Is regional variability in environmental conditions driving differences in the early body condition of endemic Australian fur seal pups?
Demelza Wall A B * , Sam Thalmann B , Simon Wotherspoon C and Mary-Anne Lea A
A B * , Sam Thalmann B , Simon Wotherspoon C and Mary-Anne Lea A
A Institute for Marine & Antarctic Studies, University of Tasmania, Hobart, Tas., Australia.
B Department of Natural Resources and Environment, Marine Conservation Program, Hobart, Tas., Australia.
C Australian Antarctic Division, Kingston, Tas., Australia.
Wildlife Research 50(12) 993-1007 https://doi.org/10.1071/WR22113
Submitted: 6 July 2022 Accepted: 7 January 2023 Published: 20 February 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Good body condition in juvenile marine mammals is crucial for survival and, therefore, population demography. Australian fur seals, endemic to Australia, recently established a breeding colony at the southern edge of their range, at The Needles, a small group of islands in south-west Tasmania (43.6614°S) and a significant distance from their core breeding range in Bass Strait.
Aims: We aimed to compare pup body condition at two breeding colonies, distinct in time since establishment and latitude. Specific aims were to: (1) establish the timing of peak pupping, to compare condition of known-age pups, and determine a baseline at The Needles; (2) investigate pup body condition over time at an established colony; and (3) gain insight into the effects of environmental conditions on pup body condition.
Methods: We conducted a colony comparison of pup body condition using condition indices at The Needles and an established breeding colony in Bass Strait, Tenth Island, for the 2019/20 and 2020/21 breeding seasons. Pup body condition was quantified at Tenth Island over 18 years (2003–2020) using a long-term morphometric dataset. To establish breeding phenology at these two colonies, we determined peak pupping date for the 2019/20 breeding season using daily pup counts. We assessed the effect of environmental parameters on body condition for the long-term dataset.
Key results: Pups from The Needles displayed significantly higher body condition than those from Tenth Island, despite similar peak pupping date. Breeding phenology was consistent with published timing for Australian fur seals. Pup body condition at Tenth Island over the 2-year colony comparison was comparable to the historical average. Environmental drivers that affect maternal foraging efficiency are linked to pup body condition.
Conclusions: Higher pup body condition at The Needles is likely underpinned by better foraging conditions resulting in increased pup provisioning levels. Our results indicate that south-west Tasmania is a region of foraging and emerging breeding importance for Australian fur seals.
Implications: Future research to monitor pup body condition, maternal foraging behaviour and ecosystem productivity at The Needles will help to provide greater understanding of likely population trajectories at this southernmost breeding site for Australian fur seals.
Keywords: adaptive management, Australian fur seal, body condition, breeding phenology, condition indices, fur seal, maternal investment, monitoring, optimal foraging, range expansion.
Introduction
Australian fur seals (Arctocephalus pusillus doriferus; AUFS), along with other species of fur seal and sea lion, were historically harvested in the Australasian region, although it is unclear how many were harvested, and how many existed pre-harvesting (Ling 1999). Similar to their conspecific, the Cape fur seal (Arctocephalus pusillus pusillus; CFS; Warneke and Shaughnessy 1985), Australian fur seal populations have undergone a recovery. However, it is estimated that current population levels of AUFS represent less than half pre-exploitation levels, and have now either stabilised or are in decline, following an increase until 2007 (McIntosh et al. 2018; McIntosh et al. 2022). Despite this overall trend, a number of new colonies were detected in 2013 and 2017 surveys (McIntosh et al. 2018, 2022), one of which was The Needles, south–west Tasmania. This site is over 300 km from Tenth Island, which is at the southern edge of their core breeding range, therefore representing a southward range shift. Prior to colonisation as a breeding colony, The Needles was known as a haul out site for AUFS (Brothers and Pemberton 1990); however, breeding was first recorded in 2017 (McIntosh et al. 2022), and pup numbers have rapidly increased since this time (S. Thalmann, unpubl. data). Conversely, Tenth Island is an established breeding colony that is currently in decline, a trend that is consistent with many other established breeding colonies in Bass Strait, including some of the largest colonies for the species (McIntosh et al. 2022).
Because The Needles is a new breeding colony, there is a unique opportunity to track trends in population health from near inception. This new colony represents a shift in the recent breeding distribution, with associated differences in environmental conditions, and so requires investigation. Tenth Island is subject to variability in pup survival due to its susceptibility to wave inundation during summer (December to February) storms, although there is a long-term morphometric dataset for pups there making it a useful site for monitoring (McIntosh et al. 2022). Understanding the mechanistic processes through which fur seals respond to their environment at each location may help identify differences in trends or give insight into the rapid expansion of breeding at the southern edge of their range.
Pup body condition and growth rates can be a useful population health parameter (Oosthuizen et al. 2016). Early-life body condition can have bottom–up effects on population demography because increased body condition improves initial postweaning survival probability (Hall et al. 2001). Good body condition can improve both development of diving skills and fasting time upon weaning while pups are still developing effective foraging skills (Gastebois et al. 2011). Furthermore, lower pup mass can be indicative of environmental stress (Oosthuizen et al. 2016).
Body condition in fur seal pups is inextricably linked to female foraging efficiency (Lunn et al. 1993). Environmental influences can decrease foraging efficiency (Speakman et al. 2020), which is crucial to maximising maternal investment and reproductive effort in lactating fur seals (Jeanniard-du-Dot et al. 2017). Increased foraging efficiency by mothers increases capacity to spend more time suckling their pups, and to produce higher quality milk, improving pup body condition, which in the long term affects population trends (Jeanniard-du-Dot et al. 2017). Therefore, a substantial inter-colony difference in pup body condition has implications for the ongoing success and population trends of each colony. Quantifying this population parameter helps us to understand the status and health of a species among colonies, and provides inferences over its whole distribution.
Breeding phenology is important for income breeding, central place foragers such as fur seals to maximise reproductive fitness (Trites and Antonelis 1994). Changes in phenology can affect fur seal pup body condition, because breeding timing is linked with prey availability and it is important to rear pups when conditions are most favourable to maximise reproductive success (Lunn and Boyd 1993). In many species, breeding phenology has changed in response to environmental changes (Cohen et al. 2018). Furthermore, latitude has been associated with differences in breeding phenology (Temte 1985; Temte and Temte 1993). Given the latitudinal difference between The Needles and Tenth Island, it is possible that this difference could be driving variabiltiy in breeding phenology. It is also possible that the associated differences in environmental conditions could be underpinning potential differences, as has been observed in chinstrap (Pygoscelis antarcticus) and gentoo penguins (P. papua) (Black et al. 2018). Additionally, assessing breeding phenology allows an approximation of pup age, because AUFS are synchronous breeders (Gibbens and Arnould 2009). This allows clearer interpretation of any differences in pup body condition among colonies, and whether age is a factor.
Marine productivity has been found to drive differences in pup body condition among colonies with geographic variation in long-nosed fur seals, (Arctocephalus. forsteri; LNFS) (Bradshaw et al. 2000; Boren et al. 2006). It is likely that environmental conditions are linked to AUFS pup body condition through regulation of maternal foraging efficiency and hence maternal investment, such as milk quality or attendance pattern (Georges and Guinet 2000). Lactating females are central place foragers due to their need to return regularly and provision their young, thus their foraging success reflects change in their local environment (Guinet et al. 2001). Gaining understanding regarding which variables affect pup body condition will help elucidate the mechanistic processes by which fur seal populations respond to their environment at The Needles and Tenth Island.
The aims of this study were to:
-
Determine if the body condition of AUFS pups differs at two breeding colonies, The Needles and Tenth Island, which vary significantly in their geographic location and population status;
-
At Tenth Island, compare 2019/20 and 2020/21 pup body condition with historical body condition;
-
Determine if breeding phenology in the 2020/21 breeding season was different between colonies, or to what has previously been observed for this species in their core breeding range in Bass Strait; and
-
Investigate which environmental variables have the strongest impact on pup body condition.
Establishing the state of pup condition at each colony will also provide useful data to enable tracking of future changes in body condition. Furthermore, having an index of the health of each colony will contribute to knowledge of fur seal populations when considering interactions with fisheries, aquaculture, and climate interactions in the regions.
Methods
Study sites
This study was conducted at two Tasmanian breeding colonies of AUFS – Tenth Island (40.942290°S, 146.985035°E) and The Needles (43.661420°S, 146.266077°E; Fig. 1). It was conducted over the 2019 and 2020 breeding seasons at both sites, and uses a long-term dataset from 2003 to 2020 for Tenth Island. The year refers to the year the Austral summer breeding season began (e.g. 2020 is the 2020/21 breeding season). Both islands are offshore rocky outcrops, with Tenth Island and The Needles reaching a height above sea level of 8 m and 284 m respectively. (McIntosh et al. 2022). Tenth Island is located in Bass Strait, which is characterised by shallow bathymetry (with average depths of 60–80 m) and low marine productivity (Gibbs et al. 1986). It is 5 km offshore and 160 km from the edge of the continental shelf. In contrast, The Needles is 13 km offshore and 40 km from the edge of the continental shelf. The surrounding bathymetry is much deeper and varied, containing canyons and deep-sea ridges (Huang et al. 2014; Heaney and Davey 2019). South-west Tasmania is situated at the meeting of the Zeehan and East Australian currents (Cresswell 2000), and primary productivity of the region is also influenced by subantarctic waters (Buchanan et al. 2014). Central southern Bass Strait is influenced by both the South Australian current and the East Australian current (Sandery and Kämpf 2007), and is relatively stagnant with long flushing times (Sandery and Kämpf 2005).
