Evaluation of lion (Panthera leo) scat as a wild dog (Lycaon pictus) deterrent on game farms
Ronja D. Haring A B * , Grant Beverley B , Peter N. Thompson
A B * , Grant Beverley B , Peter N. Thompson  A C , Andrew Taylor A D and Jacques H. O’Dell A C
A C , Andrew Taylor A D and Jacques H. O’Dell A C
A Department of Production Animal Studies, Faculty of Veterinary Science, University of Pretoria, Pretoria, South Africa.
B Carnivore Conservation Programme, Endangered Wildlife Trust, Midrand, South Africa.
C Centre for Veterinary Wildlife Research, University of Pretoria, Pretoria, South Africa.
D Wildlife in Trade Programme, Endangered Wildlife Trust, Midrand, South Africa.
Wildlife Research 50(12) 1021-1030 https://doi.org/10.1071/WR22084
Submitted: 17 May 2022 Accepted: 7 January 2023 Published: 31 January 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing
Abstract
Context: The conservation of the Endangered African wild dog (Lycaon pictus) poses a major challenge to conservationists because outside the boundaries of protected areas, wild dogs are prone to conflict with farmers. Mitigation measures appropriate for game farmers are scarce, leaving them with limited options to reduce wild dog impact. As a result, targeted persecution is a common occurrence. However, wild dogs are subject to intraguild competition with dominant competitors, often resulting in their suppression and spatial displacement. Therefore, olfactory cues of lion presence may trigger an adverse reaction in wild dogs, and could be a means to manage wild dog movements across the landscape to prevent conflict with farmers.
Aim: We aimed to evaluate whether wild dogs can be deterred by simulating lion presence.
Methods: By using translocated scent cues in the form of lion scat deployed along the perimeter of plots, lion presence was simulated on game farms where lions were absent. The rate and duration of incursions by wild dogs, collared with GPS trackers, into control and treatment plots (‘group’) were evaluated.
Key results: Wild dog incursion rate dropped by 55.5%, and duration of incursion events dropped by 72.7%, after lion scat was deposited. Control and treatment plots were equally affected with no significant effect of the grouping on wild dog movement. The magnitude of the treatment effect differed between packs.
Conclusion: The significant decline of wild dog movement after implementation of treatment suggests a deterrence effect. The insignificant effect of group on wild dog movement indicates large-scale avoidance triggered by a change in the wild dogs’ risk perception across the landscape following treatment. The fact that the magnitude of the treatment effect differed between packs indicates that the response to predator cues is likely to be context-dependent.
Implications: The findings present a novel approach to managing free-roaming wild dogs by utilising biologically relevant cues, which may benefit wild dog conservation. There is a need for further research to develop the emerging field of scent studies to provide non-lethal solutions and progress towards evidence-based large carnivore management practices.
Keywords: antipredator behaviour, conservation ecology, human–carnivore conflict, interspecific olfactory communication, landscape of fear, non-lethal mitigation measures, odour deterrent, perceived risk.
Introduction
South Africa is home to the ‘Endangered’ African wild dog (Lycaon pictus), where a free-roaming population comprising 20% of the country’s animals occurs outside of protected areas (mean 79 ± 18 (x ± s.d.) adults and yearlings) (Nicholson et al. 2020). This free-roaming population is an important stronghold for the species but is prone to anthropogenic mortality on private land. An estimated 39% of free-roaming wild dogs are killed through direct persecution that results from human–wild dog conflict (Davies-Mostert et al. 2016). Because anthropogenic mortality is additive to natural mortality, it can undermine the viability of the free-roaming population (Woodroffe et al. 2007). For populations of rare carnivores where conservation relies on the protection of individuals and groups as an intact unit, losses can be especially devastating, with impacts on the stability and persistence of social units, reproduction, genetic diversity and overall mortality (Haber 1996). For example, when an alpha female dies, packs commonly disintegrate, with no breeding taking place until a new pack is formed (Woodroffe and Sillero-Zubiri 2020). In addition, the viability of source populations is compromised if ecological traps outside of protected areas drain the source population of individuals (van der Meer et al. 2013). Consequently, hostility from landowners has led to drastic population declines in the past (Woodroffe and Ginsberg 1999) and, if ongoing, can have substantial impacts on species persistence (van der Meer et al. 2013).
Although mitigation measures that address the impacts of large carnivores on livestock farmers are well explored, the potential to mitigate the impact of large carnivores on game farms has received less attention (Shivik 2006). Game farms keep, breed and raise wildlife in sizable fenced systems for commercial purposes. With the rise of the game farming industry, game is increasingly being used for economic gain, and when consumptive wildlife utilisation dominates land use, game farmers tend to express negative attitudes towards wild dogs (Lindsey et al. 2005). Unlike livestock, game animals cannot easily be herded, rendering most mitigation measures recommended in the literature ineffective for game farmers (Thorn et al. 2015). This limits the farmer’s options to reduce wild dog impact, leading to more killing of carnivores (Fink et al. 2020).
Experimental studies under real world conditions that provide evidence of the effectiveness of non-lethal mitigation measures are scarce (Eklund et al. 2017), and manipulations of behaviour have rarely been applied to the conservation of free-roaming wildlife (Linklater 2004). The lack of scientific evidence that supports non-lethal solutions impedes progress towards evidence-based large carnivore management practices (Eklund et al. 2017) and undermines farmers’ trust in non-lethal mitigation measures (Young et al. 2019).
Wild dogs are subordinate predators and subject to intraguild interactions, involving exploitative and interference competition with dominant competitors such as lions (Panthera leo; Creel and Creel 1998; Hayward and Kerley 2008). In the Kruger National Park, South Africa, lions account for 39% of pup and at least 36% of adult deaths (Van Heerden et al. 1995), making them the single most important cause of natural mortality (Woodroffe and Ginsberg 1999). Consequently, wild dogs actively avoid lions (Webster et al. 2012) and are displaced from areas where lions are abundant (Swanson et al. 2014). Even when no wild dogs have been killed, packs actively avoid areas with suspected or known presence of lions by making use of indirect cues to asses risk (Webster et al. 2012). Previous research on cheetahs (Acinonyx jubatus) and other mesopredators has revealed that subordinate carnivores are able to avoid direct interactions with dominant competitors by using scent cues (Haswell et al. 2018; Cornhill and Kerley 2020). Olfaction is an exceptionally important and well developed sense in wild dogs (Green et al. 2012), suggesting olfactory cues might be used to assess predation risk. Odour sources that indirectly advertise lion presence might act as biologically relevant cues to wild dogs, and could be used to create a landscape of fear by altering the wild dogs’ perception of risk across space. Therefore, lion scat might hold great potential to function as a conservation tool by modifying wild dog movements across the landscape.
