Learned avoidance of trap locations in freshwater turtles
Ethan C. Hollender A B , Day B. Ligon A and Donald T. McKnight
A B , Day B. Ligon A and Donald T. McKnight  C D *
C D *
A Department of Biology, Missouri State University, 901 South National Avenue, Springfield, MO 65897, USA.
B Present address: Department of Biological Sciences, University of Arkansas, 850 West Dickson Street, Fayetteville, AR 72701, USA.
C Present address: Department of Environment and Genetics, School of Agriculture, Biomedicine, and Environment, La Trobe University, Wodonga, Vic 3690, Australia.
D College of Science and Engineering, James Cook University, One James Cook Drive, Townsville, Qld 4811, Australia.
Wildlife Research - https://doi.org/10.1071/WR21061
Submitted: 2 April 2021 Accepted: 8 July 2022 Published online: 19 August 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Understanding the effects that learned responses to being captured have on subsequent recapture rates and associated abundance estimates is important for developing accurate descriptions of populations and communities. Although variation in the willingness of individual turtles to be trapped is commonly mentioned in the literature, few studies have experimentally tested learned trap avoidance (or fondness) in turtles.
Aims: To determine whether turtles learn to avoid traps, whether repositioning traps will lead to increased capture rates, whether this effect varies among species, and whether such relocations yield more accurate depictions of community structure.
Methods: We studied a community of turtles in a small lake in south-eastern Kansas that included populations of red-eared slider turtles (Trachemys scripta elegans) and common musk turtles (Sternotherus odoratus). We trapped the lake for 35 consecutive days by using two concurrently deployed groups of traps. One group remained stationary for the duration of the study, whereas traps comprising the other group were moved to new locations on Day 14 and returned to their original locations on Day 28, thus dividing the trapping season into three periods.
Key results: For both species, capture rates declined over time. However, traps in the moved group captured more T. s. elegans than did those in the stationary group during the second period and more S. odoratus during the third period. Traps in the moved group also had higher recapture rates in the second period. Population abundance estimates based on captures from the moved group, the stationary group, and the pool of all captures were similar for T. s. elegans, but for S. odoratus the stationary group of traps produced an abundance estimate much lower than those generated from the moved group and the pool of all captures.
Conclusions: Both species exhibited learned avoidance of trap locations, but relocating traps had distinct effects on different species, and the accuracy of the observed community structure was improved by relocating traps.
Implications: The movement patterns and catchability of individuals of different species within a community must be taken into consideration when developing trapping protocols. Even high-intensity trapping over a long period may not generate an accurate sample of the community if different species use the spatial environment in substantially different ways and learn to avoid trap locations.
Keywords: abundance, behaviour, mark–recapture, population density, Sternotherus odoratus, Trachemys scripta, turtle, vertebrates.
Introduction
Effective wildlife conservation requires a thorough understanding of the population or community under consideration. This includes an accurate assessment of the population’s abundance and demographic structure, and such an assessment can only be achieved through sampling that truly represents the population. Most sampling methods are biased against certain species in a community (McKnight et al. 2015) and against certain demographic groups within a population. Size bias is common in surveys that use passive trapping and may stem from a physical aspect of the traps that precludes certain size classes from entering the traps or allows others to escape (Willson et al. 2008; Luhring et al. 2016). However, in some cases, it is unclear whether the bias stems from the physical exclusion of certain size classes or ontological shifts in behavior (Rodda et al. 2007; Hancock and Legg 2012).
Sampling methods that generate sex-biased capture rates are also quite common, presumably (and sometimes demonstrably) being the result of differences in behaviour between the sexes (Gehrt and Fritzell 1996; Vanderkist et al. 1999; Moeller et al. 2005; Altermatt et al. 2009). These kinds of biases, if not recognised, can stymy efforts to examine real biases in the sex ratio of a population (Thorbjarnarson 1997; Stoks 2001; McKnight and Ligon 2017). When a sample of a population provides an unrepresentative size distribution, sex ratio, or population density, any conclusions about demographic structure, as well as both absolute and relative abundance in a community, will likewise be biased (Ream and Ream 1966; Tesche and Hodges 2015; McKnight and Ligon 2017).
An additional source of bias can come from learned trap behaviour owing to sustained sampling in an area. For instance, learned trap avoidance in response to capture has been documented in mammals (Wood and Slade 1990; Pelton and van Manen 1996; Schipper 2007), birds (Buckland and Hereward 1982; Muraoka and Wichmann 2007), and fish (Gilbert et al. 2001). Although considerable work has been conducted to identify sources of population sampling bias, and many strategies have been developed to ameliorate them, little experimental attention has been paid to these temporally induced biases, and methods for correcting them in the field are needed.
Turtles have the ability to learn tasks and behaviours, including learning by watching other turtles (Davis 2009; Wilkinson et al. 2010), and the possibility that turtles learn to avoid traps has been suggested (Mahoney and Lindeman 2016), but no tests have been performed on learned trap avoidance in turtles. This lack of information is problematic, as many population size estimators assume equal catchability, although this assumption has historically been ignored in estimates of turtle population sizes (Lindeman 1990).