Sample sizes and fieldwork timing
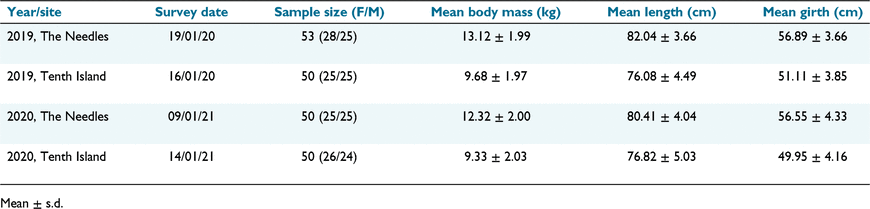
To compare body condition without age as a significant confounding factor, fieldwork was done as close to the same time as possible in each season, weather and logistics permitting (Table 1).
|
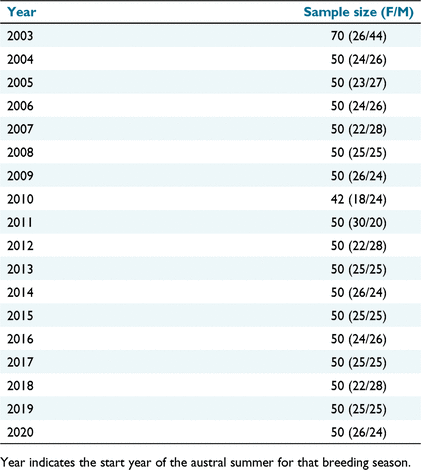
The time difference between sampling at each colony was 3 days in 2020 (2019 breeding season), and 4 days in 2021 (2020 breeding season). Over the long-term dataset, measuring date varies 25 days, from 27 December to 21 January, which was accounted for by the body condition indices used. The sample sizes for each year including the proportion of males and females are in Table 2.
|
Field methods
Pups were captured by hand, put in a hessian bag and weighed with a Salter spring scale (±0.1 kg) The bag was weighed separately after each pup to account for change in weight due to absorbing water throughout the day. Pups were sexed visually, and to ascertain straight line length, their length from nose to end of tail and axial girth were measured to the nearest 0.5 cm using a tape measure attached to square dowel. For the colony-comparison data, (2019 and 2020 breeding seasons), field personnel were the same at both islands but were different among years for the long-term Tenth Island data. To avoid biases, efforts were made to ensure pups were caught from all areas of the colonies. Pups were marked with an identifying mark to perform mark–recapture–mark population count estimates (not for this study). A T-shape was cut out of their dark natal pelage to reveal the lighter fur underneath – this made a distinct mark visible from different angles and from a distance. The marking, which ensured that measurements were not repeated on the same individuals, does not affect the pup’s ability to swim, and they lose the natal pelage when they moult in the following days.
Calculation of condition indices
Blubber layer in marine mammals is an important source of energy, therefore physical condition is indicative of the health of an animal (Pitcher 1986). Morphometric body condition indices are reliable indicators of health – they have been found to be correlated strongly with sternal blubber depth in AUFS (Arnould and Warneke 2002). For the colony comparison of pup condition, we calculated two different body condition indices (BCIs).
BCI1 was a ratio of mass over length (M/L) (Arnould 1995). Following Bradshaw et al. (2000), BCI2 was a ratio of observed mass to predicted mass from a power law relation. The predicted mass was calculated from a simple linear regression of log mass (kg) against log length (m) for all individuals in a group, and back transforming the predicted log mass for each individual. Note that this condition index is algebraically equivalent to back transforming the residuals from the regression. For the colony-comparison data in 2019 and 2020, the condition index was calculated from separate regressions for each combination of year and colony (Table 3).

|
Initially, separate regressions were also used for each sex, but long and heavy female pups at The Needles in 2021 disproportionally weighted the index. Having the same index for both sexes improved the index performance when validating against the raw data and BCI1. Fitting a common model for both sexes for BCI1 meant that differences between sexes were able to be compared for this model.
For the long-term Tenth Island data, where the timing of fieldwork was more varied, the relation between log mass and log length was found to vary from year to year, disproportionately weighting the index for years in which pups were measured later in the season. For this site and for long-term dataset, log mass was again regressed against log length, but the intercept and slope of this relation were assumed to be linear functions of the estimated days since peak pupping in that year, and a long-term condition index (BCI3) was again estimated as back transformation of the residuals from this fit.
All environmental analysis was done using BCI3. Because age varied throughout the dataset, we also calculated the average estimated total growth for the cohort. This was weight divided by their approximate age. Age was approximated as the days since peak pupping to the date of their measurement that season.
Peak pupping determination
Peak pupping date was determined for the 2020 breeding season to establish breeding phenology of AUFS from each colony. At The Needles this was done by two independent observer counts performed daily. Observations of the main breeding area on The Needles were performed using a spotting scope (Bushnell Trophy, Overland Park, Kansas, United States. 65×) from Maatsuyker Island (380 m away). The average of the two counts was used for the daily count. The median pupping date was assigned as the peak pupping date for 2020. At Tenth Island, three camera traps were deployed in early November 2020. Two of these captured the main slope of the breeding colony, in case of camera failure. The third camera was positioned in a second hidden area of the colony. All three cameras took five images and 10-s videos each day (at 0800, 1000, 1200, 1400, and 1600 hours). Two of these cameras were recovered during January 2021 field work (the third was lost either by wave wash or a large seal). The photos were used to count the number of pups each day from the first pup visible in the photos until a week after the maximum number of pups observed. Three counts were done for all photos on each camera, and the averages of these counts were taken as counts for each day, then used to assign peak pupping date.
Statistical analysis
Condition indices
For the colony-comparison dataset, all analyses were done on condition indices BCI1 and BCI2. For the long-term dataset, all analyses were done on BCI3.
All analyses were done in R studio ver. 4.0.5 (R Core Team 2021). Factorial ANOVAs were used to compare condition between The Needles and Tenth Island during 2019 and 2020, with colony, year, and sex as predictors, and condition indices BCI1 and BCI2 as response variables.
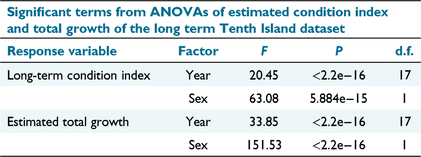
For the long-term morphometric dataset at Tenth Island, a factorial ANOVA on BCI3 and estimated total growth were done with categorical predictors of year of year and sex.
Environmental drivers of condition indices for the long-term Tenth Island dataset
To explore the influence of environmental drivers on calculated condition indices, environmental data, including sea surface temperature (SST), sea surface height anomaly (SSHa), wind strength, and chlorophyll-a (Chl-a) were extracted using ‘raadtools’ package ver. 4.1.1 in R (Sumner 2021), with the original data sources for these variables being: the Optimum Interpolation Sea Surface Temperature (OISST) for SST (Huang et al. 2021); the Copernicus Marine Environment Monitoring Service (CMEMS; Available at: https://marine.copernicus.eu/.) for SSHa; National Centers for Environmental Prediction (NCEP) for wind (Kanamitsu et al. 2002); and the Johnson improved model Chl-a estimates for Chl-a (Johnson et al. 2013). The El Niño Southern Oscillation Index (SOI), from Bureau of Meteorology (2021) and Southern annular Mode (SAM); (Marshall 2003, available at: https://legacy.bas.ac.uk/) were also sourced. Environmental parameters were selected based on variables known to affect foraging efficiency and body condition of fur seals (Speakman et al. 2020), on the premise that foraging efficiency dictates pup body condition.
For each variable, we calculated an average value for a spatial buffer around Tenth Island for two time periods: before and after pup birth. The before-birth time period spanned the month before peak pupping date, as in the last month of gestation. During this time, fur seal foetuses put on the greatest amount of mass for pregnancy, and environmental conditions during the late gestation period are known to impact birth mass (Trites 1991; Georges and Guinet 2001). The after-birth time period spanned from the published peak pupping date until mid-January, which was approximately when seals were measured, at ~50 days old. There are no data available on the foraging areas of each colony, so the area that environmental variables were extracted from was guided by published tracking data of lactating AUFS at other colonies documenting their maximum range (Kirkwood and Arnould 2011; Hoskins et al. 2015), and also from the presence of significant bathymetric and oceanographic features representing their likely foraging location. A 150-km buffer was chosen for Tenth Island because previous research has shown that lactating females stayed mostly within 150 km of the colony (Kirkwood and Arnould 2011). Also, the distance from Tenth Island to the edge of the continental shelf (a reliable, productive resource) is ~150 km.
Analysis of environmental drivers of Tenth Island long-term condition
A penalised GLM was fitted using glmnet (Friedman et al. 2010) to reduce the number of variables in the model. Because it did not have a strong effect, a stepwise generalised linear model (GLM) was conducted with condition as the dependent variable and environmental variables as the independent variables. Null, candidate and maximal models were compared and the model with the lowest AIC, and a difference of at least 2 ΔAIC from the next best model, was selected.
