 2013; Read et al. 2018).
Small mammals and reptiles are often surveyed by using pitfall traps (Thompson et al. 2003; Sutherland 2006; Hoffmann et al. 2010). Predation is a major contributor to vertebrate mortality rates in pitfall traps and can occur as a result of large predators reaching down into the pits and removing trapped animals (Ferguson and Forstner 2006), through invertebrate attack when caught in the same pit (Read and Kearney 2016), and predation by other vertebrates captured in the same pit (Karraker 2001; Waudby et al. 2019). The attack between pit co-occupants may be for consumption or as a result of boredom or defensive behaviours when in close proximity. The opportunistic predation of small reptiles by small mammals caught within the same pit can be a leading cause of vertebrate mortalities during pitfall surveys. For example, predation by Notomys was the main cause of reptile mortality (10%), during pitfall surveys in arid South Australia (Read and Kearney 2016). The incidence of small-mammal predation on reptiles is likely to be greatest in situations where small-mammal abundances are unusually high, such as within safehavens, where large mammalian predators such as cats, foxes and dingoes, and often quolls, are excluded or extinct (Legge et al. 2019). For example, following rainfall at the Arid Recovery Reserve, the rodent Pseudomys australis may be an order of magnitude more common inside the haven than outside (Read and Cunningham 2010).
Although some aspects of trap design, such as the use of drift-fences, can influence capture rates of certain taxa, pitfall trapping is largely a non-selective approach that typically catches any animal small enough to fall into the cylindrical containers (Waudby et al. 2019). However, when a specific taxon is the subject of research or monitoring, excluding non-target species from pitfall traps can enhance target-species capture rates and potentially decrease live-capture mortality rates (Edwards and Jones 2014). For example, Karraker (2001) used jute twine attached to coverboards and suspended into the pitfall traps as a means of allowing small mammals (non-target species) to climb out of the traps during reptile and amphibian surveys. This trap design successfully reduced the number of mammal captures, but also reduced trap efficacy for reptiles. The exclusion of predatory vertebrates (e.g., small mammals) may not always be feasible during surveys, particularly in situations where a wide range of taxa are to be assessed during the same survey (e.g., small mammals and reptiles). In these situations, an alternative predation-mitigation approach is required. Typically this involves the provision of refuges within pitfall traps such as litter, tubing or soil for use by prey species (Fisher and Rochester 2012; Petit and Waudby 2013; Read et al. 2015; Waudby et al. 2019) and the provision of alternative food sources for predators, such as seeds for rodents. Many of these studies do not report on the effectiveness of these approaches in reducing the number of predation events or attacks observed within the pitfall traps, making it difficult to assess their efficacy.
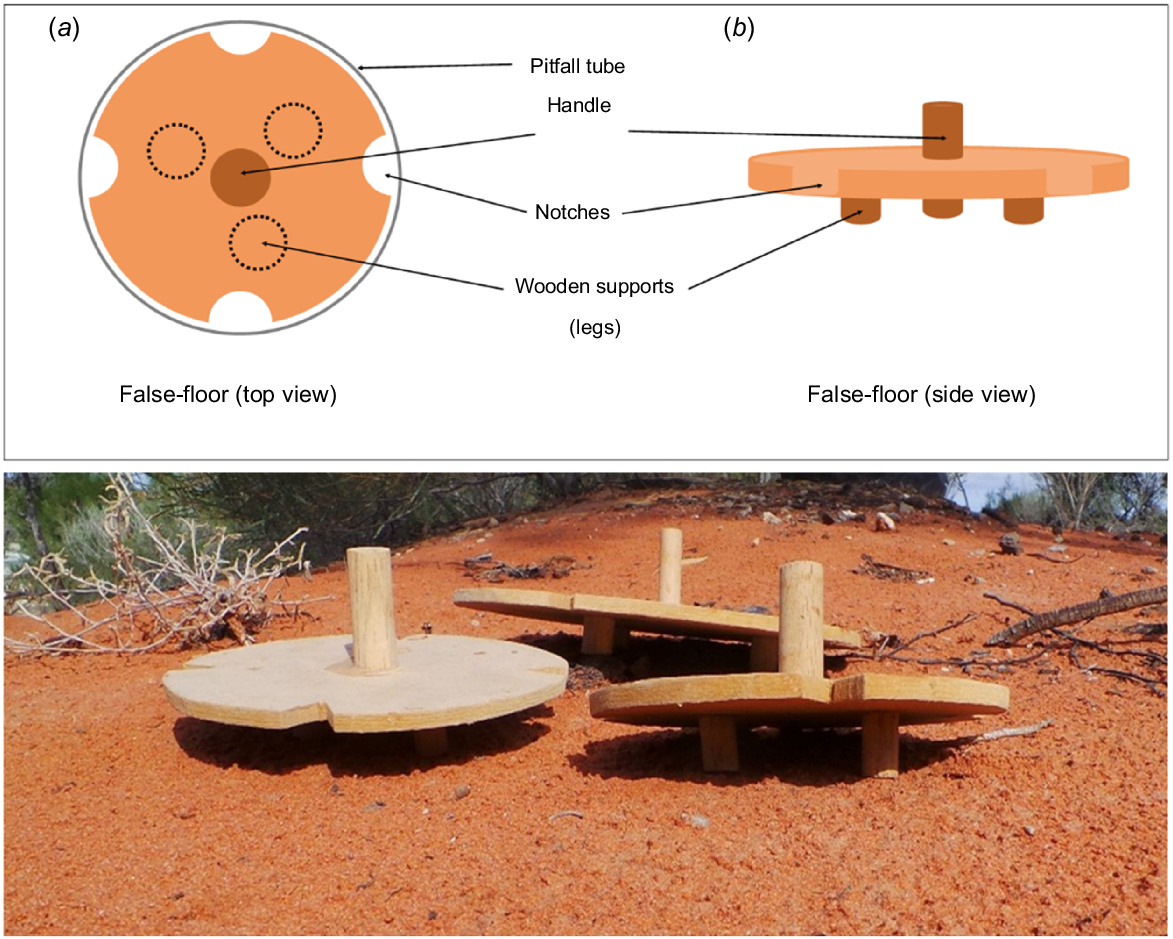
In this study, we designed and tested a false-floor for use in pitfall traps. This false-floor aims to reduce the risk of small-reptile mortality caused by small mammals captured within the same pit, without negatively affecting the capture rates of either vertebrate group. These devices were designed to sit at the bottom of the pitfall traps on short, raised legs to create a false bottom (Fig. 1). This design was used to create a physical barrier between predator and prey species. Smaller prey species are able to pass through small notches cut into the sides of the false-floor and access the refuge area underneath, whereas larger predatory species remain above the false-floor. Unlike other predator-exclusion approaches, these false-floors are designed to allow both the captured predator and prey species to be retained within the same pit and be sampled. The use of false-floors in pitfall traps as a tool to reduce predation is a novel approach and not something that has previously been reported in the literature. Greenslade (1964) briefly suggested that the predation of smaller Carabidae by larger species of Carabidae and birds can be avoided through the use of coarse gauze false-floors (4–5 mm gaps). However, this study did not explicitly test the efficacy of these gauze false-floors in reducing predation.