If turtles behave differently after they have been captured, population estimates based on capture–mark–recapture will be altered by the resultant inflation or deflation of recapture rates. Turtles may become enamoured with the free resources obtained from baited traps (trap-happy) or may become wary of entering traps in the future because of the perceived danger they pose (trap-shy). Trap-happy turtles might be drawn to the bait used in traps, but it is unclear what cues trap-shy turtles use to identify and avoid traps, and it is possible that they remember the locations of traps. Different bait types attracted unique subsets of individuals in a population of red-eared sliders (Trachemys scripta), but switching bait types did not increase recapture rates (Mali et al. 2012), suggesting that turtles that learn to avoid traps were not avoiding a particular bait odour. One possibility is that turtles can become wary of specific locations where they have been captured in the past, as has been reported in some birds and mammals (Buckland and Hereward 1982; Schipper 2007). If this is the case, the use of catch per unit effort (CPUE) as a proxy for abundance in turtle studies could be problematic. In situations where traps are deployed at the same locations over long periods of time, analyses of CPUE may artificially generate or exaggerate the appearance of temporal declines in abundance. Indeed, relocating traps to counteract decreased capture rates (presumed to be the result of learned trap-shyness) over a long session of trapping has been reported in at least one study (Selman and Qualls 2008). However, there have been no experimental investigations into whether such relocations of traps in fact increase recapture rates of turtles. Therefore, we performed an experiment to determine whether turtles learn to avoid locations at which they have previously been trapped. Specifically, we endeavoured to learn (1) whether capture rates decline over the duration of an extended bout of trapping, (2) whether relocating traps ameliorates this effect, and (3) how the effects vary among species.
Materials and methods
Study site
We executed our study at a 1.1-ha strip pit lake (37.20769, −95.04147) at Mined Land Wildlife Area (MLWA) in south-eastern Kansas. This lake was selected for its accessibility, well vegetated surroundings, moderate depth (3 m deep at the centre), lack of connectivity to surrounding bodies of water (although there are several other ponds in the area, the nearest of which is ∼50 m away), and small size. The lake is immediately adjacent to a dirt road with low vehicle traffic and has a dirt path from the road to the water’s edge. There was evidence that recreational fishing occurs, but we did not encounter anyone fishing during the course of our experiments. The lake is surrounded by woodland except for the portion of the shoreline that abuts the road, and most of the lakebed has a covering of submerged vegetation. The turtle community in this lake had not been surveyed prior to this study. We conducted this research with approval from the Missouri State University Institutional Animal Care and Use Committee (Protocol no. 17-025) and with possession of scientific collecting permit SC-071-2018 from the State of Kansas.
Trapping regime
We surveyed the perimeter of the lake and identified 33 locations with depth, slope, and woody anchors suitable for deploying traps. We marked these locations with a hand-held GPS unit and used a random-number generator to select 16 of these locations for the initial period of trap deployment (Fig. 1). On 23 June 2018, we deployed a 0.6-m-diameter, single-throated, flat-bottomed hoop net baited with canned sardines at each of these locations. Half of the traps were then randomly assigned to a stationary control group and the other half to an experimental ‘moved’ group. The traps in the control group were not moved at any point in the experiment. The traps in the experimental group were left in their initial locations for 14 days (Period 1). At the end of this period, each of the eight experimental traps was assigned to a new location randomly selected from the remaining unused potential trap locations. After another 14 days (Period 2), the experimental traps were returned to their initial locations. We continued trapping for a final 7 days (Period 3) after returning traps to their initial locations and terminated the experiment on 28 July 2018. We predicted that if turtles learn the locations of traps, (1) there should be a general decline in capture rates over the duration of the experiment, and this decline should be more pronounced in the stationary group during the second and third periods, (2) there should be significantly more captures and recaptures in the moved group than in the stationary group for the second period (after traps were moved) but not the first period (when all traps were stationary) or third period (when the moved group were back in their original positions which turtles had already had a chance to learn).
Throughout the experiment we checked traps daily and identified the species and age class (adult or juvenile) of all captured turtles, as well as the sex of adults. We marked all turtles for future identification. We marked emydids and kinosternids (hard-shelled taxa) by using a rotary tool to notch a unique series of marginal scutes, and we marked the single species of trionychid (spiny softshell turtle [Apalone spinifera]) by injecting a PIT tag in the thigh (Cagle 1939; Buhlmann and Tuberville 1998). Both methods of marking were applied to common snapping turtles (Chelydra serpentina). All recaptured animals were individually identifiable.