Results
Colony comparisons of pup body condition
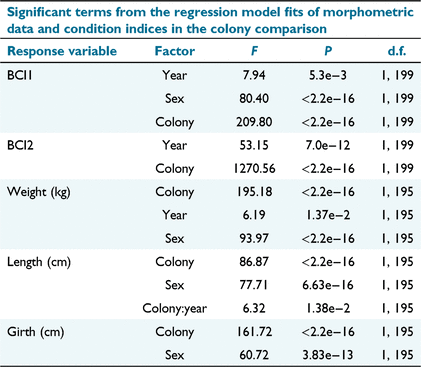
In both years, pups from The Needles were significantly heavier than those from Tenth (F1,195 = 195.175, P < 0.01; Table 4). In 2019/20, they were on average 3.4 kg heavier, 6 cm longer, and 6 cm wider across the axial girth. In 2020/21, they were on average 2.9 kg heavier, 3.6 cm longer and 6.6 cm wider (Table 1, Supplementary Fig. SM1). The between-year difference in weight was not significant at Tenth Island (Tukey’s HSD post hoc test P > 0.05), but was for weight at The Needles (Tukey’s HSD post hoc test P = 0.046). Between-colony differences in length (F1, 195 = 86.874, P < 0.01) and girth (F1, 195 = 161.718, P < 0.01) were also significant (Table 4). There was a significant interaction effect of colony and year for length (F1, 195 = 6.316, P < 0.05; Table 4). A Tukey’s test showed no significant difference between years for comparisons of Tenth:Tenth or Needles:Needles (P > 0.05) for length and girth. Between sexes, males were always significantly heavier (F1, 195 = 93.968, P < 0.01), longer (F1, 195 = 77.710, P < 0.01) and fatter (F1, 195 = 60.718, P < 0.01; Table 4).
|
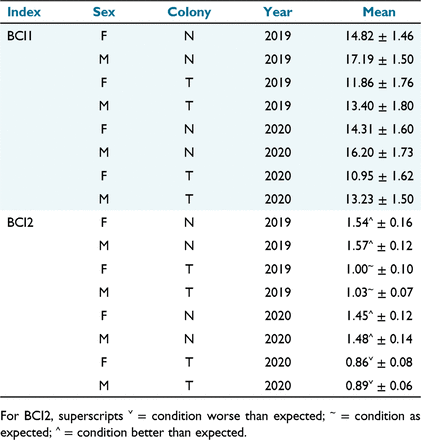
For the colony comparison of condition, BCI1 (mass/length; Fig. 2), The Needles pups were significantly heavier for their length than pups at Tenth Island in each year (F1, 199 = 209.8045, P < 0.001). Males were on average (Table 5) significantly heavier for their length than females (F1, 199 = 80.3974, P < 0.001; Table 4). There was also a significant difference (F1, 199 = 7.9416, P < 0.05) between years (Table 4), although a Tukey’s HSD post hoc test revealed that the between-year difference within colonies was not significant (P > 0.05). Pups at The Needles had a significantly higher BCI2 (observed mass/predicted mass; Bradshaw et al. 2000; Fig. 2), than those from Tenth Island, (F1, 199 = 1270.557, P < 0.001), and there was a significant difference between years (F1, 199 = 53.148, P < 0.001). Values of BCI2 equal to one indicate condition is approximately as expected. Pups from The Needles were in better condition than predicted in both years of the study (Table 5). Pups from Tenth Island were in as predicted condition in 2019/20, but in worse condition than predicted in 2020/21 (Table 5).
|
Peak pupping determination
For the 2020/21 breeding season, peak pupping date was 30 November at The Needles and 1 December at Tenth Island. Both colonies showed a similar start to pupping, with the first pup observed on 9 November at The Needles and 13 November at Tenth Island.
Long term trends in condition indices at Tenth Island
Year has a significant effect on BCI3 (F = 20.4517, P < 0.001) and estimated growth (F = 33.8517, P < 0.001; Table 6), but there is no strong trend over time (Fig. 3).
|
Environmental drivers and their effect on condition indices
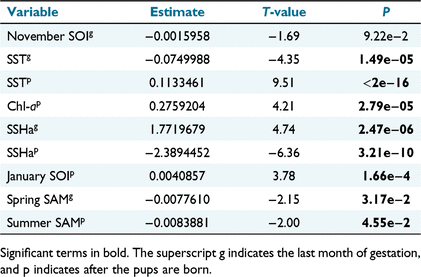
The optimal model selected using AIC for condition is summarised in Table 7. Higher sea surface height anomaly (SSHa) had a positive effect on condition before pupping and a negative effect after the pups were born. Sea surface temperature (SST) had a negative effect on the condition index during gestation, and a positive effect after pups are born.
|
Discussion
The Needles: a new breeding site
Many species are experiencing redistributions driven by prey availability or following a temperature regime shift linked to climate change (Poloczanska et al. 2007). For fur seals, density can also be a factor driving spatial redistribution via colonisation of new breeding areas (Bradshaw et al. 2000). AUFS may be in a phase of ‘recolonisation’, post exploitation, which involves re-inhabiting previous colonies, and new ones forming (Roux 1987). The core breeding range of AUFS is Bass Strait (Warneke 1982), therefore the formation of a breeding colony in south–west Tasmania represents a southward range expansion. Increased density leading to space limitation can cause seals to inhabit nearby suitable habitat. This has occurred with LNFS, with new colonies forming close to existing ones (Bradshaw et al. 2000; Boren et al. 2006). Because breeding females demonstrate breeding site fidelity (Lunn and Boyd 1991), it is likely to be animals undergoing juvenile dispersal that colonise these new areas; however, space availability is not the only factor driving density dependent redistribution.
Alongside AUFS and LNFS, CFS have also been recovering from exploitation, with their population increasing until the 1990s before stabilising. Similar to AUFS, alongside their overall population stabilising, new breeding sites of CFS have formed at the edge of their range, which has been suggested is due in part to space limitation (Kirkman et al. 2013), although many of these new colonies are further afield from existing breeding sites than has occurred in the case of LNFS in New Zealand. Kirkman et al. (2013) suggest that when new colonies are formed further away, it is also linked to food shortages.
The AUFS population is currently either stabilising or decreasing, but has experienced growth in spatial distribution (McIntosh et al. 2018; McIntosh et al. 2022). This could be due to established colonies reaching a density-dependent carrying capacity, as has occurred with LNFS in New Zealand. However, the conspecific CFS exists at much higher densities than AUFS colonies, which could indicate density-dependent resource limitation for colonies in Bass Strait, rather than absolute density influences. Furthermore, the distance new AUFS colonies have emerged from existing colonies is indicative of something more than spillover to nearby suitable habitat. For CFS, the great dispersal distance has been linked to tracking shifts in distribution of key prey species (Kirkman et al. 2013). For AUFS, The Needles is more than 300 km from Tenth Island in their core breeding range. This distance is considerable, especially considering that although some new colonies of CFS in Africa were hundreds of kilometres away from source populations, most were within 100 km (Kirkman et al. 2013). This could suggest food availability is a factor contributing to the southward expansion of AUFS. The large distance was potentially aided by fish farms on the east and west coasts of Tasmania providing reliable food sources along the way.
Although the recent AUFS range extension does represent a poleward movement as has been observed in many other species, they have also exhibited range expansion to the west, with a new colony in South Australia in 2013 (McIntosh et al. 2018). CFS have also recently expanded their range northwards (Kirkman et al. 2013), and even the southern edge of their range in South Africa is at a similar latitude to Bass Strait, indicating that they are capable of existing at such latitudes. The northward expansion of CFS is likely linked to spatial shifts in productivity of the Benguela marine ecosystem, with seals in the central and southern part of their range displaying lower breeding and foraging site fidelity (Skern-Mauritzen et al. 2009); a similar process could be occurring with AUFS.
There is some evidence for latitudinal differences in body size in mammals following Bergmann’s rule (Meiri and Dayan 2003; Adamczak et al. 2020). Indeed, female beluga whales were found to have larger body size at the poleward edge of their range in the Canadian Arctic (Ferguson et al. 2021). Although beyond the scope of this study, in light of the recent range expansion to a higher latitude in AUFS, this could potentially have an effect on adult female body size.
The range expansion of AUFS to south–west Tasmania is potentially due to a combination of space limitation and differences in food availability. This combination of factors (leading to new colonies a considerable distance from other breeding colonies) is corroborated by findings for CFS (Kirkman et al. 2013). Following the establishment of AUFS at The Needles, environmental conditions may facilitate increased population densities at this location.
Breeding phenology
The first pup was seen on 9 November at The Needles, and 13 November at Tenth Island. Although it is possible pups may have been born outside the field of view, these data are a good indication of the pupping period. Peak pupping for the two colonies was only 1 day apart – 30 November at The Needles and 1 December at Tenth Island – which would have little influence on pup growth, especially because the daily counts may not be exact, but would capture the change in births to detect a peak. Pups were measured only a couple of days apart at each colony, so were approximately the same age during fieldwork, which enabled an interpretation of the difference in body condition without age as a confounding factor. The peak pupping date recorded for the 2020/21 breeding season is consistent with previous observations for peak pupping for AUFS in Bass Strait (close to 25 November; Pemberton and Kirkwood 1994), indicating breeding phenology has not permanently shifted beyond what may be expected from inter-annual variation (Geeson et al. 2022).