Illustration and photograph of wooden false-floors used in pitfall traps to reduce mammal predation on reptiles. Each false-floor has a wooden handle to assist with the removal of the floor from the pit when checking for animals captured underneath. Illustration includes (a) top view and (b) side view of the false-floor.
To investigate the potential of our wooden false-floor design to act as an effective barrier between small reptiles and small mammals in pitfall traps, we incorporated these devices into a long-term small-vertebrate monitoring program conducted at the Arid Recovery Reserve in northern South Australia. As part of this program, pitfall-trapping surveys were conducted annually between 1998 and 2021, with false-floors being added to pitfall traps in the last 3 years of sampling (2018–2021). Two criteria were used to assess the effectiveness of false-floors. These were defined as (1) the ability to exclude the majority (>70%) of key predatory small-mammal captures from the prey refuge underneath the false-floors, and (2) to significantly reduce the number of reptile predation incidents in pits.
Materials and methods
Study area
Arid Recovery is a 123 km2 fenced, conservation reserve situated in the arid north of South Australia (30°29′S, 136°53′E; Fig. 2). The landscape consists of sand dunes dominated by tall shrubs including Acacia and Dodonaea species, and interdunal areas characterised by clay swales, gibber plains and chenopod shrublands. The region’s climate is hot and dry, with mean summer maximum temperatures of 35.8°C, mean winter minimum temperatures of 4.9°C and long-term mean rainfall of 140 mm (1998–2021; Olympic Dam Aerodome Station 016096, http://www.bom.gov.au/climate/averages/tables/cw_016096.shtml). Regional rainfall is highly variable, with mean rainfall totals met in only 46% of years (1998–2021; Olympic Dam Aerodome Station 016096). Annual rainfall totals during the false-floor study period (2018–2021) were consistently below average at 51.2 mm in 2018, 39.2 mm in 2019, 160 mm in 2020, and 143.4 mm in 2021. Ctenophorus dragons, Ctenotus skinks, Diplodactyline geckoes, Pseudomys rodents and Sminthopsis dunnarts are the dominant reptiles and small mammals found within the study area (Moseby et al. 2009; Read et al. 2012; Read and Kearney 2016).
Survey design
The pitfall-trapping method used in this study has been outlined in detail in Moseby et al. (2009) and is summarised below. Survey sites were stratified according to habitat type (dune versus swale) and inside versus outside the fenced feral-free conservation area of the Arid Recovery Reserve. Survey sites within dune and swale environments were surveyed on alternating years (with dunes surveyed on even years and swales on odd years). The dune surveys consisted of 20 sites, including 12 within the feral-free conservation area and eight outside the fenced area. The swale surveys consisted of 19 sites, including 12 within the fenced reserve and seven outside the reserve. Each site consisted of two perpendicular pitfall lines, with approximately 50 m distance between each line. Each pitfall line included alternating large and small PVC pitfall traps 10 m apart (totalling six small and six large pits per survey site). Small pitfall traps had a diameter of 15 cm and were 45 cm deep, whereas large pitfall traps were 23 cm in diameter and 60 cm deep. A drift fence made of metal flymesh was erected along each of the pitfall lines to direct animals towards the pits. Insecticide powder (Coopex™) was used sparingly around the edges of pits, to reduce the risk of captured-animal mortality from invertebrate bites and stings (e.g., scorpion, centipede and ant attack). Each annual survey consisted of four consecutive trapping days/nights in March. Additional ad hoc pitfall surveys repeating the standard trapping method were undertaken in mid-April in 2018, 2019 and 2021 and early October in 2020.