Statistical analysis
All statistical analyses were performed in R (v3.5.3; R Core Team 2019). We used negative binomial fixed-effects models to compare capture success between stationary and moved traps and to test whether capture rates declined over time. The data did not meet the assumptions of linear models, and an overdispersion test (AER package v1.2-9; Kleiber and Zeileis 2008) confirmed that the data were overdispersed for both species (Trachemys scripta elegans: z = 2.381, P = 0.009; Sternotherus odoratus: z = 1.894, P = 0.029). Additionally, for each species, we compared Akaike information criterion (AIC) values for linear, Poisson, and negative binomial models and determined that negative binomial models provided the best fit. The capture data (sum per group per day) did not include enough zeros to merit a zero-inflated model. We ran four models per species, one model with all periods combined and one for each of the three periods separately. For each model, total captures per group per day was included as the response variable, and group (stationary or moved) and day (with an interaction) were included as predictor variables (day was continuous). Significance was assessed using the Anova() function in the car package (v3.0-2) with a Type II sum of squares (Langsrud 2003; Fox and Weisberg 2019). If significant interactions were present, the trends were assessed separately for each trap group. We statistically analysed the results only for T. s. elegans and S. odoratus because of low sample sizes for other species (Table 1). To ensure that temporal autocorrelation was not present, we ran Breusch–Godfrey tests on the models with all data, by using the checkresiduals function in the forecast package (v8.16; Hyndman et al. 2022). Breusch–Godfrey tests were not significant for T. s. elegans (P = 0.5582) or S. odoratus (P = 0.3465).

|
We used total captures per group per day (sum of all traps) rather than a mixed-effects design with trap as a random effect because of a high frequency of empty traps, resulting in too many zeros for mixed-effects models to converge (traps had no T. s. elegans or no S. odoratus on 62% and 72% of all trap checks respectively). We used total captures per day rather than mean captures because model fit was poor when using means. On 5 days, there was an issue with a trap in the moved group (e.g. a hole through which trapped turtles could have escaped), resulting in only seven traps for those days. However, this would be unlikely to substantially affect the results because most traps caught zero turtles on any given day, meaning that the removal of any one trap would rarely change the daily capture total. Additionally, because these issues occurred in the moved group, any resulting bias would be away from our hypothesis that moving traps will improve capture rates, rather than toward producing a false positive.
Low recapture rates per individual prevented us from reliably assessing the effects of moving traps on individual recapture rates. Therefore, we assessed recapture patterns by focusing on turtles that were captured in the first sampling period, and later recaptured. For each trapping period, we used chi-squared tests to compare the number of recapture events for those turtles in moved and stationary traps with an expected ratio of 1:1 (i.e. for turtles of each species that were captured in the first period, we compared recapture rates in each period). If turtles learned trap locations, we expected roughly equal recapture numbers in both groups during the first period, whereas in the second period, we expected higher numbers of recaptures in the moved group than in the stationary group, because at least some of the recaptures from the first sampling period had already been exposed to the traps in the stationary group. In the third sampling period, we expected recapture rates to return to being roughly equal, because recaptures from the first period had already been exposed to trap locations for both the moved and the stationary groups (although the duration of exposure was longer for the stationary group).
To examine the effects of moving traps on population size estimates, we calculated three population size estimates for T. s. elegans and S. odoratus by using the Schumacher–Eschmeyer method (Schumacher and Eschmeyer 1943). One estimate used only the data from traps in the stationary group, another used only data from traps in the moved group, and the final used all available groups. Because of a low number of captures on any given day, we grouped the data into five 7-day capture periods for each estimate (McKnight and Ligon 2017).
Results
We captured 225 individual turtles (six species) a total of 641 times (Table 1). The majority of these were T. s. elegans (39.6% of individuals and 60.1% of captures) and S. odoratus (56.9% of individuals and 37.4% of captures).
Daily capture rates declined over the duration of the experiment for both T. s. elegans (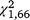 = 19.20, P < 0.001) and S. odoratus (
= 19.20, P < 0.001) and S. odoratus (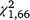 = 5.07, P = 0.024); however, for T. s. elegans the interaction between group and day was not significant (
= 5.07, P = 0.024); however, for T. s. elegans the interaction between group and day was not significant ( = 2.53, P = 0.112), whereas it was significant for S. odoratus (
= 2.53, P = 0.112), whereas it was significant for S. odoratus ( = 7.54, P = 0.006), with a negative slope for the stationary group but not the moved group. Capture rates also declined significantly within the first (
= 7.54, P = 0.006), with a negative slope for the stationary group but not the moved group. Capture rates also declined significantly within the first (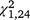 = 16.37, P < 0.001) and second (
= 16.37, P < 0.001) and second ( = 31.51, P < 0.001) trapping periods, but not the third (
= 31.51, P < 0.001) trapping periods, but not the third ( = 0.03, P = 0.868) for T. s. elegans, and within the second (
= 0.03, P = 0.868) for T. s. elegans, and within the second ( = 4.48, P = 0.034) and third (
= 4.48, P = 0.034) and third ( = 5.82, P = 0.016) but not the first (
= 5.82, P = 0.016) but not the first (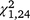 = 0.03, P = 0.861) periods for S. odoratus. No interactions were significant for individual trapping periods (for all, P ≥ 0.129).
= 0.03, P = 0.861) periods for S. odoratus. No interactions were significant for individual trapping periods (for all, P ≥ 0.129).
