The decline, fall, and rise of a large urban colonising bird
Matthew J. Hall A * , John M. Martin
A * , John M. Martin  A B , Alicia L. Burns A B and Dieter F. Hochuli
A B , Alicia L. Burns A B and Dieter F. Hochuli  A
A
A
B
Abstract
The process of urbanisation results in dramatic landscape changes with long-lasting and sometimes irreversible consequences for the biota as urban sensitive species are eliminated. The Australian brush-turkey (Alectura lathami) is a recent urban colonist despite atypical traits for an urban adapter. Contrary to observed range declines and initial reports of decreased reproductive success in cities, Australian brush-turkeys have increased their range in urban areas.
Historical atlas and present citizen science data were used to examine the changing distribution of the Australian brush-turkey at continental and city scales, and the changing land use in urban areas occupied by the species. We assess which environmental and landscape features are driving observed distribution changes over time.
We describe and map changes at the continental scale between 1839–2019. We then assessed colonisation of the cities of Brisbane and Sydney (located 900 km apart) over the period 1960–2019. At the city scale, we quantified the changing land use within Australian brush-turkey occupied areas over time using classification of satellite imagery.
The Australian brush-turkey’s geographical range has shifted over the last century, with the species receding from the western and southwestern parts of their range, while expanding in the northwest. Areas occupied in the cities of Brisbane and Sydney have expanded, with more recently occupied areas containing less vegetation and more developed land than previously occupied areas.
Our results confirm that Australian brush-turkeys are successfully colonising urban areas, including major cities, and are likely to continue moving into urban areas, despite declines elsewhere in their natural range. The species is not limited to suburbs with a high proportion of greenspace, as Australian brush-turkeys are increasingly occurring in highly developed areas with limited vegetation.
This study highlights that species which were locally extirpated from urban areas, and thought to be unlikely candidates for recolonisation, can successfully occupy human modified habitats. Successful expansion is likely to be associated with key behavioural traits, urban greening, and legal protection from human persecution.
Keywords: anthropogenic impacts, conservation, geographical range, habitat fragmentation, urban ecology.
Introduction
Urbanisation is a major land use change with often dramatic and long-lasting consequences for biodiversity (McKinney 2002; McDonald et al. 2008). Natural habitat is largely cleared and replaced by built structures, roads, and other impervious surfaces, while remaining vegetation is often highly fragmented (Grimm et al. 2008). Urban dwelling animals must also contend with chemical and sensory pollutants, altered trophic interactions, competition or predation from non-native species, and exposure to anthropogenic disturbance (Faeth et al. 2005). These conditions often result in the loss of urban sensitive animals from cities (Aronson et al. 2014; Banville et al. 2017) while a subset of species persist, leading to a depauperate and homogenised biotic community (McKinney 2006; Callaghan et al. 2019b).
While many species are unable to persist in cities, others – urban adapters – can thrive in the modified landscape. In some cases, cities can even act as refugia for rare or threatened species (Ives et al. 2016). For some species, urban environments present advantages such as new resources, heterogeneous greenspaces, high primary productivity, potential release from predators or competitors, and buffering against seasonal changes in resources (Crooks and Soulé 1999; Shochat et al. 2006; Anderies et al. 2007; Callaghan et al. 2019a). Highly successful urban species can reach higher abundances than would be possible in their natural habitat (McKinney 2002; Martin et al. 2010). Successful city dwelling species are often characterised by combinations of traits that provide an advantage in urban environments. For example, common traits in successful urban bird species include a generalist diet, canopy nesting, behavioural flexibility, high dispersal ability, a short flight distance, and high fecundity (Møller 2009; Callaghan et al. 2019b; Bressler et al. 2020). Conversely, small bodied species, ground or understory nesters, and dietary specialists, particularly insectivores, tend to decline in urban areas (Joyce et al. 2018). Contrary to earlier studies of biotic homogenisation, recent research has shown both native and exotic species can become highly abundant in urban areas (Campbell et al. 2022).
While many species can persist in cities, the return and re-establishment of formerly extirpated species in urban areas is a less frequent occurrence. Examples include mammals such as red (Cervus slaphus) and fallow (Dama dama) deer (Duarte et al. 2015), red fox (Vulpes vulpes) (Jackowiak et al. 2021), and wild boar (Sus scrofa) (Stillfried et al. 2017). The Australian brush-turkey (Alectura lathami; hereafter ‘brush-turkey’) is an example of a species that is actively recolonising urban areas in Australia, including large cities such as Brisbane and Sydney, which form part of its historic home range (Jones et al. 2004; Göth et al. 2006). The brush-turkey population was observed to be declining by the early 20th century in many popular accounts (Town and Country Journal 1881; Hanscombe 1930; Griffiths 1952), likely as a result of overhunting, habitat clearing, and predation from introduced species (Jones and Göth 2008). Brush-turkeys disappeared from most urbanised areas along the east coast, which have since greatly expanded. Despite their rarity over the past century, the species has now become a common sight in suburban areas over the last few decades since the cessation of hunting. Over time the species increased in urban areas, leading to instances of human–wildlife conflict as their foraging and nest building behaviour can damage household gardens (Jones and Everding 1991).
Although brush-turkeys show recent successes in recolonising urban areas, the future of the species remains uncertain, with concerns that cities may act as ecological traps because of poorer reproductive success (Jones and Everding 1991) and ongoing range declines in rural areas (Göth et al. 2006). Previous studies have estimated that a third of the species’ natural habitat has been lost because of land clearing (Simmonds et al. 2019) while an isolated population in the Nandewar region (New South Wales, NSW), at the far west of the species’ range, is listed as threatened under federal and state legislation (DPIE 2017). Their ground foraging behaviour, obligate ground nesting, obvious large nest mounds, poor flight ability, and lack of parental care for chicks, make brush-turkeys an atypical urban adapting bird. While human pressure through persecution of the species has largely ceased because of the introduction of laws protecting native species, previous studies have found high juvenile mortality because of predation from introduced species, such as red foxes (Vulpes vulpes) and domestic cats (Felis catus) common to urban areas (Göth and Vogel 2002). However, their omnivorous diet, boldness, and broad climatic tolerance may benefit the species in urban areas (Blumstein 2006; Jones and Göth 2008; Hall et al. 2020) as has been observed for other generalists (Croci et al. 2008; Møller 2009). Resolving the contrast between their apparent success in urban areas and possible decline in rural areas requires a greater understanding of how the brush-turkey distribution has changed over time in different regions.
In this study we investigated temporal shifts in brush-turkey distributions at multiple scales. To investigate the brush-turkey distribution at the continental scale, we mapped records and quantified changes in occupied bioregions for six time periods from 1839 to 2019. To investigate the brush-turkey distribution at the city scale, we quantified the number of occupied suburbs across four time periods from 1960 to 2019 for the cities of Brisbane and Sydney. We further investigated if changes to urban land-use at the suburb scale corresponded with changes to the urban brush-turkey distribution. We predicted that the brush-turkey distribution increased in recent time periods following bans on hunting, and that the expansion of the species in urban areas primarily occurred in suburbs with abundant greenspace and vegetation.
Methods
Study area
The study area for the continental scale assessment includes an ~3000 km tract of the Australian east coast, from the tip of Cape York, Queensland, to the Southern Highlands, New South Wales, and up to 750 km inland. The city scale assessment focuses on the cities of Brisbane and Sydney, which are the largest cities within their respective states of Queensland and NSW, as well as the two largest cities within the estimated range of the study species. Brisbane has a population of 2.56 million people (ABS 2021) and a subtropical climate. Sydney has a population of 5.37 million people (ABS 2021) and a temperate climate. Both cities are bordered by national parks to the north, west, and southeast. Both cities contain many smaller patches of natural remnant vegetation alongside managed artificial greenspaces (Queensland Museum 2003; Keith 2004; Lunney et al. 2010).
Historic and recent sightings
We downloaded all brush-turkey occurrence records from the Atlas of Living Australia, as well as a taxon-specific citizen science project, Big City Birds (formerly BrushTurkeys), on 25 March 2021. The Atlas of Living Australia (hereafter ‘Atlas’; ALA 2021) is a collaborative digital platform that compiles Australian taxon occurrence records from multiple sources, including government databases, citizen science records and museum collections, and provides information on data quality. Big City Birds is a targeted citizen science project that collected detailed ecological data on brush-turkeys, including presence, counts, sex, and behavioural observations such as nesting and roosting locations (Hall et al. 2021). These records were downloaded separately as they are not presently included in the Atlas.