Colony comparison of body condition over 2 years
Overall colony differences
Condition indices revealed that ~50-day old pups are in significantly better condition at The Needles, over both years of this study. Based on published growth rate data for AUFS, pups this age should be approximately 9–10 kg (Arnould and Hindell 2002). In this study, pup weights at The Needles averaged 13.12 kg in 2019/20 and 12.32 kg in 2020/21, which corresponds to pups ~100 days old, implying faster growth rates than previously recorded for AUFS. Pup weights at Tenth Island were comparable to published growth rates.
These results imply that mothers at The Needles have access to improved foraging conditions, leading to more maternal investment and contributing to the increased body condition of their pups, whereas mothers from Tenth Island are likely being forced to forage over a greater area, for longer. Similarly, between-colony differences in pup body condition of LNFS in New Zealand have been attributed to differences in reliability of food resources (Boren et al. 2006).
Density can also influence weaning mass and body condition (Oosthuizen et al. 2016). Bradshaw et al. (2000) found that pup density may be a contributing factor driving differences in pup body condition. Although colonies in Bass Strait may be reaching carrying capacity, the population at The Needles is still in a growth phase: pup production at The Needles in 2020 was 222 ± 31, compared with 152 ± 5 at Tenth Island (S. Thalmann, unpubl. data). Low productivity in Bass Strait may be leading to a lower carrying capacity, as Sepúlveda et al. (2014) suggested is occurring for two colonies of South American sea lions (Otaria flavescens) in Chile.
Bass Strait is characteristically shallow. In contrast, the bathymetry surrounding The Needles is deeper and more varied. The Needles is situated only ~30 km from the edge of the continental shelf, which is within the foraging range of lactating females. From Tenth Island, the distance to the edge of the continental shelf is close to being outside the foraging range that has been recorded for lactating female AUFS in Bass Strait (Kirkwood and Arnould 2011), indicating that foraging there would require significant effort and lengthen their foraging trips. There is also a deep-sea canyon ~30 km from The Needles, which could be a source of heightened marine productivity and prey availability (N. Barret, pers. comm. 2021), enabling mothers to provision pups more regularly, leading to shorter fasting intervals between feeding bouts and consequently higher growth rates. More regular provisioning and shorter fasting intervals lead to larger pups who display higher levels of aquatic activity, which is important for developing foraging skills (Gastebois et al. 2011). Furthermore, there is evidence from other species that south–west Tasmanian waters produce year-round food availability. Long-nosed fur seals breeding on Maatsuyker Island adjacent to The Needles have exhibited high pup growth rates (Lea and Hindell 1997), indicating the area is productive enough to support high levels of maternal investment for fur seals.
In Bass Strait AUFS forage benthically, and it is unknown if this is a species-specific trait, perhaps linked to their larger size compared with other fur seals that forage epipelagically, or if this is an adaptation to their environment (Arnould and Costa 2006). Until recently, AUFS have bred exclusively in the Bass Strait region. With the appearance of The Needles as a breeding colony, located in a marine environment characterised by deeper water and proximity to the shelf break, it is possible they are switching strategy and foraging pelagically in this location. However, studies of AUFS female foraging strategies at Victorian colonies close to the edge of the continental shelf indicated a preference for benthic foraging despite proximity to the shelf break (Arnould and Kirkwood 2008). Although there are no dive data from seals breeding in south-west Tasmania, this potential difference could be contributing to the pronounced disparity in pup body condition at the two colonies in this study, and site-specific investigation is required to answer this question.
Benthic foraging is associated with higher effort but higher reliability, and therefore could be a strategy to deal with poor prey availability. However, benthic foragers may also have limited ability to increase foraging effort, which could affect maternal investment (Arnould and Costa 2006). This would suggest Tenth Island mothers are restricted in their ability to increase foraging effort, hence the lessened pup condition compared with The Needles.
Conversely, if seals are feeding on pelagic prey in the south-west, this could increase vulnerability to competition with fisheries, such as the winter blue grenadier trawl fishery. Also, this strategy is associated with less predictable prey distribution, which could mean pup condition at The Needles may be more susceptible to environmental variability. For example, strong El Niño events can cause high pup mortality in Galapagos fur seals (Arctocephalus galapagoensis) (Trillmich and Limberger 1985). Furthermore, at The Needles, there is a higher spread of condition index values (Fig. 2), indicating more individual variability, perhaps due to a range of different maternal foraging strategies and success.
Pup body condition in the last 2 years at Tenth Island was very close to the long-term average, indicating the difference observed in this study is not a result of two poor years at Tenth Island. Despite the evident difference in pup body condition for the intercolony comparison in this study, there were only 2 years of data available for both locations, and it will be important to continue monitoring the body condition of seals, pups and older age classes in the south-west to determine if this difference prevails and to better understand the key drivers.
Between-year differences
At both colonies, BCI1 (the ratio of mass over length) indicated that 2020/19 was a marginally better year than 2020/21. However, although year was a statistically significant effect, the between-year but within-colony comparisons were not significant. Interestingly, BCI2 (observed/expected mass) had a significant within-colony difference between the 2 years at each site. At Tenth Island, BCI2 was below 1 (0.86 for females and 0.89 for males) in 2020/21, indicating their condition was slightly lower than expected, and in 2019/20, BCI2 was very close to 1, indicating condition was approximately as expected. These results suggest that 2019/20 was a ‘normal’ year, or that mothers were able to adequately compensate for any lack of resources, and that 2020/21 was a slightly worse year. At The Needles, the difference between the 2 years for BCI2 was ~0.1 and both years were above 1, indicating both years were ‘good years’. Colony and year both influenced pup body condition in this study, as they did for Antarctic fur seals in South Georgia (Nagel et al. 2021). Maternal investment and pup growth rates can change with prey availability, which is spatially and temporally variable (Goldsworthy 2006).
Between-sex differences
For the colony comparison, males were consistently heavier than females (BCI1). Sexes were combined for BCI2. For the long-term dataset, males were also in higher relative condition for the condition index and estimated total growth. This is consistent with higher birth weights and growth rates for male pups previously observed (Arnould and Hindell 2002). A difference in condition between males and females could indicate differential maternal investment, and male body condition is paramount for reproductive success (Trivers and Willard 1973). However, it could be due to differences in behaviour and metabolism (Kretzmann et al. 1993), because male pups direct more energy towards lean muscle mass than females (Arnould et al. 1996). From our results it is impossible to tell if males are simply born heavier than females or are in better relative condition.
Long-term trends in condition indices at Tenth Island
Compared with the long-term average, both sexes in 2019/20 were in slightly better body condition, whereas in 2020/21 both sexes were in marginally worse condition. However, in both years the difference from the long-term mean was slight, signifying that these years were average years in terms of body condition. Estimated condition correlated well with estimated growth, but less so in 2010/11 and 2011/12, 2 years where measuring occurred late. In these 2 years, relative condition was higher than average, but estimated total growth was below average. However, growth efficiency slows with age (McDonald et al. 2012), so it is difficult to interpret body condition for these years.
Although pup body condition at Tenth Island has remained relatively stable over time, it will be important to continue to maintain long-term datasets to understand what future changes in body condition mean relative to the historical baseline. Now that they are breeding in the south-west and have better pup body condition thus far, ongoing monitoring is required to see if populations continue to expand at the southern edge of their range and how this influences the total population status and recovery, which has been slower than other species that were also exploited (Kirkwood et al. 2005).
Implications of the difference in body condition
The finding that pup body condition is elevated at The Needles in comparison with Tenth Island has implications for future population demography and trends. Gaining nutritional independence is critical for juvenile mammals, because they must find enough food to survive yet lack the physiological capacity and size (Spence-Bailey et al. 2007; Orgeret et al. 2019), or the knowledge and experience of adults (de Grissac et al. 2017). Juvenile survival has a particularly important contribution to population dynamics (Sæther et al. 2013), because it affects recruitment into the breeding population (van den Hoff et al. 2014). Juvenile survival is affected by body condition at weaning, with size improving survival probability (Baker and Fowler 1992; McMahon et al. 2000, 2015). This may be because body condition improves energy reserves and the time they can spend foraging (McMahon et al. 2000), as well as impacting foraging ontogeny (Orgeret et al. 2019). In this study, there was a disparity in body condition of pups ~50 days old. This difference will likely persist until weaning, thus impacting foraging ontogeny and chance of survival, which has implications for the future breeding population size. Furthermore, seals at The Needles are potentially foraging pelagically. Benthic foraging is demanding, and takes time to learn (Fowler et al. 2006). Therefore, the difficulty of learning to forage for weaners in the south-west may be somewhat alleviated, potentially increasing survival probability. A challenging transition to independence could prolong maternal dependence, having energetic consequences for the mother, thus potentially having negative impacts on the next year’s breeding attempt (Lee 1996) and perhaps further perpetuating a disparity in body condition. Delayed weaning has been observed for Australian fur seals in Bass Strait, and higher rates of extended weaning were observed at a colony with lower pup survival rates consistent with a more exposed site (Hume et al. 2001). The proportion of juveniles to pups being suckled likely varies with environmental conditions, food availability and location (Hume et al. 2001). The Needles is likely less at risk of mass pup mortality due to wave wash, therefore studies are needed to assess the occurrence of extended maternal dependence at The Needles. Inter-colony differences in the proportion of juveniles and pups being suckled may provide further information on food availability and population health.