We investigated the effect of lion scat on wild dog movements by evaluating wild dog visitation, i.e. rate and duration of incursions, to plots whose perimeter was treated with lion scat. We hypothesised that wild dogs would be strongly averse to olfactory cues of lion presence, resulting in a reduced rate and duration of incursions after lion scat deployment.
To allow for the possibility that lion scat placement may have a wider influence on wild dog movement patterns beyond just the treatment plots, the effect of the lion scat was investigated for both treatment and control plots.
Materials and methods
Study population and area
The study was conducted on private farms and reserves within the Limpopo Province of South Africa (Fig. 1). Collared wild dog packs with an established home range in an accessible area outside of protected reserves were considered for this study. One pack, consisting of seven adult dogs, ranged freely in the Lowveld Bushveld between Acornhoek and Hoedspruit of the Mopani district (−24.541389, 31.091976). Another pack of two adult dogs occurred within the boundaries of the Mapesu Private Game Reserve (MPGR) in the Mopane Bushveld of the Vhembe district (−22.290184, 29.492373).
|
|
At both study sites, rainfall is strongly seasonal, with pronounced rainfall in the summer months between October and April (Venter et al. 2003; Rutherford et al. 2006). On average, annual rainfall varies between 500 and 700 mm in the Lowveld Bushveld (MacFadyen et al. 2018) and 300 to 400 mm in the Mopane Bushveld (Rutherford et al. 2006). Mean temperatures are generally warm all year round (Venter et al. 2003; Rutherford et al. 2006), ranging between 17.1°C and 26.3°C. The uplands of the Lowveld Bushveld are dominated by tall shrublands with Terminalia and Combretum species, whereas the bottomlands consist of dense thickets to open savannas with Senegalia nigrescens, Dichrostachys cinerea and Grewia bicolor being prominent. The Mopane Bushveld is mainly characterised by open woodland to moderately closed shrubland dominated by Colophospermum mopane (Rutherford et al. 2006).
Wild dog packs were exposed to a rich faunal assemblage at both sites, including common antelopes, megaherbivores and large predators (e.g. cheetah, leopard (Panthera pardus) and spotted hyena (Crocuta crocuta)). However, no lions occurred on the MPGR nor on any other property included in this study. The MPGR was enclosed by well-maintained ‘predator-proof’ perimeter fencing, which mostly contains the large mammals.
Pre-experimental stage
Spatial data on wild dog movements were gathered to define the area that was occupied by wild dogs. The data were derived from dogs collared with GPS trackers. One individual per pack had a GPS collar, and the movement of that individual was taken to represent the movement of the entire pack. Wild dogs are highly cohesive and move as a unit (Creel and Creel 1995). No wild dogs were specifically collared for the purpose of this study; instead, wild dogs had already been collared by the Endangered Wildlife Trust (EWT) and associated organisations (i.e. the Mapesu Private Game Reserve). Collars provided four to six GPS fixes per day at varying intervals, providing an adequate sampling frequency for a mobile species that can cover large distances daily (Pretorius et al. 2019). Two to eight weeks of movement data were used to calculate home ranges (95% isopleth) for each site prior to the experiment, after which the privately owned land within the home range was subdivided into plots. The plots were created based on linear features such as roads, rivers and fences. The size of the plots varied between 0.4 km2 and 2.65 km2, averaging ~1.20 km2, which is large enough to capture location data points but small enough to be logistically feasible. Sample plots were selected randomly and assigned to either the control or the treatment group. The group allocation of the first plot was random, after which the allocation of all following plots alternated from the first plot. To investigate the scale of the treatment effect and create spacing, control and treatment plots did not share a boundary. If the random selection of a plot would have led to a common boundary between control and treatment plots, the plot was skipped without replacement to avoid excessive clustering of plots within groups. In the event that plots assigned as treatment plots could not be used as such due to ethical considerations overruling the study design or issues of access, they persisted as potential control plots. The number of control and treatment plots was equal for all sites. Each site had five treatment and five control plots, which were separated by at least one plot.
Experimental stage
Collar data were used to determine the number of times wild dogs entered a plot (incursion events) and the amount of time they spent within the plot on each occasion, with ‘duration’ being defined as the number of consecutive GPS fixes received during incursion events. This was investigated for both the pre-test phase and the test phase. Between mid-April and the end of September ‘21, covering the denning season, each plot was monitored for four to 10 consecutive weeks during both phases. The monitoring time was determined by external factors (e.g. availability of data relating to the access to plots).
During the test phase, a natural scent barrier was created for the treatment plots by placing lion scat along the inner perimeter of the linear feature lining the plot. The lion scat was collected from wildlife sanctuaries up to twice a week and frozen (−14.5 to −20°C) inside sealed plastic containers to retain freshness until the implementation of the experiment. To create a uniform scent note, the frozen scat was pooled to allow scats of different ages to mix. The samples were defrosted 1 day before use. Along the perimeter of the treatment plots, 110 g (±5 g) of lion scat was placed every 100 m. Before placement, samples were soaked in 50 mL of water to reinforce the odour by adding moisture. The scat was replaced two to five times at 10-day intervals. Control plots neither received treatment (scat) nor were their perimeters patrolled by vehicle.
Data preparation and analysis
RStudio (ver. 3.5.3, R Core Team 2020) was used to conduct a kernel density estimation to identify the home ranges (95% isopleth) of each pack, employing the adehabitatHR package (Calenge 2006). To calculate the kernel isopleths, the reference smoothing factor href was applied, which had performed reliably in past home range calculations for wild dogs (Mbizah et al. 2014). Quantum GIS (ver. 3.14, QGIS Development Team 2021) was used to visualise estimated home ranges and if the home range included areas that were disconnected by hard boundaries (e.g. rivers), and preliminary data had shown that these areas were not utilised by the wild dogs, the home range was clipped to the hard boundary (effective home range).