Records with no latitude and longitude coordinates and records with no exact date were excluded. We included records prior to 1900 if the sighting had an exact year. We further eliminated records that were not human observations of a wild bird, nest, chicks/juveniles, eggs, or museum specimens with a collection location. Records indicating captive animals, such as those in zoos or pets, were removed. Data from one source, Queensland WildNet (hereafter ‘WildNet’), was redownloaded directly from the source website on 26 July 2021 (Queensland Government 2021) because of inaccuracies identified in the dates assigned to WildNet records in the Atlas.
To further filter the data, we mapped all remaining occurrence records using ArcMap 10.8 (Esri). We spatially filtered data by removing records over water and records where the provided location description did not match GPS coordinates. Outliers were visually identified and eliminated if they met all of the following rules: the record was from an opportunistic survey by non-experts (e.g. citizen science data), the record was outside of previously published distribution maps for the species (Göth et al. 2006; BirdLife International 2021), and there was no other sighting within the same bioregion or within 500 km for the given time period. Four remaining outliers were also eliminated: a 14 July 2000 record from Diamantina National Park was removed as it was considered a misidentification by Ley et al. (2011); two 2019 Questagame records from Adelaide, South Australia were removed because of a lack of any other sightings from a highly populated urban area indicating these sightings are likely spurious; and one 01 December 1949 record from Adelaide, was eliminated as this record was of preserved eggs only with an uncertain collection location.
Continental scale distribution time series
To investigate distribution changes at the continental scale, we assessed the number and location of brush-turkey records across bioregions. Bioregions are biologically meaningful large-scale environmental divisions of Australia based on common climate, soil, geology, and vegetation, with associated faunal communities (Thackway and Cresswell 1995; DAWE 2020). Bioregions are commonly used as coarse landscape units to guide management and conservation at the regional scale. Presence of a species in particular bioregions is informative about the habitat requirements and climatic tolerance of the species. We categorised records temporally into six time periods: pre-1900, 1900–1939, 1940–1959, 1960–1979, 1980–1999 and 2000–2019. The longer time intervals for the periods prior to 1939 reflect the scarcity of records for the species compared with the more recent time periods.
To estimate the changing brush-turkey range at the continental scale for each period, we plotted all filtered brush-turkey records in ArcMap10.8 and aggregated sighting points into a single polygon, using the minimum aggregation distance necessary for each time period to ensure all points were contained within the polygon. We used the smooth polygon tool with Berzier interpolation algorithm to eliminate sharp angles and added a 0.1 decimal degree (11.1 km) buffer to ensure sighting points were contained within the resulting polygon rather than at vertices. We refer to the resulting polygon as the ‘estimated range’.
To visualise how the number and density of records changed in each bioregion over time, the estimated range polygon was then intersected with the bioregions layer and spatially joined with the brush-turkey records point layer. We display the proportion of records occurring in each bioregion and time period as heat maps.
City scale distribution time series
To assess brush-turkey distribution changes in the major urban centres of Brisbane and Sydney, we defined the spatial extent of each city using the ‘significant urban area’ (SUA) classification used by the Australian Bureau of Statistics (ABS). The SUAs represent contiguous large urban centres or clusters of related urban centres (ABS 2017). The Brisbane and Sydney SUAs were subdivided into suburbs, representing officially recognised and named residential localities within cities and towns based on the 2019 census definition (ABS 2019). While not all suburbs present in the 2000–2019 period were developed in prior time periods, the suburban boundaries were used to define areas (polygons) used to measure changes in both brush-turkey occurrence and land use over time. As such, we present the raw number of suburbs with reported brush-turkey occurrence; we have not scaled this data based on the total number of suburbs for each period.
To map changes in brush-turkey suburb occupancy, we plotted all filtered sighting records for the two cities in ArcMap 10.8 for each time-period. We intersected the city suburbs layer with the sightings point layer to produce a map displaying a count of brush-turkey records in each suburb for each time-period. To investigate change at finer temporal scales, we further categorised records from 1960–2019 into 5-year intervals and measured the number of brush-turkey occupied suburbs for each city. Records prior to 1960 were not included due to a scarcity of records for both cities.
Land use analyses
To assess changing patterns of land use and habitat selection within brush-turkey occupied areas, we downloaded Landsat satellite imagery of the two cities from 1979, 1999, and 2019. These years were chosen as they represent the end-dates of the 1960–1979, 1980–1999, and 2000–2019 time periods used for the brush-turkey range analysis. 1979 imagery was obtained from the Landsat 2 Multispectral Scanner, 1999 imagery from the Landsat 5 Thematic Mapper, and 2019 imagery from the Landsat 8 Operational Land Imager. Images were selected from July through September to obtain comparable images with minimal cloud cover. The satellite images were imported into ArcMap 10.8 and clipped to shapefiles of the Brisbane and Sydney SUAs.
We used supervised image classification with a maximum likelihood approach to quantify Land Use/Land Cover (LULC) in each city for each year (see Hahs and McDonnell 2006; Fischer et al. 2021). Twenty training samples were manually assigned for each of the following LULC classes: commercial, residential, dense vegetation, open greenspace and bare land. We then ran the supervised image classification tool in ArcMap 10.8 to classify all images. The majority filter and boundary clean tools were used to remove isolated pixels and ragged boundaries, producing a more generalised output map. The classified images were then intersected with the brush-turkey occupied suburbs layer, to quantify the amount of each LULC type within brush-turkey occupied suburbs for each city in each year, and with the point layer of brush-turkey records to quantify LULC at each sighting point.
To test how the proportions of available habitat within occupied suburbs changed over time, we conducted Chi-squared tests of association between year and LULC classes in IBM SPSS 26. To determine how brush-turkeys preferentially used LULC classes we conducted Chi-squared tests of association between the proportion of brush-turkey records occurring in each LULC class (used habitat) to the proportion of total area within occupied suburbs covered by each class (available habitat). Post hoc Z-tests with Bonferroni corrections were conducted to determine which LULC classes had significantly different proportions between time periods and which LULC classes were used preferentially by brush-turkeys.
Results
Occurrence records
A total of 116,433 brush-turkey occurrence records were collected from 34 different sources. Following data filtering, this was reduced to 98,019 for the period 1839–2019 (Table 1). Of these, 69,671 records were located within significant urban areas. The three largest contributing sources of brush-turkey records were eBird (53.2%), Atlas of NSW Wildlife (24.6%), and Big City Birds (8.3%).
| Source | Total records | Urban records | Earliest record | Latest record | |
|---|---|---|---|---|---|
| eBird Australia | 52,093 (53%) | 30,265 (43%) | 24/08/1952 | 31/12/2019 | |
| Atlas of NSW Wildlife | 24,139 (25%) | 23,918 (34%) | 1/01/1900 | 9/12/2019 | |
| Big City Birds | 8115 (8%) | 8007 (12%) | 13/04/2008 | 31/12/2019 | |
| Birdata, BirdLife Australia | 5589 (6%) | 3086 (4%) | 29/09/1993 | 20/02/2019 | |
| WildNet | 2270 (2%) | 493 (0.7%) | 31/10/1856 | 13/10/2019 | |
| NSW Bird Atlassers | 1940 (2%) | 1937 (3%) | 1/03/1839 | 29/12/2011 | |
| Tamborine Mountain Weekly Bird Observations | 1212 (1%) | 0 | 26/07/1993 | 13/05/2019 | |
| iNaturalist Australia | 1000 (1%) | 700 (1%) | 4/05/1978 | 31/12/2019 | |
| Other Sources (n = 23) | 1661 (2%) | 1265 (2%) | 3/05/1865 | 29/12/2019 | |
| Total | 98,019 | 69,671 | 1/03/1839 | 31/12/2019 |
Total number of records, total number of urban records, date of the first record, and date of the most recent record shown for all sources with >1000 records. Date ranges presented included backdated records of sightings outside of the date ranges of the actual surveys.
Seven bioregions were continuously occupied by brush-turkeys throughout all time periods (1839–2019): Brigalow Belt North, Brigalow Belt South, Cape York Peninsula, NSW North Coast, South Eastern Queensland, Sydney Basin, and Wet Tropics (Fig. 1). Three additional bioregions were continuously occupied from 1900 to 2019: Central Mackay Coast, Nandewar, and New England Tablelands (Fig. 1b–f). These bioregions are mostly situated along the east coast of Australia, with a few located inland in Central NSW and Southern Queensland.
Australian brush-turkey records in each occupied bioregion (IBRA 7). Only parts of the bioregion containing brush-turkey records are shown (grey outlines). Darker shading indicates a greater proportion of total records for that time period (a–f) were present in the bioregion. Basemap layer credits: ESRI, USGS, NOAA.