Environmental drivers of condition indices at Tenth Island
Changes in prey availability can lead to reduced pup growth (Lea et al. 2006; McHuron et al. 2019). At two colonies of northern fur seals (Callorhinus ursinus; NFS), with opposite population trends (as in this study), differences in distance travelled from the colony and foraging trip duration of lactating females led to the colony being characterised by increasing trends of shorter foraging distances and higher pup growth rates (Banks et al. 2006). A forced expansion of foraging range can occur due to prey depletion surrounding the colony over time, and can regulate population growth (Kuhn et al. 2014). This has energetic consequences, which may restrict energy available for lactation (McHuron et al. 2019). Although our data were limited to environmental analyses at Tenth Island, this mechanism could also potentially be occurring at The Needles.
It was anticipated that environmental conditions in the last month of gestation would regulate pup body condition via maternal body condition affecting birth weight, and that this would impact condition at ~50 days old. Before birth, the environmental conditions that had an effect were SST, SSHa, and spring SAM. However more variables following birth were found to affect body condition, indicating maternal investment though milk quality and attendance has a more detectable effect on pup condition. Environmental conditions that significantly affected body condition during this time were SST, SSHa, Chl-a, January SOI, and summer SAM. These results indicate a complicated response to environmental conditions during late gestation and early life for AUFS pup condition. In late gestation, lower SST, higher SSHa and lower values of SAM were associated with higher body condition. Speakman et al. (2020) found that higher SSHa was associated with reduced foraging effort and possibly better foraging conditions in winter, corroborating with this result of higher SSHa being linked to improved body condition.
In Bass Strait, increased Chl-a concentrations were associated with reduced foraging trip duration of AUFS (Speakman et al. 2020). In this study, there was a positive effect on pup body condition of Chl-a concentrations in the first ~50 days after peak pupping. Therefore, potentially, higher Chl-a was associated with decreased maternal trip duration, which can improve pup condition (Lunn et al. 1993). During this time, lower SSHa were associated with increased body condition, which is opposite to the effect of SSHa in late gestation, and also to the results of Speakman et al. (2020). They also found current year SOI and SAM also had an effect on dive duration of adult females in Bass Strait. Shorter dive duration can indicate lower prey availability if the dive is finished after not encountering any prey (Thompson and Fedak 2001). Our results indicate January SOI and summer SAM were important for body condition, particularly SOI. January SOI had a positive effect on body condition (this study), and a positive effect on dive duration (Speakman et al. 2020). This could suggest that positive SOI values are associated with more successful dives.
Both spring and summer SAM were negatively correlated with body condition. The SAM is an important driver of weather in Tasmania (Gillett et al. 2006), and is predicted to become increasingly positive (Cai et al. 2005). SST had a significant impact on condition in both time periods, but in late gestation this was a negative effect, and in post pupping it was a positive effect. The drivers behind this are unclear.
When pups are young, thermoregulation can increase metabolism and reduce energy available for growth, and delay their entry into the water (Donohue et al. 2000; Rutishauser et al. 2004; McDonald et al. 2012). In this study, neither air temperature, rainfall, nor wind strength were correlated with pup body condition; however, pups may have died from exposure-related causes before sampling occurred because low birth weights and inadequate reserves for thermoregulation can result in death (Trites 1990). Due to site characteristics, carcass retention is low and therefore difficult to quantify (Pemberton and Kirkwood 1994).
The marine environment surrounding The Needles and Tenth Island is a completely different ‘kettle of fish’; therefore, the mechanisms via which environmental conditions dictate pup body condition may differ. The Needles is in a highly productive environment, and SST and SSHa could signify upwelling, and be a driver for pup condition in the south-west. Both these variables have high year-to-year variability in the south–west.
This study undertakes a preliminary investigation of key environmental drivers of pup body condition based off variables known to impact the foraging effort and efficiency of female Australian fur seals (Hoskins and Arnould 2014; Speakman et al. 2020), because this regulates maternal investment (Jeanniard-du-Dot et al. 2017). However, to further interrogate the environmental drivers behind pup body condition in Tasmania, colony-specific core foraging areas and dive characteristics of adult females need to be identified, especially at The Needles, because this represents a unique case in the breeding habitat of AUFS.
Conclusions
These results show that pup body condition is significantly higher at The Needles than at Tenth Island, indicating that south-west Tasmania is an area of significance for AUFS populations. Because the population at The Needles is rapidly growing, it is a valuable opportunity to monitor trends and health using key parameters and thus gain insights into drivers of population dynamics. The results of this study show that pup body condition is a useful monitoring parameter, and the greater body condition of pups at The Needles could positively influence early survivorship (Donohue et al. 2000; Rutishauser et al. 2004; Lea et al. 2009). This has implications for recruitment and population demography, thus the population at The Needles is likely to continue increasing. The differences in pup body condition observed between the two study colonies is probably linked to productivity differences of the surrounding marine environments, and the impact this has on maternal investment, and it would be valuable to conduct site-specific tracking studies of adult females (Arnould and Hindell 2001; Arnould and Costa 2006; Lea et al. 2006; Kirkwood and Arnould 2011; Speakman et al. 2020). This would also allow a more comprehensive study of environmental conditions in their core foraging areas, particularly new and emerging areas of their distribution, and provide new insight into their foraging plasticity and how these might affect population dynamics of this endemic species into the future.
Supplementary material
Supplementary material is available online.
Data availability
Data will be uploaded to the IMAS Data portal: IMAS Data Portal (utas.edu.au).
Conflicts of interest
We declare no conflicts of interest.
Declaration of funding
Funding for field work was provided by the Princess Melikoff Marine Mammal Conservation Program. All animal-handling procedures were performed under NRE staff permits for management activities.
Acknowledgements
We thank all those who have contributed to collecting data for the Tenth Island long-term time series, and the Maatsuyker Island caretakers, Heidi Krajewsky and Stephen Anstee for daily pup counts in 2020. Thank you to Sheryl Hamilton, Claire Mason and Brian Wall for helpful comments on the manuscript.
References
Adamczak, SK, Pabst, DA, McLellan, WA, and Thorne, LH (2020). Do bigger bodies require bigger radiators? Insights into thermal ecology from closely related marine mammal species and implications for ecogeographic rules. Journal of Biogeography 47, 1193–1206.| Do bigger bodies require bigger radiators? Insights into thermal ecology from closely related marine mammal species and implications for ecogeographic rules.Crossref | GoogleScholarGoogle Scholar |
Arnould, JPY (1995). Indices of body condition and body composition in female Antarctic fur seals (Arctocephalus gazella). Marine Mammal Science 11, 301–313.
| Indices of body condition and body composition in female Antarctic fur seals (Arctocephalus gazella).Crossref | GoogleScholarGoogle Scholar |
Arnould JPY, Costa DP (2006) Sea lions in drag, fur seals incognito: insights from the otariid deviants. In ‘Sea Lions of the World: Conservation and Research in the 21st Century’. pp. 309–323. (University of Alaska Fairbanks: Fairbanks, AK, USA) 10.4027/slw.2006.22
Arnould, JPY, and Hindell, MA (2001). Dive behaviour, foraging locations, and maternal-attendance patterns of Australian fur seals (Arctocephalus pusillus doriferus). Canadian Journal of Zoology 79, 35–48.
| Dive behaviour, foraging locations, and maternal-attendance patterns of Australian fur seals (Arctocephalus pusillus doriferus).Crossref | GoogleScholarGoogle Scholar |
Arnould, JPY, and Hindell, MA (2002). Milk consumption, body composition and pre-weaning growth rates of Australian fur seal (Arctocephalus pusillus doriferus) pups. Journal of Zoology 256, 351–359.
| Milk consumption, body composition and pre-weaning growth rates of Australian fur seal (Arctocephalus pusillus doriferus) pups.Crossref | GoogleScholarGoogle Scholar |
Arnould, JPY, and Kirkwood, R (2008). Habitat selection by female Australian fur seals (Arctocephalus pusillus doriferus). Aquatic Conservation: Marine and Freshwater Ecosystems 17, S53–S67.
| Habitat selection by female Australian fur seals (Arctocephalus pusillus doriferus).Crossref | GoogleScholarGoogle Scholar |
Arnould, JPY, and Warneke, RM (2002). Growth and condition in Australian fur seals (Arctocephalus pusillus doriferus) (Carnivora : Pinnipedia). Australian Journal of Zoology 50, 53–66.
| Growth and condition in Australian fur seals (Arctocephalus pusillus doriferus) (Carnivora : Pinnipedia).Crossref | GoogleScholarGoogle Scholar |
Arnould, JPY, Boyd, IL, and Socha, DG (1996). Milk consumption and growth efficiency in Antarctic fur seal (Arctocephalus gazella) pups. Canadian Journal of Zoology 74, 254–266.
| Milk consumption and growth efficiency in Antarctic fur seal (Arctocephalus gazella) pups.Crossref | GoogleScholarGoogle Scholar |
Baker, JD, and Fowler, CW (1992). Pup weight and survival of northern fur seals Callorhinus ursinus. Journal of Zoology 227, 231–238.
| Pup weight and survival of northern fur seals Callorhinus ursinus.Crossref | GoogleScholarGoogle Scholar |
Black, C, Collen, B, Lunn, D, Filby, D, Winnard, S, and Hart, T (2018). Time-lapse cameras reveal latitude and season influence breeding phenology durations in penguins. Ecology and Evolution 8, 8286–8296.