A generalised linear mixed model with a Poisson distribution and a log link was conducted in RStudio, using the lme4 package (Bates et al. 2015), to compare the rate and duration of incursions between groups (control vs treatment plots) within each phase, before and during the deployment of scat (pre-test and test phase), and between phases (pre-test vs test phase) within each group (treatment and control plots). The dependent variable was (a) the count of incursion events, and (b) the count of consecutive GPS fixes during incursion events. To adjust for the variation in the amount of opportunity that existed for each event (differences in the number of GPS fixes per day between collars and in observation days between and within phases), the natural logarithm of ‘exposure’ (GPS fixes per day multiplied by observation days) was included as an offset variable. Additionally, pack was included as a fixed effect because the two packs represented wild dogs in specific contexts, with context-specific factors likely to influence their movements and their response to lion scat. The interactions between phase and pack as well as between pack and group were added. Plots were sampled twice, therefore plot ID was included as a random factor.
A preselection of variables was conducted to construct a global model. Each explanatory variable was analysed separately to determine its effect on the dependent variable. Except for the main interaction between group and phase, and the variables of particular interest (group, phase and pack), those variables not correlated with the dependent variable (P > 0.25) were excluded from further analysis (Bendel and Afifi 1977). The optimal model was then constructed based on the procedure outlined in Zuur et al. (2009), evaluating the retained parameters in a backward stepwise manner by using the drop1 command. Statistical significance was assessed at P < 0.05.
Employing the emmeans package (Lenth 2022), contrasts of marginal linear predictions were calculated to allow for the pairwise comparison of group means. The conditional r-squared value for mixed effects models with complex random effects structures was estimated by making use of the performance package (Lüdecke et al. 2021).
Results
The Lowveld pack was sampled twice because it moved to a different area after data collection had been completed at the first site. The pack had crossed a hard boundary that provided a clear cut between sites because the pack was discouraged from crossing during everyday activities. The effective home range of the Lowveld pack spanned 35.60 km2 at the first site and 64.04 km2 at the second site, totalling 99.64 km2. The Mapesu pack covered 71.76 km2. In total, 20 plots (ncontrol = 10; ntreatment = 10) were included across sites (Fig. 2).
Rate of incursions
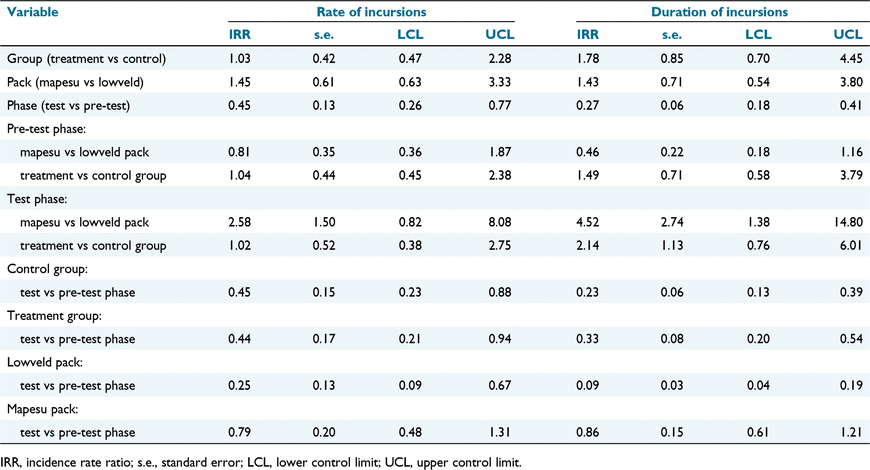
The number of incursions per group per phase averaged 2.30 ± 2.79 (x ± s.d.) over the study period. The rate of incursion was best explained by a model (Table 1) containing the variables pack (P = 0.382), group (P = 0.937) and phase (P = 0.004), as well as the interactions between phase and pack (P = 0.041) and phase and group (P = 0.972). The conditional r-squared value for the model was 0.609.
|
Neither pack (Mapesu vs Lowveld) nor group (treatment vs control) were associated with the rate of incursion (incidence rate ratio [IRR] ± s.e.: 1.45 ± 0.61, 95% CI [0.63, 3.33], P = 0.381; IRR: 1.03 ± 0.42, 95% CI [0.47, 2.28], P = 0.937), and within phases the rate of an incursions did not differ significantly between packs (pre-test phase: IRR: 0.81 ± 0.35, 95% CI [0.36, 1.87], P = 0.627; test phase: IRR: 2.58 ± 1.50, 95% CI [0.82, 8.08], P = 0.104) or between groups (pre-test phase: IRR: 1.04 ± 0.44, 95% CI [0.45, 2.38], P = 0.925; test phase: IRR: 1.02 ± 0.52, 95% CI [0.38, 2.75], P = 0.963).
Phase (test vs pre-test) had a significant effect on incursion rate. During the test phase, the incursion rate was 45% of what it was during the pre-test phase (0.45 ± 0.13, 95% CI [0.26, 0.77], P = 0.004). This effect was apparent across packs and groups. In both groups the incursion rate dropped significantly between phases and to a similar extent (Fig. 3). In the control plots, the incursion rate in the test phase was 45% of what it was in the pre-test phase (IRR: 0.45 ± 0.15, 95% CI [0.23, 0.88], P = 0.019). Similarly, the incursion rate into the treatment plots in the test phase was 44% of what it was in the pre-test phase (IRR: 0.44 ± 0.17, 95% CI [0.21, 0.94], P = 0.033). Both packs reduced their incursion rate during the test phase (Fig. 4), however, dropping by 75%; the incursion rate of the Lowveld pack (IRR: 0.25 ± 0.13, 95% CI [0.09, 0.67], P = 0.006) decreased much more than that of the Mapesu pack, where the reduction was 21% and non-significant (IRR: 0.79 ± 0.20, 95% CI [0.48, 1.31], P = 0.367).
|
|
|
|
Duration of incursions
The number of GPS fixes per group per phase averaged 5.5 ± 7.77 (x ± s.d.) over the study period. The duration of incursion events was best explained by a model (Table 1) containing the variables pack (P = 0.468), group (P = 0.225) and phase (P < 0.0001), as well as the interactions between phase and pack (P < 0.0001) and phase and group (P = 0.268). The conditional r-squared value for the model was 0.881.