The moved-trap group produced 204 captures of 78 T. s. elegans and 167 captures of 103 S. odoratus. The stationary-trap group produced 179 captures of 75 T. s. elegans and 73 captures of 44 S. odoratus. In both species, the effect of moving the traps became more pronounced after disregarding the first trapping period, for which capture rates would be expected to be similar between the two trap groups. When looking only at periods two and three, the moved-trap group produced 119 captures of 60 T. s. elegans and 104 captures of 70 S. odoratus. The stationary group produced 72 captures of 43 T. s. elegans and 32 captures of 28 S. odoratus. Among T. s. elegans, 73.8% of individuals were captured in both groups of trap. Only 15.7% of S. odoratus individuals were captured in both groups.
Over the full duration of the experiment, the moved-trap group produced significantly more captures per day for S. odoratus ( = 26.44, P < 0.001) but not T. s. elegans (
= 26.44, P < 0.001) but not T. s. elegans (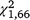 = 1.38, P = 0.240). For T. s. elegans, the moved group captured significantly more turtles in the second trapping period (
= 1.38, P = 0.240). For T. s. elegans, the moved group captured significantly more turtles in the second trapping period (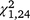 = 10.02, P = 0.002; Figs 2, 3) but not the first (
= 10.02, P = 0.002; Figs 2, 3) but not the first ( = 1.11, P = 0.293) or third (
= 1.11, P = 0.293) or third ( = 0.32, P = 0.575) trapping periods. For S. odoratus, the moved group captured significantly more turtles in the second (
= 0.32, P = 0.575) trapping periods. For S. odoratus, the moved group captured significantly more turtles in the second ( = 16.43, P < 0.001, Figs 2, 4) and third (
= 16.43, P < 0.001, Figs 2, 4) and third (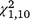 = 24.41, P < 0.001) trapping periods, but not in the first period (
= 24.41, P < 0.001) trapping periods, but not in the first period ( = 2.74, P = 0.098).
= 2.74, P = 0.098).
|
|
For the moved group of traps, similar numbers of unique T. s. elegans individuals were detected in the first period (49) and the second period (52). However, the stationary group of traps showed a steep decline from 58 individuals detected in the first period to 32 individuals detected during the second. In the third period, the number of individuals detected was much lower than for the first two periods (likely primarily due to a shorter trapping period), but similar between the moved (25) and stationary (20) groups (Fig. 5). Of 73 T. s. elegans individuals captured during the first period, 53.4% were recaptured in the moved group of traps in the second period, whereas only 41.1% were recaptured in the stationary group of traps during the second period.
|
|
For S. odoratus, the number of unique individuals detected in the moved-trap group remained fairly similar in the first period (47) and the second period (49). Only 29 individuals were captured in the moved group in the third period, but that period lasted only 7 days (compared with 14 for Periods 1 and 2). The number of individuals detected in the stationary group was also similar between the first period (30) and second period (27), before undergoing a steep drop to a mere four individuals in the third period. Of 43 individual S. odoratus captured during the first period, 39.5% were captured in the moved group of traps in the second period, whereas only 25.6% were captured again in the stationary group of traps during the second period.
When examining turtles who were captured in the first period (in either group) and later recaptured, there was no significant difference in recaptures for either species during the first period, but in the second period, the moved group had nearly twice as many recaptures as did the stationary group for T. s. elegans and almost three times as many recaptures for S. odoratus (Table 2). Both results were statistically significant (both χ2 > 11.50, P < 0.001) for the second period. In the third period, recaptures were not significantly different for T. s. elegans (χ2 = 0.35, P = 0.555), but the moved group had significantly more recaptures for S. odoratus (χ2 = 7.36, P = 0.007; Table 2).
For T. s. elegans, the moved trap group produced a population estimate (N = 87, 95% CI = 78–98) similar to that of the stationary group (N = 84, 95% CI = 74–97), both of which were congruent with the estimate obtained by pooling captures from both groups (N = 90, 95% CI = 83–98; Fig. 6). For S. odoratus, the estimate based on the moved trap group (N = 163; 95% CI = 105–363) was nearly three times higher than the estimate based on stationary traps (N = 57, 95% CI = 41–94) and agreed closely with the estimate based on pooling all captures from both trap groups (N = 167; 95% CI = 129–240).
Discussion
Our results largely matched our predictions for learned trap avoidance. For both species, capture rates declined over time, and the decline was more pronounced for stationary traps (Fig. 3), and relocating traps resulted in higher capture and recapture rates during the second capture period. The observed decline in capture rates over time suggests that T. s. elegans and S. odoratus exhibit learned trap avoidance, and the significant difference in capture and recapture rates between the moved and stationary trap groups during the second period suggests that this learned avoidance is predicated, at least in part, on having a spatial knowledge of the trap locations. This conclusion was further supported by the fact that, for both species, during the second period, recapture rates from the first period were higher in the moved group than in the stationary group.