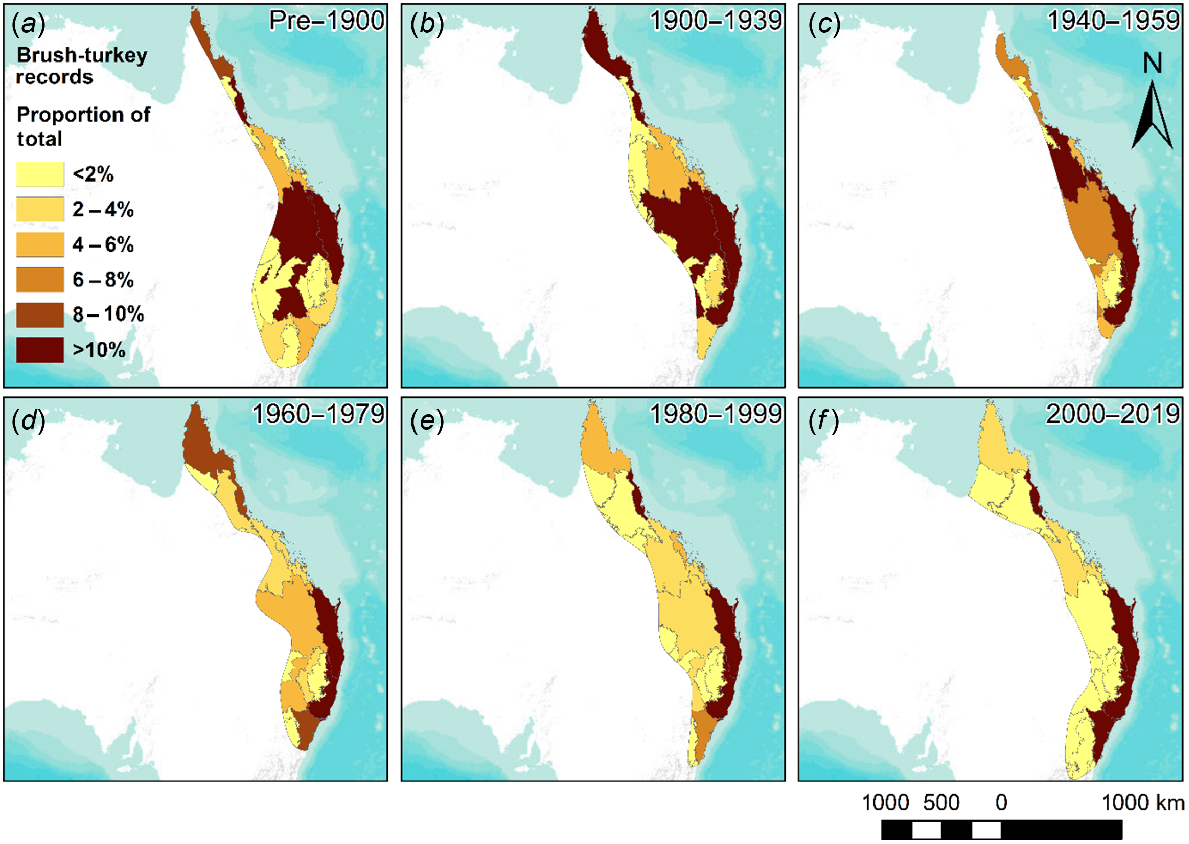
Continental scale distribution
Historical records prior to 1900 (n = 68) report brush-turkey presence in 11 bioregions, covering a total area of 1,018,348 km2. In two of these, the inland Cobar Peneplain and NSW South Western Slopes, brush-turkeys were not recorded after 1900 and were not observed again in any period (Fig. 1a). Occupied bioregions increased to 12 during the 1900–1939 period, with additional records in the Central Mackay Coast, Mitchell Grass Downs, Nandewar, and New England Tablelands. However, records ceased in the NSW South Western Slopes, South Eastern Highlands, and Cobar Peneplain bioregions (Fig. 1b). This represented an overall decrease in the brush-turkey range to 1,005,970 km2. Ten bioregions were occupied during the 1940–1959 period, with brush-turkeys no longer recorded in the inland Queensland bioregions of Einasleigh Uplands and Mitchell Grass Downs (Fig. 1c). This represented a decrease in total area of the brush-turkey range to 684,500 km2. Overall, from 1900–1959, the estimated brush-turkey range expanded in the central-western part of the species distribution but receded from the northwestern and south-western part of their pre-1900 distribution. The total area of the estimated brush-turkey range declined by 333,848 km2 during this period, or a decrease in total area of 32.8%, from the pre-1900 baseline.
Brush-turkey presence was recorded within 13 bioregions during 1960–1979, with new records from the Desert Uplands and Kanmantoo bioregions, and records resuming in the Einasleigh Uplands (Fig. 1d). This represented a total increase in area of the brush-turkey range to 956,441 km2. During the 1980–1999 period, the number of occupied bioregions increased to 16, with new records from the Mulga Lands and Gulf Plains. Records also resume in the South Eastern Highlands which had not had any records since before 1900 (Fig. 1e). This represented a further increase in total brush-turkey range area to 1,138,037 km2. Brush-turkey occupied bioregions decreased to 14 in 2000–2019, with records disappearing from the Mulga Lands and Desert Uplands (Fig. 1f). This represented a decrease in the total brush-turkey range to 1,124,483 km2.
Overall, from 1960–2019, the estimated brush-turkey range appeared to have contracted in the central-western part of their distribution but has expanded in the northwest and southeast. From 1960–2019, the total area of the estimated brush-turkey range increased by 168,042 km2, or 17.6%, and by 106,135 km2, or 10.4%, from the pre-1900 baseline.
City scale distribution
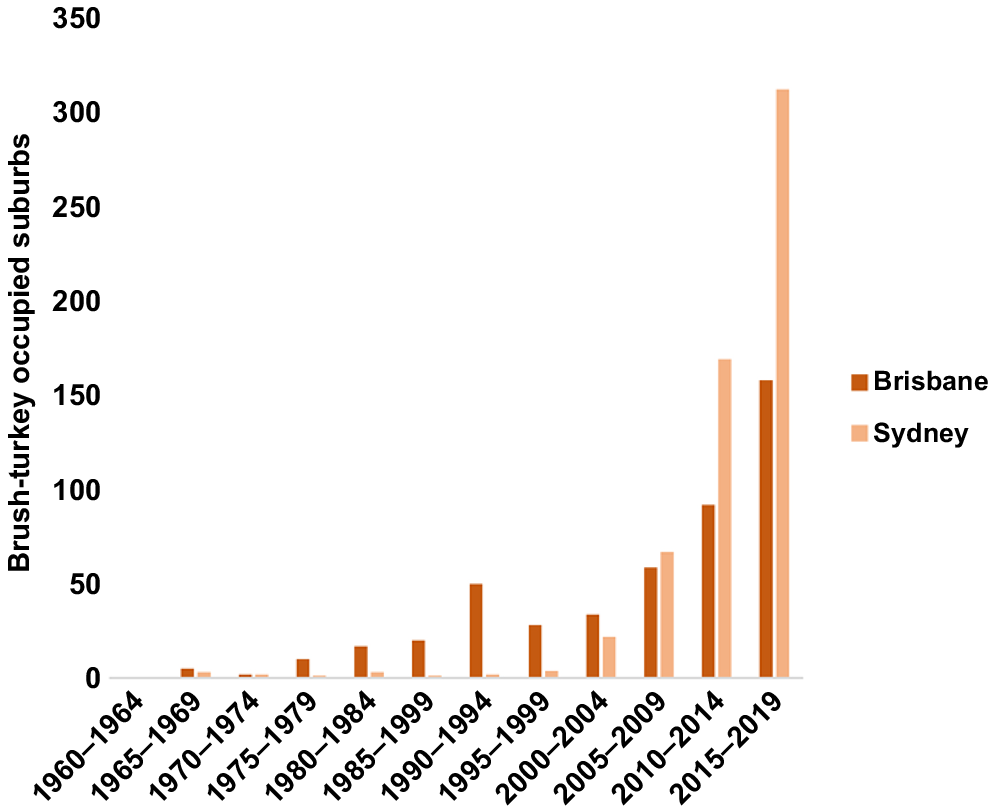
In both Brisbane and Sydney, the number of brush-turkey occupied suburbs showed a large overall increase during the period 1960–2019 (Fig. 2), with the largest increase in occupied suburbs occurring from 2010. In Brisbane, occupied suburbs increased steadily in each 5-year interval from 1965 to 1989 to a total of 50, then decreased from 1990–1994 to 28. The number of occupied suburbs then increased through all 5-year intervals to 2019, to a total of 289 suburbs. In Sydney, the number of occupied suburbs remained consistently low, fluctuating between 1 and 3 suburbs, across the 5-year time intervals from 1965 to 1994, this increased rapidly through to 2019, to a total of 310 suburbs (Fig. 2).