Neither pack (Mapesu vs Lowveld) nor group (treatment vs control) were associated with the duration of incursion events (IRR: 1.43 ± 0.71, 95% CI [0.54, 3.80], P = 0.468; IRR: 1.78 ± 0.85, 95% CI [0.70, 4.45], P = 0.225). However, within the test phase, the packs differed significantly from each other. The time the Mapesu pack spent during incursions was 4.52 times more than the time that was spent by the Lowveld pack (IRR: 4.52 ± 2.74, 95% CI [1.38, 14.80], P = 0.013). Incursion duration did not differ between groups within phases (pre-test phase: IRR: 1.49 ± 0.71, 95% CI [0.58, 3.79], P = 0.258; test phase: IRR: 2.14 ± 1.13, 95% CI [0.76, 6.01], P = 0.151).
Phase (test vs pre-test) had a significant effect on incursion duration, which dropped by 73% during the test phase (IRR: 0.27 ± 0.06, 95% CI [0.18, 0.41], P < 0.0001). This decrease was apparent across both groups (Fig. 5) and packs (Fig. 6). In the control group, the incursion duration in the test phase was 23% of what it was in the pre-test phase (IRR: 0.23 ± 0.06, 95% CI [0.13, 0.39], P < 0.0001). Similarly, the incursion duration of the treatment group in the test phase was 33% of what it was in the pre-test phase (IRR: 0.33 ± 0.08, 95% CI [0.20, 0.54], P < 0.0001). The Lowveld pack significantly reduced the duration of incursion events to 9% of what it was during the pre-test phase (IRR: 0.09 ± 0.03, 95% CI [0.04, 0.19], P < 0.0001), whereas for the Mapesu pack, the reduction was only by 14% and was non-significant (IRR: 0.86 ± 0.15, 95% CI [0.61, 1.21], P = 0.392).
Discussion
Our results indicate that wild dog movement was significantly reduced after lion scat deployment. Movement decreased in both the treatment and the control plots, with no difference detected during the test phase between treatment and control plots. Although both packs reduced their rate and duration of incursions, the decrease in wild dog movement was more pronounced in the Lowveld pack. Consequently, packs behaved significantly different from each other during the test phase when the duration of incursions was investigated.
Placement of lion scat affected the outcome of both treatment and control plots. Thus, it is likely that the placement of lion scat has a wider influence on wild dog movement patterns, leading to large-scale avoidance of lions. Lions are territorial, and the density of scats tends to increase towards the centre of territories due to a more intensive use of the core area (Zub et al. 2003). By placing a large amount of scat in a small area, as was done in this study (~30–75 g per 0.01 km2), the high lion activity found in core areas was mimicked. Because wild dogs avoid areas of high lion activity (Dröge et al. 2017), the treatment could have motivated the wild dogs to increase their distance from such plots as a safety precaution. This assumption is supported by the finding of the Waterberg Wild Dog Initiative that wild dogs moved 5 km or more between GPS points after being exposed to lion scat compared with less than 1 km prior to each instance of placing the scat (R Mooney 2021, pers. comm.). The effect of treatment on control plots was possibly exaggerated by the fact that a single farm usually accommodated both control and treatment plots. In the Lowveld, the landscape is severely fragmented, and electrified game fences separated the farms at the study site. The permeability of a hard boundary varies among taxonomically related species (Cozzi et al. 2013); whereas wild dogs are notorious for crossing fences with ease, even when electrified (Davies-Mostert et al. 2012), for lions, fences represent a nearly impassable obstacle (Cozzi et al. 2013). Apart from the physical capability of an animal to cross a barrier, the barrier’s permeability primarily depends on the animal’s perception, needs and motivation to cross (Wiens et al. 1985; Cozzi et al. 2013). The inability of lions to cross fences results in the creation of vacuum areas that are relatively lion-free and provide spatial refuges for other species. Wild dogs have an explicit perception of risk distribution across the landscape. They will, for example, seek den sites in lion vacuum areas on private land but return to protected areas daily to hunt (Cozzi et al. 2013). Possibly, the treated farms in this research were perceived as a safe refuge, but once indications of lion presence were detected, the perceived habitat quality was degraded, and the motivation of the wild dogs to cross the fence compromised, leading to reduced wild dog movement on both control and treatment plots.
After treatment had been implemented, the large decrease in wild dog movement during the test phase (56% and 73% for incursion rate and duration respectively) suggests a deterrence effect of lion scat placement on wild dogs. A decrease in wild dog movement could be a result of seasonal changes unrelated to treatment. In fact, the study period covered the denning season (Mbizah et al. 2014), during which the home ranges of wild dogs may contract by more than two thirds (Pomilla et al. 2015), and habitat selection preferences change as a result of an increased aversion to risk (O’Neill et al. 2020). In addition, wild dogs are a highly mobile species, and reduced presence later in the season might simply reflect that the wild dogs have moved out of the area. However, as it appears from the movement data, the packs did not den that season nor abandon their estimated effective home range. Moreover, based on tracks, it was noted that on multiple (>10) occasions wild dogs diverted from their original path to inspect deposited lion scat nearby (<3 m) before they continued, suggesting that lion scat has relevance to them. Mesopredators are initially attracted towards olfactory cues of apex predators. This behaviour is usually accompanied by increased vigilance and has thus been described as a trade-off between the potential risk of a lethal encounter with the apex predator and obtaining information about a potential food source in the vicinity (Wikenros et al. 2017). Wild dogs, however, rarely scavenge to avoid interactions with dominant competitors (Hayward et al. 2006). Therefore, it is questionable whether the inspection of apex predator scats fulfils the same function in wild dogs as in some of the other mesopredators. Scat conveys information about its producer, and each predator species most likely has its own very unique scent (Apfelbach et al. 2005). Lions are ambush predators (Hopcraft et al. 2005) and territorial (Mosser and Packer 2009), meaning they launch surprise attacks from a close distance and show a high site fidelity. Therefore, even aged cues may indicate the actual presence of lions and induce risk assessing and anti-predator behaviour (Bytheway et al. 2013). It should be considered that wild dogs may inspect scats of lions to assess predation risk, ultimately altering their perception of risk across the landscape.
