Is there a lizard down that spider burrow? Microhabitat influences spider burrow occupancy by the endangered pygmy bluetongue
Kimberley H. Michael A * , Ryan Baring A and Michael G. Gardner A
A * , Ryan Baring A and Michael G. Gardner A
A
Abstract
Reptiles partition their activity among their microhabitats for thermoregulatory, predatory, and refuge opportunities. We investigated whether a habitat specialist, the endangered pygmy bluetongue (Tiliqua adelaidensis), preferentially occupied vacant spider burrows in specific microhabitats in agricultural grasslands.
We investigated whether (1) microhabitat availability influenced associations of lizards occupying burrows among four populations, (2) lizard microhabitat preferences varied over time and, (3) whether a correlation was present between lizard body condition and the occupancy of spider burrows in specific microhabitats.
We assessed the microhabitat surrounding pygmy bluetongue-occupied spider burrows and unoccupied burrows that fit the criteria to be potentially suitable for pygmy bluetongue occupancy among four populations over two field seasons. We used the presence or absence of a lizard within a spider burrow to generate models to assess the probability of lizard occupancy to test whether pygmy bluetongues exhibited microhabitat preferences when occupying a spider burrow.
We found that pygmy bluetongues were strongly positively associated with burrows on an angle and were negatively associated with burrows surrounded by bare ground, rock, lichen, and that were further from vegetation. Microhabitat preferences varied among populations and time, which may have been influenced by habitat availability at each site and season. We also found that pygmy bluetongue body condition was positively associated with greater rock cover; however, rock availability did not exceed 10% cover, which suggests that it may have been an incidental association owing to the low sample size of caught lizards or was affected by above-average rainfall.
Microhabitat preferences exhibited by habitat specialists such as the pygmy bluetongue may differ when inhabiting locations that differ in their availability of high-quality habitat.
Our results have implications for selecting appropriate microhabitats when installing artificial burrows for lizards at future translocation sites and land-management implications to ensure landscape heterogeneity of benefit for successful conservation.
Keywords: burrow, conservation, landscape heterogeneity, microhabitat preference, microhabitat use, reptile, Scincidae, Tiliqua adelaidensis.
Introduction
Landscape heterogeneity is important to maintain niche diversity for fauna because species may exhibit patterns of habitat use at the macrohabitat level if they rely on a specific microhabitat component (Pizzuto et al. 2007; Baling et al. 2016). For example, reptiles will respond to their environment at a microhabitat scale (Howland et al. 2016; Bradley et al. 2022) to facilitate thermoregulation (Kerr and Bull 2004), camouflage (Baling et al. 2016; Marshall et al. 2016), and predation opportunities (Blázquez and Rodríguez-Estrella 2007). An appropriate microhabitat is particularly important for small terrestrial reptiles because they will partition their activity among different microhabitats according to their biological needs (Read 2002; Michael et al. 2010a, 2010b; Pettigrew and Bull 2011).
Heterogeneity within a landscape can be achieved through processes such as fire, and at a local scale by other fauna (Hodgson and Ritchie 2023). Species that influence ecosystem function and trophic cascades are considered ecosystem engineers, their actions having long-reaching consequences throughout the landscape (Jones et al. 1994). Ecosystem engineers alter resource availability through their direct and indirect actions, such as changing landscape and microbial heterogeneity through bioturbation (Eldridge and James 2009; Verdon et al. 2016; Decker et al. 2019; Michael et al. 2022). At a patch scale, wombat warrens generate heterogeneity and may be used by reptiles for opportunistic thermoregulation during the cooler early mornings where freshly excavated sand is subjected to greater solar radiation than is adjoining habitat (Hodgson and Ritchie 2023). At a microhabitat scale, burrowing spiders are ecosystem engineers (Prugh and Brashares 2012; Clayton et al. 2020), and their burrows may host a diverse range of taxa including other arthropods, and reptiles such as the endangered pygmy bluetongue (Tiliqua adelaidensis) (Milne and Bull 2000) and critically endangered grassland earless dragon (Tympanocryptis spp.) (Stevens et al. 2010). For lizards, spider burrows provide refuge from predators (Roznik and Johnson 2009), ambush sites for invertebrate prey (Milne and Bull 2000), and thermoregulatory opportunities (Gaudenti et al. 2021; Doucette et al. 2023). Where these burrows are located within a landscape, specifically in agricultural landscapes where livestock grazing affects the macro- and microhabitat, may also influence their occupancy by reptiles.
Livestock grazing creates a mosaic of different plant heights and inter-tussock spaces because livestock graze vegetation preferentially (Howison et al. 2017). Inter-tussock space allows a quick retreat to refuges (Kovács and Kiss 2016), affects the microclimate (Bromham et al. 1999), allows prey detection (Pettigrew and Bull 2012), and basking by ectotherms (Hacking et al. 2014). However, overgrazing can reduce vegetation cover and therefore expose spider burrows to trampling by livestock, also increasing reptile exposure to temperature extremes (Clayton and Bull 2015, 2016). By contrast, a mosaic of well-developed inter-tussock spaces provides high grass structural complexity, which has been positively associated with other grassland species such as the striped legless lizard (Delma impar) (Howland et al. 2016). However, an inappropriate grazing regime that does not facilitate inter-tussock spaces may impede visibility and accessibility of spider burrows to small terrestrial reptiles (Pettigrew and Bull 2011). Thus, spider burrows are a keystone resource in agricultural landscapes and livestock grazing affects the immediate microhabitat surrounding these burrows and their occupancy by reptiles.
Pygmy bluetongues inhabit temperate grasslands in agricultural grasslands that have been predominantly grazed by sheep. These lizards occupy vacant wolf and trapdoor spider burrows (Milne and Bull 2000; Bull et al. 2015) and will also accept an artificial burrow constructed of a hollowed wooden dowel (Souter et al. 2004). They are not able to dig their own burrow or alter a spider burrow and thus are reliant on burrowing spiders (Clayton and Bull 2015). Previous manipulative studies testing microhabitat vegetation preferences surrounding pygmy bluetongue-occupied burrows have reported complex interactions (Pettigrew and Bull 2011, 2012). Using field experiments, Pettigrew and Bull (2011) found that pygmy bluetongues would not colonise artificial burrows that had been clipped of surrounding vegetation. This was in contrast to the laboratory experiment, where lizards spent more time basking and in burrows within 20 cm of grass tussocks (Pettigrew and Bull 2011). A second field study reported that resident pygmy bluetongues increased their predation response and spent more time basking when vegetation had been clipped surrounding the burrow than did those in burrows that were not clipped of surrounding vegetation (Pettigrew and Bull 2012). Therefore, more information is required to understand microhabitat associations of pygmy bluetongues, particularly from a mensurative approach.
The density and distribution of pygmy bluetongues throughout paddocks may vary within and between adjacent paddocks that appear to have little differentiation at a landscape scale (Souter et al. 2007; Fellows et al. 2009). This may indicate that spider burrow availability and microhabitat preference influence pygmy bluetongue distribution. In this study, our aims were to (1) determine the microhabitat preferences of pygmy bluetongues found opportunistically in spider burrows on grazing properties at the southern, central, and northern range of their current distribution that experience different grazing regimes, (2) assess whether microhabitat availability or use varies among populations or over time, and (3) investigate whether lizard body condition and microhabitat are correlated. We predicted that lizards would preferentially occupy spider burrows with greater vegetation cover than bare ground as captive pygmy bluetongues will use grass tussocks for shelter (Daniell 2021); microhabitat use would vary among populations as a result of overall habitat quality affecting microhabitat availability; and microhabitat quality that facilitated basking and ambush predator attempts would be strongly correlated to higher lizard body condition. Refining our understanding of microhabitat associations exhibited by endangered habitat specialists such as pygmy bluetongues will inform threatened species management and restorative conservation programs.