Suburbs occupied by Australian brush-turkeys for each 5-year interval (1960–2019) in the cities of Brisbane and Sydney. Suburbs are defined as polygons representing named localities within each city significant urban area as of 2019.
In Brisbane, brush-turkey occupied suburbs during the 1960–1979 period were scattered across the central-western part of the city, with more isolated records in the north and southeast. The highest percentage of total records came from suburbs in the centre and central-west of the city (Fig. 3a). During the 1980–1999 period an increasing number of suburbs were occupied in the central, southwestern, and northern parts of the city, as well as the suburbs of the southeast (Fig. 3b). By 2000–2019, brush-turkeys occupied the majority of suburbs in northern and central Brisbane, with a further increase in the number of occupied suburbs in the south. The suburbs with the largest percentage of records were in the central-west part of the city, while large areas in the south of the city remained unoccupied (Fig. 3c).
Percentage of Australian brush-turkey records from each occupied suburb in Brisbane (a–c) and Sydney (d–f) from 1960–1979, 1980–1999, and 2000–2019. Darker shading indicates a greater proportion of total records occur in this suburb. White areas indicate no records. Individual records outside the city boundary are shown as blue dots. Inset map shows the location of Brisbane (black) and Sydney (white). Basemap layer credits: ESRI, USGS, NOAA.
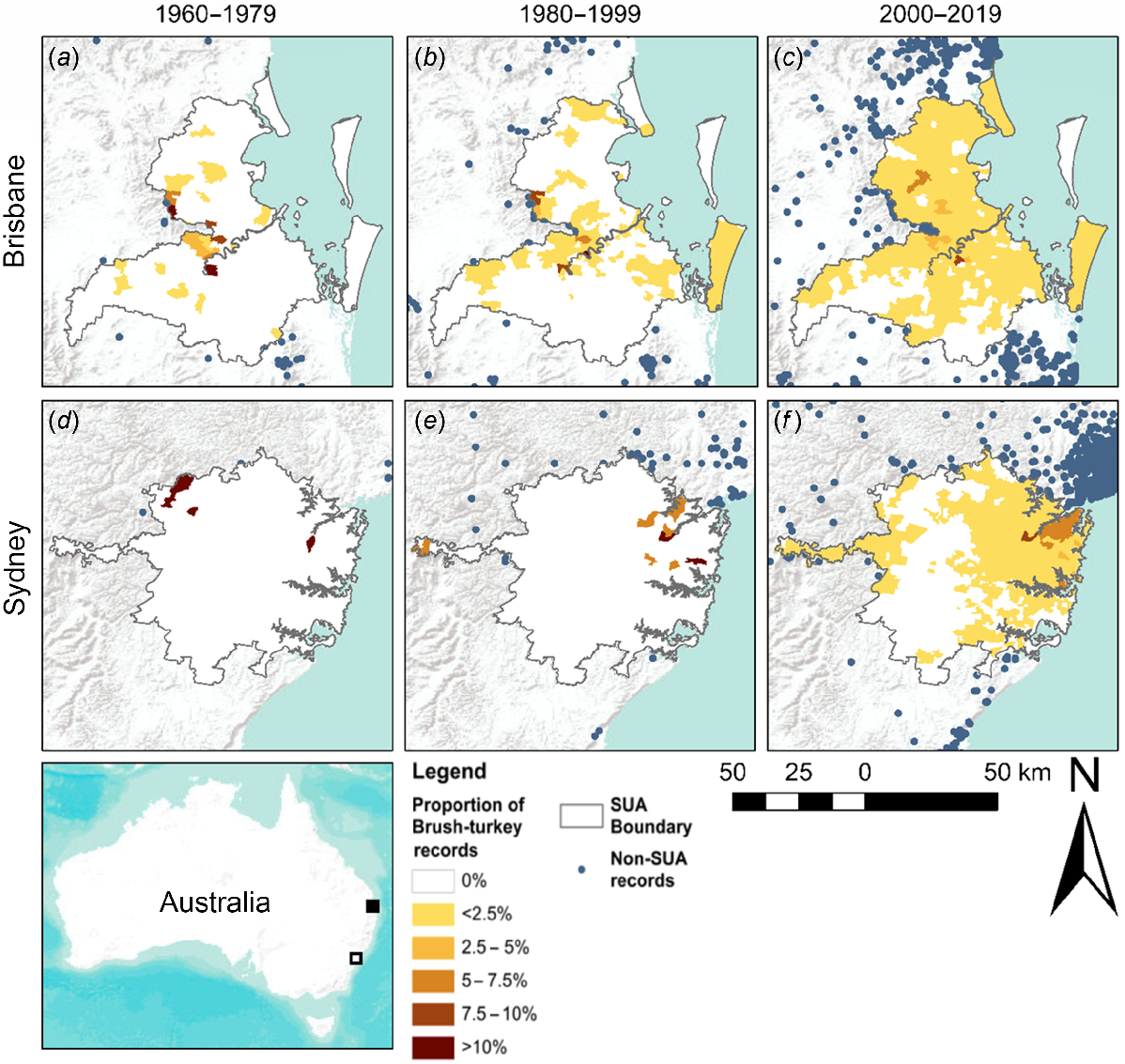
In Sydney, brush-turkey records first appeared in the north and northwest of the city during the 1960–1979 period (Fig. 3d). Increasing numbers of northern suburbs were occupied during the 1980–1999 period, as well as suburbs in the west of the city, however brush-turkeys disappeared from the north-western suburbs (Fig. 3e). All northern suburbs of Sydney were occupied by the 2000–2019 period, along with the narrow strip of suburbs in the mountainous area to the west of the city, and an increasing number of suburbs in the northwest and southeast of the city. The northern suburbs contained the highest percentage of records from 1980–2019. The majority of the western, central, and southern suburbs of the city remain unoccupied by brush-turkeys; however, suburbs to the east and west have been occupied during the 2000–2019 period (Fig. 3f).
City scale land use
The total area of brush-turkey occupied suburbs within the Brisbane SUA (available habitat area) increased from 588 km2 in 1979 to 1753 km2 in 1999, and to 4801 km2 in 2019. The total area occupied by brush-turkeys within the Sydney SUA increased from 95 km2 in 1979 to 180 km2 in 1999, and to 3132 km2 in 2019. The proportion of total area in brush-turkey occupied suburbs covered by different LULC classes was significantly different between time periods for both Brisbane ( = 434.87, P < 0.001; Fig. 4a–c) and Sydney ( = 189.87, P < 0.001; Fig. 4d–f). In Brisbane, the proportion of area in brush-turkey occupied suburbs covered by dense vegetation and bare land significantly decreased from 1979 to 1999, and from 1999 to 2019. The proportion of available area covered by open green space was significantly lower in 1999 than in either 1979 or 2019. The proportion of available area covered by residential area was not significantly different from 1979 to 1999 but increased from 1999 to 2019. The proportion of available area covered by commercial development significantly increased from 1979 to 1999 and did not significantly change between 1999 and 2019.
Used versus available habitat in Brisbane (a–c) and Sydney (d–f) from 1979–2019. Available habitat defined as the total area of each land use within brush-turkey occupied suburbs within the Brisbane and Sydney significant urban areas. Used habitat defined as the land use class at brush-turkey sighting point.