There are several possible explanations why the two packs reacted differently to the lion scat. Likely, the response to predator cues is context-dependent. For instance, a shift in habitat as a response to predation pressure is only a viable option if alternative habitat and resources are available (Ward et al. 1997). It has been found that wild dogs avoid lions via spatial partitioning, which is among others, mediated by resource distribution. As a result, territories are larger where lions and wild dogs coexist, not only to allow for the spatial avoidance of lions but also to access resources that become less available in the process (Marneweck et al. 2019). In fact, after the experiment, the effective homerange of the Lowveld pack had extended by 36% at the second location, which indicates spatial partitioning. However, unlike the free-roaming Lowveld pack, the Mapesu pack was confined to a defined area, limiting its potential to adjust their range and explore new resource patches in response to increased predation pressure. If there is no room for escape and the exposure to the risk persists, an animal has to forage in high-risk areas to meet energy demands (Hegab et al. 2015). The lack of avoidance of indirect cues associated with predators presence relates to the fitness costs of avoiding a potential food resource (Ward et al. 1997). Besides, anti-predator behaviours are not limited to spatial responses, but animals have a repertoire of potential responses to predation risk (Hegab et al. 2015). In wild dogs, behavioural plasticity is usually demonstrated on a spatial scale (Dröge et al. 2017), but they will resort to temporal avoidance if necessary (Darnell et al. 2014).
The different responses of packs to cues of lion presence may also be explained by variation in habitat structure between the two sites. When confronted with direct cues of immediate lion presence, wild dogs have been observed to condition their behaviour related to ambush risk (Webster et al. 2012; Davies et al. 2021). Where the risk of encountering lions is high, wild dogs shift to sites with a high visibility to allow for the early detection of lions and defuse situations of immediate risk (Davies et al. 2021). However, in open habitats, wild dogs are more likely to encounter and be detected by dominant competitors (Creel and Creel 1996). Accordingly, areas of dense vegetation are important refuges from competition (Davies et al. 2021). In densely vegetated areas, wild dogs are therefore more likely to display risky behaviours and only avoid the most recent location of lions (Vanak et al. 2013). In essence, risk behaviour is influenced by habitat structure (Vanak et al. 2013; Davies et al. 2021), and a mosaic of different habitat structures allows wild dogs to successfully evade lions (Davies et al. 2021). In line with these findings, Webster et al. (2012) suggest that wild dogs’ ideal habitat consists of canopied vegetation with a minimal understory (e.g. mature mopane woodlands) and occasional clearings, providing both cover and safe resting sites with an unobstructed view (Webster et al. 2012). Although an accurate assessment of landscape heterogeneity and vegetational differences was beyond the scope of this study, the landscape found in the range of the Mapesu pack resembles the description by Webster et al. (2012). It is therefore possible that the Mapesu pack was more likely to show risky behaviour than the Lowveld pack.
Furthermore, responses to predators are modulated by internal factors, such as an animal’s previous experience with predators. In some species, prior experience is necessary before effective antipredator behaviours are exhibited in response to indirect cues of predator presence (Apfelbach et al. 2005). In addition, where predators are present in the natural surroundings of an animal, the fear of predators is continuously reinforced, enhancing the responsiveness to predator cues (Ayon et al. 2017). Experiences with predators have also been shown to play a vital role in the anti-predator behaviour of wild dogs. Predator-naïve wild dogs born in captivity have been shown to underestimate the threat posed by predators, frequently resulting in failed re-introduction efforts (Frantzen et al. 2001). Whereas the range of the free-roaming Lowveld pack includes properties that keep lions, the reserve hosting the Mapesu pack is free of lions. Although the dogs of the Mapesu pack were born and raised elsewhere and likely had exposure to lions prior to capture, by the time plots were treated, the female and the male had at least spent 10 and 24 months in a lion-free environment respectively. Therefore, the lack of recent exposure to lions may have reduced the pack’s sensitivity to indirect cues of lion presence.
Predator–prey scent research has been going on for over four decades across a range of taxa, and lion faecal odours are a commonly used scent source to trigger anti-predator responses. However, research on the use of scents to direct the movement of subordinate predators and mitigate conflict is still in its infancy (Apps 2021). This study broadens the current knowledge about the responses of mesopredators to indirect cues of apex predator presence, and contributes to a slowly growing body of literature on the use of scent cues to promote human–carnivore coexistence. Notwithstanding the limitations of this study and the need for more research, the findings offer compelling evidence for the potential effectiveness of lion scat as a wild dog deterrent, and where lion scat is available, this inexpensive method of mitigation could be used to direct wild dogs away from areas where they are prone to persecution. The findings of this study could have positive conservation implications for wild dogs by supporting wildlife managers and encouraging further research in the field of scent studies.
Data availability
The data that support this study were in part obtained from the Endangered Wildlife Trust and the Mapesu Private Game Reserve by permission. Therefore, data will only be shared upon reasonable request to the corresponding author with permission from the third parties.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
Funding was provided by the Kevin Richardson Foundation. The Foundation was not involved in the preparation of the data or manuscript or the decision to submit for publication.
Author contributions
AT, JO, GB and RH conceived the ideas and designed methodology; RH collected the data; PT and RH analysed the data; RH led the writing of the manuscript and AT, PT and JO revised it. All authors contributed critically to the drafts and gave final approval for publication. Statement on inclusion: our study brings together authors of different backgrounds, including scientists based in the region where the study was carried out. All authors were engaged early on with the research and study design to ensure that the diverse sets of perspectives they represent was considered from the onset. The research was conducted in close co-operation with the landowners affected by wild dog impact, who are important stakeholders in wild dog conservation.
Acknowledgements
Apart from thanking the Kevin Richardson Foundation for their generous donation, we thank the following organisations and people who were just as vital to the success of the project: the Kevin Richardson Wildlife Sanctuary; the Lionsrock Big Cat Sanctuary; the Hoedspruit Endangered Species Centre; volunteer Konstantin Fey; and all the farm and reserve managers/owners involved. Special thanks to the Endangered Wildlife Trust for their overall support. The project was conducted with UP and EWT animal ethics approval (reference number REC140-20 and EWTEC2021_002 respectively).