Methods
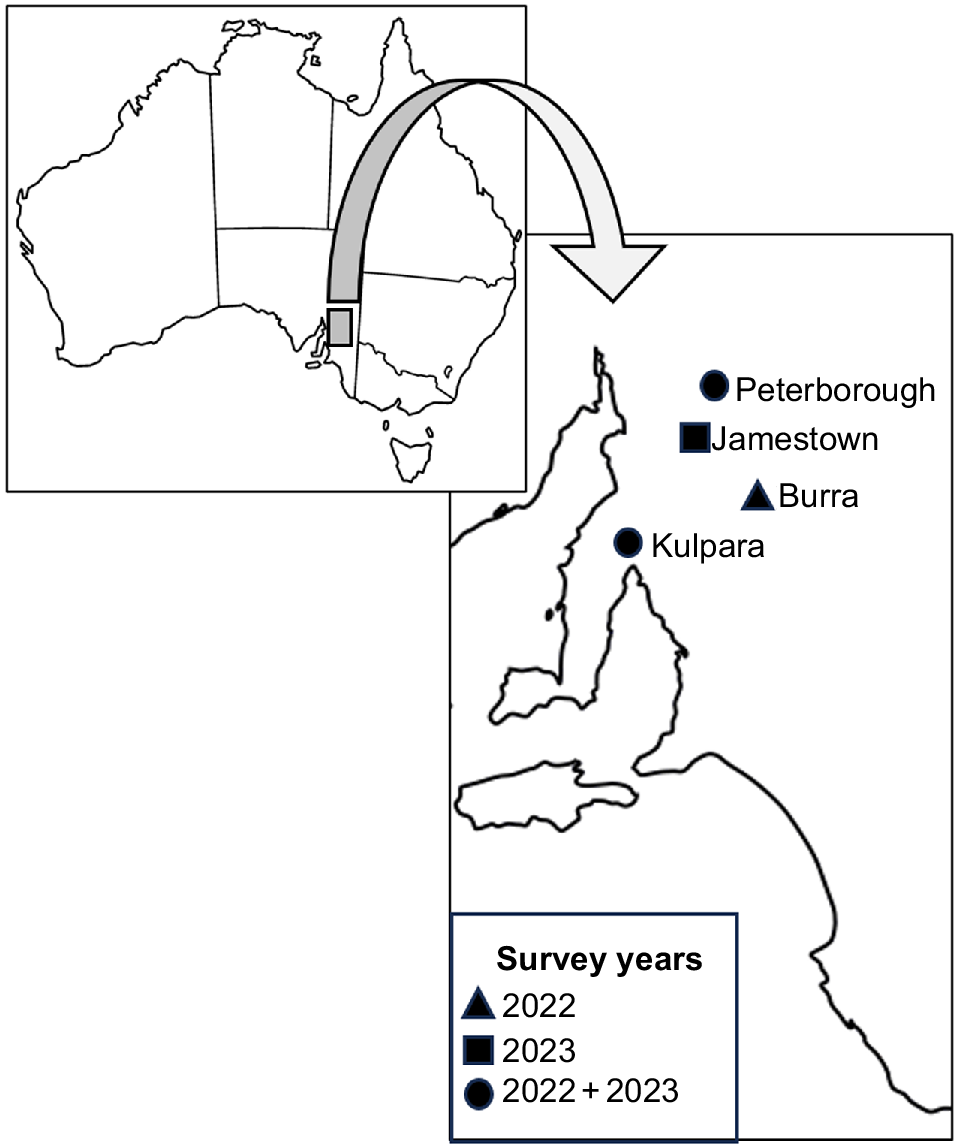
Surveys were conducted over two field seasons in April 2022 and March–April 2023. Surveys were conducted in the Austral autumn period in both years as neonates would have dispersed from their maternal burrow (Milne et al. 2003), and the sampling allows for the opportunity to catch lizards in a high body condition prior to winter brumation. Four pygmy bluetongue populations were surveyed. In 2022, Burra, Peterborough and Kulpara were surveyed and, in 2023, Kulpara and Peterborough were surveyed again, with an additional population, Jamestown, also being surveyed in 2023 (Fig. 1). The populations surveyed occurred at the southern, central, and northern-most distributional range of the pygmy bluetongue. All sites are privately owned sheep grazing properties implementing grazing with varying stock rates and varying timing of stock movements. Peterborough experienced a drought during the 2022 surveys and all populations experienced above-average rainfall prior to the 2023 surveys. Although some individual pygmy bluetongues are long-distance dispersers (e.g. >100 m), the majority of the population tends to remain within a primary burrow (Bull et al. 2015). During autumn, temperatures are cooling and lizards typically do not move burrows, with most movement occurring in the spring (Schofield et al. 2012), and will likely undergo brumation within the burrows where they were found during this study (Bull et al. 2015).
We assessed microhabitat by using a 20 cm × 20 cm quadrat surrounding pygmy bluetongue-occupied spider burrows and unoccupied burrows that appeared to be potentially suitable for pygmy bluetongue occupancy. Burrow searches were constrained to areas within 30 m of surrounding pygmy bluetongue-occupied burrows. An unoccupied burrow that was considered to be suitable was vacant of any fauna, a minimum of 120 mm deep with a minority below this depth to account for neonates, and had a smooth internal structure. Pygmy bluetongues typically prefer burrows of at least 300 mm deep (Milne and Bull 2000); however, this is a preferred burrow depth and burrows of this depth are not always available; therefore, the lizards may inhabit shallower burrows (e.g. the shallowest lizard-occupied burrow in this study was 45 mm). Within the 20 cm × 20 cm quadrat, we assessed, to the nearest 5%, cover of bare ground (bg), vegetation (veg), leaf litter (ll), rock (rock), moss (moss), and lichen (lichen), whether the burrow was on an angle or not (angle; yes or no), and the direction an angled burrow faced and whether that direction faced down the slope (face_ds; yes or no), and distance to nearest vegetation (dist_veg) to the nearest millimetre. Burrows on an angle are typically not seen from a bird’s eye perspective and so we assessed them visually; and in cases of uncertainty, gently inserted a pencil, where if the pencil immediately touched the internal chamber and fell to the side (as opposed to falling straight down the chamber if the observer let go) it was classified as angled. We used a compass to determine the direction the burrow faced if on an angle and the direction of the downward slope. Burrows within 90° of the downward slope (e.g. if downward slope faced east, then burrows facing east, north-east, south-east) were considered to face down the slope. We assumed that burrows facing down the slope would provide more optimal basking opportunities. Pygmy bluetongues remain within extremely close vicinity of their burrow outside the breeding season, and may also only partially emerge to thermoregulate or ambush passing invertebrates (Milne et al. 2003) therefore, we selected a 20 cm × 20 cm quadrat to reflect the area most used. For statistical analysis, percentage of cover, angle and face down-slope were converted into categories (Table 1). We chose to convert percentage of cover as a result of observers rounding to the nearest 5%. Variables that occurred in a smaller proportion (i.e. 1–4%) were recorded as <5%. Rounding cover percentage data is common, however, it represents somewhat ordinal classification and does not accurately reflect the actual cover percentage, for example, rounding 48% cover to 50% (Damgaard and Irvine 2019; Davis et al. 2022). We did not exclude <5% variables because some microhabitat quadrats included multiple observations of <5%, and thus are contributing to the microhabitat and may influence microhabitat quality. Therefore, converting percentage data to categories allowed us to include all observed microhabitat variables. In 2022, two 30 m × 30 m plots were erected at a minimum of 100 m apart in areas where lizards occurred at Kulpara and Peterborough, and within these plots, all spider burrows also had their width, depth, and fauna occupancy recorded.
| Microhabitat component | Category | Description | |
|---|---|---|---|
| Bare ground, vegetation, leaf litter, rock, moss, lichen (cover %) | 0 | Zero cover | |
| 1 | 1–10 | ||
| 2 | 11–25 | ||
| 3 | 26–50 | ||
| 4 | 51–75 | ||
| 5 | 76–100 | ||
| Angle, face down-slope | 0 | No | |
| 1 | Yes |
Statistical analysis
To test whether microhabitat availability differed between lizard-occupied and unoccupied burrows in different years, we used ANOVA for 2022, 2023 and pooled 2022 and 2023 data (herein referred to as pooled data). To test whether different populations exhibited different microhabitat preferences and whether preferences varied over time, we also assessed Kulpara and Peterborough populations separately because these populations were sampled in both 2022 and 2023. Because of varied sampling intensity among the 2 years and natural variations in grazing pressure, we chose to analyse each year independently to not confound our results. We also used ANOVA to test whether burrows surveyed differed significantly between these two populations or two field seasons. Student’s t-test was used to determine whether there was a difference in mean distance to nearest vegetation. We also used the mean category score of each microhabitat component and mean distance to nearest vegetation each year because the number of sampled spider burrows occupied and unoccupied differed each year.
We used the presence or absence of a lizard within a spider burrow to generate models to assess the probability of lizard occupancy to test whether pygmy bluetongues exhibited microhabitat preferences when occupying a spider burrow. Correlations among microhabitat variables were tested using Pearson’s correlation coefficient and variables with r > 0.80 were excluded because livestock grazing may increase bare ground cover while reducing vegetation cover (Naeth et al. 1991; Donovan and Monaghan 2021). We determined the most important predictor variables by using backwards step-wise multiple logistic regressions and assessed the best explanatory model of microhabitat use by pygmy bluetongues by using the Akaike information criterion (AIC). Presence or absence of pygmy bluetongue was the dependent variable, and the microhabitat components were the independent variables. We used R statistical software (R Core Team 2023) for all statistical analyses.