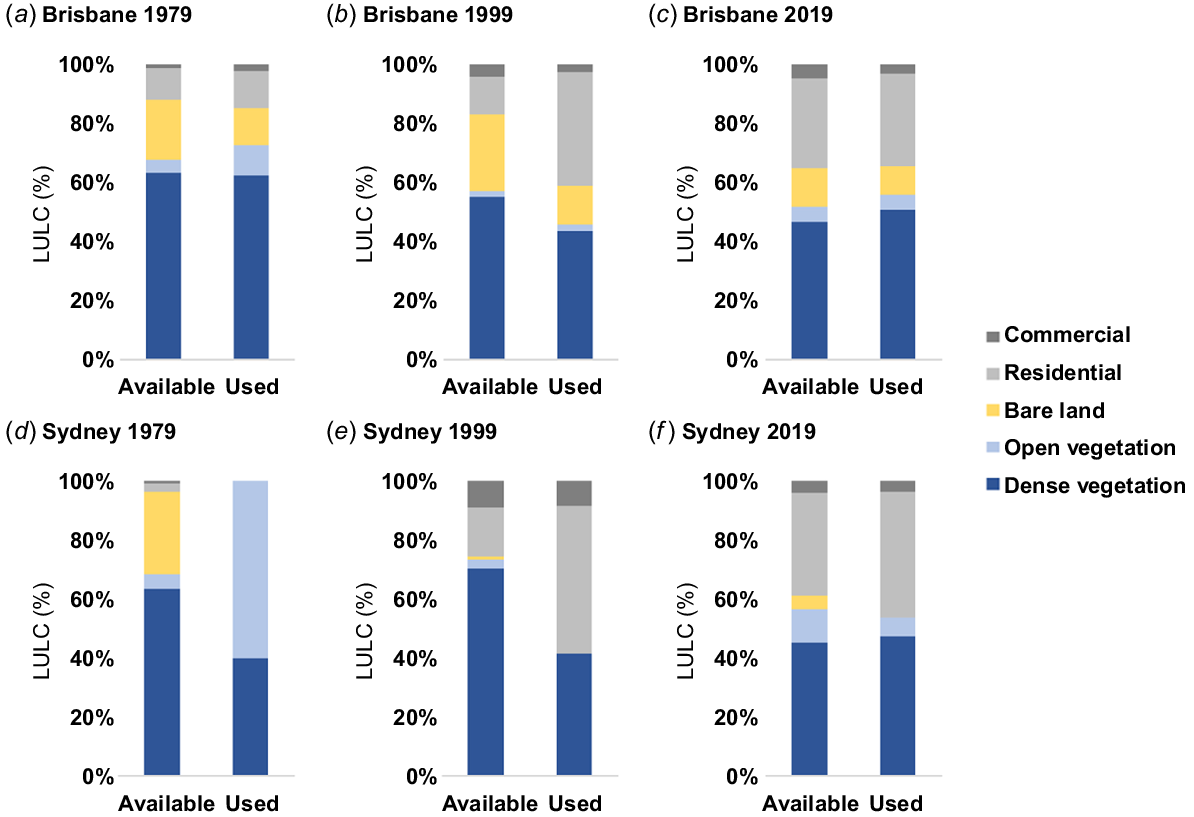
In Sydney, the proportion of available habitat area covered by dense vegetation remained constant from 1979 to 1999, and significantly decreased from 1999 to 2019. The proportion of available habitat area covered by open greenspace significantly decreased from 1979 to 1999 and significantly increased from 1999 to 2019. The proportion of available habitat area covered by bare land significantly decreased from 1979 to 1999 and remained constant from 1999 to 2019. The proportion of available habitat area covered by residential area significantly increased from 1979 to 1999 and from 1999 to 2019. The proportion of available habitat area covered by commercial development significantly increased from 1979 to 1999 and significantly decreased from 1999 to 2019 (Fig. 4d–f).
The overall proportion of brush-turkey records occurring in each LULC class was significantly different from the proportion of available area covered by each LULC class in both Brisbane ( = 304.16, P < 0.001; Fig. 4a–c) and Sydney ( = 933.71, P < 0.001 Fig. 4d–f). In Brisbane, used versus available area did not differ in 1979 (P > 0.05; Fig. 4a), but was significantly different in 1999 ( = 142.63, P < 0.001) and 2019 ( = 72.84, P < 0.001). In 1999, brush-turkey records were located in residential areas at greater-than-expected numbers, while records were located in areas of bare land and dense vegetation at lower-than-expected numbers. The proportion of records located in commercial areas and in open green space did not significantly differ from expected proportions (Fig. 4b). In 2019, brush-turkey records were located in areas of dense vegetation at greater-than-expected numbers, while records were located in bare land and commercial areas at lower-than-expected numbers. The proportion of records located in open green space and residential areas did not significantly differ from expected proportions (Fig. 4c). Across all three time periods, records were located in residential land and in open green space at greater-than-expected numbers, but at lower-than-expected numbers in bare land and commercial areas. Total record numbers in dense vegetation did not significantly differ from expected proportions.
In Sydney, used versus available habitat significantly differed in 1979 ( = 29.83, P < 0.001) and 2019 ( = 838.37, P < 0.001), but not in 1999 (P > 0.05; Fig. 4e). In 1979, brush-turkey records were located in open greenspace at greater-than-expected numbers. The proportion of records in bare land, dense vegetation, commercial, and residential areas did not significantly differ from expected proportions (Fig. 4d). In 2019, brush-turkey records were located in greater-than-expected numbers in residential areas and dense vegetation. Records were located in areas of bare land and open green space at less-than-expected numbers. The proportion of records in commercial areas did not significantly differ from expected proportions (Fig. 4f). Across all three time periods, records were located in residential areas at greater-than-expected numbers, but at lower-than-expected numbers in bare land and open green space. The number of records in dense vegetation and commercial areas did not significantly differ from expected proportions.
Discussion
After experiencing a human-mediated decline in the early 20th century, particularly in and around urban areas, brush-turkeys are actively recolonising the large cities of Brisbane and Sydney. Contrary to our original prediction, their urban expansion was not limited to suburbs adjacent to remnant vegetation and with high vegetation cover, as we found that brush-turkeys have increasingly colonised suburbs characterised by less vegetation cover and more developed land over time. The changes in the brush-turkey distribution were not limited to urban areas, with this species spreading consistently into rural and natural areas since 1960. Firstly, our assessment identified an estimated decrease in the species range of ~33% from 1900 to 1959, followed by an overall estimated increase in range of ~18% from 1960 to 2019. Most of the land area lost was in the western and southwestern portions of the species’ range, while expansion occurred in the northwest. This supported our prediction that the range of the species will have expanded following their protection under conservation legislation. All observed distributional changes were driven by records of the more common subspecies A. l. lathami, with the few A. l. purpureicollis records (47 total) confined to the Cape York Peninsula, North Queensland throughout the study.
Continental distribution trends
Brush-turkeys expanded into one new bioregion, the Gulf Plains, in the last few decades. The Gulf Plains bioregion lies along the north coast of Australia and is adjacent to the Cape York Peninsula and Einasleigh Uplands bioregions, which have been continuously and near continuously occupied by brush-turkeys respectively. Given the steady increase in records over the last four decades, it is likely that brush-turkeys naturally expanded their range into the Gulf Plains bioregion from source populations in the adjacent bioregions as these neighbouring populations grew over recent decades.
There is a general trend of increasing brush-turkey records from 1900–2019 in all continuously occupied bioregions, with the greatest increase in the number of observations occurring in the 2000–2019 period (Table S1.2). However, the number of observations increased disproportionately in coastal and urban bioregions with larger human populations. The largest increase in records was in the NSW North Coast, South Eastern Queensland, Sydney Basin, and Wet Tropics bioregions, where the total number of records increased by several orders of magnitude. While brush-turkey records increased over time in the Brigalow Belt South, Nandewar, and New England Tablelands bioregions, the number of records in these regions did not increase at a comparable rate to the coastal bioregions. This may be due to a lower human population in these areas, and hence fewer sightings, or it may indicate a smaller brush-turkey population.
Brush-turkeys ceased to be reported from multiple bioregions, indicating the long-term local extinction of this species from those areas. Three of these bioregions, Cobar Peneplain, South Western Slopes, and Riverina are at the southwestern edge of the species’ distribution. A fourth bioregion, Desert Uplands, is to the northwest of their range. Previous research suggested that brush-turkey numbers were in decline in these areas (Göth et al. 2006) including the threatened population in the Nandewar and Brigalow Belt South bioregions (DPIE 2017). Previous studies have estimated that a third of the overall suitable habitat for brush-turkeys has been cleared, with the most significant loss (61%) occurring in the Brigalow Belt North and South regions (Simmonds et al. 2019). This has largely been driven by extensive deforestation across the east-coast Australian states (Evans 2016). Predation from introduced cats and foxes, as well as overhunting by humans have also been suggested as possible explanations for brush-turkeys’ population and range decline (Göth and Vogel 2002; Jones and Göth 2008). Predation pressure on chicks is particularly high, with an 88–100% mortality observed in previous radio tracking studies (Göth and Vogel 2002). It is likely that habitat loss, predation, and hunting have all contributed to the observed range contractions. Brush-turkeys are considered to have a low vulnerability to the effects of climate change due to their broad climatic tolerance and the ability of their eggs to withstand a wide range of temperature fluctuations during incubation (Eiby and Booth 2008; Radley et al. 2018). Thus, the impact of climate change has likely had a small impact on historical distribution changes compared to other factors. However, further studies are needed to consider how the effects of climate change, particularly changing precipitation and fire regimes, may impact the future brush-turkey distribution.