References
Apfelbach, R, Blanchard, CD, Blanchard, RJ, Hayes, RA, and McGregor, IS (2005). The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neuroscience and Biobehavioral Reviews 29, 1123–1144.| The effects of predator odors in mammalian prey species: a review of field and laboratory studies.Crossref | GoogleScholarGoogle Scholar |
Apps P (2021) Saved by the smell: using chemical signals to protect predators and livestock. YouTube video. Wild Entrus, Sammamish, WA, USA. Available at https://www.youtube.com/watch?v=4oK_LDDbJGI
Ayon, RE, Putman, BJ, and Clark, RW (2017). Recent encounters with rattlesnakes enhance ground squirrel responsiveness to predator cues. Behavioral Ecology and Sociobiology 71, .
| Recent encounters with rattlesnakes enhance ground squirrel responsiveness to predator cues.Crossref | GoogleScholarGoogle Scholar |
Bates, D, Mächler, M, Bolker, B, and Walker, S (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67, 1–48.
| Fitting linear mixed-effects models using lme4.Crossref | GoogleScholarGoogle Scholar |
Bendel, RB, and Afifi, AA (1977). Regression Comparison of Stopping Rules in Forward “Stepwise” Regression. Journal of the American Statistical Association 72, 46–53.
Bytheway, JP, Carthey, AJR, and Banks, PB (2013). Risk vs. reward: how predators and prey respond to aging olfactory cues. Behavioral Ecology and Sociobiology 67, 715–725.
| Risk vs. reward: how predators and prey respond to aging olfactory cues.Crossref | GoogleScholarGoogle Scholar |
Calenge, C (2006). The package “adehabitat” for the R software: a tool for the analysis of space and habitat use by animals. Ecological Modelling 197, 516–519.
| The package “adehabitat” for the R software: a tool for the analysis of space and habitat use by animals.Crossref | GoogleScholarGoogle Scholar |
Cornhill, KL, and Kerley, GIH (2020). Cheetah communication at scent-marking sites can be inhibited or delayed by predators. Behavioral Ecology and Sociobiology 74, .
| Cheetah communication at scent-marking sites can be inhibited or delayed by predators.Crossref | GoogleScholarGoogle Scholar |
Cozzi, G, Broekhuis, F, McNutt, JW, and Schmid, B (2013). Comparison of the effects of artificial and natural barriers on large African carnivores: implications for interspecific relationships and connectivity. Journal of Animal Ecology 82, 707–715.
| Comparison of the effects of artificial and natural barriers on large African carnivores: implications for interspecific relationships and connectivity.Crossref | GoogleScholarGoogle Scholar |
Creel, S, and Creel, NM (1995). Communcal hunting and pack size in African wild dogs, Lycaon pictus. Animal Behaviour 50, 1325–1339.
| Communcal hunting and pack size in African wild dogs, Lycaon pictus.Crossref | GoogleScholarGoogle Scholar |
Creel, S, and Creel, NM (1996). Limitation of African wild dogs by competition with larger carnivores. Conservation Biology 10, 526–538.
| Limitation of African wild dogs by competition with larger carnivores.Crossref | GoogleScholarGoogle Scholar |
Creel, S, and Creel, NM (1998). Six ecological factors that may limit African wild dogs, Lycaon pictus. Animal Conservation 1, 1–9.
| Six ecological factors that may limit African wild dogs, Lycaon pictus.Crossref | GoogleScholarGoogle Scholar |
Darnell, AM, Graf, JA, Somers, MJ, Slotow, R, and Szykman Gunther, M (2014). Space use of African wild dogs in relation to other large carnivores. PLoS ONE 9, .
| Space use of African wild dogs in relation to other large carnivores.Crossref | GoogleScholarGoogle Scholar |
Davies, AB, Tambling, CJ, Marneweck, DG, Ranc, N, Druce, DJ, Cromsigt, JPGM, Le Roux, E, and Asner, GP (2021). Spatial heterogeneity facilitates carnivore coexistence. Ecology 102, .
| Spatial heterogeneity facilitates carnivore coexistence.Crossref | GoogleScholarGoogle Scholar |
Davies-Mostert, HT, Kamler, JF, Mills, MGL, Jackson, CR, Rasmussen, GSA, Groom, RJ, and Macdonald, DW (2012). Long-distance transboundary dispersal of African wild dogs among protected areas in southern Africa. African Journal of Ecology 50, 500–506.
| Long-distance transboundary dispersal of African wild dogs among protected areas in southern Africa.Crossref | GoogleScholarGoogle Scholar |
Davies-Mostert HT, Page-Nicholson S, Marneweck DG, Marnewick K, Cilliers D, Whittington-Jones B, Killian H, Mills MGL, Parker D, Power J, Rehse T, Child MF (2016) A conservation assessment of Lycaon pictus. In ‘The Red List of Mammals of South Africa, Swaziland and Lesotho’ (Eds MF Child, L Roxburgh, E Do Linh San, D Raimondo, HT Davies-Mostert). (South African National Biodiversity Institute and Endangered Wildlife Trust: South Africa), 1–13.
Dröge, E, Creel, S, Becker, MS, and M’soka, J (2017). Spatial and temporal avoidance of risk within a large carnivore guild. Ecology and Evolution 7, 189–199.
| Spatial and temporal avoidance of risk within a large carnivore guild.Crossref | GoogleScholarGoogle Scholar |
Eklund, A, López-Bao, JV, Tourani, M, Chapron, G, and Frank, J (2017). Limited evidence on the effectiveness of interventions to reduce livestock predation by large carnivores. Scientific Reports 7, .
| Limited evidence on the effectiveness of interventions to reduce livestock predation by large carnivores.Crossref | GoogleScholarGoogle Scholar |
Fink, S, Chandler, R, Chamberlain, M, Castleberry, M, Castleberry, S, and Glosenger-Thrasher, S (2020). Distribution and activity patterns of large carnivores and their implications for human–carnivore conflict management in Namibia. Human–Wildlife Interactions 14, 287–295.
| Distribution and activity patterns of large carnivores and their implications for human–carnivore conflict management in Namibia.Crossref | GoogleScholarGoogle Scholar |
Frantzen, MAJ, Ferguson, JWH, and de Villiers, MS (2001). The conservation role of captive African wild dogs (Lycaon pictus). Biological Conservation 100, 253–260.
| The conservation role of captive African wild dogs (Lycaon pictus).Crossref | GoogleScholarGoogle Scholar |
Green, PA, Van Valkenburgh, B, Pang, B, Bird, D, Rowe, T, and Curtis, A (2012). Respiratory and olfactory turbinal size in canid and arctoid carnivorans. Journal of Anatomy 221, 609–621.
| Respiratory and olfactory turbinal size in canid and arctoid carnivorans.Crossref | GoogleScholarGoogle Scholar |
Haber, GC (1996). Biological, conservation, and ethical implications of exploiting and controlling wolves. Conservation Biology 10, 1068–1081.