| Time-lapse cameras reveal latitude and season influence breeding phenology durations in penguins.Crossref | GoogleScholarGoogle Scholar |
Boren, LJ, Muller, CG, and Gemmell, NJ (2006). Colony growth and pup condition of the New Zealand fur seal (Arctocephalus forsteri) on the Kaikoura coastline compared with other east coast colonies. Wildlife Research 33, 497–505.
| Colony growth and pup condition of the New Zealand fur seal (Arctocephalus forsteri) on the Kaikoura coastline compared with other east coast colonies.Crossref | GoogleScholarGoogle Scholar |
Bradshaw, CJA, Davis, LS, Lalas, C, and Harcourt, RG (2000). Geographic and temporal variation in the condition of pups of the New Zealand fur seal (Arctocephalus forsteri): evidence for density dependence and differences in the marine environment. Journal of Zoology 252, 41–51.
| Geographic and temporal variation in the condition of pups of the New Zealand fur seal (Arctocephalus forsteri): evidence for density dependence and differences in the marine environment.Crossref | GoogleScholarGoogle Scholar |
Brothers, N, and Pemberton, D (1990). Status of Australian and New-Zealand fur seals at Maatsuyker Island, Southwestern Tasmania. Wildlife Research 17, 563–569.
| Status of Australian and New-Zealand fur seals at Maatsuyker Island, Southwestern Tasmania.Crossref | GoogleScholarGoogle Scholar |
Buchanan, PJ, Swadling, KM, Eriksen, RS, and Wild-Allen, K (2014). New evidence links changing shelf phytoplankton communities to boundary currents in southeast Tasmania. Reviews in Fish Biology and Fisheries 24, 427–442.
| New evidence links changing shelf phytoplankton communities to boundary currents in southeast Tasmania.Crossref | GoogleScholarGoogle Scholar |
Bureau of Meteorology (2021) Southern Oscillation Index (SOI) since 1876. Bureau of Meteorology, Melbourne, Vic., Australia. Available at http://www.bom.gov.au/climate/enso/soi/ [Accessed 9 September 2021]
Cai, W, Shi, G, Cowan, T, Bi, D, and Ribbe, J (2005). The response of the Southern Annular Mode, the East Australian Current, and the southern mid-latitude ocean circulation to global warming. Geophysical Research Letters 32, .
| The response of the Southern Annular Mode, the East Australian Current, and the southern mid-latitude ocean circulation to global warming.Crossref | GoogleScholarGoogle Scholar |
Cohen, JM, Lajeunesse, MJ, and Rohr, JR (2018). A global synthesis of animal phenological responses to climate change. Nature Climate Change 8, 224–228.
| A global synthesis of animal phenological responses to climate change.Crossref | GoogleScholarGoogle Scholar |
Cresswell, G (2000). Currents of the continental shelf and upper slope of Tasmania. Papers and Proceedings of the Royal Society of Tasmania 133, 21–30.
| Currents of the continental shelf and upper slope of Tasmania.Crossref | GoogleScholarGoogle Scholar |
de Grissac, S, Bartumeus, F, Cox, SL, and Weimerskirch, H (2017). Early-life foraging: behavioral responses of newly fledged albatrosses to environmental conditions. Ecology and Evolution 7, 6766–6778.
| Early-life foraging: behavioral responses of newly fledged albatrosses to environmental conditions.Crossref | GoogleScholarGoogle Scholar |
Donohue, MJ, Costa, DP, Goebel, ME, and Baker, JD (2000). The ontogeny of metabolic rate and thermoregulatory capabilities of northern fur seal, Callorhinus ursinus, pups in air and water. Journal of Experimental Biology 203, 1003–1016.
| The ontogeny of metabolic rate and thermoregulatory capabilities of northern fur seal, Callorhinus ursinus, pups in air and water.Crossref | GoogleScholarGoogle Scholar |
Ferguson, SH, Yurkowski, DJ, Hudson, JM, Edkins, T, Willing, C, and Watt, CA (2021). Larger body size leads to greater female beluga whale ovarian reproductive activity at the southern periphery of their range. Ecology and Evolution 11, 17314–17322.
| Larger body size leads to greater female beluga whale ovarian reproductive activity at the southern periphery of their range.Crossref | GoogleScholarGoogle Scholar |
Fowler, SL, Costa, DP, Arnould, JPY, Gales, NJ, and Kuhn, CE (2006). Ontogeny of diving behaviour in the Australian sea lion: trials of adolescence in a late bloomer. Journal of Animal Ecology 75, 358–367.
| Ontogeny of diving behaviour in the Australian sea lion: trials of adolescence in a late bloomer.Crossref | GoogleScholarGoogle Scholar |
Friedman, J, Hastie, T, and Tibshirani, R (2010). Regularization paths for generalized linear models via coordinate descent. Journal of Statistical Software 33, 1–22.
Gastebois, C, Viviant, M, and Guinet, C (2011). Ontogeny of aquatic behaviours in Antarctic fur seal (Arctocephalus gazella) pups in relation to growth performances at Kerguelen Islands. Polar Biology 34, 1097–1103.
| Ontogeny of aquatic behaviours in Antarctic fur seal (Arctocephalus gazella) pups in relation to growth performances at Kerguelen Islands.Crossref | GoogleScholarGoogle Scholar |
Geeson, JJ, Hobday, AJ, Speakman, CN, and Arnould, JPY (2022). Environmental influences on breeding biology and pup production in Australian fur seals. Royal Society Open Science 9, .
| Environmental influences on breeding biology and pup production in Australian fur seals.Crossref | GoogleScholarGoogle Scholar |
Georges, J-Y, and Guinet, C (2000). Maternal care in the subantarctic fur seals on Amsterdam Island. Ecology 81, 295–308.
| Maternal care in the subantarctic fur seals on Amsterdam Island.Crossref | GoogleScholarGoogle Scholar |
Georges, J-Y, and Guinet, C (2001). Prenatal investment in the subantarctic fur seal, Arctocephalus tropicalis. Canadian Journal of Zoology 79, 601–609.
| Prenatal investment in the subantarctic fur seal, Arctocephalus tropicalis.Crossref | GoogleScholarGoogle Scholar |
Gibbens, J, and Arnould, JPY (2009). Interannual variation in pup production and the timing of breeding in benthic foraging Australian fur seals. Marine Mammal Science 25, 573–587.
| Interannual variation in pup production and the timing of breeding in benthic foraging Australian fur seals.Crossref | GoogleScholarGoogle Scholar |
Gibbs, CF, Tomczak, M, and Longmore, AR (1986). The nutrient regime of Bass Strait. Marine and Freshwater Research 37, 451–466.
| The nutrient regime of Bass Strait.Crossref | GoogleScholarGoogle Scholar |
Gillett, NP, Kell, TD, and Jones, PD (2006). Regional climate impacts of the Southern Annular Mode. Geophysical Research Letters 33, .
| Regional climate impacts of the Southern Annular Mode.Crossref | GoogleScholarGoogle Scholar |
Goldsworthy, SD (2006). Maternal strategies of the New Zealand fur seal: evidence for interannual variability in provisioning and pup growth strategies. Australian Journal of Zoology 54, 31–44.
| Maternal strategies of the New Zealand fur seal: evidence for interannual variability in provisioning and pup growth strategies.Crossref | GoogleScholarGoogle Scholar |
Guinet, C, Dubroca, L, Lea, MA, Goldsworthy, S, Cherel, Y, Duhamel, G, Bonadonna, F, and Donnay, JP (2001). Spatial distribution of foraging in female Antarctic fur seals Arctocephalus gazella in relation to oceanographic variables: a scale-dependent approach using geographic information systems. Marine Ecology Progress Series 219, 251–264.
| Spatial distribution of foraging in female Antarctic fur seals Arctocephalus gazella in relation to oceanographic variables: a scale-dependent approach using geographic information systems.Crossref | GoogleScholarGoogle Scholar |
Hall, AJ, McConnell, BJ, and Barker, RJ (2001). Factors affecting first-year survival in grey seals and their implications for life history strategy. Journal of Animal Ecology 70, 138–149.
| Factors affecting first-year survival in grey seals and their implications for life history strategy.Crossref | GoogleScholarGoogle Scholar |
Heaney B, Davey C (2019) Hydrographic survey of the Freycinet, Huon and Tasman Fracture Australian Marine Parks. Project: BF2019_v01. Commonwealth Scientific and Industrial Research Organisation, Australia. Available at https://data.imas.utas.edu.au/attachments/be7daab3-8b0d-4af6-9b49-1fd8af58846f/bf2019_v01_Freycinet_Huon_TasFracture_Marine_Park.pdf
Hoskins, AJ, and Arnould, JPY (2014). Relationship between long-term environmental fluctuations and diving effort of female Australian fur seals. Marine Ecology Progress Series 511, 285–295.
| Relationship between long-term environmental fluctuations and diving effort of female Australian fur seals.Crossref | GoogleScholarGoogle Scholar |
Hoskins, AJ, Costa, DP, and Arnould, JPY (2015). Utilisation of intensive foraging zones by female Australian fur seals. PLoS ONE 10, .
| Utilisation of intensive foraging zones by female Australian fur seals.Crossref | GoogleScholarGoogle Scholar |
Huang, Z, Nichol, SL, Harris, PT, and Caley, MJ (2014). Classification of submarine canyons of the Australian continental margin. Marine Geology 357, 362–383.