Body condition
Pygmy bluetongues caught in March–April 2023 at Kulpara (n = 10), Jamestown (n = 8), and Peterborough (n = 10) were weighed to the nearest gram and snout–vent length was measured to the nearest millimetre. The body condition index was calculated using the residuals of the linear regression of natural log body mass (BM) on natural log snout–vent length (SVL), a common method for estimating condition in reptile species (loge BM, loge SVL; Shamiminoori et al. 2014; Nafus et al. 2020; Gimmel et al. 2021). Microhabitat was scored in the same way as previously described. We used backwards step-wise multiple logistic regressions and the AIC of linear models to determine the best model to explain body condition variation in lizards. Although our sample size for caught lizards was small, we did not use the corrected AIC (AICc), which is commonly used when n < 40, because the AICc may overfit the model and not necessarily provide a more accurate model (Richards 2005).
Results
In total, 177 occupied burrows (2022 n = 79; 2023 n = 98) and 100 unoccupied burrows (2022 n = 53; 2023 n = 47) were used for statistical analyses from the four populations across the two field seasons. Mean pygmy bluetongue burrow depth was 247.56 mm and mean unoccupied burrow depth was 178.21 mm. Kulpara and Peterborough populations were also analysed separately. At Kulpara, 49 occupied burrows (2022 n = 24; 2023 n = 25) and 40 unoccupied burrows (2022 n = 24; 2023 n = 16) were found, whereas at Peterborough, 43 occupied burrows (2022 n = 10; 2023 n = 33) and 31 unoccupied burrows (2022 n = 7; 2023 n = 24) were found. Burrows surveyed (occupied or unoccupied) did not significantly differ between populations (Kulpara or Peterborough) (F(1) = 0.21, P = 0.65) or years (2022 or 2023) (F(1) = 0.66, P = 0.42).
Microhabitat availability
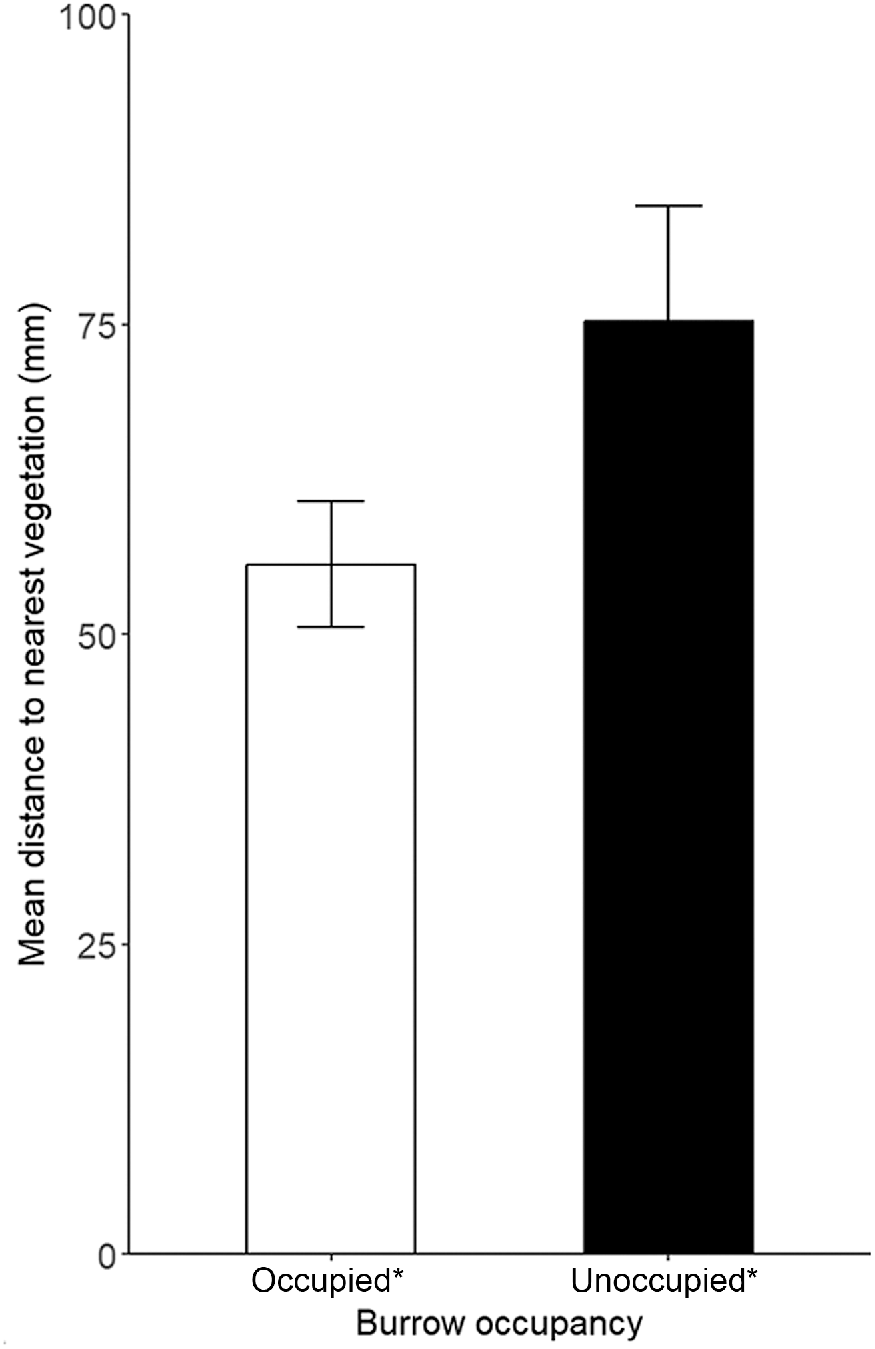
There was very strong evidence that microhabitat availability differed between pygmy bluetongue-occupied burrows and unoccupied burrows in pooled data and there was no evidence for the correlation of microhabitat variables (r > 0.80). Lizards occupied angled burrows (F(1) = 11.19, P ≤ 0.001) surrounded by higher amounts of vegetation (M score = 2.1, s.d. = 1.4) significantly more often than unoccupied burrows (M score = 1.54, s.d. = 1.1) (F(5) = 2.58), P = 0.03) (Figs 2, 3, 4). There was also moderate–weak evidence for bare ground (F(5) = 2.2, P = 0.054) being more prevalent around unoccupied burrows and that leaf litter availability (F(5) = 2.18, P = 0.057) was more prevalent around occupied burrows. There was moderate evidence (t(275) = 2.03, P = 0.043) for lizard-occupied burrows (M = 55.67 mm, s.d. = 66.95) to be closer to vegetation than were unoccupied burrows (M = 75.28 mm, s.d. = 92.1) (Fig. 5).
Mean cover (%) of microhabitat components of pygmy bluetongue-occupied spider burrows (□) and unoccupied spider burrows suitable for occupancy by a pygmy bluetongue (■). Asterisk denotes a significant difference (P < 0.05).