Estimation of the brush-turkey range, and associated changes to the population, was considerably more difficult for earlier time periods because of the scarcity of records. Older records were primarily based on opportunistic reporting, incidental observations, museum specimens, and records collected from various published and unpublished literature. The first concerted national survey of Australian birds was conducted between 1977 and 1981 for the first Atlas of Australian Birds (Blakers et al. 1984). It is likely that our data, based on records prior to this survey, underestimated the brush-turkey range for these time periods. Additionally, some uncertainty exists for records prior to 1960. While all records used in this study included an exact date and coordinates, this was often based on the central point of a grid or locality for many records prior to the introduction of GPS technology (1980s). The continental scale used in this study may also obscure population changes at a local scale. Despite these limitations, the presence and absence of records at the scale of entire bioregions can be clearly observed with the data used in this study. We can thus be confident of the broad scale changes in the brush-turkey distribution.
Urban distribution trends
Prior to the 1960s, brush-turkey records were conspicuously absent from Brisbane and Sydney, despite the existence of records from other parts of the South Eastern Queensland bioregions and Sydney Basin. Anecdotal evidence suggests that the species did occur in these areas prior to European colonisation and prior to the 20th century (Jones and Göth 2008) and this aligned with the species presence in neighbouring bioregions. This indicates that brush-turkeys had become locally extinct around these urban centres prior to their return across both cities from the mid-1960s onwards.
The rapid increase in brush-turkey observations in the suburbs of Brisbane and Sydney in the 2000–2019 period counters an earlier suggestion that cities may act as ecological traps for the species due to reduced reproductive success in urban areas (Jones and Everding 1991). The total number of suburbs occupied in both cities greatly increased over the last 20 years, continuing previously identified trends (Jones et al. 2004; Göth et al. 2006), and our results show that brush-turkeys have increasingly occupied suburbs with less vegetation cover and more residential development over time. Moreover, in both Brisbane and Sydney a greater proportion of records came from residential areas than would be expected from available habitat. Part of this change is because of changing land use within previously occupied areas as both cities expanded, however the majority of this change has been driven by a rapid, more than tenfold, increase in the total number of localities occupied by the species across both cities. This trend contrasts with observed trends elsewhere in Australia where species occupation declines as housing cover increases relative to vegetation (Humphrey et al. 2023), and this is likely to continue as brush-turkeys continue to colonise these cities.
Emigration from populations in natural areas, illegal translocations, and local recruitment from suburban populations have all been suggested as mechanisms for dispersal into suburban areas (Jones and Everding 1991). Older occupied suburbs are geographically closer to non-urban brush-turkey records in National Parks adjacent to both Brisbane and Sydney, indicating that early arrivals were migrants from populations in rural areas. However, the importance of colonisation from non-urban populations certainly diminishes in inner-city suburbs that are distant from natural areas. In inner-city suburbs, the dense urban matrix may constrain dispersal, making movement from distant areas more difficult (e.g. Fischer and Lindenmayer 2007; Canedoli et al. 2018, but see Hall et al. 2022). Brush-turkeys have been reported to have a high juvenile mortality rate (Göth and Vogel 2002) and higher rates of egg failure in urban areas compared with their natural habitat (Jones and Everding 1991). However, their continued spread across Brisbane and Sydney indicates that urban breeding success has not only maintained the population but has been sufficient to support the expansion of the population. Further surveys of the connectivity between urban and non-urban brush-turkey populations are needed to determine the importance of urban habitats as refugia for the species.
On the surface, brush-turkeys do not fit the profile of the typical successful urban dwelling bird. Their ground nesting, lack of parental care, and poor flight abilities contrast with the off-ground nesting and high dispersal ability (through flight) common to most successful urban birds (see Møller 2009; Evans et al. 2011; Bressler et al. 2020). However, they have a few traits common to other successful urban species. Brush-turkeys have a generalist omnivorous diet (Jones and Göth 2008) and have been observed feeding on novel food resources including introduced plants and anthropogenic food sources (Brookes 1919; Jones and Everding 1991; Warnken et al. 2004). Their larger body size is also consistent with the observed trend of smaller bodied species declining in urban areas (Joyce et al. 2018; Campbell et al. 2022). Brush-turkeys are also considered to be a highly disturbance tolerant species (Blumstein 2006), showing reduced fear behaviour in urban areas compared with reserves and natural bushland (Hall et al. 2020), and are capable of travelling through an urban matrix despite their poor flight capability (Hall et al. 2022). A generalist diet and increased boldness are common traits among urban birds (Callaghan et al. 2019b) and likely help the species colonise and persist in urban areas where disturbances and unnatural food sources are common. This suggests that a species may only need a few urban suitable traits to thrive in urban areas under the correct conditions; this highlights that a wider list of candidate species may be able to effectively colonise cities.
The successful colonisation of urban areas by brush-turkeys is in stark contrast to the other Australian Megapode species, the malleefowl (Leipoa ocellata) and orange-footed scrubfowl (Megapodius reinward). Orange-footed scrubfowl are known to occur in urban areas in Northern Queensland, including in the cities of Darwin, Cairns, and Port Douglas; however, the species does not appear to have penetrated urban areas to the same extent at brush-turkeys (Jones and Göth 2008). Malleefowl, in contrast, are conspicuously absent from all urban areas and have suffered significant range declines overall as a result of habitat destruction and impacts of introduced species (Jones and Göth 2008). All three species have similar foraging behaviours, diet, and incubation mound construction; however, studies on the fear behaviour or movement patterns of scrubfowl and malleefowl are presently lacking. Further research is needed to determine the traits that most contribute to the success of the brush-turkey compared with related species.
The increasing movement of brush-turkeys into less vegetated suburbs will lead to more frequent encounters with suburban residents. Proximity of wildlife and humans can occasionally lead to situations of human–wildlife conflict, particularly in urban areas (Soulsbury and White 2015). Brush-turkey presence in suburban areas has resulted in complaints from residents in response to damage to gardens caused by foraging and the construction of 3 tonne incubation mounds, both of which involve raking soil and leaf litter (Jones and Everding 1991). Complaints regarding chasing pets and small children, stealing food, noise, and fouling have also been reported (Jones and Göth 2008), leading to calls for the management of predominantly urban brush-turkey populations. While brush-turkeys are not considered to be a threatened species across their distribution, their apparent decline in the western and southwestern ends of their range necessitates careful consideration when managing their population in urban areas.
Limitations of atlas and citizen science data
The dramatic increase in the number of brush-turkey records in the new millennium is facilitated by the creation of citizen science projects and community uptake of smartphone apps and online platforms for reporting wildlife observations (Pocock et al. 2017). Data sourced from citizen science projects can contain limitations, specifically concerns around data quality, uneven participation rates, and observer bias (Tulloch et al. 2013; Brown and Williams 2019). However, there is a growing body of evidence that citizen science generated data can be of comparable quality to data generated by professionals (Gollan et al. 2012; Callaghan et al. 2020), can contain considerable amounts of ecological information (Hall et al. 2021), and can be used to answer a variety of research questions (McKinley et al. 2017; Klump et al. 2021). Two of the top three sources of brush-turkey records in this study came from citizen science projects, eBird (Sullivan et al. 2009) and Big City Birds (Hall et al. 2021), while the third, the Atlas of NSW Wildlife, receives significant contributions from citizen scientists (DPIE 2021). This trend suggests that greater insights into the brush-turkey population, including finer scale presence and habitat preferences, will be possible while citizen science participation continues and, ideally, increases.
A particular challenge of observing trends using citizen science data is disentangling a biological signal from the effects of human population and the accompanying spatial and temporal clustering of observations (Isaac et al. 2014). The greatest increase in the number of brush-turkey reports was in South East Queensland (includes Brisbane) and Sydney Basin bioregions. These areas have both the highest human population densities and highest human population growth within the brush-turkey range (ABS 2021). Thus, regional differences in the number of records may reflect human, rather than brush-turkey, population density. Similarly, the trend of brush-turkey records within cities occurring disproportionately in residential areas compared with other land uses is likely to be driven by human population density. Irrespective of the human population affecting records, it is clear from the data that brush-turkeys have spread across Brisbane and Sydney over recent decades through presence and absence data alone. Additionally, the upwards trend in the number of observations begins in some bioregions as early as the 1960s, where modern technologies to assist citizen scientists did not exist. Given the increasing availability of methods and technology to record and report observations, the complete absence of brush-turkey records from some bioregions in the 2000–2019 period is strong evidence that the species is rare or absent from these areas, including the regions where they were historically observed. Future studies should attempt to quantify observer effort to draw more detailed conclusions about the changing brush-turkey distribution at finer temporal and spatial scales.