Reptile mortalities (1998–2021)
Incidents of reptile mortality during pitfall-trapping surveys at Arid Recovery were recorded as part of Arid Recovery’s long-term dataset (1998–2021). Where possible, the cause of death was reported by survey participants (e.g., predation, ant attack, handling injuries). Predation by small mammals was recorded as the cause of death when mammals and reptiles were recorded in the same pit, when clear bite marks were observed on the reptile carcass, or when partial remains of a reptile carcass were found. In some instances, the species of small mammal involved in the predation event was also recorded.
False-floor trial
Large and small wooden false-floors (Fig. 1), with diameters of either 23.0 cm or 14.6 cm were built from 0.6 cm thick medium-density fibreboard to fit inside the two different pit diameters. When placed at the bottom of the pits, the three wooden supports on the base of the false-floors (1.8 cm high) created a small compartment underneath. This compartment was accessible only by animals small enough to fit through the notches (1.9 cm × 1.4 cm) cut into the sides of the boards, creating a potential barrier between small and large captured animals. An additional piece of wood on the top of the false-floor was used as a handle to lift it smoothly from the pitfall trap. In 2018, the false-floors were initially trialled in large pitfall traps only. In 2019 and 2021, false-floors were placed in half of the pitfall traps in each pitfall line, at sites both inside and outside the reserve (50% of large and small pits). In 2020, false-floors were installed in both the large and small pits inside the reserve where mammal-capture rates were expected to be higher. False-floors were implemented only in large pits outside the reserve in 2020, where fewer mammal captures and, consequently, a lower predation risk to reptiles, were expected. A small amount of bird seed was placed in all pits, including those with false-floors, to satiate mammal hunger and potentially reduce predation on reptiles (Read and Kearney 2016). A thin layer of sand was also added to all pits along with cardboard toilet rolls and small plastic tubes to provide additional prey refuges and shelter from the heat.
Data collection and statistical analyses
Pitfall traps were checked every morning and evening in accordance with the Guidelines for Vertebrate Surveys in South Australia (National Parks and Wildlife SA, Government of South Australia). The position of captured vertebrates relative to the false-floors (i.e. above or below) was recorded, as well as the number of live and dead animals in the pit (Fig. 3). Any evidence of a non-lethal injury was also recorded. Binomial proportion tests were used to create 95% confidence intervals for the proportion of captured vertebrates able to access the false-floor compartments. These tests were used to determine the effectiveness of false-floors in excluding small mammals from the compartments underneath or whether the position of captured animals above/below the false-floors was largely due to chance (P1 ≠ 0.5). Threshold values of P2 = 0.3 (for small mammals) and P2 = 0.7 (for small reptiles) were used to define the effectiveness of the false-floors, with the aim of excluding 70% of small-mammal captures from the compartment under false-floors, and having 70% of small-reptile captures able to utilise the compartment. Pearson’s chi-squared test was used on predation data to assess whether a capture bias was present for the three key reptile groups and mammal species of interest.
Photograph taken down a pitfall trap, showing two Pseudomys australis individuals and an Eremiascincus richardsonii co-occupying the pit. The skink is taking shelter within a plastic tube, while one of the rodents is taking shelter within a cardboard roll. Photograph by Nathan Beerkens.

The impact of false-floor presence and absence on opportunistic small-reptile predation by small mammals was assessed using Pearson’s chi-squared test. Outcomes from reptile–mammal pit co-occupancy were recorded as (a) reptile survival without injury, (b) reptile survival with injury or (c) reptile mortality. Because the number of pitfall traps with and without false-floors was not consistent between 2018 and 2021, a second chi-squared test with a binomial outcome of either reptile mortality or survivorship was applied to a subset of data collected in 2021. These data were collected during a year where 50% of all pitfall traps had false-floors present (n = 18).
Results
During the false-floor trial period (2018–2021), 1700 small-reptile and 900 small-mammal captures were recorded in the pitfall traps. Of these, 711 reptiles and 383 mammals were caught in pits with false-floors present, with 28 instances of reptile–mammal pit co-occupancy (Fig. 3). The details of these reptile–mammal encounters are outlined in Supplementary Table S1.
From 1998 to 2021, the small-mammal species captured during long-term monitoring included Leggadina forresti (2% of small mammal captures), Mus musculus (14% of small mammal captures), Notomys alexis (34%), Pseudomys australis (22%), P. bolami (18%), P. desertor (<1%), P. hermannsburgensis (<1%), Sminthopsis crassicaudata (5%), and S. macroura (5%). To assist with data interpretations and analyses (on account of low capture numbers), the smaller Pseudomys species P. bolami, P. desertor, and P. hermannsburgensis were grouped together and referred to as ‘small Pseudomys’. These species are generally <85 cm in length and <25 g in weight.
The most commonly captured small reptile species included Ctenophorus fordi, C. nuchalis, C. pictus, Tympanocryptis intima, T. tetraporophora, Diplodactylus conspicillatus, D. tessellatus, Gehyra purparescens, G. versicolor, Heteronotia binoei, Lucasium damaeum, L. stenodactylum, Nephrurus levis, Rhynchoedura eyrensis, R. ornata, Underwoodisaurus milii, Ctenotus leae, C. leonhardii, C. regius, C. schomburgkii, C. strauchii, C. taeniatus, Eremiascincus richardsonii, Lerista labialis, and Menetia greyii. Most of the small-reptile captures from 1998 to 2021 (Table 1) consisted of skinks (53%), followed by geckoes (29%), dragons (18%) and blind snakes (1%). The mean snout–vent length (SVL) of reptiles captured in pits with false-floors was similar among geckoes (49 mm ± 10.1), skinks (44 mm ± 15.1) and dragons (49 mm ± 14.4).