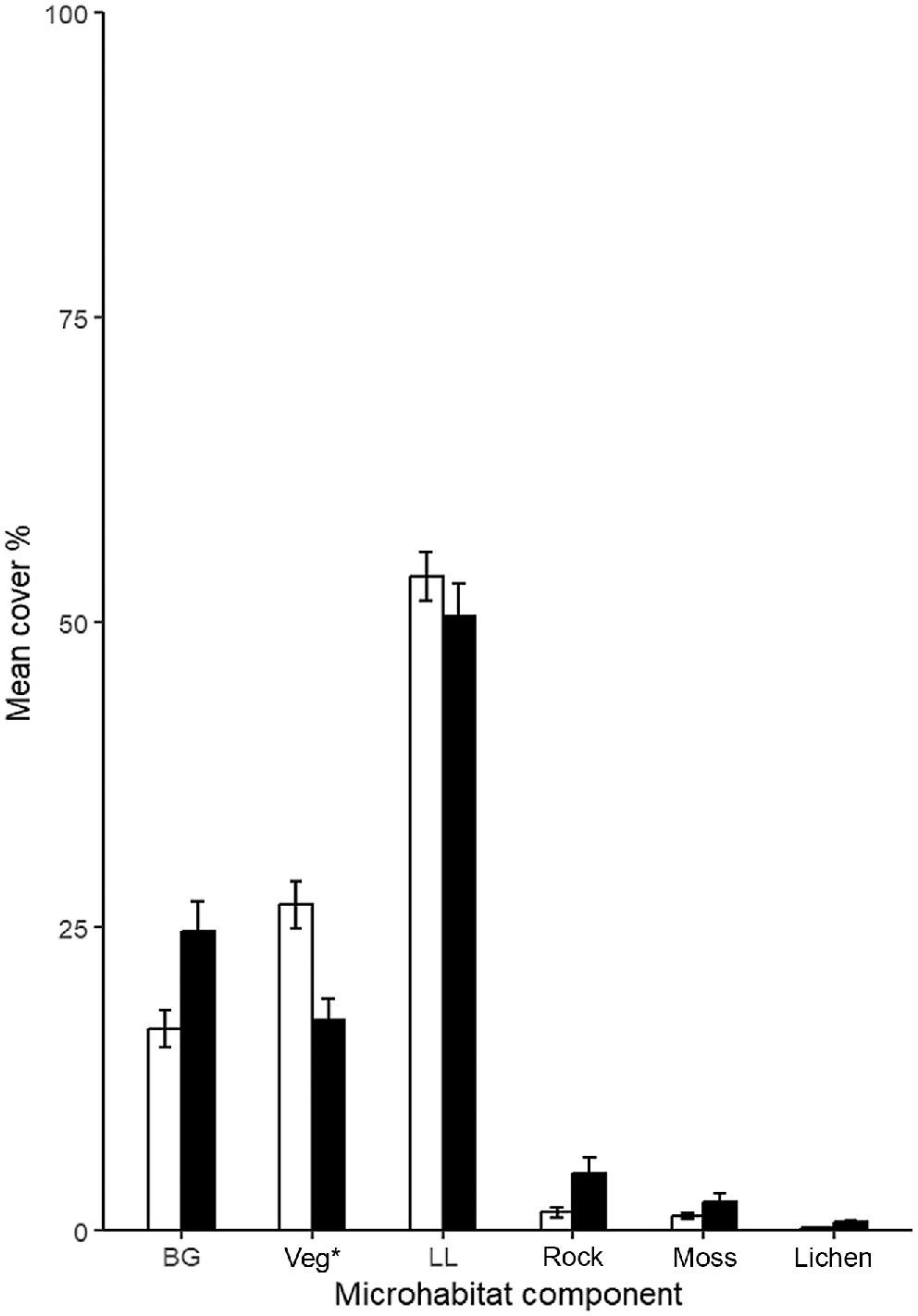
Effect plots of the logistic regressions to predict pygmy bluetongue burrow occupancy (PBT) using the best predictor variables from pooled data (Table 3). Solid blue line denotes the mean predictions and the shaded blue bands denote the 95% confidence interval.
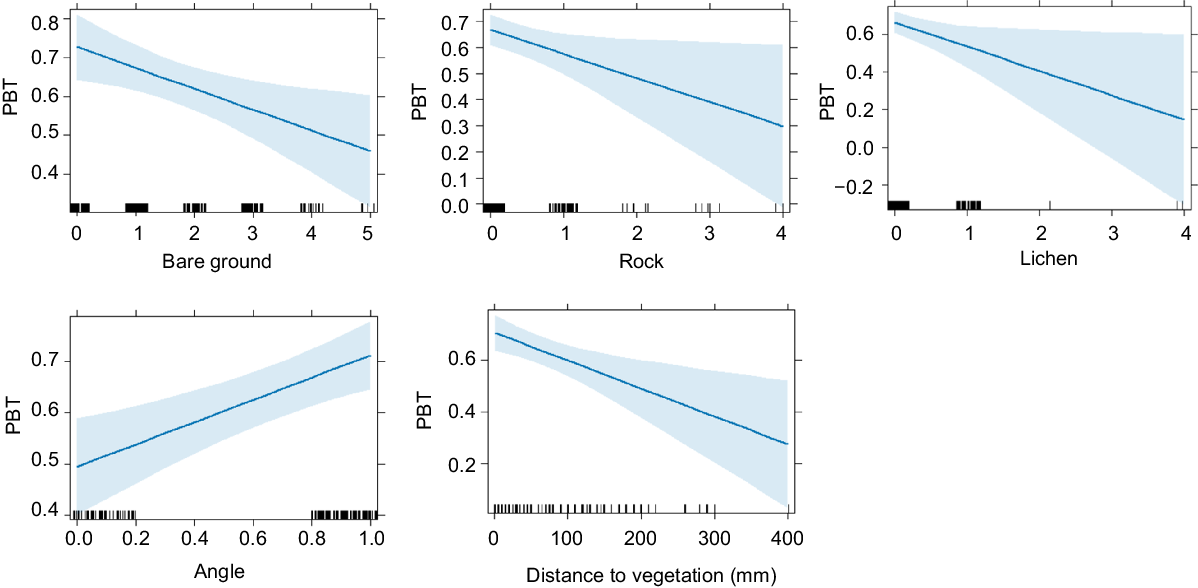
Mean score ± s.e. (expressed using category ranks in Table 1) of burrow components of pygmy bluetongue-occupied spider burrows (□) and unoccupied spider burrows suitable for occupancy by a pygmy bluetongue (■). Asterisk denotes a significant difference (P < 0.05).
Mean distance ± s.e. to the nearest vegetation (mm) of pygmy bluetongue-occupied spider burrows (□) and unoccupied spider burrows suitable for occupancy by a pygmy bluetongue (■). Asterisk denotes a significant difference (P < 0.05).
Few differences were detected in microhabitat availability when assessing 2022 and 2023 separately, and there was no evidence for the correlation of microhabitat variables (r > 0.80) in any year. There was strong evidence for less bare ground surrounding occupied burrows (M score = 1.26, s.d. = 1.2) than unoccupied burrows (M score = 2.26, s.d. = 1.4) (F(5) = 4.23, P = 0.001) in 2023. In 2023, significantly more occupied burrows were angled than unoccupied burrows (F(1) = 14.66, P ≤ 0.001). There was moderate evidence that occupied burrows were closer (M = 73.01 mm, s.d. = 68.99) to vegetation in 2022 (t(130) = 1.98, P = 0.05) than were unoccupied burrows (M = 103.47 mm, s.d. = 108.13), However, there was no evidence for a difference in distance to vegetation in 2023 (t(143) = 0.17, P = 0.87).
Microhabitat availability did not differ for the Kulpara population in any year or combined years, except for angled burrows in 2023 (F(1) = 22.05, P ≤ 0.001) and combined years (F(1) = 12.46, P ≤ 0.001). There was very strong evidence for angled burrow availability being different only in 2022 (F(1) = 19.55, P ≤ 0.001) for the Peterborough population. Rock was less prevalent around Peterborough occupied burrows (M score = 0.09, s.d. = 0.28) (F(1) = 7.24, P = 0.01) than it was around unoccupied burrows (M score = 0.42, s.d. = 0.49) and angled burrows were more prevalent in occupied burrows (F(1) = 13.17, P ≤ 0.001) in 2023. The same trend persisted in combined years with rock nearby (M score = 0.07, s.d. = 0.25) (F(1) = 5.45, P = 0.02), which was less prevalent in occupied than unoccupied burrows (M score = 0.32, s.d. = 0.47) and angled burrows more prevalent to lizards (F(1) = 25.46, P ≤ 0.001). There was no evidence for correlated microhabitat variables (r > 0.80) in any combination of year or population analysed. Availability of suitable unoccupied burrows in 2022 recorded within paired 30 m × 30 m plots at Kulpara and Peterborough was, on average, 36.5% and 6.5% respectively.