Conclusions
Understanding how species distributions have changed over time and in response to human derived land use change is foundational to inform their management and conservation, as well as predicting how they will respond to future landscape changes. Using a blend of historic records, ecological surveys, and citizen science data our study determined that the brush-turkey has undergone a complex range shift over the past century, disappearing from the edges of their range in the southwest while recolonising the heavily modified urban areas on the Australian east coast. Over the last sixty years, the species has successfully colonised more built-up and less vegetated areas of Brisbane and Sydney. The brush-turkey has become an incredibly successful urban dwelling species, despite its specialised reproductive strategy and primarily terrestrial dispersal ability, broadening scientific understanding of the traits that can characterise successful city dwelling wildlife. Future research should focus on: tracking finer scale distribution changes for the species at continental and city scales, particularly at the edges of their range; determining the drivers behind brush-turkey declines in the western parts of their range and expansion in urban areas; and quantifying brush-turkey responses to different land uses within urban areas.
Animal ethics
Research was undertaken under scientific licence SL101960 authorised by the National Parks and Wildlife Service, NSW, Australia and animal ethics approval 4a817 authorised by the Taronga Conservation Society.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Declaration of funding
The Big City Birds app was developed by Spotteron in collaboration with the University of Sydney and the Taronga Conservation society, and was funded by grants from the Australian Citizen Science Association and Birding NSW.
Acknowledgements
The authors would like to thank the volunteers and citizen scientists who contributed to the datasets used in this study, and reviewers who provided constructive comments on an earlier version of this manuscript. This manuscript forms a part of Matthew Hall’s PhD thesis Ecology of the Australian Brush-turkey in Urban Ecosystems (2022).
References
Anderies JM, Katti M, Shochat E (2007) Living in the city: resource availability, predation, and bird population dynamics in urban areas. Journal of Theoretical Biology 247, 36-49.
| Crossref | Google Scholar | PubMed |
ABS (2019) Australian Statistical Geography Standard (ASGS): Volume 3 – Non ABS Structures. Australian Bureau of Statistics. Available at https://www.abs.gov.au/ausstats/abs@.nsf/mf/1270.0.55.003 [Retrieved 25 July 2021]
ABS (2021) Regional population. Australian Bureau of Statistics. Available at https://www.abs.gov.au/statistics/people/population/regional-population/2019-20#regions [Retrieved 5 October 2021]
ALA (2021) occurrence download, Australian brush-turkey. Atlas of Living Australia. Available at https://doi.org/10.26197/ala.32c174df-6bf8-4f60-b6c7-93a1a3f76ad1 [retrieved 3 March 2021]
Aronson MFJ, La Sorte FA, Nilon CH, Katti M, Goddard MA, Lepczyk CA, Warren PS, Williams NSG, Cilliers S, Clarkson B, Dobbs C, Dolan R, Hedblom M, Klotz S, Kooijmans JL, Kühn I, MacGregor-Fors I, McDonnell M, Mörtberg U, Pyšek P, Siebert S, Sushinsky J, Werner P, Winter M (2014) A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proceedings of the Royal Society B: Biological Sciences 281, 20133330.
| Crossref | Google Scholar |
Banville MJ, Bateman HL, Earl SR, Warren PS (2017) Decadal declines in bird abundance and diversity in urban riparian zones. Landscape and Urban Planning 159, 48-61.
| Crossref | Google Scholar |
BirdLife International (2021) Species factsheet: Alectura lathami. Available at http://www.birdlife.org [Retrieved 9 October 2021]
Blumstein DT (2006) Developing an evolutionary ecology of fear: how life history and natural history traits affect disturbance tolerance in birds. Animal Behaviour 71, 389-399.
| Crossref | Google Scholar |
Bressler SA, Diamant ES, Tingley MW, Yeh PJ (2020) Nests in the cities: adaptive and non-adaptive phenotypic plasticity and convergence in an urban bird. Proceedings of the Royal Society B: Biological Sciences 287, 20202122.
| Crossref | Google Scholar |
Brookes GB (1919) Report on investigations in regard to the spread of prickly pear by the Scrubs-Turkey. Emu 18, 288-292.
| Crossref | Google Scholar |
Brown ED, Williams BK (2019) The potential for citizen science to produce reliable and useful information in ecology. Conservation Biology 33, 561-569.
| Crossref | Google Scholar | PubMed |
Callaghan CT, Bino G, Major RE, Martin JM, Lyons MB, Kingsford RT (2019a) Heterogeneous urban green areas are bird diversity hotspots: insights using continental-scale citizen science data. Landscape Ecology 34, 1231-1246.
| Crossref | Google Scholar |
Callaghan CT, Major RE, Wilshire JH, Martin JM, Kingsford RT, Cornwell WK (2019b) Generalists are the most urban-tolerant of birds: a phylogenetically controlled analysis of ecological and life history traits using a novel continuous measure of bird responses to urbanization. Oikos 128, 845-858.
| Crossref | Google Scholar |
Callaghan CT, Roberts JD, Poore AGB, Alford RA, Cogger H, Rowley JJL (2020) Citizen science data accurately predicts expert-derived species richness at a continental scale when sampling thresholds are met. Biodiversity and Conservation 29, 1323-1337.
| Crossref | Google Scholar |
Campbell CE, Jones DN, Awasthy M, Castley JG, Chauvenet ALM (2022) Big changes in backyard birds: an analysis of long-term changes in bird communities in Australia’s most populous urban regions. Biological Conservation 272, 109671.
| Crossref | Google Scholar |
Canedoli C, Manenti R, Padoa-Schioppa E (2018) Birds biodiversity in urban and periurban forests: environmental determinants at local and landscape scales. Urban Ecosystems 21, 779-793.
| Crossref | Google Scholar |
Croci S, Butet A, Clergeau P (2008) Does Urbanization filter birds on the basis of their biological traits. The Condor 110, 223-240.
| Crossref | Google Scholar |
Crooks KR, Soulé ME (1999) Mesopredator release and avifaunal extinctions in a fragmented system. Nature 400, 563-566.
| Crossref | Google Scholar |
Duarte J, Farfán MA, Fa JE, Vargas JM (2015) Deer populations inhabiting urban areas in the south of Spain: habitat and conflicts. European Journal of Wildlife Research 61, 365-377.
| Crossref | Google Scholar |
Eiby Y, Booth D (2008) Embryonic thermal tolerance and temperature variation in mounds of the Australian Brush-Turkey (Alectura Lathami). The Auk 125, 594-599.
| Crossref | Google Scholar |
Evans MC (2016) Deforestation in Australia: drivers, trends and policy responses. Pacific Conservation Biology 22, 130-50.
| Crossref | Google Scholar |
Evans KL, Chamberlain DE, Hatchwell BJ, Gregory RD, Gaston KJ (2011) What makes an urban bird? Global Change Biology 17, 32-44.
| Crossref | Google Scholar |
Faeth SH, Warren PS, Shochat E, Marussich WA (2005) Trophic dynamics in urban communities. BioScience 55, 399-407.
| Crossref | Google Scholar |
Fischer J, Lindenmayer DB (2007) Landscape modification and habitat fragmentation: a synthesis. Global Ecology and Biogeography 16, 265-280.
| Crossref | Google Scholar |
Fischer SE, Edwards AC, Weber P, Garnett ST, Whiteside TG (2021) The bird assemblage of the Darwin Region (Australia): what is the effect of twenty years of increasing urbanisation? Diversity 13, 294.
| Crossref | Google Scholar |
Gollan J, Lobry de Bruyn L, Reid N, Wilkie L (2012) Can volunteers collect data that are comparable to professional scientists? A study of variables used in monitoring the outcomes of ecosystem rehabilitation. Environmental Management 50, 969-978.
| Crossref | Google Scholar | PubMed |
Grimm NB, Faeth SH, Golubiewski NE, Redman CL, Wu J, Bai X, Briggs JM (2008) Global change and the ecology of cities. Science 319, 756-760.