As predicted, during the third period T. s. elegans capture rates were very low and there was no significant difference in capture or recapture rates between the trap groups, nor was there a significant decline in capture rates over the duration of that period. This suggests that, by the third period, most T. s. elegans individuals had discovered and were avoiding most of the original trap locations, including the initial locations of the moved trap group, even after 2 weeks, without negative reinforcement at those locations. The same pattern occurred in accumulation curves of the number of unique T. s. elegans individuals detected within each period. For the moved group of traps, similar numbers of individuals were detected in the first and second periods. However, the stationary group of traps exhibited a steep decline in the number of individuals detected between the first and second periods (Fig. 4). This suggests that, in the second period, some turtles were avoiding the locations of traps that they had learned during the first period, while still entering traps that had been moved to novel locations. The renewed parity in individuals detected by the two groups during the third period appears to be the result of traps from the moved group being placed back in locations that at least some turtles had learned about and were choosing to avoid.
Sternotherus odoratus also showed a decline in capture rates over time, with significantly higher capture and recapture rates in the moved group than in the stationary group for Period 2, suggesting that it also learned to avoid traps. However, unlike T. s. elegans, the number of unique individuals captured in the stationary group did not decline in Period 2, and, in Period 3, capture rates, recapture rates, and the number of individuals captured were higher in the moved group than in the stationary group. This could imply an inability of this species to remember trap locations long term, but more research would be needed to establish that, and we are not persuaded that this is the correct interpretation. Rather, we suspect that this result is an artifact of the much lower mobility of S. odoratus than that of T. s. elegans. Unlike T. s. elegans, S. odoratus is not particularly vagile and spends much of its time foraging by walking along the bottom. As a result, traps may have needed to be in one location for a longer period of time before most S. odoratus individuals could discover them. Thus, we propose that the number of individuals detected in the stationary group in Period 2 remained high because there were still many individuals that had not previously encountered those trap locations, and in Period 3, the moved group had significantly higher capture rates than did the stationary group because traps in the moved group had previously been in those locations only for 2 weeks, compared with 4 weeks for the stationary group (thus affording the stationary group more time for sedentary turtles to encounter and learn the trap locations). Nevertheless, even in Period 2, the number of turtles from Period 1 that were recaptured was higher in the moved group than in the stationary group, suggesting that at least some learned avoidance had already occurred by Period 2.
This interpretation is further supported by the low overlap of individual S. odoratus between the two trap groups. Only 15.7% of S. odoratus individuals were captured in both trap groups, compared with 73.8% of T. s. elegans individuals. The simplest explanation for this discrepancy is that most T. s. elegans individuals were using a larger proportion of the wetland than were S. odoratus individuals over the duration of the study. This explanation also makes sense of the fact that S. odoratus capture rates did not decline significantly during the first period but those of T. s. elegans did (i.e. there may have not been enough time for most S. odoratus individuals to discover traps).
The difference in mobility between the two species is likely to be also responsible for the interspecific difference in how population-size estimates varied with the trap group. Moving traps improved population estimates only for S. odoratus. For T. s. elegans, all three population estimates were similar, despite the differences in numbers of individuals captured and the declining capture rates over time. This, combined with the fact that nearly three-quarters of T. s. elegans individuals were detected in both trap groups, implies that for highly mobile species of turtle, it is relatively safe to assume that each trap has a nearly equal chance of catching each turtle in a sufficiently small system. This was emphatically not true of our small, sedentary species (S. odoratus), for which the stationary group severely underestimated the actual population size. On the basis of their limited mobility, it is likely that many S. odoratus individuals were simply not available for capture at stationary-trap locations, and, as a result, the population size was underestimated. However, the moved-trap group was able to provide an estimate very close to that of the full pool of traps (albeit with a lower precision) by making some traps available, at least part of the time, to a larger proportion of the population. This is important to consider because it means that generating an accurate estimate of abundance for species such as S. odoratus requires a considerably higher trapping effort than does an abundance estimate for more vagile species unless traps are relocated throughout a survey.
It is also worth noting that the estimate generated from the stationary traps underestimated the abundance of S. odoratus to a degree that rendered interpretations of the entire community structure incorrect. It is clear from the estimates based on the full complement of traps (and even from the number of individuals actually captured) that there were many more S. odoratus (N = 167) than T. s. elegans (N = 90) individuals in the lake. However, the stationary-trap group generated population estimates that indicated that S. odoratus was less abundant (N = 57) than T. s. elegans (N = 84). Given that 127 S. odoratus individuals, in total, were captured, this is clearly a gross underestimate. Nevertheless, the stationary estimate of S. odoratus abundance had considerably tighter confidence intervals than did the other two estimates, which could easily mislead investigators into believing they have produced a reliable estimate, despite the warning from Koper and Brooks (1998) that narrow confidence intervals should not necessarily be interpreted as indicative of an accurate estimate. It is already well known that obtaining representative samples of all species in turtle communities is often difficult because of the broad variation in size, diet, and habits that exist among species (Cagle 1942; Vogt 1980) and sometimes between the sexes and age classes within a species (Ream and Ream 1966; Gibbons 1969; Gamble 2006; Steen et al. 2006). Our study suggests that learned trap avoidance and interspecific differences in mobility are additional factors that researchers should take into account when sampling turtle communities.