| Classification of submarine canyons of the Australian continental margin.Crossref | GoogleScholarGoogle Scholar |
Huang, B, Liu, C, Banzon, V, Freeman, E, Graham, G, Hankins, B, Smith, T, and Zhang, H-M (2021). Improvements of the daily optimum interpolation sea surface temperature (DOISST) version 2.1. Journal of Climate 34, 2923–2939.
| Improvements of the daily optimum interpolation sea surface temperature (DOISST) version 2.1.Crossref | GoogleScholarGoogle Scholar |
Hume, F, Arnould, JPY, Kirkwood, R, and Davis, P (2001). Extended maternal dependence by juvenile Australian fur seals (Arctocephalus pusillus doriferus). Australian Mammalogy 23, 67–70.
| Extended maternal dependence by juvenile Australian fur seals (Arctocephalus pusillus doriferus).Crossref | GoogleScholarGoogle Scholar |
Jeanniard-du-Dot, T, Trites, AW, Arnould, JPY, and Guinet, C (2017). Reproductive success is energetically linked to foraging efficiency in Antarctic fur seals. PLoS ONE 12, e0174001.
| Reproductive success is energetically linked to foraging efficiency in Antarctic fur seals.Crossref | GoogleScholarGoogle Scholar |
Johnson, R, Strutton, PG, Wright, SW, McMinn, A, and Meiners, KM (2013). Three improved satellite chlorophyll algorithms for the Southern Ocean. Journal of Geophysical Research: Oceans 118, 3694–3703.
| Three improved satellite chlorophyll algorithms for the Southern Ocean.Crossref | GoogleScholarGoogle Scholar |
Kanamitsu, M, Ebisuzaki, W, Woollen, J, Yang, S, Hnilo, JJ, Fiorino, M, and Potter, GL (2002). NCEP-DOE AMIP-II reanalysis (R-2). Bulletin of the American Meteorological Society 83, 1631–1644.
| NCEP-DOE AMIP-II reanalysis (R-2).Crossref | GoogleScholarGoogle Scholar |
Kirkman, SP, Yemane, D, Oosthuizen, WH, Meÿer, MA, Kotze, PGH, Skrypzeck, H, Vaz Velho, F, and Underhill, LG (2013). Spatio-temporal shifts of the dynamic Cape fur seal population in southern Africa, based on aerial censuses (1972-2009). Marine Mammal Science 29, 497–524.
| Spatio-temporal shifts of the dynamic Cape fur seal population in southern Africa, based on aerial censuses (1972-2009).Crossref | GoogleScholarGoogle Scholar |
Kirkwood, R, and Arnould, JPY (2011). Foraging trip strategies and habitat use during late pup rearing by lactating Australian fur seals. Australian Journal of Zoology 59, 216–226.
| Foraging trip strategies and habitat use during late pup rearing by lactating Australian fur seals.Crossref | GoogleScholarGoogle Scholar |
Kirkwood, R, Gales, R, Terauds, A, Arnould, HPY, Pemberton, D, Shaughnessy, PD, Mitchell, AT, and Gibbens, J (2005). Pup production and population trends of the Australian fur seal (Arctocephalus pusillus doriferus). Marine Mammal Science 21, 260–282.
| Pup production and population trends of the Australian fur seal (Arctocephalus pusillus doriferus).Crossref | GoogleScholarGoogle Scholar |
Kretzmann, MB, Costa, DP, and Le Boeuf, BJ (1993). Maternal energy investment in elephant seal pups: evidence for sexual equality? The American Naturalist 141, 466–480.
| Maternal energy investment in elephant seal pups: evidence for sexual equality?Crossref | GoogleScholarGoogle Scholar |
Kuhn, CE, Baker, JD, Towell, RG, and Ream, RR (2014). Evidence of localized resource depletion following a natural colonization event by a large marine predator. Journal of Animal Ecology 83, 1169–1177.
| Evidence of localized resource depletion following a natural colonization event by a large marine predator.Crossref | GoogleScholarGoogle Scholar |
Lea, M-A, and Hindell, MA (1997). Pup growth and maternal care in New Zealand fur seals, Arctocephalus forsteri, at Maatsuyker Island, Tasmania. Wildlife Research 24, 307–318.
| Pup growth and maternal care in New Zealand fur seals, Arctocephalus forsteri, at Maatsuyker Island, Tasmania.Crossref | GoogleScholarGoogle Scholar |
Lea, M-A, Guinet, C, Cherel, Y, Duhamel, G, Dubroca, L, Pruvost, P, and Hindell, M (2006). Impacts of climatic anomalies on provisioning strategies of a Southern Ocean predator. Marine Ecology Progress Series 310, 77–94.
| Impacts of climatic anomalies on provisioning strategies of a Southern Ocean predator.Crossref | GoogleScholarGoogle Scholar |
Lea, M-A, Johnson, D, Ream, R, Sterling, J, Melin, S, and Gelatt, T (2009). Extreme weather events influence dispersal of naive northern fur seals. Biology Letters 5, 252–257.
| Extreme weather events influence dispersal of naive northern fur seals.Crossref | GoogleScholarGoogle Scholar |
Lee, PC (1996). The meanings of weaning: growth, lactation, and life history. Evolutionary Anthropology 5, 87–98.
| The meanings of weaning: growth, lactation, and life history.Crossref | GoogleScholarGoogle Scholar |
Ling, J (1999). Exploitation of fur seals and sea lions from Australian, New Zealand and adjacent subantarctic islands during the eighteenth, nineteenth and twentieth centuries. Australian Zoologist 31, 323–350.
| Exploitation of fur seals and sea lions from Australian, New Zealand and adjacent subantarctic islands during the eighteenth, nineteenth and twentieth centuries.Crossref | GoogleScholarGoogle Scholar |
Lunn, NJ, and Boyd, IL (1991). Pupping-site fidelity of Antarctic fur seals at Bird Island, South Georgia. Journal of Mammalogy 72, 202–206.
| Pupping-site fidelity of Antarctic fur seals at Bird Island, South Georgia.Crossref | GoogleScholarGoogle Scholar |
Lunn, NJ, and Boyd, IL (1993). Effects of maternal age and condition on parturition and the perinatal period of Antarctic fur seals. Journal of Zoology 229, 55–67.
| Effects of maternal age and condition on parturition and the perinatal period of Antarctic fur seals.Crossref | GoogleScholarGoogle Scholar |
Lunn, NJ, Boyd, IL, Barton, T, and Croxall, JP (1993). Factors affecting the growth rate and mass at weaning of Antarctic fur seals at Bird Island, South Georgia. Journal of Mammalogy 74, 908–919.
| Factors affecting the growth rate and mass at weaning of Antarctic fur seals at Bird Island, South Georgia.Crossref | GoogleScholarGoogle Scholar |
Marshall, GJ (2003). Trends in the Southern Annular Mode from observations and reanalyses. Journal of Climate 16, 4134–4143.
| Trends in the Southern Annular Mode from observations and reanalyses.Crossref | GoogleScholarGoogle Scholar |
McDonald, BI, Goebel, ME, Crocker, DE, and Costa, DP (2012). Biological and environmental drivers of energy allocation in a dependent mammal, the Antarctic fur seal pup. Physiological and Biochemical Zoology 85, 134–147.
| Biological and environmental drivers of energy allocation in a dependent mammal, the Antarctic fur seal pup.Crossref | GoogleScholarGoogle Scholar |
McHuron, EA, Sterling, JT, Costa, DP, and Goebel, ME (2019). Factors affecting energy expenditure in a declining fur seal population. Conservation Physiology 7, 1–11.
| Factors affecting energy expenditure in a declining fur seal population.Crossref | GoogleScholarGoogle Scholar |
McIntosh, RR, Kirkman, SP, Thalmann, S, Sutherland, DR, Mitchell, A, Arnould, JPY, Salton, M, Slip, DJ, Dann, P, and Kirkwood, R (2018). Understanding meta-population trends of the Australian fur seal, with insights for adaptive monitoring. PLoS ONE 13, e0200253.
| Understanding meta-population trends of the Australian fur seal, with insights for adaptive monitoring.Crossref | GoogleScholarGoogle Scholar |
McIntosh, RR, Sorrell, KJ, Thalmann, S, Mitchell, A, Gray, R, Schinagl, H, Arnould, JPY, Dann, P, and Kirkwood, R (2022). Sustained reduction in numbers of Australian fur seal pups: implications for future population monitoring. PLoS ONE 17, .
| Sustained reduction in numbers of Australian fur seal pups: implications for future population monitoring.Crossref | GoogleScholarGoogle Scholar |
McMahon, CR, Burton, HR, and Bester, MN (2000). Weaning mass and the future survival of juvenile southern elephant seals, Mirounga leonina, at Macquarie Island. Antarctic Science 12, 149–153.
| Weaning mass and the future survival of juvenile southern elephant seals, Mirounga leonina, at Macquarie Island.Crossref | GoogleScholarGoogle Scholar |
McMahon, CR, New, LF, Fairley, EJ, Hindell, MA, and Burton, HR (2015). The effects of body size and climate on post-weaning survival of elephant seals at Heard Island. Journal of Zoology 297, 301–308.
| The effects of body size and climate on post-weaning survival of elephant seals at Heard Island.Crossref | GoogleScholarGoogle Scholar |
Meiri, S, and Dayan, T (2003). On the validity of Bergmann’s rule. Journal of Biogeography 30, 331–351.