| Biological, conservation, and ethical implications of exploiting and controlling wolves.Crossref | GoogleScholarGoogle Scholar |
Haswell, PM, Jones, KA, Kusak, J, and Hayward, MW (2018). Fear, foraging and olfaction: how mesopredators avoid costly interactions with apex predators. Oecologia 187, 573–583.
| Fear, foraging and olfaction: how mesopredators avoid costly interactions with apex predators.Crossref | GoogleScholarGoogle Scholar |
Hayward, MW, and Kerley, GIH (2008). Prey preferences and dietary overlap amongst Africa’s large predators. South African Journal of Wildlife Research 38, 93–108.
| Prey preferences and dietary overlap amongst Africa’s large predators.Crossref | GoogleScholarGoogle Scholar |
Hayward, MW, O’Brien, J, Hofmeyer, M, and Kerley, GIH (2006). Prey preferences of the African wild dog Lycaon pictus (Canidae: Carnivora): ecological requirements for conservation. Journal of Mammalogy 87, 1122–1131.
| Prey preferences of the African wild dog Lycaon pictus (Canidae: Carnivora): ecological requirements for conservation.Crossref | GoogleScholarGoogle Scholar |
Hegab, IM, Kong, S, Yang, S, Mohamaden, WI, and Wei, W (2015). The ethological relevance of predator odors to induce changes in prey species. Acta Ethologica 18, 1–9.
| The ethological relevance of predator odors to induce changes in prey species.Crossref | GoogleScholarGoogle Scholar |
Hopcraft, JGC, Sinclair, ARE, and Packer, C (2005). Planning for success: Serengeti lions seek prey accessibility rather than abundance. Journal of Animal Ecology 74, 559–566.
| Planning for success: Serengeti lions seek prey accessibility rather than abundance.Crossref | GoogleScholarGoogle Scholar |
Lenth RV (2022) Emmeans: estimated marginal means, aka least-squares means. R package version 1.7.4-1. Available at https://CRAN.R-project.org/package=emmeans
Lindsey, PA, du Toit, JT, and Mills, MGL (2005). Attitudes of ranchers towards African wild dogs Lycaon pictus: conservation implications on private land. Biological Conservation 125, 113–121.
| Attitudes of ranchers towards African wild dogs Lycaon pictus: conservation implications on private land.Crossref | GoogleScholarGoogle Scholar |
Linklater, WL (2004). Wanted for conservation research: behavioral ecologists with a broader perspective. BioScience 54, 352–360.
| Wanted for conservation research: behavioral ecologists with a broader perspective.Crossref | GoogleScholarGoogle Scholar |
Lüdecke, D, Ben-Shachar, MS, Patil, I, Waggoner, P, and Makowki, D (2021). Performance: an R package for assessment, comparison and testing of statistical models. Journal of Open Source Software 6, .
| Performance: an R package for assessment, comparison and testing of statistical models.Crossref | GoogleScholarGoogle Scholar |
MacFadyen, S, Zambatis, N, Van Teeffelen, AJA, and Hui, C (2018). Long-term rainfall regression surfaces for the Kruger National Park, South Africa: a spatio-temporal review of patterns from 1981 to 2015. International Journal of Climatology 38, 2506–2519.
| Long-term rainfall regression surfaces for the Kruger National Park, South Africa: a spatio-temporal review of patterns from 1981 to 2015.Crossref | GoogleScholarGoogle Scholar |
Marneweck, C, Marneweck, DG, van Schalkwyk, OL, Beverley, G, Davies-Mostert, HT, and Parker, DM (2019). Spatial partitioning by a subordinate carnivore is mediated by conspecific overlap. Oecologia 191, 531–540.
| Spatial partitioning by a subordinate carnivore is mediated by conspecific overlap.Crossref | GoogleScholarGoogle Scholar |
Mbizah, MM, Joubert, CJ, Joubert, L, and Groom, RJ (2014). Implications of African wild dog (Lycaon pictus) denning on the density and distribution of a key prey species: addressing myths and misperceptions. Biodiversity and Conservation 23, 1441–1451.
| Implications of African wild dog (Lycaon pictus) denning on the density and distribution of a key prey species: addressing myths and misperceptions.Crossref | GoogleScholarGoogle Scholar |
Mosser, A, and Packer, C (2009). Group territoriality and the benefits of sociality in the African lion, Panthera leo. Animal Behaviour 78, 359–370.
| Group territoriality and the benefits of sociality in the African lion, Panthera leo.Crossref | GoogleScholarGoogle Scholar |
Nicholson, SK, Marneweck, DG, Lindsey, PA, Marnewick, K, and Davies-Mostert, HT (2020). A 20-year review of the status and distribution of african wild dogs (Lycaon pictus) in South Africa. African Journal of Wildlife Research 50, 8–19.
| A 20-year review of the status and distribution of african wild dogs (Lycaon pictus) in South Africa.Crossref | GoogleScholarGoogle Scholar |
O’Neill, HMK, Durant, SM, and Woodroffe, R (2020). What wild dogs want: habitat selection differs across life stages and orders of selection in a wide-ranging carnivore. BMC Zoology 5, .
| What wild dogs want: habitat selection differs across life stages and orders of selection in a wide-ranging carnivore.Crossref | GoogleScholarGoogle Scholar |
Pomilla, MA, McNutt, JW, and Jordan, NR (2015). Ecological predictors of African wild dog ranging patterns in northern Botswana. Journal of Mammalogy 96, 1214–1223.
| Ecological predictors of African wild dog ranging patterns in northern Botswana.Crossref | GoogleScholarGoogle Scholar |
Pretorius, ME, Seoraj-Pillai, N, and Pillay, N (2019). Landscape correlates of space use in the critically endangered African wild dog Lycaon pictus. PLoS ONE 14, .