| Vertebrate group | All survey years (1998–2021) | Reptile–mammal pit co-occupancy (2018–2021) | ||||
|---|---|---|---|---|---|---|
| Pitfall captures (%) | Predation incidents (%) | Incidents of pit co-occupancy (%) | Predation incidents (%) | |||
| Prey species | Blind snakes | 1 | 0 | 0 | 0 | |
| Dragons | 18 | 2 | 14 | 9 | ||
| Geckoes | 29 | 48 | 50 | 55 | ||
| Skinks | 53 | 50 | 36 | 36 | ||
| Predatory species | Leggadina forresti | 2 | 0 | 4 | 0 | |
| Mus musculus | 14 | 26 | 11 | 9 | ||
| Notomys alexis | 34 | 11 | 32 | 27 | ||
| Pseudomys australis | 22 | 44 | 18 | 27 | ||
| Small Pseudomys | 18 | 0 | 0 | 0 | ||
| Sminthopsis sp. | 10 | 19 | 18 | 9 | ||
| Unidentified mammal sp. | NA | NA | 18 | 27 | ||
Reptile mortalities (1998–2021)
The opportunistic predation of small reptiles by small mammals caught within the same pitfall trap accounted for 25% of all pitfall-trap mortalities recorded between 1998 and 2021. Most of the reptile predation events (79%) occurred at sites inside the reserve rather than outside, and this corresponded with the higher mammal captures at these sites (82% of total mammal captures).
Over the entire study period (1998–2021), reptile predation was highest for skinks and geckoes (50 and 48% respectively, of small-reptile predation records; Table 1). Skinks and geckoes were also more frequently recorded within pits with small mammals than was any other reptile group (36 and 50% of co-occupancy records respectively). A chi-squared test comparing mortality rates with incidences of co-occupancy found no selection for skinks, dragons or geckoes (X2 = 0.42 and P-value = 0.81). A large portion of the reptile predation events (35%) recorded in the long-term study period (1998–2021) did not identify the mammal species that attacked the captured reptile. Of the records where the mammal species was recorded, P. australis was identified as the primary species implicated in reptile mortalities, accounting for 22% of small-mammal captures and 44% of small-reptile predation events. This was followed by M. musculus (14% of small-mammal captures and 26% of predation events) and the Sminthopsis species (10% of small-mammal captures and 19% of predation events). A chi-squared test comparing incidents of small-reptile predation with the number of captures found no selection for P. australis, M. musculus or Sminthopsis species (X2 = 0.14 and P-value = 0.93).
False-floor usage by mammals and reptiles (2018–2021)
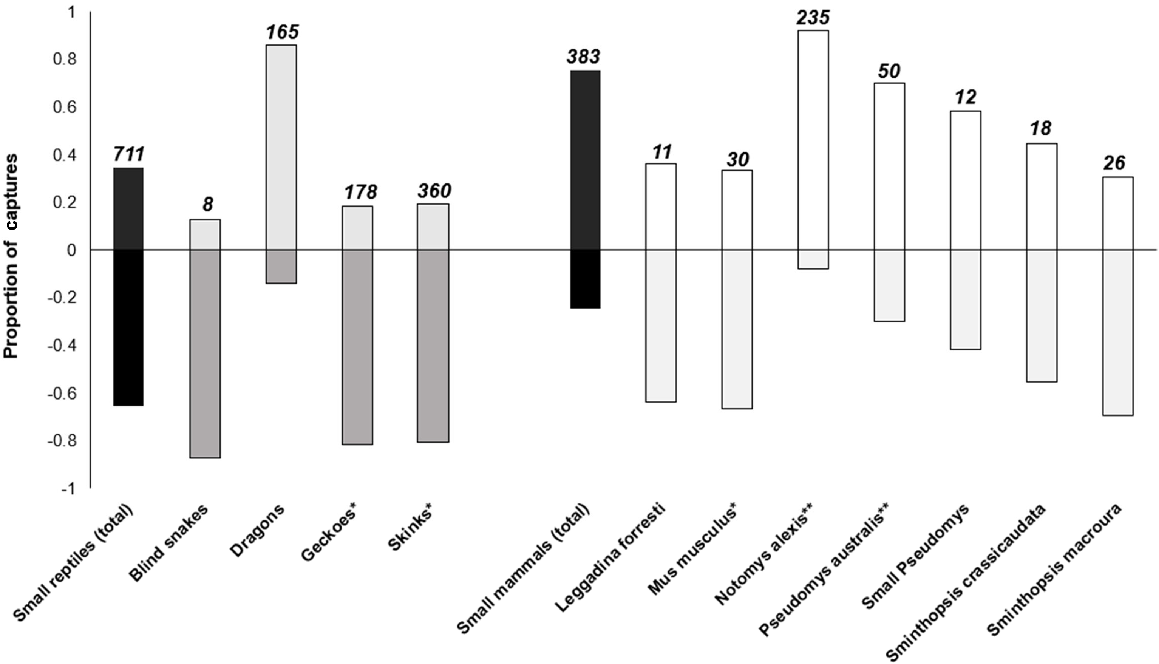
False-floors successfully separated mammal and reptile captures (Fig. 4, Table 2), with most of the small reptiles being found underneath the false-floors (66%), and most of the small mammals being observed above the false-floors (74%). The binomial proportion-test results indicated that the false-floors effectively prevented >70% of captured mammals from entering the compartment beneath them (P-value = 0.01). However, when considering mammal species separately, false-floors successfully excluded 92% of N. alexis captures (n = 235; P-value = 0.00 for P2 = 0.3) and 70% of P. australis captures (n = 50; P value = 0.56 for P2 = 0.3) from the false-floor compartment. However, a significant percentage of all other small mammal captures were able to squeeze through the notches and access the false-floor compartment (P-value > 0.05 for P2 = 0.3). For example, 67% of M. musculus captures, 42% of small Pseudomys captures and 64% Sminthopsis captures.