| On the validity of Bergmann’s rule.Crossref | GoogleScholarGoogle Scholar |
Nagel, R, Stainfield, C, Fox-Clarke, C, Toscani, C, Forcada, J, and Hoffman, JI (2021). Evidence for an Allee effect in a declining fur seal population. Proceedings of the Royal Society B: Biological Sciences 288, .
| Evidence for an Allee effect in a declining fur seal population.Crossref | GoogleScholarGoogle Scholar |
Oosthuizen, WC, de Bruyn, PJN, Wege, M, and Bester, MN (2016). Geographic variation in subantarctic fur seal pup growth: linkages with environmental variability and population density. Journal of Mammalogy 97, 347–360.
| Geographic variation in subantarctic fur seal pup growth: linkages with environmental variability and population density.Crossref | GoogleScholarGoogle Scholar |
Orgeret, F, Cox, SL, Weimerskirch, H, and Guinet, C (2019). Body condition influences ontogeny of foraging behavior in juvenile southern elephant seals. Ecology and Evolution 9, 223–236.
| Body condition influences ontogeny of foraging behavior in juvenile southern elephant seals.Crossref | GoogleScholarGoogle Scholar |
Pemberton, D, and Kirkwood, RJ (1994). Pup production and distribution of the Australian fur seal, Arctocephalus pusillus doriferus, in Tasmania. Wildlife Research 21, 341–351.
| Pup production and distribution of the Australian fur seal, Arctocephalus pusillus doriferus, in Tasmania.Crossref | GoogleScholarGoogle Scholar |
Pitcher, KW (1986). Variation in blubber thickness of harbor seals in Southern Alaska. The Journal of Wildlife Management 50, 463–466.
| Variation in blubber thickness of harbor seals in Southern Alaska.Crossref | GoogleScholarGoogle Scholar |
Poloczanska ES, Babcock RC, Butler A, Hobday AJ, Hoegh-Guldberg O, Kunz TJ, Matear R, Milton DA, Okey TA, Richardson AJ (2007) Climate change and Australian marine life. Oceanography and Marine Biology: An Annual Review 45, 407–4878. 10.1201/9781420050943.ch8
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at https://www.R-project.org/
Roux JP (1987) Recolonization processes in the Subantarctic fur seal, Arctocephalus tropicalis, on Amsterdam Island. In ‘Status, biology, and ecology of fur seals’. Proceedings of an international symposium and workshop Cambridge, England, 23-27 April 1984. (Eds Croxall JP, Gentry RL) pp. 189–194. NOAA Technical Report NMFS 51.
Rutishauser, MR, Costa, DP, Goebel, ME, and Williams, TM (2004). Ecological implications of body composition and thermal capabilities in young Antarctic fur seals (Arctocephalus gazella). Physiological and Biochemical Zoology 77, 669–681.
| Ecological implications of body composition and thermal capabilities in young Antarctic fur seals (Arctocephalus gazella).Crossref | GoogleScholarGoogle Scholar |
Sandery, PA, and Kämpf, J (2005). Winter–Spring flushing of Bass Strait, south-eastern Australia: a numerical modelling study. Estuarine, Coastal and Shelf Science 63, 23–31.
| Winter–Spring flushing of Bass Strait, south-eastern Australia: a numerical modelling study.Crossref | GoogleScholarGoogle Scholar |
Sandery, PA, and Kämpf, J (2007). Transport timescales for identifying seasonal variation in Bass Strait, south-eastern Australia. Estuarine, Coastal and Shelf Science 74, 684–696.
| Transport timescales for identifying seasonal variation in Bass Strait, south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Sepúlveda, M, Olea, D, Carrasco, P, Santos-Carvallo, M, Castillo, J, and Quiñones, RA (2014). Latitudinal variation in local productivity influences body condition of South American sea lion pups. Aquatic Biology 23, 39–47.
| Latitudinal variation in local productivity influences body condition of South American sea lion pups.Crossref | GoogleScholarGoogle Scholar |
Skern-Mauritzen, M, Kirkman, SP, Olsen, E, Bjørge, A, Drapeau, L, Meÿer, MA, Roux, J-P, Swanson, S, and Oosthuizen, WH (2009). Do inter-colony differences in Cape fur seal foraging behaviour reflect large-scale changes in the northern Benguela ecosystem? African Journal of Marine Science 31, 399–408.
| Do inter-colony differences in Cape fur seal foraging behaviour reflect large-scale changes in the northern Benguela ecosystem?Crossref | GoogleScholarGoogle Scholar |
Speakman, CN, Hoskins, AJ, Hindell, MA, Costa, DP, Hartog, JR, Hobday, AJ, and Arnould, JPY (2020). Environmental influences on foraging effort, success and efficiency in female Australian fur seals. Scientific Reports 10, .
| Environmental influences on foraging effort, success and efficiency in female Australian fur seals.Crossref | GoogleScholarGoogle Scholar |
Spence-Bailey, LM, Verrier, D, and Arnould, JPY (2007). The physiological and behavioural development of diving in Australian fur seal (Arctocephalus pusillus doriferus) pups. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology 177, 483–494.
| The physiological and behavioural development of diving in Australian fur seal (Arctocephalus pusillus doriferus) pups.Crossref | GoogleScholarGoogle Scholar |
Sumner M (2021) raadtools: Tools for synoptic environmental spatial data. R package version 4.1.1, https://github.com/AustralianAntarcticDivision/raadtools
Sæther, B-E, Coulson, T, Grøtan, V, Engen, S, Altwegg, R, Armitage, KB, Barbraud, C, Becker, PH, Blumstein, DT, Dobson, FS, Festa-Bianchet, M, Gaillard, J-M, Jenkins, A, Jones, C, Nicoll, MAC, Norris, K, Oli, MK, Ozgul, A, and Weimerskirch, H (2013). How life history influences population dynamics in fluctuating environments. The American Naturalist 182, 743–759.
| How life history influences population dynamics in fluctuating environments.Crossref | GoogleScholarGoogle Scholar |
Temte, JL (1985). Photoperiod and delayed implantation in the northern fur seal (Callorhinus ursinus). Reproduction 73, 127–131.
| Photoperiod and delayed implantation in the northern fur seal (Callorhinus ursinus).Crossref | GoogleScholarGoogle Scholar |
Temte, JL, and Temte, J (1993). Photoperiod defines the phenology of birth in captive California sea lions. Marine Mammal Science 9, 301–308.
| Photoperiod defines the phenology of birth in captive California sea lions.Crossref | GoogleScholarGoogle Scholar |
Thompson, D, and Fedak, MA (2001). How long should a dive last? A simple model of foraging decisions by breath-hold divers in a patchy environment. Animal Behaviour 61, 287–296.
| How long should a dive last? A simple model of foraging decisions by breath-hold divers in a patchy environment.Crossref | GoogleScholarGoogle Scholar |
Trillmich, F, and Limberger, D (1985). Drastic effects of El Niño on Galapagos pinnipeds. Oecologia 67, 19–22.
| Drastic effects of El Niño on Galapagos pinnipeds.Crossref | GoogleScholarGoogle Scholar |
Trites, AW (1990). Thermal budgets and climate spaces: the impact of weather on the survival of Galapagos (Arctocephalus galapagoensis Heller) and northern fur seal pups (Callorhinus ursinus L.). Functional Ecology 4, 753–768.
| Thermal budgets and climate spaces: the impact of weather on the survival of Galapagos (Arctocephalus galapagoensis Heller) and northern fur seal pups (Callorhinus ursinus L.).Crossref | GoogleScholarGoogle Scholar |
Trites, AW (1991). Fetal growth of northern fur seals: life-history strategy and sources of variation. Canadian Journal of Zoology 69, 2608–2617.
| Fetal growth of northern fur seals: life-history strategy and sources of variation.Crossref | GoogleScholarGoogle Scholar |
Trites, AW, and Antonelis, GA (1994). The influence of climatic seasonality on the life cycle of the Pribilof northern fur seal. Marine Mammal Science 10, 311–324.
| The influence of climatic seasonality on the life cycle of the Pribilof northern fur seal.Crossref | GoogleScholarGoogle Scholar |
Trivers, RL, and Willard, DE (1973). Natural selection of parental ability to vary the sex ratio of offspring. Science 179, 90–92.
| Natural selection of parental ability to vary the sex ratio of offspring.Crossref | GoogleScholarGoogle Scholar |
van den Hoff, J, McMahon, CR, Simpkins, GR, Hindell, MA, Alderman, R, and Burton, HR (2014). Bottom-up regulation of a pole-ward migratory predator population. Proceedings of the Royal Society B: Biological Sciences 281, .
| Bottom-up regulation of a pole-ward migratory predator population.Crossref | GoogleScholarGoogle Scholar |
Warneke RM (1982) The distribution and abundance of seals in the Australasian region, with summaries of biology and current research. Mammals in the seas. Report, Vol. 4. Food and Agriculture Organization, Rome, Italy.
Warneke RM, Shaughnessy PD (1985) Arctocephalus pusillus, the South African and Australian fur seal: taxonomy, evolution, biogeography, and life history. In ‘Studies of Sea Mammals in South Latitudes’. (Eds JK Ling, MM Bryden) pp. 53–77. (South Australia Museum: Adelaide, SA, Australia).