It would be instructive for future research to examine the mechanisms and cues that turtles use to remember trap locations. Intriguingly, given the large declines in capture and recapture rates we observed for the stationary group, it appears possible that some turtles are learning locations without actually entering the traps themselves. It is possible (although entirely speculative) that some turtles emit distress calls while in a trap, potentially warning other individuals. Vocalisation (often involving multiple different calls) has been documented in a wide range of turtles (Giles et al. 2009; Ferrara et al. 2013, 2014, 2017, 2019; Monteiro et al. 2019), but the context and usages of those calls have not been well established in most cases, and it is not known whether they emit distress calls. This would be a valuable line of inquiry for future research.
Conclusions
Taken together, our results suggest that both T. s. elegans and S. odoratus learn to avoid traps by using a knowledge of trap locations, and that they are capable of remembering these locations without additional reinforcement for at least 2 weeks. Considering that these species are from considerably divergent families (Joyce et al. 2013) and have quite different lifestyles, it seems likely that this is an ability common to many, if not most, turtle species. Our results further suggest that, for species with low mobility, relocating traps is important for obtaining accurate population estimates. Both results provide compelling reasons to implement periodic trap relocation as a standard part of turtle surveys. This may be particularly important for long-term studies that use CPUE to document population declines. If care is not taken to regularly relocate traps, such apparent declines may simply be an artifact of turtles learning to avoid trap locations. Nevertheless, it should be acknowledged that our study has documented only short-term memory, and additional research is needed to establish long-term memory. However, in the absence of those studies, we recommend that researchers err on the side of caution and reposition traps regularly to maximise capture success and produce accurate assessments of the populations and communities they are studying.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research was funded by Chickadee Checkoff grants from the Kansas Department of Wildlife and Parks and additional funding from Missouri State University and the Delta Foundation.
Acknowledgements
We are indebted to David Jenkins, wildlife area manager at the Kansas Department of Wildlife and Parks for granting access to the Mined Land Wildlife Area. We thank Melisa Blasky, Casey Dimond, and Craig Hollender for their assistance in the field.
References
Altermatt, F, Baumeyer, A, and Ebert, D (2009). Experimental evidence for male biased flight-to-light behavior in two moth species. Entomologia Experimentalis et Applicata 130, 259–265.| Experimental evidence for male biased flight-to-light behavior in two moth species.Crossref | GoogleScholarGoogle Scholar |
Buckland, ST, and Hereward, AC (1982). Trap-shyness of yellow wagtails Motacilla flava flavissima at a pre-migratory roost. Ringing & Migration 4, 15–23.
| Trap-shyness of yellow wagtails Motacilla flava flavissima at a pre-migratory roost.Crossref | GoogleScholarGoogle Scholar |
Buhlmann, KA, and Tuberville, TD (1998). Use of passive integrated transponder (PIT) tags for marking small freshwater turtles. Chelonian Conservation and Biology 3, 102–104.
Cagle, FR (1939). A system of marking turtles for future identification. Copeia 1939, 170–173.
| A system of marking turtles for future identification.Crossref | GoogleScholarGoogle Scholar |
Cagle, FR (1942). Turtle populations in southern Illinois. Copeia 1942, 155–162.
| Turtle populations in southern Illinois.Crossref | GoogleScholarGoogle Scholar |
Davis DM (2009) Sociality, cognition, and social learning in turtles (Emydidae). Doctoral Dissertation, University of Tennessee, Knoxville, TN, USA.
Ferrara CR, Vogt RC, Giles JC, Kuchling G (2013) Chelonian vocal communication. In ‘Biocommunication of animals’. (Ed. G Witzany) pp. 261–274. (Springer Science: London, UK)
Ferrara, CR, Mortimer, JA, and Vogt, RC (2014). First evidence that hatchlings of Chelonia mydas emit sounds. Copeia 2014, 245–247.
| First evidence that hatchlings of Chelonia mydas emit sounds.Crossref | GoogleScholarGoogle Scholar |
Ferrara, CR, Vogt, RC, Eisemberg, CC, and Doody, JS (2017). First evidence of the pig-nosed turtle (Carettochelys insculpta) vocalizing underwater. Copeia 105, 29–32.
| First evidence of the pig-nosed turtle (Carettochelys insculpta) vocalizing underwater.Crossref | GoogleScholarGoogle Scholar |
Ferrara, CR, Vogt, RC, Sousa-Lima, RS, Lenz, A, and Morales-Mávil, JE (2019). Sound communication in embryos and hatchlings of Lepidochelys kempii. Chelonian Conservation and Biology 18, 279–283.
| Sound communication in embryos and hatchlings of Lepidochelys kempii.Crossref | GoogleScholarGoogle Scholar |
Fox J, Weisberg S (2019) ‘An R companion to applied regression.’ 3rd edn. (Sage)
Gamble, T (2006). The relative efficiency of basking and hoop traps for painted turtles (Chrysemys picta). Herpetological Review 37, 308–312.