Proportion of captured vertebrates found above (positive values) and beneath false-floors (negative values) during pitfall surveys (2018–2021). Sample sizes are displayed above each corresponding bar. A single asterisk indicates the reptile groups that experienced the highest predation rates from small mammals during 1998–2021 surveys. Likewise, a single asterisk indicates the small mammals responsible for the greatest number of reptile predation events in pitfall traps in 1998–2021 (M. musculus and P. australis). A double asterisk indicates the small-mammal species that caused the greatest number of reptile predation incidents during the false-floor trials (2018–2021).
| Vertebrate group | n | Proportion below false-floors | 95% confidence interval (P2 = 0.5) | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| P2 = 0.5 | P2 = 0.7 | P2 = 0.3 | ||||||
| Small reptiles (total) | 711 | 0.66 | 0.62 | 0.69 | 0.00 | 1.00 | NA | |
| Blind snakes | 8 | 0.88 | 0.47 | 1.00 | 0.07 | 0.26 | NA | |
| Dragons | 165 | 0.14 | 0.09 | 0.20 | 0.00 | 1.00 | NA | |
| Geckoes | 178 | 0.81 | 0.75 | 0.87 | 0.00 | 0.00 | NA | |
| Skinks | 360 | 0.81 | 0.76 | 0.85 | 0.00 | 0.00 | NA | |
| Small mammals (total) | 383 | 0.25 | 0.21 | 0.29 | 0.00 | NA | 0.01 | |
| Leggadina forresti | 11 | 0.64 | 0.31 | 0.89 | 0.55 | NA | 1.00 | |
| Mus musculus | 30 | 0.67 | 0.47 | 0.83 | 0.10 | NA | 1.00 | |
| Notomys alexis | 235 | 0.08 | 0.05 | 0.12 | 0.00 | NA | 0.00 | |
| Pseudomys australis | 50 | 0.30 | 0.18 | 0.45 | 0.01 | NA | 0.57 | |
| Small Pseudomys | 12 | 0.42 | 0.15 | 0.72 | 0.77 | NA | 0.88 | |
| Sminthopsis crassicaudata | 18 | 0.56 | 0.31 | 0.78 | 0.82 | NA | 0.99 | |
| Sminthopsis macroura | 26 | 0.69 | 0.48 | 0.86 | 0.08 | NA | 1.00 | |
Binomial proportion tests were used to assess the effectiveness of false-floors in excluding mammals from the prey refuge underneath, while still being accessible by reptiles (Ha = the true probability of success for small mammals is less than 0.3 and greater than 0.7 for small reptiles). NA = not applicable.
The majority of skink and gecko captures used the false-floor prey refuge (P-value = 0.00 for P2 = 70). However, the proportion of reptiles found under the false-floors varied among taxa (Fig. 4, Table 2). In total, 80% of blind snakes (n = 8), geckoes (n = 178) and skink captures (n = 360) were found under the false-floors, compared with 14% of dragon captures (n = 165). Table S2 outlines the proportion of captured individuals of each small reptile and mammal species found underneath the false-floors.
False-floors and reptile predation (2018–2021)
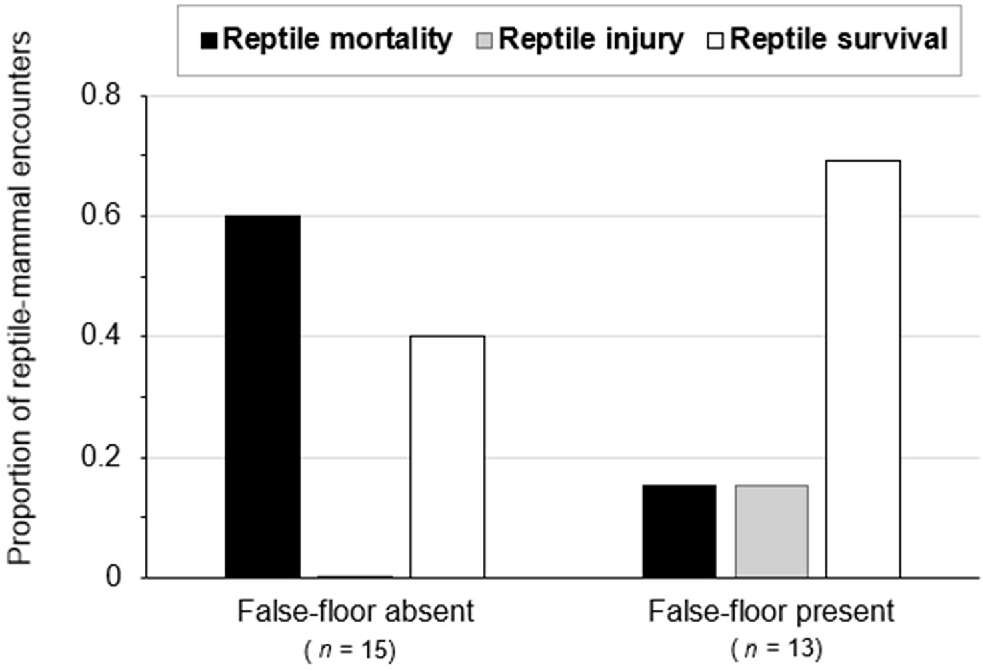
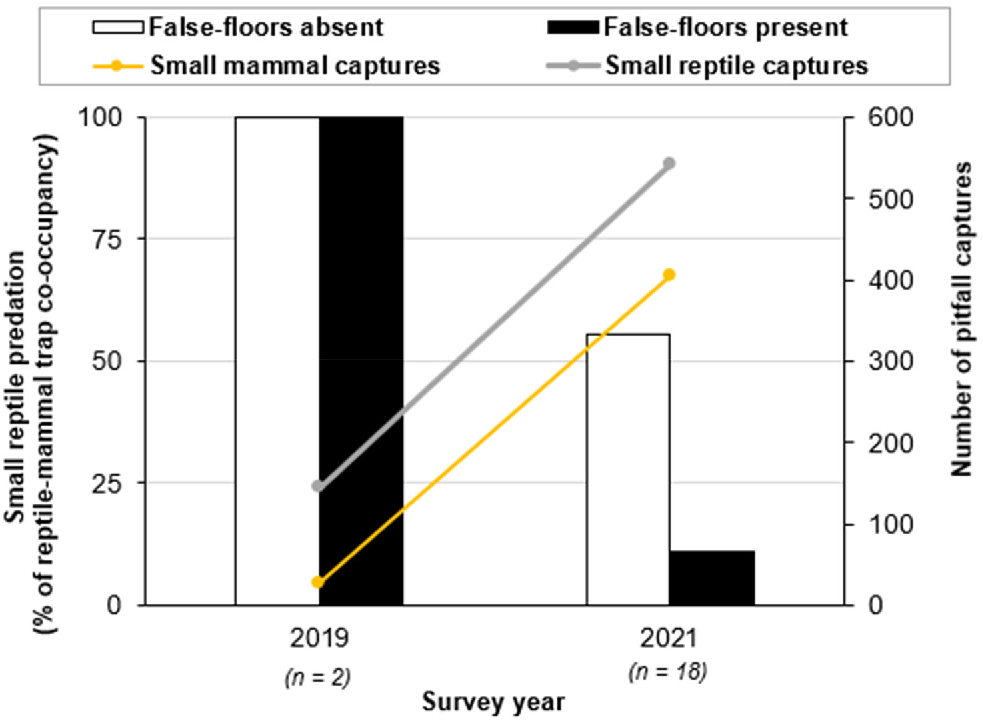