Microhabitat use by lizards
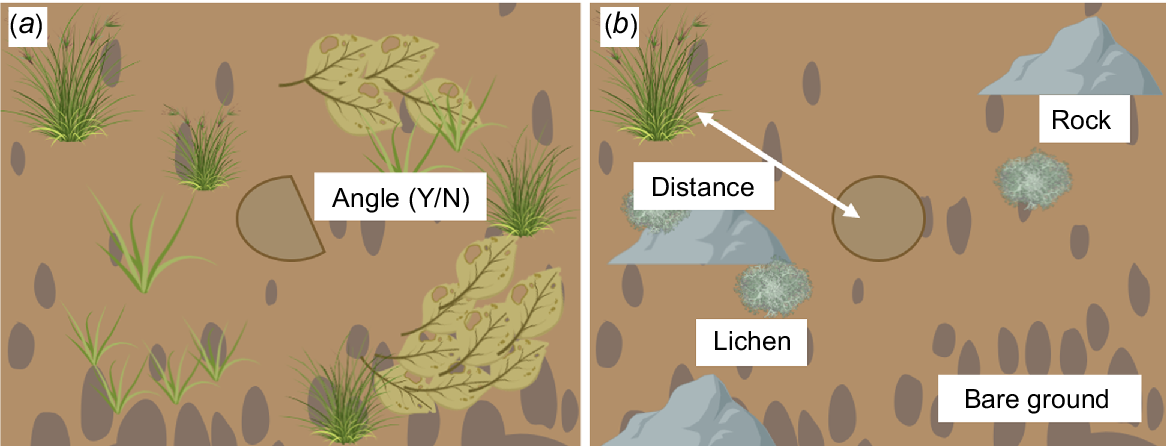
The best model to predict the occupancy of a spider burrow by a pygmy bluetongue by using pooled data (Table 2) included all five important predictor variables identified from 2022 and 2023 models. Pygmy bluetongues were negatively associated with bare ground, rock, and lichen and an increase in distance to nearest vegetation (Fig. 6). In comparison, pygmy bluetongues were strongly positively correlated to angled burrows (Fig. 6). Overall, the probability of a pygmy bluetongue occupying a spider burrow can be best predicted by Model 5 (Table 3), as follows:
| Model | Predictors | AIC | |
|---|---|---|---|
| 1 | bg, veg, ll, rock*, moss, lichen*, angle*, face_ds, dist_veg | −431.29 | |
| 2 | bg*, ll, rock*, moss, lichen*, angle*, face_ds, dist_veg* | −433.27 | |
| 3 | bg*, ll, rock*, moss, lichen, angle*, dist_veg* | −434.31 | |
| 4 | bg*, ll, rock*, lichen*, angle*, dist_veg* | −434.59 | |
| 5 | bg*, rock*, lichen*, angle*, dist_veg* | −435.22 A |
Graphic illustrating microhabitat components that are (a) positively and (b) negatively associated with pygmy bluetongues on the basis of Table 3. Created with BioRender.com.
| Microhabitat variable | Estimate | s.e. | t | P | |
|---|---|---|---|---|---|
| Intercept | 0.704 | 0.062 | 11.352 | <0.001 | |
| Bare ground | −0.054 | 0.020 | −2.644 | 0.009 | |
| Rock | −0.092 | 0.042 | −2.187 | 0.030 | |
| Lichen | −0.129 | 0.060 | −2.162 | 0.031 | |
| Angle | 0.217 | 0.058 | 3.714 | <0.001 | |
| Distance to nearest vegetation | −0.001 | 0.000 | −2.984 | 0.003 |
s.e., standard error; all microhabitat variables displayed had P < 0.05.
Both Kulpara and Peterborough populations exhibited changes in microhabitat use over time and among populations. In 2022, the model that best predicted occupancy of a spider burrow at Kulpara included bare ground, vegetation, leaf litter, rock, moss, lichen, and distance to vegetation (Supplementary Table S1). Burrow occupancy was negatively associated with leaf litter and an increase in distance to vegetation (Table S2). In 2023, the model that best predicted burrow occupancy included bare ground, angle, and face down-slope and lizard occupancy was positively associated with angled burrows (Tables S3, S4). Analysis of both years combined showed that moss, lichen, angle, and distance to vegetation best predicted burrow occupancy, with a negative association with lichen and positive association with angled burrows (Table S5, S6). The probability of a pygmy bluetongue occupying a spider burrow at Kulpara was best predicted by
In 2022, the model that best predicted burrow occupancy at Peterborough included lichen and burrow angle (Tables S7, S8). In 2023, occupancy was negatively correlated with bare ground and rock (Tables S9, S10). In both individual years, occupancy was positively correlated with angled burrows. When data from each year were combined, the best model to predict burrow occupancy at Peterborough included bare ground, rock and angle; angled burrows had a positive relationship with lizard occupancy (Tables S11, S12). The probability of a pygmy bluetongue occupying a spider burrow at Peterborough can be best predicted by
Body condition of lizards
Twenty-eight pygmy bluetongues were caught for a body-condition assessment and seven of those were neonates. The seven neonates were excluded from analysis. There was very strong evidence for a correlation between ln(mass) and ln(snout–vent length) (r2 = 0.625, P ≤ 0.001). In comparison, there was no evidence for correlation of microhabitat variables within this dataset. The best model to explain body condition variation included bare ground, rock, moss, lichen, face down-slope, and distance to vegetation (Table 4). However, no burrow was located within a microhabitat containing greater than 10% rock cover, although this factor was the most important predictor variable in Model 4 (Table 5).
| Model | Predictors | AIC | |
|---|---|---|---|
| 1 | bg, veg, ll, rock*, moss, lichen, angle, face_ds, dist_veg | −83.21 | |
| 2 | bg, veg, ll, rock*, lichen, angle, face_ds, dist_veg | −85.04 | |
| 3 | bg, veg, rock*, lichen, angle, face_ds, dist_veg | −86.76 | |
| 4 | bg, rock*, lichen, angle, face_ds, dist_veg | −88.19 A |
| Microhabitat variable | Estimate | s.e. | t | P | |
|---|---|---|---|---|---|
| Intercept | 0.012 | 0.066 | 0.182 | 0.859 | |
| Bare ground | −0.055 | 0.027 | −2.043 | 0.060 | |
| Rock | 0.123 | 0.045 | 2.735 | 0.016 | |
| Lichen | −0.081 | 0.054 | −1.501 | 0.156 | |
| Angle | 0.138 | 0.073 | 1.888 | 0.080 | |
| Face_ds | −0.999 | 0.063 | −1.592 | 0.134 | |
| Dist_veg | −0.001 | 0.0003 | −1.800 | 0.094 |
s.e., standard error; bold indicates P < 0.05.
Discussion
Spider burrows are a keystone resource in agricultural landscapes for many terrestrial reptiles, including the endangered pygmy bluetongue. Pygmy bluetongues are closely associated with spider burrows, as burrows provide thermoregulation, predation, and refuge opportunities (Milne and Bull 2000; Milne et al. 2003). We investigated microhabitat associations of lizard-occupied and unoccupied burrows and have the following three major findings: (1) pygmy bluetongues across four populations were more likely to occupy spider burrows on an angle, closer to vegetation, and in microhabitats with reduced bare ground, rock, and lichen cover; (2) in different pygmy bluetongue populations, spider burrow availability influenced occupancy and microhabitat associations by lizards and; (3) body condition may be positively associated with higher rock cover after wetter seasons such as the La Niña cycle experienced prior to body condition surveys in 2023.
First, our results suggested that pygmy bluetongues prefer burrows with a greater vegetation cover and those closer to vegetation. In this study, rock and lichen tended to co-occur with bare ground, which is likely to explain their negative association with lizard occupancy. Microhabitat availability may have influenced some microhabitat preferences, because only vegetation, angled burrow availability, and mean distance to vegetation showed strong evidence for differences between occupied and unoccupied burrows in pooled data. These findings concur with a previous study where captive lizards preferred to occupy burrows surrounded by grass tussocks rather than burrows surrounded by bare ground (Pettigrew and Bull 2011). Low vegetation and leaf-litter cover reduces microhabitat diversity and can affect the microclimate (Bromham et al. 1999). Changes in the microclimate, such as increased insolation of soil surface, can also be exacerbated by agricultural stock grazing and lead to a reduction in invertebrate diversity (Bromham et al. 1999). Any reduction in invertebrates as a prey resource, and lack of microhabitat diversity that optimises thermoregulation, may explain why pygmy bluetongues are negatively associated with bare ground and prefer to be closer to vegetation. Pygmy bluetongues are typically associated with a single burrow throughout the year, except during the breeding season in spring where males move to search for females (Milne et al. 2003; Fenner and Bull 2007; Schofield et al. 2012), and have been found to enter grass tussocks when exiting the burrow (Daniell 2021). Therefore, burrows closer to vegetation may benefit from a shading effect that optimises thermoregulatory options year round, provides more shelter from potential predators such as brown snakes (Pseudonaja textilis) (Hutchinson et al. 1994; Fenner et al. 2008; Ridley and Schlesinger 2023) as well as may be more resistant to weather related destruction such as burrow destruction following heavy rainfall (Ebrahimi et al. 2012).