Gehrt, SD, and Fritzell, EK (1996). Sex-biased response of raccoons (Procyon lotor) to live traps. American Midland Naturalist 135, 23–32.
| Sex-biased response of raccoons (Procyon lotor) to live traps.Crossref | GoogleScholarGoogle Scholar |
Gibbons, JW (1969). Ecology and population dynamics of the chicken turtle, Deirochelys reticularia. Copeia 1969, 669–676.
| Ecology and population dynamics of the chicken turtle, Deirochelys reticularia.Crossref | GoogleScholarGoogle Scholar |
Gilbert, DJ, McKenzie, JR, and Davies, NM (2001). Evidence from tag recapture experiments that fish learn to avoid fishing gear. Journal of Agricultural, Biological, and Environmental Statistics 6, 281–291.
| Evidence from tag recapture experiments that fish learn to avoid fishing gear.Crossref | GoogleScholarGoogle Scholar |
Giles, JC, Davis, JA, McCauley, RD, and Kuchling, G (2009). Voice of the turtle: the underwater acoustic repertoire of the long-necked freshwater turtle, Chelodina oblonga. The Journal of the Acoustical Society of America 126, 434–443.
| Voice of the turtle: the underwater acoustic repertoire of the long-necked freshwater turtle, Chelodina oblonga.Crossref | GoogleScholarGoogle Scholar |
Hancock, MH, and Legg, CJ (2012). Pitfall trapping bias and arthropod body mass. Insect Conservation and Diversity 5, 312–318.
| Pitfall trapping bias and arthropod body mass.Crossref | GoogleScholarGoogle Scholar |
Hyndman R, Athanasopoulos G, Bergmeir C, Caceres G, Chhay L, O’Hara-Wild M, Petropoulos F, Razbash S, Wang E, Yasmeen F (2022) forecast: forecasting functions for time series and linear models. R package version 8.16. Available at https://pkg.robjhyndman.com/forecast/
Joyce, WG, Parham, JF, Lyson, TR, Warnock, RCM, and Donoghue, PCJ (2013). A divergence dating analysis of turtles using fossil calibrations: an example of best practices. Journal of Paleontology 87, 612–634.
| A divergence dating analysis of turtles using fossil calibrations: an example of best practices.Crossref | GoogleScholarGoogle Scholar |
Kleiber C, Zeileis A (2008) ‘Applied econometrics with R.’ (Springer-Verlag: New York, NY, USA)
Koper, N, and Brooks, RJ (1998). Population-size estimators and unequal catchability in painted turtles. Canadian Journal of Zoology 76, 458–465.
| Population-size estimators and unequal catchability in painted turtles.Crossref | GoogleScholarGoogle Scholar |
Langsrud, Ø (2003). ANOVA for unbalanced data: use Type II instead of Type III sums of squares. Statistics and Computing 13, 163–167.
| ANOVA for unbalanced data: use Type II instead of Type III sums of squares.Crossref | GoogleScholarGoogle Scholar |
Lindeman, PV (1990). Closed and open model estimates of abundance and tests of model assumptions for two populations of the turtle, Chrysemys picta. Journal of Herpetology 24, 78–81.
| Closed and open model estimates of abundance and tests of model assumptions for two populations of the turtle, Chrysemys picta.Crossref | GoogleScholarGoogle Scholar |
Luhring, TM, Connette, GM, and Schalk, CM (2016). Trap characteristics and species morphology explain size-biased sampling of two salamander species. Amphibia-Reptilia 37, 79–89.
| Trap characteristics and species morphology explain size-biased sampling of two salamander species.Crossref | GoogleScholarGoogle Scholar |
Mahoney, SM, and Lindeman, PV (2016). Relative abundance and diet of spiny softshells (Apalone spinifera) in a Lake Erie population. The Canadian Field-Naturalist 130, 275–280.
| Relative abundance and diet of spiny softshells (Apalone spinifera) in a Lake Erie population.Crossref | GoogleScholarGoogle Scholar |
Mali, I, Brown, DJ, Jones, MC, and Forstner, MRJ (2012). Switching bait as a method to improve freshwater turtle capture and recapture success with hoop net traps. Southeastern Naturalist 11, 311–318.
| Switching bait as a method to improve freshwater turtle capture and recapture success with hoop net traps.Crossref | GoogleScholarGoogle Scholar |
McKnight, DT, and Ligon, DB (2017). Correcting for unequal catchability in sex ratio and population size estimates. PLoS One 12, e0184101.
| Correcting for unequal catchability in sex ratio and population size estimates.Crossref | GoogleScholarGoogle Scholar |
McKnight, DT, Harmon, JR, McKnight, JL, and Ligon, DB (2015). Taxonomic biases of seven methods used to survey a diverse herpetofaunal community. Herpetological Conservation and Biology 10, 666–678.
Moeller, BA, Hellgren, EC, Ruthven, DC, Kazmaier, RT, and Synatzske, DR (2005). Temporal differences in activity patterns of male and female Texas horned lizards (Phrynosoma cornutum) in southern Texas. Journal of Herpetology 39, 336–339.
| Temporal differences in activity patterns of male and female Texas horned lizards (Phrynosoma cornutum) in southern Texas.Crossref | GoogleScholarGoogle Scholar |
Monteiro, CC, Carmo, HMA, Santos, AJB, Corso, G, and Sousa-Lima, RS (2019). First record of bioacoustic emission in embryos and hatchlings of hawksbill sea turtles (Eretmochelys imbricata). Chelonian Conservation and Biology 18, 273–278.
| First record of bioacoustic emission in embryos and hatchlings of hawksbill sea turtles (Eretmochelys imbricata).Crossref | GoogleScholarGoogle Scholar |
Muraoka, Y, and Wichmann, G (2007). Trap response of wood sandpipers Tringa glareola. Ornis Fennica 84, 140–142.