False-floors had a statistically significant impact on the outcome of reptile–mammal co-occupancy within pitfall traps (n = 28, X2 = 6.0, P-value = 0.05), with significantly lower incidents of reptile predation by small mammals in pits with false-floors (15%) than in those without (60%; Fig. 5). Likewise, false-floors reduced incidents of reptile mortality in 2021 (n = 18, X2 = 4.0 and P-value = 0.05), when the numbers of small-mammal and reptile captures were particularly high following a wetter year (Fig. 6). In 2021, predation on reptiles was much lower in pits with false-floors (11%) than in those without (56%).
The outcomes of reptile–mammal co-occupancy in pitfall traps with and without false-floors (2018–2021). The proportion of incidents ending in reptile survival without injury is indicated with open bars. Incidents of survival with injury are shown with grey bars, and incidents of mortality are shown with black bars. False-floors had a statistically significant impact on the outcome of reptile–mammal co-occupancy in survey pits (X2 = 6.0, P-value = 0.05).
The percentage of reptile–mammal co-occupancy incidents in pitfalls resulting in reptile mortality (from predation), comparing traps with and without false-floors. Two instances of reptile–mammal pit co-occupancy were recorded in 2019 (n = 2), and 18 instances were recorded in 2021 (n = 18). A chi-squared test was applied to the 2021 dataset to assess the effectiveness of false-floors in reducing reptile mortality (X2 = 4.0, P-value = 0.05).
Issues noted with the use of false-floors
Although not directly measured, it was noted that the use of false-floors increased the time taken to set, check and reset each trap. Additionally, it was observed that there was an increased risk of captured animals experiencing crush injuries when inexperienced survey participants checked pitfall traps. This was due to participants accidentally crushing reptiles between the sides of the false-floor and the PVC pitfall trap when removing false-floors. This issue could be mitigated by the installation of stronger handles in the centre of the false-floor and additional training of participants.
Discussion
Reptile mortalities (1998–2021)
Overall, 25% of small reptile moralities were due to predation by small mammals caught in the same trap. This suggests that providing seeds as an alternative food source for captured mammals and tubing as a prey refuge are insufficient predation-mitigation measures. Skinks and geckoes had the greatest risk of opportunistic predation by small mammals, making up 50 and 48% of reptile predation records, respectively (1998–2021). In contrast, dragons only accounted for 2% of observed predation events. The higher number of predation incidents experienced by skinks and geckoes than dragons may be due to the timing of pit captures. Skinks and geckoes were predominantly caught in the late afternoon and at night, which meant they were often left in pitfall traps overnight and removed during the morning pit-checking sessions. Consequently, skinks and geckoes were more likely to be caught in a pitfall trap along with nocturnal mammals (86% of pit co-occupancy records).