We had predicted angled burrows that faced down the slope may provide more optimal basking opportunities and thus be positively associated with lizard occupancy, but found evidence only to support an association with angled burrows. The greater likelihood of pygmy bluetongue occupancy in angled burrows may allow the lizards to ambush their prey from the burrow without being detected, as their head may remain within the burrow while waiting for prey, and angled burrows coupled with moderate grass height may provide better protection from predators and weather events (Gedeon et al. 2012). Other burrow-dwelling lizards such as blunt-nosed leopard lizards (Gambelia sila) are limited in their thermal ecology when no vegetation surrounds their burrow and will spend longer within the burrow than do lizards with surrounding vegetation (Gaudenti et al. 2021). Pygmy bluetongues may exhibit a similar association with angled burrows and may be more willing to emerge to bask from angled burrows because they may be more obscured from potential predators. Angled burrows may also be particularly important during heavy-rainfall events because artificial burrows that are constructed vertically into the ground have been found to remain flooded for several days, resulting in lizards within exhibiting apnoea (Michael and Gardner 2023), which may lead to decreased survival. Although it has been established that pygmy bluetongues prefer deeper burrows that are more climatically stable (Milne and Bull 2000), further investigation is warranted to understand the thermal ecology of pygmy bluetongues in relation to microhabitat heterogeneity and burrow structure.
Second, the same species of reptile may have different ecological requirements, and therefore microhabitat preferences, when inhabiting different ecosystems or within different patches of the same landscape (Michael et al. 2010a; Mulhall et al. 2024). Lizards will respond to their habitat at multiple spatial scales; for example, social structure and occupancy pattern in saxicolous tree skinks (Egernia striolata) are significantly influenced by microhabitat heterogeneity within a landform (Michael et al. 2010a, 2010b); or sleepy lizards (Tiliqua rugosa) that will move non-randomly under a bush to maintain their optimal temperature (Kerr and Bull 2004). Both Kulpara and Peterborough pygmy bluetongue populations exhibited changes in microhabitat-use associations over time. Kulpara lizards exhibited associations with all microhabitat components at some point during the survey period, whereas Peterborough lizards exhibited similar trends over consecutive field seasons that were comparable to the overarching trends found in the pooled data. The investigation of burrow availability within 30 m × 30 m plots in 2022 showed that an average of 36.5% of spider burrows was available at Kulpara, in contrast to 6.5% average availability at Peterborough.
Our results may indicate that vegetation cover across the landscape and burrow availability may be greater determinants of which burrows pygmy bluetongues will occupy than microhabitat preferences alone. Kulpara lizards did not show a distinct microhabitat-use association over time; however, the southernly location has greater vegetation cover and higher availability of burrows of suitable depth. Kulpara lizards have greater access to more high-quality burrows with high vegetation cover throughout the landscape, and therefore have a higher likelihood of encountering a suitably deep burrow and may move burrows more often. A suitably deep burrow at Kulpara may be a greater determinant of pygmy bluetongue occupancy than the microhabitat. In contrast, Peterborough lizards occupy a more northernly location that has a higher prevalence of seasonal droughts (Bureau of Meteorology 2008, 2023; Raymond and Spoehr 2013). Grazing pressure may exacerbate the effects of drought (Staver et al. 2019), impeding vegetation cover, coupled with low availability of suitably deep spider burrows. Therefore, the microhabitat may be a greater determinant of pygmy bluetongue occupancy, because Peterborough lizards exhibited distinct microhabitat associations to favour burrows with low bare-ground cover over consecutive field seasons. Our results conform to other literature in that reptiles with specialist requirements may exhibit differing microhabitat preferences when inhabiting different locations that vary in their availability of high-quality habitat and highlight the need to incorporate variables across multiple spatial scales (Michael et al. 2010a, 2010b; Spiegel et al. 2015; Gaudenti et al. 2021).
Our third finding relates to an investigation of whether lizards in a high body condition were correlated with a specific microhabitat. Although a greater body condition is not necessarily correlated with greater survival (Cox and Calsbeek 2015), lizards in better condition may have greater ability to move throughout the landscape in search of higher-quality spider burrows. We found that body condition was positively associated with rocks; however, no lizard inhabited a microhabitat with rock cover exceeding 10%, suggesting that this may have been an incidental association owing to a low sample size of lizards caught. However, our sampling of body condition followed above-average rainfall during a La Niña cycle, which largely resulted in increased vegetation cover and therefore rocks may have provided basking sites above dense vegetation. Moreover, reduced vegetation cover has been previously associated with a decreased body condition in pygmy bluetongues (Fenner and Bull 2007). Future microhabitat studies would benefit from measuring rock sizes to further understand lizard associations with rocks.
We are careful to note the potential loss of predictive power and observer error when converting cover percentage to categories. In this study, we chose to convert data to categories because of the observer rounding to the nearest 0 or 5 (e.g. 20% or 25%), and when a microhabitat component was observed to be within 1–4%, it was recorded as <5% and not included in the final 100% cover score (e.g. a quadrat could be scored as 50% bare ground, 50% vegetation, <5% lichen). Although rounding data to easily divisible numbers is common, it is less accurate (Damgaard and Irvine 2019; Dengler and Dembicz 2023). As a result of our data-collection method, we were not able to account for variables <5% cover without conversion to a category, and highlight the consideration to estimate cover percentage directly without rounding for future studies, so as to increase statistical analysis power and data accuracy. However, we also note that from a conservation management perspective, our results are intended to inform future placement of artificial burrows. The use of categories used commonly (e.g. 26–50%) requires less training, which may lead to more uniform decision making regarding appropriate microhabitats for artificial burrow installation (Zipkin et al. 2014; Davis et al. 2022).
Livestock contribute to landscape heterogeneity and may influence pygmy bluetongue distribution, where livestock may negatively affect spider burrow availability through trampling, but also may decrease build-up of thatch and allow more basking opportunities by lizards (Clayton and Bull 2015, 2016). Although this study did not investigate livestock stocking rates, our results are important for consideration of land management implications to ensure landscape heterogeneity and maintain microhabitat diversity. Importantly, pygmy bluetongues and livestock have coexisted for decades (Milne and Bull 2000), and land management to ensure adequate vegetation cover is likely to not only benefit the lizards, as a decrease in vegetation cover also allows unpalatable exotic flora to invade (Porensky et al. 2020) and detrimentally affects livestock productivity. Other studies have shown that reptiles may coexist with livestock-induced landscape heterogeneity at low to moderate grazing intensity, because reptile responses may vary along a grazing gradient (Howland et al. 2014; Neilly et al. 2018), emphasising the importance of collaborative approaches with landowners to maintain agricultural productivity and habitat quality for inhabiting fauna.
Understanding microhabitat-use patterns within the broader macrohabitat by fauna provides insight into their distribution throughout landscapes (Vieira et al. 2005; Blázquez and Rodríguez-Estrella 2007; Pizzuto et al. 2007), particularly small terrestrial reptiles that are more vulnerable to landscape changes and need to partition their activity among the microhabitats for predation, refuge, and thermoregulatory opportunities (Toft 1985; Howland et al. 2014; Bradley et al. 2022). Our study has provided a key step forward for assessing habitat quality and the microhabitat preferences of specialist reptiles such as pygmy bluetongues where multiple populations are investigated throughout their distributional range where possible, and during different times or climates. Although many endangered species such as the pygmy bluetongue have suffered significant declines, we found that populations within 150 km of each other and within a 12-month period during a La Niña cycle, exhibited differing microhabitat preferences. Variation in microhabitat preference by pygmy bluetongues could indicate that individual lizards that have previously been primarily associated with a single burrow (Bull et al. 2015) may move more often outside the breeding season when habitat quality is higher (i.e. greater vegetation cover and spider burrow availability). Our study has long-term conservation implications for the pygmy bluetongue, as well as other reptiles with similar life histories, because translocation to more suitable climate involving the use of artificial burrows has been suggested as a management strategy (Milne and Bull 2000; Milne et al. 2003; Souter et al. 2004; Fordham et al. 2012; Delean et al. 2013). Our results have provided key insights into the best placement of artificial burrows to ensure long-term use by pygmy bluetongues and to decrease the likelihood of dispersal from a translocation site, a key determinant of translocation success (Berger-Tal et al. 2020) Our study also has land management implications for a species that coexists with grazing livestock, at current pygmy bluetongue sites to ensure that vegetation cover is not overgrazed, and at potential translocation sites to investigate spider burrow availability in relation to microhabitat availability. Understanding microhabitatuse patterns of species with niche requirements such as ectotherms will increase our ability to make informed conservation decisions in the face of future climate change-mitigation translocations.
Declaration of funding
This study was supported by the Australian Society of Herpetologists, Field Naturalists Society of South Australia, Linnean Society of New South Wales, Australian Government Research Training Program Scholarship to K. H. M. and Australian Research Council Linkage Grant (LP190100071) to M. G. G.