Pelton, MR, and van Manen, FT (1996). Benefits and pitfalls of long-term research: a case study of black bears in Great Smoky Mountains National Park. Wildlife Society Bulletin 24, 443–450.
R Core Team (2019) ‘R: a language and environment for statistical computing.’ (R Foundation for Statistical Computing, Vienna, Austria) Available at https://www.R-project.org/
Ream, C, and Ream, R (1966). The influence of sampling methods on the estimation of population structure in painted turtles. American Midland Naturalist 75, 325–338.
| The influence of sampling methods on the estimation of population structure in painted turtles.Crossref | GoogleScholarGoogle Scholar |
Rodda, GH, Savidge, JA, Tyrrell, CL, Christy, MT, and Ellingson, AR (2007). Size bias in visual searches and trapping of brown treesnakes on Guam. Journal of Wildlife Management 71, 656–661.
| Size bias in visual searches and trapping of brown treesnakes on Guam.Crossref | GoogleScholarGoogle Scholar |
Schipper, J (2007). Camera-trap avoidance by kinkajous Potos flavus: rethinking the ‘non-invasive’ paradigm. Small Carnivore Conservation 36, 38–41.
Schumacher, FX, and Eschmeyer, RW (1943). The estimation of fish populations in lakes or ponds. Journal of the Tennessee Academy of Science 18, 228–249.
Selman, W, and Qualls, C (2008). The impacts of Hurricane Katrina on a population of yellow-blotched sawbacks (Graptemys flavimaculata) in the Lower Pascagoula River. Herpetological Conservation and Biology 3, 224–230.
Steen, DA, Aresco, MJ, Beilke, SG, Compton, BW, Condon, EP, Dodd, CK, Forrester, H, Gibbons, JW, Greene, JL, Johnson, G, Langen, TA, Oldham, MJ, Oxier, DN, Saumure, RA, Schueler, FW, Sleeman, JM, Smith, LL, Tucker, JK, and Gibbs, JP (2006). Relative vulnerability of female turtles to road mortality. Animal Conservation 9, 269–273.
| Relative vulnerability of female turtles to road mortality.Crossref | GoogleScholarGoogle Scholar |
Stoks, R (2001). Male-biased sex ratios in mature damselfly populations: real or artefact? Ecological Entomology 26, 181–187.
| Male-biased sex ratios in mature damselfly populations: real or artefact?Crossref | GoogleScholarGoogle Scholar |
Tesche, MR, and Hodges, KE (2015). Unreliable population inferences from common trapping practices for freshwater turtles. Global Ecology and Conservation 3, 802–813.
| Unreliable population inferences from common trapping practices for freshwater turtles.Crossref | GoogleScholarGoogle Scholar |
Thorbjarnarson, J (1997). Are crocodilian sex ratios female biased? The data are equivocal. Copeia 1997, 451–455.
| Are crocodilian sex ratios female biased? The data are equivocal.Crossref | GoogleScholarGoogle Scholar |
Vanderkist, BA, Xue, X-H, Griffiths, R, Martin, K, Beauchamp, W, and Williams, TD (1999). Evidence of male-bias in capture samples of marbled murrelets from genetic studies in British Columbia. The Condor 101, 398–402.
| Evidence of male-bias in capture samples of marbled murrelets from genetic studies in British Columbia.Crossref | GoogleScholarGoogle Scholar |
Vogt, RC (1980). New methods for trapping aquatic turtles. Copeia 1980, 368–371.
| New methods for trapping aquatic turtles.Crossref | GoogleScholarGoogle Scholar |
Wilkinson, A, Kuenstner, K, Mueller, J, and Huber, L (2010). Social learning in a non-social reptile (Geochelone carbonaria). Biology Letters 6, 614–616.
| Social learning in a non-social reptile (Geochelone carbonaria).Crossref | GoogleScholarGoogle Scholar |
Willson, JD, Winne, CT, and Keck, MB (2008). Empirical tests of biased body size distributions in aquatic snake captures. Copeia 2008, 401–408.
| Empirical tests of biased body size distributions in aquatic snake captures.Crossref | GoogleScholarGoogle Scholar |
Wood, MD, and Slade, NA (1990). Comparison of ear-tagging and toe-clipping in prairie voles, Microtus ochrogaster. Journal of Mammalogy 71, 252–255.
| Comparison of ear-tagging and toe-clipping in prairie voles, Microtus ochrogaster.Crossref | GoogleScholarGoogle Scholar |


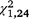 = 10.02, P = 0.002).
= 10.02, P = 0.002). = 16.43, P < 0.001) and third (
= 16.43, P < 0.001) and third (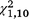 = 24.41, P < 0.001) trapping periods.
= 24.41, P < 0.001) trapping periods.