During the long-term surveys (1998–2021), P. australis and M. musculus were identified as the small-mammal species that were most likely to opportunistically or defensively attack small reptiles caught within the same pitfall trap. They accounted for 44 and 26% of small-reptile predation events respectively, and 22 and 14% of small-mammal captures. However, during the false-floor trials (2018–2021), M. musculus contributed to less reptile predation (9%) than did P. australis (27%) and N. alexis (27%). Incidents of pit co-occupancy with reptiles during this period was relatively similar among P. australis, M. musculus, and Sminthopsis sp. (18, 11 and 18%, respectively), whereas incidents of co-occupancy were highest for N. alexis (32%). During the false-floor trial, N. alexis and P. australis were the main cause of reptile predation, each accounting for 27% of predation incidents. Therefore, to be considered effective, false-floors must be designed in a way that maximises the exclusion of N. alexis, P. australis and, to a lesser extent M. musculus, as the key small-reptile predatory species.
False-floor use by mammals and reptiles (2018–2021)
The position of captured vertebrates above and below false-floors was used as an indicator of their ability and proclivity to fit through the notches in the false-floor and access the compartment underneath (prey refuge). This assessment was used to evaluate the effectiveness of false-floors at creating a barrier between captured prey (small reptiles) and the species that were likely to injure or kill reptiles (small mammals). The majority (81%) of captured geckoes and skinks were found underneath the false-floors, indicating that these refuges are easily accessible by the reptile species at greatest risk of predation. In contrast, only 14% of dragon captures were found underneath false-floors. This difference in position above and below false-floors is unlikely to be the result of differences in reptile size, as mean SVL of individuals captured in pitfall traps with false-floors was similar among all three reptile groups (44 mm for skinks and 49 mm for geckoes and dragons). The difference in false-floor usage may be the result of difference in body shape or behavioural characteristics of the dragons. For example, agamids typically prefer higher temperatures of 41.6–49.5°C, than do scincids, which typically prefer temperatures of 36.3–46.3°C (Greer 1989), and may therefore be less inclined to use the false-floors as shade.
The majority of small-mammal captures (74%) did not access the compartment under the false-floors, although this result was heavily influenced by the high proportion of captures of the two larger species, N. alexis (34% of small-mammal captures) and P. australis (22% of captures). Most N. alexis and P. australis captures (92 and 70% respectively) were found above false-floors, suggesting that they were too large to squeeze through the false-floor notches and access the prey refuge beneath. However, smaller predator species such as M. musculus, another key predatory species, along with other small-mammal species (small Pseudomys and Sminthopsis species) were readily able to access the compartment underneath false-floors (>30% of captures from these species were found underneath false-floors). False-floors are therefore likely to have considerable potential for reducing small-reptile predation from large rodents, but adjustments to the false-floor design (e.g., notch size and shape) are required to improve their capacity to reduce predation by smaller mammal species. There is a trade-off between reducing the size of the notches to prevent small mammals from accessing the refuge under the false-floors and also restricting the size of reptiles that can access the refuge. Interestingly, evidence of captured mammals chewing around false-floor notches was observed in some instances, which would have aided mammal access to the compartments below. Further research is required to determine the optimal size of the notches needed to maximise small reptile access and minimise small-mammal access. However, given that the larger rodents (N. alexis and P. australis) accounted for the majority of small-reptile predation incidents in 2018–2021 (54% collectively), the current false-floor design should suffice in reducing reptile mortality during pitfall surveys at Arid Recovery.
False-floors and reptile predation (2018–2021)
The use of false-floors improved reptile survival rates, with fewer reptile-predation incidents being recorded when false-floors were present (15% of reptile–mammal pit co-occupancy) than when they were absent (60% of pit co-occupancy). Likewise, in 2021 when capture rates of reptiles and small mammals were highest, reptile mortality in pits co-occupied by mammals was lower when false-floors were present (11%) than when they were absent (56%). This suggests that the false-floors can provide a sufficient barrier between captured individuals and reduce opportunities for reptile predation. There is a potential under-representation of predation events in our dataset because predation was documented only when evidence was found in the pit, such as dead reptile remains. Situations where the prey animal was entirely consumed could not be recorded. We also acknowledge another potential bias in our data, whereby only reptile-predation events that resulted in mortality were recorded. A few instances of reptile survivorship during pit co-occupancy with mammals may have been undocumented and this highlights the importance of recording animal mortality and sublethal injury as part of wildlife surveys.
One limitation of the study was the low number of recorded instances of co-occupancy (n = 20) during the 2 years when false-floors were utilised in 50% of pits (2019 and 2021). This not only reduced the sample size to 20, but limited the statistical comparisons that could be made. Unfortunately, the 2019 survey followed a very dry year, with only 51.2 mm total rainfall in 2018. Consequently, both small mammal and reptile abundances were low, with only 26 mammal and 145 reptile captures, resulting in only two instances of pit co-occupancy being recorded. In contrast, the 2021 survey followed a wetter year, with a total rainfall of 160 mm in 2020. This led to a large increase in small-mammal and reptile captures, with 404 small-mammal and 541 small-reptile captures in 2021. Consequently, 18 instances of reptile–mammal pit co-occupancy were recorded that year.
One drawback in using false-floors during pitfall surveys is the increased time taken to check pits, which raises ethical concerns, particularly during hot weather. Additionally, there was a risk of inexperienced trap-checkers accidentally crushing animals between the false-floor and pitfall trap wall. These are important observations when considering overall animal-welfare outcomes, and must be factored into the planning of any survey in which this method is being considered. On balance, we recommend using false-floors only in situations where the abundance of large rodents is unusually high, such as within predator-free safe havens in arid regions after significant rainfall events.