Author contributions
Kimberley Michael: conceptualisation (lead); data curation (lead); formal analysis (lead); investigation (lead); writing – original draft (lead); writing – reviewing and editing (equal). Ryan Baring: conceptualisation (equal); formal analysis (supporting); writing – reviewing and editing (equal). Michael Gardner: conceptualisation (equal); supervision (lead); writing – reviewing and editing (equal).
Acknowledgements
We thank the Nature Foundation and the Ludgate, Millard, and Robinson families for allowing access to the study sites. We are grateful to volunteers for their assistance in the field.
References
Baling M, Stuart-Fox D, Brunton DH, Dale J (2016) Habitat suitability for conservation translocation: the importance of considering camouflage in cryptic species. Biological Conservation 203, 298-305.
| Crossref | Google Scholar |
Berger-Tal O, Blumstein DT, Swaisgood RR (2020) Conservation translocations: a review of common difficulties and promising directions. Animal Conservation 23(2), 121-131.
| Crossref | Google Scholar |
Blázquez MC, Rodríguez-Estrella R (2007) Microhabitat selection in diet and trophic ecology of a spiny-tailed iguana Ctenosaura hemilopha. Biotropica 39(4), 496-501.
| Crossref | Google Scholar |
Bradley HS, Craig MD, Cross AT, Tomlinson S, Bamford MJ, Bateman PW (2022) Revealing microhabitat requirements of an endangered specialist lizard with LiDAR. Scientific Reports 12(1), 5193.
| Crossref | Google Scholar | PubMed |
Bromham L, Cardillo M, Bennett AF, Elgar MA (1999) Effects of stock grazing on the ground invertebrate fauna of woodland remnants. Australian Journal of Ecology 24(3), 199-207.
| Crossref | Google Scholar |
Bull CM, Godfrey SS, Ebrahimi M, Fenner AL (2015) Long and short term residence in refuge burrows by endangered pygmy bluetongue lizards. Amphibia-Reptilia 36(2), 119-124.
| Crossref | Google Scholar |
Bureau of Meteorology (2008) Drought statement – Issued 6th August 2008. Available at http://www.bom.gov.au/announcements/media_releases/climate/drought/20080806.shtml [accessed 19 October 2023]
Bureau of Meteorology (2023) Rainfall deficiencies and water availability – Issued 6th October 2023. Available at http://www.bom.gov.au/climate/drought/archive/20231006.archive.shtml [accessed 19 October 2023]
Clayton J, Bull CM (2015) The impact of sheep grazing on burrows for pygmy bluetongue lizards and on burrow digging spiders. Journal of Zoology 297(1), 44-53.
| Crossref | Google Scholar |
Clayton J, Bull M (2016) The impact of sheep grazing on the depth of spider burrows and of burrows selected by the pygmy bluetongue lizard (Tiliqua adelaidensis). Wildlife Research 43(8), 691-703.
| Crossref | Google Scholar |
Clayton J, Gardner MG, Fenner AL, Bull M (2020) Co-occupancy of spider-engineered burrows within a grassland community changes temporally. Austral Ecology 45(4), 454-459.
| Crossref | Google Scholar |
Cox RM, Calsbeek R (2015) Survival of the fattest? Indices of body condition do not predict viability in the brown anole (Anolis sagrei). Functional Ecology 29(3), 404-413.
| Crossref | Google Scholar |
Damgaard CF, Irvine KM (2019) Using the beta distribution to analyse plant cover data. Journal of Ecology 107, 2747-2759.
| Crossref | Google Scholar |
Davis KL, Silverman ED, Sussman AL, Wilson RR, Zipkin EF (2022) Errors in aerial survey count data: identifying pitfalls and solutions. Ecology and Evolution 12, e8733.
| Crossref | Google Scholar | PubMed |
Decker O, Eldridge DJ, Gibb H (2019) Restoration potential of threatened ecosystem engineers increases with aridity: broad scale effects on soil nutrients and function. Ecography 42(8), 1370-1382.
| Crossref | Google Scholar |
Delean S, Bull CM, Brook BW, Heard LMB, Fordham DA (2013) Using plant distributions to predict the current and future range of a rare lizard. Diversity and Distributions 19(9), 1125-1137.
| Crossref | Google Scholar |
Dengler J, Dembicz I (2023) Should we estimate plant cover in percent or on ordinal scales? Vegetation Classification and Survey 4, 131-138.
| Crossref | Google Scholar |
Donovan M, Monaghan R (2021) Impacts of grazing on ground cover, soil physical properties and soil loss via surface erosion: a novel geospatial modelling approach. Journal of Environmental Management 287, 112206.
| Crossref | Google Scholar |
Doucette LI, Duncan RP, Osborne WS, Evans M, Georges A, Gruber B, Sarre SD (2023) Climate warming drives a temperate-zone lizard to its upper thermal limits, restricting activity, and increasing energetic costs. Scientific Reports 13, 9603.
| Crossref | Google Scholar | PubMed |
Ebrahimi M, Schofield JA, Bull CM (2012) Getting your feet wet: responses of the endangered pygmy bluetongue lizard (Tiliqua adelaidensis) to rain induced burrow flooding. Herpetology Notes 5, 297-301.
| Google Scholar |
Eldridge DJ, James AI (2009) Soil-disturbance by native animals plays a critical role in maintaining healthy Australian landscapes. Ecological Management & Restoration 10, S27-S34.
| Crossref | Google Scholar |
Fellows HL, Fenner AL, Bull CM (2009) Spiders provide important resources for an endangered lizard. Journal of Zoology 279(2), 156-163.
| Crossref | Google Scholar |
Fenner AL, Bull CM (2007) Short-term impact of grassland fire on the endangered pygmy bluetongue lizard. Journal of Zoology 272(4), 444-450.
| Crossref | Google Scholar |
Fenner AL, Schofield J, Smith AL, Bull CM (2008) Observations of snake predation on the pygmy bluetongue lizard, Tiliqua adelaidensis. Herpetofauna 38, 105-109.
| Google Scholar |
Fordham DA, Watts MJ, Delean S, Brook BW, Heard LMB, Bull CM (2012) Managed relocation as an adaptation strategy for mitigating climate change threats to the persistence of an endangered lizard. Global Change Biology 18(9), 2743-2755.
| Crossref | Google Scholar | PubMed |
Gaudenti N, Nix E, Maier P, Westphal MF, Taylor EN (2021) Habitat heterogeneity affects the thermal ecology of an endangered lizard. Ecology and Evolution 11(21), 14843-14856.
| Crossref | Google Scholar | PubMed |
Gedeon CI, Boross G, Németh A, Altbäcker V (2012) Release site manipulation to favour European ground squirrel Spermophilus citellus translocations: translocation and habitat manipulation. Wildlife Biology 18(1), 97-104.
| Crossref | Google Scholar |
Gimmel A, Öfner S, Liesegang A (2021) Body condition scoring (BCS) in corn snakes (Pantherophis guttatus) and comparison to pre-existing body condition index (BCI) for snakes. Journal of Animal Physiology and Animal Nutrition 105(S2), 24-28.
| Crossref | Google Scholar |
Hacking J, Abom R, Schwarzkopf L (2014) Why do lizards avoid weeds? Biological Invasions 16(4), 935-947.
| Crossref | Google Scholar |
Hodgson MJ, Ritchie D (2023) Strange bedfellows: mammal burrow disturbances may provide thermoregulatory microsites for fossorial reptiles in densely vegetated dunes. Austral Ecology 48, 1473-1478.
| Crossref | Google Scholar |
Howison RA, Olff H, van de Koppel J, Smit C (2017) Biotically driven vegetation mosaics in grazing ecosystems: the battle between bioturbation and biocompaction. Ecological Monographs 87(3), 363-378.
| Crossref | Google Scholar |
Howland B, Stojanovic D, Gordon IJ, Manning AD, Fletcher D, Lindenmayer DB (2014) Eaten out of house and home: impacts of grazing on ground-dwelling reptiles in Australian grasslands and grassy woodlands. PLoS ONE 9(12), e105966.
| Crossref | Google Scholar | PubMed |
Howland BWA, Stojanovic D, Gordon IJ, Fletcher D, Snape M, Stirnemann IA, Lindenmayer DB (2016) Habitat preference of the striped legless lizard: implications of grazing by native herbivores and livestock for conservation of grassland biota: habitat preferences of a threatened reptile. Austral Ecology 41(4), 455-464.
| Crossref | Google Scholar |
Hutchinson MN, Milne T, Croft T (1994) Redescription and ecological notes on the pygmy bluetongue, Tiliqua adelaidensis (Squamata: Scincidae). Transactions of the Royal Society of South Australia 118, 217-226.