| Landscape correlates of space use in the critically endangered African wild dog Lycaon pictus.Crossref | GoogleScholarGoogle Scholar |
QGIS Development Team (2021) QGIS Geographic Information System. Available at http://qgis.org
R Core Team (2020) R: A language and environment for statistical computing. (R Foundation for Statistical Computing: Vienna, Austria). Available at https://www.R-project.org/
Rutherford MC, Mucina L, Lötter MC, Bredenkamp GJ, Smit JHL, Scott-Shaw CR, Hoare DB, Goodman PS, Bezuidenhout H, Scott L, Ellis F, Powrie WL, Siebert F, Mostert TH, Henning BJ, Venter CE, Camp KGT, Siebert SJ, Matthews WS, et al. (2006) bSavanna iome. In ‘The Vegetation of South Africa, Lesotho and Swaziland’. (Eds L Mucina, MC Rutherford) pp. 438–539. (South African National Biodiversity Institute: South Africa)
Shivik, JA (2006). Tools for the edge: what’s new for conserving carnivores. BioScience 56, 253–259.
| Tools for the edge: what’s new for conserving carnivores.Crossref | GoogleScholarGoogle Scholar |
Swanson, A, Caro, T, Davies-Mostert, H, Mills, MGL, Macdonald, DW, Borner, M, Masenga, E, and Packer, C (2014). Cheetahs and wild dogs show contrasting patterns of suppression by lions. Journal of Animal Ecology 83, 1418–1427.
| Cheetahs and wild dogs show contrasting patterns of suppression by lions.Crossref | GoogleScholarGoogle Scholar |
Thorn, M, Green, M, Marnewick, K, and Scott, DM (2015). Determinants of attitudes to carnivores: implications for mitigating human–carnivore conflict on South African farmland. Oryx 49, 270–277.
| Determinants of attitudes to carnivores: implications for mitigating human–carnivore conflict on South African farmland.Crossref | GoogleScholarGoogle Scholar |
van der Meer, E, Fritz, H, Blinston, P, and Rasmussen, GSA (2013). Ecological trap in the buffer zone of a protected area: effects of indirect anthropogenic mortality on the African wild dog Lycaon pictus. Oryx 48, 285–293.
| Ecological trap in the buffer zone of a protected area: effects of indirect anthropogenic mortality on the African wild dog Lycaon pictus.Crossref | GoogleScholarGoogle Scholar |
Vanak, AT, Fortin, D, Thaker, M, Ogden, M, Owen, C, Greatwood, S, and Slotow, R (2013). Moving to stay in place: behavioral mechanisms for coexistence of African large carnivores. Ecology 94, 2619–2631.
| Moving to stay in place: behavioral mechanisms for coexistence of African large carnivores.Crossref | GoogleScholarGoogle Scholar |
Van Heerden, J, Mills, MGL, van Vuuren, MJ, Kelly, PJ, and Dreyer, MJ (1995). An investigation into the health status and diseases of wild dogs (Lycaon pictus) in the Kruger National Park. Journal of the South African Veterinary Association 66, 18–27.
Venter FJ, Scholes RJ, Eckhardt HC (2003) The abiotic template and its associated vegetation pattern. In ‘The Kruger Experience: Ecology and Management of Savanna Heterogeneity’. (Eds J du Toit, H Biggs, KH Rogers) pp. 83–129. (Island Press: Washington, DC, USA)
Ward, JF, MacDonald, DW, and Doncaster, CP (1997). Responses of foraging hedgehogs to badger odour. Animal Behaviour 53, 709–720.
| Responses of foraging hedgehogs to badger odour.Crossref | GoogleScholarGoogle Scholar |
Webster, H, McNutt, JW, and McComb, K (2012). African wild dogs as a fugitive species: playback experiments investigate how wild dogs respond to their major competitors. Ethology 118, 147–156.
| African wild dogs as a fugitive species: playback experiments investigate how wild dogs respond to their major competitors.Crossref | GoogleScholarGoogle Scholar |
Wiens, JA, Crawford, CS, and Gosz, JR (1985). Boundary dynamics: a conceptual framework for studying landscape ecosystems. Oikos 45, 421–427.
| Boundary dynamics: a conceptual framework for studying landscape ecosystems.Crossref | GoogleScholarGoogle Scholar |
Wikenros, C, Jarnemo, A, Frisén, M, Kuijper, DPJ, and Schmidt, K (2017). Mesopredator behavioral response to olfactory signals of an apex predator. Journal of Ethology 35, 161–168.
| Mesopredator behavioral response to olfactory signals of an apex predator.Crossref | GoogleScholarGoogle Scholar |
Woodroffe, R, and Ginsberg, JR (1999). Conserving the African wild dog Lycaon pictus I. Diagnosing and treating causes of decline. Oryx 33, 132–142.
| Conserving the African wild dog Lycaon pictus I. Diagnosing and treating causes of decline.Crossref | GoogleScholarGoogle Scholar |
Woodroffe R, Sillero-Zubiri C (2020) African wild dog: Lycaon pictus. (amended version of 2012 assessment). In ‘The IUCN Red List of Threatened Species 2020’. (IUCN: Gland, Switzerland) Available at https://dx.doi.org/10.2305/IUCN.UK.2020-1.RLTS.T12436A166502262.en
Woodroffe, R, Davies-Mostert, H, Ginsberg, J, Graf, J, Leigh, K, McCreery, K, Robbins, R, Mills, G, Pole, A, Rasmussen, G, Somers, M, and Szykman, M (2007). Rates and causes of mortality in endangered African wild dogs Lycaon pictus: lessons for management and monitoring. Oryx 41, 215–223.
| Rates and causes of mortality in endangered African wild dogs Lycaon pictus: lessons for management and monitoring.Crossref | GoogleScholarGoogle Scholar |
Young, JK, Steuber, J, Few, A, Baca, A, and Strong, Z (2019). When strange bedfellows go all in: a template for implementing non-lethal strategies aimed at reducing carnivore predation of livestock. Animal Conservation 22, 207–209.
| When strange bedfellows go all in: a template for implementing non-lethal strategies aimed at reducing carnivore predation of livestock.Crossref | GoogleScholarGoogle Scholar |
Zub, K, Theuerkauf, J, Jędrzejewski, W, Jędrzejewska, B, Schmidt, K, and Kowalzcyk, R (2003). Wolf pack territory marking in the Bialowieza Primeval Forest (Poland). Behaviour 140, 635–648.
| Wolf pack territory marking in the Bialowieza Primeval Forest (Poland).Crossref | GoogleScholarGoogle Scholar |
Zuur AF, Ieno EN, Walker N, et al. (2009) Model selection in a GLM, in ‘Mixed effects models and extensions in ecology with R: statistics for biology and health’ (Eds M Gail et al.). pp. 220–223. (Springer: New York, USA)