False-floors are likely to afford animals some additional thermal protection from extreme heat and cold. This effect was not tested in this study, but may merit investigation, given the risk of heat stress and desiccation to animals captured within pitfall traps (Read and Kearney 2016). Further adjustments to the design of false-floors are recommended to improve their capacity to act as a barrier between captured reptiles and smaller mammals (e.g., Mus musculus).
Data availability
The data that support this study are available in the article and accompanying online supplementary material.
Acknowledgements
The authors acknowledge the Kokatha people as the traditional custodians of the lands on which this research was conducted, and pay their respects to elders past, present and emerging. The authors thank David Paton and Steve Delean from the University of Adelaide for access to the pitfall data collected by second-year university students as part of their animal-identification course. We also thank all the volunteers and researchers who have assisted with the collection of survey data used in this paper. The research was supported by Arid Recovery, a joint conservation initiative between BHP, The University of Adelaide, SA Department for Environment and Water, Bush Heritage Australia and the local community, and the University of Adelaide’s PhD Industry Engagement Program.
References
Aubry K, Stringer A (2000) Field test of the SMED, a small mammal escape device for pitfall trapping amphibians. Northwestern Naturalist 81, 69.
| Google Scholar |
Edwards KE, Jones JC (2014) Trapping efficiency and associated mortality of incidentally captured small mammals during herpetofaunal surveys of temporary wetlands. Wildlife Society Bulletin 38, 530-535.
| Crossref | Google Scholar |
Ferguson AW, Forstner MR (2006) A device for excluding predators from pitfall traps. Herpetological Review 37, 316-317.
| Google Scholar |
Greenslade PJM (1964) Pitfall trapping as a method for studying populations of Carabidae (Coleoptera). The Journal of Animal Ecology 301-310.
| Crossref | Google Scholar |
Hoffmann A, Decher J, Rovero F, Schaer J, Voigt C, Wibbelt G (2010) Field methods and techniques for monitoring mammals. In ‘Manual on field recording techniques and protocols for all taxa biodiversity inventories. Vol. 8’. (Eds J Degreef, C Häuser, JC Mohje, Y Samyn, VD Spiegel) pp. 482–529. (Abc Taxa)
Jenkins CL, McGarigal K, Gamble R (2003) Comparative effectiveness of two trapping techniques for surveying the abundance and diversity of reptiles and amphibians along drift fence arrays. Herptological Review 34, 39-42.
| Google Scholar |
Karraker NE (2001) String theory: reducing mortality of mammals in pitfall traps. Wildlife Society Bulletin 29(4), 1158-1162.
| Google Scholar |
Legge S, Ringma J, Bode M, Radford J, Woinarski J, Mitchell N, Wintle B (2019) Protecting Australian mammals from introduced cats and foxes: the current status and future growth of predator-free havens. Technical report. (Threatened Species Recovery Hub, National Environmental Science Programme) Available at https://www.Nespthreatenedspecies.Edu.Au/media/h2onftaz/safe-havens-report_web.Pdf
Moseby KE, Hill BM, Read JL (2009) Arid recovery: a comparison of reptile and small mammal populations inside and outside a large rabbit, cat and fox-proof exclosure in arid South Australia. Austral Ecology 34, 156-169.
| Crossref | Google Scholar |
Petit S, Waudby HP (2013) Standard Operating Procedures for aluminium box, wire cage, and pitfall trapping, handling, and temporary housing of small wild rodents and marsupials. Australian Journal of Zoology 60, 392-401.
| Crossref | Google Scholar |
Powell RA, Proulx G (2003) Trapping and marking terrestrial mammals for research: integrating ethics, performance criteria, techniques, and common sense. ILAR Journal 44, 259-276.
| Crossref | Google Scholar | PubMed |
Read JL, Cunningham R (2010) Relative impacts of cattle grazing and feral animals on an Australian arid zone reptile and small mammal assemblage. Austral Ecology 35, 314-324.
| Crossref | Google Scholar |
Read JL, Kearney MR (2016) Too hot to handle? Balancing increased trapability with capture mortality in hot weather pitfall trapping. Austral Ecology 41, 918-926.
| Crossref | Google Scholar |
Read JL, Kovac K-J, Brook BW, Fordham DA (2012) Booming during a bust: asynchronous population responses of arid zone lizards to climatic variables. Acta Oecologica 40, 51-61.
| Crossref | Google Scholar |
Read JL, Ward MJ, Moseby KE (2015) Factors that influence trap success of sandhill dunnarts (Sminthopsis psammophila) and other small mammals in Triodia dunefields of South Australia. Australian Mammalogy 37, 212-218.
| Crossref | Google Scholar |
Read JL, Pedler RD, Kearney MR (2018) Too much hot air? Informing ethical trapping in hot, dry environments. Wildlife Research 45, 16-30.
| Crossref | Google Scholar |
Stephens RB, Anderson EM (2014) Effects of trap type on small mammal richness, diversity, and mortality. Wildlife Society Bulletin 38, 619-627.
| Crossref | Google Scholar |
Thompson GG, Withers PC, Pianka ER, Thompson SA (2003) Assessing biodiversity with species accumulation curves; inventories of small reptiles by pit-trapping in western Australia. Austral Ecology 28, 361-383.
| Crossref | Google Scholar |
Waudby HP, Petit S, Gill MJ (2019) The scientific, financial and ethical implications of three common wildlife-trapping designs. Wildlife Research 46, 690-700.
| Crossref | Google Scholar |
Yunger JA, Brewer R, Snook RR (1992) A method for decreasing trap mortality of sovex. Canadian Field-Naturalist 106, 249-251.
| Crossref | Google Scholar |