| Google Scholar |
Jones CG, Lawton JH, Shachak M (1994) Organisms as ecosystem engineers. Oikos 69(3), 373-386.
| Crossref | Google Scholar |
Kerr GD, Bull CM (2004) Microhabitat use by the scincid lizard tiliqua rugosa: exploiting natural temperature gradients beneath plant canopies. Journal of Herpetology 38(4), 536-545.
| Crossref | Google Scholar |
Kovács D, Kiss I (2016) Microhabitat use of different age groups of snake-eyed skink and eastern green lizard. Amphibia-Reptilia 37(2), 191-198.
| Crossref | Google Scholar |
Marshall KLA, Philpot KE, Stevens M (2016) Microhabitat choice in island lizards enhances camouflage against avian predators. Scientific Reports 6(1), 19815.
| Crossref | Google Scholar |
Michael KH, Gardner MG (2023) Hold your breath: observations of the endangered pygmy bluetongue (Tiliqua adelaidensis) submerged in flooded burrows. Austral Ecology 48(6), 1200-1204.
| Crossref | Google Scholar |
Michael DR, Cunningham RB, Lindenmayer DB (2010a) Microhabitat relationships among five lizard species associated with granite outcrops in fragmented agricultural landscapes of south-eastern Australia. Austral Ecology 35(2), 214-225.
| Crossref | Google Scholar |
Michael DR, Cunningham RB, Lindenmayer DB (2010b) The social elite: habitat heterogeneity, complexity and quality in granite inselbergs influence patterns of aggregation in Egernia striolata (Lygosominae: Scincidae). Austral Ecology 35(8), 862-870.
| Crossref | Google Scholar |
Michael KH, Leonard SWJ, Decker O, Verdon SJ, Gibb H (2022) Testing the effects of ecologically extinct mammals on vegetation in arid Australia: a long-term experimental approach. Austral Ecology 47(2), 226-238.
| Crossref | Google Scholar |
Milne T, Bull CM (2000) Burrow choice by individuals of different sizes in the endangered pygmy blue tongue lizard Tiliqua adelaidensis. Biological Conservation 95(3), 295-301.
| Crossref | Google Scholar |
Milne T, Bull CM, Hutchinson MN (2003) Use of burrows by the endangered pygmy blue-tongue lizard, Tiliqua adelaidensis (Scincidae). Wildlife Research 30(5), 523-528.
| Crossref | Google Scholar |
Mulhall SJ, Di Stefano J, Dorph A, Swan M, Sitters H (2024) Do reptile responses to habitat structure and time since fire depend on landscape structure? Forest Ecology and Management 553, 121564.
| Crossref | Google Scholar |
Naeth MA, Bailey AW, Pluth DJ, Chanasyk DS, Hardin RT (1991) Grazing impacts on litter and soil organic matter in mixed prairie and fescue grassland ecosystems of Alberta. Journal of Range Management 44, 7-12.
| Crossref | Google Scholar |
Nafus MG, Yackel Adams AA, Boback SM, Siers SR, Reed RN (2020) Behavior, size, and body condition predict susceptibility to management and reflect post-treatment frequency shifts in an invasive snake. Global Ecology and Conservation 21, e00834.
| Crossref | Google Scholar |
Neilly H, O’Reagain P, Vanderwal J, Schwarzkopf L (2018) Profitable and sustainable cattle grazing strategies support reptiles in tropical savanna rangeland. Rangeland Ecology & Management 71(2), 205-212.
| Crossref | Google Scholar |
Pettigrew M, Bull CM (2011) The impact of heavy grazing on burrow choice in the pygmy bluetongue lizard, Tiliqua adelaidensis. Wildlife Research 38(4), 299-306.
| Crossref | Google Scholar |
Pettigrew M, Bull CM (2012) The response of pygmy bluetongue lizards to simulated grazing in the field during three drought years. Wildlife Research 39(6), 540-545.
| Crossref | Google Scholar |
Pizzuto TA, Finlayson GR, Crowther MS, Dickman CR (2007) Microhabitat use by the brush-tailed bettong (Bettongia penicillata) and burrowing bettong (B. lesueur) in semiarid New South Wales: implications for reintroduction programs. Wildlife Research 34, 271-279.
| Crossref | Google Scholar |
Porensky LM, McGee R, Pellatz DW (2020) Long-term grazing removal increased invasion and reduced native plant abundance and diversity in a sagebrush grassland. Global Ecology and Conservation 24, e01267.
| Crossref | Google Scholar |
Prugh LR, Brashares JS (2012) Partitioning the effects of an ecosystem engineer: kangaroo rats control community structure via multiple pathways: partitioning effects of engineers. Journal of Animal Ecology 81(3), 667-678.
| Crossref | Google Scholar | PubMed |
R Core Team (2023) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at https://www.R-project.org/
Raymond CM, Spoehr J (2013) The acceptability of climate change in agricultural communities: comparing responses across variability and change. Journal of Environmental Management 115, 69-77.
| Crossref | Google Scholar | PubMed |
Read JL (2002) Experimental trial of Australian arid zone reptiles as early warning indicators of overgrazing by cattle: arid zone reptiles as indicators of overgrazing. Austral Ecology 27(1), 55-66.
| Crossref | Google Scholar |
Richards SA (2005) Testing ecological theory using the information-theoretic approach: examples and cautionary results. Ecology 86(10), 2805-2814.
| Crossref | Google Scholar |
Ridley JCH, Schlesinger CA (2023) Activity of tjakura (great desert skinks) at burrows in relation to plant cover and predators. Ecology and Evolution 13, e10391.
| Crossref | Google Scholar | PubMed |
Roznik EA, Johnson SA (2009) Burrow use and survival of newly metamorphosed gopher frogs (Rana capito). Journal of Herpetology 43(3), 431-437.
| Crossref | Google Scholar |
Schofield JA, Fenner AL, Pelgrim K, Bull CM (2012) Male-biased movement in pygmy bluetongue lizards: implications for conservation. Wildlife Research 39(8), 677-684.
| Crossref | Google Scholar |
Shamiminoori L, Fenner AL, Bull CM (2014) Weight watching in burrows: variation in body condition in pygmy bluetongue lizards. Australian Journal of Zoology 62(4), 284-293.
| Crossref | Google Scholar |
Souter NJ, Michael Bull C, Hutchinson MN (2004) Adding burrows to enhance a population of the endangered pygmy blue tongue lizard, Tiliqua adelaidensis. Biological Conservation 116(3), 403-408.
| Crossref | Google Scholar |
Souter NJ, Bull CM, Lethbridge MR, Hutchinson MN (2007) Habitat requirements of the endangered pygmy bluetongue lizard, Tiliqua adelaidensis. Biological Conservation 135(1), 33-45.
| Crossref | Google Scholar |
Spiegel O, Leu ST, Sih A, Godfrey SS, Bull CM (2015) When the going gets tough: behavioural type-dependent space use in the sleepy lizard changes as the season dries. Proceedings of the Royal Society B: Biological Sciences 282, 20151768.
| Crossref | Google Scholar |
Staver AC, Wigley-Coetsee C, Botha J (2019) Grazer movements exacerbate grass declines during drought in an African savanna. Journal of Ecology 107, 1482-1491.
| Crossref | Google Scholar |
Stevens TA, Evans MC, Osborne WS, Sarre SD (2010) Home ranges of, and habitat use by, the grassland earless dragon (Tympanocryptis pinguicolla) in remnant native grasslands near Canberra. Australian Journal of Zoology 58(2), 76-84.
| Crossref | Google Scholar |
Toft CA (1985) Resource partitioning in amphibians and reptiles. Copeia 1985(1), 1-21.
| Crossref | Google Scholar |
Verdon SJ, Gibb H, Leonard SWJ (2016) Net effects of soil disturbance and herbivory on vegetation by a re-established digging mammal assemblage in arid zone Australia. Journal of Arid Environments 133, 29-36.
| Crossref | Google Scholar |
Vieira EM, Iob G, Briani DC, Palma ART (2005) Microhabitat selection and daily movements of two rodents (Necromys lasiurus and Oryzomys scotti) in Brazilian Cerrado, as revealed by a spool-and-line device. Mammalian Biology 70(6), 359-365.
| Crossref | Google Scholar |
Zipkin EF, Leirness JB, Kinlan BP, O’Connell AF, Silverman ED (2014) Fitting statistical distributions to sea duck count data: implications for survey design and abundance estimation. Statistical Methodology 17, 67-81.
| Crossref | Google Scholar |