| Crossref | Google Scholar | PubMed |
Göth A, Vogel U (2002) Chick survival in the megapode Alectura lathami (Australian brush-turkey). Wildlife Research 29, 503-11.
| Crossref | Google Scholar |
Göth A, Nicol KP, Ross G, Shields JJ (2006) Present and past distribution of Australian brush-turkeys Alectura lathami in New South Wales – Implications for management. Pacific Conservation Biology 12, 22-30.
| Crossref | Google Scholar |
Hahs AK, McDonnell MJ (2006) Selecting independent measures to quantify Melbourne’s urban–rural gradient. Landscape and Urban Planning 78, 435-448.
| Crossref | Google Scholar |
Hall MJ, Burns AL, Martin JM, Hochuli DF (2020) Flight initiation distance changes across landscapes and habitats in a successful urban coloniser. Urban Ecosystems 23, 785-791.
| Crossref | Google Scholar |
Hall MJ, Martin JM, Burns AL, Hochuli DF (2021) Ecological insights into a charismatic bird using different citizen science approaches. Austral Ecology 46, 1255-1265.
| Crossref | Google Scholar |
Hall MJ, Martin JM, Burns AL, Hochuli DF (2022) Unexpected dispersal of Australian brush-turkeys (Alectura lathami) in an urban landscape. Austral Ecology 47, 1544-1548.
| Crossref | Google Scholar |
Humphrey JE, Haslem A, Bennett AF (2023) Housing or habitat: what drives patterns of avian species richness in urbanized landscapes? Landscape Ecology 38, 1919-1937.
| Crossref | Google Scholar |
Isaac NJB, van Strien AJ, August TA, de Zeeuw MP, Roy DB (2014) Statistics for citizen science: extracting signals of change from noisy ecological data. Methods in Ecology and Evolution 5, 1052-1060.
| Crossref | Google Scholar |
Ives CD, Lentini PE, Threlfall CG, Ikin K, Shanahan DF, Garrard GE, Bekessy SA, Fuller RA, Mumaw L, Rayner L, Rowe R, Valentine LE, Kendal D (2016) Cities are hotspots for threatened species. Global Ecology and Biogeography 25, 117-126.
| Crossref | Google Scholar |
Jackowiak M, Gryz J, Jasińska K, Brach M, Bolibok L, Kowal P, Krauze-Gryz D (2021) Colonization of Warsaw by the red fox Vulpes vulpes in the years 1976–2019. Scientific Reports 11, 13931.
| Crossref | Google Scholar |
Jones DN, Everding SE (1991) Australian brush-turkeys in a suburban environment: implications for conflict and conservation. Wildlife Research 18, 285-297.
| Crossref | Google Scholar |
Jones DN, Sonnenburg R, Sinden KE (2004) Presence and distribution of Australian Brushturkeys in the greater Brisbane region. Sunbird: Journal of the Queensland Ornithological Society 34, 1-9.
| Google Scholar |
Joyce M, Barnes MD, Possingham HP, Van Rensburg BJ (2018) Understanding avian assemblage change within anthropogenic environments using citizen science data. Landscape and Urban Planning 179, 81-89.
| Crossref | Google Scholar |
Klump BC, Martin JM, Wild S, Hörsch JK, Major RE, Aplin LM (2021) Innovation and geographic spread of a complex foraging culture in an urban parrot. Science 373(6553), 456-460.
| Crossref | Google Scholar | PubMed |
Ley A, Tynan B, Cameron M (2011) Birds in Diamantina National Park, Queensland. Australian Field Ornithology 28, 1-208.
| Google Scholar |
Martin J, French K, Major R (2010) Population and breeding trends of an urban coloniser: the Australian white ibis. Wildlife Research 37, 230-239.
| Crossref | Google Scholar |
McDonald RI, Kareiva P, Forman RTT (2008) The implications of current and future urbanization for global protected areas and biodiversity conservation. Biological Conservation 141, 1695-1703.
| Crossref | Google Scholar |
McKinley DC, Miller-Rushing AJ, Ballard HL, Bonney R, Brown H, Cook-Patton SC, Evans DM, French RA, Parrish JK, Phillips TB, Ryan SF, Shanley LA, Shirk JL, Stepenuck KF, Weltzin JF, Wiggins A, Boyle OD, Briggs RD, Chapin SF, III, Hewitt DA, Preuss PW, Soukup MA (2017) Citizen science can improve conservation science, natural resource management, and environmental protection. Biological Conservation 208, 15-28.
| Crossref | Google Scholar |
McKinney ML (2002) Urbanization, biodiversity, and conservation: the impacts of urbanization on native species are poorly studied, but educating a highly urbanized human population about these impacts can greatly improve species conservation in all ecosystems. BioScience 52, 883-90.
| Crossref | Google Scholar |
McKinney ML (2006) Urbanization as a major cause of biotic homogenization. Biological Conservation 127, 247-260.
| Crossref | Google Scholar |
Møller AP (2009) Successful city dwellers: a comparative study of the ecological characteristics of urban birds in the Western Palearctic. Oecologia 159, 849-858.
| Crossref | Google Scholar | PubMed |
NSW Department of Planning, Industry and Environment (DPIE) (2017) Australian brush-turkey population, Nandewar and Brigalow Belt South bioregions – endangered population listing. Available at https://www.environment.nsw.gov.au/topics/animals-and-plants/threatened-species/nsw-threatened-species-scientific-committee/determinations/final-determinations/2004-2007/australian-brush-turkey-endangered-population-listing [Retrieved 9 October 2021]
NSW Department of Planning, Industry and Environment (DPIE) (2021) About BioNet Atlas. Available at https://www.environment.nsw.gov.au/topics/animals-and-plants/biodiversity/nsw-bionet/about-bionet-atlas [Retrieved 9 October 2021]
Pocock MJO, Tweddle JC, Savage J, Robinson LD, Roy HE (2017) The diversity and evolution of ecological and environmental citizen science. PLoS ONE 12, e0172579.
| Crossref | Google Scholar | PubMed |
Queensland Government (2021) WildNet Database. Available at https://www.qld.gov.au/environment/plants-animals/species-information/wildnet [Accessed 25 July 2021].
Radley PM, Davis RA, Dekker RWRJ, Molloy SW, Blake D, Heinsohn R (2018) Vulnerability of megapodes (Megapodiidae, Aves) to climate change and related threats. Environmental Conservation 45, 396-406.
| Crossref | Google Scholar |
Shochat E, Warren PS, Faeth SH, McIntyre NE, Hope D (2006) From patterns to emerging processes in mechanistic urban ecology. Trends in Ecology & Evolution 21, 186-191.
| Crossref | Google Scholar | PubMed |
Simmonds JS, Watson JEM, Salazar A, Maron M (2019) A composite measure of habitat loss for entire assemblages of species. Conservation Biology 33, 1438-1447.
| Crossref | Google Scholar | PubMed |
Soulsbury CD, White PCL (2015) Human–wildlife interactions in urban areas: a review of conflicts, benefits and opportunities. Wildlife Research 42, 541-553.
| Crossref | Google Scholar |
Stillfried M, Gras P, Börner K, Göritz F, Painer J, Röllig K, Wenzler M, Hofer H, Ortmann S, Kramer-Schadt S (2017) Secrets of success in a landscape of fear: urban wild boar adjust risk perception and tolerate disturbance. Frontiers in Ecology and Evolution 5, 157.
| Crossref | Google Scholar |
Sullivan BL, Wood CL, Iliff MJ, Bonney RE, Fink D, Kelling S (2009) eBird: A citizen-based bird observation network in the biological sciences. Biological Conservation 142, 2282-2292.
| Crossref | Google Scholar |
Thackway R, Cresswell ID (1995) An interim biogeographic regionalisation for Australia: a framework for setting priorities in the National Reserves System Cooperative Program. Available at https://www.dcceew.gov.au/sites/default/files/documents/ibra-framework-setting-priorities-nrs-cooperative-program.pdf
The Brush Turkey (1881) Australian Town and Country Journal. p. 23, Sydney, NSW, Australia. Available at https://trove.nla.gov.au/newspaper/article/70959027
Tulloch AIT, Possingham HP, Joseph LN, Szabo J, Martin TG (2013) Realising the full potential of citizen science monitoring programs. Biological Conservation 165, 128-138.
| Crossref | Google Scholar |
Warnken J, Hodgkison S, Wild C, Jones D (2004) The localized environmental degradation of protected areas adjacent to bird feeding stations: a case study of the Australian brush-turkey Alectura lathami. Journal of Environmental Management 70, 109-118.
| Crossref | Google Scholar | PubMed |


